Abstract
Background
Primary THA (THA) is a successful procedure for end-stage hip osteoarthritis. In the setting of a failed THA, revision total hip arthroplasty (rTHA) acts as a salvage procedure. This procedure has increased risks, including sepsis, infection, prolonged surgery time, blood loss, and increased length of stay. Increasing focus on understanding of demographics, comorbidities, and inpatient outcomes can lead to better perioperative optimization and post-operative outcomes. This epidemiological registry study aimed to compare the demographics, comorbidity profiles, and outcomes of patients undergoing THA and rTHA.
Methods
A retrospective review of discharge data reported from 2006 to the third quarter of 2015 using the National Inpatient Sample registry was performed. The study included adult patients aged 40 and older who underwent either THA or rTHA. A total of 2,838,742 THA patients and 400,974 rTHA patients were identified.
Results
The primary reimbursement for both THA and rTHA was dispensed by Medicare at 53.51% and 65.36% of cases respectively. Complications arose in 27.32% of THA and 39.46% of rTHA cases. Postoperative anemia was the most common complication in groups (25.20% and 35.69%). Common comorbidities in both groups were hypertension and chronic pulmonary disease. rTHA indications included dislocation/instability (21.85%) followed by mechanical loosening (19.74%), other mechanical complications (17.38%), and infection (15.10%).
Conclusion
Our data demonstrated a 69.50% increase in patients receiving THA and a 28.50% increase in rTHA from the years 2006 to 2014. The data demonstrated 27.32% and 39.46% complication rate with THA and rTHA, with postoperative anemia as the most common cause. Common comorbidities were hypertension and chronic pulmonary disease. Future analyses into preoperative optimizations, such as prior consultation with medical specialists or improved primary hip protocol, should be considered to prevent/reduce postoperative complications amongst a progressive expansion in patients receiving both THA and rTHA.
Keywords: Hip, Arthroplasty, Demographics, Epidemiology, Outcomes
Background
Primary total hip arthroplasty (THA) constitutes the standard of care for treatment of end-stage hip osteoarthritis and provides pain relief and improved joint function [1]. The prevalence of THA in the United States among adults fifty years of age or older was estimated to be 2.34% in 2010. It is predicted that demand and volume of this procedure will increase in coming years due to higher demand for improved mobility and quality of life in an aging population [2]. While THA is a successful procedure and among the top five most common and fastest-growing procedures in the United States, its failure, for reasons such as instability, infection, loosening, and implant failure or wear, poses a significant burden to patients and the national healthcare system [3]. In the setting of a failed THA, revision total hip arthroplasty (rTHA) is a salvage procedure with increased surgical risks, including sepsis, prosthetic joint infection, prolonged surgical time and exposure to anesthesia, increased blood loss, cost of care, and hospital length of stay (LOS) [4]. As the incidence of THA increases, a 43% to 70% increase in frequency of rTHA from 2014 to 2030 has been similarly projected [4].
Over the last decade, with the conceptualization of value-based care, healthcare systems witnessed a shift in focus towards improving quality while simultaneously minimizing cost of delivered care. Such efforts have gained substantial traction among high-volume and high-impact procedures, such as THA. The successful, consistent, and reproducible achievement of these targets requires implementation of evidence-based best practices through standardized care pathways. Featherall et al. demonstrated that an implemented standardized protocol of preoperative, intraoperative and postoperative care standards could effectively reduce LOS with increasing home discharge [5].
While this approach has been efficient in improving various postoperative outcomes, there remains considerable room for improvement that can be guided by more advanced perioperative optimization protocols that target the different medical and social comorbidities commonly found among arthroplasty recipients. While previous epidemiological studies focused on demographics and outcomes of THA recipients, there remains a lack of an in-depth understanding of a comprehensive list of the most common medical comorbidities found in THA and rTHA recipients.
As such, the aim of this study was to provide a better understanding of the demographics, comorbidity profile, and in-hospital outcomes of patients undergoing THA and rTHA. Further, the type of revision and the indication for rTHA performed in the United States from the year 2006 to the third quarter of 2015 were reported. In reporting these statistics, the authors aimed to provide a baseline structural understanding of common demographic and medical characteristics of THA and rTHA recipients, which might guide perioperative protocol development and improvement.
