Abstract
Infection with mycobacterial species, including Mycobacterium tuberculosis, has long been implicated in the etiopathology of rheumatoid arthritis (RA) on the basis of clinical and pathological similarities between tuberculosis and RA. Despite evidence of immune responses to mycobacterial antigens in RA patient synovial fluid, cross-reactivity between these and host joint antigens, and the presence of M. tuberculosis protein antigen in RA synovial fluid, a definite causal association with RA has not been shown. Previous studies from our laboratory using reverse transcriptase PCR (RT-PCR) of bacterial rRNAs have shown RA synovium to be colonized by a diverse range of bacteria, most of commensal origin. However, M. tuberculosis group organism (MTG) RNA sequences were found in one RA patient tissue. Since this was considered of sufficient interest to warrant further investigation, we devised a M. tuberculosis-specific nested RT-PCR test which could be used for detection of MTG in a mixed pool of bacterial crDNAs. This test was used to investigate the distribution of MTG in RA synovial tissue and also non-RA arthritis and healthy control tissues and was also used to examine the tissue distribution of MTG in an acute and chronic model of M. tuberculosis infection in the BALB/c mouse. MTG sequences were found in a high proportion of RA patient synovial tissues but also in non-RA arthritis control tissues at lower frequency. This likely reflects trafficking of persistent M. bovis BCG to inflamed joint tissue, irrespective of cause. MTG were not found in healthy synovial tissue or the tissue of patients with undifferentiated arthritis. In both the acute and chronic models of infection in BALB/c mice, M. tuberculosis was also found to have trafficked to joint tissues, however, no signs of inflammation, arthritis, or pathology associated with M. tuberculosis infection was seen. These combined results would argue against a specific causal role of MTG in RA-like arthritis; however, their role as adjuvant in immune dysfunction in an innately susceptible host cannot be excluded.
Rheumatoid arthritis (RA) is a complex, multifactorial disease in which innate susceptibility factors play a substantial role (36). However, heritable factors can account for only part of disease susceptibility, and onset of RA has long been thought to be triggered by exposure to some environmental agent. Prime candidates for this role are bacterial infectious agents, and great interest has been taken over the years in their possible role in disease onset (3, 6, 32). Their causative link with inflammatory arthritis is controversial, as early studies which sought to establish an association between disease pathology and the diverse range of bacterial species which have been isolated from diseased joints, using conventional culture methods, were unable to do so (22, 62). A number of different studies identified in RA joint material a variety of bacterial species which could not be directly associated with disease pathology, thus negating a simple, single-pathogen role for involvement of bacteria in RA. However, inflammatory arthritis can be elicited by infection with a variety of bacterial and viral species in animal models (19), and it is possible that more than one organism is involved in human disease, especially given the heterogeneous nature of RA and the variation in disease severity between individuals.
With the advent of more sensitive molecular techniques for the detection of bacteria in disease tissue (50), interest has again been revived in investigating the possibility of an infectious etiology for RA. Work from our laboratory has shown the presence of possibly live bacteria in the joints of patients with a variety of different arthropathies, using reverse transcriptase PCR (RT-PCR) for amplification of bacterial rRNAs (28). Detailed sequencing analyses of the amplified products has shown the picture to be considerably more complex that was once thought, with the detection of multiple bacterial species in each patient. These studies have been supported by those from Wilbrink and coworkers (66) and other groups (68), who have also shown that synovial tissue and fluid from patients with a number of different types of arthritis contain bacterial nucleic acids, sequence analyses again showing the presence of multiple organisms. Similar studies have also shown the presence of bacterial polysaccharide antigen (41) in RA synovial tissue and unidentified bacillus-like organisms in RA synovial explant cultures (39). Thus, evidence of live bacteria and of their nucleic acids and antigens have been found in RA synovial tissue and fluid, all of which could contribute to disease pathology. Whether bacteria and their products are instrumental in the onset or chronicity of RA remains unknown, but these studies would certainly suggest that arthritis joint material is not sterile, as thought previously.
The presence of multiple bacterial species in the tissues of patients with RA, but also patients with other arthropathies, has called into question the involvement of these organisms in the underlying pathological processes and has implied general colonization as a consequence of the compromise of chronically diseased and inflamed tissue (28). Many of the bacterial species detected were commensal organisms with low pathogenic potential, which presumably had trafficked from other body sites. Very few organisms of known pathogenic potential other than opportunistic pathogens, e.g., Staphylococcus epidermidis, were detected. However, in one RA patient we detected the presence of crDNA sequences from Mycobacterium tuberculosis group organisms (MTG) at low frequency, 2 of 48 clones sequenced in a mixed pool of bacterial crDNAs (4%). In addition to our own studies where we have detected a number of mycobacterial species in patients with arthritis including MTG, Wilbrink and coworkers have uncovered evidence of other mycobacteria in the synovial fluid of RA patients by mycobacterial species-specific nested PCR (63). Similar studies by Wu and coworkers have uncovered evidence of M. tuberculosis protein antigen in RA synovial fluid (69, 70). Thus, RA synovial tissue and fluid in addition to the other organisms identified contain mycobacterial species, some of which, e.g., MTG, have known pathogenic potential.
The detection of MTG was therefore thought to be of sufficient interest to warrant further investigation, as mycobacteria in general and M. tuberculosis in particular have long been postulated to be involved in the etiopathogenesis of RA on the basis of clinical and pathological similarities with tuberculosis (27, 34, 55, 56). However, previous studies to investigate the possible presence of mycobacteria including MTG by PCR for genomic DNA had been unsuccessful (24, 48). We thought that these negative results may have been due to low abundance of target bacterial DNA sequences, as we had also found certain bacterial sequences including MTG at low frequency.
From our previous studies, we proposed that RT-PCR of bacterial RNA is more sensitive than PCR of bacterial genomic DNA in the detection of bacterial nucleic acids in disease tissue. Enhanced detection of species-specific sequences at low abundance in a mixed pool can be facilitated by combination of universal RT-PCR and species-specific nested PCR. This has been used previously to great effect in the detection of Yersinia spp. in a patient with known Yersinia-mediated reactive arthritis (ReA) (18). We therefore used this adaptation of RT-PCR using universal oligonucleotide primers to bacterial rRNA and MTG species-specific, nested primers to investigate the prevalence of this group of organisms in the synovial tissue of three cohorts of patients with late- and early-stage RA or other forms of arthritis and in healthy controls. These studies have shown MTG organisms to be present in the tissues of patients with a number of different arthritides; however, although there appears to be trafficking of these organisms to inflamed joint tissue, there is no apparent specific association with RA.
We also used this technique to follow the in vivo tissue location of MTG in an acute and chronic model of M. tuberculosis infection in the BALB/c mouse to ascertain whether MTG are able to traffic to tissues other than those which exhibit signs of overt M. tuberculosis disease. MTG were found to have trafficked to joint tissue both in an acute and a chronic model of M. tuberculosis infection. However, the presence of M. tuberculosis in joint tissue did not appear to lead to inflammation or the development of arthritis disease pathology in this mouse model, perhaps implying no ability of this organism to cause disease in these tissues. These combined results would suggest that the presence of MTG in human and mouse joint tissue does not appear to be of significance in the primary etiopathology of inflammatory arthritis, although their role as adjuvant in the potentiation of immune dysfunction in RA cannot be excluded.
MATERIALS AND METHODS
Materials.
All general chemicals were purchased from Sigma-Aldrich Co. Ltd., Poole, Dorset, England. Media constituents were provided by Oxoid Ltd., Basingstoke, Hampshire, England. A Hybaid Ribolyser kit was purchased from Hybaid Teddington, Middlesex, England. Amplitaq Taq polymerase and buffers were supplied by Perkin-Elmer, Warrington, Cheshire, England. Superscript II reverse transcriptase and all oligonucleotide primers were purchased from GibcoBRL Life Technologies, Paisley, Scotland. Deoxyribonucleosides were purchased from Roche Diagnostics, Lewes, Sussex, England.
Growth of mycobacterial species and isolation of mycobacterial DNA.
All mycobacterial strains (Table 1) were grown on Dubos medium plus supplements (16) with the exception of M. smegmatis, which was grown on Lab-Lemco broth (pH 7.4) containing 0.3% (wt/vol) Lab-Lemco powder and 0.5% (wt/vol) peptone (Oxoid). These were incubated at 37°C, without shaking, for 7 to 21 days, according to the growth rate of the species. DNA was extracted from washed mycobacterial cell pellets recovered by centrifugation, using the guanidinium hydrochloride extraction method (25), according to the published procedure. After recovery by ethanol precipitation, mycobacterial DNA pellets were dried and then dissolved in Tris-Cl buffer containing 0.1 mM EDTA prior to use.
