Abstract
The complexity of cancer immunotherapy (CIT) demands reliable preclinical models to successfully translate study findings to the clinics. Non-human primates (NHPs; here referring to rhesus and cynomolgus macaques) share broad similarities with humans including physiology, genetic homology, and importantly also immune cell populations, immune regulatory mechanisms, and protein targets for CIT. Furthermore, NHP naturally develop cancers such as colorectal and breast cancer with an incidence, pathology, and age pattern comparable to humans. Thus, these tumor-bearing monkeys (TBMs) have the potential to bridge the experimental gap between early preclinical cancer models and patients with human cancer.
This review presents our current knowledge of NHP immunology, the incidence and features of naturally-occurring cancers in NHP, and recent TBM trials investigating CIT to provide a scientific rationale for this unique model for human cancer.
Keywords: immunotherapy; biomarkers, tumor; drug evaluation, preclinical; gastrointestinal neoplasms; breast neoplasms
The role of non-human primates in medical research
Research with NHPs has facilitated breakthrough medical research and preserved millions of human lives.1 The high level of genetic similarity to humans coupled with their outbred nature have made non-human primates (NHPs) an invaluable preclinical model. Studies of NHPs should be ethically restricted to only the most clearly necessary and well-justified work, and indeed this is the case, with NHPs accounting for less than 1% of animals in current medical research.
NHP models have proven indispensable in fields such as neuroscience, reproductive biology, and oncology. The greatest contribution of NHP models in recent years is in immunology, as exemplified by the study of simian immunodeficiency virus (SIV) as an animal model for HIV and AIDS.2
In oncology research, healthy, tumor-free NHPs are an integral part of the drug development process in dose-range finding and toxicology studies.3 4
Recent studies addressing the complexity of cancer immunotherapy (CIT) have illustrated key challenges,5 including (1) the development of preclinical models translatable to human anticancer immunity; (2) the assessment of CIT combinations in early-phase clinical studies; and (3) the prevention of off-target normal tissue injury. These challenges can be addressed by the study of NHPs with naturally occurring malignancies, so called tumor-bearing monkeys (TBMs).
This review provides an overview on NHPs as a uniquely useful model, mimicking human immunology and cancer incidence, and describes the opportunity to investigate promising CIT drug regimens in TBMs.
NHP models of human immunology
NHPs have been an indispensable resource in the dissection of pathogenic mechanisms and in testing vaccine efficacy to many human pathogens, emphasizing important similarities between the human and NHP immune systems.6
Adaptive immunity
The efficacy of the T-cell response relies on the presence of multiple major histocompatibility complex (MHC) alleles that can present a large pool of peptides for potential T-cell recognition.7 Humans have three MHC class I loci: human lymphocyte antigens (HLA) A, B, and C; and three MHC class II loci: HLA-DP, DQ, and DR and each locus can encode multiple alleles.8 Diversity within the human MHC loci is based on amino acid differences as well as the inheritance of specific sets of HLA molecules from each parent with little crossover. Rhesus macaques have two MHC class I loci: Mamu-A and Mamu-B whereas an HLA-C orthologue is missing,9 and three MHC class II loci: DP, DQ, and DR.10 Unlike humans, rhesus macaques can have several MHC class I alleles on each chromosome and significant crossover can occur which results in an increased haplotype diversity.
Human and NHP CD4 and CD8 T cells can be divided into naïve, central memory (CM) and effector memory (EM) types11 and similarly delineated using CD28 and CD95 as the primary cell surface markers.12 In both species, naïve cells are defined as CD95negCD28pos, CM cells are defined as CD95posCD28pos, and EM cells are defined as CD95posCD28neg.
Similar to humans, NHP B cells can be identified based on the CD20 and MHC class II expression. Furthermore, rhesus macaque and human B cells can be divided into naïve (IgD+CD27–), unswitched memory (IgD+CD27+), and switched memory (IgD–CD27+) B-cell subsets13 and exhibit an upregulation of CD80, CD86, and CD40 on activation.14
Innate immunity
Several immune cell subsets play a critical role in mediating innate immune responses including dendritic cells (DCs), natural killer (NK) cells, neutrophils and macrophages.
Human DCs can be divided into two distinct populations: (a) myeloid, or conventional, DC (MDC) characterized as CD11cposCD123dim and (b) plasmacytoid DC (PDC) characterized as CD11cnegCD123bright.15 MDC mainly functions to process and present antigens to naïve T cells and produce interleukin (IL)-12 on activation, whereas, PDC produces interferon (IFN)-α in response to viral infection. These two major DC subsets can be identified in rhesus macaques, using the same surface markers as for human DCs. Moreover, NHP PDC exhibits a similar cytokine and functional response as human PDC, including upregulated IFN-α, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1β, and CXCL10 expression on stimulation with herpes simplex virus. Patterns of Toll-like receptor (TLR) expression in macaque and human DCs are similar, but differ substantially from murine DCs.16 PDC from rhesus and humans express TLR7 and TLR9 but not TLR3, TLR4, and TLR8; both respond to TLR stimulation by upregulation of CD86. Rhesus and human MDC express TLR3, TLR4, TLR7, and TLR8 but not TLR9 and respond to TLR agonists by upregulation of CD40 and CD86. In contrast, murine MDC and PCD both express TLR3, TLR4, TLR7, TLR8, and TLR9.
Human NK cells are identified as CD3negCD56pos cells and can be further stratified based on the expression of CD16 and CD56. The majority of blood and spleen resident NK cells are CD16posCD56dim, and are primarily cytotoxic. The remaining NK cells are CD16negCD56bright, and are less focused on directly killing target cells but rather secrete large amounts of inflammatory cytokines.17 Similarly, NK cells in rhesus macaques are CD3neg and can be divided into a major CD16highCD56neg cytolytic population and a minor CD16lowCD56pos cytokine-producing population.18 Similar to human NK cells, all rhesus NK cell subsets express CD2, CD11a, and NKG2A.
NK cells recognize missing and altered self through inhibitory and activating receptors. In humans, recognition of MHC molecules is mediated by the killer inhibitory receptor, a CD94/NKG2 complex. Homologues of the human CD94/NKG2 family members have been identified in rhesus macaques, and have similar stimulatory and inhibitory functions as their human counterparts.19
Considerable discrepancies exist in the phenotypic characterization of macrophages across species, anatomic sites, and disease models, and there are conflicting reports regarding the delineation of myeloid cell subsets.20 Characterization of NHP macrophages, defined as HLA-DR+CD11b+CD68+ leukocytes, has been limited by the availability of cross-reactive reagents and the autofluorescence inherent to macrophages. Macrophages vary widely in phenotype and pro-inflammatory capacity by anatomic location.21 This heterogeneity likely reflects responses to the microenvironments encountered by macrophages in each tissue.
Interestingly, human and NHP macrophages share a primate specific evolutionary-conserved regulation of cathelicidin antimicrobial peptide (CAMP) gene by vitamin D response elements (VDRE).22 In murine monocytes and macrophages, TLR signaling is principally mediated by nitric oxide.23 In contrast, human macrophages upregulate vitamin D receptor and vitamin D-1-hydroxylase to induce CAMP production on TLR activation. The VDRE in the CAMP promoter region is present in humans and NHP and absent in mice, rats and dogs, and is illustrative of the differences in immune system regulation when comparing mice, NHPs, and humans.22
A brief summary on the similarities and differences of the innate and adaptive immune system and immunoregulatory mechanisms in humans and rhesus macaques can be found in table 1.
Table 1.
Comparative immunology reveals widespread similarities between human and rhesus macaque immune cell populations and regulatory mechanisms
| Adaptive immunity | Ref | |
| MHC class I loci | Human: HLA-A, HLA-B, HLA-C. Rhesus macaque: Mamu-A and Mamu-B (no HLA-C orthologue) increased haplotype diversity due to several MHC class I alleles on each chromosome. |
9 |
| MHC class II loci | Human: HLA-DP, HLA-DQ, HLA-DR. Rhesus macaque: Mamu-DP, Mamu-DQ, Mamu-DR. |
10 |
| CD4 and CD8 T cells | Similar classification into naïve (CD95−CD28+), central memory (CD95+CD28+), and effector memory (CD95+CD28−) cells. | 11 12 |
| B cells | Similar identification by CD20 and classification using CD27 and IgD into naïve (IgD+CD27−), unswitched memory (IgD+CD27+), and switched memory (IgD−CD27+) B cells. | 13 |
| Similar upregulation of CD80, CD86, and CD40 on activation. | 14 | |
| Innate immunity | ||
| DCs | Similar DC cell subsets—myeloid (CD11cposCD123dim) and plasmacytoid (CD11cnegCD123bright)—with similar cytokine and functional response. | 15 |
| TLR on DC | Similar TLR expression in PDC (TLR7/9) and MDC (TLR3/4/7/8). | 16 |
| NK cells | Similar populations of a majority of cytolytic (CD16highCD56neg) and a minority of cytokine producing (CD16lowCD56pos) NK cells. | 18 |
| Human and rhesus NK cells express CD2, CD11a, and NKG2A and are CD3neg. | 19 | |
| CD94/NKG2 complex triggers similar stimulatory and inhibitory functions. | 19 | |
| Macrophages | Similar classification as HLA-DR+CD11b+CD68+. | 21 |
| Similar primate-exclusive regulation of cathelicidin antimicrobial peptide by vitamin D response element via vitamin D receptor and vitamin D-1-hydroxylase upregulation. | 22 |
DC, dendritic cells; HLA, human leukocyte antigen; MHC, major histocompatibility complex; NK, natural killer; PDC, plasmacytoid DC; TLR, Toll-like receptor.