Methods
A retrospective analysis of discharge data from 2006 to the third quarter of 2015 using the NIS registry was undertaken. This database accounts for roughly 20% of inpatient stays across the United States, and includes information such as demographics, comorbidity profiles, in-hospital outcomes, as well as the type and reason for rTHA. The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) was used for procedure and diagnosis codes within the NIS during the study period. As the NIS transitioned to ICD-10 coding in the fourth quarter of 2015 we elected to exclude that quarter to maintain homogeneity of the implemented methodology. The inclusion criteria for this study were defined as patients aged at least 40 years who underwent either THA or rTHA. The age of at least 40 years was chosen to include a cohort representative of the elective joint replacement population that would be typically encountered in practice. These patients were identified with ICD-9 code 81.51 (total hip replacement) or 81.53 (revision of total hip replacement). Reasons for revision were also included with the use of ICD-9 codes: 996.42 (dislocation/instability), 996.41 (mechanical loosening), 996.66 (infection), 996.43 (implant failure), 996.45 (periprosthetic osteolysis), 996.44 (periprosthetic fracture), 996.46 (bearing surface wear), 996.47 (other mechanical problems), and 996.49 (other mechanical complications). We elected to combine “other mechanical problems” and “other mechanical complications” into one category in the results. The type of revision was also included with ICD-9 codes: 00.70 (all components), 00.71 (acetabular component), 00.72 (femoral component), 00.73 (acetabular liner and/or femoral head only), 80.05 (arthrotomy for removal of prosthesis), and 81.53 (other not otherwise specified). The variables assessed included demographics, in-hospital outcomes, Elixhauser medical comorbities profile, reason for rTHA, and type of rTHA.
Demographic data collected includes total number of discharges (corresponding to the number of cases), patient age, sex, primary payor, race, calendar year of discharge, hospital bed size, location/teaching status, hospital geographic location, as well as elective or non-elective nature of admission for rTHA.
In-hospital outcome data collected includes patient complications during the inpatient stay. This dataset includes a variable, any complication, as a measure referring to any cardiac, respiratory, peripheral vascular disease (PVD), hematoma/seroma, wound dehiscence, postoperative infection, gastrointestinal complication, genitourinary complication, deep vein thrombosis, pulmonary embolism or postoperative anemia complication. In addition, this data set does include the average cost and length of stay for both primary and revision total hip arthroplasty.
A comorbidity profile was created using the Elixhauser comorbidity index. This index is used frequently in database studies to evaluate patient comorbidities. The index allows for better controlling of potential confounding effects of preexisting diseases [6]. Although this study did not aim to directly compare any variables, presenting the entire Elixhauser comorbidity profile allows for a more comprehensive understanding of the major medical comorbidities of this patient population.
Results
Demographic data
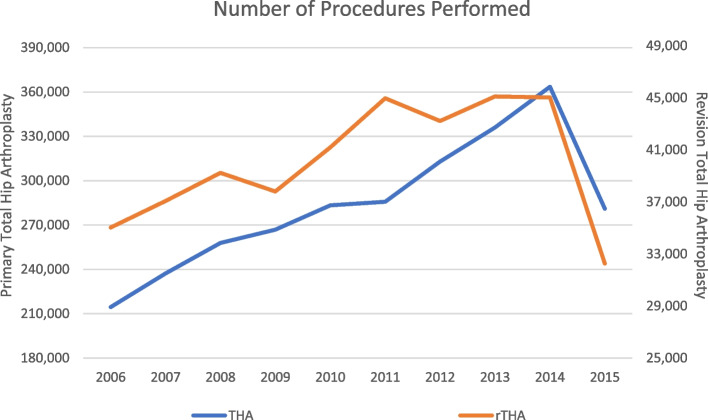
A total of 2,838,742 THA patients and 400,974 rTHA patients were included in this study. Average age in THA and rTHA groups was 65.98 and 68.54 years, respectively. The major primary payor for both THA and rTHA was Medicare, at 53.51% and 65.36% of cases, respectively, followed by private payor. In terms of race, non-Hispanic whites were by far the majority recipients of THA and rTHA, at 74.80% and 74.40% of discharges, respectively. In terms of number of discharges per year, which can be translated to number of procedures performed per year, according to our results there has been a consistent increase every year between 2006 and 2014 (Fig. 1). Of note, the procedure volume for the year 2015 showed a slight decrease in cases in both cohorts, which can be explained by the exclusion of the data from the 4th quarter from that year as per the study design. The demographic variables of the study population are detailed in Table 1.
Fig. 1.
Number of procedures performed. This figure displays the amount of patient undergoing THA and rTHA by year
Table 1.