TABLE 1.
Bacterial species and strains used in this study
| Species | Sourcea |
|---|---|
| Mycobacterium tuberculosis H37Rv | NCTC 7416 |
| M. tuberculosis H37Ra | NCTC 7417 |
| M. bovis BCG | NCTC 5692 |
| M. microti | NCTC 8710 |
| M. habana | M. J. Colston, NIMR |
| M. marinum | NCTC 2275 |
| M. marinum | NCTC 10009 |
| M. malmoense | ATCC 29571 |
| M. ulcerans | ATCC 19423 |
| M. chelonii | NCTC 10882 |
| M. fortuitum | NCTC 10394 |
| M. kansasii | NCTC 10268 |
| M. scrofulaceum | NCTC 10803 |
| M. scrofulaceum | ATCC 19073 |
| M. paratuberculosis | ATCC 43544 |
| M. avium sub sp. silvaticum | F. Portaels, ITM |
| M. avium chromogenicum | F. Portaels, ITM |
| M. intracellulare | NCTC 10425 |
| M. intracellulare | NCTC 10682 |
| M. avium | ATCC 25291 |
| M. avium | NCTC 8559 |
| M. vaccae | NCTC 15483 |
| M. vaccae | NCTC 23005 |
| M. lufu | M. J. Colston, NIMR |
| M. phlei | NCTC 8151 |
| M. gordonae | NCTC 10267 |
| M. aurum | NCTC 10437 |
| M. smegmatis | NCTC 8159 |
| M. smegmtis MC2 155 | N. Stoker, LSHTM |
| Streptomyces coelicolor | ATCC 10147 |
ATCC, American Type Culture Collection, Manassas, Va; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London NW9 5HT, United Kingdom; NIMR, National Institute for Medical Research, London, United Kingdom; ITM, Mycobacteriology Unit, Institute of Tropical Medicine, Antwerp, Belgium; LSHTM, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Acute and chronic M. tuberculosis infection models of BALB/c mice.
To ascertain (i) the potential of M. tuberculosis to traffic to joint tissue and (ii) the technical feasibility of MTG-specific PCR to detect live organisms in infected tissue samples, a BALB/c mouse model of acute and chronic M. tuberculosis infection was established. Tissues from infected mice were then assayed for the presence of MTG by nested, specific RT-PCR and also by conventional bacteriological staining. Female BALB/c mice (weighing approximately 20 g, Charles River) were maintained in isolators under ACDP category 3 conditions. These were infected intravenously with 200-μl volumes of M. tuberculosis (H37Rv) which had been prepared to the appropriate dilution (Table 2) from stocks stored at −80°C. To achieve a long-term (118-day) chronic infection a challenge concentration of approximately 4 × 104 CFU was administered to each mouse. To establish a more acute infection, we used a higher challenge level (approximately 5 × 106 CFU) for which the survival time was between 28 and 40 days. Mice with either chronic (day 118) or acute (28-day) infections were sacrificed by cervical dislocation; tissues were then removed aseptically for RT-PCR and histological analyses. After debridement of external tissue from excised joints, all tissues were either (i) processed for RNA extraction by being placed immediately into 500 μl of guanidinium isothiocyanate extraction buffer (GIEB) medium and then stored at −80°C or (ii) fixed in 10% neutral buffered formalin for histological evaluation. After fixation, the joints were decalcified in EDTA, processed to paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin or Ziehl-Neelson acid fuschin. All samples were examined microscopically using a Leica DM Research microscope and were scored for inflammation, granuloma formation, and the presence of acid-fast bacteria. A total of 11 joints from five chronically infected mice and 13 joints from five acutely infected mice were collected along with uninfected joints, eight control joints from three uninfected mice. In addition, lung and spleen tissues from one uninfected and one acutely infected mouse were removed for use as positive controls (Table 2).
TABLE 2.
Details of M. tuberculosis-infected and control BALB/c mouse tissues used in this study
| Mouse tissue | Infection | Tissue type |
|---|---|---|
| 1a | Control, uninfected | Knee |
| 1b | Control, uninfected | Wrist |
| 2a | Control, uninfected | Knee |
| 2b | Control, uninfected | Wrist |
| 2c | Control, uninfected | Hip |
| 3a | Low-inoculum (3.6 × 104), 118-day chronic infection | Wrist |
| 3b | Low-inoculum (3.6 × 104), 118-day chronic infection | Knee |
| 3c | Low-inoculum (3.6 × 104), 118-day chronic infection | Hip |
| 4a | Low-inoculum (3.6 × 104), 118-day chronic infection | Wrist |
| 4b | Low-inoculum (3.6 × 104), 118-day chronic infection | Hip |
| 5a | Low-inoculum (3.6 × 104), 118-day chronic infection | Wrist |
| 5b | Low-inoculum (3.6 × 104), 118-day chronic infection | Knee |
| 6a | Low-inoculum (3.6 × 104), 118-day chronic infection | Wrist |
| 6b | Low-inoculum (3.6 × 104), 118-day chronic infection | Hip |
| 7a | Low-inoculum (3.6 × 104), 118-day chronic infection | Wrist |
| 7b | Low-inoculum (3.6 × 104), 118-day chronic infection | Knee |
| 8a | Control, uninfected | Lung |
| 8b | Control, uninfected | Spleen |
| 8c | Control, uninfected | Wrist |
| 8d | Control, uninfected | Knee |
| 8e | Control, uninfected | Hip |
| 9a | High-inoculum (5 × 106), 28-day acute infection | Lung |
| 9b | High-inoculum (5 × 106), 28-day acute infection | Spleen |
| 9c | High-inoculum (5 × 106), 28-day acute infection | Wrist |
| 9d | High-inoculum (5 × 106), 28-day acute infection | Knee |
| 9e | High-inoculum (5 × 106), 28-day acute infection | Hip |
| 10a | High-inoculum (5 × 106), 28-day acute infection | Wrist |
| 10b | High-inoculum (5 × 106), 28-day acute infection | Knee |
| 10c | High-inoculum (5 × 106), 28-day acute infection | Hip |
| 11a | High-inoculum (5 × 106), 28-day acute infection | Wrist |
| 11b | High-inoculum (5 × 106), 28-day acute infection | Knee |
| 11c | High-inoculum (5 × 106), 28-day acute infection | Hip |
| 12a | High-inoculum (5 × 106), 28-day acute infection | Knee |
| 12b | High-inoculum (5 × 106), 28-day acute infection | Hip |
| 13a | High-inoculum (5 × 106), 28-day acute infection | Wrist |
| 13b | High-inoculum (5 × 106), 28-day acute infection | Knee |
| 13c | High-inoculum (5 × 106), 28-day acute infection | Hip |
Tissue handling and RNA isolation.
RA, osteoarthritis (OA), and other disease (undifferentiated arthritis [UA]) synovial tissue specimens were collected with patient consent at surgery for joint replacement with a few exceptions: in cohort 1, the RA patient 2 specimen, which was obtained by needle biopsy, and OA patient 20 specimen, which was tissue removed at first metatarsal phalangeal surgery. Healthy synovial tissues were collected from patients 22 and 23 by needle biopsy and from patient 21 at arthroscopy for unexplained knee pain, (clinical details of patients in cohort 1 are given in Table 3). Healthy controls were not age and sex matched to the RA patient group; however, the trauma specimens were unlikely to have features of joint disease pathology in common with arthritis patients of many years duration. Full microbiological analyses of patients from cohort 1 have been published in detail elsewhere (28). Synovial tissue was obtained in cohort 2 patients with from late-stage RA and OA, plus patient controls with arthritis due to other causes (Table 4), either at surgery for joint replacement, from power tool washings for debridement of inflamed synovium, or by arthroscopic biopsy. Conditions of all late-stage RA patients according to the American College of Rheumatology criteria (5) were classified as late-stage disease, i.e., disease of many year's duration with joint destruction requiring arthroplasty. Synovial tissues from patients in cohort 3 (Table 5) with early RA or reactive arthritis (ReA), i.e., categorized as having disease of less than 1 year's duration, were obtained with permission by needle biopsy.
TABLE 3.
