Abstract
Immunization(s) fostering the induction of genital mucosa-targeted immune effectors is the goal of vaccines against sexually transmitted diseases. However, it is uncertain whether vaccine administration should be based on the current assumptions about the common mucosal immune system. We investigated the relationship between mucosal sites of infection, infection-induced inflammation, and immune-mediated bacterial clearance in mice using the epitheliotropic pathogen Chlamydia trachomatis. Chlamydial infection of the conjunctival, pulmonary, or genital mucosae stimulated significant changes in tissue architecture with dramatic up-regulation of the vascular addressin, VCAM, a vigorous mixed-cell inflammatory response with an influx of α4β1+ T cells, and clearance of bacteria within 30 days. Conversely, intestinal mucosa infection was physiologically inapparent, with no change in expression of the local MAdCAM addressin, no VCAM induction, no histologically detectable inflammation, and no tissue pathology. Microbial clearance was complete within 60 days in the small intestine but bacterial titers remained at high levels for at least 8 months in the large intestine. These findings are compatible with the notion that VCAM plays a functional role in recruiting cells to inflammatory foci, and its absence from the intestinal mucosa contributes to immunologic homeostasis at that site. Also, expression of type 1 T cell-mediated immunity to intracellular Chlamydia may exhibit tissue-specific variation, with the rate and possibly the mechanism(s) of clearance differing between enteric and nonenteric mucosae. The implications of these data for the common mucosal immune system and the delivery of vaccines against mucosal pathogens are discussed.
The existence of a mucosal immune system distinct from that operative at systemic tissue sites was suggested by the unique ability of mucosal epithelial cells to elaborate secretory IgA, a function dependent upon plasma cells and epithelial cell specializations (27). The revelation that antigens introduced via the intestinal mucosa elicited sIgA responses at distant as well as local mucosal sites suggested a functional linkage between these epithelialized tissues to form a common mucosal immune system against mucosal pathogens (26). Analyses of lymphocyte trafficking patterns supported this concept in that mucosally derived B lymphoblasts homed preferentially to mucosal and not peripheral lymph nodes (23). Subsequent definition of MAdCAM as a mucosal addressin expressed constitutively by vascular endothelia of the mesenteric lymph nodes and intestinal lamina propria, and of α4β7 as the relevant lymphocyte receptor, provided a molecular basis for a functionally distinct mucosal lymphocyte trafficking system (2, 42). Conversely, lymphocyte homing to peripheral lymph nodes was attributed to the interaction of l-selectin with the peripheral lymph node addressin, PNAd, and trafficking to systemic sites of inflammation was attributed to binding of α4β1+ lymphocytes to the inducible vascular cell adhesion molecule, VCAM (4, 40).
The relative failure of parenteral immunization schemes to induce a common mucosal sIgA response (17, 22, 24, 32) indicated that access to the mucosal immune system may be restricted. Indeed, while intestinal priming stimulated widespread sIgA production, immunization at nonintestinal mucosae drove only local or regional sIgA responses and parenteral immunization schemes provided no mucosal response (17, 22, 24, 32). Based upon these differential immune patterns, compartmentalization of inductive and effector sites within the common mucosal immune system was theorized (28, 49). Besides, current strategies for delivery of vaccines against mucosal pathogens rely heavily upon the theories and clinical implications of such a system, even though comparable data on the mucosal homing properties of T cells are lacking.
The present studies utilized the obligate intracellular pathogen Chlamydia trachomatis to investigate the cellular and molecular basis for expression of immunity within distinct mucosal tissues. The tropism of this bacterium for mucosal epithelial cells and dependence upon type 1 T cell-mediated immunity for clearance (30, 37, 48) provided an ideal system to dissect the nature of T-cell reactivity at intestinal versus nonintestinal mucosae. Using a murine model system, it was reported previously that not all routes of immunization against infection with Chlamydia lead to the establishment of protective immunity (11). However, all immunization routes leading to protection caused the induction of a high intensity of specific Th1 cells in the genital mucosa (11). Other studies revealed that chlamydial infection enhanced VCAM expression in the female reproductive tract and stimulated an influx of α4β7+, α4β1+ T cells and α4β7+, α4β1− B cells (13, 38). This contrasted with findings for the intestine, where constitutive expression of MAdCAM was unchanged by infection, VCAM was not induced, and resident T cells were of the α4β1− phenotype (38). To determine which of these profiles was most representative of mucosal tissues in general, our studies were extended to two additional mucosae susceptible to chlamydial infection, specifically to those of the eye and lung. Results indicated that the conjunctival and pulmonary mucosae were both characterized by infection-induced expression of VCAM but not MAdCAM and by α4β1+ inflammatory T cells, in keeping with the profile identified at the genital mucosa. The functional expression of T cell-mediated immunity was also similar at the conjunctival, pulmonary, and genital mucosae in that Chlamydia was cleared within 30 days of infection. In contrast, chlamydial clearance was delayed or absent in the small and large intestine, respectively. These findings have implications for the differential expression of T- versus B-cell immunity within the common mucosal immune system and for the delivery of vaccines against mucosal pathogens.
MATERIALS AND METHODS
Abbreviations.
