Abstract
High copy number, damage prone, and lean on repair mechanisms are unique features of mitochondrial DNA (mtDNA) that are hard to reconcile with its essentiality for oxidative phosphorylation, the primary function ascribed to this maternally inherited component of our genome. We propose that mtDNA is also a genotoxic stress sentinel, as well as a direct second messenger of this type of cellular stress. Here, we discuss existing evidence for this sentinel/effector role through the ability of mtDNA to escape the confines of the mitochondrial matrix and activate nuclear DNA damage/repair responses via interferon-stimulated gene products and other downstream effectors. However, this arrangement may come at a cost, leading to cancer chemoresistance and contributing to inflammation, disease pathology, and aging.
Beyond OXPHOS, a stress signaling role for mtDNA
Since its discovery in the early 1960s, mtDNA has fascinated biologists. In vertebrates, including humans, the ~16.5 kb, circular mtDNA is maternally inherited and provides the wiring diagram for the oxidation phosphorylation (OXPHOS) system by encoding 11 essential protein subunits of the mitochondrial electron transport chain and two subunits of the ATP synthase, as well as 22 tRNAs and two rRNAs needed for mRNA translation in the mitochondrial matrix. The remaining >1000 mitochondrial proteins are encoded by nuclear DNA (nDNA) and targeted to or imported into the organelle, including the multitude of factors needed for mtDNA replication, repair, and gene expression [1]. Thus, there is a complex relationship between mitochondria and the nucleus that is orchestrated by signaling pathways between these organelles, including retrograde signaling (see Glossary) that relays mitochondrial stress to the nucleus to promote gene expression changes to maintain mitochondrial homeostasis [2]. Herein, we discuss mtDNA as a genotoxic stress sentinel and second messenger that relays retrograde signals to the nucleus to increase expression of genes required for enhanced nDNA repair.
Properties of mtDNA consistent with a genotoxic stress sentinel function
High copy number
Unlike nDNA, which is organized as pairs of homologous chromosomes in most cells, mtDNA is present at many (usually thousands) copies/cell. Each cell type has a characteristic mtDNA copy number [3,4], which appears to be maintained within a narrow range under basal conditions by cellular and mitochondrial deoxynucleoside triphosphate (dNTP) pools [5–7], the expression level/activity of factors required for mtDNA packaging and replication [8,9], and probably other regulatory pathways [10]. The selective pressure for maintaining a high copy number of mtDNA remains unclear, but has been speculated to ensure optimal segregation during cell and mitochondrial division [11,12] or as a crude mechanism to provide high gene dosage of rDNA for mitochondrial ribosome biogenesis in the absence of more sophisticated transcriptional regulatory mechanisms [13]. There may also be cells and tissues where mtDNA copy number is limiting for the activity of certain OXPHOS complexes [14]. However, these explanations do not account for the fact that the amount of mtDNA appears to be in excess of that needed to maintain OXPHOS capacity. This is perhaps best exemplified by: (i) the ‘threshold effect’ observed with inherited pathogenic mtDNA mutations, where the percentage of mutant mtDNA must reach very high levels before major cell and tissue dysfunction manifests to promote disease pathology [15]; and (ii) mice heterozygous for the TFAM gene (Tfam+/–), which have 50–70% depletion of mtDNA in tissues [16] and yet are alive and have seemingly normal lifespans under typical laboratory conditions. Thus, we hypothesize that an alternative reason for high mtDNA copy number is to allow mtDNA to act as a genotoxic stress sensor.
The high copy number of mtDNA could enable a sensor function in several ways. First, the many copies make it unlikely that every molecule can be damaged or mutated to an extent to overcome the threshold effect. Second, damaged and dysfunctional mtDNA can be repaired in some cases (see later) or degraded by mitochondrial nucleases [17,18]. Third, the ability of mitochondria to fuse and divide provides a mechanism for genetic (i.e., trans) complementation or, if mtDNA damage is extensive, to segregate out dysfunctional mitochondria (and the damaged mtDNA they contain) for removal by mitophagy or bulk autophagy [19]. Finally, new mitochondrial biogenesis can restore the network to reestablish mtDNA copy number to the original set point [20]. Thus, unlike nDNA, which has efficient DNA repair as its primary option to remove damage, mitochondria have a highly redundant and dynamic system to cope with mtDNA damage and restore the network after stress.
Fewer repair systems
Almost 50 years ago, it was determined that UV-induced pyrimidine dimers in mtDNA cannot be repaired in mammalian cells [21], the first demonstration of the now generally accepted lack of a canonical nucleotide excision repair pathway in mitochondria [22]. While there are reports of limited direct reversal, mismatch, and double-strand break repair activities in mammalian mitochondria, the repertoire of DNA repair activities in the organelle is limited compared with that for nDNA [22]. However, mitochondria do have a robust base excision repair (BER) pathway for removal of oxidative damage that utilizes mitochondria-localized versions of many of the same BER enzymes in the nucleus [22]. These mitochondrial isoforms are generated by alternative splicing or other mechanisms [23]. BER is clearly needed to cope with the highly oxidative environment in the mitochondrial matrix where mtDNA resides. Perhaps the high copy number and other considerations relayed in the last section explain why evolution has not selected for additional or more robust DNA repair pathways in mitochondria, but we propose that this arrangement is in line with a genotoxic stress sentinel function for mtDNA (i.e., by allowing mtDNA damage to occur more readily and persist longer to act as a stress signal). In fact, even oxidative damage may occur more readily and be repaired more slowly in mtDNA than nDNA, despite active mitochondrial BER pathways [24–26].