Total hip arthroplasty demographics
| THA | rTHA | |
|---|---|---|
| Discharges (n = 2,838,742) | Discharges (n = 400,974) | |
| Age of Patient (Years) | ||
| Mean (Standard Error) | 65.98 (0.05) | 68.54 (0.08) |
| Biological Sex of Patient | ||
| Male | 1,239,107 (43.65%) | 172,950 (43.14%) |
| Female | 1,599,635 (56.35%) | 228,024 (56.87%) |
| Expected Primary Payor | ||
| Medicare | 1,519,119 (53.51%) | 262,084 (65.36%) |
| Medicaid | 95,921 (3.38%) | 15,860 (3.96%) |
| Private | 1,124,965 (39.63%) | 108,030 (26.94%) |
| Self-Pay | 20,617 (0.73%) | 2,824 (0.70%) |
| No Charge | 3,707 (0.13%) | 480 (0.12%) |
| Other Insurance | 74,412 (2.62%) | 11,697 (2.92%) |
| Race of Patient | ||
| Non-Hispanic White | 2,123,402 (74.80%) | 298,324 (74.40%) |
| Non-Hispanic Black | 171,191 (6.03%) | 22,680 (5.66%) |
| Hispanic | 76,260 (2.69%) | 12,396 (3.09%) |
| Other Race | 467,889 (16.48%) | 67,574 (16.85%) |
| Calendar Year of Discharge | ||
| 2006 | 214,451 (7.55%) | 35,034 (8.74%) |
| 2007 | 237,237 (8.36%) | 37,075 (9.25%) |
| 2008 | 257,853 (9.08%) | 39,243 (9.79%) |
| 2009 | 266,904 (9.40%) | 37,808 (9.43%) |
| 2010 | 283,275 (9.98%) | 41,194 (10.27%) |
| 2011 | 285,683 (10.06%) | 44,980 (11.22%) |
| 2012 | 312,910 (11.02%) | 43,230 (10.78%) |
| 2013 | 335,900 (11.83%) | 45,110 (11.25%) |
| 2014 | 363,575 (12.81%) | 45,030 (11.23%) |
| 2015 | 281,000 (9.90%) | 32,270 (8.05%) |
| Bedsize of Hospital | ||
| Small | 542,124 (19.10%) | 65,044 (16.22%) |
| Medium | 740,181 (26.07%) | 95,687 (23.86%) |
| Large | 1,547,591 (54.52%) | 238,109 (59.38%) |
| Unknown | 8,846 (0.31%) | 2,134 (0.53%) |
| Location/Teaching Status of Hospital | ||
| Rural | 277,772 (9.79%) | 28,180 (7.03%) |
| Urban Nonteaching | 1,153,501 (40.63%) | 143,218 (35.72%) |
| Urban Teaching | 1,398,623 (49.27%) | 227,442 (56.72%) |
| Unknown | 8,846 (0.31%) | 2,134 (0.53%) |
| Region of Hospital | ||
| Northeast | 566,838 (19.97%) | 72,122 (17.99%) |
| Midwest | 746,641 (26.30%) | 98,410 (24.54%) |
| South | 939,107 (33.08%) | 144,753 (36.10%) |
| West | 586,156 (20.65%) | 85,688 (21.37%) |
| Elective Admission | ||
| Non-Elective | n/a | 114,244 (28.49%) |
| Elective | n/a | 285,841 (71.29%) |
| Unknown | n/a | 888 (0.22%) |
A comprehensive list of demographic data collected from the NIS database of patients undergoing THA and rTHA from 2006 to the third quarter of 2015
Elixhauser comorbidity profile
There was a slight variation in the incidence of different comorbidities between the THA and rTHA groups. Hypertension appeared to be the most common comorbidity in both THA and rTHA cohorts, at 60.46% and 59.74%, respectively. The five most prevalent comorbidities in the THA group were hypertension (60.46%), obesity (15.11%), chronic pulmonary disease (14.37%), hypothyroidism (13.68%), and uncomplicated diabetes (13.67%). The five most prevalent comorbidities in the rTHA group included hypertension (59.74%), deficiency anemia (17.51%), chronic pulmonary disease (16.92%), fluid/electrolyte disorders (15.74%), and hypothyroidism (14.81%). The complete Elixhauser comorbidity profile for both THA and rTHA is summarized in Table 2.
Table 2.
