Abstract
Inevitable exposure to high-LET ionizing radiation (IR) present in galactic cosmic radiation (GCR) could enhance gastrointestinal (GI) cancer incidence among astronauts undertaking deep space exploration and GI-cancer mortality has been predicted to far exceed NASA’s limit of < 3% REID (Radiation exposure-induced death) from cancer. Therefore, the development of countermeasure agents against high-LET radiation-induced GI cancer is needed to safeguard astronauts during and after an outer space mission. The cyclooxygenase-2/prostaglandin E2 (COX2/PGE2) mediated activation of pro-inflammatory and oncogenic signaling has been reported to play an important role in persistent inflammation and GI-tumorigenesis after high-LET radiation exposure. Therefore, aspirin, a well-known inhibitor of the COX/PGE2 pathway, was evaluated as a potential countermeasure against 28Si-induced PGE2 and tumorigenesis in Apc1638N/+, a murine model of human GI-cancer. Animals were fed either standard or aspirin supplemented diet (75, 150, or 300 mg/day of human equivalent dose) starting at the age of 4 weeks and continued till the end of the study, while mice were exposed to 28Si-ions (300 MeV/n; 69 keV/μm) at the age of 8 weeks. Serum PGE2 level, GI tumor size (>2mm2), number, and cluster (>5 adjoining tumors) were analyzed at 150 days post-exposure. Aspirin led to a significant reduction in PGE2 in a dose-dependent manner but did not reduce 28Si-induced GI tumorigenesis even at the highest (300 mg/day) dose. In summary, this study suggests that aspirin could reduce high-LET IR-induced pro-inflammatory PGE2 levels, however, lacks the ability to reduce high-LET IR-induced GI tumorigenesis in Apc1638N/+ mice.
Keywords: High-LET radiation, Tumorigenesis, Aspirin, Chemoprevention, Cyclooxygenase, Prostaglandin E2
1. Introduction
Exposure to ionizing radiation (IR) is associated with a significantly greater risk of gastrointestinal (GI) tumorigenesis, therefore inevitable cosmic radiation exposure in the range of 0.1 to 0.2 Gy/y during outer-space exploration is a primary health concern for astronauts (Cragle et al., 1988; Preston et al., 2007; Boice et al., 2006; Dupree-Ellis et al., 2000; Cucinotta et al., 2017; Sakata et al., 2019). In contrast to low energy transfer (LET) radiation (γ or x-rays), exposure to densely ionizing high-LET heavy-ions present in the galactic cosmic radiation (GCR) poses greater cancer risk with relative biological effectiveness (RBE) in the range of 3.7 to 8 for GI-tumorigenesis (Shuryak et al., 2017). Furthermore, human cancer risk prediction have also indicated a higher GI-cancer risk that far exceeds the current NASA limit of < 3% risk of exposure-induced death (REID) from cancers (Cucinotta et al., 2017; Shuryak et al., 2017). Although using appropriate radiation shielding is the best protective approach, current space craft shielding provides only modest protection from high-LET radiation (Naito et al., 2020; Giraudo et al., 2018). Therefore, there is an unmet need to develop safe and effective countermeasure agents against high-LET radiation-induced GI-tumorigenesis.
Molecular mechanisms implicated in high-LET ionizing radiation (IR)-induced GI-tumorigenesis includes chronic oxidative stress, persistent DNA damage, accelerated aging phenotype, and increased senescence-inflammatory response (SIR) (Datta et al., 2012; Cheema et al., 2014; Datta et al., 2016; Suman et al., 2018; Kumar et al., 2018b; Kumar et al., 2019; Suman et al., 2020). SIR signaling is known to increase inflammation and tumorigenesis in many tissues, including the GI tract (Ruhland et al., 2016; Frey et al., 2018; Kumar et al., 2018b). Notably, higher levels of pro-inflammatory SIR factor, PGE2 (prostaglandin E2), and its precursor enzyme cyclooxygenase-2 (COX2) has been observed in mouse GI mucosa after high-LET radiation exposure (Cheema et al., 2014) and higher PGE2 is also known to increase GI-cancer incidence in humans (Eberhart et al., 1994; Kargman et al., 1995; Sano et al., 1995).
Aspirin (acetylsalicylic acid or ASA) is a well-studied drug with long-history of human use that effectively reduces PGE2 levels via inhibition of COX (both COX-1 and 2) enzymes and its chemo-preventive potential against GI-cancer has been noted in both human epidemiological and animal studies (Drew et al., 2016). Low-dose daily aspirin use in long-term human clinical trials was found to be safe and effective in reducing chronic systemic inflammation and mortality due to GI cancer (Ishikawa et al., 2014; Morris et al., 2009). Moreover, the association between increasing dose and duration of aspirin use with a reduction in human GI-cancer incidence has also been observed (Bosetti et al., 2020). In this study, we evaluated the GI-cancer preventive effects of human equivalent doses of aspirin (75, 150, and 300 mg/day) against high-LET radiation in Apc1638N/+, a well-characterized murine model of human GI-cancer. These mice develop 3–5 tumors throughout the GI tract and mimic sporadic human colorectal cancers where the loss of functional Apc is prevalent in ~80% of the tumors (Bakker et al., 2013; Suman et al., 2017). The overall purpose of this study was to evaluate the dose-dependent effects of dietary aspirin on high-LET radiation-induced GI-tumorigenesis and serum PGE2 levels in Apc1638N/+ mice.
2. Materials and Methods
2.1. Mouse colony, study groups and radiation exposure
Mice were bred and genotyped, as reported earlier (Suman et al., 2017) and male Apc1638N/+ mice (n=20/group) were randomly assigned to the experimental groups, as described in Table-1. Aspirin supplemented diet in applicable groups was initiated at 4 weeks of age and continued till the end of the study (Fig. 1). At seven weeks of age, all experimental mice including the control group were shipped to Brookhaven National Laboratory (BNL) and after one week of acclimation, mice were exposed to 28Si-ion (10 or 50 cGy dose; 69 keV/μm; 300 MeV/n) at the NASA Space Radiation Laboratory (NSRL) in BNL, as described previously (Suman et al., 2017). All animals were group-housed in a well-ventilated cage with easy access to standard or aspirin supplemented diet and water. Humidity (~50%), temperature (22 °C), and 12-h dark-light cycle were kept consistent and the health of all animals was regularly observed as per our approved institutional animal protocol at GU and NSRL/BNL.