Oncology-related studies in ‘tumor-free’ NHPs
The congruencies in physiology, genetics, and immune system make ‘tumor-free’ NHPs an essential model to determine pharmacodynamics (PD) and pharmacokinetics (PK), define dose ranges, and conduct safety studies of common chemotherapeutics and CIT drugs.
Early NHP studies included assessment of small molecule cancer therapies such as vinca alkaloids in rhesus macaques.24 Drug half-life and clearance rates showed strong similarities to those reported in humans demonstrating the utility of the model. A study focusing on irinotecan found that despite a lower metabolic conversion in rhesus macaques, the systemic clearance of irinotecan and the maximum area-under-curve of its metabolite were less different between humans and NHPs than between humans and mice.25
More specific targeting strategies followed, including many antibodies or antibody-like drugs, for example, the PD characterization of the colony stimulating factor 1 receptor (CSF-1R)-targeting antibody emactuzumab in cynomolgus macaques,4 including PK/PD modeling to determine the dosing regimen for translation to a human phase I clinical study. Emactuzumab efficiently reduced CSF-1Rpos macrophages in the liver and the colon of the monkeys, in line with the expected mode-of-action (MoA) of the compound. A dose dependent reduction of dermal macrophages in emactuzumab-treated NHPs further supported the overall PD and MoA of emactuzumab and influenced the design of the human biomarker strategy.
Another key example is the study of CD45 targeting for leukemia and lymphoma therapy in cynomolgus macaques.26 Directly radiolabeled CD45 antibodies demonstrated higher off-target dose deposition due to extended systemic exposure to the radioligand than a pre-targeting approach with anti-CD45 streptavidin fusion protein followed by radio-DOTA-biotin 48 hours later; reducing the radioligand’s time in circulation. This study was a continuation of data generated in pig-tailed macaques given Y90-labeled anti-CD45, which illustrated improved dose deposition to marrow in comparison to liver and lung.27
A recent study investigating a bispecific anti-huPD-1-huGITR-L not only emphasized the high sequence identity between human and cynomolgus macaque programmed cell death protein-1 (PD-1) and glucocorticoid-induced tumor necrosis factor receptor (GITR) but also similar receptor numbers and expression levels in activated T cells in peripheral blood mononuclear cells (PBMCs) and tissues.28 Furthermore, a similar binding affinity and signaling transduction via nuclear factor-kB was observed accompanied by cynomolgus PBMC expansion on treatment with anti-huPD-1-huGITR-L.
Another high-impact study was the testing of an anti-CD20/CD3 T-cell dependent bispecific antibody (BTCT4465A) in tumor-free cynomolgus macaques as prospective treatment for B-cell malignancies.29 NHP were exposed to different doses and application patterns, sampled at multiple time points, and versatile statistical models fitted to the data. PD assessment in NHP illustrated a decline of CD4 and CD8 T cells within 1 hour and reappearance in the blood within 48 hours. This rapid recovery of T cell counts suggested an activation-induced margination while B-cell depletion persisted, suggesting T-cell-mediated killing. Ultimately, data obtained by this NHP study allowed prospective clinical simulations of human plasma PKs.
Another highly relevant anticancer drug extensively tested in macaques is trastuzumab (Herceptin) which was administered to study toxicity on numerous organ systems and included combinatorial regimens.3 A specific justification for NHPs in these studies is the high degree of homology of the erbB2 receptor (HER2), the target of trastuzumab, as demonstrated in cynomolgus macaques. Due to the species-specific action of trastuzumab, neither mice nor dogs could be employed—emphasizing the need for NHPs for toxicity and pharmacologic studies.
Immune checkpoint inhibitors have changed the paradigm of cancer therapy and drastically improved patient outcomes for numerous cancers. Importantly, tumor-free NHP fostered the development and approval of agents such as PD-1-targeting pembrolizumab,30–32 nivolumab,33–36 dostarlimab,37–39 and cemiplimab,40 programmed death ligand-1 (PD-L1)-targeting atezolizumab,41 durvalumab,42 and avelumab,43 44 and cytotoxic T-lymphocytes-associated protein 4-targeting ipilimumab36 45 and tremelimumab.46 These studies and preclinical trials demonstrated similar binding affinities, tissue distribution, and the necessity to use NHP as the only relevant and pharmacologically responsive model.34 For example, primate-specific conserved amino acids (eg, Arg95) in PD-L1 are crucial for binding of durvalumab and are absent in rodents.42
In general, the increasing need for NHP studies reflects the increasing specificity of cancer treatments. Less-specific chemotherapeutics such as vinca alkaloids, topoisomerase inhibitors, or anthracyclines act comparably in different species. However, the future of oncology is precision medicine aiming for disease-specific or even patient-specific targets to eradicate malignant cells, consequently demanding more complex models that can better reflect the activity of the clinical candidate as compared with the use of surrogates in less relevant animal models.
Cancer risk factors in NHP
Similarities between NHPs and humans extend to almost all aspects of anatomy, physiology, neurology, endocrinology, immunology and age-related disease. Macaques are the most commonly used long-lived primates in biomedical research47 with approximately 70,000 rhesus macaques living in the USA. Rhesus macaques age at roughly three times the rate of humans, and a significant increase in cancer incidence can be observed in animals above 20 years of age, similar to humans 60+ years old.47 Neoplasia was involved in more than half of all deaths in rhesus macaques older than 26 years. Thus, while cancer is a low-frequency disease, it can be found reliably in aging populations of NHPs, providing an opportunity to study its natural history and treatment.
Immune competence
The reduced ability to resist infectious disease in the elderly human population is well-known.48 Over 90% of influenza-associated deaths in the USA occur in persons aged 65+ years,49 and the incidence of severe sepsis increases over 100-fold in persons 85+ years old compared with children.50 Over 80% of the COVID-19-associated deaths in Italy, Spain, and Japan occurred in humans 70+ years old.51 This age-related reduction in immune competence in humans52 is similarly found in NHPs as illustrated by a comparable loss of naïve CD4 and CD8 T cells and a concomitant accumulation of memory T cells, limiting the T-cell repertoire.6 53 Elderly NHPs also have a reduced immune response to influenza vaccination54 and a poorer CD8 T cell and B-cell response on vaccination with modified vaccinia strain Ankara.55 Temporally, the increase in tumor frequency coincides with reduced immune competence in aging NHPs, resembling the situation in patients with human cancer.
Viral infection
NHPs are similar to humans in their susceptibility to virus-induced neoplasms. For example, Kaposi’s sarcoma-associated herpesvirus which is common in patients with immune-deprived HIV, also termed HHV8, has a relative in the form of rhesus rhadinovirus (RRV).56 RRV is associated with a mesenchymal malignancy termed retroperitoneal fibromatosis, resembling Kaposi’s sarcoma in SIV-infected rhesus macaques.57
Additionally, oncogenic Epstein-Barr virus (EBV) has an analogous rhesus lymphocryptovirus (rhLCV).56 rhLCV has a high degree of homology with human EBV, encoding for an identical viral gene repertoire, and parallels EBV-associated disease including B-cell lymphomas and hairy leukoplakia in SIV-infected macaques.58 Furthermore, the T-cell specific and humoral response to EBV in human and rhLCV in rhesus macaques share similarities, illustrating the potential for studies on EBV and EBV-associated malignancies in rhesus macaques.
