Abstract
Background
Osteoporosis (OP) and diabetes mellitus (DM) are two major healthcare issues in the world. Numerous population based-studies have reported an increased prevalence of OP among individuals with DM, though, estimates vary significantly.
Purpose
The objective of this study is to estimate the prevalence of OP in patients with DM.
Methods
To identify relevant literature, PubMed, Embase, Medline, CBM and Cochrane Library were searched for studies published from inception till July 2022, The search was conducted, and studies were included without countries and language restrictions. For full-text articles included in the study, the references were also independently searched. Random inverse variance-weighted models were used by Stata version 17.0 to estimate the prevalence of OP in patients with diabetes across studies. The heterogeneity was examined with I2 via the χ2 test on Cochrane’s Q statistic. Subgroup analysis and meta-regression were used to explore potential sources of heterogeneity. Egger’s test was used to assess publication bias.
Results
A high OP prevalence of 27.67% (95% confidence interval (CI) 21.37-33.98%) was found in a pooled analysis of 21 studies involving 11,603 T2DM patients. Methodological value of the included articles was high, with only three medium-quality studies and no low-quality studies. A significantly high heterogeneity (I2 = 98.5%) was observed.
Conclusions
Worldwide, a high prevalence of OP was found in patients with T2DM. Therefore, strong measures to prevent and treat osteoporosis in diabetic patients are required.
Trial registration
This study has been registered on PROSPERO, number CRD42021286580.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-022-01260-8.
Keywords: Osteoporosis, Diabetes mellitus, Systematic review, Meta-analysis, Observational studies
Introduction
Osteoporosis (OP), one of the most common metabolic skeletal disorders, is characterized by decreased bone mass and increased destruction of bone microstructure, which consequently increases bone fragility and fracture risk [1]. OP tends to occur in older people and individuals with predisposing health condition [2] and has been considered as a serious public health concern attributable to its high morbidity, mortality, and healthcare costs. Some risk factors for OP have been identified, some of which are complex owing to the multiple mechanisms involved, such as diabetes mellitus (DM).
DM has developed into a global health concern that poses a major hazard to human health. And it is associated with an increased risk of fracture, particularly the hip fracture, despite normal or high bone mineral density (BMD). Nowadays, the incidences and prevalence of DM are rising sharply around the world. According to the International Diabetes Federation (IDF), the global prevalence of DM was estimated at 9.3% in 2019, and is expected to increase to 10.2% in 2030 and 10.9% in 2045 [3] Thus emphasizing the need to pay more attention and consideration to this disease.
In the past few years, multiple research has focused on the relationship between type 2 diabetes mellitus (T2DM) and OP [4, 5]. In China, a meta-analysis reported that the prevalence of OP in type 2 diabetics was 44.8 and 37.0% in women and men respectively [6] and a cross-sectional study demonstrated that the prevalence of OP was 5.0 and 20.6% among men and women, respectively in individuals aged over 40 years [7]. Estimates vary significantly.
The prevalence of OP has been assessed in postmenopausal women or elderly men with T2DM in a few regions and countries, However, studies on the prevalence of OP in diabetes patients worldwide have not been well documented. We conducted a systematic review and meta-analysis of the prevalence of OP in patients with DM worldwide. The purpose of this study was to provide clinical guidance for prevention, diagnosis, and control strategies in light of the persistently rising prevalence of OP and DM.
Methods
Searching strategy and selection criteria
The study was conducted in accordance with the systematic review and meta-analysis of observational studies. The prevalence of OP in patients with T1DM and T2DM was estimated. The study has been registered on the International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/PROSPERO/), with registration number CRD 42021286580.
To identify primary studies on the prevalence of OP in patients with DM, two investigators did a comprehensive search of PubMed, Embase, Medline, CBM and Cochrane Library databases. The studies published from database inception to July 2022 without any country and language barrier. Medical subject headings (MESH), keywords, and free words were used in the retrieval strategy including “osteoporosis”, “bone losses”, “post-traumatic osteoporosis”, “senile osteoporosis”, “age-related osteoporosis”, “involutional osteoporosis”, “diabetes mellitus”, “diabetes”, “hyperglycemia”, “observational study”, “cross-sectional studies”, and “cohort studies”. To evaluate further studies, a manual search was done on the reference list of all selected. The retrieval strategy in PubMed is applicable to other databases, as shown in details in Additional file 1 Appendix a.
