Abstract
Introduction:
Encouraging the appropriate use of staging imaging in patients with newly diagnosed prostate cancer remains a challenge. Assessing the effects of national efforts may help guide future initiatives in curtailing low-value care. The purpose of this study was to determine the impact of the Choosing Wisely campaign on imaging utilization among men with prostate cancer.
Methods:
Surveillance, Epidemiology, and End Results – Medicare data were used to complete a longitudinal population-based study of men diagnosed with prostate cancer from 2007 to 2015. An interrupted time series analysis evaluated the impact of the Choosing Wisely campaign on trends of imaging utilization.
Results:
From 2007-2015 imaging utilization in low-risk patients decreased, with computed tomography (CT) usage declining from 45.0% to 34.4% (p<0.001) and nuclear medicine bone scan (NMBS) from 27.8% to 11.7% (p<0.001). Choosing Wisely likely contributed to an absolute reduction of 2.9% (p=0.03) in utilization of NMBS in the low-risk population. Imaging usage for all modalities increased in the high-risk population, but with 32.8% continuing to not receive guideline-supported imaging.
Conclusions:
In 2012, the Choosing Wisely campaign sought to decrease inappropriate staging imaging for men with low-risk prostate cancer and encourage stewardship of medical resources. Overall decreases in staging imaging trends suggest a move towards higher value care. However, this study found that the Choosing Wisely recommendations had a modest impact on utilization of NMBS, but not CT or PET scans. These results may help inform future efforts to promote guideline concordant imaging.
Introduction
Encouraging the appropriate use of staging imaging in patients with newly diagnosed prostate cancer remains a challenge1. Before the era of prostate-specific antigen (PSA) screening the majority of incident prostate cancer cases presented at advanced stages2. For this reason, clinicians across the field uniformly accepted diagnostic imaging as a method for staging among men newly diagnosed with prostate cancer2,3. However, with the widespread adoption of PSA screening, now greater than 90% of incident cases present at a localized stage4. As a result, clinical guidelines on the utilization of diagnostic imaging have dramatically changed5–7. In the mid-1990s numerous professional societies and policy organizations reached a consensus: imaging evaluation of low-risk disease is unnecessary, and clinicians should reserve staging imaging for patients with high-risk disease2,3,6–8. To help diminish overuse of imaging, several organizations such as the National Comprehensive Cancer Network (NCCN) and the American Urological Association (AUA) published clear guidelines9,10.
Despite these longstanding efforts and recommendations, a large number of patients continue to face the repercussions of guideline-discordant imaging11–13. The overuse of imaging often leads to wasteful spending and false-positive test results, which can result in overall patient harm through anxiety and stress from evaluation of incidental findings and increased costs14,15. The importance of curtailing this persistent trend was highlighted again in 2012-2013 when organizations such as the AUA and the American Society of Clinical Oncology (ASCO) identified inappropriate prostate cancer imaging among their top priorities for Choosing Wisely (CW)2. The CW campaign, a nationwide effort to encourage stewardship of medical resources, issued statements aimed at reducing guideline-discordant computed tomography (CT), positron emission tomography (PET), and nuclear medicine bone scan (NMBS) imaging among men with low-risk prostate cancer. Few studies have provided updates on recent imaging trends and the effects of national efforts such as CW. Of those reported findings, national imaging trends continue to show poor compliance with these guidelines16.
The purpose of our study is to determine the impact of the CW campaign recommendations on imaging utilization among men with low-risk prostate cancer. Further, we evaluate imaging use in men with newly diagnosed prostate cancer by modality and risk group, with a focus on implications for low-value care.
Methods
Study population
Diagnostic imaging used to stage cases of incident prostate cancer were extracted from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database from 2007 to 2015. The SEER–Medicare data set comprises of patient demographics, cancer diagnosis, treatment-related information, and cause of death for participants in 18 geographic regions that comprises approximately 26% of the general population in the United States17. Enrollment data of Medicare beneficiaries is then linked to SEER registries18. Analysis using the SEER Medicare database meets criteria for non-human subjects’ research by our institutional review board, excluding requirement for review.
Selection of Study Subjects
We identified a total of 79,801 men with prostate cancer from 2007-2015. Selection was limited to men age 66 and older with newly diagnosed, localized or locally advanced prostate cancer (Stage ≤ III). Subjects must have had continuous Medicare Part A and B coverage in the period spanning 12 months before to 12 months after diagnosis (aged, OASI). Exclusion criteria included prostate cancer diagnosis prior to 2007, PSA at time of diagnosis greater than or equal to 50 ng/mL, and diagnosis made at time of death.
Variables
We stratified patients into three groups: low-, intermediate-, and high-risk prostate cancer. Risk level was determined by a modified version of the NCCN prostate cancer guidelines9. The risk stratification was defined as reported in Table 1.
Table 1.
Risk stratification parameters and imaging classification by appropriateness
| Risk Group | PSA (ng/ml) | Gleason Score | T Stage | NCCN Imaging Guidelines | Low-Value Imaging |
|---|---|---|---|---|---|
| Low 1 | ≤ 10 | ≤ 6 | ≤ T2 | None3 | Any imaging3 |
| High 2 | ≥ 20 | ≥ 8 | ≥ T3 | NMBS and Pelvic+/− Abdominal imaging Or PET | No imaging completed OR Any other combination differing from what is defined as appropriate |
Notes.