Human papilloma virus (HPV) is a highly relevant risk factor for cervical, anal, vulvar, and head and neck cancer in humans.59 Vaccinating rhesus macaques with a DNA vaccine encoding a fusion protein of HPV18 EV6/EV760 or an inexpensive trivalent HPV16/18/58 vaccine produced in Escherichia coli61 both produced successful immunization as determined by the emergence of neutralizing antibodies. Replication incompetent HPV16 pseudovirions with a red fluorescent protein (RFP) reporter system were successfully used in rhesus macaques for visualization of the traumatic effect of Pap smear collection as an infection enhancing factor as well as iota-carrageenan as a potential HPV microbicide.62 Interestingly, an oncogenic rhesus specific papillomavirus, RhPV-1, was identified in a monkey with penile carcinoma in a breeding colony.63 Seventy-one per cent of his mating partners (22/31) had clinical, histopathological, or molecular evidence of infection. The lesions included warty, dysplastic lesions and/or acetowhitening in 35% (11/31) of his mating partners, and two cervical carcinomas. This finding of sexual transmissibility and the oncogenic potential of RhPV-1 is similar to high-risk HPVs in humans. Corroborating results were generated in cynomolgus macaques where cervical intraepithelial neoplasia was associated with papillomavirus infection.64 Notably, the most prevalent type, RhPV-d, is phylogenetically related to oncogenic HPV16 and was transferred via cervical cytobrush samples, resulting in new infections in 4/12 animals. Moreover, within 18 weeks 1/4 infected animals developed abnormal cytology and histology. In addition to captive colonies, papillomavirus DNA was also found in 75.2% (88/117) of wild rhesus macaques roaming the Kam Shan Country Park in Hong Kong.65 Phylogenetic analysis demonstrated the parallel evolution of papillomaviruses in monkeys and similar disease mechanisms and affected body sites. Cynomolgus macaque-associated MfPV3 degrades host p53 by virally-encoded E6 protein, as does HPV16. Moreover, alpha papillomaviruses detected in the cervicovaginal region of NHPs and humans include α9 (HPV16), α7 (HPV18) and α12 (MfPV3) species,66 demonstrating close evolutionary parallels and providing a promising tool to investigate HPV persistence and carcinogenesis in an animal model.
Inherited genetic mutations
Recent studies demonstrated elevated rates of colorectal cancer (CRC) in related rhesus macaques67 68 resembling the autosomal dominant inheritance of human hereditary non-polyposis colorectal cancer (HNPCC).69 70 These tumors frequently lacked expression of MLH1 or PMS2, two key proteins of DNA mismatch repair (MMR). In addition to a similar histopathology, sequencing revealed deletions in the MLH1 promoter region, de novo stop codons in MLH1, and deleterious missense mutations in MSH6 leading to a microsatellite-instable phenotype, closely resembling human HNPCC. Moreover, these heritable CRC cases could be traced back to three founding males with some exonic non-synonymous single nucleotide polymorphisms (SNPs) resembling those described in patients with human Lynch syndrome.71
There are still a broad variety of human inherited risk factors which are not yet described in NHPs, such as BRCA1/2 mutations. The number of fully sequenced NHPs is still relatively low, and these translationally-relevant mutations may yet emerge in cross-institutional initiatives such as the Macaque Genotype and Phenotype resource (mGAP) developed and maintained at the Oregon National Primate Research Center72 73 which links phenotypes observed in large, pedigreed rhesus colonies by DNA sequencing to detect naturally occurring genetic variations.
Transgenic or gene-edited NHP models have been successfully employed to model neurological disease including Parkinson’s disease, Huntington’s disease, or Rett syndrome.74 Similarly, cancer-promoting genetic variants detected in the NHP population could be intentionally introduced and propagated by selective breeding. Both approaches bear a great potential for research on cancer therapy and particularly cancer prevention but would come with substantial ethical concerns, as well as practical concerns for the long-term commitment to study such animals. Needless to say, a thorough and diligent ethical evaluation including comprehensive ethics boards would need to precede the establishment of such a model for heritable cancer. We consider the induction of cancer in NHPs to be both ethically and practically questionable. However, the treatment of animals with naturally-occurring disease provides a benefit to both the science of cancer treatment, and to the animal.
Cancer incidence in NHPs
Reports on malignancies in rhesus macaques range from case reports75–78 to studies including full breeding colonies investigating a total of 2660 individuals.47 68 79 Drawing epidemiologic conclusions from current literature reports is difficult and restricted to studies with larger animal numbers in combination with a particular focus on cancer diagnosis. Other issues to consider when evaluating cancer incidence and prevalence rates in rhesus macaques include euthanasia before geriatric age, transfer of animals, and reporting bias due to low suspicion of cancer.67 In contrast, factors positively affecting cancer diagnosis could be a closer clinical surveillance than certain human populations and the higher likelihood of a full postmortem examination and tissue biobanking on death. As a result, both underdiagnosis or overdiagnosis could occur in comparison to the human situation.
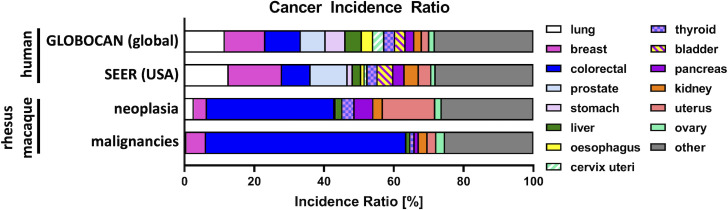
The most common reported malignancy in men is lung cancer, which is closely linked to smoking; this risk factor is absent in NHP. About 50% of all malignancies in rhesus macaques are gastrointestinal malignancies with ileocecal/colonic adenocarcinomas as most frequent types (see figure 1).47 79 In humans of both sexes, CRC is the third most common cancer accounting for 9.96% of new cancer cases and 10.1% of the reported cancers deaths, making it second in terms of absolute cancer death tolls.80 81 Differences in cancer incidence between rhesus macaques and humans likely reflects a combination of genetic risk and relative risk factor exposures.80
Figure 1.
Proportion of cancer types in the global81 and USA93 human population in all sexes in comparison to neoplasia and malignancies in a rhesus macaque cohort of 2660 individuals.47 Lung, breast, colorectal, and prostate cancer are the most common malignancies in humans. In rhesus macaques, colorectal adenocarcinoma is the most observed neoplasm and malignancy. The second most frequent neoplasm in rhesus macaques is benign uterine leiomyoma. The second most frequent malignancy in rhesus macaques is breast cancer.
High incidence rates for CRC in humans are seen in countries with a high Human Development Index (HDI).80 Reduced CRC incidence rate in low HDI countries may be due to a more restricted diet with less processed foods, higher physical activity, underdiagnosis, and reduced life expectancy. Similar patterns of a reduced incidence of CRC, breast cancer (BC), and other malignancies are present in rhesus macaque colonies where diet and behavior commonly more closely resemble the natural environment, where cancer diagnostics are limited, and lifespan observation to geriatric age is uncommon.
In women, breast and cervix cancer dominate the ranking; these tumor types are increased by reproductive and hormonal factors including earlier age of menarche, later age at first birth and lower parity80 but also physical inactivity, excessive body weight, and smoking.82 These factors potentially also influence and explain the reported lower incidence rate of BC in rhesus macaques.
Additionally, most reported neoplasms in rhesus macaques tend to be found at death, as exemplified by the Wisconsin survey in which only 14/217 TBMs were diagnosed on biopsy.47 In contrast, elderly humans do not regularly undergo extensive autopsy on death and thus many of these lesions identified in the abovementioned rhesus macaque cohort would not be identified in human patients.
In the framework of the Primate Cancer Initiative at Wake Forest University, we have evaluated 40 NHP cancer candidates over the past decade; 25 were diagnosed with naturally occurring cancers, with referral numbers steadily increasing. These NHPs originate from our network of close collaborators throughout the USA, allowing us to concentrate our cross-institutional efforts on becoming a center of excellence for cancer research and therapy.
As a result of our efforts, we have recruited primarily rhesus macaques with CRC and BC for preclinical trials assessing CIT drug candidates.83–85 Hence, we will focus on these two malignancies in more detail.
CRC in NHPs
Typical clinical signs of CRC in rhesus macaques include progressive weight loss, palpable abdominal mass, intermittent diarrhea, hypoproteinemia, fecal occult blood, and microcytic anemia.47 75 77 78 Suspected cases require diagnostic confirmation by ultrasound, CT, positron emission tomography, or laparotomy to exclude other gastrointestinal diseases such as diverticulitis. Histology generally reveals right-sided, mucinous adenocarcinoma, with dense, constrictive stroma. Precancerous lesions and polyps are not a reported feature of CRC in macaques.79 Metastasis to local lymph nodes, cases of miliary peritoneal carcinomatosis, or distant metastasis to spleen, lung, or spine are described in rhesus macaques but liver metastasis are not as frequently observed as in patients with human CRC.86
As mentioned above, Lynch-syndrome-like loss of MLH1 due to germline mutations is described for rhesus macaques and accompanied by an increased incidence of MMR deficient (MMRd) CRC.67 68 These familial cases in NHP demonstrate the role of MMRd and its parallels to human HNPCC cases. In contrast, mice with constitutive knockdown of MLH1 or MSH2 as models for MMRd do not develop CRC, but predominantly develop T-cell lymphomas.87
Moreover, MMRd and microsatellite instability suggest a broad range of tumor mutational burden (TMB) and neoantigens. TMB is an established positive response predictor for immune checkpoint blockade.88 89 Pembrolizumab received Food and Drug Administration-approval in 2020 as first-line and single-agent checkpoint inhibitor against MMRd highly-advanced CRC90 and promising data was recently obtained in a phase II trial assessing dostarlimab in MMRd, locally advanced rectal cancer.91 Similar patterns of MMRd CRC in NHPs might extend our understanding of the underlying processes in CRC, and the basis for observed responses to treatment.
