Abstract
Nitric oxide (NO) is a well-known gaseous mediator that maintains vascular homeostasis. Extensive evidence supports that a hallmark of endothelial dysfunction, which leads to cardiovascular diseases, is endothelial NO deficiency. Thus, restoring endothelial NO represents a promising approach to treating cardiovascular complications. Despite many therapeutic agents having been shown to augment NO bioavailability under various pathological conditions, success in resulting clinical trials has remained elusive. There is solid evidence of diverse beneficial effects of the treatment with low-power near-infrared (NIR) light, defined as photobiomodulation (PBM). Although the precise mechanisms of action of PBM are still elusive, recent studies consistently report that PBM improves endothelial dysfunction via increasing bioavailable NO in a dose-dependent manner and open a feasible path to the use of PBM for treating cardiovascular diseases via augmenting NO bioavailability. In particular, the use of NIR light in the NIR-II window (1000-1700 nm) for PBM, which has reduced scattering and minimal tissue absorption with the largest penetration depth, is emerging as a promising therapy. In this review, we update recent findings on PBM and NO.
Keywords: cardiovascular diseases, endothelial cells, near-infrared light, nitric oxide, photobiomodulation
Introduction
Nitric oxide (NO) is a well-known gaseous mediator that maintains vascular homeostasis as a vasodilator and antithrombotic factor, inducing disaggregation of pre-aggregated platelets [1; 2; 3] by enhancing endogenous fibrinolysis [4; 5]. NO is enzymatically synthesized from L-arginine by NO synthases (NOS) in vivo. Endothelial (eNOS), inducible (iNOS), and neural (nNOS) isoforms of NOS have been identified [6; 7]. Amongst NOSs isoforms, eNOS is expressed in endothelial cells (ECs) and plays a critical role in maintaining the endothelial lining in a quiescent, unactivated state via NO [6; 7]. It is established that impairment of eNOS activity is associated with the pathogenesis of cardiovascular diseases [1; 8; 9; 10; 11]. A hallmark of endothelial dysfunction leading to cardiovascular complications is suppressed eNOS activity with concomitant NO deficiency [1; 10]. Thus, restoring endothelial NO via activation of eNOS represents a promising approach for treating and preventing cardiovascular events. In addition, eNOS has been shown to be expressed in red blood cells (RBCs) in humans [12; 13] and mice [13; 14]. Leo et al. demonstrated that eNOS in both ECs and RBCs independently contributed to blood pressure homeostasis and NO reservoir using EC- or RBC-specific eNOS knockout and knock-in mouse models [15]. The significance of eNOS in RBCs in cardiovascular diseases is an active topic in NO research.
Alternatively, it has also been well appreciated that NOS-independent pathways to produce NO exist. First, elevated nitrite reductase activity is reported under hypoxic conditions. The inorganic anion nitrite (NO2−) is regarded as an inert intermediate product of NO metabolism or residues in the food chain. Still, several lines of evidence support the nitrite reduction to NO under hypoxic conditions, in which several hemeproteins, including myoglobin, hemoglobin, cytoglobin, neuroglobin, globin X, xanthine oxidase, cytochrome c oxidase (Complex IV, CcO) acquire a nitrite-reductase activity [16]. NO from the nitrite reduction has been shown to modulate hypoxic vasodilation, platelet activation, and mitochondrial respiration [17; 18] and mediate cytoprotection in a number of animal models of ischemia-reperfusion [19]. Second, thiol groups can be nitrosated and are capable of transporting NO [20]. S-nitrosothiols (SNO) are formed by S-nitrosylation of hemoglobin with NO and represents one of the mechanisms to pool NO. Cysteine residues within the beta chains of hemoglobin (Cysβ 93) are favored to release NO from deoxyhemoglobin and are considered the primary source of red blood cell-derived NO [20].
Pharmacological interventions that are known to improve endothelial function are often used for therapeutic purposes in the context of cardiovascular events [7; 21]. Thus, restoring endothelial NO is a promising approach for treating cardiovascular events. This can be achieved by direct application of NO itself, but the beneficial pharmacological properties of inhaled NO are not easy to separate from its side effects [22]. The medical use of NO gas requires special equipment and highly skilled medical workers, which would be expensive and challenging for therapeutic applications in a large patient population. Instead, the therapeutic agents, including NO donors, aspirin, statins, dipyridamole, calcium channel blockers, inhibitors of angiotensin-converting enzymes, or phosphodiesterase-3 have been shown to augment NO bioavailability under a variety of pathological conditions [8; 9]. For example, in preclinical studies, NO donors reduced lesion size and maintained cerebral blood flow in stroke [23]. In a subgroup analysis of the large ENOS trial, transdermal glyceryl trinitrate (GTN), an NO donor, was safe and associated with improved functional outcomes and fewer deaths. Still, it is effective only when administered within 6 hours of stroke onset [24], posing the same severe limitation as the conventional approaches, including intravenous thrombolysis and endovascular thrombectomy to establish reperfusion. These therapeutic modalities need to be administered within 4.5-6 h of stroke onset with a high risk of bleeding, labor-intensive monitoring, special equipment and skills requirement, and the risk of complications associated with invasive interventions [25; 26]. Overall, success in resulting clinical trials using pharmacological agents has remained elusive [27]. Therefore, a novel approach to improve endothelial NO bioavailability is desired to reduce morbidity and mortality from cardiovascular diseases.
Near-infrared (NIR) light exposure to increase endothelial NO bioavailability
There is solid evidence for diverse biological effects of the treatment with low-power near-infrared (NIR) light (630-900 nm) in the NIR-I window, including analgesia, tissue regeneration, inflammation reduction, bone healing, and addressing symptoms of neurological and neuropsychiatric disorders [28; 29; 30; 31; 32; 33; 34; 35; 36], which are broadly defined as photobiomodulation (PBM) [30]. Light-based therapy using a physical parameter has numerous advantages over conventional pharmacological approaches. It is free from cold-chain storage, hypodermic needles, biohazardous sharp waste, irreversible formulation with other ingredients, undesirable biodistribution in vital organs, or unknown long-term toxicity [37; 38; 39]. Thus, PBM with NIR light has been explored for a wide array of therapeutic purposes, including treating cardiovascular diseases [30]. For example, low-power 808 nm laser irradiation attenuated myocardial infarction size in rats and dogs [40]. When an 808 nm laser was applied transcranially, it reduced stroke injury and long-term neurological deficits in rats [41]. Low-power 630 nm laser has been shown to improve memory [42] and conditions associated with traumatic brain injury (TBI), depression, and ischemic stroke in patients [43]. In the past decades, a series of evidence describes the use of non-invasive NIR laser-based therapy to improve endothelial function via augmenting NO bioavailability [44]. In response to this, treatment of acute stroke in patients using the 810 nm NIR-I laser was addressed in three clinical trials, “Neurothera Effectiveness and Safety Trials” (NEST-1) [45], NEST-2 [46; 47], and NEST-3 [48], with a discussion of potential mechanisms including mitochondrial pathways and NO signaling [49; 50]. Although NEST-1 (120 patients, 40 to 85 years of age) and NEST-2 (660 patients, aged 40 to 90) found a significantly improved outcome in moderate and moderate-severe stroke patients [45; 48], the last clinical trial (NEST-3) was prematurely terminated for futility at 566 completed patients [51]. The relatively long interval between stroke onset and laser treatment, insufficient light penetration into the affected brain tissue, an inappropriate scale used to assess stroke severity, inadequate number of treatments, and failure in precise exposure of the anatomical location of the stroke were possible reasons [49]. These results indicate there is room for improvement in PBM regarding its application site, treatment schedule, and laser modes and doses for the treatment of cardiovascular diseases. In addition, uncertainties about the clinical trial protocols and precise mechanisms of action of PBM at the molecular and cellular levels have held back its wide acceptance in the stroke community.
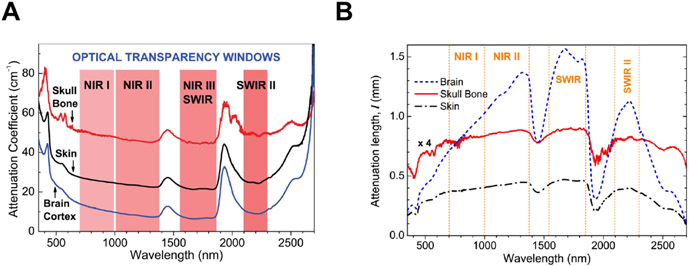
Researchers, including our group, have further demonstrated that NIR light in the NIR-II window (1000-1700 nm) shows PBM effects. Importantly, NIR-II light has many advantages over NIR-I light. The absorption coefficient or the light scattering coefficient in the skin and other tissues steadily decreases with the increase of wavelength and reaches the lowest around 1125 nm [52; 53]. As compared to the traditional laser therapy with NIR-I, NIR light in the NIR-II window represents the absence of carcinogenic or mutagenic properties [54], reduced scattering, and minimal tissue absorption [38; 52; 55], thus achieving the penetration depth of 4-8 cm [56] as compared to that of NIR-I (~3.2 cm) [57] (Figure 1).
Figure 1. NIR-I and NIR-II optical windows.
(A) Spectra of attenuation coefficient (how easily a beam of light can penetrate a volume of material) versus wavelength for head tissues: brain cortex, cranial bone, and skin in the range of wavelength from 350 to 2,700 nm. Four optical transparency windows are indicated: ~700 to 1000 nm near-infrared window I (NIR-I), ~1000 to 1350 nm (NIR-II), ~1550 to 1870 nm (NIR-III or short-wave infrared, SWIR), and ~2100 to 2300 nm (SWIR-II). (B) Attenuation length lt spectra for rat brain cortex, cranial bone, and skin in the range of wavelength from 350 to 2,700 nm. Passing the rat head and slice of brain, light remains the most intense in SWIR and NIR-II windows, while light permeability in SWIR-II and NIR-I is smaller. (A-B) Adapted from Golovynskyi et al. 2018 [53] with permission from John Wiley and Sons.