Elixhauser comorbidity profiles
| THA | rTHA | ||
|---|---|---|---|
| Discharges (n = 2,838,742) | Discharges (n = 400,974) | ||
| Hypertension | 1,716,272 (60.46%) | Hypertension | 239,561 (59.74%) |
| Obesity | 428,814 (15.11%) | Deficiency Anemias | 70,229 (17.51%) |
| Chronic Pulmonary Disease | 407,858 (14.37%) | Chronic Pulmonary Disease | 67,859 (16.92%) |
| Hypothyroidism | 388,394 (13.68%) | Fluid and Electrolyte Disorders | 63,133 (15.74%) |
| Diabetes (Uncomplicated) | 388,170 (13.67%) | Hypothyroidism | 59,399 (14.81%) |
| Deficiency Anemias | 387,928 (13.67%) | Obesity | 59,202 (14.76%) |
| Depression | 318,317 (11.21%) | Depression | 57,911 (14.44%) |
| Fluid and Electrolyte Disorder | 261,545 (9.21%) | Uncomplicated Diabetes | 54,259 (13.53%) |
| Renal Failure | 119,673 (4.22%) | Other Neurological Disorders | 28,123 (7.01%) |
| Valvular Heart Disease | 113,545 (4.00%) | Renal Failure | 27,955 (6.97%) |
| Rheumatoid Arthritis/Collagen Vascular Disease | 108,372 (3.82%) | Rheumatoid Arthritis/Collagen Vascular Disease | 27,602 (6.88%) |
| Other Neurological Disorders | 105,927 (3.73%) | Congestive Heart Failure | 24,152 (6.02%) |
| Congestive Heart Failure | 80,299 (2.83%) | Valvular Disease | 20,709 (5.17%) |
| Peripheral Vascular Disorders | 68,802 (2.42%) | Coagulopathy | 16,292 (4.06%) |
| Coagulopathy | 64,920 (2.29%) | Peripheral Vascular Disorders | 13,677 (3.41%) |
| Psychoses | 53,880 (1.90%) | Psychoses | 12,344 (3.08%) |
| Chronic Blood Loss Anemias | 50,668 (1.79%) | Weight Loss | 12,123 (3.02%) |
| Alcohol Abuse | 48,069 (1.69%) | Alcohol Abuse | 9,816 (2.45%) |
| Diabetes (Complicated) | 36,013 (1.27%) | Chronic Blood Loss Anemias | 9,295 (2.32%) |
| Liver Disease | 30,597 (1.08%) | Liver Disease | 8,051 (2.01%) |
| Pulmonary Circulation Disorders | 24,838 (0.88%) | Complicated Diabetes | 6,718 (1.68%) |
| Drug Abuse | 20,699 (0.73%) | Pulmonary Circulation Disorders | 6,716 (1.68%) |
| Weight Loss | 16,756 (0.59%) | Drug Abuse | 5,708 (1.42%) |
| Solid Tumor without Metastasis | 16,463 (0.58%) | Paralysis | 3,848 (0.96%) |
| Lymphoma | 10,662 (0.38%) | Solid Tumor without Metastasis | 3,028 (0.76%) |
| Paralysis | 10,199 (0.36%) | Lymphoma | 2,261 (0.56%) |
| Metastatic Cancer | 8,225 (0.29%) | Metastatic Cancer | 2,240 (0.56%) |
| Acquired Immune Deficiency Syndrome (AIDS) | 3,644 (0.13%) | Acquired Immune Deficiency Syndrome (AIDS) | 640 (0.16%) |
| Peptic Ulcer Disease Excluding Bleeding | 494 (0.02%) | Peptic Ulcer Disease Excluding Bleeding | 96 (0.02%) |
Elixhauser comorbidity profile collected from the NIS database of patients undergoing THA and rTHA from 2006 to the third quarter of 2015
In-hospital outcomes
The outcomes collected from the NIS database aimed to highlight the complications during the in-hospital stay of patients undergoing pTHA and rTHA. The rate of sustaining any complication was 27.32% for THA and 39.46% for rTHA. In terms of specific complications, the most common complication for both pTHA and rTHA was postoperative anemia, at 25.72% and 35.69%, respectively. In terms of economic postoperative outcomes, THA had a total cost of $53,324 with an average length of stay (LOS) of 3.30 days, while rTHA had a total cost of $75,037 with an average LOS of 5.37 days. Table 3 summarizes all in-hospital outcomes collected for both cohorts.
Table 3.
Total hip arthroplasty in hospital outcomes
| THA | rTHA | ||
|---|---|---|---|
| Discharges (n = 2,838,742) | Discharges (n = 400,974) | ||
| Any Complications | 775,599 (27.32%) | Any Complication | 158,235 (39.46%) |
| Postoperative Anemia | 730,134 (25.72%) | Postoperative Anemia | 143,120 (35.69%) |
| Hematoma/Seroma | 22,521 (0.79%) | Hematoma/Seroma | 11,539 (2.88%) |
| Cardiac Complication | 19,042 (0.67%) | Postoperative Infection | 4,337 (1.11%) |
| Genitourinary Complication | 16,046 (0.57%) | Wound Dehiscence | 4,194 (1.05%) |
| Gastrointestinal Complication | 9,198 (0.32%) | Cardiac Complication | 3,806 (0.95%) |
| Deep Vein Thrombosis | 5,752 (0.20%) | Died During Hospitalization | 3,127 (0.78%) |
| Pulmonary Embolism | 5,348 (0.16%) | Deep Vein Thrombosis | 3,034 (0.76%) |
| Respiratory Complication | 4,556 (0.16%) | Genitourinary Complication | 2,358 (0.59%) |
| Died During Hospitalization | 4,234 (0.15%) | Respiratory Complication | 1,870 (0.47%) |
| Postoperative Infection | 3,246 (0.11%) | Pulmonary Embolism | 1,589 (0.40%) |
| Wound Dehiscence | 1,903 (0.07%) | Gastrointestinal Complication | 1,511 (0.38%) |
| Peripheral Vascular Disease Complication | 1,242 (0.04%) | Peripheral Vascular Disease Complication | 369 (0.09%) |
| Total Charges, $, (Standard Error) | $53,324 ($478) | Total Charges, $, (Standard Error) | $75,037 ($901) |
| Length of Stay, Days, (Standard Error) | 3.30 (0.01) | Length of Stay, Days, (Standard Error) | 5.37 (0.04) |
In hospital outcomes collected from the NIS database of patients undergoing THA and rTHA from 2006 to the third quarter of 2015
Reason/type of rTHA
In the study time period, dislocation and instability (21.85%) appeared to be the most common reason for rTHA. This was followed by mechanical loosening (19.74%), other mechanical complications (17.38%), and then infection (15.10%). In terms of type of revision, the majority of patients underwent revision of all components (42.33%). The femoral component (15.66%) appeared to be revised more often than the acetabular component (14.36%). The findings for reason and type of rTHA are summarized in Tables 4 and 5, respectively.