BC in NHPs
BC has a predicted lifetime incidence of about 6% in female macaques compared with approximately 13% in American women.76 92 93 NHP are seldom maintained for their entire potential life span, limiting BC detection. Moreover, multiparity, ovariectomy, as well as a generally lower rate of obesity may contribute to lower BC in NHP.80
Humans and macaques share similar mammary gland anatomy, physiology, and comparable patterns of development, regression, and sex steroid receptor expression.94 Sex hormones including estrogen, progesterone, luteinizing hormone, and follicle stimulating hormone exhibit a similar pattern in women as in cynomolgus and rhesus macaques.95
Breast neoplasms diagnosed in macaques resemble those seen in humans, including precursor lesions such as atypical hyperplasia; ductal carcinoma in situ; and invasive ductal carcinomas. Moreover, metastasis of high-grade lesions to the axillary lymph nodes, lung, chest wall, and other sites has been documented.76 Notably, the full range of hormone receptor (progesterone receptor and estrogen receptor) and HER2 receptor positivity is observed in rhesus macaque BCs by immunohistochemistry (IHC)94 resulting in similar molecular subtypes including predominantly Luminal A (HR+/HER2−), lower numbers of Luminal B (HR+/HER2+), HER2 (HR−/HER2+), and a small proportion of triple-negative cancers (HR−/HER2−) as described in patients with human BC.76 94 In addition, TP53 positive BC is reported in rhesus macaques suggesting non-synonymous TP53 mutations as observed in 20–35% of sporadic human BC.92
A typical DNA repair deficiency associated with hereditary BC in humans and putative driver for high TMB is homologous recombination repair deficiency by heterozygous loss-of-function mutations in BRCA1 or BRCA2. While extensive studies were conducted to compare BRCA1 and BRCA2 in primate species and their respective evolution,96 there is no detailed knowledge on their potential involvement in heritable BC in NHPs at present.
A brief summary on the comparative oncology in between rhesus macaques and humans addressing cancers in general and CRC and BC in particular can be found in table 2.
Table 2.
Comparative oncology reveals widespread similarities in cancer development and pathology in between human and rhesus macaques
| Cancer in rhesus macaques | Ref | |
| Age | Similar increase in cancer incidence in aging/geriatric population. | 47 |
| Immune competence | Similar reports of declining immune competence and TCR repertoire with age, resembling the immune situation in aging patients with human cancer. | 6 52 53 |
| Viral infections | Rhesus rhadinovirus in SIV-infected rhesus macaques is similarly associated with mesenchymal malignancies as Kaposi’s sarcoma-associated herpesvirus in HIV-infected humans. | 56 57 |
| Epstein-Barr virus homologue rhesus lymphocryptovirus is associated with B-cell lymphomas and hairy leukoplakia in SIV-infected macaques. | 58 | |
| Papilloma virus is present in wild rhesus macaques (88/117) and is sexually transmitted in breeding colonies. | 63–65 | |
| HPV analog RhPV-1 is associated with penile carcinoma, dysplastic lesions, acetowhitening (35%), and two cervical carcinomas in a breeding colony (n=31, transmission rate 71%). | 63 | |
| Inherited mutations | Rhesus equivalent of HNPCC/Lynch syndrome is linked to germline MLH1 promoter deletion, de novo stop codon in MLH1, and deleterious missense mutations in MSH6. | 67 68 |
| No description of BRCA1/2 involvement in NHP yet, potentially due to the limited germline/phenotype information. | ||
| Colorectal cancer | ||
| Prevalence | Neoplasia of the gastrointestinal system is the most commonly diagnosed in rhesus macaques with adenocarcinoma of the large intestines as most prevalent tumor. | 47 |
| Symptoms | Similar clinical signs as in humans including weight loss, intermittent diarrhea, hypoproteinemia, fecal occult blood, and microcytic anemia. | 47 75 77 78 |
| Location | Humans: Throughout the ascending/transverse/descending/sigmoid colon and rectum. Rhesus macaques: Mostly right-sided (ileocecal junction, ascending colon). |
|
| Precancerous lesions | Humans: Colonic polyps are well established as precancerous lesions. Rhesus macaques: Polyps are not reported and CRC usually infiltrates within colonic wall. |
79 |
| Progression and metastatic spread | Humans: Metastatic spread is common in late stages and a major factor for CRC mortality, frequently to liver and other distant organs such as lung. Rhesus macaques: Constriction of the colonic lumen by the primary lesion and blocking passage is the major cause for a humane endpoint in rhesus macaques, treatment resistant anemia (iron/B12 supplement) can be frequently observed, local lymph node metastasis, distant metastatic spread (eg, bone/spine), and abdominal carcinomatosis (eg, omentum, uterus, stomach, pancreas) is observed in certain cases. |
86 |
| Mismatch repair deficiency | MMRd in rhesus CRCs results in microsatellite instability as reported in human CRCs. | 67 68 |
| Breast cancer | ||
| Lifetime incidence | Six per cent in female macaques compared with 13% in American women (note: under-reporting and difference in risk factor exposure in macaques). |
76 92 93 |
| Histology | Similar mammary gland anatomy, development patterns, regression, and sex steroid receptor expression. | 94 |
| Sex hormones | Similar patterns of estrogen, progesterone, luteinizing hormone, and follicle stimulation hormone in human, cynomolgus, and rhesus macaques. | 95 |
| Histopathology | Similar range of atypical hyperplasia, DCIS, and invasive ductal carcinomas reported. | 76 |
| Progression and metastatic spread | Similar progression and metastatic spread to axillary lymph nodes, lung, and chest wall can be observed in rhesus macaques and humans. | 47 76 |
| Receptor expression in BC | Subtypes described in humans are similarly observed in rhesus macaques incl. Luminal A (HR+/HER2–), HER2 (HR–/HER2+), and triple-negative BC (HR–/HER2–). | 76 94 |
BC, breast cancer; CRC, colorectal cancer; DCIS, ductal carcinoma in situ; HER2, human epidermal growth factor receptor 2; HNPCC, hereditary non-polyposis colorectal cancer; HPV, human papilloma virus; NHP, non-human primates; RhPV, rhesus specific papillomavirus; SIV, simian immunodeficiency virus; TCR, T cell receptor.
Interventional CIT studies in TBMs
General considerations and benefits
TBMs provide a study population of treatment-naïve patients of a comparable life stage, with naturally-occurring tumors, and an unaltered immune status, fostering and accelerating the application of first-line CIT agents. Translation from preclinical experiments to clinical phase I studies in humans currently relies on recruitment of patients with late-stage cancer who have usually undergone multiple cycles of one or more treatment regimens and/or have recurring treatment-resistant clones. Ethical concerns in human trials influence the study design and require combination studies of the prospective agent with the standard-of-care regimen in cases with good to intermediate clinical prognosis,97 98 limiting single-agent studies to cases with a poor prognosis.99–102
Altruistic participation of patients with cancer is a crucial keystone of clinical research and has fostered a multitude of advancements but may not mimic the clinical situation of untreated patients in which first-line CIT approaches proved beneficial.90 103 104 Certain established and emerging CITs can potentially shift from a late-stage salvage therapy to an approved and promising first-line treatment option, fostered by preceding TBM trials.
Needless to say, NHP trials demand nothing less than the gold standards in care, animal welfare, treatment, and study design to follow the principle of the 3 Rs105 meticulously and cause as little discomfort as necessary to generate as much translational data and benefits from this animal model as feasible.
