Abstract
Introduction
Chronic hepatitis B (CHB) patients with metabolic syndrome (MetS) may present increased risk of liver-related outcomes (LROs), but prior studies were limited by small sample size and/or conflicting results. Using a systematic review and meta-analytic approach, we aimed to determine the association between MetS and LROs in CHB.
Methods
Two researchers independently screened studies from the PubMed, Embase, Web of Science, and Cochrane Library databases from inception to January 21, 2020, and extracted the data. Estimates were pooled using a random-effects model.
Results
We screened 2,228 articles and included 10 eligible studies (18,360 CHB patients, 2,557 with MetS). MetS was significantly associated with LROs overall (odds ratio = 2.45, 95% confidence interval = 1.39–4.32) but not the individual LRO components but subgroup analyses were limited by small study numbers.
Discussion/Conclusion
MetS is associated with almost 3-folds higher risk of LROs in CHB and should be considered in management decisions. However, additional studies are needed.
Keywords: Hepatocellular carcinoma, Liver fibrosis, Prognosis, Mortality, Diabetes
Introduction
Affecting more than 250 million individuals worldwide, chronic infection with the hepatitis B virus (HBV) is a leading cause of adverse liver-related outcomes (LROs) [1]. Potential risk factors for the development and progression of cirrhosis and hepatocellular carcinoma (HCC) in chronic hepatitis B (CHB) depend on several patients and viral characteristics [2, 3]. Long-term therapy with nucleos(t)ide analogues is effective in suppressing viral replication and reduce but not eliminate adverse LROs in CHB patients, especially in certain higher risk patient subgroups [4]. With the rising obesity epidemic, metabolic syndrome (MetS), comprised of central obesity, hypertension, glucose intolerance, and dyslipidemia, has become one of the most challenging public health problems around the globe. MetS affects not only the cardiovascular system but also the liver [5]. Taken together, both CHB and MetS are important health problems across the world. The prevalence of combined CHB and MetS in the general population is 0.99–1.74% [6, 7, 8], but it varies geographically depending on whether the area is endemic for HBV infection and/or MetS.
While the mechanisms of the interaction between MetS and CHB remain uncertain, studies in different CHB populations have demonstrated that hepatic steatosis (HS), increased body mass index, diabetes, or a combination of different metabolic risk factors have suggested an increased risk of HCC and/or cirrhosis among CHB patients with MetS [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. However, prior studies were of mostly small sample size or of conflicting results. Recent meta-analyses have described the relationships between MetS and HCC and liver-related events in the general population or in mixed populations with different liver disease etiologies, though none have focused specifically on the relationship between MetS and CHB [22, 23]. Therefore, we aimed to use a systematic review and meta-analytic approach to evaluate the associations between MetS and LROs including liver fibrosis, HCC, and mortality among patients with CHB.
Methods
Study Design and Search Strategy
We conducted this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (online suppl. Table 1; see www.karger.com/doi/10.1159/000521768 for all online suppl. material). All literature search, article screening, data extraction, and quality assessment were performed independently by two researchers. Discrepancies were resolved by discussion and consensus; and if necessary, a third reviewer was involved.
We searched PubMed, EMBASE, Web of Science, and the Cochrane Library databases from inception to January 21, 2020, and without language restriction for relevant studies. We used search strategies based on hepatitis B and MetS as developed in collaboration with a medical librarian (CDS) from the Stanford Lane Library (Palo Alto, CA, USA) as below.
PubMed
(“hepatitis b” [mesh] OR hbv [ti] OR “hepatitis b” [tw]) AND (“metabolic syndrome” [mesh] OR “metabolic syndrome” [tw] OR “syndrome x” [tw] OR “Metabolic X Syndrome” [tw] OR “Metabolic Cardiovascular Syndrome” [tw] OR “insulin resistance” [tw] OR “insulin resistance” [mesh] OR diabet* [ti] OR diabet* [ot] OR “diabetes Mellitus” [mesh]) NOT (“animals” [mesh] NOT “Humans” [mesh]) NOT “letter” [pt] NOT “case reports” [pt].
Embase
(“hepatitis b”/exp OR “hepatitis b”:ti, kw OR “hepatitis b”:ti, kw OR hbv*:ti) AND (“metabolic syndrome X”/exp OR “metabolic syndrome”:ti, ab, kw OR “syndrome x”:ti, ab, kw OR “Metabolic X Syndrome”:ti, ab, kw OR “Metabolic Cardiovascular Syndrome”:ti, ab, kw OR “insulin resistance”:ti, ab, kw OR diabet*:ti, kw OR ′insulin resistance”/exp OR ′diabetes mellitus”/exp) NOT (“case report”/exp OR “letter”/exp) AND ([embase]/lim NOT ([embase]/lim AND [medline]/lim) OR ([embase classic]/lim NOT ([embase classic]/lim AND [medline]/lim))) AND “human”/de.