Details of disease tissues from cohort 1 patients used in this study
| Patient | Diagnosis | Sex/age (yr)/tissue detailsa |
|---|---|---|
| 1 | RA | DNG/DNG/TKR |
| 2 | RA | M/DNG/needle biopsy (knee) |
| 3 | RA | F/58/TKR |
| 4 | RA | M/84/THR |
| 5 | RA | F/63/TKR |
| 6 | RA | M/54/TKR |
| 7 | RA | M/72/THR |
| 8 | RA | F/60/TKR |
| 9 | RA | F/60/TKR |
| 10 | OA | F/76/THR |
| 11 | OA | M/64/TKR |
| 12a | OA | M/79/TKR |
| 12b | OA | RA |
| 12c | OA | RA |
| 12d | OA | RA |
| 12e | OA | RA |
| 12f | OA | RA |
| 12g | OA | RA |
| 13 | OA | M/57/TKR |
| 14 | OA | F/64/THR |
| 15 | OA | M/77/THR |
| 16 | OA | F/28/1st MTP surgery |
| 17 | UA | M/79/TKR |
| 18 | UA | F/71/TKR |
| 19 | UA | F/88/TKR |
| 20 | UA | M/66/TKR |
| 21 | Normal synovium | M/DNG/knee trauma, arthroscopic biopsy |
| 22 | Normal synovium | M/DNG/normal needle biopsy (knee) |
| 23 | Normal synovium | M/DNG/normal needle biopsy (knee) |
DNG, details not given; TKR, total knee replacement, M, male; F, female; THR, total hip replacement, 1st MTP, first metatarsal phalangeal surgery.
TABLE 4.
Details of disease tissues from cohort 2 patients used in this study
| Patient | Diagnosis | Sex/age (yr)/tissue details/ inflammatory statusa |
|---|---|---|
| 1 | OA | DNG/72/TKR |
| 2 | OA | M/73/TKR |
| 3a | OA | F/63/TKR (inflamed) |
| 3b | OA | F/63/TKR (noninflamed) |
| 4 | OA | F/56/PTW |
| 5a | OA | DNG/65/PTW (inflamed) |
| 5b | OA | DNG/65/PTW (noninflamed) |
| 6 | OA | DNG/72/TKR |
| 7 | OA | DNG/58/TKR |
| 8 | OA | DNG/51/PTW |
| 9 | OA | F/75/TKR |
| 10 | OA | M/72/THR |
| 11 | OA | F/69/THR |
| 12 | OA | F/80/THR |
| 13 | OA | F/65/THR |
| 14 | OA | M/69/THR |
| 15 | OA | F/72/THR |
| 16 | OA | DNG/72/TKR |
| 17 | RA | F/67/MTP surgery |
| 18 | RA | F/40/TKR |
| 19 | RA | M/59/TKR |
| 20 | RA | F/37/arthroscopic total synovectomy |
| 21a | RA | DNG/72/TKR (inflamed) |
| 21b | RA | DNG/72/TKR (noninflamed) |
| 25 | RA | F/70/TKR |
| 26 | RA | F/51/TKR |
| 27 | RA | F/DNG/arthroscopic biopsy |
| 28 | RA | M/65/TKR |
| 29 | RA | M/54/TER |
| 30 | RA | M/67/THR |
| 31 | RA | F/52/THR |
| 32 | RA | F/37/TKR |
| 33 | RA | M/46/TKR |
| 34 | RA | M/65/MTP surgery |
| 35 | Crystal arthritis | DNG/DNG/PTW |
| 36 | Suspected septic arthritis | M/20/severe synovitis, PTW |
| 37 | Psoriatic arthritis | M/DNG/arthroscopic biopsy |
DNG, details not given; TKR; total knee replacement; M, male; F, female; PTW, power tool washings, THR, total hip replacement; MTP; metatarsal phalangeal surgery; TER; total elbow replacement.
TABLE 5.
Details of disease tissues from cohort 3 patients used in this studya
| Patient | Diagnosis | Sexb/age (yr)/tissue details/ disease status | Disease duration (wks) |
|---|---|---|---|
| 1 | Early RA | M/61/needle biopsy/persistent | 20 |
| 2 | Early RA | M/67/needle biopsy/persistent | 24 |
| 3 | Early RA | M/22/needle biopsy/persistent | 24 |
| 4 | Early RA | M/62/needle biopsy/persistent | 28 |
| 5 | Early RA | F/32/needle biopsy/persistent | 40 |
| 6 | Early RA | M/50/needle biopsy/persistent | 52 |
| 7 | Early ReA | M/16/needle biopsy/remission | 3 |
| 8 | Early ReA | M/27/needle biopsy/remission | 24 |
| 9 | Early ReA | M/21/needle biopsy/remission | 8 |
| 10 | Early ReA | M/29/needle biopsy/remission | 12 |
| 11 | Early ReA | M/34/needle biopsy/remission | 1 |
| 12 | Early ReA | M/27/needle biopsy/remission | 10 |
All tissues were obtained by needle biopsy.
M, male; F, female.
Resected synovial tissue samples collected at surgery were immediately frozen on dry ice and then stored at −80°C prior to use. Total RNA was isolated from late- and early-stage RA, OA, and ReA synovial tissue and from control and mouse tissues using a modification of the Hybaid RiboLyser, guanidinium isothiocyanate-acid phenol extraction method, in which buffer A was substituted with fresh GIEB (38). In short, approximately 0.1 g of tissue was thawed in 500 μl of GIEB, chopped finely (mouse joint material excepted, due to the high bone content), and then extracted by shear lysis in the presence of 500 μl of phenol (pH 4.0) and 100 μl of chloroform isoamyl alcohol in a Hybaid Ribolyser, according to the manufacturer's instructions. Total RNA was recovered by precipitation with 2-propanol, dried, and then dissolved in diethyl pyrocarbonate-treated water containing 0.1 mM EDTA. Bacterial RNAs were recovered from washed Escherichia coli and M. tuberculosis cell pellets, using the same protocol with minor modifications based on the protocol of Mangan and coworkers, i.e., Divolab (pH 4.0) added to 1% to GIEB prior to shear lysis (37).
Specific detection of MTG by rDNA-specific PCR of mycobacterial DNAs.
rDNA fragments were amplified from mycobacterial genomic DNAs by PCR amplification using universal, bacterial rRNA-specific oligonucleotide primers R1 and R2 (i.e., PCR1; all oligonucleotide sequences are given in Table 6). Genomic DNA (5 fg) was used as template in a PCR mix containing 1× Amplitaq PCR buffer, 0.2 μM deoxynucleoside triphosphates (dNTPs), 0.2 μM PCR primers R1 and R2, 1.5 mM MgCl2, and 2.5 U of Amplitaq Taq polymerase. Amplication was performed as follows: 94°C for 4 min, followed by 30 cycles of 58°C for 1 min, 72°C for 3 min, and 94°C for 1 min. MTG-specific nested PCR (PCR2) was then performed on amplified bacterial rDNA fragments by the following means. One microliter of the PCR1 product was used as the template in a PCR mix containing 1 × Amplitaq PCR buffer, 0.2 μM dNTPs, 0.2 μM PCR primers TB1 and TB2, 1.5 mM MgCl2 and 2.5 U of Amplitaq Taq polymerase. The amplification program consisted of 94°C for 4 min, followed by 30 cycles of 72°C for 3 min and 94°C for 1 min. All PCR products were visualized by electrophoresis on a 2% agarose gel.
TABLE 6.
Details of oligonucleotide primers used in this study
| Oligonucleotide primer | Sequence | Position in M. tuberculosis rRNA gene sequence |
|---|---|---|
| R1 | AGAGTTTGATCCTGGCTCAG | 10–29 |
| R2 | ACTGCTGCCTCCCGTAGGAG | 337–356 |
| TB1 | CGAACGGAAAGGTCTCTTCGGAGA | 65–88 |
| TB2 | ACCACAAGACATGCATCCCGTGGT | 179–202 |
Amplification of bacterial crDNAs and M. tuberculosis-specific nested PCR of mouse and human tissue RNA by Reverse T-PCR.
Arthritis and control tissue and bacterial RNAs were reverse transcribed by the same method in 4 μl of buffer containing 50 mM Tris-Cl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 40 μM dNTPs, approximately 25 to 100 ng of RNA, and 20 μM primer R2. This mixture was heated to 65°C for 1 min and then cooled to room temperature for 3 min; 200 U of Superscript reverse transcriptase was added, and the mix was incubated at 37°C for 1 h. The reaction was stopped by incubation at 65°C for 10 min. General bacterial ribosomal crDNA fragments were amplified from total cDNA by the procedure outlined above for PCR1 of DNA templates; MTG-specific nested PCR2 was then performed on RNA-derived, PCR1-amplified crDNA fragments as outlined above. Amplified MTG-specific products from human tissue samples and bacterial RNAs were confirmed, either by Southern blotting using established techniques (38) and hybridization using 32P-labeled, gel-purified M. tuberculosis-specific PCR2 fragment or by gel purification and sequencing.
RESULTS
Specific detection of MTG from closely related bacteria by nested PCR.