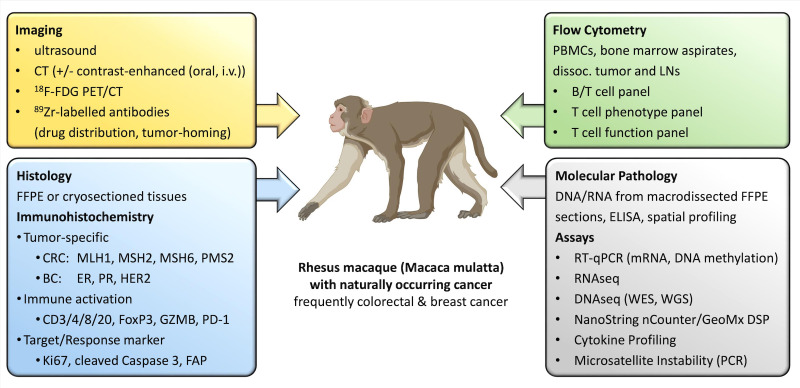
Ongoing efforts by the Primate Cancer Initiative at Wake Forest University, the only program in the USA particularly dedicated to this field, aim to facilitate this model by broad collaborations to recruit NHPs with suspected cancers for prospective treatment studies. Using this approach, the timing of spontaneous cancer cases, the exact cancer location, or the histologic subtype cannot be controlled as in induced or grafted animal models; rather, a screening and recruitment approach is needed. Another key factor for TBM studies is the requirement for specialized veterinary staff and a close affiliation to core resources providing imaging and established NHP-specific IHC and flow cytometry procedures for follow-up biomarker analysis. In addition to improved clinical imaging, an array of NHP-specific assays has been established to provide the same biomarker and tumor characterization pipelines in TBM trials as in their human counterparts (see figure 2). Moreover, a repository of tissues and body fluids at numerous time points and at necropsy is made available to the scientific community on request.
Figure 2.
Diagnostics, biomarkers, and assays currently employed in TBMs. A multitude of methods can be used to diagnose cancer in TBM, classify the suspicious lesion, and follow-up treatment response. This includes versatile imaging methods, tumor-specific Immunohistochemistry panels, molecular pathological tests, as well as the follow-up of the immunological response in the tumor bed, lymph nodes, and peripheral blood by flow cytometry (created with BioRender). BC, breast cancer; CRC, colorectal cancer; DNAseq, DNA sequencing; ER, estrogen receptor; FAP, fibroblast activation protein; FDG, fluorodeoxyglucose; FFPE, formalin fixed paraffin embedded; i.v., intravenous; LNs, lymph nodes; mRNA, messenger RNA; PBMCs, peripheral blood mononuclear cells; PD-1, programmed cell death protein-1; PET, positron emission tomography; PR, progesterone receptor; RNAseq, RNA sequencing; RT-qPCR, real time quantitative polymerase chain reaction; TBM, tumor-bearing monkey; WES, whole exome sequencing; WGS, whole genome sequencing.
Previous CIT studies in TBM: drug candidates, imaging, and biomarkers
The earliest studies on CIT in TBM focused on fibroblast activation protein α (FAP) which is a dimeric Type II transmembrane glycoprotein with proteolytic activity.106 FAP is scarce in normal, healthy adult tissues but is highly expressed on the surface of cancer-associated fibroblasts in >90% human epithelial malignancies.107 The high and restricted expression on reactive stroma in the tumor microenvironment makes FAP a promising target for tumor treatment, particularly for FAP-targeted bispecific antibodies.83 84 108
In mouse tumor models, FAP expression does not always reflect the expression pattern observed in human patients. In contrast, FAP expression observed in TBM better mimics the situation in humans and is particularly abundant in CRC. De facto, extensive FAP expression has been observed in TBM in the tumor-adjacent stroma (and to a lesser extent in lymphoid fibroreticular cells within adjacent lymph nodes), but not in tumor cell nests,84 a typical expression pattern observed in human tumors.109 Additionally, in both humanized mice and immune competent mouse models, FAP is expressed by murine (and not human) fibroblasts, which consequently requires the employment of murine surrogate FAP-targeted bispecific antibodies to perform murine in vivo studies. On the other hand, FAP-targeted bispecific antibodies employed in TBMs are the same as the compounds later employed in human patients (eg, FAP-4-1BBL), making the use of murine surrogates unnecessary and clearly indicating that TBMs are an ideal animal model for translational research on FAP-targeted CIT compounds.
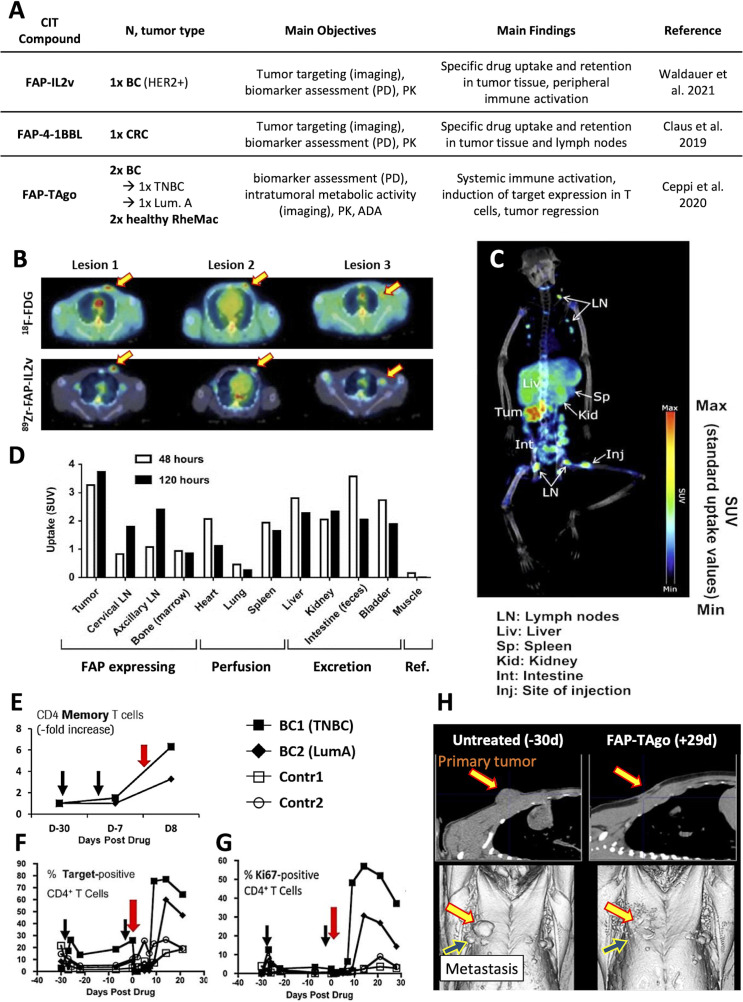
So far, three FAP-targeted CIT compounds have been tested in TBMs: FAP-IL2v, FAP-4-1BBL and FAP-TAgo (as illustrated in figure 3A).83–85 Of note, the low number of patients (n=1–4) in each of the TBM studies conducted to date emphasizes the limited, but translational, ‘proof of concept’ nature of the obtained data and observations.
Figure 3.
Preclinical studies on CIT agents performed in TBM. (A) Several novel bispecifics were tested in TBM and confirmed tumor-homing of the CIT agent as shown in (B) for FAP-IL2v (lesions highlighted by arrows) in a BC-bearing rhesus-macaques. (C) tumor-homing was also demonstrated for FAP-4–1-BBL (lesion highlighted by an arrow) in a CRC-bearing rhesus macaque and (D) the tissue distribution over time determined. (E) Diphtheria/pertussis/tetanus vaccination in combination with FAP-TAgo induced an expansion of tumorous CD4+ memory T cells in BC-bearing rhesus macaques and a strong expansion of peripheral target-positive (F) CD4+ T cells and (G) Ki67+CD4+ T cells. (H) a strong radiographic response was observed in BC2 with no nodules regrowing >12 months later. BC1 had lung metastases at the time of treatment which responded initially (not shown) but reached her humane endpoint soon after. (Illustrations adapted from83–85). ADA, anti-drug antibody; BC, breast cancer; CIT, cancer immunotherapy; CRC, colorectal cancer; FAP, fibroblast activation protein; FDG, fluorodeoxyglucose; LNs, lymph nodes; PD, pharmacodynamics; PK, pharmacokinetics; RheMac, rhesus macaques; SUV, standardized uptake value; TBM, tumor-bearing monkey; TNBC, triple-negative breast cancer.
FAP-IL2v
Simlukafusp alfa (FAP-IL2v, RO6874281/RG7461) is an immunocytokine targeting FAP and bearing an IL-2 variant with retained affinity for IL-2 Rβγ and abolished binding to IL-2Rα.83 FAP expression in tumor stroma of a HER2+ BC-bearing rhesus macaque was confirmed by IHC and 3× fluorodeoxyglucose (FDG)-avid lesions identified by PET scans. Based on postmortem histology, 2/3 FDG-avid lesions were confirmed as tumor nodules while the remaining FDG-avid axillary lymph node was most likely active due to an inflammatory response. The monkey was given 0.5 mg/kg of FAP-IL2v and tracer amounts of 89Zr-labeled FAP-IL2v intravenously with PET demonstrating selective accumulation in all three lesions identified by FDG-PET scans within 67 hours and 154 hours (see figure 3B). Furthermore, an immune cell infiltration was observably induced in tumor biopsies obtained 3 days post FAP-IL2v administration.