The following abbreviations are used in this paper: FACS, fluorescence-activated cell sorting; ICAMs, intercellular adhesion molecules; IFU, inclusion-forming units; IgA, immunoglobulin A; MAbs, monoclonal antibodies; MAdCAM, mucosal cellular adhesion molecule; MoPn, mouse pneumonitis; PBS, phosphate-buffered saline; sIgA, secretory IgA; STD, sexually transmitted disease; VCAM, vascular cell adhesion molecule.
Chlamydia stocks and antigens.
Stocks of the C. trachomatis agent of MoPn used to infect mice were prepared by propagating elementary bodies in McCoy cells, as previously described (43). Stocks were titered by infecting McCoy cells with varying dilutions of elementary bodies, and the infectious titer was expressed as IFU per milliliter.
Animals, infection protocols, and chlamydial isolation.
Female C57BL/6 mice, 5 to 8 weeks old, were obtained from The Jackson Laboratory, Bar Harbor, Maine. All animals were provided with food and water ad libitum and were maintained in laminar flow racks under pathogen-free conditions and 12-h light/dark cycles. Mice were infected intranasally, conjunctivally, vaginally, or orally with 1.0 to 1.5 × 103 IFU of MoPn per mouse in a volume of 30 μl of PBS while under methoxyflurane anesthesia (38). Clearance of chlamydial infections was monitored by enumeration of IFU per gram of minced tissue on susceptible HeLa cell monolayers using indirect immunofluorescence for the major outer membrane protein (30).
Immunohistochemistry.
Tissues collected from infected mice were mounted in OCT medium (Miles Inc., Elkhart, Ind.), frozen in liquid nitrogen, and stored at −80°C. Tissues were cut into 5-μm sections on a cryostat microtome, transferred to slides, air dried, and fixed in 4°C acetone for 30 min. After washing in PBS, tissues were stained with primary rat MAbs recognizing murine ICAM-1 (CD54, clone YN1/1.7.4; ATCC, Rockville, Md.), VCAM-1 (CD106, clone M/K2.7; ATCC), or MadCAM-1 (clone MECA-367; PharMingen, San Diego, Calif.), washed in PBS, and developed using mouse-Ig-absorbed tetramethyl rhodamine-conjugated goat anti-rat Ig (Southern Biotechnology Associates, Birmingham, Ala.), as previously described (38). Slides were taken with Nikon Microphot Provia 400 daylight film. Images were digitalized using a Polaroid SprintScan 35-mm slide scanner, and figures were assembled in gray scale without further manipulation using Adobe Photoshop, version 4.0.
Assessment of inflammation.
Tissues were collected from at least three mice per group at 10 to 225 days after infection and fixed in 10% buffered formalin. Inflammation in coded samples was evaluated by an independent laboratory (Histopath of America, Millersville, Md.).
FACS analysis.
At the indicated time periods after infection, lymphocytes were collected by collagenase disruption of infected tissues as previously described (38). Single cell preparations from the indicated organs and tissues were costained with fluorochrome-labeled MAbs directed against T cells (CD3), B cells (B220), and selected integrin and adhesion markers (PharMingen) and were analyzed by flow cytometry as previously described (38) on a FACScan flow cytometer (Becton Dickinson, Sunnyvale, Calif.). Controls were stained with isotype-matched irrelevant antibodies. Fluorochrome-conjugated, mouse-reactive MAbs used to stain isolated lymphocytes were as follows: CD3ɛ (clone 145-2C11), CD11a (integrin αL chain, clone 2D7), CD18 (integrin β2 chain, clone C71/16), CD29 (integrin β1 chain, clone Ha2-5), CD44 (clone IM7), CD49a (integrin α1 chain, clone Ha31/8), CD49d (integrin α4 chain, clone R1-2), CD62L (l-selectin, clone MEL-14), CD103 (integrin αE chain), and integrin β7 chain (clone M293). Binding of biotinylated MAbs was detected with streptavidin-RED613 (Life Technologies, Grand Island, N.Y.). Results are expressed as the percentage of positively stained cells in each cell preparation and are representative of data obtained in two to four separate experiments.
RESULTS
Integrin profiles of T and B lymphocytes recovered from Chlamydia-infected mucosae.
