Abstract
Background
Patients with critical illness can lose more than 15% of muscle mass in one week, and this can have long-term detrimental effects. However, there is currently no synthesis of the data of intensive care unit (ICU) muscle wasting studies, so the true mean rate of muscle loss across all studies is unknown. The aim of this project was therefore to systematically synthetise data on the rate of muscle loss and to identify the methods used to measure muscle size and to synthetise data on the prevalence of ICU-acquired weakness in critically ill patients.
Methods
We conducted a systematic literature search of MEDLINE, PubMed, AMED, BNI, CINAHL, and EMCARE until January 2022 (International Prospective Register of Systematic Reviews [PROSPERO] registration: CRD420222989540. We included studies with at least 20 adult critically ill patients where the investigators measured a muscle mass-related variable at two time points during the ICU stay. We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and assessed the study quality using the Newcastle–Ottawa Scale.
Results
Fifty-two studies that included 3251 patients fulfilled the selection criteria. These studies investigated the rate of muscle wasting in 1773 (55%) patients and assessed ICU-acquired muscle weakness in 1478 (45%) patients. The methods used to assess muscle mass were ultrasound in 85% (n = 28/33) of the studies and computed tomography in the rest 15% (n = 5/33). During the first week of critical illness, patients lost every day −1.75% (95% CI −2.05, −1.45) of their rectus femoris thickness or −2.10% (95% CI −3.17, −1.02) of rectus femoris cross-sectional area. The overall prevalence of ICU-acquired weakness was 48% (95% CI 39%, 56%).
Conclusion
On average, critically ill patients lose nearly 2% of skeletal muscle per day during the first week of ICU admission.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04253-0.
Keywords: Intensive care unit, Critical illness, Muscle wasting, Muscle atrophy, ICU-acquired weakness, ICU-AW
Introduction
Critical illness is defined as the deterioration of an illness resulting in a deranged homeostasis. This leads to life-threatening organ dysfunction requiring advanced organ support techniques, and therefore it is associated with high morbidity and mortality. Both the underlying disease and the causes for the unfavourable course of the disease are diverse and often end in further secondary organ dysfunctions, which are referred to as multi-organ failure. Organ dysfunction, sepsis, prolonged mechanical ventilation, and immobility are risk factors for muscle wasting which leads to ICU-acquired weakness (ICU-AW) [1].
ICU-AW is an umbrella term that describes a bundle of neuromuscular disorders that develop due to admission in the intensive care unit and severe illness [2]. The pathophysiology of ICU-AW is incompletely understood; however, ICU-AW appears to be triggered by critical illness and its severity during the ICU is independent of the underlying primary condition [3–5]. The hallmarks of the ICU-AW are an inflammatory response, bioenergetic dysfunction, altered protein balance, neuronal axon degeneration, changes in muscle histology, and muscle wasting [6, 7]. During critical illness, factors such as immobilisation and altered neuroendocrine responses cause muscle wasting by making protein balance negative [8]. On the other hand, muscle dysfunction is caused by multiple factors including microcirculatory disturbances reducing oxygen supply, bioenergetic mitochondria impairment causing reduced ATP production, and disruptions in the ion channels membrane [9]. These conditions in addition to the patients immobilisation and malnutrition make muscle wasting the dominant phenotype of acquired muscle weakness in critically ill [10].
Patients with critical illness lose muscle mass and muscle function with limited treatment options. Specifically, muscle wasting starts early in the first week of critical illness and patients with multi-organ failure lose more muscle mass than other patients [11]. Observational studies have reported that muscle wasting is associated with a longer stay on ICU [12, 13], and higher ICU [14] and hospital mortality [15]. They also noted that muscle wasting is associated with acquired weakness [16, 17]. However, to date there is no study that has summarised published data on the daily amount of muscle that is lost in ICU patients, the methods used to monitor muscle size in those patients, and on the prevalence of ICU-AW in critically ill patients.
To address this issue, we carried out a systematic review and meta-analysis aiming to answer the following research questions:
What is the rate of muscle wasting in critically ill patients?
What are the methods used to assess changes in muscle mass in critically ill patients?
What is the incidence of ICU-AW in critically ill patients?
What are the outcomes (i.e. mortality, mechanical ventilation time, and length of stay) associated with muscle wasting?
Methods
The study protocol was registered and published on 13 January 2022 on the International Prospective Register of Systematic Reviews (PROSPERO) of the National Institute for Health Research (NIHR) under the ID CRD42022298954. We conducted this systematic review and meta-analysis in accordance with the Joanna-Briggs Institute (JBI) Reviewer’s Manual for Systematic Reviews of Literature [18] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [19, 20].
Definitions
The measurement of the rectus femoris is taken by placing the transducer perpendicular to the long axis of the tight on its superior aspect, three-fifth of the distance from the anterior superior iliac spine to the superior patella border. This is the highest point in the tight that the entire rectus femoris cross section can be visualised in a single field. The cross-sectional area is calculated by a planimetric technique after the inner echogenic line of the rectus femoris is outlined by a movable cursor on a frozen image [21].
The quadriceps femoris muscle include vastus medialis, vastus lateralis, vastus intermedius, and rectus femoris.
Search strategy and selection criteria
Search strategy
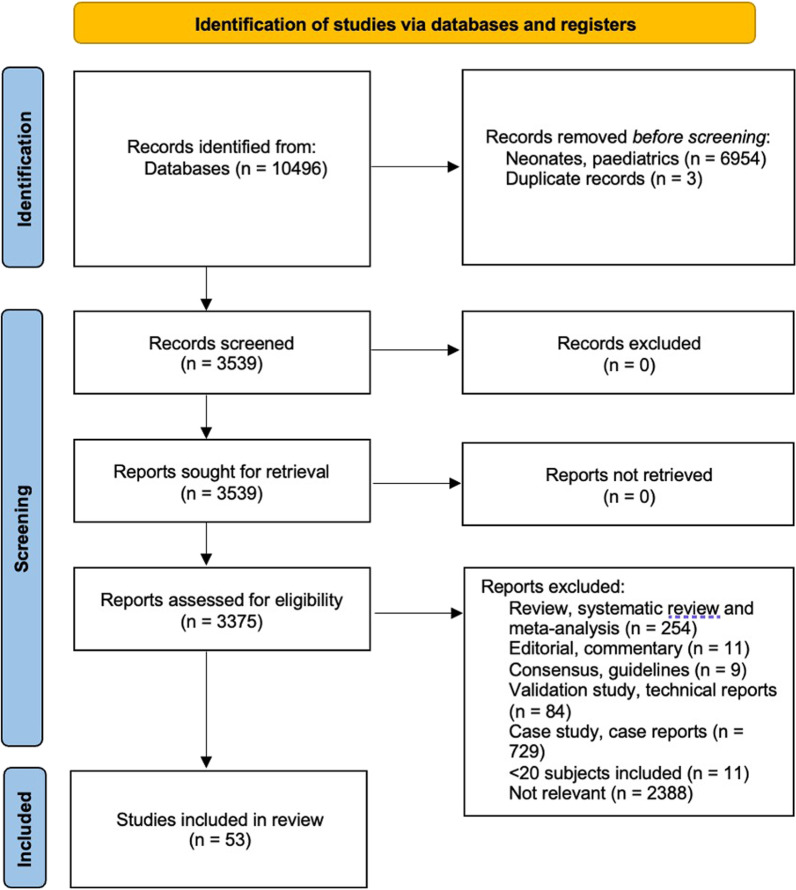
We conducted our search on MEDLINE (National library of medicine: Bethesda, MD) and AMED, BNI, CINAHL, and EMCARE. Studies were also identified and retrieved by citation searching from the references of each relevant study, as reported in Fig. 1.
Fig. 1.
Flow diagram of selected studies according to the PRISMA guidelines
For the initial literature search, we used a combination of MeSH terms and key terms including (muscle mass OR muscle atrophy OR muscle wast* OR muscle loss OR muscle weakness OR muscle strength OR muscle function OR intensive care unit acquired weakness OR ICU-AW) AND (critical* ill* OR critical care OR intensive care unit OR ICU).
We included studies that were published between 1 January 2000 and 31 January 2022.
The references of all included papers, review articles, commentaries, and editorials on this topic were reviewed to identify other relevant studies which were missed during the primary search. If necessary, we contacted the corresponding authors to obtain data necessary for our study. No language restrictions were applied. Three investigators (BF, TM, and CC) independently screened title and abstracts in duplicate for selection of full-text review. If a decision was not achieved from reading the title and abstract alone, the full text was reviewed. The reviewers also independently reviewed the full text of relevant studies and decided on eligibility. Inter-rater disagreements in the study selection were resolved by consensus or if necessary, by consultation with a senior author (HW). A flow chart of the whole process is presented according to PRISMA guidelines 2020 in Fig. 1.
Study inclusion and exclusion criteria
Eligible studies included adult women and men (age > 18 years old) admitted to any critical care facility (ICU/high dependency unit [HDU]) receiving invasive or non-invasive mechanical ventilation. Large treatment effects have been reported in studies including fewer patients [22]; hence, we included all peer-reviewed studies enrolling leastwise 20 critically ill patients who had assessment of muscle mass or ICU-AW at any time point after the day of admission or at two time points during ICU stay. We excluded (i) studies published prior 1 January 2000, (ii) reviews and meta-analysis, (iii) book chapters, comments, editorials, (iv) guidelines and consensus report, (v) protocol studies, and (vi) case studies, case reports.