Observational studies: cross-sectional and cohort studies related to OP prevalence in patients with DM were fetched. OP is defined as follows: a bone mineral density (BMD) T-score < = − 2.5 SD in one or more of the following regions: lumbar spine, femoral neck, or total hip, or T-score < = − 2.5 SD plus a history of fracture. Case-control studies were excluded because they were unable to provide any information about the incidence or prevalence of disease as no measurements are made in a population-based sample. Case series with a small sample size (less than 50 participants), reviews, conference abstracts, and articles without primary data or explicit description of methods, or both, were also excluded. For studies published in more than one report (duplicate), the most comprehensive study with the largest sample capacity and influence was considered. In research abstracts without full text available, relevant data was included in the abstract, an attempt was made to email the first author or corresponding author to obtain the full text for information extraction and quality assessment of the specification. Articles without a response from the author were discarded. For final inclusion, two investigators independently examined the titles and abstracts of studies retrieved through the literature search. The full texts of possibly eligible publications were collected. All duplicate articles were removed during the screening process.
Two investigators, X. Y Liu and F. H Chen screened all potential studies, and verified key data, including all data on OP prevalence in patients with DM. Disagreements were resolved through discussions until a consensus was achieved.
Data extraction
Two investigators, X. Y Liu and F. H Chen, independently retrieved relevant data from separate investigations using a planned and standardized data extraction form. Data information was extracted including the first author’s name, year of publication, country, study design, sample size, OP prevalence in diabetes, measuring site, diagnostic criteria for OP, and contained participants (type of diabetes, age, gender, BMI, and sources of participants). Discrepancies were worked out through discussions until a consensus was reached.
Data processing
The criteria established by Hoy and his teammates in 2012, which consists of ten items, were used to assess the methodological quality of the observational studies [8]. Each item was given a score of 1 (yes) or 0 (no), and the values were added together to obtain an overall quality score that ranged from 0 to 10. The study was divided into three grades based on its total quality scores, with a low (> 8), moderate (6-8), and high (≤ 5) risk of bias respectively [9]. X. Y Liu and F. H Chen independently assessed the methodological quality of the included studies, and disagreements were resolved through discussion.
To summarize the prevalence data, data analysis was conducted by Stata version 17.0 software. In order to minimize the influence of studies with extreme prevalence on the overall estimate, the proportion was first stabilized through the Freeman-Tukey Transformed method before pooling proportion using the random-effect model meta-analysis [9–11]. Heterogeneity was assessed by Cochran’s Q, I2, and H statistics [9]. The percentage of differences across studies to total variation was described by I2, where an I2 value greater than 50% suggests the presence of between-studies heterogeneity [10]. In this study, the pooled OP prevalence in patients with DM was evaluated using a random-effect model with a forest plot with a 95% confidence interval (95%CI). Subgroup analyses were performed to identify the potential heterogeneity between included studies based on age (<= 60 years vs. > 60 years), gender (proportion of female <= 60% vs. > 60%), BMI (obesity vs. non-obesity), study quality (high vs. moderate), published country (China vs. non-China), and DM duration (<= 10 years vs. > 10 years). BMI was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as BMI ≥ 28 kg/m2 in China and ≥ 30 kg/m2 in other countries [12, 13]. Of 21 studies, two were excluded from the analysis because they did not mention BMI [14, 15].
Further, a meta-regression was conducted to test the association of OP prevalence in diabetics based on age, gender (proportion of female), sample size (number of study participants), and published year. These all factors may potentially explain differences in OP prevalence. At last, Egger’s test was used to assess publication bias, with P < 0.10 suggesting significant publication bias [16].