Prone to damage
In addition to the obvious liabilities stemming from the paucity of DNA repair mechanisms, mtDNA is also intrinsically more susceptible to damage than nDNA because of how it is replicated and packaged. A prominent mode of mtDNA replication is via an asymmetric, strand-displacement or displacement-loop (D-loop) mechanism [27]. In this mode, the displaced strand (at times up to 2/3 full-genome length) spends a significant amount of time single-stranded and hence more prone to damage and breakage [28]. Furthermore, leading-strand (also called the heavy or H-strand) DNA synthesis is frequently arrested at specific sites downstream and the nascent H-strand, forming the 7S DNA (ranging from 500 to 615 nucleotides in mouse and human cells [29]) that remains bound to the template strand, forming a stable three-stranded, D-loop structure [30]. In this state, again the displaced (non-template) strand is single-stranded and prone to damage. Finally, mtDNA is packaged in protein-nucleic complexes called nucleoids [31], which is facilitated by TFAM, the abundant HMG-box protein and transcriptional activator in mitochondria [32]. While this packaging helps protect mtDNA from damage to a certain extent [33], the amount of protection is likely much less than that afforded to nDNA via histones and higher-order chromatin structures. Again, we propose these properties are consistent with a genotoxic stress sentinel function by allowing mtDNA to be significantly more prone to damage than nDNA.
mtDNA as an intracellular second messenger of genotoxic stress
Having argued earlier that mtDNA has properties consistent with a sentinel function, we now turn to the critical point of how it might relay signals of genotoxic stress to serve a prophylactic or protective role for the cell. Later, we discuss how mtDNA might be a direct second messenger of genotoxic stress via its ability to be released into the cytoplasm and enact specific signaling responses. We start with the now well-established premise that mtDNA does indeed get released into the cytoplasm of cells. There have been several mechanisms proposed for how this happens (Box 1), but this is an active area of research and likely multiple routes exist that are cell type-and/or context-dependent. From this starting point, we also discuss how cytoplasmic mtDNA signals. Given the space limits for this article, we focus primarily on the cyclic GMP-AMP synthase (cGAS)-STING (stimulator of interferon genes) innate immune signaling pathway and how it relates to the proposed sentinel function of mtDNA, but mtDNA also activates Toll-like receptor (TLR)9, inflammasomes (most notably Nlrp3), and other cytoplasmic nucleic acid sensors (e.g., Zbp1) [25,34,35].
Box 1. Multiple routes to mitochondrial DNA (mtDNA) release into the cytoplasm.
A key underlying premise to our proposal that mtDNA acts directly as a signaling second messenger of genotoxic stress is its ability to be released (or escape) from mitochondria and enter the cytoplasm. Early reports [74–76] indicated that fragments of mtDNA can be released from mitochondria in a manner requiring the mitochondrial permeability transition pore (mPTP), a calcium- and oxidant-regulated pore in the inner mitochondrial membrane, the precise composition of which is still not fully resolved, that is inhibited by cyclosporin A (CsA) [77]. More recently, these observations were apparently linked to mtDNA binding directly to voltage-dependent anion channel 1 (VDAC1) in the outer mitochondrial membrane, which facilitates release of mtDNA fragments in a CsA-, calcium-, and reactive oxygen species (ROS)-dependent fashion [78], and in response to mitochondrial localization of TDP-43, a protein involved in amyotrophic lateral sclerosis [79]. How mtDNA release via the mPTP is linked mechanistically to VDAC1 and whether this is always the case, or if other pores in the outer membrane can also be involved, remains unclear. Interestingly, VDAC1 was originally postulated to be a physical component of the mPTP, but subsequently shown not to be required for this activity [77]. With regard to the involvement of additional pores in mtDNA release, at least two others appear to be active in different contexts. First, during apoptosis, BAX/BAK oligomerization forms macropores that allow the inner mitochondrial membrane to extrude (i.e., herniate) into the cytoplasm to release mtDNA [12,80]. In contrast to VDAC1-mediated release, BAX/BAK-induced mtDNA release is independent of mPTP opening. BAK/BAX pores also appear to mediate some mtDNA release in response to influenza A virus that is dependent on calcium, but not ROS [39]. Interestingly, mtDNA release in response to dengue virus infection is both mPTP- and ROS-dependent [81]. Second, internalization of lipopolysaccharides in endothelial cells induces caspase11-dependent cleavage of Gasdermin D, N-terminal fragments of which then localize to mitochondria and form pores through which mtDNA is released [82]. Finally, NLRP3 inflammasome activation can also instigate mtDNA release by an unknown mechanism [83] and, downstream of this or TLR activation in macrophages, oxidized mtDNA is released into the cytoplasm, where it binds NLRP3 as an effector [35,84]. From these examples, which are not exhaustive, we conclude that there are multiple mechanisms through which mtDNA can be released into the cytoplasm and signal, and that multiple downstream and upstream effectors allow mtDNA-sentinel signaling to be cell type- and context-specific (e.g., differentially affected by specific cellular stress conditions or pathogens).