Table 1.
Description of experimental groups. Mice were randomly assigned to 12 groups with either standard diet, three aspirin groups, or two IR groups as noted below.
| IR\Diet | Standard diet | Aspirin 75 mg/day | Aspirin 150 mg/day | Aspirin 300 mg/day |
|---|---|---|---|---|
| Sham | Control | ASA 75 | ASA 150 | ASA 300 |
| 28Si 10 cGy | 10 cGy | ASA 75 + 10 cGy | ASA 150 + 10 cGy | ASA 300 + 10 cGy |
| 28Si 50 cGy | 50 cGy | ASA 75 + 50 cGy | ASA 150 + 50 cGy | ASA 300 + 50 cGy |
Figure 1.
Schematic summary of the experimental approach to test chemo-preventive efficacy of dietary aspirin on high-LET radiation-induced GI-tumorigenesis.
2.2. Aspirin dose calculation and administration regimen
The physician recommended human dose of aspirin range from 75 to 325 mg/day, where 75 mg represents the low-dose aspirin and the 325 mg represents adult aspirin dose. We calculated mouse aspirin dose equivalent to human aspirin dose based on established dose calculation guidelines (Reagan-Shaw et al., 2008; Bachmanov et al., 2002). Briefly, to calculate human equivalent dose in mouse, we used mean human daily dose for 60 kg adult i.e., 1.25 mg/kg/day and mouse and human Km factor of 3 and 37, respectively. Finally, we used 61.5 mg, 123 mg, or 246 mg of aspirin per kg of diet so that mice will have the human equivalent of daily 75, 150, or 300 mg aspirin. Aspirin formulated diet was obtained from ENVIGO (www.envigo.com) in a color-coded form for each different aspirin dose. No difference in food consumption and body weight among control and aspirin diet groups were noted during the course of the study.
2.3. Serum prostaglandin E2 (PGE2) measurements
Animals were euthanized using an asphyxiation chamber attached to a carbon dioxide cylinder with a flow rate of 30 to 70 % chamber volume per minute. Blood was collected through cardiac puncture, and BD Microtainer SST™ serum separator tubes were used to isolate serum, as per the manufacture’s recommendation. Finally, serum samples were aliquoted and flash-frozen in liquid nitrogen and later stored at −80 °C until PGE2 ELISA assay. Serum samples (n=6/group) were diluted (1:10) in assay buffer and relative changes in PGE2 levels were measured using a competitive ELISA kit, as per manufacturer’s instructions (Cat # ab133021, Abcam, Cambridge, MA, USA). Optical density (O.D.) was recorded at 405 nm with a multi-well plate reader (Molecular Devices, CA, USA) and was normalized using blank samples. Finally, the percentage change in PGE2 levels over control samples was calculated. This ELISA assay has the sensitivity to detect 13.4 pg/ml of PGE2 and is recommended for detection of species independent PGE2 levels in the range of 39.1 to 2500 pg/ml.
2.4. Tumor quantification
GI tumor number, size (>2 mm2), and clusters (>5 adjoining tumors) were quantified, as described previously (Suman et al., 2017). Briefly, after euthanasia small and large intestines were surgically obtained, cleaned with phosphate-buffered saline, and opened longitudinally to expose the GI tumors. Finally, tumors were observed and counted by individuals blinded to the experimental groups using Leica MZ6 dissecting microscope scope.
2.5. Statistics and data analysis
All statistical analyses were performed in GraphPad Prism v6.0a (La Jolla, CA). Initially, data normality was analyzed using Shapiro-Wilk test and p<0.05 indicated a non-normal distribution. Therefore, non-parametric one-way ANOVA (Kruskal-Wallis test) followed by post-hoc Dunn’s multiple comparison test was performed to detect any significant difference between the groups. All values presented as a bar graph for each group show means and error bars are presented as SEM. Statistically significant differences among groups were considered when the p-value was ≤ 0.05.
3. Results
3.1. High-LET radiation exposure causes a dose-dependent increase in GI-tumorigenesis
Exposure to both 10 cGy and 50 cGy 28Si-ions led to significantly higher GI tumorigenesis at 150 days post-exposure, relative to the control group (Fig. 2). A dose-dependent increase in intestinal and colonic tumors were observed, where 10 cGy (intestinal tumor 5.9 ± 0.28; colon tumor 0.18 ± 0.08; p<0.05) and 50 cGy (intestinal tumor 7.95 ± 0.47; colon tumor 0.23 ± 0.11; p<0.05) led to a statistically significant increase in intestinal (Fig. 2A) and colonic tumor counts (Fig. 2B), relative to control (intestinal tumor 3.85 ± 0.28; and no colon tumor). Moreover, a statistically significant increase in mean tumor cluster (Fig. 2C) and mean count of large tumors (Fig. 2D) were observed among control (cluster 0.045 ± 0.045, large tumor 1.63 ± 0.21), 10 cGy (cluster 0.11 ± 0.07, large tumor 2.73 ± 0.24; p<0.05 relative to control) and 50 cGy (cluster 0.30 ± 0.12, large tumor 4.23 ± 0.58; p<0.05 relative to control) animals. The highest GI tumorigenesis was observed after 50 cGy of 28Si-ion, where mean tumor count (Fig. 2A) and intestinal clusters (Fig. 2D) both were significantly higher than the 10 cGy dose.
Figure 2.