Due to its significant therapeutic advantage, reports of PBM using NIR-II are steadily increasing over the past decade. The 22 independent clinical studies demonstrated that low-level 1064 nm laser treatment on knee arthropathies, spine, shoulder or elbow, wound, gynecological, or osteoporosis effectively reduced pain, increased range-of-motion (ROM), increased functional scores, and increased the quality of life (QOL) for knee osteoarthritis and spinal disorders [58]. Wang et al. demonstrated that brief exposure of human forearms with low-power 1064 nm NIR light increases hemoglobin oxygenation [59]. We have shown that exposure to NIR-II laser between 1061-1301 nm activated innate immunity and augmented the immune response to the vaccine [37; 38; 39; 60]. In these studies, we have shown that non-pulsed NIR-II laser activates innate responses of CD103+ migratory dendritic cells (migDCs) in the skin [60; 61; 62], which are critical for early and long-term adaptive memory responses [63; 64; 65; 66; 67], and augments the efficacy of the intradermal vaccine without side effect [68; 69]. Treatment with a pulsed NIR-II laser that generated the same degree of heat in the tissue showed no effect, which indicates that these effects were not mediated by the photothermal effect [62]. In addition, we have recently demonstrated that exposure to dual NIR-II laser of 1064 + 1270 nm improves cytotoxic T cell function and augments the effects of cancer immunotherapy [70]. It should be noted that NIR-II laser has been extensively explored in the field of transcranial PBM (tPBM), a non-invasive laser therapy delivering NIR laser transcranially to modulate biochemical changes within neural cells and to treat neurodegenerative diseases. Since the NIR-II laser can reach the human cerebral cortex up to ~3 cm below the human scalp [71], it is a preferred wavelength for transcranial applications. tPBM using light-emitting diodes (LEDs) with a bandwidth of 1064 to 1080 nm was reported to improve the executive functioning of dementia patients [72]. Another series of studies showed that tPBM using a 1064 nm laser enhanced cognitive performance in rats [73] and human subjects [74; 75; 76; 77; 78]. Intriguingly, this effect has been shown to be not mediated by heat [79].
Recently, we have demonstrated that two distinct wavelengths of low-power NIR-II laser (1064 and 1270 nm) induce NO release [80], suggesting that the effect of tPBM is mediated not only by the direct effect of NIR-II light on oxygen metabolism in neuronal cells but also improved blood flow via augmented NO generation in endothelial cells. These observations collaborate well with clinical studies of tPBM with a 1064 nm laser, which showed a significant increase in cerebral blood oxygenation in human subjects [81; 82; 83]. Remarkably, tPBM using low-level 1267 nm augmented the drainage and clearing function of the meningeal lymphatic vessels [84] and alleviated neurocognitive deficits associated with the accumulation of beta-amyloid (Aβ) in mice [85; 86]. These results collectively suggest that the beneficial effects of tPBM on the cerebral drainage system [87] are also mediated by NO in lymphatic endothelial cells.
The primary site of NIR-I absorption is identified as CcO in the mitochondria [88; 89]. NIR-I absorption promotes changes in the redox state of enzymes in the mitochondrial respiratory chain, increases ATP synthesis [90], and activates mitochondrial retrograde signaling, including mitochondrial ROS and NO, ultimately resulting in broad beneficial effects [31]. The mechanisms involved in PBM with NIR-II seem to be the same as that of NIR-I. Ravera et al. demonstrated that 1064 nm laser light modulated the transmembrane mitochondrial complexes I, III, and Complex IV (CcO) [91]. We and others have also shown that the mechanisms of action involve the photochemical effects of the generation of ROS [62; 92], activation of mitochondrial retrograde signaling [80; 91], and subsequent expression of immunostimulatory cytokines and chemokines [61; 62; 68; 69; 93]. Dolgova et al. showed that a short-term increase in ROS levels, in turn, activates the cell antioxidant system [94], which ultimately exerts broad beneficial effects. In addition, Schroeder et al. demonstrated that broadband NIR light (760-1440 nm) induced mitochondrial ROS generation and increased redox potential in cultured human dermal fibroblasts [95]. Khokhlova et al. consistently observed mitochondrial ROS generation with 1265 nm laser radiation [96]. We consistently observed a reduction of cellular ROS levels with 1064 + 1270 nm NIR-II treatment in T cells [92; 97].
In short, NIR-II light might be able to resolve historical issues associated with PBM using NIR-I light and represents a non-invasive and promising therapeutic approach for cardiovascular diseases (Figure 2). In this manuscript, we perform an updated review of recent findings on PBM and NO in preclinical (Table 1) and clinical settings (Table 2) and provide future perspectives on the use of NIR-II light to augment endothelial NO bioavailability.
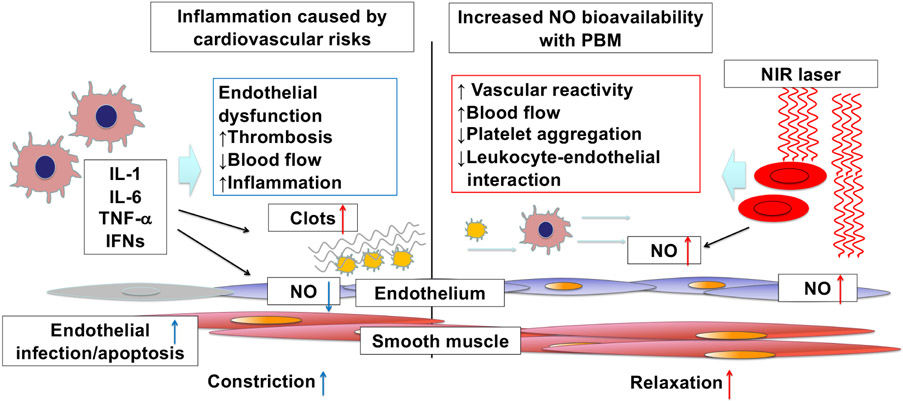
Figure 2. Endothelial dysfunction excerpts cardiovascular disorders that can be alleviated by increased nitric oxide (NO) bioavailability upon photobiomodulation (PBM) with near-infrared (NIR) light.
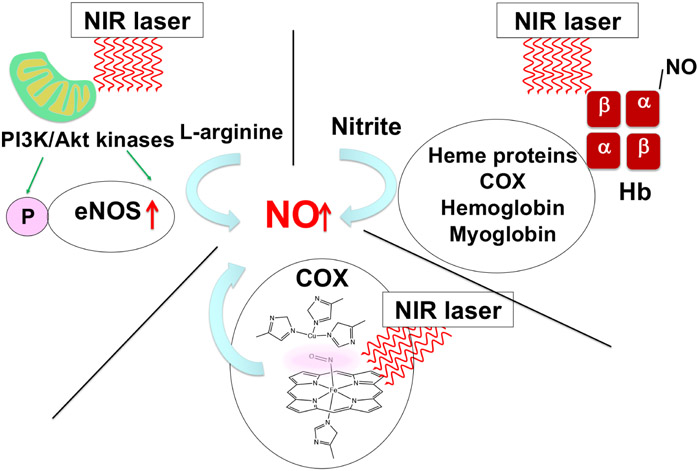
Nitric oxide (NO) is a gaseous mediator to maintain vascular homeostasis. A hallmark of endothelial dysfunction and subsequent pro-thrombotic events is endothelial NO synthase (eNOS) suppression with concomitant NO deficiency, resulting in vasoconstriction, inflammation, platelet activation, hypercoagulation, and cardiovascular diseases. Photobiomodulation (PBM) with near-infrared (NIR) light exposure has the potential to increase NO bioavailability via multiple mechanisms.
Table 1.
In vitro and preclinical studies on UVA, blue light and photobiomodulation (PBM), and nitric oxide (NO) bioavailability.