Cochrane Library
(“hepatitis b” OR “hepatitis b” OR hbv*) AND (“metabolic syndrome” OR “syndrome x” OR “Metabolic X Syndrome” OR “Metabolic Cardiovascular Syndrome” OR “insulin resistance” OR diabet*)
Web of Science
(“hepatitis b” OR “hepatitis b” OR hbv*) AND (“metabolic syndrome” OR “syndrome x” OR “Metabolic X Syndrome” OR “Metabolic Cardiovascular Syndrome” OR “insulin resistance” OR diabet*)
Study Selection
The inclusion criteria for studies were as follows: (1) the studies were original human research studies published as full-length articles; (2) the study participants were 18 years of age or older who had CHB defined with positive hepatitis B surface antigen for at least 6 months and without other viral coinfection at baseline; (3) the study included participants with MetS defined by the International Diabetes Federation or National Cholesterol Education Program ATP-III criteria [24, 25], and (4) the study reported LROs such as HS, liver fibrosis, cirrhosis, HCC, and death.
The exclusion criteria for studies were as follows: (1) the study was irrelevant as it failed to match the inclusion criteria such as animal study, cell study, pediatric study, meeting abstract, case report, clinical trial, letter, author's reply, editorial, systematic review/meta-analysis, review article, and guideline; (2) the study did not exclude other causes of liver disease (e.g., viral hepatitis C or human immunodeficiency virus coinfection, autoimmune hepatitis, Wilson's disease); (3) the study did not report screening for excess alcohol consumption; (4) duplicate publications from the same cohort; and (5) the study sample size was less than 25. For cohort with overlapping populations, we included the article that included the largest cohort, the most recent publication, and/or the publication with the most detailed and relevant data.
Data Extraction
From each retrieved article, the following data were extracted and recorded within a case report form developed for this study: name of the first author, year of publication, country where the study was performed, study period, study center, sample size, population characteristics (e.g., mean/median age, sex distribution, comorbidities), number of cases, definition of MetS, specific outcomes and risk estimates and their 95% confidence intervals (CIs) (presence vs. absence of MetS), and study outcome measurements.
Quality Assessment
We used a scale based on the Newcastle-Ottawa quality assessment scale for assessing the quality of nonrandomized studies in a meta-analysis [26] to assess the quality of individual reports. The Newcastle-Ottawa quality assessment scale is a scoring tool comprising 7 items with 9 scores that assesses how well the investigators selected their participants (score ranges from 0 to5), how comparable their results may be (score ranges from 0 to 1), and how applicable the outcomes are (score ranges from 0 to 2). The higher the score, the greater the quality of the study, the lower the risk of bias study and lower the risk of bias. We categorized the studies as good quality if the total score was 7 or more, fair if the score was 4–6, and poor if the score was <4 (online suppl. Table 2).
Statistical Analysis
The random-effect model was used to calculate the pooled odds ratio (OR) and 95% CI for the association between MetS and risk of liver-related complications. The Cochran's Q test and I2 statistics were used to evaluate the heterogeneity among the included studies [27]. Heterogeneity with I2 of 50% or more was considered significant. Potential publication bias was assessed by visual inspection of the funnel plots, where an asymmetric plot suggests possible publication bias.