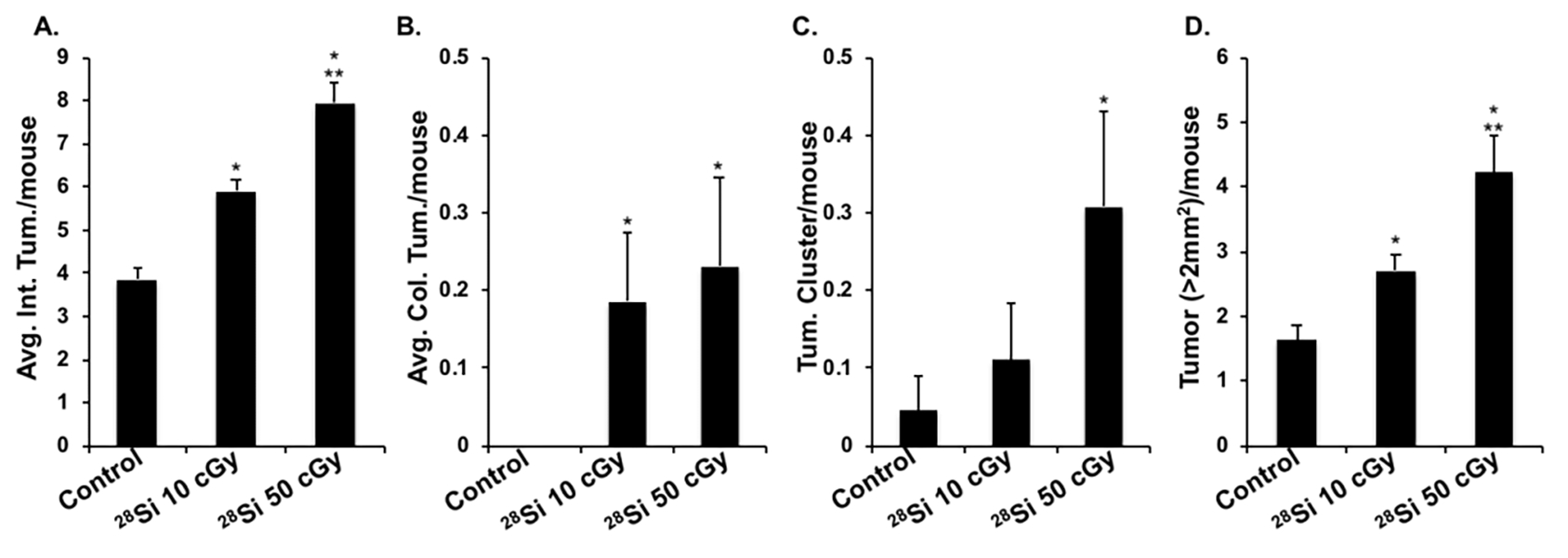
High-LET IR-induced GI-tumorigenesis in Apc1638N/+ mice. A) Mean intestinal tumor per mouse. B) Mean colonic tumor/mouse. C) Mean tumor clusters/mouse. D) Mean large size tumor/mouse in control, and 28Si-ion (10 and 50 cGy) irradiated animals. Bars represent mean ± SEM and * indicates p<0.05 compared to control whereas ** indicates p<0.05 between irradiated groups.
3.2. Effect of dietary aspirin on serum prostaglandin levels
Dietary aspirin use was associated with marked reductions in serum level of PGE2 at all tested doses and the highest effect was observed with the 300 mg daily dose. A dose-dependent reduction (% to control) in serum PGE2 was observed at 75 mg/day (88.48 ± 1.84; p<0.05); 150 mg/day (75.49 ± 1.52; p<0.05) and 300 mg/day (69.44 ± 2.55; p<0.05) aspirin doses (Fig. 3A), while a dose-dependent increase in high-LET IR-induced serum PGE2 was observed in 28Si-ion exposed mice, compared to the control group (Fig. 3B). A significant reduction in radiation-induced serum PGE2 levels was also observed in all ASA treated groups, however, the 150 and 300 mg human equivalent ASA dose was highly effective in reducing 28Si-induced PGE2 levels, relative to 75 mg dose (Fig. 3C–D). Therefore, our results clearly showed that aspirin is effective in reducing basal as well as high-LET radiation-induced PGE2 levels.
Figure 3.
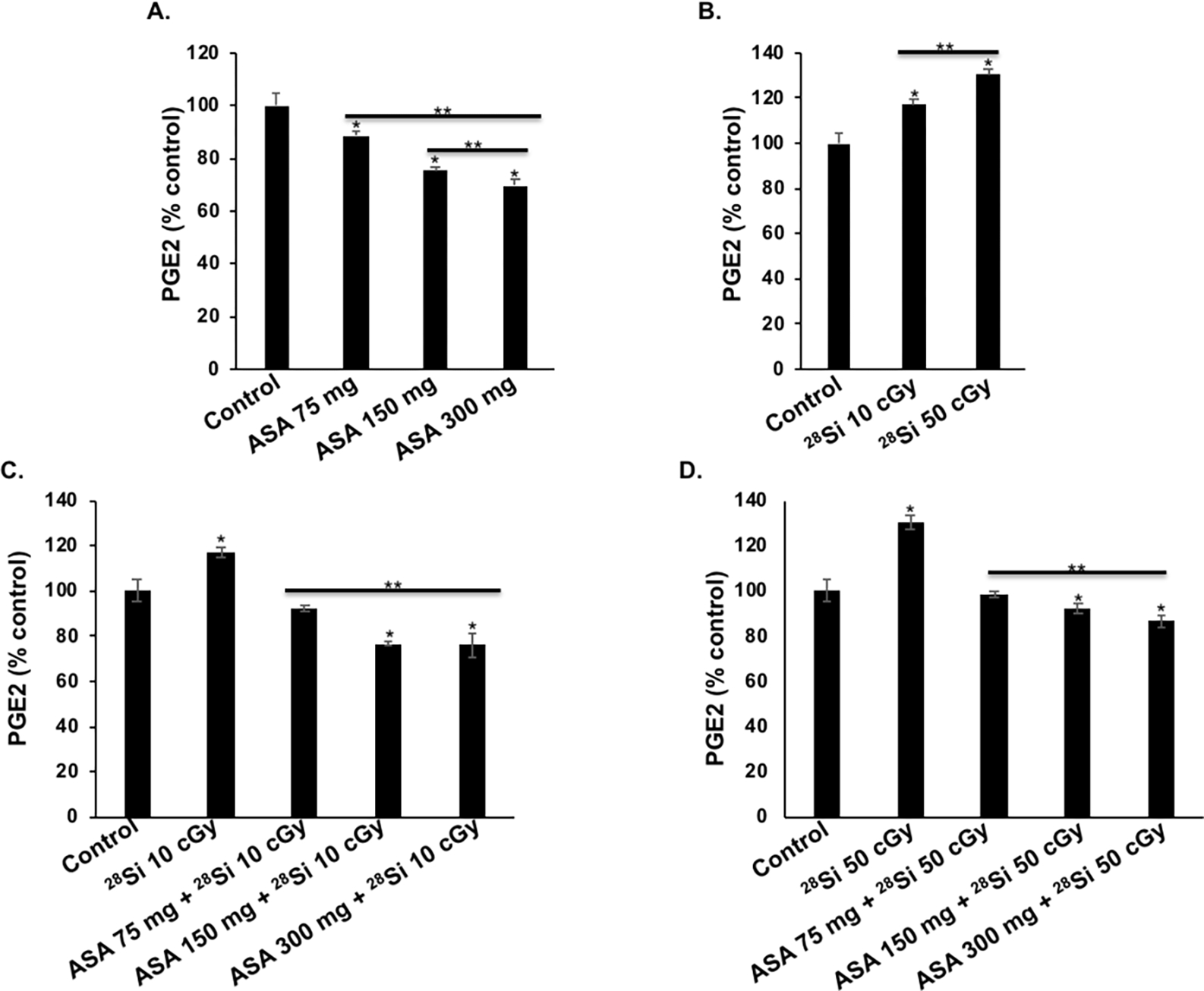
Effects of dietary aspirin (ASA) on serum prostaglandin E2 (PGE2) levels in Apc1638N/+ mice (n=6/group). A) Percentage change in PGE2 after human daily dose equivalent of 75 mg, 150 mg, and 300 mg ASA. B) Percentage change in PGE2 after whole body 28Si-ion exposure. C) Percentage change in PGE2 in 10 cGy 28Si-ion exposed mice and effect of dietary ASA. D) Percentage change in PGE2 in 50 cGy 28Si-ion exposed mice and effect of dietary ASA. Bars represent mean ± SEM and * indicates p<0.05 compared to control whereas ** indicates p<0.05 between irradiated groups.