Results
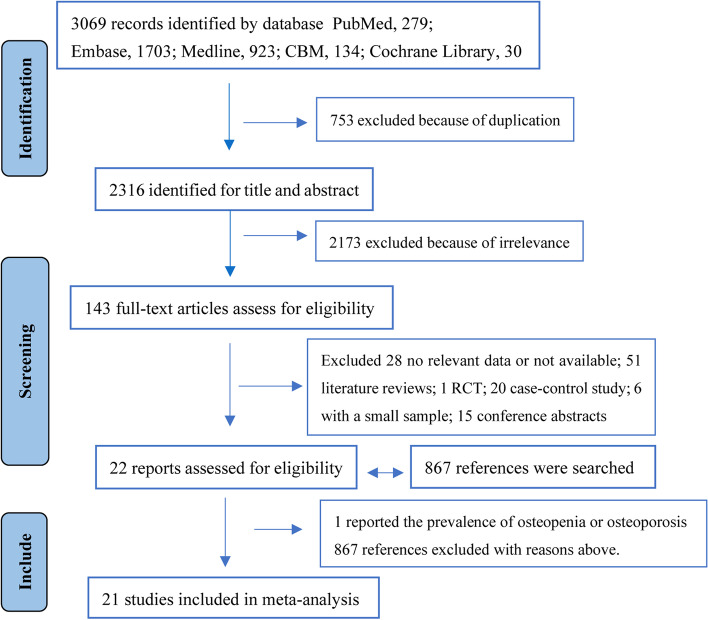
The original search began with 3069 studies, of which 753 were excluded due to redundancy. After preliminary screening of titles and abstracts, 2173 non-relevant articles were excluded. The remaining 143 full-text articles were assessed for eligibility; however, a large fraction was excluded for various reasons. Excluded articles comprised 28 articles with no relevant data or not available, 51 literature reviews, 1 randomized controlled trial (RCT), 20 case-control studies, 6 with a small sample size (less than 50 participants), and 15 conference abstracts. The remaining 22 reports were assessed for eligibility, of which one literature reported the prevalence of osteopenia or osteoporosis. From these reports, 867 references were independently searched; however, all articles were excluded because of no relevant data, reviews, not available, and duplicates. Finally, 21 studies including 11,603 individuals with DM, were considered in the meta-analysis [14, 15, 17–34]. The study selection process has been presented in Fig. 1.
Fig. 1.
Flowchart of study selection process
Table 1 show the characteristics and OP prevalence in DM patients, for all studies the included studies. Of these only one study included both type 1 and type 2 diabetes [35], and the rest of 21 studies focused on type 2 diabetes and were included in the standardized analysis. The prevalence of OP in patients with DM varied from 7.29 to 53.71% across studies. The methodological quality of included studies was high, with only three medium-quality [17, 20, 24] and no low-quality studies (Table 2).
Table 1.