Data extraction
Three reviewers (BF, TM, and CC) extracted the following information from each publication into an Excel file: date of publication, country, study design, number of included patients, age, gender, clinical features, laboratory findings, severity and outcome of the disease (including health-related quality of life, cognitive status, mental health, physical function, muscle and/or nerve function, and pulmonary function). Data extraction was performed in duplicate by three authors acting independently (BF, TM, and CC). A flow chart of the whole process is presented according to PRISMA guidelines 2021 in Fig. 1. The characteristics of the studies included are presented in Table 1.
Table 1.
Characteristics of included studies assessing muscle wasting
| Author/References | Design/Country/Setting | No. of Population | Inclusion criteria | Tool | Body site | Timing | Muscle mass loss | Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1. Lambell [45] |
RC single centre Australia ICU |
32 Trauma, medical, surgical |
> 18 yo, who had CT scans before admission with routine care and a second or multiple CT scan ≥ 7 days later. Patients included if both CT scans were appropriate for analysis of SMA and if the predominant nutrition route was enteral and/or parenteral (planned > 70% requirements), due to oral intake not being routinely recorded in a quantifiable manner | CT | Skeletal muscle CSA at L3 level | CT scan at week 1 (day 0–7) and second CT scan ≥ 7 days later |
SMA loss in 7 days MD: −21.9 [−29.9 to −13.9] cm2, (149.9 ± 38.8 vs. 127.9 ± 38.4 cm2), p < 0.001 %SMA change per day: −1.27 ± 0.88% cm2 |
Not reported |
| 2. Lee [15, 69] |
PC Single Centre Malaysia ICU |
86 Cardiovascular, respiratory, gastrointestinal, neurology, sepsis, trauma, metabolic, renal, immunocompromised |
Consecutive patients > 17 years old, expected to stay > 96 h on ICU. Patients with ‘normal’ baseline muscle status | USS | Quadriceps muscle layer thickness (QMLT), RF CSA, VI, pennation angle (PA) and fascicle length (FL) | Day 1 (within first 48 h), 7, 14, 22 of ICU admission |
%QMLT compared to baseline: at day 7: −8.61 ± 19.44 at day 14: −15.63 ± 23.75 %RF CSA compared to baseline: at day 7: −9.81 ± 19 at day 14: −22.73 ± 20 |
Every 1% loss of QMLT over the first week of critical illness was associated with 5% increase in 60-day mortality (Adjusted odds ratio [AdjOR] 0.950 for every 1% less QMLT loss, 95% CI 0.90,0.99; p = 0.023 |
| 3. Toledo [14] |
PC single centre Brazil ICU |
74 Sepsis, stroke, lung transplant, cardiac insufficiency |
Patients > 18 years old, needing mechanical ventilation for ≥ 48 h | USS | Quadriceps muscle thickness | Day 1, 3, 7 |
% Quadriceps muscle thickness decrease from day 1 to day 7 Right leg: 15% (± 19.5%) Left leg: 12.7% (± 16%) |
Predictor of survival: cut-off value in muscle thickness of ≤ 1.64 cm on day 7 (HR = 0.7, 95% CI, 0.582–0.801, sensitivity 81%, specificity 63%). Higher probability to remain on mechanical ventilation in patients with 1.64 cm loss of thigh muscle thickness on day 7, HR: 2.1 (95% CI, 1.1–3.8) higher than their counterparts (P = 0.017). Greater loss of thigh muscle thickness on day 7 for worst ICU survival (HR: 3.7; 95% CI, 1.2–11.5) and hospital survival (HR: 4.5; 95% CI, 1.5–13.7) |
| 4. Zhang [26] |
PC Single centre China ICU |
37 Sepsis, pneumonia, severe pancreatitis, liver failure, renal failure, cardiac dysfunction, surgical |
Patients aged ≥ 18 years with an anticipated ICU stay of at least 2 days | USS | RF thickness and CSA, VI and BB muscles | Day 1, 4, 7, 10 | % not reported | Not reported |
| 5. Borges [27] |
PC Single centre Brazil ICU |
45 Severe and septic shock |
Patients > 18 yo with sepsis or severe septic shock within 24 h of admission | USS | RF CSA | Day 2, 4, 6, at ICU discharge and hospital discharge |
RF CSA: day 2: 5.11 ± 0.85cm2 versus day 6: 4.49 ± 0.84cm2; P = 0.001 Daily RF CSA loss: 1.2% During ICU stay average muscle loss of 13.5% compared to baseline |
RF CSA in patients who underwent mechanical ventilation versus those without mechanical ventilation, P = 0.080. RF CSA during hospital stay in mechanically ventilated 17.25% versus patients without ventilation 10.76%, P = 0.001 |
| 6. Dimopoulos [13] |
PC Single centre Greece ICU |
165 Cardiac surgery |
Patients > 18 yo admitted to cardiac ICU within 24 h of cardiac surgery | USS | RF thickness | Day 1, 3, 5, 7 |
RF mass (cm): D1: 1.37 ± 0.25 D3: 1.2 ± 0.5 D5: 1.25 ± 0.52 RF + VI mass(cm): D1: 2.58 ± 0.34 D3: 2.41 ± 0.94 D5: 2.37 ± 0.8 In 5 days RF mass loss by 2.2% [(95%CI: −0.21 to 0.15), P = 0.729] and RF + VI mass loss by 3.5% [(95% CI: −0.4 to 0.22), P = 0.530] |
RF + VI mass < 2.5 cm on D1: longer ICU length of stay (47 ± 74 h vs 28 ± 46 h, P = 0.02) and ventilator time (17 ± 9 h vs 14 ± 9 h, P = 0.05). ICU-AW versus no ICU-AW on D3: longer ventilation (44 ± 14 h vs 19 ± 9 h, P = 0.006) and ECMO (159 ± 91 min vs 112 ± 71 min, P = 0.025) |
| 7. Kemp [12] |
PC Single centre UK ICU |
20 Cardiac surgery |
Adults > 18 yo with elective aortic operation requiring admission to the ICU as identified by the surgical team | USS | RF CSA | Day before surgery and day 1, 3, 7 after surgery |
RF CSA (cm2) at D0: 6.85 ± 1.45 (5.4–8.3) D7: 6.3 ± 1.45 (4.85–7.75) RF CSA mass loss: 8% (6.6–10.2) |
Muscle loss > 10% was associated with longer ICU length of stay (P = 0.038), hospital length of stay (P = 0.014), mechanical ventilation time (P = 0.05) |
| 8. Mayer [16] |
PC Single centre USA ICU |
41 Sepsis or acute respiratory failure |
Adults > 18 yo, diagnosis of acute respiratory failure or sepsis of any origin anticipated to survive and spend > 3 days on ICU, enrolled within 48 h of admission | USS | RF and TA CSA, muscle thickness (mT), echo intensity (EI) | Day 1, 3, 5, 7 |
RF mT: D1 0.98 ± 0.3 versus D7 0.81 ± 0.27, P = 0.0316 RF CSA: D1 2.99 ± 0.99 versus D7 2.47 ± 0.88; P = 0.0253 RF EI: D1 91 ± 24.9 versus D7 99.1 ± 27.6; P = 0.081 TA mT: D1 2.01 ± 0.36 versus D71.82 ± 0.31, P < 0.001 TA CSA: D1 5.3 ± 0.89 versus D7 4.71 ± 0.95, P < 0.001 TA EI: D1 82.7 ± 21.2 versus D7 96.7 ± 22.6; P = 0.002 Changes from D1 to 7 RF mT: 20.1% (12–26) RF CSA: 18.5% (11–23) RF EI: 10.5% (5–20) TA mT: 9.1% (5–12) TA CSA: 8.1% (5–15) TA EI: 15.4% (7–28) |
RF EI in first 7 days of ICU admission predictor of ICU-AW (area under curve = 0.912) |
| 9. McNelly [44] |
RCT Multi-centre UK ICU |
121 mechanically ventilated patients | Adult (> 18 years), expected to be intubated and ventilated for ≥ 48 h; requiring enteral nutrition via nasogastric tube; multi-organ failure (Sequential Organ Failure Assessment [SOFA] score > 2 in ≥ 2 domains at admission); likely ICU stay ≥ 7 days and likely survival ≥ 10 days | USS | RF CSA | At Day 1, 7, 10 in both groups |
Intermittent feed Day 7 −12.9%(95%CI −17.1 to –8.7) Day 10 −18.7% (95% CI −29.8 to −7.6) Continuous feed Day 7: −14.7% (95% CI −19.5 to 9.9) Day 10: −20.6% (95% CI −31.0 to 10.2) P value P = 0.431 P = 0.337 |
Safety profiles, gastric intolerance, physical function milestones, and discharge destinations did not differ between groups |
| 10. Nakamura [46] |
RCT Single centre Japan ICU |
117 Medical and surgical |
Patients admitted to ICU | CT | Femoral muscle volume | Day 1 and 10 | Femoral muscle volume loss was 12.9 ± 8.5% in the high-protein group and 16.9 ± 7.0% in the medium-protein group, with significant difference (p = 0.0059) | For critical care, high-protein delivery provided better muscle volume maintenance, but only with active early rehabilitation |
| 11. Nakanishi [28] |
PC Multi-centre Japan ICU |
56 Respiratory failure, heart failure, sepsis, cardiac arrest, trauma, neurologic |
Consecutive adult > 18 yo, expected to remain in ICU > 5 days. Patients were prospectively recruited within 12 h of ICU admission | USS | RF CSA | Day 1, 3, 5, 7 |