3.3. Aspirin administration has no effect on GI-tumorigenesis in Apc1638N/+ mice
The intestinal tumor count in Apc1638N/+ mice (3.85 ± 0.28) was not altered in the ASA-treated group and no statistical difference was observed between 75 mg/day (4.15 ± 0.26), 150 mg/day (4.2 ± 0.32), and 300 mg/day (4.25 ± 0.23) aspirin groups (Fig. 4A). The mean colonic tumor count (Fig. 4B) and the number of intestinal tumor clusters (Fig. 4C) were rare in all experimental groups. Similar to tumorigenesis, no significant difference was observed in count of large (>2mm2) size tumors between control (1.63 ± 0.21) and aspirin 75 mg/day (1.48 ± 0.25), 150 mg/day (1.72 ± 0.25) and 300 mg/day (1.62 ± 0.30) aspirin groups (Fig. 4D). Altogether, our results show no effect of dietary aspirin on GI tumorigenesis in the 75–300 mg dose range.
Figure 4.
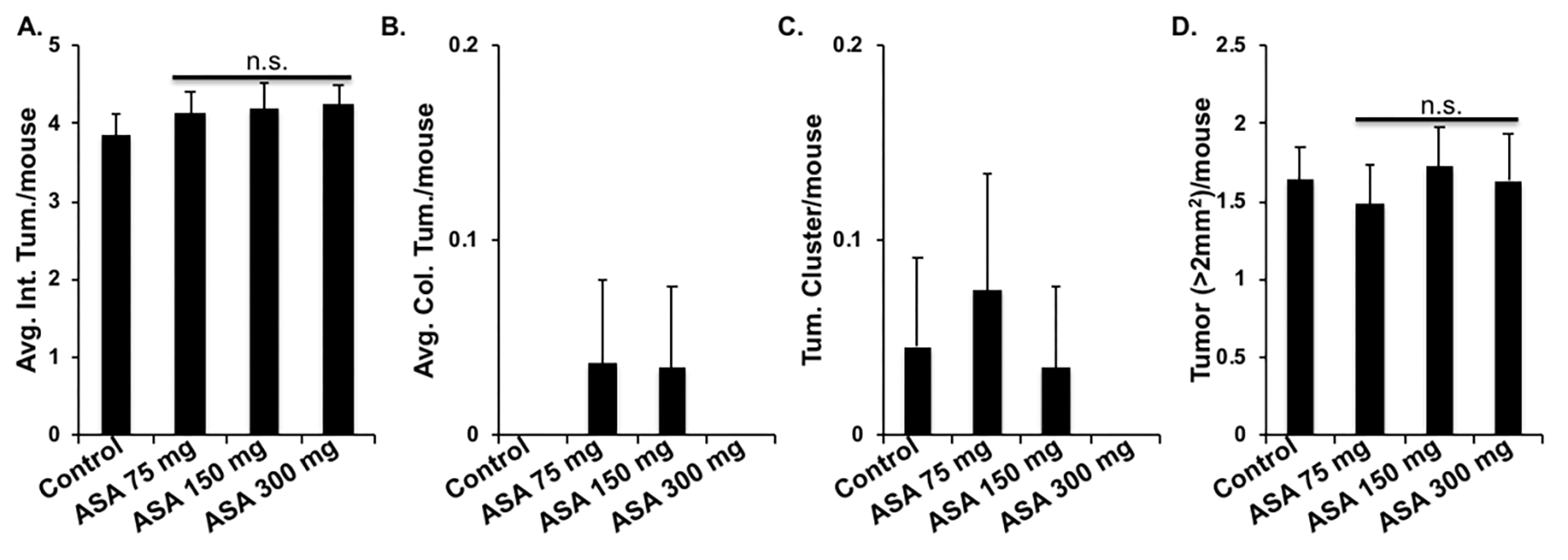
Effect of dietary aspirin (ASA) on GI tumorigenesis in Apc1638N/+ mice (n=20/group). A) Intestinal tumor count/mouse. B) Colonic tumor count/mouse. C) Mean tumor clusters/mouse. D) Mean large size tumor/mouse in control, ASA 75, 150, and 300 groups. Bars represent mean ± SEM and n.s. indicates no statistically significant difference among experimental groups.
3.4. Dietary aspirin does not confer protection against high-LET radiation-induced GI-tumorigenesis in Apc1638N/+ mice
Aspirin administration did not show a significant reduction in high-LET IR-induced radiogenic GI tumorigenesis as well as on the number of tumor clusters and size (Fig. 5). In control, IR-exposed, aspirin only, and aspirin + IR-exposed groups the distribution of tumors throughout GI-tract was similar. The mean number of radiogenic GI tumors at 10 cGy (5.90 ± 0.28) was not significantly affected in aspirin-treated mice i.e. 75 mg/day (5.75±0.31), 150 mg/day (5.95±0.34), and 300 mg/day (5.30±0.29) (Fig. 5A–C). In addition, the mean number of radiogenic GI tumors at 50 cGy (7.95 ± 0.47) was also not affected among aspirin-treated mice i.e., 75 mg (7.35±0.55), 150 mg (7.05±0.58), and 300 mg (7.40±0.43) (Fig. 5D–F). Similar to tumor count, no statistical difference was observed in the number of large tumors and clusters between 28Si exposed and aspirin + 28Si exposed groups at all aspirin doses (Fig. A-F). Altogether, we demonstrate no significant effect of aspirin on high LET IR-induced GI tumorigenesis in male Apc1638N/+mice at the 75–300 mg/day dose range.