General information and features of the included 22 studies
| Study | Country | Study design | Skeletal site | DC for OP | N | n | Percent (%) | Participants and sources |
|---|---|---|---|---|---|---|---|---|
| Kanazawa,2019 [26] | Japan | cross-sectional study | The thoracic and LS | T score | 309 | 166 | 53.70 | T2DM, 196 male, 113 female; age 65.3 years; BMI 25.2 kg/m2, from hospital; |
| Maíra Viégas,2011 [20] | Maceió | cross-sectional study | LS and FN | T score | 148 | 45 | 30.40 | T2DM, postmenopausal women, age 61.87 ± 7.85 (41–80 years); from outpatient clinic, Hospital |
| Abdulameer,2018 [25] | Malaysia | cross-sectional study | The heel bone | T score and QUS | 450 | 100 | 22.20 | T2DM, 231(51.3%)male, 219(48.7%)female; age 62.67 ± 9.24 years; BMI 26.36 ± 4.39 kg/m2, from the outpatient diabetes clinic, hospital; |
| Maryam Ghodsi,2021 [31] | Iran | cross-sectional study | LS (L2–L4), hip, and FN | T score or Z score | 165 | 23 | 13.92 | T2DM, 63 male,102 female; age, total 50.86 (11.17), male 51.02 (12.85), female 50.76(10.07); BMI 29.40 (4.32) kg/m2, from community. |
| AL-Homood,2017 [23] | Arabia | cross-sectional study | LS (L2–L4) and FN | T score | 170 | 50 | 29.41 | T2DM, females patients; age 56.3 ± 8.5 years; BMI 32.2 ± 6.2 kg/m2; from hospital. |
| Yan Guo,2020 [27] | China | cross-sectional study | The heel bone | T score | 1073 | 434 | 40.45 | T2DM, male 382, female 691(64.4%); age 69.09 ± 6.53; BMI 25.29 ± 3.31; from communities. |
| Afshinnia, 2007 [18] | USA | cross-sectional study | LS (L2–L4), TH | T score | 159 | 54 | 33.96 | T2DM, 26 male, 133 female; age 72.3 ± 10.4 years; BMI 28.5 ± 5.4 kg/m2; from hospital |
| Yaturu, 2009 [19] | USA | cross-sectional study | LS and FN | T score | 550 | 97 | 17.63 | T2DM, 550 male,age 67.5 ± 0.38 (50 to 76) years; BMI 30.08 ± 0.2; from hospital |
| Schwartz, 2005 [14] | USA | cohort study | FN and TH | T score | 480 | 66 | 13.75 | T2DM, 222 (46.25%) female; age 70-90 years; from university |
| Roma’n, 2004 [17] | Spain | cross-sectional study | The calcaneal | T score | 92 | 20 | 21.70 | T2DM, 56 females and 36 males; age 63.3 ± 9.1 years; BMI 29.6 ± 6.1 kg/m2; from a tertiary care hospital. |
| Lingna Fang, 2021 [30] | China | cross-sectional study | LS and hip | T score | 187 | 58 | 31.02 | T2DM, 82 male, 105 female; age male 65.23 ± 9.34, female 65.08 ± 8.28 years; BMI male 24.9 ± 3.99, female 25.61 ± 3.62 kg/m2; from hospital. |
| Jianbo Li, 2014 [22] | China | cross-sectional study | LS (L1–L4) and FN | T or Z score | 258 | 133 | 51.55 | T2DM, postmenopausal women; age non-OP 59.6 (48.7, 65.3), OP 63.3 (50.2, 67.7); BMI non-OP 23.7 (2.2), OP 22.4 (2.7); from hospital. |
| Karimifar, 2012 [21] | Iran | cross sectional study | FN and LS (L2-L4) | T score | 200 | 78 | 39 | T2DM; postmenopausal women; age 66.91 ± 5.78 years; BMI 31.65 ± 4.42 kg/m2; from hospital. |
| Bruckner, 2014 [35] | Germany | cross-sectional study | Self-reported | T score | 398 | T1, 14 T2, 43 | T1, 9.03 T2, 17.7 | 155 T1DM, 243 T2DM; T1DM, 139 (71 male, 68 female), T2DM, 243 (115 male, 128 female) age T2DM male, 62.7 ± 8.5 female, 62.9 ± 8.5 BMI T1D male 25.2 ± 3.3, female 24.6 ± 2.9, T2D male 28.9 ± 4.5,female 29.7 ± 5.3 kg/m2; from hospital. |
| Yufeng Li, 2020 [28] | USA | cross-sectional study | LS (L2-4) | QCT | 629 | 106 | 16.85 | T2DM, male 342, female 287(46%); age 55.12 ± 9.75 years; BMI 27.55 ± 3.87 kg/m2; from communities. |
| Junyan Li, 2021 [32] | China | cross-sectional study | LS (L1–L4) and left hip | T score | 164 | 65 | 39.63 | T2DM, postmenopausal women; age 45-84 years, NBG 55.17 ± 6.59, OAG 62.17 ± 7.71, OPG 65.78 ± 8.1 years; from hospital. |
| Min Qiu, 2020 [29] | China | cross sectional study | LS (L1–L4) and FN | T score | 147 | 26 | 17.69 | T2DM, 84 male, 63 female; age NBG 53.4 ± 3.2, OAG 55.7 ± 4.1 OPG 57.6 ± 5.0 years; BMI NBG 25.3 ± 2.1,OAG 25.0 ± 1.7, OPG 24.9 ± 1.7 kg/m2; from hospital. |
| Shuangling Xiu, 2019 [5] | China | cross-sectional study | LS (2–4), FN, and the hip | T score | 370 | 121 | 32.70 | T2DM, male 176, female 194; age 67.6 ± 5.78 years; BMI 25.81 ± 3.66; from hospital. |