| Wavelength | Irradiance | Light source |
Model | Mechanisms / Outcome |
References |
|---|---|---|---|---|---|
| 320-420 nm | 34mW/cm2 | Mercury arc lamp | Human skin tissue | NOS-independent NO↑ Photo-decomposition of RSNO and nitrite |
[115] |
| 441.6 or 632.8 nm | ND | He-Cd (441.6 nm) and He-Ne (632.8 nm) lasers | HbNO solution | NO↑ Photodissociation of HbNO by He-Cd laser but not He-Ne laser |
[118] |
| 447, 532, 635 or 808 nm | 800 ± 17.9μW/cm2 | LD CW |
Cultured hTERT-RPE cells | Increase NOS-independent, but the substrate source of electrons entering the electron transport chain dependent NO generation Sequential or simultaneous exposures at two different wavelengths enhanced NO generation |
[114] |
| 590 ± 14 nm | up to 10W/m2 | LED CW |
Isolated mitochondria from yeast and mouse brain | Nitrite-dependent NO release by CCO↑ NO production increased in hypoxia 509-691 nm was stimulatory, but 820-880 nm was inhibitory in mouse brain mitochondria |
[123] |
| 632.5 nm | 0.26J/cm2 | CW He-Ne laser |
Cultured HUVECs | Increase NO generation via enhanced eNOS gene expression and eNOS phosphorylation at S1177 via Akt phosphorylation | [131] |
| 670nm | 170mW/cm2 for 5 min (51 J/cm2) | LED CW |
Mice | NO release from HbNO and MbNO Cardioprotection from ischemia and reperfusion injury in normal and diabetic mice, independent of NOS No effect of 740 and 830 nm was observed |
[126] |
| 670 nm | 5-50 mW/cm2 (1.5-15 J/cm2) | LED CW |
Cultured neonatal rat ventricular myocytes, cardiac muscle cell line HL-1 cells | NOS independent and dependent NO↑ Cellular ATP↑ Protect myocytes from hypoxia and reoxygenation-induced apoptosis |
[113] |
| 670nm | 60 mW/cm2 for 10 min (36 J) for 14 days | LED CW |
Mice, Rabbits, HUVECs | An increase in collateralization and blood flow in a hindlimb ischemia model in rabbits and mice HUVEC produced NO independently of NOS and increased proliferation and tube formation |
[125] |
| 670nm | 60 mW/cm2 for 3 min (11 J) | LED CW |
Rabbits | Increased NO release from HbNO or MbNO Reduction of nitrite to form NO in the anaerobic condition via the nitrite reductase activity of deoxyhemoglobin Cardioprotection in cardiac ischemia and reperfusion injury model in rabbits |
[124] |
| 670nm | 10 mW/cm2 for 5 min | LED CW |
Isolated facialis arteries of mice HMVEC-d |
Increased vasodilation of isolated arteries in an endothelium-dependent manner Induced NO release from the endothelium, suggestive from S-nitrosothiols or non-heme iron nitrosyl complexes |
[127] |
| 780nm | 300 J/cm2 10 consecutive days |
GaAlAs laser PW (300 Hz) |
Isolated femora from rats | NO↑ Stimulating bone healing of femora ex vivo |
[33] |
| 804nm | 8 mW/cm2 for 2 min (0.96 J/cm2) | GaAs laser CW | Rats | Increased iNOS expression and angiogenesis and decreased infarct size in a myocardial infarction model | [132] |
| 1064 and 1270nm | 10 mW/cm2 for 5 min | InGaAs laser CW |
HUVECs | NO↑ Enhancing HUVEC migration in vitro |
[80] |
UVA, ultraviolet-A; NO, nitric oxide; GaAs, Gallium-arsenide; Gallium-aluminum-arsenide, GaAlAs; RSNO, S-nitrosothiols; ND, Not described; He-Cd, helium-cadmium; He-Ne, heliumneon; HbNO, nitrosyl hemoglobin; LD, laser diode; CW, continuous wave; hTERT-RPE, human telomerase reverse transcriptase transformed retinal pigment epithelium; NOS, nitric oxide synthase; LED, light-emitting diode; CCO, Cytochrome c oxidase; HUVECs, human umbilical vein endothelial cell; HMVEC-d, Human dermal microvascular endothelial cells; eNOS, endothelial nitric oxide; MbNO, nitrosyl myoglobin; PW, pulse wave; iNOS, inducible nitric oxide.
Table 2.
Clinical studies on UVA, blue light, and PBM and NO bioavailability.
| Wavelength | Irradiance | Light source |
Model | Mechanisms / Outcome |
References |
|---|---|---|---|---|---|
| 320-410 nm | 20 J/cm2 for 22 min | UVA bulbs CW (Waldmann GH-8 ST unit) |
Human volunteers (adult 18 men, 6 women) | UVA exposure of the forearm releases NO from storage in the epidermis, lowered blood pressure, and increased blood flow independently of NOS | [116] |
| 350-400 nm | 10mW/cm2 for 60 min (60 J/cm2) | UVA bulbs (Philips TLK 40 W/10R R-UVA) | Human volunteers (adult 4 men, 14 women) | Increased NO-related products in the skin, including (nitrate, nitrite, and RSNOs and RNNOs) | [117] |
| 450 ± 5 nm | 42mW/cm2 for 30 min (72 J/cm2) | UV-free blue light LED CW (Philips Light & Health) |
14 healthy male subjects | UVA increased plasma NOx (nitrite, nitrate, and RXNOs) and blood flow and decreased blood pressure | [119] |
| 455, 660, and 850 nm | 50 mW/cm2 for 15 min | LED (RoseLab, Montreal, Canada) | Human forearms (adult male) | 455 and 850 nm increased NO release from the treated skin | [122] |
| 650 and 880 nm | 30 min treatment per day, 3 times per week for 8 weeks | GaAs LED PW 584 Hz (RevitaMe d Infrared Light Therapy RL-1001SP device) |
22 patients with the clinical diagnosis of type 1 (N = 2) or type 2 (N = 20) diabetes mellitus | NIR therapy improved peripheral protective sensation No change in plasma NO metabolite levels (nitrates, nitrites, and nitrosothiols) |
[142] |
| 890 nm | 7 mW/cm2 (636mW/90 cm2) for 30 min | GaAlAs diode PW (292 Hz, 50% duty cycle) (Anodyne therapy, Tampa, FL) |
Human arms of 15 healthy subjects (adult 10 men, 5 women) | Plasma NO metabolites (nitrite/nitrate) levels↑ The peak increase in NO occurred 5 mins into the treatment | [130] |
UVA, ultraviolet-A; CW, continuous wave; GaAs, Gallium-arsenide; NO, nitric oxide, NOS, nitric oxide synthase; RSNOs, S-nitrosothiols; RNNOs, N-nitrosamines; LED, light-emitting diode; NOx, NO species; RXNOs, nitroso compounds; ND, not described; PW, pulse wave.
1. Photo-dissociation of NO from heme proteins upon PBM
NO binds to heme a3 in competition with oxygen and acts as a reversible inhibitor of CcO at low concentrations [98; 99], modulating activities of the electron transport chain and activating retrograde signaling [100; 101; 102; 103], ultimately diverting the fate of oxygen into ROS generation [104; 105; 106; 107] and activating ROS- or NO-mediated retrograde signaling via forkhead box Os (FOXOs), NF-κB, activator protein-1 (AP-1), Myc [90]. These mechanisms have been shown to regulate tissue oxygen gradients and induce cytoprotective effects [108; 109]. NIR light has been shown to induce photo-dissociation of NO from heme proteins. Boelens et al. demonstrated that 640 and < 400 nm light displaced NO from CuB+2 using cryogenic electron paramagnetic resonance (EPR) [107; 110]. Sarti et al. showed that white light, including NIR light, displaced NO bound to the heme a3 in CcO subunit I [111]. This photo-dissociation has a physiological consequence; it is proposed that during hypoxia, NO modulates energy metabolism by reducing oxygen consumption by inhibiting CcO [112]. Consistently, low level (up to 50 mW/cm2) 670 nm has been demonstrated to increase ATP synthase activity and NO generation, and protect cultured cardiomyocytes from hypoxia and reoxygenation-induced apoptosis [113], suggesting possible involvement of modulation of NO-CcO upon NIR exposure in these effects. Importantly, these effects were dependent upon NO derived from NOS and non-NOS sources in this study.
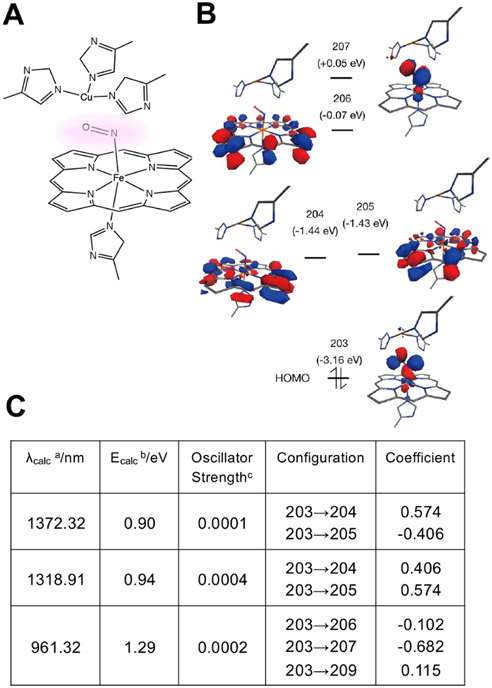
In order to explore the possible use of NIR-II for PBM, we further performed time-dependent density functional theory (TDDFT) computation of the binding of NO to CcO molecule (Figure 3) [92]. There were three multispectral absorbance peaks in the NIR range: 961, 1319, and 1372 nm, which indicates that the treatment with NIR-II light could photo-dissociate NO from CcO, exerting PBM in the deep thickness of exposed tissue due to its large penetration depth of this range of NIR light in biological tissue [38].
Figure 3. Time-dependent density functional theory (TDDFT) analysis of NO binding to cytochrome c oxidase (CcO).
(A) A region around copper ion - NO - iron ion (Cu-(NO)-Fe) binding with porphyrin complex and histidine (His) was extracted from the whole CcO molecule (PDB: 5X1F). A copper ion is surrounded by three histidines, while NO binds to an iron ion coupled with a porphyrin structure and a histidine. (B-C) The time-dependent density functional theory (TDDFT) method was performed to calculate the excited states relevant to the NIR light absorption. (C) There are three multispectral absorbance peaks, which derive from the electronic transition from molecular orbital (MO) 203 (Highest Occupied Molecular Orbital: HOMO) to low-lying unoccupied orbitals (MOs 204-209). a: Absorption maxima, b: Corresponding transition energy calculated by TDDFT method, c: Oscillator strength. These results indicate that tissue treatment with NIR light leads to the NO release from CcO. Modified from Katagiri et al. 2020 [92] with permission from The International Society for Optical Engineering (SPIE).