Figure 5.
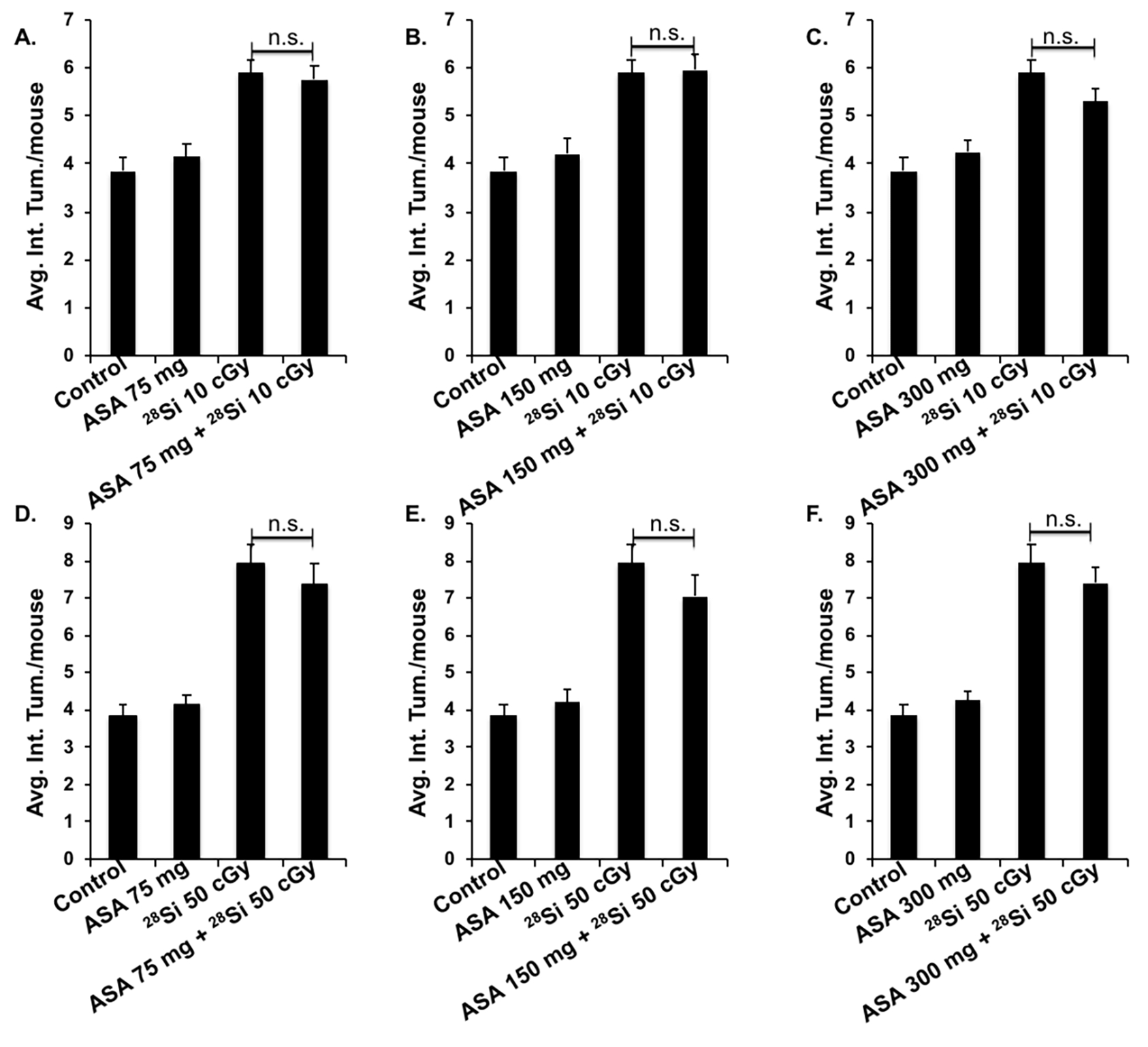
Effect of aspirin on high-LET IR-induced GI tumorigenesis in Apc1638N/+ mice (n=20/group). A) Intestinal tumor count/mouse in control, ASA 75 mg, 28Si-ion (10 cGy), and ASA 75 + 28Si-ion exposed groups. B) Intestinal tumor number/mouse in control, ASA 150 mg, 28Si-ion (10 cGy), and ASA 150 + 28Si-ion exposed groups. C) Intestinal tumor number/mouse in control, ASA 300 mg, 28Si-ion (10 cGy), and ASA 300 + 28Si-ion exposed groups. D) Intestinal tumor number/mouse in control, ASA 75 mg, 28Si-ion (50 cGy), and ASA 75 + 28Si-ion exposed groups. B) Intestinal tumor number/mouse in control, ASA 150 mg, 28Si-ion (50 cGy), and ASA 150 + 50 cGy 28Si-ion exposed groups. C) Intestinal tumor number/mouse in control, ASA 300 mg, 28Si-ion (50 cGy), and ASA 300 + 28Si-ion exposed groups. All bars represent mean ± SEM and n.s. indicates no statistically significant difference.
4. Discussion
Anti-inflammatory and GI-cancer preventive effects of aspirin are attributed to its COX inhibitory activity (Stolfi et al., 2013; Wang & DuBois, 2013; Sostres et al., 2014), whereas high-LET radiation-induced persistent inflammation and higher GI-cancer incidence are in part ascribed to higher intestinal COX2 expression and accumulation of its pro-inflammatory product PGE2 (Cheema et al., 2014; Kumar et al., 2018b). This study demonstrates that aspirin was able to reduce radiation-induced serum PGE2. However, aspirin had no effects on either on background or on IR-induced GI-tumorigenesis.