Results
Study Selection and Study Characteristics
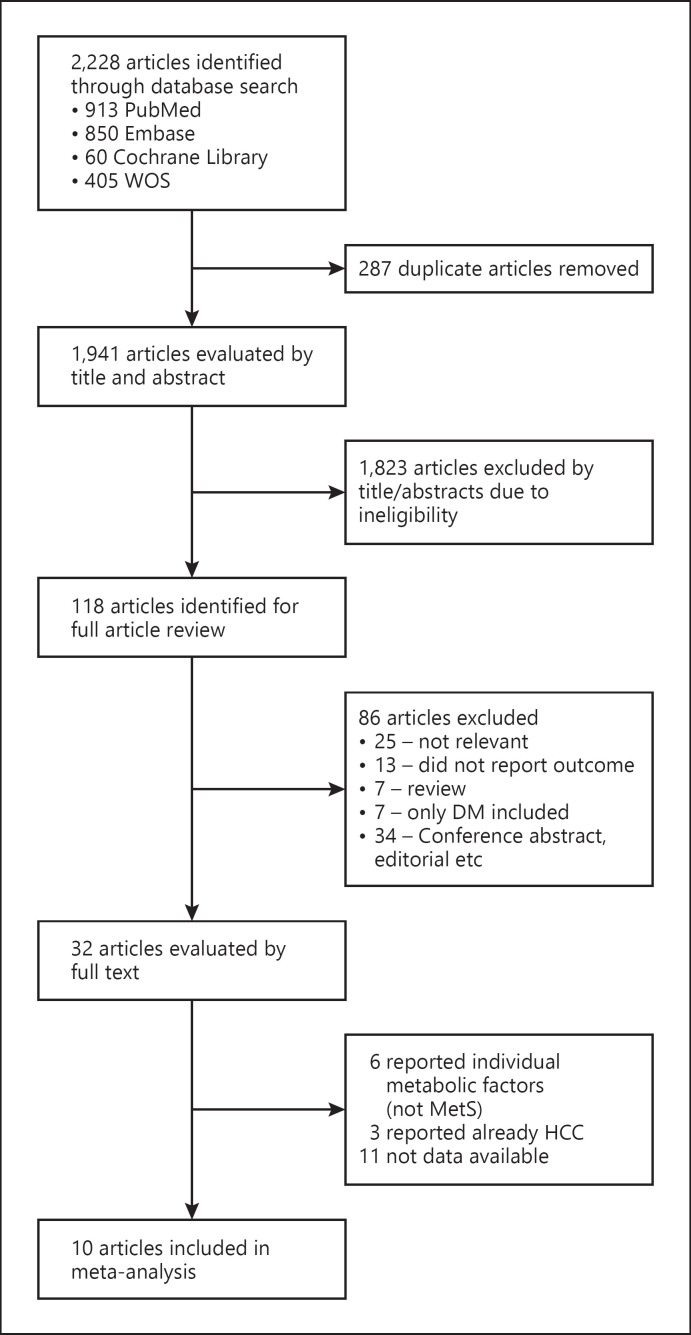
A total of 2,228 articles were identified by the initial literature search (PubMed: 931 articles; Embase: 1,941 articles; Cochrane Library: 60 articles; and Web of Science: 405 articles). As shown in the flow diagram (Fig. 1), 287 duplicate articles were excluded. We reviewed titles and abstracts of 1,941 articles and excluded 1,823 articles that did not meet our study inclusion/exclusion criteria, leaving a total of 118 articles for full-text screening. Of these, we excluded 86 studies that did not meet our study inclusion/exclusion criteria, leaving a total of 32 articles for full-text review and data extraction. During this process, 22 studies were excluded: 11 due to insufficient data, 3 due to inclusion of patients with existing HCC, 6 without sufficient data on MetS, and 2 for overlapping cohorts.
Fig. 1.
Systematic literature search and screening for outcomes of CHB in patients with MetS.
In total, we included and analyzed data from 10 eligible studies [7, 18, 28, 29, 30, 31, 32, 33, 34, 35] involving 18,360 CHB patients, and of these, 2,557 (13.9%) individuals had MetS. Detailed characteristics of the 10 studies are summarized in the supplemental file (Table 1). Briefly, four studies were retrospective studies, four studies were prospective studies, one study was cross-sectional study, and one study had a mixed study design. Five studies were conducted in an outpatient setting, one study was conducted in an inpatient setting, one study was conducted in a mixed setting, and study setting was not reported for three studies. Nine studies were conducted in Asia (China, Taiwan, Korea, Hong Kong), and one study was conducted in Europe (Spain). The individual study sample sizes were less than 1,000 in five studies and greater than 1,000 in the remaining five studies. The pooled patient mean age was 45.81 years, and the pooled proportion of male participants was 54.91.
Table 1.
Characteristics of included studies
| Authors (year) | Country/region | Study design | Setting | Outcomes | Sample size | Mean age | Male, % | Definition of MetS | Criteria for alcohol use exclusion |
|---|---|---|---|---|---|---|---|---|---|
| Cal [28] | China | Retrospective | Unknown | Liver fibrosis | 1,236 | 42.71 | 72 | IDF | >30 g/day |
| Liver steatosis | |||||||||
| Chen [29] | Taiwan | Retrospective | Unknown | HCC | 5,606 | − | 34.25 | NCEP | Exact amount not mention |
| Kim [30] | Korea | Retrospective | Outpatient | HCC | 1,696 | 50.00 | 40.92 | NCEP | Exact amount not mention |
| Mena [31] | Spain | Cross-sectional | Outpatient | Liver fibrosis | 96 | 46.00 | 66.67 | NCEP | >30 g/day |
| Cirrhosis | |||||||||
| Seto [18] | Hong Kong | Prospective | Outpatient | Fibrosis regression | 123 | 56.9 | 77.24 | IDF | >20 g/day |
| Shi [32] | China | Retrospective | Inpatient | Liver fibrosis | 136 | 34.52 | 75.74 | NCEP | Alcoholic liver disease was excluded |
| Wong [7] | Hong Kong | Prospective | Outpatient | Liver cirrhosis | 1,466 | 46.00 | 64.05 | IDF | >30 g/day |
| Yoon [33] | Korea | Mixed | Mixed | Liver fibrosis | 850 | 43.2 | 63.29 | IDF | ≥20 g/day |
| Kim [34] | Korea | Retrospective | Unknown | Liver-related death | 587 | 48.2 | 65.42 | IDF | ≥20 g/day |
| Tan [35] | China | Prospective | Outpatient | HCC | 6,564 | 45.42 | 67.85 | IDF | >80 g/day 3 times a wk |
| All studies | − | − | − | − | 18,360 | 45.81 | 54.91 | − | − |
IDF, International Diabetes Federation; NCEPT, National Cholesterol Education Program.