Activation of cGAS-STING innate immune signaling by cytoplasmic mtDNA
In a seminal triad of reports, we and others demonstrated that, when released into the cytoplasm, mtDNA activates the cGAS-STING innate immune signaling pathway [36–38]. Two of these studies showed that the lack of caspase-mediated cell death unveiled mtDNA-mediated type I interferon (IFN) signaling, demonstrating that one role of apoptosis is to prevent proinflammatory innate immune signaling [36,38]. The third showed that mtDNA stress alone (e.g., in Tfam+/– cells) results in a basal increase in mtDNA release, cGAS-STING activation, and enhanced viral resistance [37], a phenotype shared by activation of the mtDNA-cGAS-STING pathway by knockout of caspase 3 or caspase 9 in endothelial and hematopoietic cells [36].
Activation of cGAS-STING by cytoplasmic mtDNA has now been observed under many other physiological and pathological conditions and in response to certain bacterial and viral infections [34,35,39]. But what is the relationship of these observations to the proposed sentinel role of mtDNA? We argue a major new insight comes from our more recent analysis of mtDNA stress-mediated cGAS-STING activation, where we showed that chronic Tfam-deficiency elicits a cGAS-STING response that differs from a typical antiviral response [40] (Figure 1). That is, these conditions elicit enhanced basal expression of a subset of IFN-stimulated genes (ISGs), but not induction of IFN genes themselves or activation of NF-κB typical of activation of cGAS-STING by viral DNA. This ISG subset overlaps significantly with that termed the IFN-related DNA-damage resistance signature (IRDS), associated with resistance of cancer cells to DNA damage induced by chemotherapy and radiation [41]. Highlighting the relevance of this correlation, we showed that TFAM-depleted cells exhibit resistance to DNA-damaging agents by enhancing nDNA damage and repair responses and that mtDNA damage alone can elicit this response [40]. Thus, mtDNA damage and stress can elicit a protective response to preserve nDNA integrity, entirely consistent with the proposed genotoxic stress sentinel/effector role.
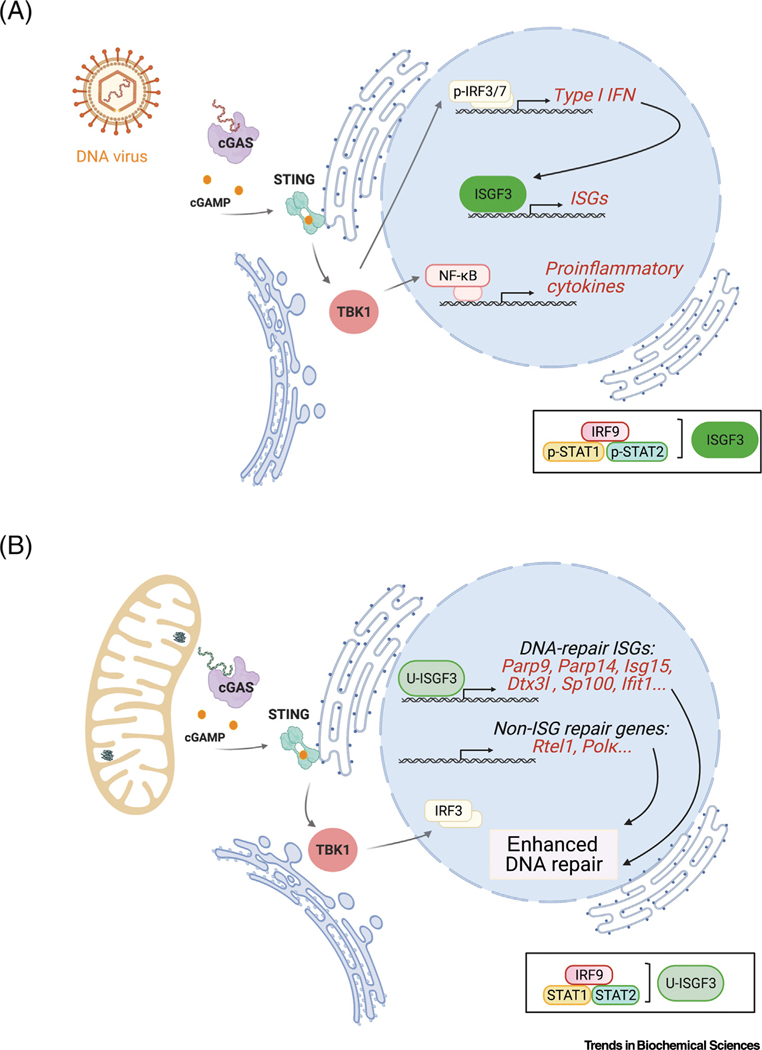
Figure 1. DNA-mediated innate immune and mtDNA-mediated genotoxic stress signaling.
(A) Upon DNA virus infection, the cellular DNA sensor cyclic GMP-AMP synthase (cGAS) (purple) binds the viral DNA and produces the small cyclic dinucleotide, 2′−3′-cyclic GMP-AMP (cGAMP) (small circle). Stimulator of interferon genes (STING) [shown at the endoplasmic reticulum (ER)] binds cGAMP and subsequently activates TBK1 (at the ER and Golgi intermediate compartment), resulting in the nuclear localization of IRF3, IRF7, and NF- B to activate transcription of type I interferon (IFN) and proinflammatory cytokines. Activation of type I IFN signaling leads to the formation of the ISGF3 transcription factor complex (green), composed of phosphorylated STAT1 (p-STAT1), phosphorylated STAT2 (p-STAT2), and IRF9 (see box below blue nucleus). ISGF3 induces expression of many IFN-stimulated genes (ISGs) as part of the antiviral response.