Aspirin dose and duration of its use are often associated with its efficacy and side effects in humans (Ishikawa et al., 2014; Morris et al., 2009; Bosetti et al., 2020). We used a spectrum of human equivalent doses of aspirin i.e., low (75 mg/day), intermediate (150 mg/day), and high (300 mg/day) to test its efficacy against spontaneous as well as 28Si-induced GI-tumorigenesis. Aspirin did not alter 28Si-induced tumor count, cluster, or size in the irradiated animals; however, this study is limited to continuous aspirin dosing and one time-point. In concurrence, other reports on aspirin’s effect on GI-tumorigenesis in Apc1638N/+ mice also displayed no appreciable prevention from GI-tumorigenesis (Williamson et al., 1999). The aspirin’s GI-cancer prevention studies using other similar mouse models of CRC i.e. ApcMin/+ mice and also in human clinical trials have also shown mixed results (Reuter et al., 2002; Chiu et al., 2000; Barnes & Lee, 1998; McNeil et al., 2021; Drew et al., 2020; Burn et al., 2020; Qiao et al., 2018). Since the duration of aspirin use for effective GI-cancer prevention is also not established in mouse or human studies (Bosetti et al., 2020) therefore further studies might be required to optimize aspirin timing and duration to achieve desired chemoprevention.
In another Apc-based GI-cancer model i.e., ApcMin/+ mice up to 50% chemoprevention was achieved but with a bolus aspirin dose (Barnes & Lee, 1998), which is many-fold higher than the adult human dose. However, in ApcMin/+ mice it was noted that in-utero aspirin use is more effective compared to aspirin use after weaning (Perkins et al., 2003; Rohwer et al., 2020). Unlike ApcMin/+, the mutation in Apc1638N/+ is localized outside the β-catenin interaction domain therefore WT and truncated protein forms could suppress oncogenic β-catenin, therefore might reflect mutation-specific differential aspirin responses. Moreover, GI-mucosal physiology of Apc1638N/+ mice is very similar to WT mice as WT Apc allele is often maintained even in the GI-polyps derived from Apc1638N/+ mice, and loss of the WT allele is not detected until the development of frank tumors (Haigis et al. 2002; Wang et al. 2002). Additionally, ~50% decrease in WT APC protein expression in Apc1638N/+ mice, indicates a primary role of Apc haploinsufficiency as a plausible mechanism of tumorigenesis in this model (Wang et al. 2002), rather than inactivating mutation.
A significant reduction in serum levels of PGE2 was noted at all tested aspirin dose. In concurrence, aspirin mediated effective reductions in mucosal as well as serum PGE2 levels with reduced sign of systemic inflammation have been observed both in mouse and human studies (Morris et al., 1985; Cryer et al., 1990; Montrose et al., 2015; Liu et al., 2013; Boutaud et al., 2016; Kumar et al., 2018a). PGE2 is known to exert pleiotropic effect on many of the hallmarks of cancer (Greenhough et al., 2009) including activation of the β-Catenin signaling and mechanisms involved in tumor promotion, maintenance and progression (Buchanan & DuBois, 2006). Studies using Ptgs-2 (gene coding for COX-2) knock-out mice have indicated resistance to colorectal carcinogenesis (Chulada et al., 2000), while Ptgs-2 transgenic mice have revealed that COX-2/PGE 2 pathway activation alone is insufficient to induce tumorigenesis (Oshima et al., 1996). Therefore, activation of COX/PGE2 pathway in the tumor promotion process is more defined rather than its role in tumor initiation process.
Greater expression of PGE2 have shown a good correlation with larger tumor size in both human and mouse model studies (Kettunen et al., 2003; Yang et al., 1998). While no correlation for aspirin use and tumor size is available for comparison, and we did not observe any significant reduction in tumor size in aspirin treated mice. Aspirin mediated reductions in COX/PGE2 is important anti-cancer mechanism, however mechanisms independent of COX/PGE2 might also be important in 28Si-induced GI-tumorigenesis; as no appreciable effect of aspirin on 28Si-induced GI-tumorigenesis and size was noted in Apc1638N/+ mice. While higher COX2/PGE2 level was observed after high-LET IR (Cheema et al., 2014), but other pro-inflammatory/proliferative factors (IGF1, IL-8 and IL-6) (Kumar et al., 2018b; Kumar et al., 2019; Suman et al., 2016a) have also been found to accumulate in GI-tissues after high-LET IR exposure and might function as alternate signaling to drive high-LET IR-induced GI-tumorigenesis, rendering aspirin mediated reductions in PGE2 levels ineffective. Therefore, we postulate that aspirin at levels used in this study is reducing COX-related inflammation but other pro-inflammatory and pro-proliferative responses independent of COX/PGE2 are also being elicited after high-LET exposure to promote GI-tumorigenesis in Apc1638N/+ mice. However, further studies in the WT or Apc gene independent model of GI-cancer using various aspirin doses and duration against high-LET radiation are required for validation and to rule out any genotype-specific bias that may prevail in this study.
5. Conclusions
In summary, this study suggests that aspirin has no preventive effect against high-LET radiation-induced GI tumorigenesis in Apc1638N/+ mice. Although an effective reduction in serum PGE2 level was noted in aspirin treated group, however, deemed insufficient to reduce high-LET radiation-induced tumorigenesis due to its limited role in the tumor initiation process (Oshima et al., 1996). A multitude of molecular alterations caused by high-LET IR exposure such as, increase in chronic oxidative stress, persistent DNA damage and accumulation of pro-oncogenic SIR factors (Datta et al., 2012; Cheema et al., 2014; Datta et al., 2016; Suman et al., 2018; Kumar et al., 2018b; Kumar et al., 2019; Suman et al., 2020) might undermine aspirin’s efficacy in preventing radiation-induced GI tumorigenesis. However, reductions in 28Si-induced PGE2 levels with aspirin use is encouraging and warrants further experimentation to optimize aspirin’s preventive efficacy in a drug combination setting to simultaneously target alternate tumorigenic pathways induced after high-LET IR exposure.
Highlights.
High-LET ionizing radiation (IR) causes a dose-dependent increase in GI-tumorigenesis.
Dietary aspirin reduces serum PGE2 levels in a dose-dependent manner.
Dietary aspirin reduces high-LET IR-induced serum PGE2 levels.
Dietary aspirin has no preventive effect against high-LET IR-induced GI-tumorigenesis.