No studies were graded as poor quality. One study had fair quality score of 6 and the remaining had good quality scores of 8–9 (online suppl. Table 2).
Association between MetS and LROs
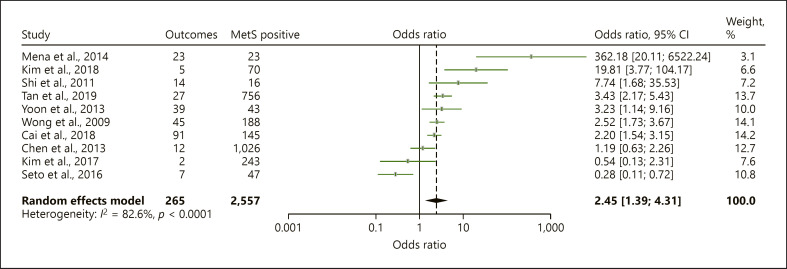
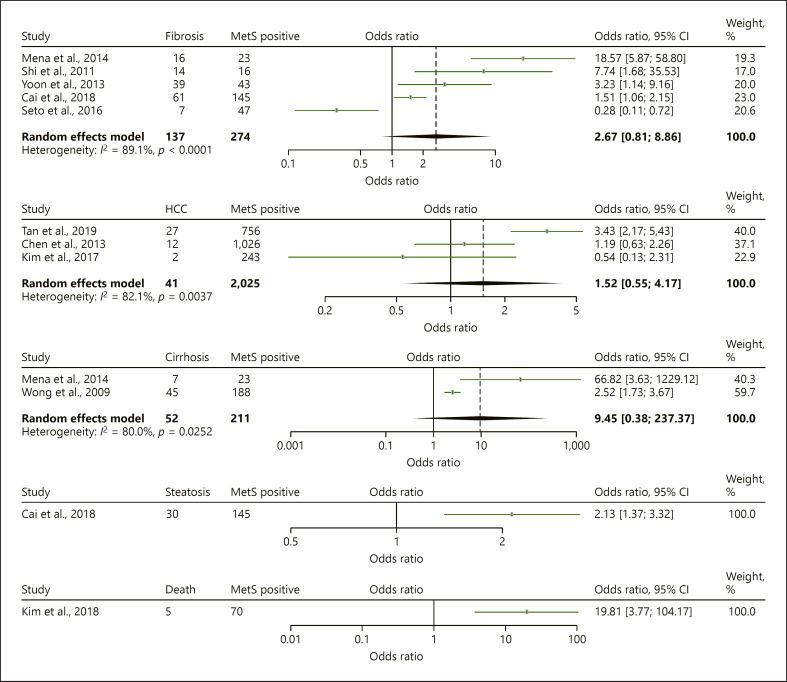
In the overall analysis, the presence of MetS in CHB patients was significantly associated with 2.45-folds increased risk of LROs (OR = 2.45, 95% CI = 1.39–4.32) though the heterogeneity of the pooled analysis was considerable (I2 = 82.6%, p < 0.0001) (Table 2, Fig. 2). Subgroup analysis was performed to assess pooled ORs for the association between MetS and the individual liver outcomes with results described as below (Table 2; Fig. 3). The overall association between MetS and LROs remains significant after a less severe outcome such as steatosis was removed (OR, 2.66, 95% CI 1.29–5.48, I2 84.5, 9 studies, 17,124 patients). Heterogeneity of the individual subgroup analyses was also considerable (Table 2).
Table 2.
Overall and subgroup analyses for association between MetS and LROs in patients with CHB
| LROs | Studies (total patients), n | Test of association |
Test of heterogeneity |
||
|---|---|---|---|---|---|
| OR | 95%CI | I2 (%) | p value | ||
| Overall | 10 (18,360) | 2.45 | 1.39–4.32 | 82.6 | <0.0001 |
| Fibrosis | 5 (2,441) | 2.67 | 0.81–8.86 | 89.1 | <0.0001 |
| HCC | 3 (13,866) | 1.52 | 0.55–4.17 | 82.1 | 0.0037 |
| Cirrhosis | 2 (1,562) | 9.45 | 0.38–237.37 | 80.0 | 0.025 |
| Steatosis | 1 (1,236) | 2.13 | 1.37–3.32 | N/A | N/A |
| Death | 1 (587) | 19.81 | 3.7–104.17 | N/A | N/A |
Fig. 2.