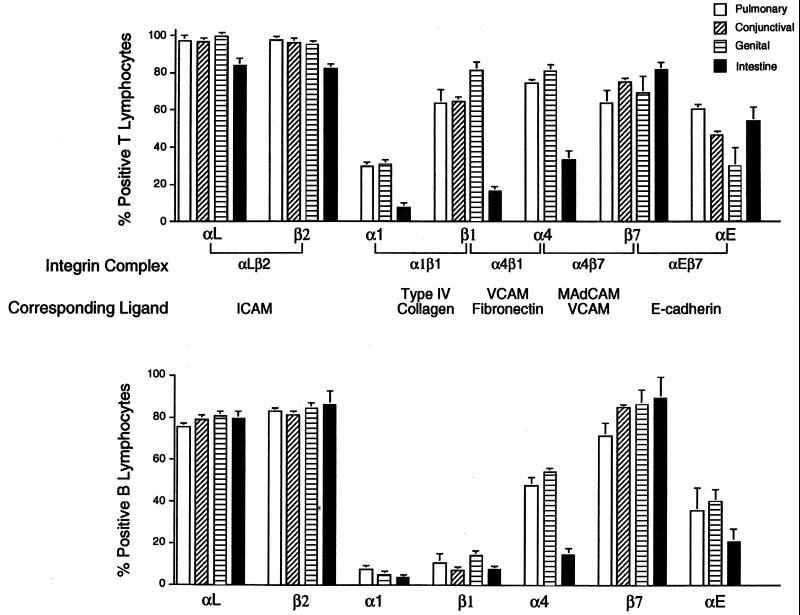
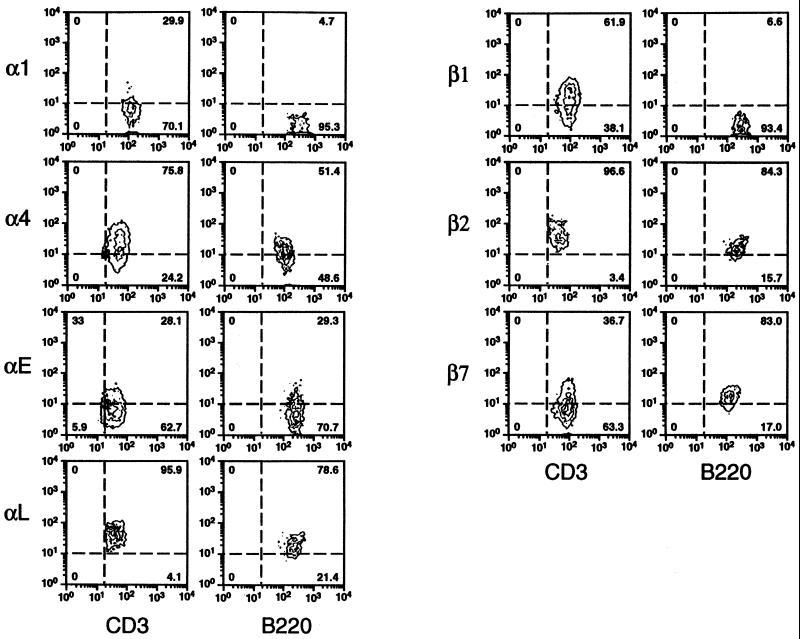
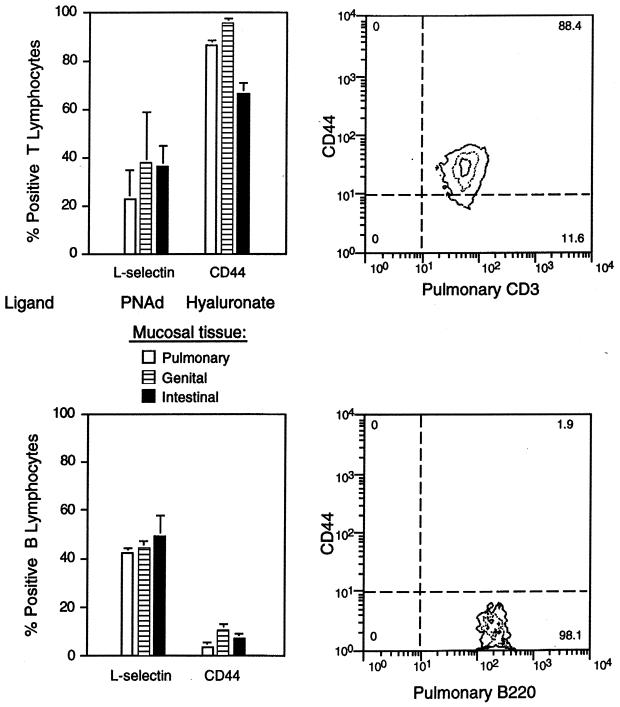
Infections of the conjunctival, pulmonary, genital, or intestinal mucosae were established by ocular, intranasal, intravaginal, or oral delivery of C. trachomatis, respectively. Ten to twelve days later, infiltrating lymphocytes recovered by enzymatic digestion of infected tissues and cells were analyzed by flow cytometry for expression of integrins and other adhesion molecules that contribute to site-specific lymphocyte homing. Most lymphocytes from all sites expressed the αLβ2 integrin (i.e., lymphocyte function antigen-1) that serves as a receptor for the ICAMs, a related set of immunoglobulin superfamily glycoproteins expressed on endothelial cells as well as on macrophages, lymphocytes, and other cell types (40) (Fig. 1 and 2). A majority of lymphocytes from all sites also expressed the β7 integrins α4β7 and/or αEβ7, which mediate binding to the mucosal vascular ligand, MAdCAM, and the epithelial ligand, E-cadherin (7), respectively. This suggested that immunization via these routes is likely to generate lymphocytes capable of homing to other mucosal sites that express MadCAM. However, while expression of β7 integrins was consistently highest on intestinally derived cells, the relative expression of β1 integrins most reliably distinguished the lymphocyte tissue source. T cells but not B cells from the conjunctival, pulmonary, and genital mucosae expressed high levels of the β1 integrin chain that complexes with α1 or α4 to form receptors for type IV collagen or fibronectin, respectively (Fig. 1 and 2). In contrast, β1 integrins were only weakly expressed by intestinal T cells. T cells but not B cells from all sites also uniformly expressed the Pgp-1 isoform of CD44 (Fig. 3), a hyaluronate receptor required for migration into inflammatory foci (8). l-Selectin, a carbohydrate receptor expressed at high levels on lymph node cells that encounter the peripheral lymph node addressin (40), was expressed on less than 50% of T or B lymphocytes derived from the pulmonary, genital, and intestinal mucosae (Fig. 3). Collectively, these integrin profiles distinguished lymphocyte subpopulations from intestinal and nonintestinal sources in that CD44 connective tissue receptors were up-regulated only on T cells and β1 receptors only on T cells from nonintestinal Chlamydia-infected mucosae.