6. Acknowledgments
We are grateful to Dr. Peter Guida, Dr. Adam Rusek, and all the support staff at the NASA Space Radiation Laboratory (NSRL) for providing excellent support to conduct 28Si-ion exposures. We are also thankful to the Georgetown University (GU) and Brookhaven National Lab (BNL) animal facility members for their help in the management and transportation of our mouse colony.
7. Funding
This study is supported in part by NASA grant NNX09AU95G.
Abbreviations
- ASA
Acetyl salicylic acid (Aspirin)
- LET
Linear energy transfer
- GCR
Galactic cosmic radiation
- NSAID
Non-steroidal anti-inflammatory drug
- Apc
Adenomatous polyposis coli gene
- REID
Risk of radiation exposure-induced death
8. References
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG (2002) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker ERM, Hoekstra E, Franken PF, Helvensteijn W, van Deurzen CHM, van Veelen W, Kuipers EJ, Smits R (2013) β-Catenin signaling dosage dictates tissue-specific tumor predisposition in Apc-driven cancer. Oncogene 32, 4579–4585. [DOI] [PubMed] [Google Scholar]
- Barnes CJ, Lee M (1998) Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology 114, 873–877. [DOI] [PubMed] [Google Scholar]
- Boice JD, Cohen SS, Mumma MT, Dupree Ellis E, Eckerman KF, Leggett RW, Boecker BB, Brill AB, Henderson BE (2006) Mortality among radiation workers at Rocketdyne (Atomics International), 1948–1999. Radiat Res 166, 98–115. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C (2020) Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol 31, 558–568. [DOI] [PubMed] [Google Scholar]
- Boutaud O, Sosa IR, Amin T, Oram D, Adler D, Hwang HS, Crews BC, Milne G, Harris BK, Hoeksema M, Knollmann BC, Lammers PE, Marnett LJ, Massion PP, Oates JA (2016) Inhibition of the Biosynthesis of Prostaglandin E2 By Low-Dose Aspirin: Implications for Adenocarcinoma Metastasis. Cancer Prev Res (Phila) 9, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, DuBois RN (2006) Connecting COX-2 and Wnt in cancer. Cancer Cell 9, 6–8. [DOI] [PubMed] [Google Scholar]
- Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, Möslein G, McRonald FE, Bertario L, Evans DG, Gerdes AM, Ho JWC, Lindblom A, Morrison PJ, Rashbass J, Ramesar R, Seppälä T, Thomas HJW, Pylvänäinen K, Borthwick GM, Mathers JC, Bishop DT, CAPP2 I (2020) Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet 395, 1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema AK, Suman S, Kaur P, Singh R, Fornace AJ, Datta K (2014) Long-term differential changes in mouse intestinal metabolomics after γ and heavy ion radiation exposure. PLoS One 9, e87079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CH, McEntee MF, Whelan J (2000) Discordant effect of aspirin and indomethacin on intestinal tumor burden in Apc(Min/+)mice. Prostaglandins Leukot Essent Fatty Acids 62, 269–275. [DOI] [PubMed] [Google Scholar]
- Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R (2000) Genetic disruption of Ptgs-1, as well as of Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer research 60, 4705–4708. [PubMed] [Google Scholar]
- Cragle DL, McLain RW, Qualters JR, Hickey JL, Wilkinson GS, Tankersley WG, Lushbaugh CC (1988) Mortality among workers at a nuclear fuels production facility. Am J Ind Med 14, 379–401. [DOI] [PubMed] [Google Scholar]
- Cryer B, Goldschmiedt M, Redfern JS, Feldman M (1990) Comparison of salsalate and aspirin on mucosal injury and gastroduodenal mucosal prostaglandins. Gastroenterology 99, 1616–1621. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, To K, Cacao E (2017) Predictions of space radiation fatality risk for exploration missions. Life Sci Space Res (Amst) 13, 1–11. [DOI] [PubMed] [Google Scholar]
- Datta K, Suman S, Kallakury BV, Fornace AJ (2012) Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS One 7, e42224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K, Suman S, Kumar S, Fornace AJ (2016) Colorectal Carcinogenesis, Radiation Quality, and the Ubiquitin-Proteasome Pathway. J Cancer 7, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew DA, Cao Y, Chan AT (2016) Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 16, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew DA, Schuck MM, Magicheva-Gupta MV, Stewart KO, Gilpin KK, Miller P, Parziale MP, Pond EN, Takacsi-Nagy O, Zerjav DC, Chin SM, Mackinnon Krems J, Meixell D, Joshi AD, Ma W, Colizzo FP, Carolan PJ, Nishioka NS, Staller K, Richter JM, Khalili H, Gala MK, Garber JJ, Chung DC, Yarze JC, Zukerberg L, Petrucci G, Rocca B, Patrono C, Milne GL, Wang M, Chan AT (2020) Effect of Low-dose and Standard-dose Aspirin on PGE2 Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial. Cancer Prev Res (Phila) 13, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree-Ellis E, Watkins J, Ingle JN, Phillips J (2000) External radiation exposure and mortality in a cohort of uranium processing workers. Am J Epidemiol 152, 91–95. [DOI] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN (1994) Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107, 1183–1188. [DOI] [PubMed] [Google Scholar]
- Frey N, Venturelli S, Zender L, Bitzer M (2018) Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat Rev Gastroenterol Hepatol 15, 81–95. [DOI] [PubMed] [Google Scholar]
- Giraudo M, Schuy C, Weber U, Rovituso M, Santin G, Norbury JW, Tracino E, Menicucci A, Bocchini L, Lobascio C, Durante M, Tessa C (2018) Accelerator-Based Tests of Shielding Effectiveness of Different Materials and Multilayers using High-Energy Light and Heavy Ions. Radiat Res 190, 526–537. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30, 377–386. [DOI] [PubMed] [Google Scholar]
- Haigis KM, Hoff PD, White A, Shoemaker AR, Halberg RB, Dove WF (2004) Tumor regionality in the mouse intestine reflects the mechanism of loss of Apc function. Proc Natl Acad Sci USA 101, 9769–9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Mutoh M, Suzuki S, Tokudome S, Saida Y, Abe T, Okamura S, Tajika M, Joh T, Tanaka S (2014) The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut 63, 1755–1759. [DOI] [PubMed] [Google Scholar]
- Kargman SL, O’Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S (1995) Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 55, 2556–2559. [PubMed] [Google Scholar]
- Kettunen HL, Kettunen AS, Rautonen NE (2003) Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res 63, 5136–5142. [PubMed] [Google Scholar]