Forest plot for association of HBV patients with MetS and LROs.
Fig. 3.
Forest plot for subgroup analysis.
Association between MetS and Liver Fibrosis
There were five studies reporting the relationship between MetS and liver fibrosis [18, 28, 31, 32, 33]. Two studies involving 1332 CHB patients reported that the proportion of fibrosis increased with an increasing number of components of MetS [28, 31]. Fibrosis was assessed with liver stiffness measurement through transient elastography and considered significant fibrosis (≥F2) at liver stiffness of >7.4 kPa in one study and >7.5 kPa in the other study. One of them was cross-sectional study including CHB inactive carriers. The two retrospective studies described MetS was associated with severity of fibrosis in histology finding [32, 33]. Seto et al. [18] studied 123 nucleoside analogue-treated CHB patients with severe fibrosis and performed follow-up assessment for fibrosis regression measured with transient elastography. Fibrosis regression to liver stiffness of <6 kPa, defined as minimal hepatic fibrosis, was the primary outcome of the study. The authors defined severe liver fibrosis as liver stiffness >9–12 kPa with normal ALT and with ALT >1–5 × ULN, liver stiffness >12–13.4 kPa. Liver stiffness >12 kPa with normal ALT or >13.4 kPa with ALT >1–5 × ULN was defined as cirrhosis. The mean age of this study was 56.9 years and the majority of patients were male (72%). The study found the lower rate of fibrosis regression in patients with MetS. Although the first four studies were shown the positive association between MetS and the risk of liver fibrosis, the combination of 5 studies resulted in no significant association between MetS with risk of liver fibrosis (OR = 2.67, 95% CI = 0.81–8.86), probably due to opposite outcome measurement of the Seto et al. [18] study.
Association between MetS and HCC
Two retrospective and one prospective cohorts were included for the outcome of HCC development among CHB patients with or without MetS [29, 30, 35]. The number of participants of the two retrospective studies was 5,606 and 1,696 with HCC development of 1.01% and 1.4%, respectively [29, 30]. Both studies were conducted in health checkup clinics, and both studies found that MetS was not a risk factor for HCC development in CHB patients.
In the prospective study of Tan et al. [35] after a 45,668.0 person-year follow-up of 6,564 subjects (mean 76.0 ± 30.8 months) from an initial cohort of 105,397 civil servants, 89 incident HCC cases were identified. The study cohort included 4,454 (67.9%) males and had an average age of 45.4 years. In this study, 780 individuals had cirrhosis (11.9%). This study found that MetS was independently associated with more than 2-fold increased HCC risk (HR, 2.25; 95% CI, 1.41–3.60) after adjusting for age (in 1-year increments), sex, cigarette smoking, alcohol consumption, liver cirrhosis, and elevated aspartate aminotransferase levels (≥40 U/L) [35]. As a result, the pooled estimate of these three studies showed MetS had no significant association with the risk of HCC development (OR = 1.52, 95% CI = 0.55–4.17).
Association between MetS and Cirrhosis
We found only two studies that reported on the risk of cirrhosis development [7, 31]. One was a prospective study and one was cross-sectional study. The total number of patients for this subgroup analysis was 1,562 CHB patients. The studies defined probable cirrhosis as liver stiffness measurement ≥13.4 kPa and >11.8 kPa with transient elastography, respectively. Each study stated that MetS was associated with cirrhosis development, but the pooled estimate showed that the presence of MetS was not significantly associated with the development of cirrhosis (OR = 9.45, 95% CI = 0.38–237.37). This is likely because the pooled estimate was based on data from only two studies and thus unreliable. One study provided stratified data for HBeAg status which found that the MetS was significantly associated with cirrhosis regardless of HBeAg status [7].
Association between MetS and Other Outcomes
We identified only one study that used transient elastography to assess HS which was defined as controlled attenuation parameter values >310 dB/m [28]. Controlled attenuation parameter was found to increase progressively with the number of MetS components (OR = 2.13, 95% CI = 1.37–3.32).
There was also only one study reporting retrospectively the overall survival in 587 CHB patients who started oral nucleos(t)ide analogue [34]. The mean age of the study population was 48.2 (+/−10.4) years, and the proportion of male patients was 65.4%. Overall survival was significantly shorter in CHB patients with MetS than in those without MetS (aHR, 12.29, 95% CI = 2.25–67.24, p < 0.001). There were 7 liver-related deaths in the study cohort during the study observation period (5 in CHB patients with MetS and 2 in CHB patients without MetS), and they were all due to hepatic failure. The cumulative occurrence rates of viral breakthrough, genotypic resistance, HCC, disease progression, and/or overall adverse outcomes were also significantly higher in CHB patients with MetS than in those without MetS (p < 0.001).