Although the detailed mechanism of action is not clear, Pope et al. showed that a single or combination of NIR light of 447, 532, 635, or 808 nm induced NO generation in cultured human telomerase reverse transcriptase transformed retinal pigment epithelium (hTERT-RPE) cells [114], which was not dependent on NOS but the substrate source of electrons entering the electron transport chain. This work suggests that the simple model of the light-mediated release of NO from CcO still represents one of the mechanisms of action of PBM, but it cannot explain the wide variety of PBM effects reported in the literature entirely [114]. Thus, the possible involvement of this pathway needs to be further clarified in each PBM system.
2. NO release via reductase activities of heme proteins upon PBM
It has been well recognized that UV light induces NO generation via the photo-decomposition of photo-reactive nitrogen oxides. Paunel et al. showed NO increase in the human skin tissue sample by photo-decomposition of S-nitrosothiol and nitrite upon exposure to 320 to 420 nm UV light from a mercury arc lamp [115]. Consistently, Liu et al. demonstrated in 24 volunteers that 320-410 nm ultraviolet A (UVA) irradiation enhanced the release of NO from storage in the epidermis, lowered blood pressure, and increased blood flow independently of NOS [116]. Mowbray et al. further showed that in human volunteers, 350-400 nm UVA was also able to increase NO-related products in the skin, possibly derived from vessels [117]. Similarly, blue light has also been shown to release NO from photolabile NO metabolites. Borisenko et al. described 441.6 nm, but not 632.8 nm laser increased NO due to photodissociation of nitrosyl hemoglobin (HbNO) [118]. Stern et al. demonstrated in human volunteers that whole-body irradiation with 445-455 nm blue light increased plasma NOx (nitrite, nitrate, and RXNOs) and blood flow and decreased systolic blood pressure [119], suggesting NO release from photolabile NO metabolites into circulation.
PBM using red-NIR light has also been shown to increase NO bioavailability from intracellular stores, but the mechanism of action is distinct from that of UVA or blue light and involves nitrite reductase activity of heme proteins, including CcO, hemoglobin, or myoglobin. Upon its synthesis via NOS, NO embarks in reactions with biomolecular targets, being ultimately oxidized into nitrite [120]. Within tissues, nitrosyl-heme proteins can generate NO under both hypoxia or low pH and normoxic conditions [121]. Barolet et al. tested the impact of 455 nm blue light as well as 660 and 850 nm NIR light on NO release in the forearms of a human volunteer. In this work, 455 and 850 nm, but not 660 nm, increased NO levels, although the precise mechanism of NO release action for each condition was unclear [122]. Ball et al. showed that 590 ± 14 nm red light stimulated nitrate reductase activity of CcO and induced NO release under the hypoxic condition from pre-existing nitrite tissue stores in isolated mitochondria [123]. Interestingly, while wavelengths 509-691 nm were stimulatory, those above 820 nm (up to 880 nm) were rather inhibitory in mouse brain mitochondria. It is proposed that 590 ± 14 nm irradiation enhances the NO off rate from CcO, hence the nitrite on rate, resulting in NO production [121]. Lohr et al. demonstrated that low-level irradiation with 670 nm NIR light facilitated NO release from HbNO or nitrosyl myoglobin (MbNO) and reduced nitrite to form NO via the nitrite reductase activity of deoxyhemoglobin in the anaerobic condition [124]. Low-level (60 mW/cm2 for 3 min) exposure of the heart with 670 nm NIR light conferred cardioprotection in cardiac ischemia and reperfusion injury model in rabbits [124]. They consistently demonstrated that low-level 670 nm NIR light-induced NOS-independent NO release in endothelial cells and enhanced collateralization and blood flow in a hindlimb ischemia model in rabbits and mice [125]. Keszler et al. demonstrated that 670 nm light similarly released NO from HbNO and MbNO and protected normal and diabetic mice from ischemia and reperfusion injury, independent of NOS [126]. Remarkably, among tested wavelengths (670, 740, and 830 nm), 660 nm yields the highest release of NO and conferred cardioprotection. NO production in response to 670 nm NIR light exposure has been reported to occur in endothelium [127]. Consistently, Keszler et al. observed increased vasodilation of isolated arteries via NO release in the endothelium in the pressure myography system [127]. Together, these results suggest that PBM using red-NIR light increases NO bioavailability from intracellular stores via nitrite reductase activity of heme proteins. Further study on the effect of NIR-II on this pathway is warranted.
3. Augmentation of NO production from NOS upon PBM
PBM has been shown to increase NO production by eNOS. eNOS is the primary source of NO in endothelium and is phosphorylated at serine 1177 (S1177) by Akt kinase, representing a major activation mechanism of eNOS to increase NO production [128; 129]. NO is oxidized into nitrite after its synthesis via eNOS [120]. Mitchell et al. also demonstrated that low-level 890 nm NIR laser treatment on an area of the skin increased plasma nitrite levels in venous blood draining from treated tissue, suggesting increased generation of endothelial NO in the treated area [130]. He-Ne laser irradiation at 632.5 nm has been reported to promote proliferation, migration, NO generation, and angiogenesis of human umbilical vein endothelial cells (HUVECs) via activation of the PI3K/Akt/eNOS pathway [131]. Alternatively, this pathway has also been shown to increase eNOS protein and gene expression in HUVEC in vitro [131]. In addition, other isoforms of NOS could contribute to an increase in NO bioavailability upon PBM. Tuby et al. showed that low-level 804 nm laser treatment increased iNOS expression, facilitated angiogenesis, and decreased infarct size in a myocardial infarction model in rats [132]. The augmented angiogenesis was mediated by increased expression of vascular endothelial growth factor (VEGF) by NO.
Our group has further demonstrated that two distinct wavelengths of NIR-II laser (1064 and 1270 nm) at an irradiance of 10 mW/cm2 induce NO release from endothelial cells. These lasers enhanced Akt phosphorylation at serine 473 and eNOS phosphorylation at S1177 [80]. Consistent with PBM with NIR-I, the NO release and phosphorylation of eNOS involved mitochondrial retrograde signaling. Interestingly, the induction of NO was highly dose-dependent (Figure 4). This is consistent with a report by Ravera et al. showing that 1064 nm laser modulated the transmembrane mitochondrial complexes I, III, and Complex IV (CcO) with a narrowed range of tolerability [91]. Importantly, these two wavelengths of NIR-II laser induced no appreciable NO generation in cultured neuronal cells expressing nNOS [80]. Golovynska et al. also demonstrated that 650 and 808 nm red and NIR-I light promoted intracellular calcium elevation in cultured neuronal cells [133] via stimulation of N-methyl-D-aspartate receptors (NMDARs) [134], while 1064 nm NIR-II laser light did not evoke any changes in intracellular calcium in cultured cells expressing NMDARs [133]. Since NMDARs are involved in the excitotoxicity induced by hypoxia-ischemia [135], and nNOS-derived NO plays a neurotoxicity role in ischemic stroke [136], the use of NIR-II would be a favorable option for the treatment of ischemic stroke with PBM.
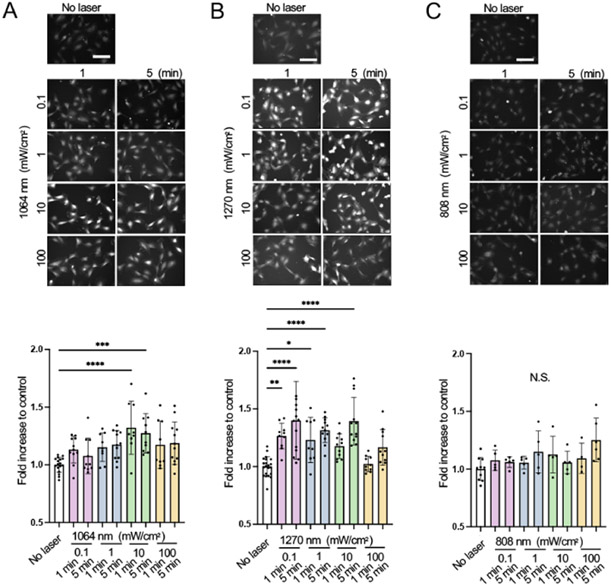
Figure 4. Dose- and time-dependent nitric oxide (NO) generation of human umbilical vein endothelial cells (HUVECs) and neuronal cells induced by NIR-II exposure.
(A-C) We irradiated cultured human umbilical vein endothelial cells (HUVECs) with 808, 1064, or 1270 nm laser at an irradiance of 0.1-100 mW/cm2 for 1-5 min. We determined NO generation upon laser irradiation using NO-sensitive fluorophore 4,5-diaminofluorescein diacetate (DAF-2 DA). The fold change in the fluorescent signals at each time point over the no-laser control group was calculated for each group. (A-C, top) Representative fluorescence images of DAF-2T signal loaded in HUVECs in each experimental group and (A-C, bottom) the fold changes of the fluorescence signal of (A), 1064 (B), 1270 and (C), 808 nm laser-treated group. Adapted from Yokomizo et al. 2022 [80] with permission from John Wiley and Sons.
eNOS is also expressed in RBCs [12; 13; 14] and plays a critical role in blood pressure homeostasis and NO reservoir [15]. Nagarajan et al. demonstrated that the mechanical stimuli phosphorylated eNOS at the S1177 in human RBCs and activated the enzyme activity, induced nitrosylation of RBC proteins, and regulated structural flexibility for the passage of RBCs through the microvasculature [137]. These results warrant further investigation of the impact of PBM on eNOS in RBCs.
In short, these lines of evidence suggest that PBM with NIR light increases NO bioavailability in situ via distinct mechanisms of action (Figure 5) and could be used to improve endothelial dysfunction and treat cardiovascular diseases.
Figure 5. Increased NO release and production upon NIR laser treatment.