Low risk requires all PSA, Gleason Score, and T stage be within the definition for categorization;
Intermediate and high risk only require one factor be true for categorization. Intermediate risk must have no high-risk factors;
MRI use among low-risk patients was permitted and not considered low-value.
Abbreviations: PSA- Prostate Specific Antigen, NCCN- National Comprehensive Cancer Network, NMBS- Nuclear Medicine Bone Scan
Using a modified approach of a previously described strategy based on CPT and ICD-9/10 codes, we determined whether patients had undergone the following imaging modalities newly diagnosed prostate cancer: abdominal/pelvic CT, NMBS, pelvic magnetic resonance imaging (MRI), and PET19.
We used the NCCN guidelines, and the ASCO CW campaign to define low-value or guideline discordant imaging6, 9,10,20(Table 1). Any imaging obtained in low-risk patients was considered low-value. MRI was omitted from the value analysis due to the growing utilization of MRI for prostate cancer screening and treatment decision making in low-risk patients. Intermediate-risk patients were not evaluated regarding value given the broad spectrum of disease presentation and variability of imaging indicated in each clinical scenario. High-risk patients receiving any combination other than NMBS and any pelvic +/− abdominal imaging, or PET scan, were considered low-value imaging.
Statistical Analysis
Descriptive statistics of patient factors were stratified by risk group. To minimize bias due to missing data, we generated five imputations using the Amelia II R package, which uses an expectation maximization with bootstrapping algorithm21,22. Amelia II has features to make valid and accurate imputations for time series and cross-sectional data by allowing level change over time and shifts across locations23. Imputed variables were clinical T stage, Gleason score, and PSA, which were necessary for risk stratification. Year of diagnosis, SEER registry, race/ethnicity, marital status, and initial treatment were used as supplementary covariates for the multiple imputation model. We treated the SEER registry as a cross-sectional variable and year of diagnosis as a time-series variable. PSA was log transformed21. Race/ethnicity, initial treatment, and marital status were entered as nominal variables, and clinical T stage and Gleason score were inputted as ordered variables in the model.
To evaluate the potential impact of the CW campaign on imaging use in low-risk prostate cancer patients, an interrupted time series analysis was completed using segmented Poisson regression. The pre-intervention period spanned 2007-2011, while the post-intervention period spanned 2012-2015, with a 6-month washout period around the implementation of CW recommendations. The primary outcome was change in utilization of advanced imaging among patients with low-risk disease. This model was adjusted for race/ethnicity, Charlson Comorbidity Index score (CCI), and age of diagnosis. Within our baseline models we adjusted for race/ethnicity, CCI, and age of diagnosis, while keeping time as an adjustable variable. We used the likelihood ratio test to measure the goodness-of-fit with the implementation of CW. Measures of goodness-of-fit were evaluated as a level, slope, or both level and slope change. Models were evaluated for autocorrelation and overdispersion. Following interrupted time series we performed a sensitivity analysis using Poisson generalized estimating equations clustering on provider ZIP code.
Imaging utilization in low- and high-risk prostate cancer patients was plotted by imaging modality over time (years) in each risk group. The association of receipt of low-value staging imaging for low- and high-risk patients was evaluated for all encounters using multivariable logistic regression models adjusted for patient factors. Analyses were performed in R (version 4.0.2). P values less than 0.05 were considered as statistically significant.
Results
The study included 25,368 low-risk, 33,522 intermediate-risk, and 20,911 high-risk men diagnosed with prostate cancer. Descriptive statistics of the cohort are available in Table 2. Of the total population 77.5% were white, 47.1% had a CCI of 3 or greater, and 61.4% had clinical stage 1 disease. Incidence of prostate cancer diagnosis decreased over the study period. The number of high-risk diagnoses remained largely unchanged, with diagnoses decreasing in both the intermediate-risk and low-risk groups.
Table 2.