RF CSA loss: –8.6 ± 4.9% on D3, –13.8 ± 5.9% on D5, –18.2 ± 5.6% on D7, respectively (p < 0.01) |
Not reported |
| 12. Nakanishi [29] |
PC Multi-centre Japan ICU |
64 Respiratory failure, sepsis, post-cardiac surgery, heart failure, cardiac arrest, trauma, neurologic |
Expected mechanical ventilation > 48 h, stay in ICU > 5 days | USS | BB CSA, RF CSA | Day 1, 3, 5, 7 and ICU discharge |
BB CSA decreased by 6.0% (95% CI, 4.4–7.6%) D3, 11.0% (95% CI, 9.3–12.7%) D5, and 15.6% (95% CI, 13.5– 17.6%) D7 (p < 0.01) RF CSA decreased by 6.2% (95% CI, 3.3%–9.1%) D3, 12.9% (95% CI, 9.8–15.9%) D5, and 17.1% (95% CI, 13.4%– 20.7%) D7; (p < 0.01) BB CSA loss: 2.24% per day, BB CSA loss: 15.6% per week, RF CSA loss: 2.44% per day, RF CSA: 17.1% per week |
BB and RF muscle atrophy did not predict in-hospital mortality on day 3 (P = 0.70 and P = 0.53, respectively). BB muscle loss predicted mortality on days 5 (P = 0.02) and 7 (P = 0.01). RF muscle atrophy on days 5 and 7 predicted mortality (P = 0.02 and P = 0.01, respectively) |
| 13. Borges [30] |
PC Single centre Brazil ICU |
37 Severe sepsis or septic shock |
Patients > 18 yo diagnosed with severe sepsis or septic shock within 24 h of evolution | USS | RF CSA | Day 2, 4, 6, ICU discharge and hospital discharge |
RF CSA loss: −5.20 ± 0.47 on D2, −4.4 ± 0.45 on ICU discharge and 4.36 ± 0.42 on hospital discharge, (P < 0.05) RF CSA: −1.45% per day; −14.5% ± 7.6 in 10 days |
No difference in RF CSA between patients who underwent mechanical ventilation and in those without; P = 0.08 |
| 14. Dusseaux [47] |
RC Single centre France ICU |
25 Sepsis, septic shock, acute pancreatitis, cardiac arrest, pneumonia, endocarditis |
> 18yo, in ICU for at least 7 days, required mechanical ventilation during their ICU stay, and had abdominal CT scans within the first 48 h of admission to ICU (CT 1: initial assessment) and 7 to 14 days after (CT 2: late assessment CT 2) | CT | Skeletal muscle radiodensity, skeletal muscle mass CSA at L3 vertebra | CT 1: within the first 48 h of admission CT 2: 7 to 14 days later |
SMM (cm2/m2): CT 1 48.73 ± 12.57; CT 2 46.64 ± 10.64 SMD: CT 1 34.86 ± 10.46, CT 2 33.56 ± 7.67 SMM loss: −2.09 (± 6.96); p = 0.183 over 7–14 days SMD loss: −1.3 ± 8.53 over 7–14 days |
No significant correlation was observed between mortality outcome and SMM [P = 0.289; OR 95% CI: 0.93 (0.81–1.060)] or SMD [P = 0.091; OR 95% CI: 1.12 (0.98–1.28)] |
| 15. Haines [48] |
RC Single centre UK ICU |
10 7 Trauma |
All trauma admissions admitted to the adult ICU either directly or via the operating theatre |
Urea/creatinine ratio CT |
Total abdominal muscle CSA measured at the level of the third lumbar (L3) vertebrae, and psoas muscle CSA was calculated at the L4 level |
CT 1: on admission CT 2: within 1–9 days or after 10 days of ICU stay |
At the second CT urea/creatinine ratio negatively correlated with L4 psoas and L3 muscle cross-sectional areas (R2 0.39, p < 0.001) | |
| 16. Nakanishi [31] |
PC Single centre Japan ICU |
21 surgical, non-surgical | Adults > 18yo expected mechanical ventilation > 48 h, stay in ICU > 5 days | USS, BIA | Combined BB and RF CSA | Day 1, 3, 5, 7, 10 |
Muscle mass: on D3 −9.2% (95% CI, 5.9–12.5%), on D5 −12.7% (95% CI, 9.3–16.1%), on D7 −18.2% (95% CI, 14.7–21.6%), on D10 −21.8% (95% CI, 17.9–25.7%) (P < 0.01) Muscle loss: −2.6% per day; −18.2% per week |
Not reported |
| 17. Trung [32] |
PC Single centre Vietnam ICU |
79 Tetanus |
Patients ≥ 16 y of age with a clinical diagnosis of generalized tetanus and within 48 h of ICU admission | USS | RF CSA | Day 1, 7, 14, at hospital discharge |
RF CSA loss: −7.43 ± 3.17 at D7, −11.59 ± 4.52 at D14, −13.2 ± 5.4 at discharge Muscle loss between admission and discharge P < 0.01 |
Not reported |
| 18. Wandrag [33] |
PC Multi-centre UK ICU |
43 Pneumonia, cardiology/cardiac surgery, neurology/neurosurgery, sepsis, septic shock, major trauma, traumatic brain injury, gastroenterology, gastrointestinal surgery, HIV, multi-organ failure, renal failure |
Patients > 18yo, anticipated to be ventilated > 48 h | USS | Combined muscle depth BB, forearm (flexor compartment of muscle) and thigh (rectus femoris and vastus intermedius) | Day 1, 3, 7 and 14 | Total muscle depth (cm): D1: 7.6 ± 3.7, D7: 6.5 ± 3.1; MD (cm): −1.1 (1.5–0.7), P < 0.0001 | |
| 19. Hadda [34] |
PC Single centre India ICU |
70 Sepsis |
Adults > 18 years old, diagnosis of sepsis (non-surgical) | USS | BB thickness and quadriceps muscles | Day 1, 3, 5, 7, 10, 14 and then weekly until discharge or death |
On day 7 percentage muscle thickness loss [median (IQR)] BB: 7.61 (− 1.51, 32.05) %; P < 0.001 Quadriceps: 10.62 (− 1.48, 32.06) %, p < 0.001); P < 0.001 |
Decline in muscle thickness was significantly higher among patients with worse outcome at 90 days |
| 20. Hayes [35] |
PC Single centre Australia ICU |
25 ARDS, bridge to transplant, pulmonary hypertension, cardiac failure/infarction, cardiac arrest |
Patients > 18 yo expected to be on ECMO > 24 h or > 5 days in ICU prior to recruitment | USS | RF CSA | At baseline, day 10, day 20 |
RF CSA loss: 4.2 ± 1.3 at D1, 3.4 ± 1.1 at D10 compared to baseline: RF CSA −19.2% [95% CI, − 13.7 to − 24.8%], P < 0.001 at day 10; −30.5% [95% CI, − 24.1 to − 36.9], P < 0.001 at day 20 |
Not reported |
| 21. Katari [36] |
PC Single centre India ICU |
100 Mixed medical and surgical |
Patients 18–90 years old, anticipated ICU stay > 7 days | USS | Total anterior thigh thickness, RF thickness, and combined thickness of VI and RF | Day 1, 3, 7 |
RF thickness: D1: 1.37 ± 0.41, (0.96) D3: 1.26 ± 0.41, D7: 1.22 ± 0.47; P < 0.001 respectively RF thickness: −11 (± 38.5)% at D7 compared to baseline |
Not reported |
| 22. Nakanishi [37] |
PC Single centre Japan ICU |
28 Mixed ICU patients |
Expected mechanical ventilation > 48 h, stay in ICU > 5 days | USS | BB and RF thickness and CSA | Day 1, 3, 5, 7 |
Loss compared to baseline: BB thickness at D7 −13.2%; P < 0.01 BB CSA at D7-16.9%; P < 0.01 RF thickness at D7: −18.8% RF CSA at D7: −20.7% |
Not reported |
| 23. Palakshappa [17] |
PC Single centre USA ICU |
29 Medical patients with sepsis and shock or respiratory failure |
Admitted to the medical ICU with a diagnosis of sepsis complicated by respiratory failure or shock requiring vasopressors for a minimum of 6 h, and an anticipated ICU length of stay > 48 h | USS |
RF CSA Quadricep muscle thickness |
On Day 0 and Day 7 |
RF CSA decreased by 23.2% Quadriceps thickness decreased by 17.9% |
Quadriceps muscle thickness shows a weak correlation with the strength RF CSA depicts a moderate correlation with the strength |
| 24. Pardo [24] |
PC Single centre France ICU |
29 Mixed ICU patients |
> 18 years old, expected ICU stay > 7 days, patients to receive muscle US as part of usual care | USS | Quadriceps femori muscle thickness | Day 1, 3, 5, 7, 21 |
Quadriceps femori at admission: 1.72 [95% CI, 1.62; 2.13], D7: 1.45 [95% CI, 1.24; 1.665] P < 0.01, D21: 1.30 [95% CI, 0.80; 1.48] P < 0.01 Quadriceps femori loss: 16% over a week |
Not reported |
| 25. Silva [38] |
PC Single centre Brazil ICU |
22 TBI |
Patients 18–60 yo and mechanically ventilated | USS | TA, BB and RF muscle thickness | Day 1, 7, 14 |
Muscle wasting at D14 compared to baseline: RF: −22% P = 0.0001, TA: −19% P = 0.0001, BB: −12% P = 0.0004 |
Not reported |
| 26. Annetta [39] |
PC Single centre Italy ICU |
38 Trauma |
Trauma patients with an injury severity score (ISS) exceeding 25, admitted to ICU within few hours after the injury. Only well-nourished, previously healthy subjects, aged 18–59 yo | USS | RF and TA CSA | Admission day, 5, 10, 15, 20 |