PBM with NIR light exposure augments NO bioavailability via multiple mechanisms. First, PBM rests on its ability to photo-dissociate NO from cytochrome c oxidase (CcO) in mitochondria. Second, PBM increases NO bioavailability from intracellular stores, especially engaging heme proteins, including CcO, hemoglobin (Hb), or myoglobin. The schematics show hemoglobin composed of a heme prosthetic group, two α-globin, and two β-globin chains. Third, PBM has been shown to increase NO production from NOS via increasing the expression level and phosphorylation.
Perspectives: Study tools for PBM on NO bioavailability
A deficiency of bioactive NO is implicated in arterial thrombosis in animal models and patients with endothelial dysfunction [3; 138]. A hallmark of endothelial dysfunction is considered to be endothelial NO deficiency [1; 10]. Therefore, research tools to study the relationship between NIR exposure and eNOS are critical to advancing our understanding of the impact of PBM on NO signaling.
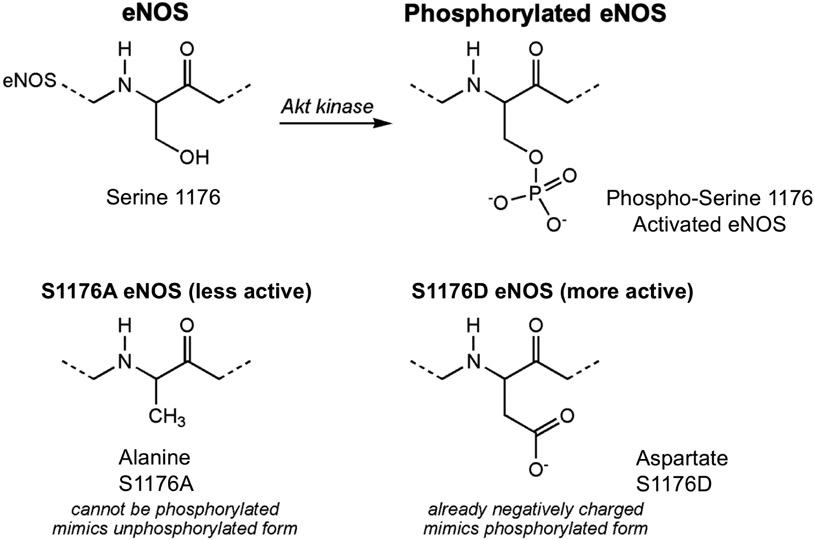
eNOS knockout mice have been extensively used as an animal model of endothelial dysfunction [139] and for mechanistic studies of PBM to determine the source of NO [125; 126]. However, this is an extreme model for endothelial dysfunction because they have no endothelial NO. Phosphorylation of eNOS at S1177 is a clinically important mechanism for the regulation of eNOS enzyme activity. S1177 phosphorylation is deficient in patients with diabetes, hypertension, and hypercholesterolemia [1; 10; 11]. As outlined above, NIR light exposures have been shown to stimulate a major regulator of NO bioavailability, eNOS phosphorylation [131]; a research tool that can determine the role of eNOS phosphorylation in vivo would be useful. In response, mutant mice carrying mutations at the S1176 phosphorylation site (equivalent to S1177 in humans and S1179 in bovines) were generated [140; 141]. The S1176D mutation contains an aspartate in place of the serine, with a carboxyl side group that mimics a negatively charged phosphate group (constitutively active, high NO production), and the S1176A mutation contains an alanine in place of the serine, so it cannot be phosphorylated (not activatable, low NO production) (Figure 6).
Figure 6. Mouse models of S1176D and S1176A eNOS mutations.
The S1176D mutation contains an aspartate in place of the serine, with a carboxyl side group that mimics a negatively charged phosphate group (constitutively active, high NO production), and the S1176A mutation contains an alanine in place of the serine, so it cannot be phosphorylated (not activatable, low NO production).
Unlike eNOS knockout mice, the S1176D and S1176A mice are more pathophysiologically relevant because they do have eNOS activity, yet they mimic the biological actions of phosphorylated and unphosphorylated states. These models have been successfully used to determine the importance of eNOS phosphorylation in endothelial function in vasoconstrictor and vasodilator responses of vessels from the leptin-deficient mice with S1176A or S1176D mutations in a myograph system ex vivo and stroke model in vivo in the context of diabetes [141]. These mice are perspective tools to study mechanisms of the PBM action on endothelial NOS-connected pathways in vivo.
Conclusion
The recent mechanistic studies solidified evidence that PBM with NIR-I light can augment NO bioavailability in multiple mechanisms of action. A recently expanded list of the effects of PBM on cardiovascular diseases, along with the matured medical laser industry, is expected to facilitate the initiation of the clinical translation of laser-based technology. Especially, a clinically relevant molecular target of PBM, including CcO, heme proteins, and eNOS, holds promise in the treatment of cardiovascular diseases. Exploration of further applications of PBM using NIR-II light with the largest penetration depth using in vivo mouse models with modified eNOS activity is expected to be optimal for expanding such an opportunity and following this path in the near future.
Highlights.
Photobiomodulation (PBM) augments nitric oxide (NO) bioavailability via multiple mechanisms
PBM reverses endothelial dysfunction via augmenting NO bioavailability and can be used for the therapy of cardiovascular diseases
Near-infrared (NIR) light in the NIR-II window (1000-1700 nm), which penetrates biological tissue deeper, shows PBM
NIR-II light augments NO bioavailability via activation of endothelial NO synthase (eNOS)
Funding:
This work was supported by the US NIH grants NIAID #R21AI144103 (SK), NINDS #R01NS096237 (DNA), NHLBI/NINDS #R01NS112808 (DEB), a study abroad scholarship of CMI Inc., Tokyo, Japan (SY), and Massachusetts General Hospital Executive Committee On Research (ECOR) Interim Support Funding (SK). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript, and the contents of this paper are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest:
All authors: No reported conflicts.
References
- [1].Atochin DN, Huang PL, Role of endothelial nitric oxide in cerebrovascular regulation, Curr Pharm Biotechnol 12 (2011) 1334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stamler JS, Vaughan DE, Loscalzo J, Synergistic disaggregation of platelets by tissue-type plasminogen activator, prostaglandin E1, and nitroglycerin, Circ Res 65 (1989) 796–804. [DOI] [PubMed] [Google Scholar]
- [3].Loscalzo J, Nitric oxide insufficiency, platelet activation, and arterial thrombosis, Circ Res 88 (2001) 756–62. [DOI] [PubMed] [Google Scholar]
- [4].Kawabata A, Evidence that endogenous nitric oxide modulates plasma fibrinogen levels in the rat, Br J Pharmacol 117 (1996) 236–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C, The role of nitric oxide on endothelial function, Curr Vasc Pharmacol 10 (2012) 4–18. [DOI] [PubMed] [Google Scholar]
- [6].Sobrevia L, Ooi L, Ryan S, Steinert JR, Nitric Oxide: A Regulator of Cellular Function in Health and Disease, Oxid Med Cell Longev 2016 (2016) 9782346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reichenbach G, Momi S, Gresele P, Nitric oxide and its antithrombotic action in the cardiovascular system, Curr Drug Targets Cardiovasc Haematol Disord 5 (2005) 65–74. [DOI] [PubMed] [Google Scholar]
- [8].Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KT, The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside, Exp Neurol 263 (2015) 235–43. [DOI] [PubMed] [Google Scholar]
- [9].Srivastava K, Bath PM, Bayraktutan U, Current therapeutic strategies to mitigate the eNOS dysfunction in ischaemic stroke, Cell Mol Neurobiol 32 (2012) 319–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang PL, Endothelial nitric oxide synthase and endothelial dysfunction, Curr Hypertens Rep 5 (2003) 473–80. [DOI] [PubMed] [Google Scholar]
- [11].Atochin DN, Huang PL, Endothelial nitric oxide synthase transgenic models of endothelial dysfunction, Pflugers Arch 460 (2010) 965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiss C, Kroncke KD, Hogg N, Feelisch M, Kelm M, Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease, Blood 120 (2012) 4229–37. [DOI] [PubMed] [Google Scholar]
- [13].Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M, Red blood cells express a functional endothelial nitric oxide synthase, Blood 107 (2006) 2943–51. [DOI] [PubMed] [Google Scholar]
- [14].Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, Raghavachari N, Boehm M, Kato GJ, Kelm M, Gladwin MT, Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis, Arterioscler Thromb Vasc Biol 33 (2013) 1861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, Piragine E, Schneckmann R, Hutzler B, Good ME, Fernandez BO, Vornholz L, Rogers S, Doctor A, Grandoch M, Stegbauer J, Weitzberg E, Feelisch M, Lundberg JO, Isakson BE, Kelm M, Cortese-Krott MM, Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure, Circulation 144 (2021) 870–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shiva S, Gladwin MT, Shining a light on tissue NO stores: near infrared release of NO from nitrite and nitrosylated hemes, J Mol Cell Cardiol 46 (2009) 1–3. [DOI] [PubMed] [Google Scholar]
- [17].Corti P, Tejero J, Gladwin MT, Evidence mounts that red cells and deoxyhemoglobin can reduce nitrite to bioactive NO to mediate intravascular endocrine NO signaling: commentary on "Anti-platelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex", Free Radic Biol Med 65 (2013) 1518–1520. [DOI] [PubMed] [Google Scholar]
- [18].Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A, Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex, Free Radic Biol Med 65 (2013) 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ, Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver, J Clin Invest 115 (2005) 1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singel DJ, Stamler JS, Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin, Annu Rev Physiol 67 (2005) 99–145. [DOI] [PubMed] [Google Scholar]
- [21].Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T, Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease, Circulation 104 (2001) 2673–8. [DOI] [PubMed] [Google Scholar]
- [22].Weinberger B, Laskin DL, Heck DE, Laskin JD, The toxicology of inhaled nitric oxide, Toxicol Sci 59 (2001) 5–16. [DOI] [PubMed] [Google Scholar]