Patient and Treatment Characteristics by Cancer Risk
| All Patients | Low | Intermediate | High | |
|---|---|---|---|---|
| Patient Characteristic | 79,801 (100%) | 25,368 (31.8%) | 33,522 (42.0%) | 20,911 (26.2%) |
|
| ||||
| Age of Diagnosis, mean (IQR) | 72 (68-75) | 71 (68-74) | 72 (68-76) | 74 (69-79) |
| Race/Ethnicity | ||||
| Non-Hispanic, White | 61,826 (77.5%) | 20,111 (79.3%) | 26,064 (77.8%) | 15,651 (74.8%) |
| Non-Hispanic, Black | 6,826 (8.6%) | 1,850 (7.3%) | 2,988 (8.9%) | 1,988 (9.5%) |
| Hispanic | 4,532 (5.7%) | 1,413 (5.6%) | 1,834 (5.5%) | 1,285 (6.1%) |
| Non-Hispanic, Asian | 3,683 (4.6%) | 963 (3.8%) | 1,492 (4.5%) | 1,228 (5.9%) |
| Other | 2,934 (3.7%) | 1,031 (4.1%) | 1,144 (3.4%) | 759 (3.6%) |
| Marital Status | ||||
| Married | 51,915 (65.1%) | 16,753 (66.0%) | 22,240 (66.3%) | 12,922 (61.8%) |
| Single, Unmarried | 5,218 (6.5%) | 1,548 (6.1%) | 2,156 (6.4%) | 1,514 (7.2%) |
| Widowed | 4,680 (5.9%) | 1,189 (4.7%) | 1,815 (5.4%) | 1,676 (8%) |
| Other | 17,988 (22.5%) | 5,878 (23.2%) | 7,311 (21.8%) | 4,799 (22.9%) |
| CCI | ||||
| 0 | 9,690 (12.1%) | 3,364 (13.3%) | 3,919 (11.7%) | 2,407 (11.5%) |
| 1 | 988 (1.2%) | 358 (1.4%) | 329 (1%) | 301 (1.4%) |
| 2 | 31,565 (39.6%) | 10,697 (42.2%) | 13,591 (40.5%) | 7,277 (34.8%) |
| 3+ | 37,558 (47.1%) | 10,949 (43.2%) | 15,683 (46.8%) | 10,926 (52.3%) |
| Year of Diagnosis | ||||
| 2007 | 10,268 (12.9%) | 3,537 (13.9%) | 4,338 (12.9%) | 2,393 (11.4%) |
| 2008 | 9,556 (12.0%) | 3,189 (12.6%) | 4,068 (12.1%) | 2,299 (11.0%) |
| 2009 | 9,352 (11.7%) | 2,827 (11.1%) | 3,614 (10.8%) | 2,911 (13.9%) |
| 2010 | 9,239 (11.6%) | 3,220 (12.7%) | 3,757 (11.2%) | 2,262 (10.8%) |
| 2011 | 9,634 (12.1%) | 3,322 (13.1%) | 3,973 (11.9%) | 2,339 (11.2%) |
| 2012 | 7,890 (9.9%) | 2,482 (9.8%) | 3,338 (10.0%) | 2,070 (9.9%) |
| 2013 | 7,868 (9.9%) | 2,450 (9.7%) | 3,403 (10.2%) | 2,015 (9.6%) |
| 2014 | 7,741 (9.7%) | 2,171 (8.6%) | 3,400 (10.1%) | 2,170 (10.4%) |
| 2015 | 8,253 (10.3%) | 2,170 (8.6%) | 3,631 (10.8%) | 2,452 (11.7%) |
| Grade Group | ||||
| 1 | 31,917 (40.0%) | 25,368 (100%) | 4,765 (14.2%) | 1,784 (8.5%) |
| 2 | 21,100 (26.4%) | 0 (0%) | 19,398 (57.9%) | 1,702 (8.1%) |
| 3 | 10,873 (13.6%) | 0 (0%) | 9,359 (27.9%) | 1,514 (7.2%) |
| 4 | 14,212 (17.8%) | 0 (0%) | 0 (0%) | 14,212 (68%) |
| 5 | 1,699 (2.1%) | 0 (0%) | 0 (0%) | 1,699 (8.1%) |
| Clinical Stage | ||||
| I | 49,013 (61.4%) | 17,686 (69.7%) | 20,995 (62.6%) | 10,332 (49.4%) |
| II | 28,559 (35.8%) | 7,682 (30.3%) | 12,527 (37.4%) | 8,350 (39.9%) |
| III | 2,229 (2.8%) | 0 (0%) | 0 (0%) | 2,229 (10.7%) |
| PSA, mean (IQR) | 8.6 (4.8-9.8) | 5.6 (4.3-7.1) | 8.3 (5.1-11.0) | 13.9 (6.2-20.0) |
| Imaging | ||||
| CT | 37,151 (46.6%) | 8,791 (34.7%) | 15,523 (46.3%) | 12,837 (61.4%) |
| PET | 1,605 (2.0%) | 288 (1.1%) | 640 (1.9%) | 677 (3.2%) |
| MRI | 10,395 (13.0%) | 2,647 (10.4%) | 4,554 (13.6%) | 3,194 (15.3%) |
| NMBS | 30,782 (38.6%) | 4,916 (19.4%) | 13,088 (39.0%) | 12,778 (61.1%) |
| Primary Treatment | ||||
| Prostatectomy | 18,042 (22.6%) | 5,274 (20.8%) | 8,619 (25.7%) | 4,149 (19.8%) |
| ADT | 820 (1.0%) | 103 (0.4%) | 263 (0.8%) | 454 (2.2%) |
| Cryotherapy | 1,339 (1.7%) | 395 (1.6%) | 614 (1.8%) | 330 (1.6%) |
| Orchiectomy | 102 (0.1%) | 11 (0%) | 37 (0.1%) | 54 (0.3%) |
| Radiotherapy | 30,842 (38.6%) | 8,249 (32.5%) | 13,795 (41.2%) | 8,798 (42.1%) |
| Watchful Waiting/Active Surveillance | 27,356 (34.3%) | 10,869 (42.8%) | 9,708 (29%) | 6,779 (32.4%) |
Across the entire study period, we found that NMBS use among low-risk patients decreased from 29.9% to 12.5%, CT imaging decreased from 43.6% to 33.4%, and PET imaging increased from 1.0% to 1.5% (Figure A). However, 38.0% of men with low-risk disease continued to receive at least one low-value imaging modality at the end of the study period. We identified evidence of a significant level shift (p=0.029) and slope change (p=0.020) in utilization of NMBS, as usage declined in conjunction with the CW campaign. We did not find that CW was significantly associated with changes, either in level or slope, in use of CT, PET, or any imaging in low-risk prostate cancers (Table 3). The significant level shift and slope change suggest that CW contributed to an absolute decline of 2.9% in NMBS.