Table 4.
Reason for revision total hip arthroplasty
| Discharges (n = 400,974) | |
|---|---|
| Dislocation/Instability | 87,605 (21.85%) |
| Mechanical Loosening | 79,156 (19.74%) |
| Other Mechanical Complications | 69,684 (17.38%) |
| Infection | 60,562 (15.10%) |
| Periprosthetic Osteolysis | 27,668 (6.90%) |
| Periprosthetic Fracture | 26,332 (6.57%) |
| Implant Failure | 23,103 (5.76%) |
| Bearing Surface Wear | 22,306 (5.56%) |
Listed reason for patients undergoing rTHA collected from the NIS database of patients undergoing THA and rTHA from 2006 to the third quarter of 2015
Table 5.
Type of revision total hip arthroplasty
| Discharges (n = 400,974) | |
|---|---|
| All Components | 169,743 (42.33%) |
| Femoral Component | 62,808 (15.66%) |
| Acetabular Liner and/or Femoral Head Only | 57,875 (14.43%) |
| Acetabular Component | 57,589 (14.36%) |
| Arthrotomy for Removal of Prosthesis | 36,422 (9.08%) |
| Other, Not Otherwise Specified | 20,592 (5.14%) |
Listed types of rTHA collected from the NIS database of patients undergoing THA and rTHA from 2006 to the third quarter of 2015
Discussion
The rates of THA are projected to increase between 43% and 70% from 2014 to 2030, with a corresponding proportional increase in rTHA [4]. Our analysis of 2006 to 2014 data demonstrated a 69.50% increase in patients receiving THA and a 28.50% increase in rTHA. 2015 data were not included in these calculations as they do not contain data from the full year. The increase in patients receiving these procedures may be indicative of expanding indications and increasing demand for THA, potentially driven by an aging population, higher rates of diagnosis and treatment of osteoarthritis, increased demand for improved quality of life [2], and generational improvements in implant design and longevity. As the volume of both index and revision procedures increases yearly, and as reimbursement systems shift towards value-based models, a heightened focus has been placed on resource utilization and quality of care delivery. In the setting of THA, this transition translates into a focus on limiting wasteful resource consumption while simultaneously improving perioperative outcomes. A failed THA results in a rTHA performed in an inpatient setting, with a reported average cost of $75,037 per procedure, totaling over $30,000,000,000 from 2006–2015. In order to decrease this revision burden, there is a need for continuously improving the technical aspects associated with the procedure in addition to perioperative medical and social optimization of THA recipients. Such optimization efforts would be substantially challenging without an in-depth understanding of the major demographic and medical comorbidities of this patient population.
While primary THA is considered one of the most successful procedures in medicine, its failure and subsequent revisions pose a significant negative impact on patient quality of life and the healthcare system. In this study, an increase in the rate of rTHA was noted between 2006 and 2015, with dislocation constituting the most common reason for revision during that time period. Previously reported literature also reported similar findings for reasons for rTHA [7, 8]. Ulrich et al. reported that in a cohort of 237 rTHA, 50% of patients had the revision surgery within five years of the index procedure. Furthermore, most revisions were performed for instability (33%) and infection (24%) [7]. The most crucial independent variable which may predispose patients to dislocation is implant position, specifically positioning of the acetabular shell [9, 10]. Robotic assisted THA, which helps with implant positioning, has been shown to help reduce dislocation rates compared to conventional THA. Shaw et al. found, in their cohort of 2247 patients, that the dislocation rate 0.60% with robotic assisted THA vs. 2.50% in the conventional THA cohort [11]. In addition to technical factors, patient characteristics and comorbidities also play a role in dislocation rates. Obesity, dementia, depression, Parkinson’s disease, chronic lung disease and inflammatory arthritis, amongst other factors, serve as independent risk factors to dislocation following THA [12, 13]. Preoperative optimization of these independent risk factors as well as taking care intraoperatively to ensure appropriate implant position may serve to decrease overall need for rTHA.