(B) Similar to viral DNA, mtDNA released into the cytoplasm binds cGAS and activates STING and TBK1. However, in Tfam+/– cells, where this pathway was unveiled, this instead leads to IRF3-dependent formation of the U-ISGF3 complex (composed of unphosphorylated STAT1, STAT2, and IRF9, see box below blue nucleus), which drives expression of a unique subset of ISGs (e.g., Parp9, Parp14, Isg15, Dtx3l, sp100, and Ifit1) that enhance nDNA repair. Other DNA repair-associated genes (e.g., Rtel1 and Pol ) are also upregulated in Tfam+/– cells, that we propose, along with the DNA repair-associated ISGs, form an ‘mtDNA damage response regulon’ that enhances nDNA repair as part of the genotoxic stress sentinel function of mtDNA.
ISGs in nDNA damage signaling and repair
Discovery of the IRDS led Minn and colleagues to assess the role of specific ISGs in promoting resistance to DNA-damaging agents in cancer cells. They showed that ISG15 and IFIT1 each contributed to the overall resistance observed, but clearly the phenotype must require cooperative actions of several ISGs [41]. The requirement of ISG15 is explained by its multiple roles in the cellular responses to genotoxic stress as a ubiquitin-like modifier (via ISGylation of proteins) and beyond [42]. This led us to also query the other ISGs induced by mtDNA stress [37] for those with potential connections to nDNA repair pathways (Table 1). In addition to ISG15, which is also upregulated in Tfam+/– cells, three poly(ADP-ribose) polymerase (PARP) family members (PARP9, 12, and 14) initially caught our eye, given the clear connection of PARP1 and 2 to nDNA repair and as anticancer therapy targets [43]. PARP9 has a role in chromatin remodeling that, along with its closely linked and coexpressed partner [44] DTX3L (an E3 ubiquitin ligase), facilitates PARP1 and BRCA function during nDNA double-strand break repair [45–47]. Similar to the results with ISG15 and IFIT1 in cancer cells, we showed that PARP9 is partially responsible for the enhanced nDNA damage resistance imparted by mtDNA stress [40]. PARP14 has reported roles in homologous recombination (HR) and its deletion results in hypersensitivity to nDNA-damaging agents and is synthetic lethal with loss of the ATR-CHK1 DNA damage response pathway [48,49]. SP100 is an ISG that regulates promyelocytic leukemia protein (PML) nuclear body (NB) formation and nDNA repair [50,51]. Both viral infection and genotoxic stress stimulate the formation of PML-NBs, many of which localize closely to DNA damage sites [52,53]. Moreover, crucial DNA repair factors (e.g., MRE11, RAD50, ATR, and CHK2) reside in PML-NBs, perturbations of which contribute to defective HR DNA repair and exacerbated DNA damage [52,54–56]. Thus, we conclude that these PARPs and other ISGs activated by mtDNA release with known or proposed roles in nDNA and mtDNA repair (Table 1) together constitute an effector regulon downstream of the sentinel function of mtDNA to protect the genome (Figure 1). A similar ISG-mediated effect may underlie the reported ability of IFN to enhance nDNA repair responses in macrophages in a STING-dependent fashion [57].
Table 1.
Mitochondrial DNA (mtDNA) stress-activated DNA repair factors
| Protein | ISG | Known or postulated DNA repair function | Refs |
|---|---|---|---|
| ATM | Ya | DNA-damage and mitochondrial stress signaling kinase | [85,86] |
| BRCA2 | Y | DNA double-strand break repair via HR and other functions needed for genome stability | [87] |
| CMPK2 | Y | Nucleotides for mtDNA repair/replication | [84] |
| DNA2 | Y | DNA-dependent ATPase and structure-specific nuclease/helicase with multiple roles in nDNA and mtDNA repair/replication | [88] |
| DTX3L | Y | Ubiquitin ligase involved in chromatin remodeling at DNA damage sites; works with PARP9 | [40,45] |
| EIF2AK2 | Y | IFN-inducible PKR | [89] |
| IFIT1 | Y | Chemoresistance/IRDS | [41] |
| ISG15 | Y | Post-translational modification of multiple DNA damage response, repair, and replication factors | [42] |
| PARP9 | Y | PARP family member involved in chromatin remodeling at DNA damage sites; works with DTX3L | [40,45] |
| PARP14 | Y | PARP family member that promotes HR and protects from DNA replication stress | [48,49] |
| POLK | N | Error-prone DNA lesion bypass | See main text |
| RTEL1 | N | Telomere maintenance and HR | See main text |
| SP100 | Y | PML nuclear bodies, genomic stability, and telomeres | See main text |
| TONSL | Y | HR replication stress recovery | [90] |
| UBA7 | Y | ISG15 E1 enzyme | [42] |
| UBE2L6 | Y | Ubiquitin and ISG15 E2 enzyme involved in chromatin remodeling in response to UV damage | [42,91] |
Abbreviations: PKR, protein kinase R; N, no; Y, yes.
We showed that the enhanced resistance to genotoxic stress in Tfam+/– cells is largely STAT1-dependent [40], which is consistent with ISGs being the primary drivers of this phenotype.
However, we also observed upregulation of nDNA repair proteins that have not been classified as ISGs [37] (Table 1 and Figure 1). For example, RTEL1 plays a crucial role in the maintenance of telomeres and prevents aberrant HR to promote genome stability [58,59], and POLκ is an error-prone DNA polymerase that aids in the continuation of nDNA replication in the presence of DNA lesions [60,61]. Thus, non-ISGs upregulated in response to mtDNA stress may also be part of the mtDNA stress sentinel regulon (Figure 1).