- [23].Willmot M, Gray L, Gibson C, Murphy S, Bath PM, A systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow, Nitric Oxide 12 (2005) 141–9. [DOI] [PubMed] [Google Scholar]
- [24].Woodhouse L, Scutt P, Krishnan K, Berge E, Gommans J, Ntaios G, Wardlaw J, Sprigg N, Bath PM, E. Investigators, Effect of Hyperacute Administration (Within 6 Hours) of Transdermal Glyceryl Trinitrate, a Nitric Oxide Donor, on Outcome After Stroke: Subgroup Analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) Trial, Stroke 46 (2015) 3194–201. [DOI] [PubMed] [Google Scholar]
- [25].Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA, Ischaemic stroke, Nat Rev Dis Primers 5 (2019) 70. [DOI] [PubMed] [Google Scholar]
- [26].Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, H. collaborators, Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials, Lancet 387 (2016) 1723–31. [DOI] [PubMed] [Google Scholar]
- [27].O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW, 1,026 experimental treatments in acute stroke, Ann Neurol 59 (2006) 467–77. [DOI] [PubMed] [Google Scholar]
- [28].Huang YY, Chen AC, Carroll JD, Hamblin MR, Biphasic dose response in low level light therapy, Dose Response 7 (2009) 358–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang YY, Sharma SK, Carroll J, Hamblin MR, Biphasic dose response in low level light therapy - an update, Dose Response 9 (2011) 602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Karu T, Is it time to consider photobiomodulation as a drug equivalent?, Photomed Laser Surg 31 (2013) 189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Karu T, Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP, Photomed Laser Surg 28 (2010) 159–60. [DOI] [PubMed] [Google Scholar]
- [32].Karu TI, Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation, IUBMB Life 62 (2010) 607–10. [DOI] [PubMed] [Google Scholar]
- [33].Guzzardella GA, Fini M, Torricelli P, Giavaresi G, Giardino R, Laser stimulation on bone defect healing: an in vitro study, Lasers Med Sci 17 (2002) 216–20. [DOI] [PubMed] [Google Scholar]
- [34].Liebert A, Bicknell B, Laakso EL, Heller G, Jalilitabaei P, Tilley S, Mitrofanis J, Kiat H, Improvements in clinical signs of Parkinson's disease using photobiomodulation: a prospective proof-of-concept study, BMC Neurol 21 (2021) 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liebert A, Bicknell B, Markman W, Kiat H, A Potential Role for Photobiomodulation Therapy in Disease Treatment and Prevention in the Era of COVID-19, Aging Dis 11 (2020)1352–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liebert AD, Bicknell BT, Adams RD, Protein conformational modulation by photons: a mechanism for laser treatment effects, Med Hypotheses 82 (2014) 275–81. [DOI] [PubMed] [Google Scholar]
- [37].Kashiwagi S, Laser adjuvant for vaccination, FASEB J 34 (2020) 3485–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kashiwagi S, Brauns T, Gelfand J, Poznansky MC, Laser vaccine adjuvants. History, progress, and potential, Hum Vaccin Immunother 10 (2014) 1892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kashiwagi S, Brauns T, Poznansky MC, Classification of Laser Vaccine Adjuvants, J Vaccines Vaccin 7 (2016) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S, Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation, Lasers Surg Med 28 (2001) 204–11. [DOI] [PubMed] [Google Scholar]
- [41].Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M, Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits, Stroke 37 (2006) 2620–4. [DOI] [PubMed] [Google Scholar]
- [42].Zhang J, Yue X, Luo H, Jiang W, Mei Y, Ai L, Gao G, Wu Y, Yang H, An J, Ding S, Yang X, Sun B, Luo W, He R, Jia J, Lyu J, Tong Z, Illumination with 630 nm Red Light Reduces Oxidative Stress and Restores Memory by Photo-Activating Catalase and Formaldehyde Dehydrogenase in SAMP8 Mice, Antioxid Redox Signal 30 (2019) 1432–1449. [DOI] [PubMed] [Google Scholar]
- [43].Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J, Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer's and Parkinson's Disease, Front Neurosci 9 (2015) 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Colombo E, Signore A, Aicardi S, Zekiy A, Utyuzh A, Benedicenti S, Amaroli A, Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review, Biomedicines 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U, Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1), Stroke 38 (2007) 1843–9. [DOI] [PubMed] [Google Scholar]
- [46].Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J, NeuroThera E, Safety Trial I, Effectiveness and safety of transcranial laser therapy for acute ischemic stroke, Stroke 40 (2009) 1359–64. [DOI] [PubMed] [Google Scholar]
- [47].Huisa BN, Stemer AB, Walker MG, Rapp K, Meyer BC, Zivin JA, Nest, investigators, Transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2, Int J Stroke 8 (2013) 315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zivin JA, Sehra R, Shoshoo A, Albers GW, Bornstein NM, Dahlof B, Kasner SE, Howard G, Shuaib A, Streeter J, Richieri SP, Hacke W, N.-. investigators, NeuroThera(R) Efficacy and Safety Trial-3 (NEST-3): a double-blind, randomized, sham-controlled, parallel group, multicenter, pivotal study to assess the safety and efficacy of transcranial laser therapy with the NeuroThera(R) Laser System for the treatment of acute ischemic stroke within 24 h of stroke onset, Int J Stroke 9 (2014) 950–5. [DOI] [PubMed] [Google Scholar]
- [49].Hamblin MR, Shining light on the head: Photobiomodulation for brain disorders, BBA Clin 6 (2016) 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR, Brain Photobiomodulation Therapy: a Narrative Review, Mol Neurobiol 55 (2018) 6601–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lapchak PA, Boitano PD, Transcranial Near-Infrared Laser Therapy for Stroke: How to Recover from Futility in the NEST-3 Clinical Trial, Acta Neurochir Suppl 121 (2016) 7–12. [DOI] [PubMed] [Google Scholar]
- [52].Cao J, Zhu B, Zheng K, He S, Meng L, Song J, Yang H, Recent Progress in NIR-II Contrast Agent for Biological Imaging, Front Bioeng Biotechnol 7 (2019) 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Golovynskyi S, Golovynska I, Stepanova LI, Datsenko OI, Liu L, Qu J, Ohulchanskyy TY, Optical windows for head tissues in near-infrared and short-wave infrared regions: Approaching transcranial light applications, J Biophotonics 11 (2018) e201800141. [DOI] [PubMed] [Google Scholar]
- [54].Khan I, Tang E, Arany P, Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress, Scientific reports 5 (2015) 10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chinnathambi S, Shirahata N, Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging, Sci Technol Adv Mater 20 (2019) 337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dang X, Bardhan NM, Qi J, Gu L, Eze NA, Lin CW, Kataria S, Hammond PT, Belcher AM, Deep-tissue optical imaging of near cellular-sized features, Sci Rep 9 (2019) 3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen G, Shen J, Ohulchanskyy TY, Patel NJ, Kutikov A, Li Z, Song J, Pandey RK, Agren H, Prasad PN, Han G, (alpha-NaYbF4:Tm(3+))/CaF2 core/shell nanoparticles with efficient near-infrared to near-infrared upconversion for high-contrast deep tissue bioimaging, ACS Nano 6 (2012) 8280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Penberthy WT, Vorwaller CE, Utilization of the 1064 nm Wavelength in Photobiomodulation: A Systematic Review and Meta-Analysis, J Lasers Med Sci 12 (2021) e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang X, Tian F, Soni SS, Gonzalez-Lima F, Liu H, Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser, Sci Rep 6 (2016) 30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yokomizo S, Katagiri W, Maki Y, Sano T, Inoue K, Fukushi M, Atochin DN, Kushibiki T, Kawana A, Kimizuka Y, Kashiwagi S, Brief exposure of skin to near-infrared laser augments early vaccine responses, Nanophotonics 10 (2021) 3187–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Morse K, Kimizuka Y, Chan MPK, Shibata M, Shimaoka Y, Takeuchi S, Forbes B, Nirschl C, Li B, Zeng Y, Bronson RT, Katagiri W, Shigeta A, Sirbulescu RF, Chen H, Tan RYY, Tsukada K, Brauns T, Gelfand J, Sluder A, Locascio JJ, Poznansky MC, Anandasabapathy N, Kashiwagi S, Near-Infrared 1064 nm Laser Modulates Migratory Dendritic Cells To Augment the Immune Response to Intradermal Influenza Vaccine, J Immunol 199 (2017) 1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kimizuka Y, Katagiri W, Locascio JJ, Shigeta A, Sasaki Y, Shibata M, Morse K, Sirbulescu RF, Miyatake M, Reeves P, Suematsu M, Gelfand J, Brauns T, Poznansky MC, Tsukada K, Kashiwagi S, Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response, J Immunol 201 (2018) 3587–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kitano M, Yamazaki C, Takumi A, Ikeno T, Hemmi H, Takahashi N, Shimizu K, Fraser SE, Hoshino K, Kaisho T, Okada T, Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node, Proc Natl Acad Sci U S A 113 (2016) 1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Alexandre YO, Ghilas S, Sanchez C, Le Bon A, Crozat K, Dalod M, XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses, J Exp Med 213 (2016) 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H, Garbi N, Kaisho T, Germain RN, Kastenmuller W, Robust Anti-viral Immunity Requires Multiple Distinct T Cell-Dendritic Cell Interactions, Cell 162 (2015) 1322–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gerner MY, Torabi-Parizi P, Germain RN, Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens, Immunity 42 (2015) 172–85. [DOI] [PubMed] [Google Scholar]