FAP-4-1BBL
FAP-4-1BBL is a bispecific antibody-like fusion protein that simultaneously targets FAP and 4-1BB, a member of the tumor necrosis factor receptor super family (TNFRSF) transiently expressed on activated T cells.84 To confirm the intended MoA and elucidate the systemic distribution in a translatable animal model, a rhesus macaque (male, 21 years old, 8.5 kg) with spontaneous CRC at the ileocecal junction was recruited for this study (see figure 3C, D). The monkey was given 0.5 mg/kg FAP-4–1-BBL mixed with tracer amounts of 89Zr-labeled FAP-4–1-BBL (165MBq) intravenously and PET scans recorded after 48 hours and 120 hours. Importantly, persistent tissue accumulation within the rhesus CRC was observed initially at the 48 hours time point and increased towards the 120 hours time point. These NHP results paved the way for a phase I clinical study110 111 and a separate clinical imaging substudy.112 Importantly, the data initially obtained in the TBM, emphasizing tumor-specific uptake and the expected biodistribution pattern of 89Zr- FAP-4–1-BBL, could be recapitulated in the human clinical setting.112
FAP-TAgo
Another combined imaging/biomarker study in TBM investigated a second FAP-targeted TNFRSF agonist, termed FAP-TAgo.85 Two BC-bearing rhesus monkeys (1× triple-negative, 1× Luminal A) were first pre-immunized with a diphtheria/pertussis/tetanus vaccine, and then exposed to a single administration of FAP-TAgo (100 mg/kg, intravenous). In both TBMs, we detected the induction of the TNFRSF agonist in the periphery as well as a strong systemic immune activation. This increase in target-positive CD4+ and Ki67+CD4+ T cells was exclusive for TBM and only marginally observed in the healthy control animals (figure 3E–G). Consistently, the induction of CD4+ memory T cells was observed in the tumor of both TBMs after FAP-TAgo treatment which most importantly resulted in extensive tumor regression in both TBM (figure 3H). These results are even more impressive, when considering that FAP-TAgo was administered only once. Hence, these observations validated the applied pre-immunization strategy to induce the TNFRSF protein expression in T cells and confirmed the target to be pursued in the clinical setting. The investigation of FAP-TAgo in TBM and the resulting data analysis is currently being finalized with a novel successor trial.
Potential for expansion of TBM studies
The National Institutes of Health (NIH) in 2018 reported that there are over 100,000 nonhuman primates in the USA of which approximately 2/3 are rhesus macaques and 15% are cynomolgus macaques,113 114 and this number has generally increased over time. Within this total there are substantial aging populations, supported by the NIH through the National Primate Research Centers and other funding sources. Existing inter-institutional collaborations115 could provide a blueprint for development of a national Primate Cancer Network. We believe there is the potential nationwide to study over 100 NHPs per year with malignancies, primarily colorectal, breast and cervix, and substantially more if an effort were made to detect precancerous lesions of the cervix and vagina. Optimal development of this underused aspect of naturally-occurring disease in NHPs would require funding for establishment of a collaborative network enabling diagnostic screening, and support for local or referral-based treatment of patients with NHP cancer at academic medical centers or primate research centers with appropriate expertize and facilities in veterinary medicine, pathology, comparative oncology, and imaging.
Conclusion
Tumor-bearing NHPs are emerging as a valuable translational model to test CIT agents. The assessment of drug candidates in TBMs mimics the analysis performed in human oncology patients since tumor targeting, biodistribution, PK and PD activity, immunogenicity, intratumoral metabolic activity and tumor regression can all be similarly investigated in TBMs. Because of the shared target protein expression patterns between humans and rhesus macaques, TBMs are well-suited to preclinically test biomarkers and efficacy of for example, FAP-targeting agents. Multiple institutes are invested in overcoming current challenges of this model including (1) limited population genomics (mGAP); (2) limited molecular characterization of NHP cancers (retranslation of human cancer characterization pipelines); and (3) the limited number of NHP patients due to undiagnosed disease (improved imaging and cancer screening by liquid biopsy/cell-free DNA). We consider TBMs as an outstanding and increasingly relevant opportunity and model for the early generation of biomarker insights including proof-of-mechanism for CIT drug candidates, in small but very informative ‘signal-seeking’ experiments. Consequently, we anticipate that testing CIT compounds in TBMs could be of high predictability for clinical behavior in the human oncology setting.
Acknowledgments
The authors are grateful to Drs Matthew Starost (National Institutes of Health), Brandy Dozier and Cassandra Cullin (Oregon National Primate Research Center), Charlotte Hotchkiss (Washington National Primate Research Center), Emily Romero and Francois Villinger (New Iberia Research Center), Armando Burgos (Caribbean Primate Research Center), and Greg Dugan and Melaney Gee (Wake Forest School of Medicine) for case referrals and clinical consultations. We are also grateful to the caretakers, veterinary staff, radiation and environmental enrichment technicians who substantially contributed to the care and well-being of the animals.
Footnotes
Contributors: SD, BG, JC, MC, and JMC wrote this review.
Funding: This work was enabled by the Wake Forest University Cancer Center Support Grant (P30CA012197). SD is a beneficiary of the Roche Postdoctoral Fellowship program (RPF-ID541). JC and BG are employees of Roche Pharma Research and Early Development (pRED). MC was an employee of Roche pRED and is currently employed at iTeos Therapeutics (Gosselies, Belgium).
Competing interests: SD is a beneficiary of the Roche Postdoctoral Fellowship (RPF) program. JC and BG are employees of Roche Pharma Research and Early Development (pRED). MC was an employee of Roche pRED and is currently employed at iTeos Therapeutics (Gosselies, Belgium). JMC has received research support from Roche pRED.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Foundation for biomedical research. Available: https://fbresearch.org/
- 2.Van Rompay KKA. Tackling HIV and AIDS: contributions by non-human primate models. Lab Anim 2017;46:259–70. 10.1038/laban.1279 [DOI] [PubMed] [Google Scholar]
- 3.EMA . Initial scientific discussion for the approval of Herceptin, 2004. Available: https://www.ema.europa.eu/en/documents/scientific-discussion/herceptin-epar-scientific-discussion_en.pdf
- 4.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014;25:846–59. 10.1016/j.ccr.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 5.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020;52:17–35. 10.1016/j.immuni.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 6.Messaoudi I, Estep R, Robinson B, et al. Nonhuman primate models of human immunology. Antioxid Redox Signal 2011;14:261–73. 10.1089/ars.2010.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiina T, Blancher A, Inoko H, et al. Comparative genomics of the human, macaque and mouse major histocompatibility complex. Immunology 2017;150:127–38. 10.1111/imm.12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janeway CA, et al. The major histocompatibility complex and its functions, in immunobiology: the immune system in health and disease. New York: Garland Science, 2001. [Google Scholar]
- 9.Doxiadis GGM, de Groot N, Otting N, et al. Genomic plasticity of the MHC class I a region in rhesus macaques: extensive haplotype diversity at the population level as revealed by microsatellites. Immunogenetics 2011;63:73–83. 10.1007/s00251-010-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doxiadis GGM, de Groot N, Otting N, et al. Haplotype diversity generated by ancient recombination-like events in the MHC of Indian rhesus macaques. Immunogenetics 2013;65:569–84. 10.1007/s00251-013-0707-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol 2002;168:29–43. 10.4049/jimmunol.168.1.29 [DOI] [PubMed] [Google Scholar]
- 12.Mahnke YD, Brodie TM, Sallusto F, et al. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 2013;43:2797–809. 10.1002/eji.201343751 [DOI] [PubMed] [Google Scholar]
- 13.Gujer C, Sundling C, Seder RA, et al. Human and rhesus plasmacytoid dendritic cell and B-cell responses to Toll-like receptor stimulation. Immunology 2011;134:257–69. 10.1111/j.1365-2567.2011.03484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teleshova N, Kenney J, Williams V, et al. CpG-C ISS-ODN activation of blood-derived B cells from healthy and chronic immunodeficiency virus-infected macaques. J Leukoc Biol 2006;79:257–67. 10.1189/jlb.0205084 [DOI] [PubMed] [Google Scholar]