RF CSA (cm2): D0: 6.1 [5.1–7.3], D5: 5.9 [4.8–6.3], D10: 5.1 [4.3–6.2], D15: 4.6 [3.8–5.3], D20: 3.5 [3.2–4.7] AT CSA (cm2): D0: 5.6 [4.5–6.4], D5: 4.8 [3.7–5.6], D10: 4.0 [3.7–5.2], D15: 4.0 [3.3–4.8], D20: 4.2 [3.4–4.7] Overall 45% reduction in RF CSA during the first 20 days of ICU stay; 15% loss from day 5 to 10, 12% from day 10 to 15, 21% from day 15 to 20 TA CSA 22% loss during the overall ICU stay, P = 0.30 |
Not reported |
| 27. Puthucheary [40] |
PC Multi-centre UK ICU |
43 Surgical and medical |
All patients were recruited within 24 h of admission to a university hospital and a community hospital and were expected to survive intensive care unit (ICU) admission after being invasively ventilated for over 48 h and in the ICU longer than 7 days | USS | RF thickness and RF CSA | Day 1, 7, 10 |
RF thickness Day 7: −5.88 (−11.69, −0.06) Day 10: −9.65 (−15.43, −3.84 (P = 0.031) RF CSA Day 7: −13 (−16.5, −9.48) Day 10: −17.72 (−21.15; −14.29) (P = 0.004 |
ΔRFCSA was greater in those with knee extensor weakness than those without (20.7% [95% CI, 13.7–27.7] vs. 8.4% [95% CI, 2.5–14.3], respectively; P = 0.012). ΔThickness did not differ between these groups (12.6% [95% CI, 0.94–24.2] vs. 12.1 [95% CI, 2.7–21.5], respectively; P = 0.95). In a bivariable logistical regression, ΔRFCSA was associated with knee extensor weakness (odds ratio, 1.101 [95% CI, 1.011–1.199]; P = 0.027), but Δthickness was not (odds ratio, 1.001 [95% CI, 0.960–1.044]; P = 0.947) |
| 28. Segaran [41] |
PC Single centre UK ICU |
39 Surgical, medical, trauma |
Patients > 18 yo, BMI > 19 kgm−2, expected to be mechanically ventilated > 48 h, and artificially fed | USS | Muscle depth of BB, forearm and thigh | Day 1, 3, 5, 7, 12, 14 | Muscle loss per day:2.93%, at D7: 20.53% | Not reported |
| 29. Turton [42] |
PC Single centre UK ICU |
22 Mechanically ventilated critically ill patients |
Patients who > 18 years of age who were assented within 24 h of being intubated and admitted to the participating intensive care units were included in the study | USS |
Pennation Angle and Fascicle Length and Muscle thickness Upper Limb: Right Elbow Flexor Compartment Lower Limb: Right Vastus Lateralis The right medial head of the gastrocnemius |
On days 1, 5 and 10 |
Elbow flexor compartment and gastrocnemius muscle thickness did not significantly change Vastus Lateralis pennation angle and muscle thickness significantly reduced by day 5 Fascicle length did not significantly change for all three muscle groups |
Muscle thickness and architecture of vastus lateralis undergo rapid changes during the early phase of admission to a critical care environment |
| 30. Parry [25] |
PC Single centre Australia ICU |
22 Mixed medical and surgical |
Adults ventilated > 48 h, remain at least 4 days in ICU | USS | RF thickness, vastus lateralis thickness, VI thickness, RF CSA | Baseline (day 1), day 3, day 5, day 7, day 10 |
Compared to baseline: RF Thickness: D3: −8.7%, D5: −16.6%, D7: −24.9%, D10: −30.4%.; VI Thickness D3: −1.3%, D5: −18.1%, D7: −20.0%, D10: −29.7% VL thickness D3: −0.2%, D5: −5.7%, D7: −6.0%, D10: −14.1% RF CSA D3: −1.0%, D5: −11.8%, D7: −16.8%, D10: −29.9% |
Correlation between ICU discharge and RF, VI, VL thickness (P < 0.05) |
| 31. Jung [49] |
RC Single centre France ICU |
23 Mixed ICU patients |
Admitted to ICU and had CT scan before admission, CT scan during ICU, at least one measure of diaphragmatic contractility | CT scan | Psoas volume, CSA of skeletal muscles at L3 vertebra examination with 64-section spiral CT | Baseline and 25 days after ICU admission |
Psoas volume baseline:272 ± 116, D25: 233 ± 108; P < 0.01 Skeletal muscle CSA cm2/m2 baseline: 17.1 ± 5.4, D25: 16.1 ± 5.2 Psoas loss: 14.34% skeletal muscle CSA loss: 5.85% |
Not reported |
| 32. Puthucheary [11] |
PC Single centre UK ICU |
63 Sepsis, trauma, intracranial bleeding, acute liver failure, cardiogenic shock |
Patients > 18 yo, anticipated to be intubated > 48 h, spend > 7 days in critical care, and to survive ICU stay | USS; 28 patients were assessed by USS, ration protein DNA, histopathological analysis | RF CSA, biopsy, histological samples | Day 1, 3, 7, 10 |
RF CSA mm2 at D1: 514 (464–566), D3: 495 (442–549), D7: 450 (402–498), D10: 423 (378–469) From days 1 to 7 (− 12.5% [95% CI, − 15.8% to − 9.1%]; P = 0.002), and to day 10 (− 17.7% [95% CI, − 20.9% to − 4.8%]; P < 0.001) In 28 patients assessed by all 3 methods on days 1 and 7, the rectus femoris cross-sectional area decreased by 10.3% (95% CI, 6.1% to 14.5%), the fibre cross-sectional area by 17.5% (95 CI%, 5.8% to 29.3%), and the ratio of protein to DNA by 29.5% (95% CI, 13.4% to 45.6%) |
Not reported |
| 33. Reid [43] |
PC Single centre UK ICU |
50 Sepsis, cardiac, respiratory failure, multiple trauma, head injury, head injury, medical |
Patients > 18yo admitted to the ICU for ventilatory support > for 5 days or longer | USS | Mid-upper arm circumference and muscle thickness | 1–3-day intervals between 5 and 39 days (median 7 days) |
Muscle thickness at baseline: 4.5(2.6–6.8); change at D7: −0.57(0.2–2.3) Muscle loss: 1.6%(0.2–5.7) per day, 12.05%(0–46.7) |
Not reported |
PC prospective cohort, RC retrospective cohort, RCT randomised controlled trial, ICU intensive care unit, CT computed tomography, USS ultrasound sonography, CSA cross-sectional area, SMA skeletal muscle area, MD median difference, RF rectus femoris, VI vastus intermedius, BB bicep brachii, TA tibia anterior, mT muscle thickness, D day, ICU-AW intensive care unit acquired weakness
Risk of bias assessment
Three reviewers (BF, TM, and CC) independently assessed the risk of bias using the Newcastle–Ottawa Scale (NOS) for observational studies [14], and the Cochrane Risk of Bias tool (ROB2) was used for assessing randomised controlled trial [23]. Risk of bias across studies was assessed using the approach outlined by the Grading of Recommendations Assessment Development and Evaluation (GRADE) working group [15, 16]. Any disagreements were recorded and resolved by involvement of an additional reviewer.
Data synthesis and analysis
A narrative and tabular synthesis of the findings from the included studies was provided. Data were grouped into the main outcomes above specified. Numerical data on the long-term outcomes above specified were collected for quantitative analysis.
Statistical analysis
Mean and standard deviation (SD) or median and interquartile range (1st quartile to 3rd quartile) were used for numerical data if appropriate, while odds ratio (OR) with 95% confidence interval (CI) was used for categorical data. For data presenting median and interquartile range (IQR) or median and range, mean and standard deviation (SD) were transformed according to standard equations [17–19]. The studies included for meta-analysis were pooled together using the random-effects model accounting for the incidence. The results were presented in forest plots. Heterogeneity among studies was evaluated using the Tau2 test, I2 statistics, and Cochrane Q. A p value < 0.05 was considered as evidence of publication bias. Analysis of data was performed using the statistical software packages Review Manager 5.4 (RevMan 5.4.1®) and OpenMeta [Analyst]®.
Results
We identified 10,496 studies through our literature search. After removing duplicates and publications that did not fit our inclusion criteria, we were left with 53 publications. Of these, 33 quantify muscle wasting over time with 4 studies measuring muscle wasting and ICU-AW and 20 studies assess ICU-AW only. See Fig. 1 to appreciate the flow diagram of the studies included.
Overall, the publications reported data on 3251 patients, 1773 (55%) on muscle wasting, and 1478 (45%) for ICU-acquired weakness. We found 1 randomised controlled trial and 43 single-centre and 8 multi-centre observational studies across Australia, Asia, USA, South America, and Europe. Studies’ characteristics are summarised in Tables 1 and 2.
Table 2.