Figure.
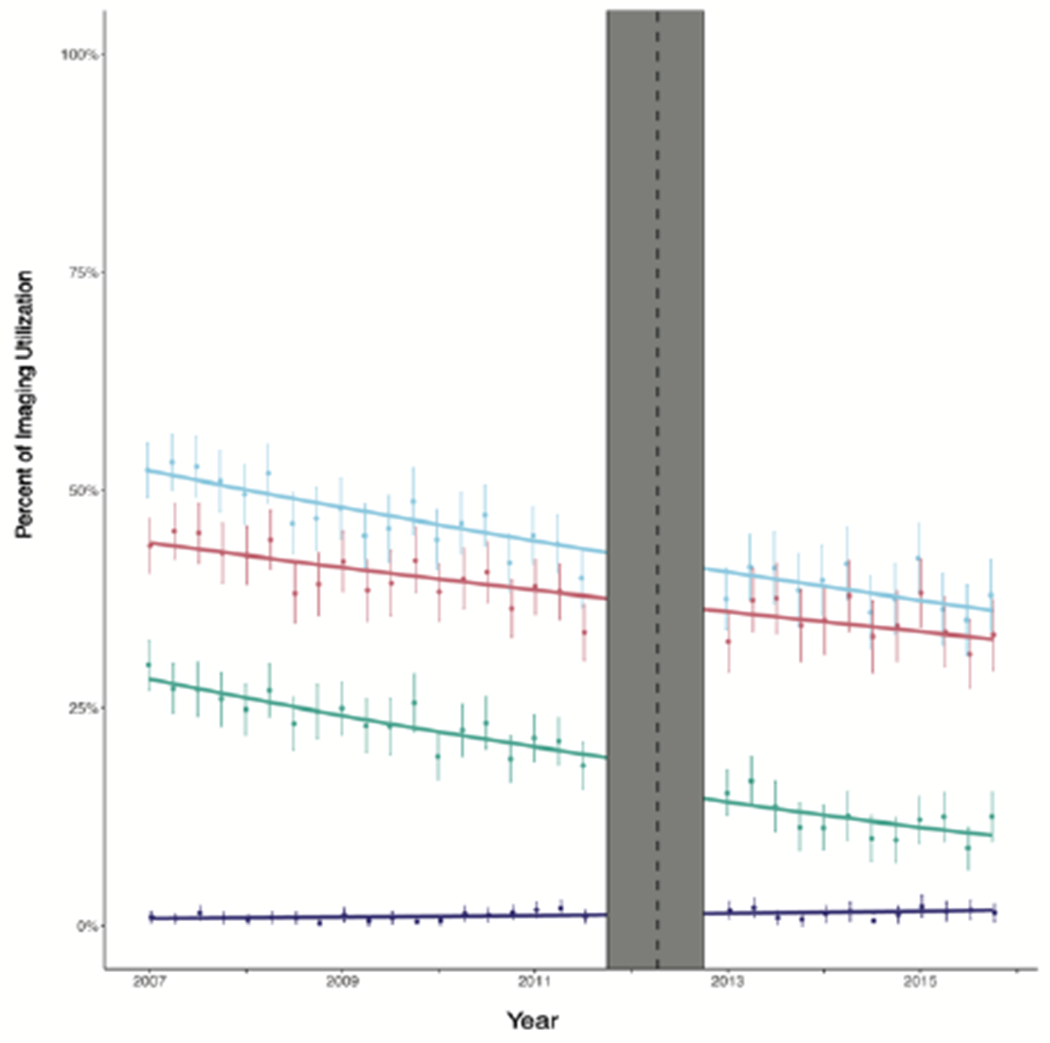
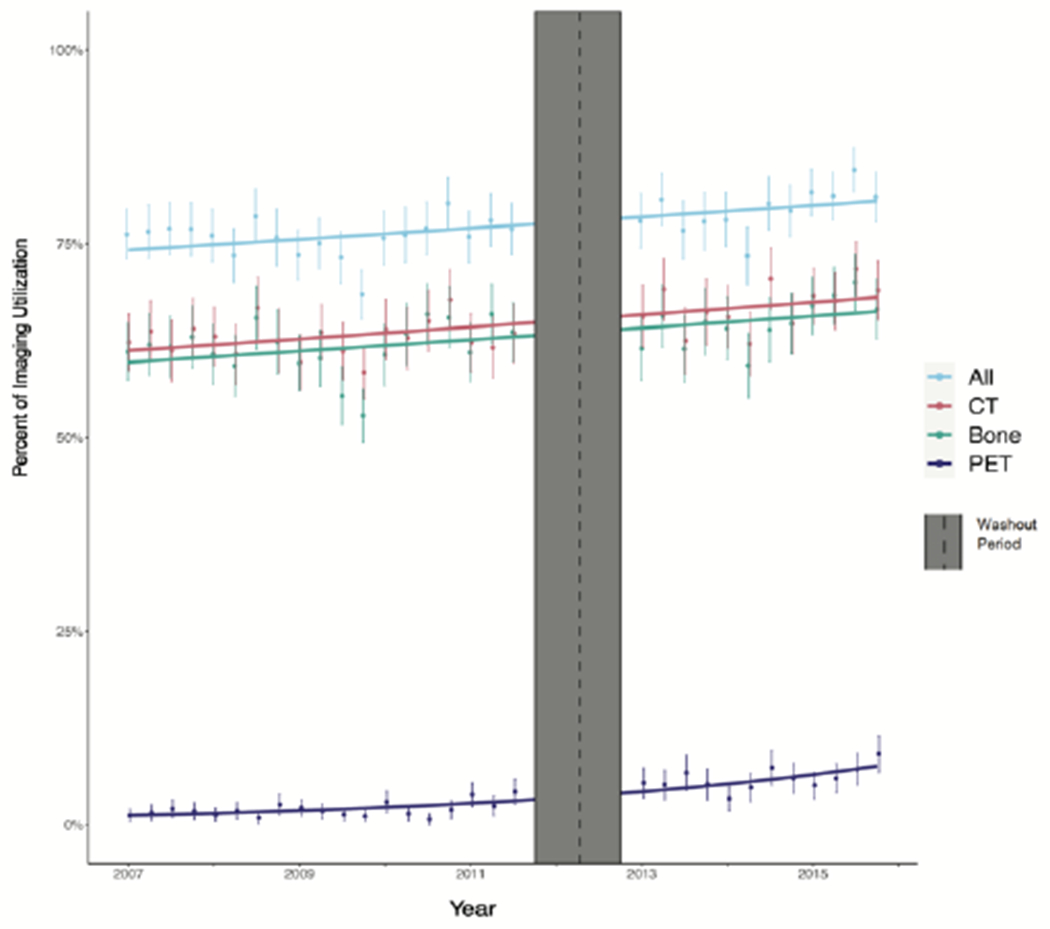
A: Trends in Imaging Use for Low-Risk Prostate Cancer Patients Before and Alter the Publication of the choosing wisely campaing
B: Trends in Imaging Use lor High-Risk Prostate Cancer Patients Before and After the Publication of the Choosing Wisely campaing
Table 3.
Statistical Significance of Models in Interrupted Time Series Analysis of Choosing Wisely Impact on Imaging Utilization in High- and Low-Risk Prostate Cancer
| Alternative Model | ||||
|---|---|---|---|---|
|
| ||||
| Level Change | Slope Change | |||
|
| ||||
| Null Model | Low Risk | NMBS | p=0.029 | p=0.020 |
| CT | p=0.379 | p=0.308 | ||
| PET | p=0.526 | p=0.432 | ||
| Any Imaging | p=0.524 | p=0.414 | ||
|
|
||||
| High Risk | NMBS | p=0.925 | p=0.807 | |
| CT | p=0.613 | p=0.463 | ||
| PET | p=0.336 | p=0.423 | ||
| Any Imaging | p=0.712 | p=0.528 | ||
Interventions such as CW can have off-target effects. Appropriate imaging among men with high-risk cancer increased across the study period (Figure B). Interrupted time series analysis indicates that CW likely had no effect on imaging in this group (Table 3, Figure B). Nonetheless, utilization of guideline-supported imaging remains suboptimal with 32.8% of men with high-risk disease not receiving a NMBS, CT, or both.
In order to further understand the use of low-value imaging among prostate cancer patients, we explored the relationship between imaging and other patient and treatment related characteristics. Logistic regression models found that in patients with low-risk cancer, risk for low-value imaging increased with increasing CCI (Table 4). Patients who were placed on watchful waiting/active surveillance were less likely to receive low-value imaging as compared to those undergoing prostatectomy (OR 0.53), while those undergoing radiotherapy were more likely to receive low-value imaging (OR 1.26).
Table 4.