FIG. 1.
Integrin profiles of T and B lymphocytes recovered from Chlamydia-infected pulmonary, conjunctival, genital, and intestinal mucosae. Four groups of female C57BL/6 mice were each infected intranasally, conjunctivally, vaginally, or orally with 1.0 to 1.5 × 103 IFU of the MoPn agent. Lymphocytes collected by collagenase disruption of infected tissues were costained with a panel of MAbs recognizing murine CD3, B220, or the indicated integrin chains and were analyzed by flow cytometry as previously described (10, 35). Data summarized from 10 separate experiments represent the mean percent positive T cells (top panel) or B cells (lower panel) for each integrin marker as determined by quadrant analyses of FACS plots. Note the heightened expression of β1 integrins on T but not B lymphocytes from nonintestinal mucosae.
FIG. 2.
FACS plots of integrin chains expressed by T and B lymphocytes infiltrating Chlamydia-infected pulmonary mucosae. Graphic representation of the relative fluorescent intensity of staining for the various integrin markers and the proportion of cells falling within each quadrant, for cells gated for CD3 or B220 expression. Markers such as α4, β1, and β7 are expressed on all cells at variable levels. For example, note the differential induction of β1 integrin chains on T versus B lymphocytes.
FIG. 3.
Nonintegrin adhesion molecules expressed by T and B cells infiltrating mucosal sites of Chlamydia infection. CD44 is up-regulated on T but not B cells from the pulmonary, genital, and intestinal mucosae, as determined by flow cytometric analyses of cells recovered from collagenase-digested tissues (see legend to Fig. 1). l-Selectin was variably expressed on cells from either subset. l-Selectin was detected using the CD62L-specific MAb clone MEL-14, and CD44 was detected using MAb clone IM7, which recognizes the Pgp-1 isoform (PharMingen).
Profiles of vascular addressin expression in Chlamydia-infected mucosae.
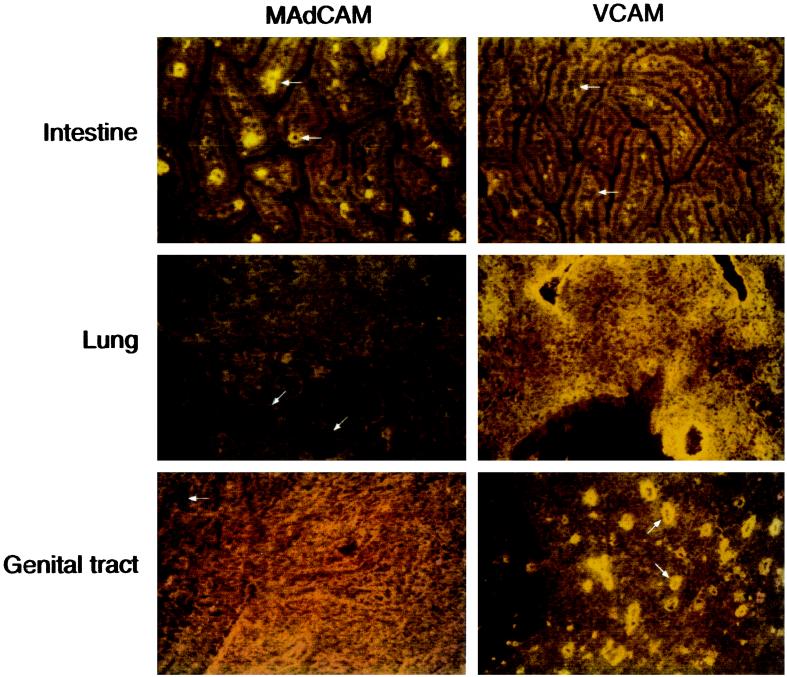
To evaluate the relationship between the profile of lymphocyte integrins detected and the expression of corresponding vascular addressins at mucosal sites, immunohistochemical studies were conducted and site-specific expression of specific vascular addressins were compared in intestinal and nonintestinal mucosae. Immunofluorescent staining of frozen tissue sections revealed expression of MAdCAM on multiple small vessels within the lamina propria of the small intestine, as expected (42) (Fig. 4). VCAM-1 was not detected in the small or large intestine of infected mice, similar to findings in other models of intestinal disease (3). In contrast, endothelial cells at infected conjunctival, pulmonary, and genital mucosae displayed the reciprocal profile with vascular expression of VCAM but low MAdCAM (Fig. 4). ICAM-1 was expressed at high levels by endothelial as well as stromal cells in all tissues examined, in keeping with the pervasive distribution of the complementary αLβ2 lymphocyte receptor (Fig. 1 and data not shown). Overall, it appeared that induction of ICAM-1 was a common tissue response to chlamydial infection but that up-regulation of VCAM-1 was restricted to mucosae outside the gastrointestinal tract (38). MAdCAM, on the other hand, was detected mainly in intestinal tissues where expression was constitutive (42) and apparently unaffected by infection.