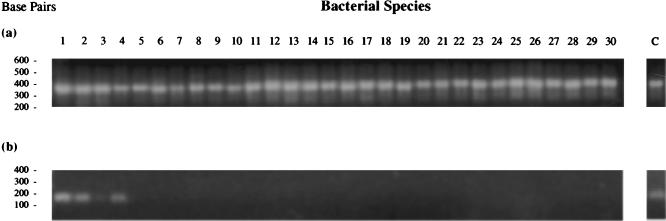
MTG could be distinguished from closely related bacteria (a total of 25 other species and substrains) by nested PCR using primers R1 and R2 in a primary mix (PCR1) and primers TB1 and TB2 in a subsequent nested reaction (PCR2) (Fig. 1). PCR1 amplified fragments of the same size from all bacterial DNAs tested, as expected (primers R1 and R2 are generic and will amplifiy rDNA fragments from most bacterial species [Fig. 1a]). However, PCR2 amplified only a band of approximately 150 bp in MTG, M. tuberculosis subsp. H37Rv (virulent type strain), M. tuberculosis H37 Ra (avirulent strain), M. bovis BCG (vaccine strain), and M. microti (vole bacillus) (Fig. 1b). Under less stringent annealing conditions (less than 70°C), PCR2 weakly amplifies fragments of similar size in strains of M. marinum (data not shown); however, under the stringent PCR conditions used (annealing temperature of 72°C), PCR2 is MTG specific.
FIG. 1.
Results of PCR1 using 5 fg of bacterial genomic DNA as template (a) and MTG-specific nested PCR 2 using 1 μl of PCR1 product as template (b).
To establish the efficacy of the nested MTG-specific PCR test on bacterial crDNAs, 50-ng aliquots of E. coli or M. tuberculosis total RNAs were reverse transcribed as described below, and PCR1 and PCR2 were carried out using 10 fg of E. coli DNA as control (Fig. 2). Gel electrophoresis showed a band for PCR 1 (Fig. 2a) in all samples as expected (in sample 2, due to trace amounts of contaminating DNA) but a band of the appropriate size for PCR2 only in the M. tuberculosis RNA sample (Fig. 2b). Southern blotting using 32P-labeled, gel-purified M. tuberculosis PCR2 product confirmed these observations.
FIG. 2.
Results of PCR1 (a) and PCR2 (b) on whole bacterial RNAs lanes: 1, 10 fg of E. coli DNA; 2, 50 ng of E. coli total RNA, no reverse transcription; 3, 50 ng of E. coli RNA reverse transcribed to cDNA; 4, 50 ng of M. tuberculosis total RNA, reverse transcribed to cDNA. Amplification products from PCR1 and PCR2 were visualized on a 2.0% agarose gel, transferred to nylon membranes using standard techniques, and then hybridized with 32P-labeled MTG-specific PCR2 amplification product using M. tuberculosis genomic DNA as template.
Use of specific nested RT-PCR for detection of MTG in M. tuberculosis-infected mouse tissues.
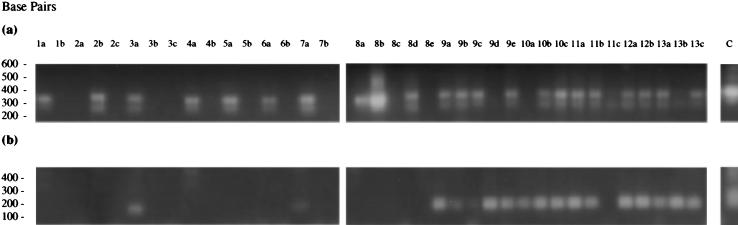
PCR1 and PCR2 were conducted on joint and control mouse tissues recovered from either chronically or acutely infected BALB/c mice and controls (Fig. 3). Seven of the mouse tissues from the M. tuberculosis chronically infected mice and control mouse 1 were positive for bacteria using PCR1 (Fig. 3a). Two of these tissues from infected mice were additionally positive for MTG using specific nested PCR2 (Fig. 3b); these were both wrist joints (tissues 3a and 7a). These results indicate that M. tuberculosis administered intravenously at low dose can traffic to the joints of infected BALB/c mice and can be detected using MTG-specific nested RT-PCR. Interestingly, the results also suggest some low-grade non-M. tuberculosis infection in the remaining PCR1 positive joints; these were again all wrist joints.
FIG. 3.
Results of PCR1 and PCR2 on reverse-transcribed mouse tissue RNAs from M. tuberculosis-infected BALB/c mice and controls (see Table 2 for tissue details). (a) PCR1; (b) MTG-specific PCR2.
In acutely infected animals, tissues from all control and test animals (with the exception of tissue 8e, hip joint from control mouse 8), were positive for bacteria using PCR1 (8c, 9d, 10a, 11c, and 13b were weakly positive). All tissues from the M. tuberculosis-infected mice (except 11c) were additionally positive for MTG using PCR2, while the control tissues were not. The control mice in both groups showed some signs of laboratory-derived bacterial infection and pneumonitis, which would perhaps explain the positive signal obtained in tissues from these mice using PCR1.
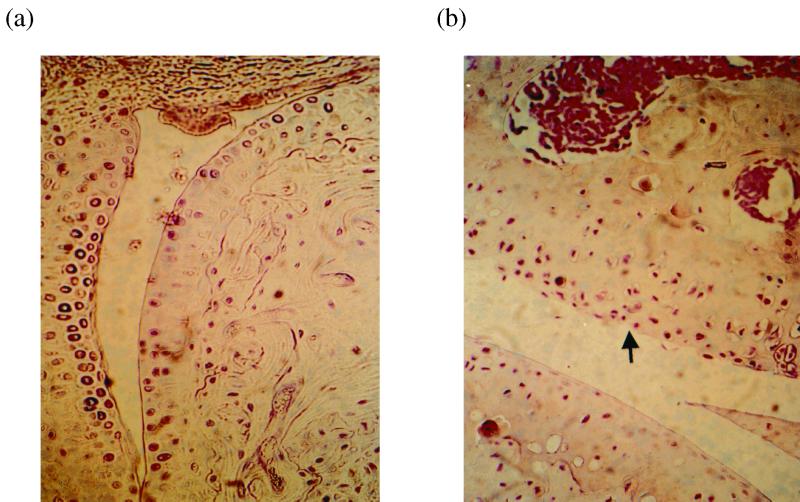
Thus, M. tuberculosis given intravenously at low and high doses appears to traffic to body tissues in addition to those which show clear evidence of disease pathology, e.g., lung and spleen (data not shown). However, all chronically and acutely infected animals with evidence of MTG in joint tissue showed no overt signs of joint inflammation. Parallel joints taken from chronically infected animals showed no evidence of pathology associated with M. tuberculosis infection, such as granuloma formation, or any gross histopathological evidence of joint damage; some infected joints showed evidence of minor damage to the cartilage surface compared with control joints (Fig. 4). Thus, despite the presence of live organisms in a number of mouse joint tissues in this animal model, there appear to be no overt signs of pathology associated with M. tuberculosis infection, joint inflammation, or arthritis.
FIG. 4.
Sections of mouse joints stained with hemotoxylin and eosin. (a) Wrist joint from uninfected mouse 1; (b) wrist joint from M. tuberculosis-infected mouse 3. Arrow indicates area of cartilage disruption in M. tuberculosis-infected mouse joint.
Detection of MTG in human arthritis tissue from patient cohorts 1 and 2 by Specific, Nested RT-PCR.
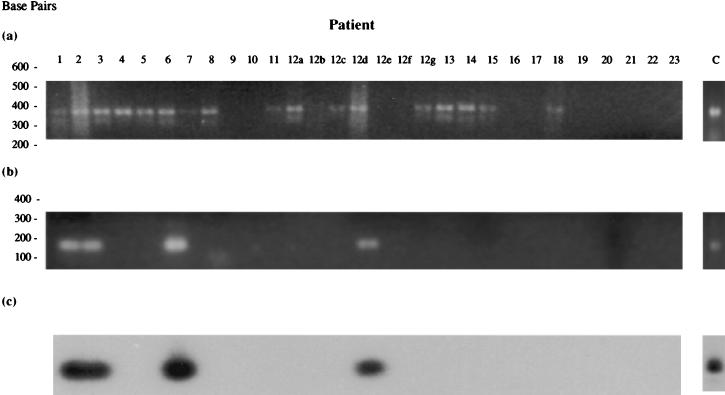
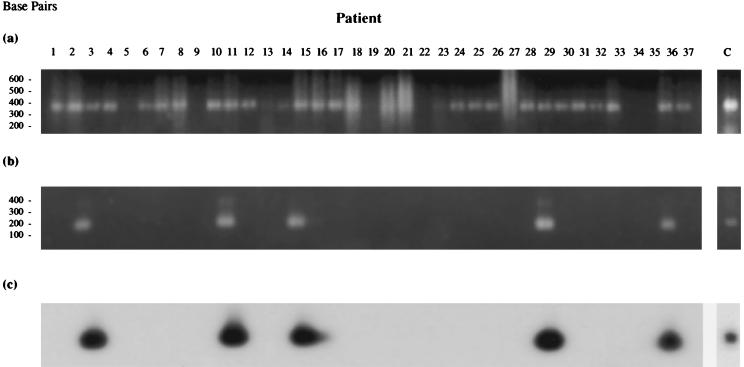
Synovial tissue RNAs of patients from cohorts 1 and 2 were reverse transcribed and subjected to analysis for bacteria and the presence of MTG using PCR1 and PCR2 (Fig. 5 and 6 and Table 7). PCR1 showed the presence of bacterial rRNA in many of the joint tissues of patients in both study groups (complete analysis of the bacterial species present in the joints of patients from cohort 1 has been published in detail elsewhere [28]).