- 15.Chung E, Amrute SB, Abel K, et al. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin Diagn Lab Immunol 2005;12:426–35. 10.1128/CDLI.12.3.426-435.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketloy C, Engering A, Srichairatanakul U, et al. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Vet Immunol Immunopathol 2008;125:18–30. 10.1016/j.vetimm.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Caligiuri MA. Human natural killer cells. Blood 2008;112:461–9. 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology 2005;115:206–14. 10.1111/j.1365-2567.2005.02147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labonte ML, Letvin NL. Variable NKG2 expression in the peripheral blood lymphocytes of rhesus monkeys. Clin Exp Immunol 2004;138:205–12. 10.1111/j.1365-2249.2004.02625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol 2011;89:557–63. 10.1189/jlb.0710409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz AM, DiNapoli SR, Brenchley JM. Macrophages are phenotypically and functionally diverse across tissues in simian immunodeficiency virus-infected and uninfected Asian macaques. J Virol 2015;89:5883–94. 10.1128/JVI.00005-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009;4:1151–65. 10.2217/fmb.09.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, et al. Toll-Like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006;311:1770–3. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 24.Sethi VS, Surratt P, Spurr CL. Pharmacokinetics of vincristine, vinblastine, and vindesine in rhesus monkeys. Cancer Chemother Pharmacol 1984;12:31–5. 10.1007/BF00255905 [DOI] [PubMed] [Google Scholar]
- 25.Inaba M, Ohnishi Y, Ishii H, et al. Pharmacokinetics of CPT-11 in rhesus monkeys. Cancer Chemother Pharmacol 1998;41:103–8. 10.1007/s002800050715 [DOI] [PubMed] [Google Scholar]
- 26.Green DJ, Pagel JM, Nemecek ER, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood 2009;114:1226–35. 10.1182/blood-2009-03-210344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemecek ER, Hamlin DK, Fisher DR, et al. Biodistribution of yttrium-90-labeled anti-CD45 antibody in a nonhuman primate model. Clin Cancer Res 2005;11:787–94. 10.1158/1078-0432.787.11.2 [DOI] [PubMed] [Google Scholar]
- 28.Chan S, Belmar N, Ho S, et al. An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat Cancer 2022;3:337–54. 10.1038/s43018-022-00334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferl GZ, Reyes A, Sun LL, et al. A preclinical population pharmacokinetic model for Anti-CD20/CD3 T-cell-dependent bispecific antibodies. Clin Transl Sci 2018;11:296–304. 10.1111/cts.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchins B, Starling GC, McCoy MA, et al. Biophysical and Immunological Characterization and In Vivo Pharmacokinetics and Toxicology in Nonhuman Primates of the Anti-PD-1 Antibody Pembrolizumab. Mol Cancer Ther 2020;19:1298–307. 10.1158/1535-7163.MCT-19-0774 [DOI] [PubMed] [Google Scholar]
- 31.Li W, Wang Y, Rubins D, et al. PET/CT Imaging of 89Zr-N-sucDf-Pembrolizumab in Healthy Cynomolgus Monkeys. Mol Imaging Biol 2021;23): :250–9. 10.1007/s11307-020-01558-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EMA . Assessment report: Keytruda, 2015. Available: https://www.ema.europa.eu/en/documents/assessment-report/keytruda-epar-public-assessment-report_en.pdf
- 33.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846–56. 10.1158/2326-6066.CIR-14-0040 [DOI] [PubMed] [Google Scholar]
- 34.EMA . Assessment report: nivolumab, 2015. Available: https://www.ema.europa.eu/en/documents/assessment-report/nivolumab-bms-epar-public-assessment-report_en.pdf
- 35.Cole EL, Kim J, Donnelly DJ, et al. Radiosynthesis and preclinical PET evaluation of 89Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorg Med Chem 2017;25:5407–14. 10.1016/j.bmc.2017.07.066 [DOI] [PubMed] [Google Scholar]
- 36.Selby MJ, Engelhardt JJ, Johnston RJ, et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One 2016;11:e0161779. 10.1371/journal.pone.0161779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EMA . Assessment report: Jemperli, 2021. Available: https://www.ema.europa.eu/en/documents/assessment-report/jemperli-epar-public-assessment-report_en.pdf
- 38.Kumar S, Ghosh S, Sharma G, et al. Preclinical characterization of dostarlimab, a therapeutic anti-PD-1 antibody with potent activity to enhance immune function in in vitro cellular assays and in vivo animal models. MAbs 2021;13:1954136. 10.1080/19420862.2021.1954136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markham A. Dostarlimab: first approval. Drugs 2021;81:1213–9. 10.1007/s40265-021-01539-5 [DOI] [PubMed] [Google Scholar]
- 40.Markham A, Duggan S. Cemiplimab: first global approval. Drugs 2018;78:1841–6. 10.1007/s40265-018-1012-5 [DOI] [PubMed] [Google Scholar]
- 41.EMA . Assessment report: Tecentriq, 2017. Available: https://www.ema.europa.eu/en/documents/assessment-report/tecentriq-epar-public-assessment-report_en.pdf
- 42.EMA . Assessment report: Imfinzi, 2018. Available: https://www.ema.europa.eu/en/documents/assessment-report/imfinzi-epar-public-assessment-report_en.pdf
- 43.Jin H, D'Urso V, Neuteboom B, et al. Avelumab internalization by human circulating immune cells is mediated by both Fc gamma receptor and PD-L1 binding. Oncoimmunology 2021;10:1958590. 10.1080/2162402X.2021.1958590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EMA . Assessment report: Bavencio, 2017. Available: https://www.ema.europa.eu/en/documents/assessment-report/bavencio-epar-public-assessment-report_en.pdf
- 45.EMA . Assessment report for Yervoy (ipilimumab), 2011. Available: https://www.ema.europa.eu/en/documents/assessment-report/yervoy-epar-public-assessment-report_en.pdf
- 46.Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ. Tremelimumab: research and clinical development. Onco Targets Ther 2016;9:1767–76. 10.2147/OTT.S65802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons HA, Mattison JA. The incidence of spontaneous neoplasia in two populations of captive rhesus macaques (Macaca mulatta). Antioxid Redox Signal 2011;14:221–7. 10.1089/ars.2010.3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckman I, Dimopoulos K, Xu XN, et al. T cell activation in the elderly: evidence for specific deficiencies in T cell/accessory cell interactions. Mech Ageing Dev 1990;51:265–76. 10.1016/0047-6374(90)90076-R [DOI] [PubMed] [Google Scholar]
- 49.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet 2003;362:1733–45. 10.1016/S0140-6736(03)14854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 51.Omori R, Matsuyama R, Nakata Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci Rep 2020;10:16642. 10.1038/s41598-020-73777-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drijvers JM, Sharpe AH, Haigis MC. The effects of age and systemic metabolism on anti-tumor T cell responses. Elife 2020;9. 10.7554/eLife.62420. [Epub ahead of print: 10 Nov 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janković V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood 2003;102:3244–51. 10.1182/blood-2003-03-0927 [DOI] [PubMed] [Google Scholar]
- 54.Coe CL, Lubach GR, Kinnard J. Immune senescence in old and very old rhesus monkeys: reduced antibody response to influenza vaccination. Age 2012;34:1169–77. 10.1007/s11357-011-9356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Čičin-Šain L, Smyk-Paerson S, Currier N, et al. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J.i. 2010;184:6739–45. 10.4049/jimmunol.0904193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Estes JD, Wong SW, Brenchley JM. Nonhuman primate models of human viral infections. Nat Rev Immunol 2018;18:390–404. 10.1038/s41577-018-0005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estep RD, Wong SW. Rhesus macaque rhadinovirus-associated disease. Curr Opin Virol 2013;3:245–50. 10.1016/j.coviro.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F. Nonhuman primate models for Epstein-Barr virus infection. Curr Opin Virol 2013;3:233–7. 10.1016/j.coviro.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ault KA, Brown DR. Advancing human papillomavirus research with a rhesus monkey model. J Natl Cancer Inst 2011;103:703. 10.1093/jnci/djr125 [DOI] [PubMed] [Google Scholar]
- 60.Yan J, Harris K, Khan AS, et al. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine 2008;26:5210–5. 10.1016/j.vaccine.2008.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin F, Wang Y, Chen N, et al. A novel trivalent HPV 16/18/58 vaccine with anti-HPV 16 and 18 neutralizing antibody responses comparable to those induced by the Gardasil quadrivalent vaccine in rhesus macaque model. Papillomavirus Res 2017;3: :85–90. 10.1016/j.pvr.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts JN, Kines RC, Katki HA, et al. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst 2011;103:737–43. 10.1093/jnci/djr061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ostrow RS, McGlennen RC, Shaver MK, et al. A rhesus monkey model for sexual transmission of a papillomavirus isolated from a squamous cell carcinoma. Proc Natl Acad Sci U S A 1990;87:8170–4. 10.1073/pnas.87.20.8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood CE, Chen Z, Cline JM, et al. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol 2007;81:6339–45. 10.1128/JVI.00233-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Z, Long T, Wong PY, et al. Non-Human primate papillomaviruses share similar evolutionary histories and niche adaptation as the human counterparts. Front Microbiol 2019;10:p. 2093. 10.3389/fmicb.2019.02093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z, Schiffman M, Herrero R, et al. Classification and evolution of human papillomavirus genome variants: alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and alpha-3 (HPV61). Virology 2018;516: :86–101. 10.1016/j.virol.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dray BK, Raveendran M, Harris RA, et al. Mismatch repair gene mutations lead to Lynch syndrome colorectal cancer in rhesus macaques. Genes Cancer 2018;9:142–52. 10.18632/genesandcancer.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brammer DW, Gillespie PJ, Tian M, et al. MLH1-rheMac hereditary nonpolyposis colorectal cancer syndrome in rhesus macaques. Proc Natl Acad Sci U S A 2018;115:2806–11. 10.1073/pnas.1722106115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei W, Liu F, Liu L, et al. Distinct mutations in MLH1 and MSH2 genes in hereditary non-polyposis colorectal cancer (HNPCC) families from China. BMB Rep 2011;44:317–22. 10.5483/BMBRep.2011.44.5.317 [DOI] [PubMed] [Google Scholar]