Distracting nuclear cGAS?
In a manner that requires enzymatic 2′−3′-cyclic GMP-AMP (cGAMP) production, cGAS inhibits nDNA repair by binding chromatin and blocking the role of PARP1 in HR [62]. At first glance, this seems contradictory to released mtDNA, which activates cGAS, acting as a sentinel to protect nDNA. However, this function requires direct actions of cGAS in the nucleus, localization of which is regulated by tyrosine phosphorylation by B-lymphoid tyrosine kinase 1 (TBK1). Thus, it is possible that mtDNA release leads to exclusion of cGAS from the nucleus to allow it to bind and respond to mtDNA stress in the cytoplasm, thus preventing its inhibition of nDNA repair. Consistent with this hypothesis, West et al. [37] observed less nuclear localization and more association of cGAS with mtDNA nucleoids in Tfam+/– cells undergoing mtDNA stress.
Role of Nlrp3 and other cytokines
As already mentioned, cytoplasmic mtDNA also activates the Nlrp3 inflammasome, with an apparent preference for oxidized mtDNA. This pathway leads to production of interleukin (IL)-1β and IL-18, but whether this also leads to enhanced nDNA damage and repair responses remains to be determined. However, in this regard, it is noteworthy that IL-18 provides resistance to UV damage in keratinocytes, probably by enhancing a nucleotide excision repair pathway [63] and IL-1β induces cGAS-STING signaling via mtDNA release in myeloid, fibroblast, and epithelial cells [64]. Thus, activation of Nlrp3 could enact similar nDNA protection responses via ISGs, as described earlier. Finally, although IL-1α is not activated by inflammasomes and has no known connection to mtDNA-mediated innate immune signaling, it is related to IL-1β and activates the same plasma membrane receptor. Interestingly, Cohen et al. [65] reported that IL-1α can bind to sites of nDNA damage before being secreted and hence provide a genotoxic stress signaling role. It is also possible that IL-1α is sensitive to mtDNA signals in keratinocytes [66]. This begs the question of whether IL-1β or other cytokines downstream of mtDNA release play a similar role in sensing and relaying mtDNA or nDNA stress as part of the mtDNA sentinel regulon.
Liabilities to the mtDNA-sentinel function
The benefits of the mtDNA sentinel role are obvious: early detection of genotoxic stress to elicit warning signals to protect nDNA and enhanced resistance to certain infections. In addition, that a sentinel role for mtDNA may extend to cell–cell communication of ‘alarm’ signals during lung injury has been postulated [67,68]. However, despite the clear advantages of such a system, this situation may have unintended negative consequences as well. For example, like nDNA, mtDNA is a direct target of chemotherapeutic drugs and radiation. In fact, as already discussed, mtDNA is likely even more prone to these due to fewer DNA repair mechanisms. Thus, these cancer interventions may prompt the sentinel function of mtDNA to promote chemoresistance by enhancing nDNA repair capacity in tumor cells [40]. The mtDNA-sentinel function may also be an example of antagonistic pleiotropy. That is, while this function is beneficial when organisms are younger by providing resistance to stress and infection, it becomes a liability with aging when repair systems break down further, allowing mtDNA to accumulate damage and mutations that contribute to age-related cell and tissue dysfunction and chronic inflammation. How mtDNA-mediated inflammatory signaling leads to pathology has been reviewed by us and others [35,67].
Additional modes of mtDNA–nDNA crosstalk
It is important to point out that not all forms of mtDNA stress lead to enhanced nDNA repair or beneficial outcomes. For example, cells with aberrantly increased mtDNA replication rates can deplete cellular dNTP pools, leading to nDNA replication stress and instability [69]. In addition, cells exposed to mitochondrial singlet-oxygen stress induce mitochondrial reactive oxygen species (ROS)-mediated double-strand breaks in telomeres [70]. There is also signaling of nDNA status to mitochondria. For example, the telomere shelterin complex protein, Tin2, localizes to mitochondria, where it regulates OXPHOS and ROS production [71], suggesting signaling of telomere status to mitochondria. In addition, nDNA damage/repair responses, through activation of PARP enzymes, can deplete cellular NAD+ and acetyl-CoA pools that can influence mitochondrial metabolism [72]. Finally, it has recently been suggested that nDNA damage synergizes with double-strand mtDNA breaks to fully activate type I IFN responses to cellular genotoxic stress [73]. Interestingly, in this case, mtDNA double-strand breaks lead to the release of mtRNA into the cytoplasm that activates type I IFN signaling, indicating that the mtDNA-sentinel function can use both mtDNA and mtRNA as second messengers. Clearly, there remains much more to learn about pathways that connect mtDNA and nDNA stress.
Concluding remarks
We propose that mtDNA has evolved multiple, unique properties that allow it to serve as a cellular sentinel of genotoxic stress. In some contexts, its enhanced susceptibility to damage may promote cell death to avoid inflammation and other deleterious consequences. However, we argue herein that some forms of mtDNA stress result in its release into the cytoplasm to promote cell survival and other beneficial adaptive responses. In particular, we highlight cell-intrinsic signaling pathways that upregulate ISGs and other factors that can enhance nDNA repair to protect the genome of a cell. However, this sentinel function may complicate cancer treatments and/or ultimately prove to be a liability that contributes to pathology and aging. Last, we raise some key outstanding questions regarding the proposed sentinel function of mtDNA that will be exciting to address going forward (see Outstanding questions).