| Xiaojuan Xu,2021 [34] | China | cross-sectional study | LS (L1–L4), FN, and the hip | T score | 933 | 220 | 23.58 | T2DM, 535 male> = 50 years and 398 postmenopausal women); age male 63.0 (59.0, 67.0), female 64.0 (59.0, 70.0); BMI male 24.58 ± 3.29, female 24.58 (22.03, 27.30); from hospital. |
| Yang Wu, 2021 [33] | China | cross-sectional study | LV, FN, TH | T score | 631 | 374 | 59.27A | T2DM, male; age 57.3 ± 12.0 years; BMI 24.9 ± 3.6 kg/m2; from hospital. |
| L. Zhou, 2017 [24] | China | cross-sectional study | LS (L1–L4), proximal femur | T score | 99 | 27 | 27.30 | T2DM, postmenopausal women; age 62 ± 8 years; BMI 27.6 ± 4.2 kg/m2; from hospital. |
| Xueyu Li, 2021 [15] | China | cross-sectional study | Existing database | T score | 4777 | 348 | 7.30 | T2DM, male 52% (2482); age 64.2 ± 14.2 years, from hospital |
Study: the first author‘s name or first name, published year
Continuous variables are expressed as means ± standard deviation or medians with interquartile range
Categorical variables are expressed as numbers with percentages
T1, type 1 diabetes mellitus; T2, type 2 diabetes mellitus; LS, lumbar spine; FN, femoral neck; LV, lumbar vertebra, FN, femoral neck; TH, total hip;
QUS, Quantitative Ultrasound Scan
NBG, normal BMD group; OAG, osteopenia group; OPG, osteoporosis group;
OP, osteoporosis; N, sample size of diabetic patients; n, the number of OP in diabetes; DC, diagnostic criteria; BMI, body mass index
A, the prevalence of osteopenia or osteoporosis
Table 2.
Quality assessment of the 22 included studies
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | TS | QG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kanazawa,2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Maíra Viégas,2011 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | moderate |
| Abdulameer,2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Maryam Ghodsi,2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| AL-Homood,2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Yan Guo,2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Afshinnia MD, 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Yaturu,2009 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Schwartz,2004 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Roma’n,2004 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | moderate |
| Lingna Fang,2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Jianbo Li,2014 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Karimifar,2012 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Bruckner,2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Yufeng Li,2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Junyan Li,2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Min Qiu,2020 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| Shuangling Xiu,2019 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Xiaojuan Xu,2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
| Yang Wu, 2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | high |
| L. Zhou, 2017 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | moderate |
| Xueyu Li, 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | high |
Study: first author, Published year; TS, total scores; QG, quality grade;
External validity: Q1-Q4; Internal validity: Q5-Q10
Q1: Was the study’s target population a close representation of the national population in relation to relevant variables?
Q2: Was the sampling frame a true or close representation of the target population?
Q3: Was some form of random selection used to select the sample, or was a census undertaken?
Q4: Was the likelihood of nonresponse bias minimal?
Q5: Were data collected directly from the subjects (as opposed to a proxy)?
Q6: Was an acceptable case definition used in the study?
Q7: Was the study instrument that measured the parameter of interest shown to have validity and reliability?
Q8: Was the same mode of data collection used for all subjects?
Q9: Was the length of the shortest prevalence period for the parameter of interest appropriate?