Publication Bias
Funnel plot was performed to assess the publication bias in the meta-analysis. Funnel plots' shape of all contrasts revealed asymmetry (online suppl. Fig. 1). Thus, the result suggested there was some publication bias.
Discussion/Conclusion
In this meta-analysis, we were able to identify only 10 publications, all but one was good quality. Overall pooled estimate showed the presence of MetS in CHB patients to significantly associate with higher risk of liver-related complications when compared with CHB patients without MetS. Given the known association between MetS and nonalcoholic fatty liver disease (NAFLD) and the association between NAFLD and LROs, the association between MetS and LROs in patients with an existing liver disease such as CHB is highly relevant, as both CHB and NAFLD are highly prevalent globally. However, there was no association between MetS and individual outcome in subgroup analysis. This could be due to smaller number of patients in each specific outcome and heterogenicity of the studies. Studies also tend to show conflicting results.
Regarding the outcome of liver fibrosis, four studies [28, 31, 32, 33] reported that MetS were associated with liver fibrosis, and one study [18] identified the presence of MetS was associated with lower rate of fibrosis regression than those without MetS. On the other hand, some studies found the more components present in the CHB patient, the higher the potential for liver fibrosis [31]. Therefore, viral factors are not the only factors influencing the progression to fibrosis. Even in active HBV patients taking antivirals for HBV, fibrosis regression rates were also lower among patients with presence of MetS than without MetS from univariate analysis. Thus, clinicians may need to also monitor intervention for MetS in CHB patients with MetS because virologic suppression via nucleoside analogue therapy alone may not be sufficient to improve the LRO in CHB patients with MetS.
Regarding HCC outcome, while the pooled analysis did not find a significant association between MetS and HCC, this finding was based on limited data. In this analysis, while two retrospective studies did not find significant association [29, 30], one large prospective study of 6,564 patients found a 2.25-fold increased HCC risk in CHB individuals with MetS relative to those without MS after adjusting for age, sex, cigarette smoking, habitual alcohol consumption, elevated AST levels, and cirrhosis (HR = 2.12, CI = 1.16–3.89) [35]. Therefore, further studies are needed to better evaluate the association between MetS and HCC in CHB patients since if positively assocaited, MetS is a potentially modifiable so active intervention for MetS should become part of CHB management alongside antiviral therapy in the prevention of HCC.
As also noted earlier, while the association between MetS and LROs overall was statistically significant, the association between MetS and the individual liver outcome was not though the measurement of association all tended toward a positive association between MetS and each outcome with OR >1.0. Except for HCC, both the number of patients and number of studies were few and may be underpowered to identify a statistically significant association even if the association may have been “true.” Even in the case of HCC, while the total number of patients was large, these patients came from only 3 studies that were significantly heterogeneous, thus limiting its conclusion. As such, further studies are needed to evaluate the association between MetS and the individual liver outcome.
Recent data have suggested that patients with CHB have aged globally in the past decade [36, 37, 38, 39, 40], and as such, the prevalence of CHB patients with comorbidities including MetS has increased substantially, furthering highlighting the need for further research in this area. Additionally, data regarding the underlying biologic mechanisms between chronic HBV infection and MetS remains sparse. The possible mechanisms are as follows: the connective tissue growth factor induced by hyperglycemia and hyperinsulinemia in MetS could potentially encourage the development of fibrosis and cirrhosis with direct stimulation of liver stellate cells resulting accumulation of extracellular matrix [41]. Hyperinsulinemia may also increase the risk of HCC, via the effects insulin-like growth factor and by increasing the production of cytokines and mitogens, increasing fibrosis, and promoting angiogenesis [42].
Limitations of This Study
The number of the included cohort studies was rather few. Therefore, results of subgroup analyses should be interpreted with caution, and large-scale cohort studies are needed to confirm these findings. Second, although most-adequately adjusted results were pooled, we could not exclude other factors, such as the treatment against the components of MetS, nonsignificant alcohol consumption as well as the effect of antiviral therapy for CHB, which may confound the association between MetS and increased risk of complications. There was also publication bias as well as significant heterogeneity in most analysis. Additionally, nine of the studies were from Asia and only 1 was outside Asia, and this may limit the generalizability of the study since the prevalence of MetS differs geographically around the world. Finally, study level data were heterogeneous, and the results of the current study should be interpreted with caution.
Discussion/Conclusion
Results of our meta-analysis showed that the presence of MetS is significantly associated with increased risk of liver-related complications when compared with those without MetS at baseline. For CHB patients with MetS, modification of lifestyle and potential therapeutic intervention for MetS can help reduce the risk of progression to end stage liver stage. However, our results should be interpreted with caution due to the small number of available studies in the existing literatures and the heterogeneity of the included study cohorts. Further research is needed to better characterize the association between MetS and long-term outcomes of CHB.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
Mindie Nguyen has served as a consultant or in advisory board for Spring Bank, Novartis, Gilead, Intercept, Exact Sciences, Laboratory of Advanced Medicine, Eisai, and Bayer and has received grant/research support from Gilead, Pfizer, Enanta, Vir, National Cancer Institute, B. K. Kee Foundation, and Glycotest. All others: none to declare.