Adjusted Odds Receiving Inappropriate Imaging
| Low Risk | High Risk | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Age Group | ||||
| Less than 75 | 1 (REF) | 1 (REF) | ||
| 75-79 | 1.25 (1.16, 1.35) | <0.001 | 0.97 (0.89, 1.05) | 0.435 |
| 80+ | 1.54 (1.38, 1.72) | <0.001 | 1.15 (1.06, 1.26) | 0.001 |
| Race/Ethnicity | ||||
| non-Hispanic White | 1 (REF) | 1 (REF) | ||
| non-Hispanic Black | 1.02 (0.9, 1.14) | 0.786 | 1.08 (0.96, 1.21) | 0.218 |
| Hispanic | 1.08 (0.95, 1.22) | 0.248 | 1.06 (0.92, 1.23) | 0.417 |
| non-Hispanic Asian | 0.92 (0.78, 1.07) | 0.273 | 1.03 (0.9, 1.18) | 0.675 |
| Other | 0.98 (0.83, 1.14) | 0.751 | 1.24 (1.04, 1.49) | 0.019 |
| Marital Status | ||||
| Married | 1 (REF) | 1 (REF) | ||
| Single, Unmarried | 1.08 (0.96, 1.22) | 0.202 | 1.01 (0.89, 1.16) | 0.846 |
| Widowed | 1.18 (1.03, 1.35) | 0.016 | 1.16 (1.02, 1.31) | 0.019 |
| Other | 1.13 (1.05, 1.22) | 0.002 | 1.04 (0.96, 1.13) | 0.347 |
| CCI | ||||
| 0 | 1 (REF) | 1 (REF) | ||
| 1 | 4.69 (3.52, 6.25) | <0.001 | 0.39 (0.28, 0.55) | <0.001 |
| 2 | 5.33 (4.59, 6.19) | <0.001 | 0.11 (0.09, 0.14) | <0.001 |
| 3+ | 8.66 (7.43, 10.09) | <0.001 | 0.09 (0.07, 0.1) | <0.001 |
| Year of Diagnosis | ||||
| 2007 | 1 (REF) | 1 (REF) | ||
| 2008 | 0.86 (0.77, 0.97) | 0.01 | 0.99 (0.86, 1.13) | 0.838 |
| 2009 | 0.8 (0.71, 0.9) | <0.001 | 1.19 (1.04, 1.35) | 0.009 |
| 2010 | 0.73 (0.65, 0.82) | <0.001 | 0.9 (0.78, 1.03) | 0.136 |
| 2011 | 0.69 (0.62, 0.77) | <0.001 | 0.99 (0.87, 1.14) | 0.942 |
| 2012 | 0.63 (0.56, 0.71) | <0.001 | 0.8 (0.69, 0.92) | 0.002 |
| 2013 | 0.64 (0.57, 0.72) | <0.001 | 0.75 (0.64, 0.87) | <0.001 |
| 2014 | 0.64 (0.56, 0.73) | <0.001 | 0.73 (0.64, 0.84) | <0.001 |
| 2015 | 0.63 (0.56, 0.71) | <0.001 | 0.58 (0.5, 0.66) | <0.001 |
| Grade Group | ||||
| 1 | 1 (REF) | |||
| 2 | 0.73 (0.61, 0.87) | <0.001 | ||
| 3 | 0.59 (0.48, 0.73) | <0.001 | ||
| 4 | 0.4 (0.34, 0.46) | <0.001 | ||
| 5 | 0.27 (0.22, 0.32) | <0.001 | ||
| Clinical Stage | ||||
| I | 1 (REF) | 1 (REF) | ||
| II | 0.99 (0.93, 1.05) | 0.701 | 0.91 (0.84, 0.98) | 0.01 |
| III | 0.75 (0.66, 0.86) | <0.001 | ||
| PSA | 1.02 (1, 1.03) | 0.011 | 0.98 (0.97, 0.98) | <0.001 |
| Primary Treatment | ||||
| Prostatectomy | 1 (REF) | 1 (REF) | ||
| ADT | 2.25 (1.35, 3.75) | 0.002 | 2.42 (1.92, 3.06) | <0.001 |
| Cryotherapy | 0.9 (0.72, 1.11) | 0.325 | 1.16 (0.91, 1.47) | 0.223 |
| Orchiectomy | 1.5 (0.44, 5.13) | 0.521 | 2.38 (1.31, 4.32) | 0.004 |
| Radiotherapy | 1.26 (1.17, 1.36) | <0.001 | 0.65 (0.59, 0.72) | <0.001 |
| Watchful Waiting/Active Surveillance | 0.53 (0.49, 0.58) | <0.001 | 2.55 (2.27, 2.87) | <0.001 |
In men diagnosed with high-risk cancer, the likelihood of low-value imaging decreased with increasing CCI, Gleason Grade Group, and clinical stage. Men undergoing androgen deprivation therapy or those placed on watchful waiting/active surveillance were at higher risk for low-value imaging (OR 2.42 and 2.55, respectively). Men undergoing radiotherapy were less likely to have low-value imaging (OR 0.65). In both risk groups there was no trend in risks associated with year of diagnosis.
Discussion
In this study, we evaluated trends in staging imaging use in men with newly diagnosed prostate cancer with a focus on understanding whether observed changes were attributable to the CW campaign. We identified a decline in the usage of CT and NMBS among men with low-risk prostate cancer across the study period, but were only able to attribute the observed decline in NMBS to the CW campaign. We did not observe an impact of the CW campaign on use of CT scans among low-risk patients.