FIG. 5.
Result of PCR1 and PCR2 on reverse-transcribed synovial RNAs from cohort 1 patients (see Table 3 for tissue details). (a) PCR1; (b) MTG-specific PCR2; (c) Southern blot analysis of PCR2 amplification products probed with 32P-labeled MTG-specific PCR2 amplification product using M. tuberculosis genomic DNA as template.
FIG. 6.
Result of PCR1 and PCR2 on reverse-transcribed synovial RNAs from cohort 2 patients (see Table 4 for tissue details). (a) PCR1; (b) MTG-specific PCR2; (c) Southern blot analysis of PCR2 amplification products probed with 32P-labeled MTG-specific PCR2 amplification product using M. tuberculosis genomic DNA as template.
TABLE 7.
Summary of results of PCR1 and nested MTG-specific PCR2 on patient tissues RNAs from cohorts 1, 2, and 3
| Patient | Diagnosis | No. of MTG-specific tests | No. of tests positive | Total no. of patients in group | Total MTG positive (%) |
|---|---|---|---|---|---|
| Cohort 1 | |||||
| 1 | RA | 3 | 3 | 9 | |
| 2 | RA | 3 | 3 | ||
| 6 | RA | 3 | 3 | ||
| 8 | RA | 3 | 1 | 44.44 | |
| 12c | OA | 3 | 1 | 7 | 14.3 |
| UA | 3 | 0 | 3 | 0 | |
| Control | 3 | 0 | 3 | 0 | |
| Cohort 2 | |||||
| 18 | RA | 3 | 2 | 17 | |
| 19 | RA | 3 | 1 | ||
| 22 | RA | 3 | 2 | ||
| 23 | RA | 3 | 1 | ||
| 24 | RA | 3 | 2 | ||
| 25 | RA | 3 | 2 | ||
| 26 | RA | 3 | 3 | ||
| 27 | RA | 3 | 2 | ||
| 28 | RA | 3 | 2 | ||
| 29 | RA | 3 | 1 | ||
| 31 | RA | 3 | 1 | ||
| 33 | RA | 3 | 1 | 70.6 | |
| 1 | OA | 3 | 2 | 17 | |
| 6 | OA | 3 | 2 | ||
| 7 | OA | 3 | 3 | ||
| 8 | OA | 3 | 2 | ||
| 9 | OA | 3 | 3 | ||
| 10 | OA | 3 | 1 | ||
| 11 | OA | 3 | 1 | ||
| 14 | OA | 3 | 1 | 47 | |
| 36 | Suspected septic arthritis | 3 | 2 | 1 | 100 |
| 37 | Psoriatic arthritis | 3 | 2 | 1 | 100 |
| Cohort 3 | |||||
| 1 | Early RA | 1 | 1 | 6 | |
| 5 | Early RA | 1 | 1 | 33.3 | |
| 8 | Early RA | 1 | 1 | 6 | 16.67 |
Initial early analyses using PCR2 to detect the presence of MTG in the tissue of cohort 1 patients revealed evidence of these organisms in the joints of four of nine RA patients (44.4%) and one of seven OA patients (14.3%), with none in the UA or control subjects (Fig. 5b and Table 7). This result suggested a more specific association of MTG with RA inflammatory arthritis compared with the other groups, particularly the OA group. However, due to the small sample size of patients in each of the study groups, the significance of the results could not be fully ascertained. PCR1 and PCR2 were therefore reapplied to tissue RNAs from a larger study group, cohort 2. The results showed the presence of MTG sequences in a number of patient tissues (Fig. 6b and Table 7), including 12 of 17 RA samples (70.6%), 8 of 17 OA specimens (47%), and one each of suspected septic arthritis and psoriatic arthritis controls. These results suggest no specific association of MTG sequences with late-stage RA, although their prevalence in this patient group appears to be higher. This could be due merely to the greater degree of chronic inflammation found in these patients, leading to a general increased trafficking of bacteria, perhaps carried to this area by immune cells continuously recruited to the site of inflammation.
Detection of MTG in synovial tissue from early RA and ReA patients.
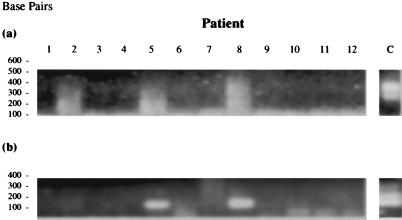
It has been shown previously by our group that later-stage RA and OA tissues are colonized by a variety of bacteria and the joint tissues usually contain a number of different species (28). MTG could be merely a part of this general bacterial milieu, most likely opportunistically colonizing tissue which is already diseased and compromised. Therefore, to look for any association of MTG with early RA, we applied MTG-specific PCR1 and PCR2 to a third cohort of tissues from patients with early-stage RA and ReA (Fig. 7). The PCR signal generated with primers R1 and R2 were rather weak, and the products show evidence of prior nucleic acid degradation. Upon cloning and sequencing, these products were found to be bacterial in origin (data not shown), although of short length. However, this does not appear to obviate their utility as template for nested PCR2, where a clear signal was generated in a small number of patient samples. Two of six (33.3%) of the early RA and only one of six (16.67%) of the early ReA patients were found to have MTG sequences in their synovium (Fig. 7b and Table 7); this implied no specific association of MTG with early RA. In addition, only a small proportion of early RA patients showed evidence of MTG in their synovium, perhaps also suggesting no etiopathological link of this group of organisms with disease.
FIG. 7.
Result of PCR1 and PCR2 on reverse-transcribed synovial RNAs from cohort 3 patients (see Table 5 for tissue details). (a) PCR1; (b) MTG-specific PCR2. PCR2 amplification products were verified by gel purification and sequence analysis.
DISCUSSION
The results presented in this study demonstrate that PCR1 and PCR2 are able to differentiate MTG from closely related bacteria. This nested-PCR test can be used for detection of MTG in mouse tissues and also in human synovial tissue from patients with a variety of arthropathies. In the acute and chronic BALB/c mouse model of infection, MTG sequences could be detected in tissues which show clear signs of pathology, e.g., lung and spleen, but were also found in joint tissue, indicating trafficking of these organisms to peripheral tissues. The presence of MTG in the joint did not appear to be associated with signs of pathology associated with M. tuberculosis infection, inflammation, or overt symptoms of arthritis; however, some minor cartilage damage was observed. These observations would imply that joint tissue infection with live M. tuberculosis does not lead to RA-like inflammatory arthritis in this animal model. This result was not unexpected, as no evidence of M. tuberculosis-associated joint pathology has been found in BALB/c mice, despite years of investigation using this mouse strain as the host for this infectious organism. To our knowledge, this is the first report of trafficking of live MTG to mouse joint tissues.
As this study revealed no signs of joint disease in the animals analyzed, it does not support MTG as etiological agents in inflammatory joint disease in this model. However, the BALB/c mouse may be an inappropriate strain for these types of studies, as its genetic background may not confer any susceptibility to RA-like inflammatory arthritis. In certain rodent strains such as the Lewis rat, arthritis can be elicited by immunization with heat-killed M. bovis BCG emulsified in oil (Freund's complete adjuvant) (7). However, this phenomenon is clearly dependent on the specific genetic configuration of the model animal, as disease cannot be initiated by such means in the closely related Fisher (F344) strain. Freund's complete adjuvant is also commonly used in a number of other animal models of arthritis (47, 61), without which the antigen challenge used to elicit joint inflammation is less effective. Therefore, mycobacteria have strong immunopotentiating properties and in a susceptible host could contribute to immune dysregulation. In susceptible animals and in humans genetically predisposed to inflammatory joint disease, a combination of factors could contribute to disease onset by way of a complex interplay between infectious agent and host resulting in joint inflammation. For future studies, it would be of interest to repeat these challenge experiments in susceptible rodent strains or transgenic for factors conferring susceptibility to RA such as HLA-DR4.