FIG. 4.
Expression of VCAM and MAdCAM at mucosal sites of Chlamydia infection. Tissues collected from mice infected as described in Table 1 were mounted in OCT, frozen in liquid nitrogen, and stored at −80°C. Five-micrometer sections were cut on a cryostat microtome, transferred to slides, air dried, and fixed in 4°C acetone for 30 min. Tissues were stained with primary rat MAbs recognizing murine ICAM-1, VCAM-1, or MAdCAM-1, washed in PBS, and developed using mouse-Ig-absorbed tetramethylrhodamine-conjugated goat anti-rat Ig as previously described (10, 35). Slides were viewed with a Nikon Microphot SA epifluorescence microscope, and photomicrographs were taken with Fujichrome Provia 400 daylight film. Images were digitized using a Polaroid SprintScan 35-mm slide scanner, and figures were assembled in gray scale without further manipulation using Adobe Photoshop, version 4.0. Although the intense autofluorescence of murine conjunctival tissues precluded satisfactory photographic documentation, the staining profile was similar to that of other nonintestinal mucosae in that VCAM-1 but not MAdCAM-1 was detected. Arrows indicate vascular structures in each tissue.
Inflammation and clearance of Chlamydia from infected mucosal tissues.
Detection of consistent differences in the profile of homing ligands and receptors expressed at intestinal versus nonintestinal mucosae prompted a comparison of functional parameters of immunity at these same sites. Infections of the pulmonary or genital mucosae with 1.0 to 1.5 × 103 IFU of C. trachomatis were cleared within 4 weeks with peak recoveries of 5 to 10 × 106 organisms per gram of tissue (Table 1). Conjunctival infections were cleared more rapidly with minimal chlamydial shedding over a 7- to 10-day period (data not shown), which accords with previous reports (44, 47). Infections of the small intestine were cleared more slowly, requiring up to 2 months before organisms could no longer be cultured from tissues. Surprisingly, infections of the large intestine were maintained for the entire 8-month observation period at a fairly constant level of 5 × 105 bacteria per gram of tissue. Similar results were achieved when animals were inoculated orally with 102 to 108 IFU of C. trachomatis, suggesting a replication plateau at that level (data not shown). The persistence of Chlamydia in the large intestine precluded attempts to measure expression of acquired immunity in the small intestine, since chronic Chlamydia shedding from colonic epithelia could potentially colonize adjacent cells of the ileum and, possibly, the jejunum to confound results. Acquired immunity to chlamydial infection has been documented in the genital (5, 6) and pulmonary (51) mucosae and appears to exist at the conjunctival mucosa as well (46).
TABLE 1.
Chlamydia-induced inflammation and clearance at distinct mucosal tissues
| Mucosal site of infection | Infectious dose (IFU)a | Mean duration of infection (days) | Peak Chlamydia recovery (IFU) | Inflammation as a response to infection |
|---|---|---|---|---|
| Conjunctiva | 103 | <25 | NDb | Yes |
| Lung | 103 | 25 | 1 × 107 | Yes |
| Genital tract | 1.5 × 103 | 25 | 5 × 106 | Yes |
| Intestine | 103 | |||
| Small | 50 | 5 × 105 | No | |
| Large | >260 | 5 × 105 | No |
C57BL/6 female mice were infected with C. trachomatis as described in the Fig. 1 legend and were monitored for bacterial clearance by enumeration of IFU per gram of minced tissue on susceptible Hela cell monolayers using indirect immunofluorescence for the major outer membrane protein (30). Inflammation in coded samples was evaluated by an independent laboratory (Histopath of America, Millersville, Md.) and was not apparent in intestinal tissues over a wide range of infectious challenges (102 to 108 IFU/mouse) when measured between 7 and 260 days postinfection.
ND, not done.