We also examined the possibility of unintended consequences of CW on the use of appropriate imaging in patients with high-risk prostate cancer patients. In our analysis, CW did not alter the rates of guideline concordant imaging usage of CT, PET, or in men with high-risk prostate cancer.
While we only identified a link between CW and NMBS usage among low-risk prostate cancer patients, we did observe important overall changes in imaging use over the study period that differed by risk group. Compared to imaging utilization in 2007, low- and high-risk patients received less low-value imaging in 2015; however, substantial low-value practices remain with 38.0% of low-risk patients continuing to receive low-value imaging and 32.8% of high-risk patients not receiving guideline concordant imaging at the end of the study period.
Exploratory analyses revealed important relationships between low-risk prostate cancer patients and treatment characteristics in relation to low-value imaging practices. Logistic regression models found increased risk for low-value imaging in patients as CCI increased, consistent with other studies12,24. Risk for receipt of low-value imaging was also related to primary treatment options. Those choosing watchful waiting or active surveillance had a lower risk, while those undergoing radiotherapy were at a higher risk. It may be that providers recommending conservative management also follow a less aggressive approach to imaging. In contrast, patients undergoing radiotherapy may be more likely to receive low-value imaging as most radiotherapy practices have advanced imaging capabilities in facility and financial incentives to use them. This study did not account for changes in trends of primary treatment in low-risk prostate cancer, but with the observed increase in active surveillance and decrease in definitive therapy in this population, a portion of this decrease in imaging use may likely be from these changes in management.
Previous studies have focused on identifying patient and provider factors that affect the risk for receipt of guideline-discordant staging12,16,19,24,25. These factors are important to identify targets for intervention to decrease low-value care. Despite identifying target populations for the last decade, guideline-discordant staging still exists. To date, the medical system depends on professional guidelines for the education and dissemination of updated information to providers. The rate of change in practice patterns with this method is slow and heterogeneous between healthcare systems and regions. We sought to understand the impact of a large value-based initiative on practice patterns, finding a significant decline in NMBS use in low-risk populations in conjunction with the CW campaign. While this finding is meaningful, demonstrating a possible positive impact on the value of care, in line with the initiative’s goals, there was no other correlation of other imaging types with the launch of the initiative. Lack of significant impact of the CW campaign has also been demonstrated in other regions and urologic subspecialties26,27.
Changes in medical practice occur slowly, and while guidelines and value-based campaigns may directly facilitate change, it appears to be a process over years. In addition to understanding more about factors which impact low-value care delivery, healthcare may benefit from identifying processes that can be utilized to rapidly shift practice patterns on a systematic level. Such processes would allow individual clinicians to keep up with increasingly complex guidelines and provide the best patient care with a focus on quality. Recent work found that when comparing passive propagation of the CW campaign’s recommendations with single or multicompetent active interventions targeting clinicians, active interventions were more likely to generate high-value care and diminish low-value care28. For instance, electronic medical record based clinical decision support has been shown to improve guideline-concordant use of PSA as a screening test29. It is not a trivial task to improve the quality of healthcare, but it is our responsibility to ensure the highest quality for our patients. Thinking outside the box and using alternative methods aimed at clinicians’ daily workflows in addition to issuing periodic guideline updates may be part of the answer.
This study has limitations. While allowing for a large population analysis, the SEER-Medicare population contains specific patient and coverage demographics that may not be generalizable to other populations. The population excludes men <65 years of age for whom this information is important as their younger age at diagnosis increases their cumulative risk of down-stream effects from low-value imaging. Large claims databases often come with missing data, which we accounted for with statistical imputations. Lastly, the nature of claims database analysis does not allow it to account for specific patient or provider decision making in individual situations, such as when imaging is done due to concerning symptoms.
Future studies to understand the trends in the rate of changing imaging use may benefit from incorporating provider-specific, regional, or health system variables as subsequent programs and interventions can be more specifically targeted from a regional or care level. This study also found an increase in MRI and PET scan use over time. The most significant increase in MRI utilization was in the low-risk population, which may account for screening/biopsy planning as opposed to use as cross-sectional imaging in place of CT scans. There are limited recommendations for these newer, more expensive tests. Understanding present utilization patterns could be important for efficiently reorienting clinicians to use them appropriately as guidelines change. Finally, these data demonstrate the slow nature of changes occurring in the healthcare system and highlight an opportunity to improve the pace at which changes are made. Further interventional studies grounded in implementation science are needed to assess the best methods for improving provider efficiency in practicing updated guideline-concordant testing.