Characteristics of included studies assessing ICU-acquired weakness
| Author/References | Design/Country/Setting | No. of Population | Inclusion criteria | Exclusion criteria | Tool | Score | Prevalence of ICU-AW |
|---|---|---|---|---|---|---|---|
| 1. Van Aerde et al. [50] |
RC Single centre Belgium ICU |
50 COVID-19 |
Adult patients (> 18 years old) requiring mechanical ventilation | N/A | Clinical examination | MRC sum score | (36/50) 72% |
| 2. Ballve et al. [51] |
PC Single centre Brasil |
111 | Adult patients (> 18 years old), mechanically ventilated for ≥ 24 h | Pre-existing neurological conditions (central or peripheral nervous system disease, stroke), orthopaedic or traumatic limitations | Clinical examination | MRC sum score | (66/111) 59% |
| 3. Nguyen et al. [52] |
PC Single centre Vietnam |
133 | > 15 years old, residents of ICU for at least 10 days | N/A | Clinical examination | MRC sum score and neuropathy limitation scale (ONLS) | (73/133) 55% |
| 4. Parry et al. [25] |
PC Single centre Australia |
60 | Mechanical ventilation for at least 48 h | Pre-existing neurological conditions (central or peripheral nervous system disease, stroke) | Clinical examination |
MRC sum score and a new 4-point scoring system as well as handgrip dynamometry Diagnosis: MRC-SS: < 48/60, MRC 4-point score: < 24/36 |
(25/60) 42% |
| 5. Hough et al. [53] |
PC Single centre USA |
30 | > 3 days of mechanical ventilation | Pre-existing neurological conditions (central or peripheral nervous system disease, stroke), language barriers | Clinical examination |
MRC sum score Diagnosis: MRC < 48/60 |
(6/30) 20% |
| 6. Brunello et al. [54] |
PC Single centre Switzerland |
39 | Systemic Inflammatory Response Syndrome (SIRS) diagnosis, Mechanical Ventilation for > 2 days | Pre-existing neurological conditions, paediatric patients | Clinical examination | Physical and Neurological examination: assessment of 10 muscle groups, skin sensorimotor response and tendon reflexes Diagnosis: Modified MRC Score of < 35/50 | (13/39) 33% |
| 7. Carstens et al. [55] |
PC Single centre Germany |
56 | Patients on mechanical ventilation with a SAPS II score of ≥ 20 | < 18 years old, patients diagnosed with other known myopathies or neuropathies, thrombocytopenia | Electrophysiological examination | Diagnosis: CMAP < 3 mV in at least one investigation before awakening | (34/56) 61% |
| 8. Sharshar et al. [57] |
PC Multi-centre France |
115 | Mechanical ventilation for > 7 days | Pre-existing neuromuscular conditions, or other myopathies | Clinical examination |
MRC sum score Diagnosis: MRC < 48/60 |
(75/115) 65% |
| 9. Nanas et al. [58] |
PC Single centre Greece |
185 | Mechanical ventilation for at least 10 days | Muscle weakness before ICU admission, muscle relaxant administration, pre-existing neuromuscular conditions | Clinical examination |
MRC sum score Diagnosis: MRC < 48/60 |
(44/185) 23.8% |
| 10. Ali et al. [59] |
PC Multi-centre USA |
136 | Age ≥ 18 years old, mechanical ventilation for ≥ 5 days | Mechanically ventilated before referral to ICU, limb amputation ≥ 2 parts, subject unable to communicate | Clinical examination |
MRC-ss and handgrip dynamometry Diagnosis: MRC < 48/60 |
(35/136) 25.5% |
| 11. Latronico et al. [60] |
PC Multi-centre Italy |
92 | > 15 years old, score 35–70 in SAPS II | Pre-existing neuromuscular conditions, multiple organ failure, amputations, fractures, oedema in legs | Electrophysiological examination | CMAP or SNAP amplitude reduced by > 2 Standard Deviations (SD) of normal limits | (28/92) 30.4% |
| 12. Villar et al. [61] |
PC Single centre Spain |
30 | Mechanical ventilation for >or = 48 h, IV corticosteroids (> or = 240 mg methylprednisolone) during admission, admitted as a result of COPD exacerbation | > 80 years old, comorbidities of cardiogenic, renal or pulmonary origin | Electrophysiological examination |
Electromyography after weaning from ventilation Muscle biopsy obtained if the patients diagnosed with myopathy from the electrophysiological examination |
(9/30) 34.6% |
| 13. Bednarik et al. [62] |
PC Single centre Czech Republic |
51 | SOFA score grades 3 or 4 in two organ systems, admission in ICU within 24 h of critical illness | Pre-existing neuromuscular conditions | Clinical examination and electrophysiological examination |
Clinical examination: daily from the day of admission until day 28 Performance of electrophysiological analysis twice: the first week of admission and the fifth week Diagnosis: MRC grade ≤ 2 in examined muscles CIPM diagnosis if there are fibrillation potentials, reduced CMAP amplitude |
Clinical examination: (17/51) 27.9% Electrophysiological examination: (35/51) 57.4% |
| 14. Montero et al. [64] |
PC Single centre Spain |
26 | Patients diagnosed with septic shock, mechanically ventilated for at least one week | Between 18 and 80 years old, pre-existing neuropathies or myopathies, infected with HIV, renal failure | Electrophysiological examination once the patient weans from mechanical ventilation | reduction in CMAP and SNAP amplitudes | (34/64) 53.1% |
| 15. Bercker et al. [65] |
RC Single centre Germany |
45 | Patient diagnosed with ARDS | Pre-existing neuromuscular conditions | Clinical examination and electrophysiological examination |
Clinical assessment using MRC-SS Electrophysiological examination at early days of admission |
(27/45) 60% |
| 16. Jonghe et al. [66] |
PC Multi-centre France |
95 | Mechanical ventilation for ≥ 1 week | Pre-existing neuromuscular conditions, language barrier | Clinical examination and electrophysiological examination |
Clinical assessment using MRC-SS once patient awake Electrophysiological examination at day 10 Diagnosis: MRC-SS < 48, Reduced CMAP |
(24/95) 25.3% |
| 17. Letter et al. [3] |
PC Single centre Netherlands |
98 | Mechanical ventilation for at least 4 days | Pre-existing spinal cord injuries or pre-existing diagnosed myopathy | Clinical examination and electrophysiological examination |
Clinical examination twice weekly during admission Electrophysiological nerve conduction studies on days 4, 11, 25 after initiation of mechanical ventilation Diagnosis: Motor sum score < 26 with absent tendon reflexes, CMAP < 2.6 mV (peroneal nerve) and CMAP < 4.2 mV (ulnar nerve) |
(32/98) 33% |
| 18. Druschky et al. [67] |
PC Single centre Germany |
28 | Mechanical ventilation for > or = 4 days | Pre-existing neuromuscular conditions or other known myopathies | Clinical examination and electrophysiological examination |
Examinations on days 4,8 and 14 after initiation of mechanical ventilation Clinical examination: functional disability score (FDS) calculated Electrophysiological examination: electromyography Diagnosis: Reduced Compound Muscle and sensory nerve action potentials with fibrillation potentials and positive sharp waves, low FDS |
(16/28) 57% |
| 19. Montero et al. [64] |
PC Single centre Spain |
73 | Septic patients with evidence of multi-organ dysfunction and mechanical ventilation for ≥ ten days | < 18 years or > 80 years old, comorbidities such as other known myopathies, infection with HIV, renal failure, liver cirrhosis | Electrophysiological examination |
Electrophysiological examinations on day 10 and day 21 from initiation of mechanical ventilation Diagnosis: reduced CMAP and SNAP amplitudes with fibrillation potentials |
(50/73) 69% |
| 20. Tepper et al. [69] |
PC Single centre Netherlands |
25 | Diagnosis of septic shock | Age > 80 years old, pre-existing neuromuscular conditions, neuropathies/myopathies, renal disease, diabetes, alcohol abuse | Electrophysiological examination |
Electrophysiological examination within 72 h of admission Diagnosis: Reduced velocity, CMAP and spontaneous activity presence |
(19/25) 76% |
PC prospective cohort, RC retrospective cohort, RCT randomised controlled trial, ICU intensive care unit, MRC sum muscle power assessment scale, SAPS simplified acute physiology score
The observational studies assessed with the Newcastle–Ottawa Scale were found to have relatively low risk of bias being all good quality (6*). The randomised controlled study was assessed with the ROB scale, and we found fair risk of bias specifically about blinding of participants and personnel and blinding of outcome assessment (refer to the Additional File 1).
Outcomes
Assessment methods
We analysed the methods used to assess muscle wasting and found that of the 33 studies that measured muscle size, 28 (85%) studies used ultrasound [11–17, 24–31, 31–44] and 5 studies (15%) used computed tomography (CT) [45–49] at different time points. Additional methods used in conjunction with ultrasound and CT were the ratio of protein to DNA and histopathological analyses [11], bioelectrical impedance analysis [31], and the urea-to-creatinine ratio in blood [48]. This reveals a high degree of inconsistency in assessing muscle mass as different studies analyse different muscles at different time points during critical illness. The main muscles assessed using ultrasound were rectus femoris, quadriceps muscle, and biceps brachii with measurements taken for cross-sectional area or thickness. The areas measured on CT were the skeletal muscle cross-sectional area at the third vertebrae (L3) level and the cross-sectional area of the femoral muscle volume using sagittal direction integration.
Changes in muscle mass
During the first week of critical illness, patients lost on average every day −1.75% (95% CI −2.05, −1.45) of their rectus femoris thickness and −2.10% (95% CI −3.17, −1.02) of their rectus femoris cross-sectional area, respectively. Quadriceps muscle thickness decreased by −1.82% (95% CI −2.97, −0.66) each day. The daily loss in biceps brachii muscle cross-sectional area was −2.23% (95% CI −2.60, −1.80) and −1.64% (95% CI −3.09, 0.19) for biceps brachii thickness.
Four studies measured [16, 25, 37, 40] both rectus femoris cross-sectional area and thickness and highlighted that thickness measurement can significantly underestimate muscle loss compared with cross-sectional area (p < 0.001). This was also similar for bicep brachii [37]. The loss in muscle mass for all the muscles measured over the course of ICU stay is presented in Fig. 2.
Fig. 2.