Outstanding questions.
How many mitochondrial DNA (mtDNA) release mechanisms are there and how does the exact nature of the released mtDNA (e.g., types of damage or specific sequences) determine signaling outcomes?
Does the mtDNA copy number, packaging (nucleoid) state, or repair capacity (e.g., expression of specific mtDNA repair factors) of specific cell types allow them to act as sentinel cells?
Are other cytokines downstream of mtDNA release (e.g., IL-1β) part of the mtDNA sentinel regulon and is there co-operation between the many pathways activated by cytoplasmic mtDNA to preserve nDNA integrity?
Can extracellular and circulating cell-free mtDNA communicate genotoxic stress to neighboring cells and systemically, respectively?
Is the transfer of mitochondria between cells a response to mtDNA damage in the recipient cell that is signaled through the mtDNA sentinel function?
Does the sentinel function of mtDNA become a liability with aging that causes mitochondrial dysfunction and chronic inflammation?
Can blocking mtDNA release or sentinel signaling make cancer cells more susceptible to chemotherapy or radiation and reduce chemoresistance?
Is mtDNA-mediated DNA-damage signaling involved in other forms of drug resistance?
Highlights.
Mitochondrial DNA (mtDNA) is present at high cellular copy number and more prone to damage than nuclear DNA (nDNA), characteristics consistent with a genotoxic stress sentinel function.
mtDNA can be released from mitochondria into the cytoplasm and can therefore act as a second messenger signaling molecule.
Cytoplasmic mtDNA engages innate immune nucleic acid sensors and upregulates interferon-stimulated (and other) genes that have roles in nuclear DNA repair.
While clearly beneficial in terms of adaptive stress responses and genome stability, an mtDNA genotoxic stress sentinel function may contribute to cancer chemoresistance, chronic inflammation, and aging.
Acknowledgments
The authors wish to acknowledge the following funding sources: National Institutes of Health (NIH) R01 AR069876 and the Audrey Geisel Chair in Biomedical Science to G.S.S., NIH F31 AG062099 to A.G.S., and support from the Chinese Scholarship Counsel to Z.W.
Glossary
- Base excision repair (BER)
a DNA repair mechanism involving the excision of oxidized bases (and other types of damaged bases) that do not distort the DNA helix to a large extent from DNA
- cGAMP
(2′−3′-cyclic GMP-AMP) an unusual cyclic dinucleotide produced by cGAS that acts as a second messenger to activate innate immune signaling by binding to STING
- cGAS
(cyclic GMP-AMP synthase) an innate immune nucleic acid sensor that primarily binds DNA and produces the cyclic dinucleotide cGAMP
- Genotoxic stress
instability of DNA due to exposure to toxic endogenous or environmental agents
- Homologous recombination (HR)
exchange between two similar or identical DNA strands, which is one mechanism to repair double-strand DNA breaks
- Inflammasomes
cytoplasmic multiprotein complexes of the innate immune system that promote the production of mature interleukin (IL)-1β and IL-18
- Nucleotide excision repair
a DNA repair pathway for removal of bulky, helix-distorting lesions
- Retrograde signaling
the transmission of signals from mitochondria to the nucleus to modulate expression of nuclear genes required to maintain mitochondrial homeostasis or change mitochondrial biogenesis and/or function in response to physiological cues or stress conditions
- STING
(stimulator of interferon genes) an endoplasmic reticulum-associated protein of the innate immune system that binds to cyclic dinucleotides, such as cGAMP, to mediate the production of type I interferons and proinflammatory cytokines
Footnotes
Declaration of interests
No interests are declared.
References
- 1.Gustafsson CM et al. (2016) Maintenance and expression of mammalian mitochondrial DNA. Annu. Rev. Biochem 85, 133–160 [DOI] [PubMed] [Google Scholar]
- 2.Quiros PM et al. (2016) Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol 17, 213–226 [DOI] [PubMed] [Google Scholar]
- 3.Moraes CT (2001) What regulates mitochondrial DNA copy number in animal cells? Trends Genet. 17, 199–205 [DOI] [PubMed] [Google Scholar]
- 4.Veltri KL et al. (1990) Distinct genomic copy number in mitochondria of different mammalian organs. J. Cell. Physiol 143, 160–164 [DOI] [PubMed] [Google Scholar]
- 5.Rotig A.and Poulton J.(2009) Genetic causes of mitochondrial DNA depletion in humans. Biochim. Biophys. Acta 1792, 1103–1108 [DOI] [PubMed] [Google Scholar]
- 6.Tang Y.et al. (2000) Rearrangements of human mitochondrial DNA (mtDNA): new insights into the regulation of mtDNA copy number and gene expression. Mol. Biol. Cell 11, 1471–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor SD et al. (2005) The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell 16, 3010–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekstrand MI et al. (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet 13, 935–944 [DOI] [PubMed] [Google Scholar]
- 9.Tyynismaa H.et al. (2004) Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum. Mol. Genet 13, 3219–3227 [DOI] [PubMed] [Google Scholar]
- 10.Fukuoh A.et al. (2014) Screen for mitochondrial DNA copy number maintenance genes reveals essential role for ATP synthase. Mol. Syst. Biol 10, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebedeva MA and Shadel GS (2007) Cell cycle- and ribonucleotide reductase-driven changes in mtDNA copy number influence mtDNA Inheritance without compromising mitochondrial gene expression. Cell Cycle 6, 2048–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McArthur K.et al. (2018) BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 [DOI] [PubMed] [Google Scholar]
- 13.Bendich AJ (1987) Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays 6, 279–282 [DOI] [PubMed] [Google Scholar]
- 14.Van den Bogert C.et al. (1993) Regulation of the expression of mitochondrial proteins: relationship between mtDNA copy number and cytochrome-c oxidase activity in human cells and tissues. Biochim. Biophys. Acta 1144, 177–183 [DOI] [PubMed] [Google Scholar]