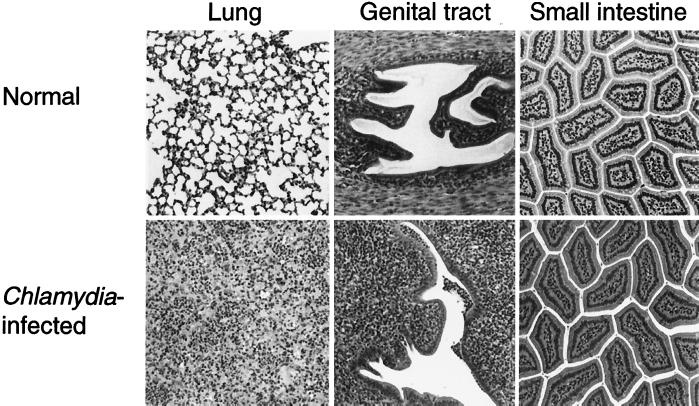
Chlamydial infection of the conjunctival, pulmonary, or genital mucosa has been shown to induce a vigorous host inflammatory response, characterized by a mixed cellular infiltrate comprised of neutrophils and lymphoid cells (34, 36). Inflammatory responses in the Chlamydia-infected intestine were monitored by histological analysis of normal versus infected tissue samples collected at several time points throughout the infection period (days 3, 10, 20, 60, 180, and 240). No differences were detected between infected and uninfected samples of small or large intestinal tissues (Fig. 5). Densities of intraepithelial and lamina propria lymphocytes were indistinguishable, consistent with the failure of infection to induce an increase in the number of lymphocytes recoverable from this site (38). Neither was there any evidence for intestinal tissue pathology, hyperplasia of the Peyer's patches being the only apparent response to infection (data not shown). The development of hydrosalpinx and infertility is a common result of genital chlamydia infections (36), while pulmonary pathology is largely related to inflammation and alveolar congestion (50). Overall, the response of the intestinal tissues to chlamydial infection appears to be quite distinct from that of other mucosal tissues.
FIG. 5.
Inflammatory responses within Chlamydia-infected pulmonary, genital, and intestinal mucosae. Mice were infected as described in Fig. 1, and tissues were collected 10 to 18 days later. Mixed infiltrates comprised of neutrophils and mononuclear cells were present within infected (bottom row) but not in normal (top row) pulmonary, genital, and conjunctival (not shown) mucosae, whereas no evidence of inflammation was detected within infected intestinal mucosa.
DISCUSSION
Targeting the host immune response to specific mucosal tissues is critical to the control of Chlamydia, human immunodeficiency virus, and other pathogens that utilize a mucosal route of entry. Since definition of the common mucosal immune system was based essentially on the trafficking pattern of mucosal B cells and the distribution of mucosal sIgA antibody responses, it is uncertain whether those findings are directly relevant to mucosal T-cell function. This is particularly relevant since several mucosally encountered pathogens, including C. trachomatis, are controlled by T cell-mediated immunity requiring the induction and recruitment of Th1 cells into a specific mucosa microenvironment. To address this issue, the ability of C. trachomatis to induce expression of vascular addressins, inflammation, and immune-mediated clearance was compared for four distinct mucosal tissues. Collectively, the results reveal two distinct profiles of reactivity to mucosal Chlamydia infections. In the mucosae of the eye, lung, and genital tract, infection stimulated an α4β1-VCAM T-cell homing pathway, vigorous inflammation, and reasonably efficient bacterial clearance. Within the intestine, T cells homed via an α4β7-MAdCAM pathway and bacteria persisted for longer periods yet no host inflammatory response was evoked. It would therefore appear that immunological events defined for the intestinal mucosa are not broadly applicable to all mucosal tissues, at least in the system under study.
The absence of infection-induced inflammation in the intestinal mucosa was unexpected given the exquisite sensitivity of other mucosal tissues to the presence of Chlamydia. Within the genital tract, inflammation was induced even during infections with nonmurine isolates of Chlamydia (peak recoveries of less than 104 IFU), yet infection of the intestine with up to 108 IFU of a murine strain failed to stimulate a local tissue response (data not shown). It seems likely that suppression of infection-induced inflammation and the persistence of viable Chlamydia in intestinal epithelial cells are consequences of the tissue-specific evolutionary adaptations that developed at this site to dampen T-cell responsiveness against commensal bacteria and food antigens (1, 14). The implication of this finding is that oral immunization may not be suitable for inducting immune T cells targeted to the genital mucosa.
Expression of VCAM at sites of mucosal inflammation and of MAdCAM at noninflammatory locations suggests that vascular addressins may serve a functional role in lymphocyte trafficking. In this respect, it is conceivable that VCAM-mediated selection of an α4β1-rich lymphocyte population that has dual binding specificity for extracellular matrix proteins (fibronectin) provides a pool of T cells capable of interacting with the surrounding tissue during migration to sites of epithelial infection or during the tissue remodeling that probably occurs during infection and recovery. On the other hand, MAdCAM-based selection of an α4β7-rich lymphocyte population with diminished expression of the β1 family of connective tissue receptors may contribute directly or indirectly to the down-regulation of T-cell reactivity within the highly complex intestinal immune system. Identification of these vascular ligands as direct or indirect regulators of immune function rather than as markers of systemic versus mucosal tissues requires further experimental verification. However, this interpretation is consistent with the different profiles of inflammatory reactivity at MAdCAM- versus VCAM-rich mucosae and the broad tissue distribution of both ligands, which encompasses mucosal as well as systemic sites (4, 9, 16, 40, 41).