- Kumar D, Rahman H, Tyagi E, Liu T, Li C, Lu R, Lum D, Holmen SL, Maschek JA, Cox JE, VanBrocklin MW, Grossman D (2018a) Aspirin Suppresses PGE2 and Activates AMP Kinase to Inhibit Melanoma Cell Motility, Pigmentation, and Selective Tumor Growth In Vivo. Cancer Prev Res (Phila) 11, 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suman S, Fornace AJ, Datta K (2018b) Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc Natl Acad Sci U S A 115, E9832–E9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suman S, Fornace AJ, Datta K (2019) Intestinal stem cells acquire premature senescence and senescence associated secretory phenotype concurrent with persistent DNA damage after heavy ion radiation in mice. Aging (Albany NY) 11, 4145–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Laidlaw TM, Katz HR, Boyce JA (2013) Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A 110, 16987–16992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil JJ, Gibbs P, Orchard SG, Lockery JE, Bernstein WB, Cao Y, Ford L, Haydon A, Kirpach B, Macrae F, McLean C, Millar J, Murray AM, Nelson MR, Polekhina G, Reid CM, Richmond E, Rodríguez LM, Shah RC, Tie J, Umar A, Londen GJV, Ronaldson K, Wolfe R, Woods RL, Zalcberg J, Chan AT, ASPREE IG (2021) Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J Natl Cancer Inst 113, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose DC, Nakanishi M, Murphy RC, Zarini S, McAleer JP, Vella AT, Rosenberg DW (2015) The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat 116–117, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HG, Sherman NA, McQuain C, Goldlust MB, Chang SF, Harrison LI (1985) Effects of salsalate (nonacetylated salicylate) and aspirin on serum prostaglandins in humans. Ther Drug Monit 7, 435–438. [DOI] [PubMed] [Google Scholar]
- Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW (2009) Effects of low-dose aspirin on acute inflammatory responses in humans. The Journal of Immunology 183, 2089–2096. [DOI] [PubMed] [Google Scholar]
- Naito M, Kodaira S, Ogawara R, Tobita K, Someya Y, Kusumoto T, Kusano H, Kitamura H, Koike M, Uchihori Y, Yamanaka M, Mikoshiba R, Endo T, Kiyono N, Hagiwara Y, Kodama H, Matsuo S, Takami Y, Sato T, Orimo SI (2020) Investigation of shielding material properties for effective space radiation protection. Life Sci Space Res (Amst) 26, 69–76. [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–809. [DOI] [PubMed] [Google Scholar]
- Perkins S, Clarke AR, Steward W, Gescher A (2003) Age-related difference in susceptibility of Apc(Min/+) mice towards the chemopreventive efficacy of dietary aspirin and curcumin. Br J Cancer 88, 1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K (2007) Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168, 1–64. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, Lu Z (2018) Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer 18, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22, 659–661. [DOI] [PubMed] [Google Scholar]
- Reuter BK, Zhang XJ, Miller MJ (2002) Therapeutic utility of aspirin in the ApcMin/+ murine model of colon carcinogenesis. BMC Cancer 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N, Kühl AA, Ostermann AI, Hartung NM, Schebb NH, Zopf D, McDonald FM, Weylandt KH (2020) Effects of chronic low-dose aspirin treatment on tumor prevention in three mouse models of intestinal tumorigenesis. Cancer Med 9, 2535–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland MK, Coussens LM, Stewart SA (2016) Senescence and cancer: An evolving inflammatory paradox. Biochim Biophys Acta 1865, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata R, Preston DL, Brenner AV, Sugiyama H, Grant EJ, Rajaraman P, Sadakane A, Utada M, French B, Cahoon EK, Mabuchi K, Ozasa K (2019) Radiation-Related Risk of Cancers of the Upper Digestive Tract among Japanese Atomic Bomb Survivors. Radiat Res 192, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T (1995) Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 55, 3785–3789. [PubMed] [Google Scholar]
- Shuryak I, Fornace AJ, Datta K, Suman S, Kumar S, Sachs RK, Brenner DJ (2017) Scaling Human Cancer Risks from Low LET to High LET when Dose-Effect Relationships are Complex. Radiat Res 187, 476–482. [DOI] [PubMed] [Google Scholar]
- Sostres C, Gargallo CJ, Lanas A (2014) Aspirin, cyclooxygenase inhibition and colorectal cancer. World J Gastrointest Pharmacol Ther 5, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi C, De Simone V, Pallone F, Monteleone G (2013) Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci 14, 17972–17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman S, Kumar S, Fornace AJ, Datta K (2016a) Space radiation exposure persistently increased leptin and IGF1 in serum and activated leptin-IGF1 signaling axis in mouse intestine. Sci Rep 6, 31853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suman S, Kumar S, Fornace AJ, Datta K (2018) The effect of carbon irradiation is associated with greater oxidative stress in mouse intestine and colon relative to γ-rays. Free Radic Res 52, 556–567. [DOI] [PubMed] [Google Scholar]
- Suman S, Kumar S, Moon BH, Fornace AJ, Datta K (2017) Low and high dose rate heavy ion radiation-induced intestinal and colonic tumorigenesis in APC1638N/+ mice. Life Sci Space Res (Amst) 13, 45–50. [DOI] [PubMed] [Google Scholar]
- Suman S, Jaruga P, Dizdaroglu M, Fornace AJ Jr, Datta K (2020) Heavy ion space radiation triggers ongoing DNA base damage by downregulating DNA repair pathways. Life Sciences in Space Research 27, 27–32. [DOI] [PubMed] [Google Scholar]
- Wang D, DuBois RN (2013) An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J 19, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. 2002. Wang D, Pezo RC, Corner G, Sison C, Lesser ML, Shenoy SM, Mariadason JM, Singer RH, Augenlicht LH (2010) Altered dynamics of intestinal cell maturation in Apc1638N/+ mice. Cancer Res 70, 5348–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SL, Kartheuser A, Coaker J, Kooshkghazi MD, Fodde R, Burn J, Mathers JC (1999) Intestinal tumorigenesis in the Apc1638N mouse treated with aspirin and resistant starch for up to 5 months. Carcinogenesis 20, 805–810. [DOI] [PubMed] [Google Scholar]
- Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, Robinson CR, Giardiello FM (1998) Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res 58, 1750–1753. [PubMed] [Google Scholar]