- 70.Worthley DL, Walsh MD, Barker M, et al. Familial mutations in PMS2 can cause autosomal dominant hereditary nonpolyposis colorectal cancer. Gastroenterology 2005;128:1431–6. 10.1053/j.gastro.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 71.Brieger A, Engels K, Schaefer D, et al. Malignant fibrous histiocytoma is a rare Lynch syndrome-associated tumor in two German families. Fam Cancer 2011;10:591–5. 10.1007/s10689-011-9455-9 [DOI] [PubMed] [Google Scholar]
- 72.Rogers J. Genomic resources for rhesus macaques (Macaca mulatta). Mamm Genome 2022;33:91–9. 10.1007/s00335-021-09922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bimber BN, Yan MY, Peterson SM, et al. mGAP: the macaque genotype and phenotype resource, a framework for accessing and interpreting macaque variant data, and identifying new models of human disease. BMC Genomics 2019;20:176. 10.1186/s12864-019-5559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt JK, Jones KM, Van Vleck T, et al. Modeling genetic diseases in nonhuman primates through embryonic and germline modification: considerations and challenges. Sci Transl Med 2022;14:eabf4879. 10.1126/scitranslmed.abf4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DePaoli A, McClure HM. Gastrointestinal neoplasms in nonhuman primates: a review and report of eleven new cases. Vet Pathol Suppl 1982;7:104–25. 10.1177/030098588201907s08 [DOI] [PubMed] [Google Scholar]
- 76.Wood CE, Usborne AL, Starost MF, et al. Hyperplastic and neoplastic lesions of the mammary gland in macaques. Vet Pathol 2006;43:471–83. 10.1354/vp.43-4-471 [DOI] [PubMed] [Google Scholar]
- 77.Valverde CR, Tarara RP, Griffey SM, et al. Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.). Comp Med 2000;50:540–4. [PubMed] [Google Scholar]
- 78.Lang CD, Daviau JS, Merton DA, et al. Adenocarcinoma of the ileocolic junction and multifocal hepatic sarcomas in an aged rhesus macaque (Macaca mulatta). Comp Med 2013;63:361–6. [PMC free article] [PubMed] [Google Scholar]
- 79.Uno H, Alsum P, Zimbric ML, et al. Colon cancer in aged captive rhesus monkeys (Macaca mulatta). Am J Primatol 1998;44:19–27. [DOI] [PubMed] [Google Scholar]
- 80.IARC-WHO . World Cancer Report - Cancer research for cancer prevention 2020.
- 81.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 82.Torre LA, Islami F, Siegel RL, et al. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev 2017;26:444–57. 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 83.Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs 2021;13:1913791. 10.1080/19420862.2021.1913791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Claus C, Ferrara C, Xu W, et al. Tumor-Targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med 2019;11. 10.1126/scitranslmed.aav5989. [Epub ahead of print: 12 Jun 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ceppi M, Hettich M, Teichgräber V, et al. Abstract 6135: tumor-bearing non-human primates: an unrivaled model for translational cancer immunology research. Cancer Res 2020;80:6135. 10.1158/1538-7445.AM2020-6135 [DOI] [Google Scholar]
- 86.Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. 10.1038/srep29765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee K, Tosti E, Edelmann W. Mouse models of DNA mismatch repair in cancer research. DNA Repair 2016;38:140–6. 10.1016/j.dnarep.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–608. 10.1158/1535-7163.MCT-17-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med 2020;383:1328–39. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 90.FDA . FDA approves pembrolizumab for first-line treatment of MSI-H/dMMR colorectal cancer, 2020. Available: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer
- 91.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 2022;386:2363–76. 10.1056/NEJMoa2201445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beck AP, Brooks A, Zeiss CJ. Invasive ductular carcinoma in 2 rhesus macaques (Macaca mulatta). Comp Med 2014;64:314–22. [PMC free article] [PubMed] [Google Scholar]
- 93.National_Cancer_Institute_(NCI) . Cancer Stat facts (derived from SEER 13). Available: https://seer.cancer.gov/statfacts/
- 94.Dewi FN, Cline JM. Nonhuman primate model in mammary gland biology and neoplasia research. Lab Anim Res 2021;37:3. 10.1186/s42826-020-00053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weinbauer GF, Niehoff M, Niehaus M, et al. Physiology and endocrinology of the ovarian cycle in macaques. Toxicol Pathol 2008;36:7S–23. 10.1177/0192623308327412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lou DI, McBee RM, Le UQ, et al. Rapid evolution of BRCA1 and BRCA2 in humans and other primates. BMC Evol Biol 2014;14:155. 10.1186/1471-2148-14-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galsky MD, Arija José Ángel Arranz, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1547–57. 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 98. Hoffman-LaRoche. A safety and pharmacology study of Atezolizumab (MPDL3280A) administered with Obinutuzumab or Tazemetostat in participants with relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma, 2014. Available: https://clinicaltrials.gov/ct2/show/NCT02220842?term=NCT02220842&draw=2&rank=1
- 99.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537–46. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 100.Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 poplar and phase 3 oak clinical trials. J Thorac Oncol 2021;16:140–50. 10.1016/j.jtho.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 101.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 102.McFarlane JJ, Kochenderfer MD, Olsen MR, et al. Safety and efficacy of nivolumab in patients with advanced clear cell renal cell carcinoma: results from the phase IIIb/IV CheckMate 374 study. Clin Genitourin Cancer 2020;18:469–76. 10.1016/j.clgc.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 103.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41. 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emens LA, Cruz C, Eder JP, et al. Long-Term clinical outcomes and biomarker analyses of Atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019;5:74–82. 10.1001/jamaoncol.2018.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hubrecht RC, Carter E. The 3Rs and humane experimental technique: implementing change. Animals 2019;9. 10.3390/ani9100754. [Epub ahead of print: 30 09 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu R, Li H, Liu L, et al. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther 2012;13:123–9. 10.4161/cbt.13.3.18696 [DOI] [PubMed] [Google Scholar]
- 107.Kratochwil C, Flechsig P, Lindner T, et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J Nucl Med 2019;60:801–5. 10.2967/jnumed.119.227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brünker P, Wartha K, Friess T, et al. RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and tumor cell apoptosis. Mol Cancer Ther 2016;15:946–57. 10.1158/1535-7163.MCT-15-0647 [DOI] [PubMed] [Google Scholar]
- 109.Dziadek S. A86: extensive FAP expression analysis in 23 tumor indications and potential application in defining the patient population in FAP-targeting cancer immunotherapies. SITC 36th annual meeting, Washington, DC, 2021. [Google Scholar]
- 110.Moreno V. 370 - Pharmacodynamic assessment of a novel FAP-targeted 4-1BB agonist, administered a single agent and in combination with atezolizumab to patients with advanced solid tumors. SITC 35th Annual Meeting, 2020. [Google Scholar]
- 111.Melero I. 1025MO - First-in-human (FIH) phase I study of R07122290 (RO), a novel FAP-targeted 4-1BB agonist, administered as single agent and in combination with atezolizumab (ATZ) to patients with advanced solid tumors. ESMO Virtual Congress 2020, 2020. [Google Scholar]
- 112.Velloso MJG. 286 - Tumor targeting and tissue biodistribution of RO7122290, a novel FAP-targeted 4-1BB (CD137) agonist, in patients with advanced solid tumors, using [89Zr]-RO7122290 as a PET tracer. SITC 35th Annual Meeting, 2020. [Google Scholar]
- 113.Feister AJ. Nonhuman Primate Evaluation and Analysis - Part 1: Analysis of Future Demand and Supply, 2018. Available: https://orip.nih.gov/sites/default/files/508%20NHP%20Evaluation%20and%20Analysis%20Final%20Report%20-%20Part%201%20Update%2030Oct2018_508.pdf
- 114.Grimm D. Record number of monkeys being used in U.S. research, 2018. Available: https://www.science.org/content/article/record-number-monkeys-being-used-us-research
- 115.NIH-ORIP . National Primate Research Centers Consortium. Available: https://orip.nih.gov/resource-directory/national-primate-research-centers