There are currently about 46 million geriatric adult patients living in the United States. This number is expected to grow to about 90 million geriatric adults based on a 2050 projection estimate [14]. In the years between 2020 and 2030 alone, the projection estimates another 18 million geriatrics adults [14]. Our study data revealed that the average age of patients undergoing pTHA was 65.98 and 68.54 for rTHA. As the population ages and “baby boomers” reach geriatric age, and the reported average age of THA is about 65 years, there is a projected significant increase in the amount of THA and corresponding increase in rTHA procedures. Increasing age would also account for an increased amount of comorbidities which would further impact rates of complications in hip arthroplasty patients.
With regards to other demographic variables, the findings of this study were in line with previously reported literature in terms of average age, gender distribution, and primary payor type, among other variables [15]. In terms of hospital location, we noted a higher rate of procedures performed in the urban setting, with a total of 89.90% for THA and 92.44% for rTHA. More interestingly, teaching institutions constituted a notably different rate between the procedures, with 56.72% for rTHA compared to a 49.27% for THA. While referral patterns of complex revisions for reasons such as lack of expertise or resources at smaller non-teaching centers may in part explain these differences, there are likely also financial incentives at play [16]. Previous literature has demonstrated that contemporary reimbursement models fail to adequately compensate for the additional resource utilization and costs that rTHA requires relative to THA [17, 18]. As such, non-teaching institutions may be incentivized to “cherry pick” primary procedures while “lemon dropping” the more complex and less financially rewarding revision procedures, consequently offloading the additional revision burden to teaching institutions [17, 19, 20]. Such financial disincentives have the potential to lead to access to care issues, and our findings support the idea that the Current Procedural Terminology (CPT) coding system and RVU allocation for revision procedures should be reexamined to better align incentives [21].
This study reported on all comorbidities that constitute the Elixhauser comorbidity index, which has been extensively utilized in epidemiological studies and proven to be a superior tool for outcome prediction at the population level [6, 22, 23]. Understanding the medical comorbidity profile of THA and rTHA recipients might allow for improved preoperative optimization protocols and for development of appropriate risk stratification models that can subsequently guide establishment of fairer reimbursement models. A study by Dlott et al. reported reduced LOS and less subsequent emergency department visits among patients who underwent preoperative optimization [24]. While arthroplasty literature assessing correlation of various medical comorbidities with postoperative outcomes is quite extensive, a complete delineation of the impact of major comorbidities and their interaction on postoperative outcomes remains lacking. The data noted the most common comorbidities in both THA and rTHA groups to be hypertension, obesity, chronic pulmonary disease, hypothyroidism, uncomplicated diabetes, deficiency anemia, fluid/electrolyte imbalance and depression.
Obesity, one of the most studied comorbidities in arthroplasty, was found to be the second highest comorbidity in the THA group and the sixth highest in the rTHA group. Prior studies have shown that increasing BMI is associated with increased LOS, costs, and intraoperative blood loss, which in turn can lead to a variety of postoperative complications [25].
Other comorbidities such as diabetes have been shown to affect postoperative outcomes. Lovecchio et al. elucidated the increased risk of medical complications in both insulin-dependent and non-insulin-dependent diabetics [26]. Insulin dependence was also described to be associated with higher readmission rates. Additionally, hypertension has a variable effect on cardiac complications. A systematic review by Elsiwy et al. discussed four independent articles evaluating the effect of hypertension on hip and knee joint arthroplasty outcomes, with two showing a positive correlation and two exhibiting no effect. The authors concluded that history of cardiac disease bore the strongest association with postoperative cardiac complications [27].
Optimization from these pre-existing comorbidities may lead to decreased length of stay duration, decreased post-discharge ED visits, and provide value by reducing cost burden to the health care system.
More recently, the implementation of advanced data analysis tools, such as machine learning algorithms, to better understand impact of a combination of various medical comorbidities on postoperative outcomes has been popularized [28–30]. Harris et al. demonstrated an accurate predictive model for mortality and complications following joint arthroplasty with patient-specific variables [31]. Such efforts would benefit from a better understanding of critical comorbidities, which could potentially be incorporated as predictive variables for these algorithms and would allow for the development of risk-stratification models with increasing accuracy. Once such models are available, and once postoperative outcomes can be predicted with relatively high accuracy based on comorbidity profiles, patient-specific payment models with a more distributed reimbursement system can be implemented. Ramkumar et al. utilized a preliminary Bayesian machine learning model trained with patient factors to forecast LOS and to quantify patient risk. The algorithm proposed a staggered payment model that reimburses based on patient risk level to reduce patient selection bias and promote access [32]. Such reimbursement models would diminish concerns for provider “cherry-picking” through fairly accounting for the heightened risk undertaken by surgeons performing THA and rTHA on a more complex population.