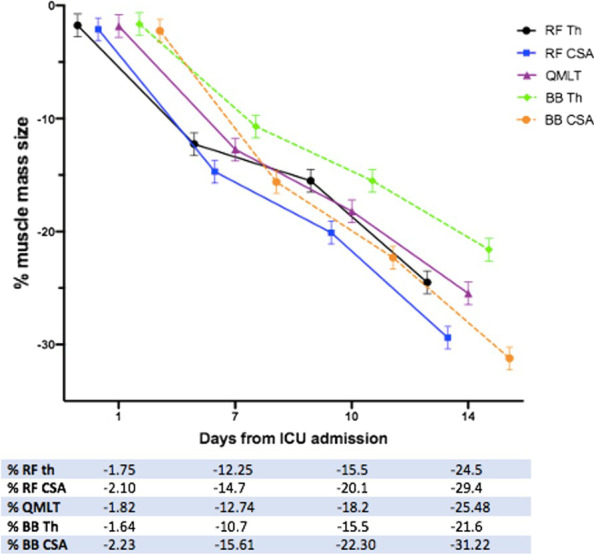
Loss in muscle mass from day 1 to day 14 of ICU admission. Abbreviations: percentage, %; rectus femoris: RF; cross-sectional area: CSA, thickness: Th, quadriceps muscle layer thickness: QMLT; biceps brachii: BB
Four studies assessed skeletal muscle mass cross-sectional area at lumbar 3 level on CT scans differently. One study [45] found a reduction of −21.9 (−29.9 to −13.9) cm2 [(149.9 ± 38.8 cm2 versus 127.9 ± 38.4 cm2), p < 0.001] equal to 15% loss in muscle mass during the first week in ICU. The second study [47] reported a change of −2.09 (± 6.96) cm2/m2 (CT 1 48.73 ± 12.57 cm2/m2 versus CT 2 46.64 ± 10.64 cm2/m2; p = 0.183), equal to 4.29% loss over 7 to 14 days of admission. The third study [49] noted a skeletal muscle cross-sectional area reduction of 5.85% at 25 days equal to a difference of −1.00 (−1.32, 3.32) cm2/m2 (baseline: 17.1 ± 5.4 vs. day 25: 16.1 ± 5.2) from baseline. The fourth study compared an initial CT on admission versus a repeated CT taken within 1–9 days or after 10 days of ICU stay and measured the urea/creatinine ratio [48].
The L4 psoas and L3 muscle cross-sectional area both progressively decrease over time (R2 0.64 and 0.59, respectively), and the skeletal muscle wasting is accompanied by elevated urea/creatinine ratio.
One RCT [46] assessing femoral muscle volume in patients receiving high-protein versus medium-protein intake found that muscle volume loss at day 10 (assessed using CT scan) was significantly lower in patients receiving high-protein (high-protein group: 12.9 ± 8.5% versus medium-protein group: 16.9 ± 7.0%, p = 0.0059). Total energy delivery was around 20 kcal/kg/day in both groups, but protein delivery was 1.5 g/kg/day and 0.8 g/kg/day. Early active rehabilitation was also provided to both groups. A second RCT [44] comparing the effect of continuous versus intermittent feeding found that muscle loss at day 10 (i.e. rectus femoris muscle cross-sectional area determined by ultrasound) was similar between arms (−1.1% [95% CI, −6.1% to −4.0%]; p = 0.676). Intermittently fed patients received 80% or more of target protein (OR, 1.52 [1.16–1.99]; p < 0.001) and energy (OR, 1.59 [1.21–2.08]; p = 0.001).
Prevalence of ICU-acquired weakness
Twenty studies analysed the prevalence of ICU-acquired muscle weakness [3, 25, 50–61, 63–69], of these 9 used the MRC sum score, which is a validated clinical examination for assessment of muscle strength and power of upper and lower extremities, 6 used electrophysiological examination, and 5 used both. The overall prevalence of ICU-AW in the twenty selected studies is 48% (95% CI 39%, 56%). This varied across studies, from 43% (95% CI 31%, 55%) in those using the MRC sum score clinical examination alone to 55% (95% CI 41%, 69%) in studies using solely electrophysiological examination. Studies using MRC sum score clinical examination combined with electrophysiological examination had a prevalence of ICU-AW equal to 48% (95% CI 31%, 65%).
Outcomes associated with muscle wasting
A meta-analysis of outcomes associated with muscle wasting was not possible as studies assessed various outcomes differently. For example, the outcome of mortality was not equally assessed and in a study was evaluated 60-day mortality [70], or in-hospital mortality [28], or mortality in ICU [71].
A study noted that patients with multi-organ failure lost muscle mass early and that the loss was more severe when compared to patients with single organ failure [11]. In patients with sepsis and septic shock, the changes of rectus femoris cross-sectional area were reported to be significantly higher (17.5%) in mechanically ventilated patients compared to those without ventilation (10.76%), p = 0.001 [27]. Early decline in biceps brachii mass was found a predictor for mortality [28]. Additionally, a study noted that over the first week of critical illness, every 1% loss of quadriceps femoris muscle thickness was associated with 5% increase in 60-day mortality [adjusted OR 0.95 (95% CI 0.90, 0.99) p = 0.023] [15]. A logistic regression analysis noted that patients who lost more than 10% of quadriceps femoris muscle thickness at day 7 had higher probability to remain on mechanical ventilation [HR: 2.1 (95% CI, 1.1, 3.8); p = 0.017] [14]. During the first week in intensive care, more than 10% loss of rectus femoris cross-sectional area was associated with longer ICU length of stay (p = 0.038), hospital length of stay (p = 0.014), and mechanical ventilation time (p = 0.05) [12].
In patients with sepsis and acute respiratory distress syndrome, muscle wasting during the first 7 days of ICU was found to be a predictor for ICU-acquired weakness (area under the curve = 0.912) [16]. Patients presenting with muscle wasting and ICU-acquired weakness on day 3 had longer mechanical ventilation time (p = 0.006) and ECMO (p = 0.025) compared to those with no ICU-AW [13].
Discussion
In this systematic review, we pooled results from 53 studies from international settings including 3251 critically ill patients. Our main findings are that (1) 85% of studies used ultrasound to assess muscle mass with measurements taken at the rectus femoris, quadriceps muscle and biceps brachii cross-sectional area or thickness; (2) during the first week of critical illness, patients lose roughly 2% of muscle mass per day, and muscle mass decreases over the course of the ICU stay; and (3) half of the critically ill patients have ICU-acquired weakness.
There is no consensus on how to quantify muscular changes in critically ill patients. In our analysis, ultrasound was the most frequently used method. This is probably because ultrasound devices are portable and can be used directly at the bedside of the patient. In contrast, CT requires transferring the patient to the scanner, which is risky and may not always be possible depending on the clinical stability of the critically ill patient.
The use of ultrasound is reliable (intraclass correlation coefficient, > 0.75 for all comparisons) when considering interobserver correlation for quantitative analysis of muscle parameters in critically ill patients [72]. Use and interpretation of ultrasound measurements are not without challenges, as there is considerable methodological variability in the measurement technique to quantify muscle mass. Specifically, the cross-sectional area and muscle layer thickness are different measurements and do not account for the same volume. It has been shown that for assessment of rectus femoris measuring the muscle layer thickness significantly underestimated ICU muscle wasting compared with cross-sectional area [40]. Furthermore, ultrasound-based quadriceps muscle layer thickness (QMLT) did not accurately estimate muscle loss when compared to quantifications of computed tomography (CT)-based muscle cross-sectional area (CSA) [73]. A study found that measuring cross-sectional area may be a more reliable proxy for muscle strength and could be used as a biomarker for proximal lower-limb muscle loss and knee extensor weakness during early critical illness in settings where volitional and non-volitional muscle strength measurements are challenging [40].
The incidence rate of ICU-AW was high (48%), and our findings may be an under-representation of the actual prevalence, as this depends upon the diagnostic evaluation used. We noted that electrophysiological examination resulted in the detection of more individuals with ICU-AW. This is potentially attributed to the fact that clinical examinations have a certain extent of subjectivity, and the diagnosis is partially determined by the clinician’s decision. On the other hand, electrophysiological assessments are standardised with clear cut-off values and instructions for the diagnosis. However, this difference can also result from methodological dissimilarity in assessing ICU-AW, such as the timing of diagnosis, the lack of homogeneity between patient populations and variable assessment frequency.
Strength and limitations
Our systematic review is the first to quantify the overall rate of muscle loss in critically ill, but has limitations. Firstly, there was a high degree of inconsistency in assessing muscle mass since studies assessed different muscles and different measurements methods at different time points during critical illness. Therefore, a differentiation of muscle loss for individual illnesses was not possible and thus remained unanswered. Second, the pre-admission baseline characteristics and patient functional state were limited. Consequently, it was not possible to assess the impact on muscle loss by severity of critical illness or pre-existing comorbidities. Finally, the studies inconsistency in assessing outcomes made a meta-analysis of outcomes associated with muscle wasting not possible. The recent CONCISE Delphi consensus should provide further guidance for authors assessing outcomes related to muscle wasting [74].
Conclusion
Critically ill patients suffer from early and marked muscle wasting. Ultrasound is the most used assessment tool in evaluating loss in muscle mass over time. The muscle mass is about 2% per day, but this rate is different between muscles and depends upon the measurement taken. The prevalence of ICU-AW is 50% amongst critically ill and those have worst outcomes.
Supplementary Information
Additional file 1. Table 1. Quality and risk of bias assessment using the Newcastle-Ottawa Scale (NOS) for observational studies assessing muscle wasting. Table 2. Quality and risk of bias assessment using the Newcastle-Ottawa Scale (NOS) for observational studies assessing ICU-acquired weakness. Figure 1. Risk of Bias.