- 15.Rossignol R.et al. (2003) Mitochondrial threshold effects. Biochem. J 370, 751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson NG et al. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet 18, 231–236 [DOI] [PubMed] [Google Scholar]
- 17.Bayona-Bafaluy MP et al. (2005) Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc. Natl. Acad. Sci. U. S. A 102, 14392–14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C.et al. (2018) Structural insights into DNA degradation by human mitochondrial nuclease MGME1. Nucleic Acids Res. 46, 11075–11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra P.and Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol 15, 634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickles S.et al. (2018) Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol 28, R170–R185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton DA et al. (1974) The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc. Natl. Acad. Sci. U. S. A 71, 2777–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheibye-Knudsen M.et al. (2015) Protecting the mitochondrial powerhouse. Trends Cell Biol. 25, 158–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang D.and Hamasaki N.(2002) Maintenance of mitochondrial DNA integrity: repair and degradation. Curr. Genet 41, 311–322 [DOI] [PubMed] [Google Scholar]
- 24.Ballinger SW et al. (2000) Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ. Res 86, 960–966 [DOI] [PubMed] [Google Scholar]
- 25.Szczesny B.et al. (2018) Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci. Rep 8, 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yakes FM and Van Houten B.(1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. U. S. A 94, 514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton DA (2003) Mitochondrial DNA replication: what we know. IUBMB Life 55, 213–217 [DOI] [PubMed] [Google Scholar]
- 28.Chan K.et al. (2012) Base damage within single-strand DNA underlies in vivo hypermutability induced by a ubiquitous environmental agent. PLoS Genet. 8, e1003149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillum AM and Clayton DA (1978) Displacement-loop replication initiation sequence in animal mitochondrial DNA exists as a family of discrete lengths. Proc. Natl. Acad. Sci. U. S. A 75, 677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shadel GS and Clayton DA (1997) Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem 66, 409–435 [DOI] [PubMed] [Google Scholar]
- 31.Bogenhagen DF (2012) Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta 1819, 914–920 [DOI] [PubMed] [Google Scholar]
- 32.Bonawitz ND et al. (2006) Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol. Cell 24, 813–825 [DOI] [PubMed] [Google Scholar]
- 33.O’Rourke TW et al. (2002) Mitochondrial dysfunction due to oxidative mitochondrial DNA damage is reduced through cooperative actions of diverse proteins. Mol. Cell. Biol 22, 4086–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley JS and Tait SW (2020) Mitochondrial DNA in inflammation and immunity. EMBO Rep. 21, e49799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West AP and Shadel GS (2017) Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol 17, 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rongvaux A.et al. (2014) Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West AP et al. (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White MJ et al. (2014) Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriyama M.et al. (2019) Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun 10, 4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z.et al. (2019) Mitochondrial DNA stress signalling protects the nuclear genome. Nat. Metab 1, 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weichselbaum RR et al. (2008) An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. U. S. A 105, 18490–18495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandy Z.et al. (2020) More than meets the ISG15: emerging roles in the DNA damage response and beyond. Biomolecules 10, 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azarm K.and Smith S.(2020) Nuclear PARPs and genome integrity. Genes Dev. 34, 285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juszczynski P.et al. (2006) BAL1 and BBAP are regulated by a gamma interferon-responsive bidirectional promoter and are overexpressed in diffuse large B-cell lymphomas with a prominent inflammatory infiltrate. Mol. Cell. Biol 26, 5348–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Q.et al. (2009) BBAP monoubiquitylates histone H4 at lysine 91 and selectively modulates the DNA damage response. Mol. Cell 36, 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Q.et al. (2013) BAL1 and its partner E3 ligase, BBAP, link Poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA repair independent of ATM, MDC1, and RNF8. Mol. Cell. Biol 33, 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang CS et al. (2017) Ubiquitin modification by the E3 ligase/ ADP-ribosyltransferase Dtx3L/Parp9. Mol. Cell 66, 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhoonmoon A.et al. (2020) Genome-wide CRISPR synthetic lethality screen identifies a role for the ADP-ribosyltransferase PARP14 in DNA replication dynamics controlled by ATR. Nucleic Acids Res. 48, 7252–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolae CM et al. (2015) A novel role for the mono-ADP-ribosyltransferase PARP14/ARTD8 in promoting homologous recombination and protecting against replication stress. Nucleic Acids Res. 43, 3143–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang HR et al. (2018) The functional roles of PML nuclear bodies in genome maintenance. Mutat. Res 809, 99–107 [DOI] [PubMed] [Google Scholar]
- 51.Xu P.and Roizman B.(2017) The SP100 component of ND10 enhances accumulation of PML and suppresses replication and the assembly of HSV replication compartments. Proc. Natl. Acad. Sci. U. S. A 114, E3823–E3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu SB et al. (2017) PML silencing inhibits cell proliferation and induces DNA damage in cultured ovarian cancer cells. Biomed. Rep 7, 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vancurova M.et al. (2019) PML nuclear bodies are recruited to persistent DNA damage lesions in an RNF168–53BP1 dependent manner and contribute to DNA repair. DNA Repair (Amst) 78, 114–127 [DOI] [PubMed] [Google Scholar]