Similar to most large database cross-sectional observational studies, this study has several limitations. While the NIS supplies a large amount of healthcare and resource utilization data at the population level, it remains prone to frequent errors due to reliance on suboptimal coding systems and human manual entry of data [33]. Despite this inherent potential weakness, the database has been validated for complication and comorbidity data, and is considered an excellent tool to conduct population-based observational epidemiological studies. Additionally, the NIS is strictly limited to inpatient data, and hence NIS does not allow for a complete assessment of postoperative clinical and economic outcomes beyond the immediate in-hospital period. While this was not an initial aim for this study, a better understanding of the long-term postoperative course would further help in improving optimization efforts, and subsequent studies could focus on shedding further light onto that aspect of the episode of care. Furthermore, the NIS registry does not provide information such as operative technique, preoperative diagnostic information or intraoperative complications. This data would be helpful in further highlighting reasons behind complications following hip arthroplasty.
Despite its inherent limitations, the present study has considerable strengths in design and analysis. To the authors’ knowledge, this study constitutes the largest available comprehensive report at the population level delineating the notable medical comorbidities of THA and rTHA recipients. The substantially large volume of data and long duration of the study provide a generalizable cross-sectional understanding of the demographics, comorbidities, clinical and economic outcomes following THA and rTHA, in addition to the type and reason for rTHA. This knowledge can potentially empower clinicians with a deeper understanding of perioperative conditions that might impact postoperative outcomes and allow for development and implementation of perioperative optimization pathways geared to target these conditions.
Conclusion
In conclusion, this study assessed the demographic and epidemiological characteristics of THA and rTHA recipients between 2006–2015 and reported significant medical comorbidities and postoperative outcomes associated with each cohort. In an era of value-based care delivery where focus is placed on achieving higher quality while simultaneously optimizing the efficiency of an episode of care, developing an understanding of the patient-specific comorbidities is paramount to designing personalized care protocols that subsequently improve outcomes. While some medical comorbidities prevalent among these cohorts have been extensively studied, this study identified gaps that will allow future studies to further assess additional comorbidities that were noted to be commonly encountered among THA and rTHA recipients.
Acknowledgements
Not applicable.
Abbreviations
- THA
Total Hip Arthroplasty
- rTHA
Revision Total Hip Arthroplasty
- LOS
Length of Stay
- CPT
Current Procedural Terminology
Authors’ contributions
All authors (I.P., F.N., A.Z., M.E.) contributed to the analysis and interpretation of data as well as manuscript preparation. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the National Inpatient Sample repository, https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hussein IH, Zalikha AK, Tuluca A, et al. Epidemiology of obese patients undergoing revision total knee arthroplasty: understanding demographics, comorbidities, and propensity weighted analysis of inpatient outcomes. J Am Acad Orthop Surg Glob Res Rev. 2022;6:20220216. doi: 10.5435/JAAOSGlobal-D-21-00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003–2012. 2014 Dec. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb–. Statistical Brief #186. PMID: 25695123.
- 4.Schwartz AM, Farley KX, Guild GN, et al. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35:S79–S85. doi: 10.1016/j.arth.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Featherall J, Brigati DP, Faour M, et al. Implementation of a total hip arthroplasty care pathway at a high-volume health system: effect on length of stay, discharge disposition, and 90-day complications. J Arthroplasty. 2018;33:1675–1680. doi: 10.1016/j.arth.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Ondeck NT, Bohl DD, Bovonratwet P, et al. Discriminative ability of Elixhauser’s comorbidity measure is superior to other comorbidity scores for inpatient adverse outcomes after total hip arthroplasty. J Arthroplasty. 2018;33:250–257. doi: 10.1016/j.arth.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich SD, Seyler TM, Bennett D, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32:597–604. doi: 10.1007/s00264-007-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upfill-Brown A, Hsiue PP, Sekimura T, et al. Instability is the most common indication for revision hip arthroplasty in the United States: national trends from 2012 to 2018. Arthroplast Today. 2021;11:88–101. doi: 10.1016/j.artd.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrack RL. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003;11:89–99. doi: 10.5435/00124635-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23:237–248. doi: 10.1016/S0030-5898(20)31734-X. [DOI] [PubMed] [Google Scholar]
- 11.Shaw JH, Rahman TM, Wesemann LD, et al. Comparison of postoperative instability and acetabular cup positioning in robotic-assisted versus traditional total hip arthroplasty. J Arthroplasty. 2022;37:S881–S889. doi: 10.1016/j.arth.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Gausden EB, Parhar HS, Popper JE, et al. Risk factors for early dislocation following primary elective total hip arthroplasty. J Arthroplasty. 2018;33:1567–1571.e1562. doi: 10.1016/j.arth.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Haverkamp D, Klinkenbijl MN, Somford MP, et al. Obesity in total hip arthroplasty--does it really matter? A meta-analysis. Acta Orthop. 2011;82:417–422. doi: 10.3109/17453674.2011.588859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colby SL, Jennifer M. Projections of the Size and Composition of the U.S. Population: 2014 to 2060, Current Population Reports, P25-1143. Washington, DC: U.S. Census Bureau; 2014.