Author contributions
BF, TM, and CC wrote the manuscript draft. All authors have edited and contributed for intellectual content. All authors approved the final version. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
Supplementary materials are available and can be accessed online.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
SJS received grants and non-financial support from Reactive Robotics GmbH (Munich, Germany), ASP GmbH (Attendorn, Germany), STIMIT AG (Biel, Switzerland), ESICM (Geneva, Switzerland), grants, personal fees and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes and Advanz Pharma GmbH (Bielefeld, Germany), non-financial support from national and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work. Dr. Schaller holds stocks in small amounts from Alphabeth Inc., Bayer AG and Siemens AG; these holdings have not affected any decisions regarding his research or this study. All other authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zudin Puthucheary and Henning Wackerhage: Joint last authors
Contributor Information
Brigitta Fazzini, Email: brigitta.fazzini@nhs.net.
Henning Wackerhage, Email: henning.wackerhage@tum.de.
References
- 1.Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, Hopkins RO, Hough CL, Kress JP, Latronico N, Moss M, Needham DM, Rich MM, Stevens RD, Wilson KC, Winkelman C, Zochodne DW, Ali NA; ATS Committee on ICU-acquired Weakness in Adults; American Thoracic Society. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–46. doi:10.1164/rccm.201411-2011ST. [DOI] [PubMed]
- 2.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1(2):147–157. doi: 10.1007/s13539-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Letter MA, Schmitz PI, Visser LH, Verheul FA, Schellens RL, Op de Coul DA, et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit Care Med. 2001;29(12):2281–6. [DOI] [PubMed]
- 4.Eikermann M, Koch G, Gerwig M, Ochterbeck C, Beiderlinden M, Koeppen S, Neuhäuser M, Peters J. Muscle force and fatigue in patients with sepsis and multiorgan failure. Intensive Care Med. 2006;32(2):251–259. doi: 10.1007/s00134-005-0029-x. [DOI] [PubMed] [Google Scholar]
- 5.Weber-Carstens S, Deja M, Koch S, Spranger J, Bubser F, Wernecke KD, Spies CD, Spuler S, Keh D. Risk factors in critical illness myopathy during the early course of critical illness: a prospective observational study. Crit Care. 2010;14(3):R119. doi: 10.1186/cc9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. The sick and the weak: neuropathies/myopathies in the critically Ill. Physiol Rev. 2015;95(3):1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crossland H, Skirrow S, Puthucheary ZA, Constantin-Teodosiu D, Greenhaff PL. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end-points. J Physiol. 2019;597(5):1259–1270. doi: 10.1113/JP275444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puthucheary ZA, Astin R, Mcphail MJW, Saeed S, Pasha Y, Bear DE, Constantin D, Velloso C, Manning S, Calvert L, Singer M, Batterham RL, Gomez-Romero M, Holmes E, Steiner MC, Atherton PJ, Greenhaff P, Edwards LM, Smith K, Harridge SD, Hart N, Montgomery HE. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–935. doi: 10.1136/thoraxjnl-2017-211073. [DOI] [PubMed] [Google Scholar]
- 10.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 11.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 12.Kemp PR, Paul R, Hinken AC, Neil D, Russell A, Griffiths MJ. Metabolic profiling shows pre-existing mitochondrial dysfunction contributes to muscle loss in a model of ICU-acquired weakness. J Cachexia Sarcopenia Muscle. 2020;11(5):1321–1335. doi: 10.1002/jcsm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimopoulos S, Raidou V, Elaiopoulos D, Chatzivasiloglou F, Markantonaki D, Lyberopoulou E, et al. Sonographic muscle mass assessment in patients after cardiac surgery. World J Cardiol. 2020;12(7):351–361. doi: 10.4330/wjc.v12.i7.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toledo DO, Freitas BJ, Dib R, Pfeilsticker F, Santos DMD, Gomes BC, et al. Peripheral muscular ultrasound as outcome assessment tool in critically ill patients on mechanical ventilation: an observational cohort study. Clin Nutr ESPEN. 2021;43:408–414. doi: 10.1016/j.clnesp.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee ZY, Ong SP, Ng CC, Yap CSL, Engkasan JP, Barakatun-Nisak MY, et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: a single-center prospective observational study. Clin Nutr. 2021;40(3):1338–1347. doi: 10.1016/j.clnu.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, Pastva AM, Dupont-Versteegden EE, Parry SM, et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24(1):637. doi: 10.1186/s13054-020-03355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palakshappa JA, Reilly JP, Schweickert WD, Anderson BJ, Khoury V, Shashaty MG, et al. Quantitative peripheral muscle ultrasound in sepsis: muscle area superior to thickness. J Crit Care. 2018;2018(47):324–330. doi: 10.1016/j.jcrc.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aromataris E, Munn Z (Editors). JBI manual for evidence synthesis. JBI, 2020. Available from https://synthesismanual.jbi.global. 10.46658/JBIMES-20-01.
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 22.Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304. doi: 10.1136/bmj.f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
- 24.Pardo E, El Behi H, Boizeau P, Verdonk F, Alberti C, Lescot T. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol. 2018;18(1):205. doi: 10.1186/s12871-018-0647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Wu J, Gu Q, Gu Y, Zhao Y, Ge X, et al. Changes in muscle ultrasound for the diagnosis of intensive care unit acquired weakness in critically ill patients. Sci Rep. 2021;11(1):18280. doi: 10.1038/s41598-021-97680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borges RC, Barbeiro HV, Barbeiro DF, Soriano FG. Muscle degradation, vitamin D and systemic inflammation in hospitalized septic patients. J Crit Care. 2020;56:125–131. doi: 10.1016/j.jcrc.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi N, Oto J, Tsutsumi R, Akimoto Y, Nakano Y, Nishimura M. Upper limb muscle atrophy associated with in-hospital mortality and physical function impairments in mechanically ventilated critically ill adults: a two-center prospective observational study. J Intensive Care. 2020;8(1):87. doi: 10.1186/s40560-020-00507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi N, Tsutsumi R, Hara K, Takashima T, Nakataki E, Itagaki T, et al. Urinary titin is a novel biomarker for muscle atrophy in nonsurgical critically Ill patients: a two-center, prospective observational study. Crit Care Med. 2020;48(9):1327–1333. doi: 10.1097/CCM.0000000000004486. [DOI] [PubMed] [Google Scholar]
- 30.Borges RC, Soriano FG. Association between muscle wasting and muscle strength in patients who developed severe sepsis and septic shock. Shock. 2019;51(3):312–320. doi: 10.1097/SHK.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi N, Tsutsumi R, Okayama Y, Takashima T, Ueno Y, Itagaki T, et al. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care. 2019;7:61. doi: 10.1186/s40560-019-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trung TN, Duoc NVT, Nhat LTH, Yen LM, Hao NV, Truong NT, et al. Functional outcome and muscle wasting in adults with tetanus. Trans R Soc Trop Med Hyg. 2019;113(11):706–713. doi: 10.1093/trstmh/trz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wandrag L, Brett SJ, Frost GS, Bountziouka V, Hickson M. Exploration of muscle loss and metabolic state during prolonged critical illness: Implications for intervention? PLoS ONE. 2019;14(11):e0224565. doi: 10.1371/journal.pone.0224565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadda V, Kumar R, Khilnani GC, Kalaivani M, Madan K, Tiwari P, et al. Trends of loss of peripheral muscle thickness on ultrasonography and its relationship with outcomes among patients with sepsis. J Intensive Care. 2018;6:81. doi: 10.1186/s40560-018-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes K, Holland AE, Pellegrino VA, Mathur S, Hodgson CL. Acute skeletal muscle wasting and relation to physical function in patients requiring extracorporeal membrane oxygenation (ECMO) J Crit Care. 2018;48:1–8. doi: 10.1016/j.jcrc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Katari Y, Srinivasan R, Arvind P, Hiremathada S. Point-of-care ultrasound to evaluate thickness of rectus femoris, vastus intermedius muscle, and fat as an indicator of muscle and fat wasting in critically Ill patients in a multidisciplinary intensive care unit. Indian J Crit Care Med. 2018;22(11):781–788. doi: 10.4103/ijccm.IJCCM_394_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanishi N, Oto J, Tsutsumi R, Iuchi M, Onodera M, Nishimura M. Upper and lower limb muscle atrophy in critically ill patients: an observational ultrasonography study. Intensive Care Med. 2018;44(2):263–264. doi: 10.1007/s00134-017-4975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva PE, Maldaner V, Vieira L, de Carvalho KL, Gomes H, Melo P, et al. Neuromuscular electrophysiological disorders and muscle atrophy in mechanically-ventilated traumatic brain injury patients: new insights from a prospective observational study. J Crit Care. 2018;44:87–94. doi: 10.1016/j.jcrc.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Annetta MG, Pittiruti M, Silvestri D, Grieco DL, Maccaglia A, La Torre MF, et al. Ultrasound assessment of rectus femoris and anterior tibialis muscles in young trauma patients. Ann Intensive Care. 2017;7(1):104. doi: 10.1186/s13613-017-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puthucheary ZA, McNelly AS, Jai R, Connolly B, Sidhu PS, Rowlerson A, et al. Rectus femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am J Respir Crit Care Med. 2017;195(1):136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segaran E, Wandrag L, Stotz M, Terblanche M, Hickson M. Does body mass index impact on muscle wasting and recovery following critical illness? A pilot feasibility observational study. J Hum Nutr Diet. 2017;30(2):227–235. doi: 10.1111/jhn.12401. [DOI] [PubMed] [Google Scholar]