- 54.Conlan LA et al. (2004) Proteasome-dependent dispersal of PML nuclear bodies in response to alkylating DNA damage. Oncogene 23, 307–310 [DOI] [PubMed] [Google Scholar]
- 55.di Masi A.et al. (2016) PML nuclear body disruption impairs DNA double-strand break sensing and repair in APL. Cell Death Dis. 7, e2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voisset E.et al. (2018) PML nuclear body disruption cooperates in APL pathogenesis and impairs DNA damage repair pathways in mice. Blood 131, 636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales AJ et al. (2017) A type I IFN-dependent DNA damage response regulates the genetic program and inflammasome activation in macrophages. Elife 6, e24655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barber LJ et al. (2008) RTEL1 maintains genomic stability by suppressing homologous recombination. Cell 135, 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vannier JB et al. (2013) RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science 342, 239–242 [DOI] [PubMed] [Google Scholar]
- 60.Bavoux C.et al. (2005) Adaptation to DNA damage and stimulation of genetic instability: the double-edged sword mammalian DNA polymerase kappa. Biochimie 87, 637–646 [DOI] [PubMed] [Google Scholar]
- 61.Ohashi E.et al. (2000) Fidelity and processivity of DNA synthesis by DNA polymerase kappa, the product of the human DINB1 gene. J. Biol. Chem 275, 39678–39684 [DOI] [PubMed] [Google Scholar]
- 62.Liu H.et al. (2018) Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136 [DOI] [PubMed] [Google Scholar]
- 63.Schwarz A.et al. (2006) IL-18 reduces ultraviolet radiation-induced DNA damage and thereby affects photoimmunosuppression. J. Immunol 176, 2896–2901 [DOI] [PubMed] [Google Scholar]
- 64.Aarreberg LD et al. (2019) Interleukin-1beta induces mtDNA release to activate innate immune signaling via cGAS-STING. Mol. Cell 74, 801–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen I.et al. (2015) IL-1alpha is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Sci. Rep 5, 14756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corsini E.et al. (1999) Sodium arsenate induces overproduction of interleukin-1alpha in murine keratinocytes: role of mitochondria. J. Invest. Dermatol 113, 760–765 [DOI] [PubMed] [Google Scholar]
- 67.Schumacker PT et al. (2014) Mitochondria in lung biology and pathology: more than just a powerhouse. Am. J. Physiol. Lung Cell Mol. Physiol 306, L962–L974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan YB et al. (2017) Pharmacologic protection of mitochondrial DNA integrity may afford a new strategy for suppressing lung ischemia-reperfusion injury. Ann. Am. Thorac Soc 14, S210–S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamalainen RH et al. (2019) Defects in mtDNA replication challenge nuclear genome stability through nucleotide depletion and provide a unifying mechanism for mouse progerias. Nat. Metab 1, 958–965 [DOI] [PubMed] [Google Scholar]
- 70.Qian W.et al. (2019) Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc. Natl. Acad. Sci. U. S. A 116, 18435–18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen LY et al. (2012) Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control. Mol. Cell 47, 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang EF et al. (2016) Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol 17, 308–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tigano M.et al. (2021) Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature 591, 477–481 [DOI] [PubMed] [Google Scholar]
- 74.Garcia N.and Chavez E.(2007) Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci. 81, 1160–1166 [DOI] [PubMed] [Google Scholar]
- 75.Garcia N.et al. (2005) The permeability transition pore as a pathway for the release of mitochondrial DNA. Life Sci. 76, 2873–2880 [DOI] [PubMed] [Google Scholar]
- 76.Patrushev M.et al. (2004) Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell. Mol. Life Sci 61, 3100–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernardi P.et al. (2015) The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev 95, 1111–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J.et al. (2019) VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu CH et al. (2020) TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183, 636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riley JS et al. (2018) Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai JH et al. (2018) Infection with the dengue RNA virus activates TLR9 signaling in human dendritic cells. EMBO Rep. 19, e46182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang LS et al. (2020) mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52, 475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakahira K.et al. (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol 12, 222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhong Z.et al. (2018) New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paull TT (2015) Mechanisms of ATM activation. Annu. Rev. Biochem 84, 711–738 [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y.et al. (2018) Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci. Signal 11, eaaq0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fradet-Turcotte A.et al. (2016) BRCA2 functions: from DNA repair to replication fork stabilization. Endocr. Relat. Cancer 23, T1–T17 [DOI] [PubMed] [Google Scholar]
- 88.Zheng L.et al. (2020) Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases. Nucleic Acids Res. 48, 16–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng X.et al. (2015) PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice. Blood 126, 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donnell L.et al. (2010) The MMS22L-TONSL complex mediates recovery from replication stress and homologous recombination. Mol. Cell 40, 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shibata E.et al. (2011) Selective ubiquitylation of p21 and Cdt1 by UBCH8 and UBE2G ubiquitin-conjugating enzymes via the CRL4Cdt2 ubiquitin ligase complex. Mol. Cell. Biol 31, 3136–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]