MTG sequences were found in the synovial tissue of some individuals in all groups studied with the exception of patients with UA and healthy controls. The prevalence in late-stage RA synovium was found to be higher than for the other disease groups, but this may be due in part to the generally higher degree of inflammation seen in these patients. However, the observation that MTG are not specifically associated with the RA group, despite our initial encouraging observations for cohort 1, argues against a simple etiopathological role of these organisms in late-stage RA. In addition, MTG were not found in all early RA patient samples, although these results may be influenced by the small size of the biopsy material taken from these patients, which may have contributed to sampling errors. Even taking this fact into consideration, the relative abundance of MTG in these tissues would have to be extremely low to be overlooked. Overall, its seems unlikely that these organisms play a central role in the onset or progression of inflammatory arthritis, although they may contribute to ongoing inflammatory processes and chronicity, alongside other species in the general bacterial colonization of synovial tissue. The observation that the RA group seem to be positive for MTG at a higher frequency may also perhaps be explained by the fact that RA patients are more susceptible to bacterial infection in general (57). In addition, they may also be more susceptible to mycobacterial infection, as evidenced by case reports of RA patient infection with mycobacterial species which are usually weakly pathogenic for humans (13, 17, 32) and those which are pathogenic to humans, such as M. tuberculosis (28).
M. tuberculosis is known to cause a number of types of arthritis in human hosts, including septic arthritis and a form of sterile ReA (Poncet's disease), although these are relatively rare and the occurence of arthritic manifestations in patients with pulmonary tuberculosis is generally low (14). This again suggests that in the absence of other contributory factors, M. tuberculosis infection does not usually contribute to onset of inflammatory arthritis. However, workers using antimycobacterial therapies in the treatment of RA (9, 46) have obtained intriguing results, perhaps suggesting from our studies that elimination of colonizing bacteria can lead to amelioration of symptoms in some individuals. However, whether these effects are due to the anti-inflammatory properties of the antimycobacterials used, or a function of bacterial clearance remains unknown (28).
Recognition of mycobacterial antigens by synovial T cells and antibody cross-reactivity between mycobacterial antigens and human proteins including cartilage components has also been observed in RA patient synovial fluid and blood (2, 12, 13, 23, 33, 45, 52, 54, 59). However, it is a subject of some controversy as to whether responses to common bacterial antigens, e.g. heat shock protein 65, are specific to the mycobacterial homologues or are part of a more generalized immune reactivity to such proteins by memory cells recruited to inflammatory lesions (21, 51). However, there is some evidence that HLA-DR4, one of the susceptibility factors in RA, may confer increase responsiveness to mycobacterial antigens (44, 45), and as discussed previously mycobacteria and their antigens appear to have more potent adjuvant properties than those from other bacterial species. These could contribute to local inflammatory effects on specific cell types in bone and joint infections. Mycobacterial heat shock protein 10 has been found to stimulate bone resorption in a bone explant model, which is proposed to be a contributary factor in degenerative M. tuberculosis infection of the spine (Potts disease) (40). Immunization with the same antigen can modulate adjuvant arthritis in the rat model, suggesting some central role for this antigen in adjuvant arthritis (49). Other mycobacterial antigens can also stimulate RA patient-derived mononuclear cells to cartilage proteoglycan depletion (67). These observations again suggest that mycobacterial antigens have strong immunopotentiating properties, which in an appropriate tissue setting and subject to predisposing conditions could elicit strong reactivity from resident or infiltrating inflammatory cells. Whether such mechanisms are at play in RA remains a topic for future study.
Overall, the results presented here provide evidence of a previously unsuspected presence of MTG in inflamed synovial tissue irrespective of cause. As this test cannot differentiate between different members of the MTG complex because their rRNA sequences are 99.9% conserved (29), these observations most likely reflect persistence of M. bovis BCG. Most of these these individuals are highly likely to have received BCG vaccination, and it is unlikely that these results are due to infection with virulent M. tuberculosis; however, recrudescence of previous M. tuberculosis infection cannot be excluded. Whatever the origin of these MTG sequences, the organisms are likely to have been carried to the site of disease by inflammatory cells, as is probably likely with the many other organisms found in these diseased tissues (28). M. bovis BCG is known to persist in vaccinated individuals for long periods of time, as has been suggested from emerging case reports on individuals with human immunodeficiency virus who after progression to AIDS succumb to recrudescent M. bovis BCG infection some considerable time after receiving vaccination (4, 8, 53, 60, 64).
If latent organisms are present in patients with inflammatory arthropathies, it is also likely that immunosuppressive therapies used in the control of inflammatory joint disease could lead to reactivation and reemergence of these bacilli. However, they may be unconnected to disease pathology, and their presence may be incidental. Persistence and colonization of joint tissues could also be contributed to by deficiencies in host immune surveillance mechanisms leading to impaired bacterial clearance. This hypothesis is supported by previous observations that patients with RA often exhibit signs of impairment of inflammatory cell function. These defects are particularly found in cells associated with bacterial killing, e.g., professional phagocytes and T cells (1, 17, 26, 30, 43, 58, 65), and may be associated with cytokine hyperstimulation as a consequence of the chronic inflammatory nature of the disease (10, 11). RA patients also show defects in mucosal integrity (42), which could lead to increased bacterial trafficking from other body sites already heavily colonized by commensal organisms. Whether these factors could combine to promote accumulation of opportunistic bacterial colonizers in diseased tissue from either resident or environmental sources is unclear. However, these observations may contribute to our understanding of the complex mechanisms at work in the pathology of inflammatory arthritis and may also influence our strategy approaches to patient therapy. Future strategies could employ antibacterial treatment in conjunction with conventional therapies, which if their combined effects prove beneficial may provide clues as to the role of these colonizing bacteria in disease pathology.
ACKNOWLEDGMENTS
We thank Gabriel Panayi, Dorian Haskard, Peter Sewell, George Southgate, Gill Pountain, Andrew Hassell, Robin Strachan, Neil Hunt, and Sally Roberts for the kind provision of clinical material for use in this work. We also thank Anthony Savage for assistance in analysis of histological sections.
We thank Glaxo Wellcome UK for sponsoring this project and for financial support of Charles J. Cox.