Differential expression of VCAM- and MAdCAM-based trafficking pathways at enteric and nonenteric mucosae may alter current concepts of the common mucosal immune system and the practical options for delivery of vaccines against mucosal pathogens. Trafficking of intestinally primed α4β7+ T and B lymphocytes is theoretically unrestricted since the α4β7 integrin carries binding sites for MAdCAM as well as the related VCAM molecule (15, 52), allowing homing of intestinal lymphocytes to any tissue or organ that expresses at least one of these ligands. The tendency of intestinally primed mice to generate significant serum IgG titers may be one reflection of this broad trafficking potential (12, 18, 20, 25, 45). In contrast, parenterally primed T cells expressing high levels of the α4β1 integrin may be restricted to tissues expressing the VCAM ligand, thus ensuring the absence of undesirable inflammatory responses within the VCAM-negative mucosa of the intestine. The overall result of the proposed trafficking schemes is the generation of systemic as well as mucosal T and B cell immune responses to mucosally introduced pathogens, with the intestine being excluded from the recirculation pathway of parenterally primed, VCAM-restricted T cells. The generation of mucosal as well as systemic immune responses to mucosal pathogens is probably one mechanism utilized by the immune system to deter potential dissemination of these organisms to deeper tissues.
The reciprocal situation that involves the development of mucosal immune responses following systemic priming requires further evaluation. It has been demonstrated repeatedly that sIgA responses do not develop following systemic immunization (17, 22, 24, 32), consistent with the irrelevance of a surface antibody response to defense against a systemic pathogen. Unlike the B cell, however, there is no apparent specialization of T-cell function in mucosal tissues, at least at VCAM-rich mucosae. Data are compatible with the notion that conventional T cells utilizing an α4β1-VCAM homing pathway are capable of migrating into systemic or mucosal sites of inflammation (intestine excluded), suggesting that there may be fewer barriers to parenteral induction of a mucosal T-cell response than were predicted by measures of mucosal antibody induction. Indeed, available evidence suggests that systemic immunization can induce protective T cell-mediated immunity against several mucosal pathogens (17, 19, 29, 33, 53).
Admittedly, division of host tissues into VCAM-, MAdCAM-, and peripheral lymph node addressin-expressing sites is an oversimplification of a complex set of interactions that ultimately result in site-specific lymphocyte homing. No account has been made for the existence of vascular addressin splice variants (21, 39), the extensive promiscuity of relevant receptor-ligand interactions (15, 31, 52), or the potential existence of undiscovered adhesion molecules that could contribute to mucosal versus systemic tissue trafficking. Nevertheless, it appears that expression of the MAdCAM addressin in mucosal tissues may be limited, which would suggest that it may not serve as a vascular marker for a common mucosal immune system. It is equally apparent that T lymphocytes trafficking to nonintestinal sites of mucosal infection utilize homing signals that are shared at systemic sites of inflammation. Thus, the rules established for B-cell trafficking within the common mucosal immune system may not be strictly applicable to T cells migrating to mucosal sites of bacterial infection.
ACKNOWLEDGMENTS
This study was partially supported by institutional research support from PHS grants AI41231 and RR03034 from the National Institutes of Health.
Special appreciation is extended to Scott Hughes for expert technical assistance, Bob Evans for graphic illustrations, and Mark Jutila for exchange of information and advice.
REFERENCES
- 1.Abreu-Martin M T, Targan S R. Regulation of immune response of the intestinal mucosa. Crit Rev Immunol. 1996;16:277–309. doi: 10.1615/critrevimmunol.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 2.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 3.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy L M, Butcher E C, Kassam N, Mackay C R, Newman W, Ringler D J. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 5.Byrne G I. The host cell, host immune responses, and the intracellular growth of chlamydia. In: Moulder J W, editor. Intracellular parasitism. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 35–49. [Google Scholar]
- 6.Byrne G I. Immunity to Chlamydia. In: Stephens R S, Byrne G I, Christiansen G, Clarke I N, Grayston J T, Rank R G, Ridgway G L, Saikku P, Schachter J, Stamm W E, editors. Chlamydial infections, 1998. Berkeley, Calif: University of California; 1998. pp. 365–374. [Google Scholar]
- 7.Cepek K L, Shaw S K, Parker C M, Russell G J, Morrow J S, Rimm D L, Brenner M B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 8.DeGrendele H C, Estess P, Siegelman M H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 9.Hanninen A, Taylor C, Streeter P R, Stark L S, Sarte J M, Shizuru J A, Simell O, Michie S A. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Investig. 1993;92:2209–2215. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison H R, Lee S M, Lucas D O. Chlamydia trachomatis pneumonitis in the C57BL/KsJ mouse: pathologic and immunologic features. J Lab Clin Med. 1982;100:953–962. [PubMed] [Google Scholar]
- 11.Igietseme J U, Uriri I M, Kumar S N, Ananaba G A, Ojior O O, Momodu I A, Candal D H, Black C M. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S L, Barone K S, Flanagan M P, Michael J G. Activation patterns of murine B cells after oral administration of an encapsulated soluble antigen. Vaccine. 1996;14:42–48. doi: 10.1016/0264-410x(95)00158-w. [DOI] [PubMed] [Google Scholar]
- 13.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoo U Y, Proctor I E, Macpherson A J. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal antigens. J Immunol. 1997;158:3626–3634. [PubMed] [Google Scholar]