- 15.Jimenez-Garcia R, Villanueva-Martinez M, Fernandez-de-Las-Penas C, et al. Trends in primary total hip arthroplasty in Spain from 2001 to 2008: evaluating changes in demographics, comorbidity, incidence rates, length of stay, costs and mortality. BMC Musculoskelet Disord. 2011;12:43. doi: 10.1186/1471-2474-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illgen RL, Lewallen DG, Yep PJ, et al. Migration patterns for revision total hip arthroplasty in the United States as reported in the American joint replacement registry. J Arthroplasty. 2021;36:1401–1406. doi: 10.1016/j.arth.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Fang CJ, Shaker JM, Ward DM, et al. Financial burden of revision hip and knee arthroplasty at an orthopedic specialty hospital: higher costs and unequal reimbursements. J Arthroplasty. 2021;36:2680–2684. doi: 10.1016/j.arth.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Tokarski AT, Deirmengian CA, Lichstein PM, et al. Medicare fails to compensate additional surgical time and effort associated with revision arthroplasty. J Arthroplasty. 2015;30:535–538. doi: 10.1016/j.arth.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Feng JE, Anoushiravani AA, Schoof LH, et al. Barriers to revision total hip service lines: a surgeon’s perspective through a deterministic financial model. Clin Orthop Relat Res. 2020;478:1657–1666. doi: 10.1097/CORR.0000000000001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humbyrd CJ. The ethics of bundled payments in total joint replacement: “cherry picking” and “lemon dropping”. J Clin Ethics. 2018;29:62–68. doi: 10.1086/JCE2018291062. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Oladipo V, Kerzner B, et al. A retrospective review of reimbursement in revision total hip arthroplasty: a disparity between case complexity and RVU compensation. J Arthroplasty. 2022;37:S807–S813. doi: 10.1016/j.arth.2022.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Menendez ME, Neuhaus V, van Dijk CN, et al. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472:2878–2886. doi: 10.1007/s11999-014-3686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlott CC, Moore A, Nelson C, et al. Preoperative risk factor optimization lowers hospital length of stay and postoperative emergency department visits in primary total hip and knee arthroplasty patients. J Arthroplasty. 2020;35:1508–1515.e1502. doi: 10.1016/j.arth.2020.01.083. [DOI] [PubMed] [Google Scholar]
- 25.Hijazi A, Padela M, Sayeed Z, et al. Review article: Patient characteristics that act as risk factors for intraoperative complications in hip, knee, and shoulder arthroplasties. J Orthop. 2019;17. 10.1016/j.jor.2019.06.022. [DOI] [PMC free article] [PubMed]
- 26.Lovecchio F, Beal M, Kwasny M, et al. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472:3570–3575. doi: 10.1007/s11999-014-3891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsiwy Y, Jovanovic I, Doma K, et al. Risk factors associated with cardiac complication after total joint arthroplasty of the hip and knee: a systematic review. J Orthop Surg Res. 2019;14:15. doi: 10.1186/s13018-018-1058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Othmani MM, Zalikha AK, Shah RP. Comparative analysis of the ability of machine learning models in predicting in-hospital postoperative outcomes after total hip arthroplasty. J Am Acad Orthop Surg. 2022 20220809. 10.5435/JAAOS-D-21-00987. [DOI] [PubMed]
- 29.Ramkumar PN, Karnuta JM, Navarro SM, et al. Deep learning preoperatively predicts value metrics for primary total knee arthroplasty: development and validation of an artificial neural network model. J Arthroplasty. 2019;34:2220–2227.e2221. doi: 10.1016/j.arth.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Sniderman J, Stark RB, Schwartz CE, et al. Patient factors that matter in predicting hip arthroplasty outcomes: a machine-learning approach. J Arthroplasty. 2021;36:2024–2032. doi: 10.1016/j.arth.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 31.Harris AHS, Kuo AC, Weng Y, et al. Can machine learning methods produce accurate and easy-to-use prediction models of 30-day complications and mortality after knee or hip arthroplasty? Clin Orthop Relat Res. 2019;477:452–460. doi: 10.1097/CORR.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramkumar PN, Navarro SM, Haeberle HS, et al. Development and validation of a machine learning algorithm after primary total hip arthroplasty: applications to length of stay and payment models. J Arthroplasty. 2019;34:632–637. doi: 10.1016/j.arth.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Pass HI. Medical registries: continued attempts for robust quality data. J Thorac Oncol. 2010;5:S198–S199. doi: 10.1097/JTO.0b013e3181dcf957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the National Inpatient Sample repository, https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.