REFERENCES
- 1.Abdou N I, Lindsley H B, Racela L S, Pascual E, Hassanein K M. Suppressor T cell dysfunction and anti-suppressor cell antibody in active early rheumatoid arthritis. J Rheumatol. 1981;8:9–18. [PubMed] [Google Scholar]
- 2.Aguas A, Esaguy N, Sunkel C E, Silva M T. Cross-reactivity and sequence homology between the 65-kilodalton mycobacterial heat shock protein and human lactoferrin, transferrin, and DR beta subsets of major histocompatibility complex class II molecules. Infect Immun. 1990;58:1461–1470. doi: 10.1128/iai.58.5.1461-1470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albani S, Carson D A. A multistep molecular mimicry hypothesis for the pathogenesis of rheumatoid arthritis. Immunol Today. 1996;17:466–470. doi: 10.1016/0167-5699(96)20029-g. [DOI] [PubMed] [Google Scholar]
- 4.Armbruster C, Junker W, Vetter N, Jaksch G. Disseminated bacille Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J Infect Dis. 1990;162:1216. doi: 10.1093/infdis/162.5.1216. [DOI] [PubMed] [Google Scholar]
- 5.Arnett F C, Edworth S M, Bloch D A, Shane D J, Fries J F, Cooper N S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 6.Behar S M, Porcelli S A. Mechanisms of autoimmune disease induction: the role of the immune response to microbial pathogens. Arthritis Rheum. 1995;38:458–476. doi: 10.1002/art.1780380403. [DOI] [PubMed] [Google Scholar]
- 7.Billingham M E J. Adjuvant arthritis: the first model. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press Ltd.; 1995. pp. 25–46. [Google Scholar]
- 8.Blondon H, Guez T, Paul G, Truffot-Pernot C, Sicard D. BCG adenitis 6 years after vaccination in AIDS. Presse Med. 1991;20:1091. [PubMed] [Google Scholar]
- 9.Caruso I. Antituberculous drugs in rheumatoid arthritis. J Rheumatol. 1993;20:199–200. [PubMed] [Google Scholar]
- 10.Cope A P, Londei M, Randall Chu N, Cohen S B A, Elliot M J, Brennan F M, Maini R N, Feldmann M. Chronic exposure to tumour necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Investig. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cope A P, Liblau R S, Yang X-D, Conglia M, Laudanna C, Schreiber R D, Probert L, Kollias G, McDevitt H O. Chronic tumour necrosis factor alters T cell responses by attenuating T cell receptor signalling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crick F D, Gatenby P A. Limiting-dilution analysis of T cell reactivity to mycobacterial antigens in peripheral blood and synovium from rheumatoid arthritis patients. Clin Exp Immunol. 1992;88:424–429. doi: 10.1111/j.1365-2249.1992.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Graeff-Meeder E R, Voorhorst M, van Eden W, Schuurman H J, Huber J, Barkley D, Maini R N, Kuis W, Rijkers G T, Zegers B J. Antibodies to the mycobacterial 65-kDa heat shock protein are reactive with synovial tissue of adjuvant arthritic rats and patients with rheumatoid arthritis and osteoarthritis. Am J Pathol. 1990;137:1013–1017. [PMC free article] [PubMed] [Google Scholar]
- 14.Dlugovitky D, Torres A, Hourquescos M C, Svetaz M J, Quagliato N, Valentini E, Amigot B, Molteni O, Bottasso O. Low occurance of arthritic manifestations in patients with pulmonary tuberculosis. T cell subsets and humoral studies. Mem Inst Oswald Cruz (Rio de Janeiro) 1995;90:623–628. doi: 10.1590/s0074-02761995000500016. [DOI] [PubMed] [Google Scholar]
- 15.Dreisin R B, Scoggin C, Davidson P T. The pathogenicity of Mycobacterium fortuitum and Mycobacterium chelonii in man: a report of seven cases. Tubercle. 1976;57:49–57. doi: 10.1016/0041-3879(76)90017-9. [DOI] [PubMed] [Google Scholar]
- 16.Dubos R J, Davis B D. Factors affecting the growth of tubercle bacilli in liquid media. J Exp Med. 1946;43:409–423. [PubMed] [Google Scholar]
- 17.Emery P, Panayi G S, Nouri A M E. Interleukin-2 reverses deficient cell-mediated immune responses in rheumatoid arthritis. Clin Exp Immunol. 1984;57:123–129. [PMC free article] [PubMed] [Google Scholar]
- 18.Gaston J S H, Cox C, Granfors K. Clinical and experimental evidence for persistent Yersinia infection in reactive arthritis. Arthritis Rheum. 1999;42:2239–2242. doi: 10.1002/1529-0131(199910)42:10<2239::AID-ANR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths M M. Arthritis induced by bacteria and viruses. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press Ltd.; 1995. pp. 411–430. [Google Scholar]
- 20.Hernandez-Cruz B, Cardiel M H, Villa A R, Alcocer-Varela J. Development, recurrence, and severity of infections in Mexican patients with rheumatoid arthritis. A nested case-control study. J Rheumatol. 1998;25:1900–1907. [PubMed] [Google Scholar]
- 21.Hirata D, Hirai I, Iwamoto M, Yoshio T, Takeda A, Masuyama J I, Mimori A, Kano S, Minota S. Preferential binding with Escherichia coli HSP60 of antibodies prevalent in sera from patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1997;82:141–148. doi: 10.1006/clin.1996.4280. [DOI] [PubMed] [Google Scholar]
- 22.Hollander J L. History of rheumatoid arthritis: an American perspective. In: Utsinger P D, Zvaifler N J, Erlich G E J B, editors. Rheumatoid arthritis: etiology: diagnosis: management. Philadelphia, Pa: Lippincott Co.; 1985. pp. 11–17. [Google Scholar]
- 23.Holoshitz J, Drucker I, Yaretzky A, van Eden W, Klajman A, Lapidot Z, Frenkel A, Cohen I. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986;2:305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 24.Jalal H, Millar M, Linton C, Dieppe P. Absence of Mycobacterium tuberculosis DNA in synovial fluid from patients with rheumatoid arthritis. Ann Rheum Dis. 1994;53:695–698. doi: 10.1136/ard.53.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji Y-E, Kempsell K E, Colston M J, Cox R A. Nucleotide sequences of the spacer-1, spacer-2 and trailer regions of the rrn operons and secondary structures of precursor 23S rRNAs and precursor 5S rRNAs of slow-growing mycobacteria. Microbiology. 1994;140:1763–1773. doi: 10.1099/13500872-140-7-1763. [DOI] [PubMed] [Google Scholar]
- 26.Katona I M, Ohura K, Allen J B, Wahl L M, Chenoweth D E, Wahl S M. Modulation of monocyte chemotactic function in inflammatory lesions. Role of inflammatory mediators. J Immunol. 1991;146:708–714. [PubMed] [Google Scholar]
- 27.Keat A. TB or not TB?: that is the question. Br J Rheumatol. 1993;32:824–826. doi: 10.1093/rheumatology/32.9.769. [DOI] [PubMed] [Google Scholar]
- 28.Kempsell K E, Cox C J, Hurle M, Wong T, Wilkie S, Zanders E D, Gaston J S H, Crowe J S. Reverse transcriptase-PCR analysis of bacterial rRNA for detection and characterization of bacterial species in arthritis synovial tissue. Infect Immun. 2000;68:6012–6026. doi: 10.1128/iai.68.10.6012-6026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempsell K E, Ji Y-E, Estrada I C E, Colston M J, Cox R A. The nucleotide sequence of the promoter, 16S rRNA and spacer region of the ribosomal RNA operon of Mycobacterium tuberculosis and comparison with Mycobacterium leprae precursor rRNA. J Gen Microbiol. 1992;138:1717–1727. doi: 10.1099/00221287-138-8-1717. [DOI] [PubMed] [Google Scholar]
- 30.Kitas G D, Salmon M, Farr M, Young S P, Bacon P A. T-cell functional defects in rheumatoid arthritis: intrinsic or extrinsic? J Autoimmun. 1988;1:339–351. doi: 10.1016/0896-8411(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 31.Kogure T, Fijinaga H, Niizawa A, Shimada Y, Itoh Y, Ochiai H, Terasawa K. Rheumatoid arthritis complicated by Mycobacterium tuberculosis: are there characteristics predisposing to this condition. J Clin Rheumatol. 1999;5:17–21. doi: 10.1097/00124743-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Krause A, Kamradt T, Burmester G. Potential infectious agents in the induction of arthritides. Curr Opin Rheumatol. 1996;8:203–209. doi: 10.1097/00002281-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Li S G, Quayle A J, Shen Y, Kjeldsen-Kragh J, Oftung F, Gupta R S, Natvig J B, Førre Ø T. Mycobacteria and human heat shock protein-specific cytotoxic T lymphocytes in rheumatoid synovial inflammation. Arthritis Rheum. 1992;35:270–281. doi: 10.1002/art.1780350305. [DOI] [PubMed] [Google Scholar]
- 34.Lydyard P M, Rook G A, Tsoulfa G, Sharif M, Smith M. Is there a role for mycobacteria in the etiopathogenesis of rheumatoid arthritis. Immunol Rev. 1991;121:137–154. doi: 10.1111/j.1600-065x.1991.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 35.Macfarlane J D, Dieppe P A, Rigden B G, Clark T J. Pulmonary and pleural lesions in rheumatoid arthritis. Br J Dis Chest. 1978;72:288–300. [PubMed] [Google Scholar]