- 42.Turton P, Hay R, Taylor J, Mcphee J, Welters I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care e an observational study using ultrasound. BMC Anesth. 2016;16(119):1–8. doi: 10.1186/s12871-016-0269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid CL, Campbell IT, Little RA. Muscle wasting and energy balance in critical illness. Clin Nutr. 2004;23(2):273–280. doi: 10.1016/S0261-5614(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 44.McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, Rooney K, Cupitt J, Carr B, Koelfat K, Damink SO, Atherton PJ, Hart N, Montgomery HE, Puthucheary ZA. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158(1):183–194. doi: 10.1016/j.chest.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 45.Lambell KJ, Tierney AC, Wang JC, Nanjayya V, Forsyth A, Goh GS, et al. Comparison of ultrasound-derived muscle thickness with computed tomography muscle cross-sectional area on admission to the intensive care unit: a pilot cross-sectional study. JPEN J Parenter Enteral Nutr. 2021;45(1):136–145. doi: 10.1002/jpen.1822. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura K, Nakano H, Naraba H, Mochizuki M, Takahashi Y, Sonoo T, Hashimoto H, Morimura N. High protein versus medium protein delivery under equal total energy delivery in critical care: a randomized controlled trial. Clin Nutr. 2021;40(3):796–803. doi: 10.1016/j.clnu.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 47.Dusseaux MM, Antoun S, Grigioni S, Béduneau G, Carpentier D, Girault C, et al. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS ONE. 2019;14(6):e0216991. doi: 10.1371/journal.pone.0216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haines RW, Zolfaghari P, Wan Y, Pearse RM, Puthucheary Z, Prowle JR. Elevated urea-to-creatinine ratio provides a biochemical signature of muscle catabolism and persistent critical illness after major trauma. Intensive Care Med. 2019;45(12):1718–1731. doi: 10.1007/s00134-019-05760-5. [DOI] [PubMed] [Google Scholar]
- 49.Jung B, Nougaret S, Conseil M, Coisel Y, Futier E, Chanques G, et al. Sepsis is associated with a preferential diaphragmatic atrophy: a critically ill patient study using tridimensional computed tomography. Anesthesiology. 2014;120(5):1182–1191. doi: 10.1097/ALN.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 50.Van Aerde N, Meersseman P, Debaveye Y, Wilmer A, Gunst J, Casaer MP, Bruyninckx F, Wouters PJ, Gosselink R, Van den Berghe G, Hermans G. Five-year impact of ICU-acquired neuromuscular complications: a prospective, observational study. Intensive Care Med. 2020;46(6):1184–1193. doi: 10.1007/s00134-020-05927-5. [DOI] [PubMed] [Google Scholar]
- 51.Diaz Ballve LP, Dargains N, Urrutia Inchaustegui JG, Bratos A, Milagros Percaz M, Bueno Ardariz C, et al. Weakness acquired in the intensive care unit. Incidence, risk factors and their association with inspiratory weakness. Observational cohort study. Rev Bras Ter Intensiva. 2017;29(4):466–75. [DOI] [PMC free article] [PubMed]
- 52.Nguyen The L, Nguyen HuuC. Critical illness polyneuropathy and myopathy in a rural area in Vietnam. J Neurol Sci. 2015;357(1–2):276–281. doi: 10.1016/j.jns.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15(1):R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunello AG, Haenggi M, Wigger O, Porta F, Takala J, Jakob SM. Usefulness of a clinical diagnosis of ICU-acquired paresis to predict outcome in patients with SIRS and acute respiratory failure. Intensive Care Med. 2010;36(1):66–74. doi: 10.1007/s00134-009-1645-7. [DOI] [PubMed] [Google Scholar]
- 55.Weber-Carstens S, Koch S, Spuler S, Spies CD, Bubser F, Wernecke KD, et al. Nonexcitable muscle membrane predicts intensive care unit-acquired paresis in mechanically ventilated, sedated patients. Crit Care Med. 2009;37(9):2632–2637. doi: 10.1097/CCM.0b013e3181a92f28. [DOI] [PubMed] [Google Scholar]
- 56.Ahlbeck K, Fredriksson K, Rooyackers O, Maback G, Remahl S, Ansved T, et al. Signs of critical illness polyneuropathy and myopathy can be seen early in the ICU course. Acta Anaesthesiol Scand. 2009;53(6):717–723. doi: 10.1111/j.1399-6576.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 57.Sharshar T, Bastuji-Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37(12):3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 58.Nanas S, Kritikos K, Angelopoulos E, Siafaka A, Tsikriki S, Poriazi M, et al. Predisposing factors for critical illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand. 2008;118(3):175–181. doi: 10.1111/j.1600-0404.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 59.Ali NA, O’Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 60.Latronico N, Bertolini G, Guarneri B, Botteri M, Peli E, Andreoletti S, et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Crit Care. 2007;11(1):R11. doi: 10.1186/cc5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amaya-Villar R, Garnacho-Montero J, Garcia-Garmendia JL, Madrazo-Osuna J, Garnacho-Montero MC, Luque R, et al. Steroid-induced myopathy in patients intubated due to exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2005;31(1):157–161. doi: 10.1007/s00134-004-2509-9. [DOI] [PubMed] [Google Scholar]
- 62.Bednarik J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol. 2005;252(3):343–51. [DOI] [PubMed]
- 63.Bednarik J, Vondracek P, Dusek L, Moravcova E, Cundrle I. Risk factors for critical illness polyneuromyopathy. J Neurol. 2005;252(3):343–351. doi: 10.1007/s00415-005-0654-x. [DOI] [PubMed] [Google Scholar]
- 64.Garnacho-Montero J, Amaya-Villar R, Garcia-Garmendia JL, Madrazo-Osuna J, Ortiz-Leyba C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit Care Med. 2005;33(2):349–354. doi: 10.1097/01.CCM.0000153521.41848.7E. [DOI] [PubMed] [Google Scholar]
- 65.Bercker S, Weber-Carstens S, Deja M, Grimm C, Wolf S, Behse F, et al. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(4):711–715. doi: 10.1097/01.CCM.0000157969.46388.A2. [DOI] [PubMed] [Google Scholar]
- 66.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 67.Druschky A, Herkert M, Radespiel-Troger M, Druschky K, Hund E, Becker CM, et al. Critical illness polyneuropathy: clinical findings and cell culture assay of neurotoxicity assessed by a prospective study. Intensive Care Med. 2001;27(4):686–693. doi: 10.1007/s001340100890. [DOI] [PubMed] [Google Scholar]
- 68.Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, et al. Critical illness polyneuropathy: risk factors and clinical consequences: a cohort study in septic patients. Intensive Care Med. 2001;27(8):1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 69.Tepper M, Rakic S, Haas JA, Woittiez AJ. Incidence and onset of critical illness polyneuropathy in patients with septic shock. Neth J Med. 2000;56(6):211–214. doi: 10.1016/S0300-2977(00)00019-X. [DOI] [PubMed] [Google Scholar]
- 70.Lee ZY, Ong SP, Ng CC, Yap CSL, Engkasan JP, Barakatun-Nisak MY, Heyland DK, Hasan MS. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: a single-center prospective observational study. Clin Nutr. 2021;40(3):1338–1347. doi: 10.1016/j.clnu.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Dusseaux MM, Antoun S, Grigioni S, Béduneau G, Carpentier D, Girault C, Grange S, Tamion F. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS ONE. 2019;14(6):e0216991. doi: 10.1371/journal.pone.0216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sarwal A, Parry SM, Berry MJ, Hsu FC, Lewis MT, Justus NW, Morris PE, Denehy L, Berney S, Dhar S, Cartwright MS. Interobserver reliability of quantitative muscle sonographic analysis in the critically Ill population. J Ultrasound Med. 2015;34(7):1191–1200. doi: 10.7863/ultra.34.7.1191. [DOI] [PubMed] [Google Scholar]
- 73.Paris MT, Mourtzakis M, Day A, Leung R, Watharkar S, Kozar R, Earthman C, Kuchnia A, Dhaliwal R, Moisey L, Compher C, Martin N, Nicolo M, White T, Roosevelt H, Peterson S, Heyland DK. Validation of bedside ultrasound of muscle layer thickness of the quadriceps in the critically Ill patient (VALIDUM study) JPEN J Parenter Enteral Nutr. 2017;41(2):171–180. doi: 10.1177/0148607116637852. [DOI] [PubMed] [Google Scholar]
- 74.Davies TW, van Gassel RJJ, van de Poll M, Gunst J, Casaer MP, Christopher KB, Preiser JC, Hill A, Gundogan K, Reintam-Blaser A, Rousseau AF, Hodgson C, Needham DM, Castro M, Schaller S, McClelland T, Pilkington JJ, Sevin CM, Wischmeyer PE, Lee ZY, Govil D, Li A, Chapple L, Denehy L, Montejo-González JC, Taylor B, Bear DE, Pearse R, McNelly A, Prowle J, Puthucheary ZA. Core outcome measures for clinical effectiveness trials of nutritional and metabolic interventions in critical illness: an international modified Delphi consensus study evaluation (CONCISE) Crit Care. 2022;26(1):240. doi: 10.1186/s13054-022-04113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table 1. Quality and risk of bias assessment using the Newcastle-Ottawa Scale (NOS) for observational studies assessing muscle wasting. Table 2. Quality and risk of bias assessment using the Newcastle-Ottawa Scale (NOS) for observational studies assessing ICU-acquired weakness. Figure 1. Risk of Bias.
Data Availability Statement
Supplementary materials are available and can be accessed online.