- [67].Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, Iizuka A, Sato K, Tanaka T, Hoshino K, Kaisho T, Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1, J Immunol 190 (2013) 6071–82. [DOI] [PubMed] [Google Scholar]
- [68].Kashiwagi S, Yuan J, Forbes B, Hibert ML, Lee EL, Whicher L, Goudie C, Yang Y, Chen T, Edelblute B, Collette B, Edington L, Trussler J, Nezivar J, Leblanc P, Bronson R, Tsukada K, Suematsu M, Dover J, Brauns T, Gelfand J, Poznansky MC, Near-infrared laser adjuvant for influenza vaccine, PLoS One 8 (2013) e82899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kimizuka Y, Callahan JJ, Huang Z, Morse K, Katagiri W, Shigeta A, Bronson R, Takeuchi S, Shimaoka Y, Chan MP, Zeng Y, Li B, Chen H, Tan RY, Dwyer C, Mulley T, Leblanc P, Goudie C, Gelfand J, Tsukada K, Brauns T, Poznansky MC, Bean D, Kashiwagi S, Semiconductor diode laser device adjuvanting intradermal vaccine, Vaccine 35 (2017) 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Katagiri W, Yokomizo S, Ishizuka T, Yamashita K, Kopp T, Roessing M, Sato A, Iwasaki T, Sato H, Fukuda T, Monaco H, Manganiello S, Nomura S, Ng MR, Feil S, Ogawa E, Fukumura D, Atochin DN, Choi HS, Kashiwagi S, Dual near-infrared II laser modulates the cellular redox state of T cells and augments the efficacy of cancer immunotherapy, FASEB J 36 (2022) e22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tedford CE, DeLapp S, Jacques S, Anders J, Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue, Lasers Surg Med 47 (2015) 312–22. [DOI] [PubMed] [Google Scholar]
- [72].Berman MH, Halper JP, Nichols TW, Jarrett H, Lundy A, Huang JH, Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition, J Neurol Neurosci 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Banqueri M, Martinez JA, Prieto MJ, Cid-Duarte S, Mendez M, Arias JL, Photobiomodulation rescues cognitive flexibility in early stressed subjects, Brain Res 1720 (2019) 146300. [DOI] [PubMed] [Google Scholar]
- [74].Hwang J, Castelli DM, Gonzalez-Lima F, Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise, Lasers Med Sci 31 (2016) 1151–60. [DOI] [PubMed] [Google Scholar]
- [75].Barrett DW, Gonzalez-Lima F, Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans, Neuroscience 230 (2013) 13–23. [DOI] [PubMed] [Google Scholar]
- [76].Blanco NJ, Maddox WT, Gonzalez-Lima F, Improving executive function using transcranial infrared laser stimulation, J Neuropsychol 11 (2017) 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Blanco NJ, Saucedo CL, Gonzalez-Lima F, Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans, Neurobiol Learn Mem 139 (2017) 69–75. [DOI] [PubMed] [Google Scholar]
- [78].Wang X, Dmochowski JP, Zeng L, Kallioniemi E, Husain M, Gonzalez-Lima F, Liu H, Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms, Neurophotonics 6 (2019) 025013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wang X, Wanniarachchi H, Wu A, Gonzalez-Lima F, Liu H, Transcranial photobiomodulation and thermal stimulation induce distinct topographies of EEG alpha and beta power changes in healthy humans, Sci Rep 11 (2021) 18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yokomizo S, Roessing M, Morita A, Kopp T, Ogawa E, Katagiri W, Feil S, Huang PL, Atochin DN, Kashiwagi S, Near-infrared II photobiomodulation augments nitric oxide bioavailability via phosphorylation of endothelial nitric oxide synthase, FASEB J 36 (2022) e22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang X, Tian F, Reddy DD, Nalawade SS, Barrett DW, Gonzalez-Lima F, Liu H, Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study, J Cereb Blood Flow Metab 37 (2017) 3789–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tian F, Hase SN, Gonzalez-Lima F, Liu H, Transcranial laser stimulation improves human cerebral oxygenation, Lasers Surg Med 48 (2016) 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pruitt T, Wang X, Wu A, Kallioniemi E, Husain MM, Liu H, Transcranial Photobiomodulation (tPBM) With 1,064-nm Laser to Improve Cerebral Metabolism of the Human Brain In Vivo, Lasers Surg Med 52 (2020) 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Semyachkina-Glushkovskaya O, Abdurashitov A, Dubrovsky A, Klimova M, Agranovich I, Terskov A, Shirokov A, Vinnik V, Kuzmina A, Lezhnev N, Blokhina I, Shnitenkova A, Tuchin V, Rafailov E, Kurths J, Photobiomodulation of lymphatic drainage and clearance: perspective strategy for augmentation of meningeal lymphatic functions, Biomed Opt Express 11 (2020) 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Semyachkina-Glushkovskaya O, Klimova M, Iskra T, Bragin D, Abdurashitov A, Dubrovsky A, Khorovodov A, Terskov A, Blokhina I, Lezhnev N, Vinnik V, Agranovich I, Mamedova A, Shirokov A, Navolokin N, Khlebsov B, Tuchin V, Kurths J, Transcranial Photobiomodulation of Clearance of Beta-Amyloid from the Mouse Brain: Effects on the Meningeal Lymphatic Drainage and Blood Oxygen Saturation of the Brain, Adv Exp Med Biol 1269 (2021) 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zinchenko E, Navolokin N, Shirokov A, Khlebtsov B, Dubrovsky A, Saranceva E, Abdurashitov A, Khorovodov A, Terskov A, Mamedova A, Klimova M, Agranovich I, Martinov D, Tuchin V, Semyachkina-Glushkovskaya O, Kurts J, Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: breakthrough strategies for non-pharmacologic therapy of Alzheimer's disease, Biomed Opt Express 10 (2019) 4003–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Salehpour F, Khademi M, Bragin DE, DiDuro JO, Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System, Int J Mol Sci 23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Amaroli A, Ferrando S, Benedicenti S, Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue, Photochem Photobiol 95 (2019) 455–459. [DOI] [PubMed] [Google Scholar]
- [89].Quirk BJ, Whelan HT, What Lies at the Heart of Photobiomodulation: Light, Cytochrome C Oxidase, and Nitric Oxide-Review of the Evidence, Photobiomodul Photomed Laser Surg (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hamblin MR, Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation, Photochem Photobiol 94 (2018) 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ravera S, Ferrando S, Agas D, De Angelis N, Raffetto M, Sabbieti MG, Signore A, Benedicenti S, Amaroli A, 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes, J Biophotonics 12 (2019) e201900101. [DOI] [PubMed] [Google Scholar]
- [92].Katagiri W, Lee G, Tanushi A, Tsukada K, Choi HS, Kashiwagi S, High-throughput single-cell live imaging of photobiomodulation with multispectral near-infrared lasers in cultured T cells, J Biomed Opt 25 (2020) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gelfand JA, Nazarian RM, Kashiwagi S, Brauns T, Martin B, Kimizuka Y, Korek S, Botvinick E, Elkins K, Thomas L, Locascio J, Parry B, Kelly KM, Poznansky MC, A pilot clinical trial of a near-infrared laser vaccine adjuvant: safety, tolerability, and cutaneous immune cell trafficking, FASEB J 33 (2019) 3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dolgova D, Abakumova T, Gening T, Poludnyakova L, Zolotovskii I, Stoliarov D, Fotiadi A, Khokhlova A, Rafailov E, Sokolovski S, Anti-inflammatory and cell proliferative effect of the 1270 nm laser irradiation on the BALB/c nude mouse model involves activation of the cell antioxidant system, Biomed Opt Express 10 (2019) 4261–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schroeder P, Pohl C, Calles C, Marks C, Wild S, Krutmann J, Cellular response to infrared radiation involves retrograde mitochondrial signaling, Free Radic Biol Med 43 (2007) 128–35. [DOI] [PubMed] [Google Scholar]
- [96].Khokhlova A, Zolotovskii I, Stoliarov D, Vorsina S, Liamina D, Pogodina E, Fotiadi AA, Sokolovski SG, Saenko Y, Rafailov EU, The Photobiomodulation of Vital Parameters of the Cancer Cell Culture by Low Dose of Near-IR Laser Irradiation, IEEE Journal of Selected Topics in Quantum Electronics 25 (2019) 1–10. [Google Scholar]
- [97].Katagiri W, Yokomizo S, Ishizuka T, Yamashita K, Kopp T, Roessing M, Sato A, Iwasaki T, Sato H, Fukuda T, Monaco H, Manganiello S, Nomura S, Ng MR, Feil S, Ogawa E, Fukumura D, Atochin DN, Choi HS, Kashiwagi S, Dual near-infrared II laser modulates the cellular redox state of T cells and augments the efficacy of cancer immunotherapy, FASEB J (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Brown GC, Cooper CE, Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase, FEBS Lett 356 (1994) 295–8. [DOI] [PubMed] [Google Scholar]
- [99].Mason MG, Nicholls P, Wilson MT, Cooper CE, Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase, Proc Natl Acad Sci U S A 103 (2006) 708–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bohovych I, Khalimonchuk O, Sending Out an SOS: Mitochondria as a Signaling Hub, Front Cell Dev Biol 4 (2016) 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chandel NS, Evolution of Mitochondria as Signaling Organelles, Cell Metab 22 (2015) 204–6. [DOI] [PubMed] [Google Scholar]