- 36.Maini R N, Chu C Q, Feldmann M. Aetiopathogenesis of rheumatoid arthritis. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press Ltd.; 1995. pp. 25–46. [Google Scholar]
- 37.Mangan J A, Sole K M, Mitchison D A, Butcher P D. An effective method of RNA extraction from bacteria refractory to disruption, including mycobacteria. Nucleic Acids Res. 1997;25:675–676. doi: 10.1093/nar/25.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 39.Medrano J M M, Galbete J C V. Evidencia morfológica dela presencia de un bacilo en cultivos celulares de membrana sinovial de enfermos de artritis reumatoide. Rev Clin Esp. 1990;187:329–333. [PubMed] [Google Scholar]
- 40.Meghji S, White P A, Nair S P, Reddi K, Heron K, Henderson B, Zaliani A, Fossati G, Mascagni P, Hunt J F, Roberts M M, Coates A R M. Mycobacterium tuberculosis chaperonin 10 stimulates bone resorption: a potential contributory factor in Pott's disease. J Exp Med. 1997;186:1241–1246. doi: 10.1084/jem.186.8.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melief M J, Hoijer M A, Van Paassen H C, Hazenberg M P. Presence of bacterial flora-derived antigen in synovial tissue macrophages and dendritic cells. Br J Rheumatol. 1995;34:1112–1116. doi: 10.1093/rheumatology/34.12.1112. [DOI] [PubMed] [Google Scholar]
- 42.Mielants H, De Vos M, Goemaere S, Schelstraete K, Cuvelier C, Goethals K, Maertens M, Ackerman C, Veys E M. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J Rheumatol. 1991;18:394–400. [PubMed] [Google Scholar]
- 43.Nilsson E, von Stedingk L V, Biberfeld G. T-cell helper activity and B-cell function of synovial and blood lymphocytes from patients with rheumatoid arthritis or other forms of chronic arthritis. Scand J Immunol. 1986;24:721–728. doi: 10.1111/j.1365-3083.1986.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 44.Ottenhoff T H, Torres P, de las Aguas J T, Fernandez R, van Eden W, de Vries R R, Stanford J L. Evidence for an HLA-DR4-associated immune-response gene for Mycobacterium tuberculosis. A clue to the pathogenesis of rheumatoid arthritis. Lancet. 1986;2:310–313. doi: 10.1016/s0140-6736(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 45.Palacios-Boix A A, Estrada-G I, Joseph Colston M, Panayi G S. HLA-DR4 restricted lymphocyte proliferation to a Mycobacterium tuberculosis extract in rheumatoid arthritis and healthy subjects. J Immunol. 1987;140:1844–1850. [PubMed] [Google Scholar]
- 46.Panush R S, Longley S. Therapies of potential but unproven benefit. In: Utsinger P D, Zvaifler N J, Erlich G E J B, editors. Rheumatoid arthritis: etiology: diagnosis: management. Philadelphia, Pa: Lippincott Co.; 1985. pp. 695–709. [Google Scholar]
- 47.Pettifer E R, Blake S. Antigen-induced arthritis. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid arthritis. London, England: Academic Press Ltd.; 1995. pp. 457–470. [Google Scholar]
- 48.Pras E, Schumacher H R, Kastner D L, Wilder R L. Lack of evidence of mycobacteria in synovial tissue from patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:2080–2081. doi: 10.1002/art.1780391221. [DOI] [PubMed] [Google Scholar]
- 49.Ragno S, Winrow V R, Mascagni P, Lucietto P, di Pierro F, Morris C J, Blake D R. A synthetic 10-kDa heat shock protein (hsp 10) from Mycobacterium tuberculosis modulates adjuvant arthritis. Clin Exp Immunol. 1996;103:384–390. doi: 10.1111/j.1365-2249.1996.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Relman D A. The search for unrecognised pathogens. Science. 1999;284:1308–1310. doi: 10.1126/science.284.5418.1308. [DOI] [PubMed] [Google Scholar]
- 51.Res P C M, Telgt D, van Laar J M, Pool M O, Breedveld F C, de Vries R R P. High antigen reactivity in mononuclear cells from sites of chronic inflammation. Lancet. 1990;336:1406–1408. doi: 10.1016/0140-6736(90)93104-w. [DOI] [PubMed] [Google Scholar]
- 52.Res P C, Schaar C G, Breedveld F C, van Eden W, van Embden J D, Cohen I R, de Vries R R. Synovial fluid T cell rectivity against 65 kDa heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988;2:478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 53.Reynes J, Perez C, Lamaury I, Janbon F, Bertrand A. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J Infect Dis. 1989;160:727. doi: 10.1093/infdis/160.4.727. [DOI] [PubMed] [Google Scholar]
- 54.Rich T, Gruneberg U, Trowsdale J. Heat shock proteins, HLA-DR and rheumatoid arthritis. Nat Med. 1998;4:1210–1211. doi: 10.1038/3172. [DOI] [PubMed] [Google Scholar]
- 55.Rook G, Lydyard P, Stanford J. Mycobacteria and rheumatoid arthritis. Arthritis Rheum. 1990;33:431–435. doi: 10.1002/art.1780330319. [DOI] [PubMed] [Google Scholar]
- 56.Rook G, McCulloch J. HLA-DR4, mycobacteria, heat shock proteins and rheumatoid arthritis. Arthritis Rheum. 1992;35:1409–1412. doi: 10.1002/art.1780351202. [DOI] [PubMed] [Google Scholar]
- 57.Sawitzke A D, Ward J R. An infected rheumatoid joint. In: Klippel J H, Dieppe P A, editors. Rheumatology. 2nd ed. 1, Sect. 5. London, England: Mosby International; 1998. pp. 16.22–16.24. [Google Scholar]
- 58.Seitz M, Napierski I, Kirchner H. Depressed PPD and tetanus toxoid presentation by monocytes to T lymphocytes in patients with rheumatoid arthritis: restoration by interferon gamma. Rheumatol Int. 1988;8:189–196. doi: 10.1007/BF00269194. [DOI] [PubMed] [Google Scholar]
- 59.Sioud M, Kjeldsen-Kragh J, Quayle A J, Wiker H G, Sørskaar D, Natvig J B, Førre Ø. Immune responses to 18.6 and 30-kDa mycobacterial antigens in rheumatois patients and Vβ usage by specific synovial T-cell lines and fresh T cells. Scand J Immunol. 1991;34:803–812. doi: 10.1111/j.1365-3083.1991.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 60.Smith E, Thybo S, Bennedsen J. Infection with Mycobacterium bovis in a patient with AIDS: a late complication of BCG vaccination. Scand J Infect Dis. 1992;24:109–110. doi: 10.3109/00365549209048409. [DOI] [PubMed] [Google Scholar]
- 61.Trentham D E, Dynesius-Trentham R. Collagen-induced arthritis. In: Henderson B, Edwards J C W, Pettipher E R, editors. Mechanisms and models in rheumatoid Arthritis. London, England: Academic Press Ltd.; 1995. pp. 447–456. [Google Scholar]
- 62.Utsinger P D, Zvaifler N J, Weiner S B. Etiology. In: Utsinger P D, Zvaifler N J, Erlich G E J B, editors. Rheumatoid arthritis: etiology: diagnosis: management. Philadelphia, Pa: Lippincott Co.; 1985. pp. 21–48. [Google Scholar]
- 63.Van der Heijen I M, Wilbrink B, Schouls L M, van Embden J D, Breedveld F C, Tak P P. Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology. 1999;38:547–553. doi: 10.1093/rheumatology/38.6.547. [DOI] [PubMed] [Google Scholar]
- 64.van Deutekom H, Smulders Y M, Roozendaal K J, van Soolingen D. Bacille Calmette-Guerin (BCG) meningitis in an AIDS patient 12 years after vaccination with BCG. Clin Infect Dis. 1996;22:870–871. doi: 10.1093/clinids/22.5.870. [DOI] [PubMed] [Google Scholar]
- 65.Verwilghen J, Corrigall V, Pope R M, Rodrigues R, Panayi G S. Expression and function of CD5 and CD28 in patients with rheumatoid arthritis. Immunology. 1993;80:96–102. [PMC free article] [PubMed] [Google Scholar]
- 66.Wilbrink B, van der Heijden I M, Schouls L M, van Embden J D A, Hazes J M W, Breedveld F C, Tak P P. Detection of bacterial DNA in joint samples from patients with undifferentiated arthritis and reactive arthritis using polymerase chain reaction with universal 16S ribosomal RNA primers. Arthritis Rheum. 1998;41:535–543. doi: 10.1002/1529-0131(199803)41:3<535::AID-ART20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 67.Wilbrink B, Bilsma J W, Huber-Bruning O, Van Roy J L, Den Otter W, van Eden W. Mycobacterial antigens stimulate rheumatoid mononuclear cells to cartilage proteoglycan depletion. J Rheumatol. 1990;17:532–537. [PubMed] [Google Scholar]
- 68.Wilkinson N Z, Kingsley G H, Jones H W, Sieper J, Braun J, Ward M E. The detection of DNA from a range of bacterial species in the joints of patients with a variety of arthritides using a nested, broad-range polymerase chain reaction. Rheumatology. 1999;38:260–266. doi: 10.1093/rheumatology/38.3.260. [DOI] [PubMed] [Google Scholar]
- 69.Wu C-H, Lan J-L. Detection of Mycobacterium tuberculosis antigen in synovial fluid of patients with rheumatoid arthritis. Brit J Rheumatol. 1992;31:615–618. doi: 10.1093/rheumatology/31.9.615. [DOI] [PubMed] [Google Scholar]
- 70.Wu C-H, Jeng K-C, Lan J-L. Mycobacterium tuberculosis antigen, interleukin 2 and interleukin 2 inhibitor in patients with rheumatoid arthritis. Immunol Investig. 1995;24:957–964. doi: 10.3109/08820139509060720. [DOI] [PubMed] [Google Scholar]