Funding Sources
There are no funding sources to declare.
Author Contributions
Guarantor of article: Mindie H. Nguyen. Study concept and study supervision: Mindie H. Nguyen and Ramsey C. Cheung. Study design: Khin Naing Thin, Ramsey C. Cheung, and Mindie H. Nguyen. Data collection and/or data interpretation: all authors. Data analysis: Eunice Yewon Lee, Andrew Tran, Ramsey C. Cheung, and Mindie H. Nguyen. Manuscript drafting: Khin Naing Thin, Andrew Tran, Ramsey C. Cheung, and Mindie H. Nguyen. Manuscript edition and final approval: all authors. All authors identified above have critically reviewed the paper and approved the final version of this paper, including the authorship statement.
Data Availability Statement
All relevant data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgment
The authors wish to thank B. K. Kee Foundation for their support of the Myanmar Liver Scholar Fellowship (KNT).
Funding Statement
There are no funding sources to declare.
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386((10003)):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003 Dec;362((9401)):2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 4.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381((9865)):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135((1)):111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Xin Li JZ, Ren N, Ling W, Chen F, Zang W, Fan X, et al. Adiponectin and its receptors in chronic hepatitis B patients with metabolic syndrome in China. Hepatogastroenterology. 2012;59((118)):1735–1743. doi: 10.5754/hge10717. [DOI] [PubMed] [Google Scholar]
- 7.Wong GLH, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, et al. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58((1)):111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 8.Wong VWS, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, et al. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56((3)):533–540. doi: 10.1016/j.jhep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5((1)):95. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiang JC, Gane EJ, Bai WW, Gerred SJ, John C, Gane EJ, et al. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol. 2015;30((3)):591–599. doi: 10.1111/jgh.12790. [DOI] [PubMed] [Google Scholar]
- 11.Huang YW, Wang TC, Lin SC, Chang HY, Chen DS, Hu JT, et al. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin Infect Dis. 2013;57((12)):1695–702. doi: 10.1093/cid/cit603. [DOI] [PubMed] [Google Scholar]
- 12.Hui RWH, Seto WK, Cheung KS, Mak LY, Liu KSH, Fung J, et al. Inverse relationship between hepatic steatosis and hepatitis B viremia: results of a large case-control study. J Viral Hepatitis. 2018;25((1)):97–104. doi: 10.1111/jvh.12766. [DOI] [PubMed] [Google Scholar]
- 13.Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology. 2017;65((3)):828–835. doi: 10.1002/hep.28917. [DOI] [PubMed] [Google Scholar]
- 14.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66((5)):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Khalili M, Lombardero M, Chung RT, Terrault NA, Ghany MG, Kim WR, et al. Diabetes and prediabetes in patients with hepatitis B residing in North America. Hepatology. 2015;62((5)):1364–1374. doi: 10.1002/hep.28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol Metab. 2015 Mar;26((3)):153–161. doi: 10.1016/j.tem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serfaty L. Metabolic manifestations of hepatitis C virus: diabetes mellitus, dyslipidemia. Clin Liver Dis. 2017;21((3)):475–486. doi: 10.1016/j.cld.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Seto WK, Fung J, Cheung KS, Mak LY, Hui RW, Liu KS, et al. Body-mass index is associated with fibrosis regression during long-term nucleoside analogue therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2016;44((10)):1071–1079. doi: 10.1111/apt.13804. [DOI] [PubMed] [Google Scholar]
- 19.Seto WK, Hui RWH, Mak LY, Fung J, Cheung KS, Liu KSH, et al. Association between hepatic steatosis, measured by controlled attenuation parameter, and fibrosis burden in chronic hepatitis B. Clin Gastroenterol Hepatol. 2018;16((4)):575–83.e2. doi: 10.1016/j.cgh.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Wong GLH, Chan HL, Yu Z, Chan AW, Choi PC, Chim AM, et al. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B: a prospective cohort study with paired transient elastography examinations. Aliment Pharmacol Ther. 2014;39((8)):883–893. doi: 10.1111/apt.12658. [DOI] [PubMed] [Google Scholar]
- 21.Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26((34)):5576–5582. doi: 10.1200/JCO.2008.16.1075. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Li X, Wu S, Ye W, Lou L. Metabolic syndrome and the incidence of hepatocellular carcinoma: a meta-analysis of cohort studies. Onco Targets Ther. 2018;11:6277–6285. doi: 10.2147/OTT.S154848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren H, Wang J, Gao Y, Yang F, Huang W. Metabolic syndrome and liver-related events: a systematic review and meta-analysis. BMC Endocr Disord. 2019;19((1)):40. doi: 10.1186/s12902-019-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.“Consensus statements.”. Accessed: 2021 Jan 2. [Online]. Available: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html.