- [102].da Cunha FM, Torelli NQ, Kowaltowski AJ, Mitochondrial Retrograde Signaling: Triggers, Pathways, and Outcomes, Oxid Med Cell Longev 2015 (2015) 482582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Karu TI, Kolyakov SF, Exact action spectra for cellular responses relevant to phototherapy, Photomed Laser Surg 23 (2005) 355–61. [DOI] [PubMed] [Google Scholar]
- [104].Borutaite V, Budriunaite A, Brown GC, Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols, Biochim Biophys Acta 1459 (2000) 405–12. [DOI] [PubMed] [Google Scholar]
- [105].Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, Otterbein LE, Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species, FASEB J 21 (2007) 1099–106. [DOI] [PubMed] [Google Scholar]
- [106].Moncada S, Bolanos JP, Nitric oxide, cell bioenergetics and neurodegeneration, J Neurochem 97 (2006) 1676–89. [DOI] [PubMed] [Google Scholar]
- [107].Boelens R, Wever R, Van Gelder BF, Rademaker H, An EPR study of the photodissociation reactions of oxidised cytochrome c oxidase-nitric oxide complexes, Biochim Biophys Acta 724 (1983) 176–83. [DOI] [PubMed] [Google Scholar]
- [108].Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr., The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2, Proc Natl Acad Sci U S A 98 (2001) 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sarti P, Forte E, Giuffre A, Mastronicola D, Magnifico MC, Arese M, The Chemical Interplay between Nitric Oxide and Mitochondrial Cytochrome c Oxidase: Reactions, Effectors and Pathophysiology, Int J Cell Biol 2012 (2012) 571067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Boelens R, Rademaker H, Pel R, Wever R, EPR studies of the photodissociation reactions of cytochrome c oxidase-nitric oxide complexes, Biochim Biophys Acta 679 (1982) 84–94. [DOI] [PubMed] [Google Scholar]
- [111].Sarti P, Giuffre A, Forte E, Mastronicola D, Barone MC, Brunori M, Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation, Biochem Biophys Res Commun 274 (2000) 183–7. [DOI] [PubMed] [Google Scholar]
- [112].Umbrello M, Dyson A, Feelisch M, Singer M, The key role of nitric oxide in hypoxia: hypoxic vasodilation and energy supply-demand matching, Antioxid Redox Signal 19 (2013)1690–710. [DOI] [PubMed] [Google Scholar]
- [113].Zhang R, Mio Y, Pratt PF, Lohr N, Warltier DC, Whelan HT, Zhu D, Jacobs ER, Medhora M, Bienengraeber M, Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism, J Mol Cell Cardiol 46 (2009) 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pope NJ, Powell SM, Wigle JC, Denton ML, Wavelength- and irradiance-dependent changes in intracellular nitric oxide level, J Biomed Opt 25 (2020) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Paunel AN, Dejam A, Thelen S, Kirsch M, Horstjann M, Gharini P, Murtz M, Kelm M, de Groot H, Kolb-Bachofen V, Suschek CV, Enzyme-independent nitric oxide formation during UVA challenge of human skin: characterization, molecular sources, and mechanisms, Free Radic Biol Med 38 (2005) 606–15. [DOI] [PubMed] [Google Scholar]
- [116].Liu D, Fernandez BO, Hamilton A, Lang NN, Gallagher JMC, Newby DE, Feelisch M, Weller RB, UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase, J Invest Dermatol 134 (2014) 1839–1846. [DOI] [PubMed] [Google Scholar]
- [117].Mowbray M, McLintock S, Weerakoon R, Lomatschinsky N, Jones S, Rossi AG, Weller RB, Enzyme-independent NO stores in human skin: quantification and influence of UV radiation, J Invest Dermatol 129 (2009) 834–42. [DOI] [PubMed] [Google Scholar]
- [118].Borisenko GG, Osipov AN, Kazarinov KD, Vladimirov Yu A, Photochemical reactions of nitrosyl hemoglobin during exposure to low-power laser irradiation, Biochemistry (Mosc) 62 (1997) 661–6. [PubMed] [Google Scholar]
- [119].Stern M, Broja M, Sansone R, Grone M, Skene SS, Liebmann J, Suschek CV, Born M, Kelm M, Heiss C, Blue light exposure decreases systolic blood pressure, arterial stiffness, and improves endothelial function in humans, Eur J Prev Cardiol 25 (2018) 1875–1883. [DOI] [PubMed] [Google Scholar]
- [120].Shiva S, Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function, Redox Biol 1 (2013) 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Poyton RO, Ball KA, Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase, Discov Med 11 (2011) 154–9. [PubMed] [Google Scholar]
- [122].Barolet AC, Cormack G, Lachance G, Auclair M, Barolet D, In vivo quantification of nitric oxide (NO) release from intact human skin following exposure to photobiomodulation wavelengths in the visible and near-infrared spectrum. in: Hamblin MR, Carroll J, Arany P, (Eds.), Proc of SPIE, SPIE; 2019. [Google Scholar]
- [123].Ball KA, Castello PR, Poyton RO, Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy, J Photochem Photobiol B 102 (2011) 182–91. [DOI] [PubMed] [Google Scholar]
- [124].Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N, Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection, J Mol Cell Cardiol 47 (2009) 256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lohr NL, Ninomiya JT, Warltier DC, Weihrauch D, Far red/near infrared light treatment promotes femoral artery collateralization in the ischemic hindlimb, J Mol Cell Cardiol 62 (2013)36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Keszler A, Brandal G, Baumgardt S, Ge ZD, Pratt PF, Riess ML, Bienengraeber M, Far red/near infrared light-induced protection against cardiac ischemia and reperfusion injury remains intact under diabetic conditions and is independent of nitric oxide synthase, Front Physiol 5 (2014) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Keszler A, Lindemer B, Weihrauch D, Jones D, Hogg N, Lohr NL, Red/near infrared light stimulates release of an endothelium dependent vasodilator and rescues vascular dysfunction in a diabetes model, Free Radic Biol Med 113 (2017) 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Namkoong S, Lee SJ, Kim CK, Kim YM, Chung HT, Lee H, Han JA, Ha KS, Kwon YG, Kim YM, Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells, Exp Mol Med 37 (2005) 588–600. [DOI] [PubMed] [Google Scholar]
- [129].Uruno A, Sugawara A, Kanatsuka H, Arima S, Taniyama Y, Kudo M, Takeuchi K, Ito S, Hepatocyte growth factor stimulates nitric oxide production through endothelial nitric oxide synthase activation by the phosphoinositide 3-kinase/Akt pathway and possibly by mitogen-activated protein kinase kinase in vascular endothelial cells, Hypertens Res 27 (2004) 887–95. [DOI] [PubMed] [Google Scholar]
- [130].Mitchell UH, Mack GL, Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: a single-blind, randomized clinical trial of efficacy, Am J Phys Med Rehabil 92 (2013) 151–6. [DOI] [PubMed] [Google Scholar]
- [131].Chen CH, Hung HS, Hsu SH, Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway, Lasers Surg Med 40 (2008) 46–54. [DOI] [PubMed] [Google Scholar]
- [132].Tuby H, Maltz L, Oron U, Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis, Lasers Surg Med 38 (2006) 682–8. [DOI] [PubMed] [Google Scholar]
- [133].Golovynska I, Golovynskyi S, Stepanov YV, Stepanova LI, Qu J, Ohulchanskyy TY, Red and near-infrared light evokes Ca(2+) influx, endoplasmic reticulum release and membrane depolarization in neurons and cancer cells, J Photochem Photobiol B 214 (2021) 112088. [DOI] [PubMed] [Google Scholar]
- [134].Golovynska I, Golovynskyi S, Stepanov YV, Garmanchuk LV, Stepanova LI, Qu J, Ohulchanskyy TY, Red and near-infrared light induces intracellular Ca(2+) flux via the activation of glutamate N-methyl-D-aspartate receptors, J Cell Physiol (2019). [DOI] [PubMed] [Google Scholar]
- [135].Zhang X, Peng K, Zhang X, The Function of the NMDA Receptor in Hypoxic-Ischemic Encephalopathy, Front Neurosci 14 (2020) 567665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen ZQ, Mou RT, Feng DX, Wang Z, Chen G, The role of nitric oxide in stroke, Med Gas Res 7 (2017) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nagarajan S, Raj RK, Saravanakumar V, Balaguru UM, Behera J, Rajendran VK, Shathya Y, Ali BM, Sumantran V, Chatterjee S, Mechanical perturbations trigger endothelial nitric oxide synthase activity in human red blood cells, Sci Rep 6 (2016) 26935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Freedman JE, Loscalzo J, Nitric oxide and its relationship to thrombotic disorders, J Thromb Haemost 1 (2003) 1183–8. [DOI] [PubMed] [Google Scholar]
- [139].Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC, Hypertension in mice lacking the gene for endothelial nitric oxide synthase, Nature 377 (1995) 239–42. [DOI] [PubMed] [Google Scholar]
- [140].Kashiwagi S, Atochin DN, Li Q, Schleicher M, Pong T, Sessa WC, Huang PL, eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity, Biochem Biophys Res Commun 431 (2013) 284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Li Q, Atochin D, Kashiwagi S, Earle J, Wang A, Mandeville E, Hayakawa K, d'Uscio LV, Lo EH, Katusic Z, Sessa W, Huang PL, Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size, Stroke 44 (2013) 3183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Arnall DA, Nelson AG, Lopez L, Sanz N, Iversen L, Sanz I, Stambaugh L, Arnall SB, The restorative effects of pulsed infrared light therapy on significant loss of peripheral protective sensation in patients with long-term type 1 and type 2 diabetes mellitus, Acta Diabetol 43 (2006) 26–33. [DOI] [PubMed] [Google Scholar]