- 15.Kilger G, Clements J, Holzmann B. Amino acid residues required for binding of vascular cell adhesion molecule-1 to integrin alpha 4 beta 7. Int Immunol. 1997;9:219–226. doi: 10.1093/intimm/9.2.219. [DOI] [PubMed] [Google Scholar]
- 16.Kraal G, Schornagel K, Streeter P R, Holzmann B, Butcher E C. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147:763–771. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuklin N A, Daheshia M, Marconi P C, Krisky D M, Rouse R J, Glorioso J C, Manican E, Rouse B T. Modulation of mucosal and systemic immunity by enteric administration of nonreplicating herpes simplex virus expressing cytokines. Virology. 1998;240:245–253. doi: 10.1006/viro.1997.8926. [DOI] [PubMed] [Google Scholar]
- 19.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 20.Langermann S, Palaszynski S, Sadziene A, Stover C K, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 21.Leung E, Greene J, Ni J, Raymond L G, Lehnert K, Langley R, Krissansen G W. Cloning of the mucosal addressin MAdCAM-1 from human brain: identification of novel alternatively spliced transcripts. Immunol Cell Biol. 1996;74:490–496. doi: 10.1038/icb.1996.81. [DOI] [PubMed] [Google Scholar]
- 22.Lue C, van den Wall Bake A, Prince S J, Julian B A, Tseng M L, Radl J, Elson C O, Mestecky J. Intraperitoneal immunization of human subjects with tetanus toxoid induces specific antibody-secreting cells in the peritoneal cavity and in the circulation, but fails to elicit a secretory IgA response. Clin Exp Immunol. 1994;96:356–363. doi: 10.1111/j.1365-2249.1994.tb06567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott M R, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 24.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 25.Medaglini D, Pozzi G, King T P, Fischetti V A. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc Natl Acad Sci USA. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 27.Mestecky J, McGhee J R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 28.Mestecky J, Moldoveanu Z, Novak M, Compans R W. Mucosal immunity and strategies for novel microbial vaccines. Acta Paediatr J. 1994;36:537–544. doi: 10.1111/j.1442-200x.1994.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 29.Morrison L A, Da Costa X J, Knipe D M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 30.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newham P, Craig S E, Clark K, Mould A P, Humphries M J. Analysis of ligand-induced and ligand-attenuated epitopes on the leukocyte integrin alpha4betal: VCAM-1, mucosal addressin cell adhesion molecule-1, and fibronectin induce distinct conformational changes. J Immunol. 1998;160:4508–4517. [PubMed] [Google Scholar]
- 32.Ogra P L, Ogra S S. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973;110:1307–1311. [PubMed] [Google Scholar]
- 33.Pal S, Theodor I, Peterson E M, de la Maza L M. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton D L. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985;7:746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- 35.Patton D L, Landers D V, Schachter J. Experimental Chlamydia trachomatis salpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J Infect Dis. 1989;159:1105–1110. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- 36.Patton D L, Rank R G. Animal models for the study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–111. [Google Scholar]
- 37.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 38.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 39.Sampaio S O, Li X, Takeuchi M, Mei C, Francke U, Butcher E C, Briskin M J. Organization, regulatory sequences, and alternatively spliced transcripts of the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) gene. J Immunol. 1995;155:2477–2486. [PubMed] [Google Scholar]
- 40.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 41.Steffen B J, Breier G, Butcher E C, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- 42.Streeter P R, Berg E L, Rouse B T, Bargatze R F, Butcher E C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 43.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor H R, Johnson S L, Prendergast R A, Schachter J, Dawson C R, Silverstein A M. An animal model of trachoma. II. The importance of repeated reinfection. Investig Ophthalmol Vis Sci. 1982;23:507–515. [PubMed] [Google Scholar]
- 45.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 46.Whittum-Hudson J A, Ann L L, Saltzman W M, Prendergast R A, MacDonald A B. Oral immunization with an anti-idiotypic antibody to the exoglycolipid antigen protects against experimental Chlamydia trachomatis infection. Nat Med. 1996;2:1116–1121. doi: 10.1038/nm1096-1116. [DOI] [PubMed] [Google Scholar]
- 47.Whittum-Hudson J A, O'Brien T P, Prendergast R A. Murine model of ocular infection by a human biovar of Chlamydia trachomatis. Investig Ophthalmol Vis Sci. 1995;36:1976–1987. [PubMed] [Google Scholar]
- 48.Williams D M, Grubbs B G, Pack E, Kelly K, Rank R G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H-Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Brunham R C. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 51.Yang X, Brunham R C. Role of T cell-mediated immunity in host defense against Chlamydia trachomatis and its implication for vaccine development. Can J Infect Dis. 1998;9:99. doi: 10.1155/1998/395297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Cardarelli P M, Lehnert K, Rowland S, Krissansen G W. LPAM-1(integrin alpha 4 beta 7)-ligand binding: overlapping binding sites recognizing VCAM-1, MAdCAM-1 and CS-1 are blocked by fibrinogen, a fibronectin-like polymer and RGD-like cyclic peptides. Eur J Immunol. 1998;28:995–1004. doi: 10.1002/(SICI)1521-4141(199803)28:03<995::AID-IMMU995>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y P, Loosmore S M, Underdown B J, Klein M H. Nasopharyngeal colonization with nontypeable Haemophilus influenzae in chinchillas. Infect Immun. 1998;66:1973–1980. doi: 10.1128/iai.66.5.1973-1980.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]