- 25.National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii), “third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec;106((25)):3143–421. [PubMed] [Google Scholar]
- 26.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. Clin Epidemiol. 2014;7 [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep;327((7414)):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai S, Ou Z, Liu D, Liu L, Liu Y, Wu X, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United European Gastroenterol J. 2018 May;6((4)):558–566. doi: 10.1177/2050640617751252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CT, hen JY, Wang JH, Chang KC, Tseng PL, Kee KM, et al. Diabetes mellitus, metabolic syndrome and obesity are not significant risk factors for hepatocellular carcinoma in an HBV- and HCV-endemic area of Southern Taiwan. Kaohsiung J Med Sci. 2013;29((8)):451–459. doi: 10.1016/j.kjms.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Sinn DH, Gwak GY, Kang W, Paik YH, Choi MS, et al. Insulin resistance and the risk of hepatocellular carcinoma in chronic hepatitis B patients. J Gastroenterol Hepatol. 2017;32((5)):1100–1106. doi: 10.1111/jgh.13647. [DOI] [PubMed] [Google Scholar]
- 31.Mena Á, Pedreira JD, Castro Á, López S, Vázquez P, Poveda E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol. 2014 Jan;29((1)):173–178. doi: 10.1111/jgh.12432. [DOI] [PubMed] [Google Scholar]
- 32.Shi JP, Xun YH, Su YX, Jiang YM, Zhang L, Hu CB, et al. Metabolic syndrome and severity of fibrosis in patients with chronic viral hepatitis B infection or non-alcoholic fatty liver disease. AJMR. 2011 Mar;5((5)):481–485. [Google Scholar]
- 33.Yoon H, Lee JG, Yoo JH, Son MS, Kim DY, Hwang SG, et al. Effects of metabolic syndrome on fibrosis in chronic viral hepatitis. Gut Liver. 2013 Jul;7((4)):469–474. doi: 10.5009/gnl.2013.7.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim NH, Cho YK, Kim BI, Kim HJ. Effect of metabolic syndrome on the clinical outcomes of chronic hepatitis B patients with nucleos(t)ide analogues treatment. Dig Dis Sci. 2018;63((10)):2792–2799. doi: 10.1007/s10620-018-5165-6. [DOI] [PubMed] [Google Scholar]
- 35.Tan Y, Zhang X, Zhang W, Tang L, Yang H, Yan K, et al. The influence of metabolic syndrome on the risk of hepatocellular carcinoma in patients with chronic hepatitis B infection in Mainland China. Cancer Epidemiol Biomarkers Prev. 2019 Dec;28((12)):2038–2046. doi: 10.1158/1055-9965.EPI-19-0303. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen MH, Lim JK, Burak Ozbay A, Fraysse J, Liou I, Meyer N, et al. Advancing age and comorbidity in a US insured population-based cohort of patients with chronic hepatitis B: hepatology. Hepatology. 2019 Mar;69((3)):959–973. doi: 10.1002/hep.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong GL, Wong VW, Yuen BW, Tse YK, Luk HW, Yip TC, et al. An Aging population of chronic hepatitis B with increasing comorbidities: a territory‐wide study from 2000 to 2017. Hepatology. 2020 Feb;71((2)):444–455. doi: 10.1002/hep.30833. [DOI] [PubMed] [Google Scholar]
- 38.Yotsuyanagi H, Kurosaki M, Yatsuhashi H, Lee IH, Ng A, Brooks-Rooney C, et al. Characteristics and healthcare costs in the aging hepatitis B population of Japan: a nationwide real-world analysis. Dig Dis. 2021 Mar;40((1)):68–77. doi: 10.1159/000515854. [DOI] [PubMed] [Google Scholar]
- 39.Oh H, Jun DW, Lee IH, Ahn HJ, Kim BO, Jung S, et al. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment Pharmacol Ther. 2020 Jul;52((2)):371–381. doi: 10.1111/apt.15867. [DOI] [PubMed] [Google Scholar]
- 40.Tseng CH, Hsu YC, Ho HJ, Nguyen MH, Wu CY. Increasing age and nonliver comorbidities in patients with chronic hepatitis b in taiwan: a nationwide population-based analysis. Dig Dis. 2021;39((3)):266–274. doi: 10.1159/000511585. [DOI] [PubMed] [Google Scholar]
- 41.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001 Oct;34((4)):738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 42.Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015 Oct;35((10)):2203–2217. doi: 10.1111/liv.12903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All relevant data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.