Source of Funding:
This research was supported by the National Cancer Institute of the National Institutes of Health(NIH) under Award Number K08CA234431. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Footnotes
Conflict of Interest: None
References
- 1.Makarov DV, Loeb S, Ulmert D, Drevin L, Lambe M, Stattin P. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst. 2013;105(17):1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wollin DA, Makarov DV. Guideline of Guidelines: Imaging of Localized Prostate Cancer. BJU Int. 2015;116(4):526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarov DV, Sedlander E, Braithwaite RS, et al. A qualitative study to understand guideline-discordant use of imaging to stage incident prostate cancer. Implement Sci. 2016;11(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton RG, Thompson IM, Austenfeld MS, et al. Prostate Cancer Clinical Guidelines Panel Summary report on the management of clinically localized prostate cancer. The American Urological Association. J Urol. 1995;154(6):2144–2148. [PubMed] [Google Scholar]
- 6.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724. [DOI] [PubMed] [Google Scholar]
- 7.Aus G, Abbou CC, Pacik D, et al. EAU guidelines on prostate cancer. Eur Urol. 2001;40(2):97–101. [DOI] [PubMed] [Google Scholar]
- 8.Miller DC, Murtagh DS, Suh RS, Knapp PM, Dunn RL, Montie JE. Establishment of a urological surgery quality collaborative. J Urol. 2010;184(6):2485–2490. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Prostate Cancer (Version 2, 2021). Available at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 10.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018;199(3):683–690. [DOI] [PubMed] [Google Scholar]
- 11.Skolarus TA, Chan S, Shelton JB, et al. Quality of prostate cancer care among rural men in the Veterans Health Administration. Cancer. 2013;119(20):3629–3635. [DOI] [PubMed] [Google Scholar]
- 12.Makarov DV, Desai RA, Yu JB, et al. The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the medicare population. J Urol. 2012;187(1):97–102. [DOI] [PubMed] [Google Scholar]
- 13.Lavery HJ, Brajtbord JS, Levinson AW, Nabizada-Pace F, Pollard ME, Samadi DB. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77(2):274–278. [DOI] [PubMed] [Google Scholar]
- 14.Drangsholt S, Walter D, Ciprut S, et al. Quantifying downstream impact of inappropriate staging imaging in a cohort of veterans with low- and intermediate-risk incident prostate cancer. Urol Oncol. 2019;37(2):145–149. [DOI] [PubMed] [Google Scholar]
- 15.Falchook AD, Salloum RG, Hendrix LH, Chen RC. Use of bone scan during initial prostate cancer workup, downstream procedures, and associated Medicare costs. Int J Radiat Oncol Biol Phys. 2014;89(2):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarov DV, Hu EY, Walter D, et al. Appropriateness of Prostate Cancer Imaging among Veterans in a Delivery System without Incentives for Overutilization. Health Serv Res. 2016;51(3):1021–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD, /, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Available from: https://seer.cancer.gov/csr/1975_2016. [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):Iv-3–18. [DOI] [PubMed] [Google Scholar]
- 19.Makarov DV, Soulos PR, Gold HT, et al. Regional-Level Correlations in Inappropriate Imaging Rates for Prostate and Breast Cancers: Potential Implications for the Choosing Wisely Campaign. JAMA Oncol. 2015;1(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Panel on Urologic I, Coakley FV, Oto A, et al. ACR Appropriateness Criteria((R)) Prostate Cancer-Pretreatment Detection, Surveillance, and Staging. J Am Coll Radiol. 2017;14(5S):S245–S257. [DOI] [PubMed] [Google Scholar]
- 21.Jeong CW, Washington SL 3rd, Herlemann A, Gomez SL, Carroll PR, Cooperberg MR. The New Surveillance, Epidemiology, and End Results Prostate with Watchful Waiting Database: Opportunities and Limitations. Eur Urol. 2020;78(3):335–344. [DOI] [PubMed] [Google Scholar]
- 22.Honaker J, King G, Blackwell M. Amelia II: A Program for Missing Data. Journal of Statistical Software. 2011;45(7):1–47. [Google Scholar]
- 23.Honaker J, King G. What to Do about Missing Values in Time-Series Cross-Section Data. American Journal of Political Science. 2010;54(2):561–581. [Google Scholar]
- 24.Salloum RG, O’Keeffe-Rosetti M, Ritzwoller DP, Hornbrook MC, Lafata JE, Nielsen ME. Use of Evidence-Based Prostate Cancer Imaging in a Nongovernmental Integrated Health Care System. J Oncol Pract. 2017;13(5):e441–e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipitz-Snyderman A, Sima CS, Atoria CL, et al. Physician-Driven Variation in Nonrecommended Services Among Older Adults Diagnosed With Cancer. JAMA Intern Med. 2016;176(10):1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welk B, Winick-Ng J, McClure JA, Lorenzo AJ, Kulkarni G, Ordon M. The Impact of the Choosing Wisely Campaign in Urology. Urology. 2018;116:81–86. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter CP, Johnston D, Tourville E, Sharadin C, Alzubaidi AN, Giel DW. Inappropriate imaging for management of cryptorchidism: Has the choosing Wisely(R) recommendation reduced occurrence? J Pediatr Urol. 2020;16(4):462 e461–462 e466. [DOI] [PubMed] [Google Scholar]
- 28.CLIFF BQ, AVANCEÑA ALV, HIRTH RA, LEE S-YD. The Impact of Choosing Wisely Interventions on Low-Value Medical Services: A Systematic Review. The Milbank Quarterly. n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah A, Polascik TJ, George DJ, et al. Implementation and Impact of a Risk-Stratified Prostate Cancer Screening Algorithm as a Clinical Decision Support Tool in a Primary Care Network. J Gen Intern Med. 2021;36(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