Q10: Were the numerator(s) and denominator(s) for the parameter of interest appropriate?
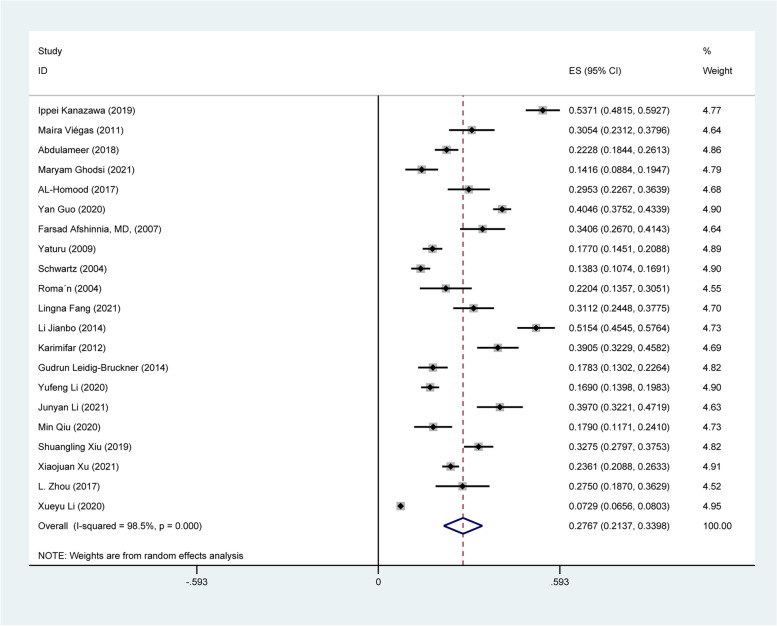
A pooled prevalence of 27.67% (95% CI 21.37-33.98%) of OP was found in patients with DM (Fig. 2). The heterogeneity tests indicated significant differences between individual studies (I2 = 98.5%, P < 0.001). To explore the potential source of heterogeneity, several subgroups were defined and a meta-regression was carried out.
Fig. 2.
The forest plot of pooled OP prevalence in patients with DM
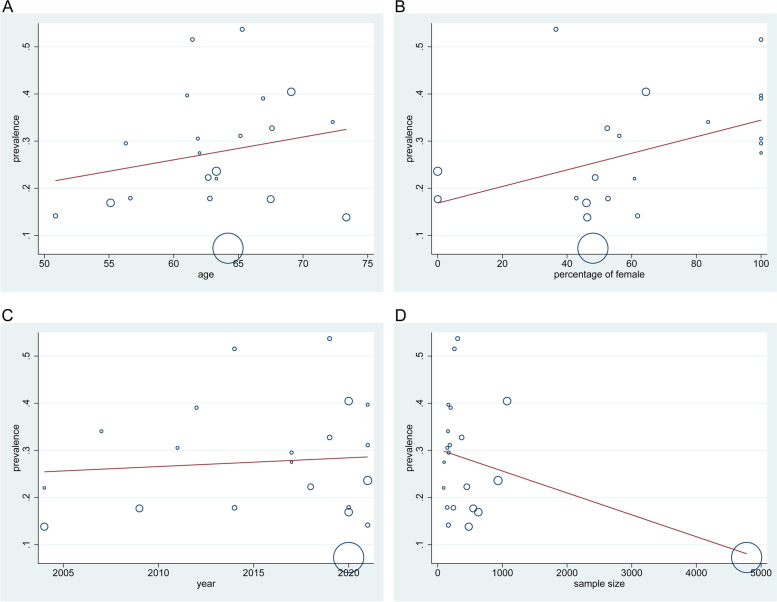
Age. The prevalence of OP in diabetes patients aged 60 or younger was lower (19.17% [95% CI 13.79-24.56%]), compared to the older individuals (29.61% [95% CI 21.97-37.24%]) (Fig. 3A). Fig. 4a shows that there was no significant relationship between age at testing for OP prevalence in diabetes patients (P = 0.354).
Fig. 3.
A. The forest plot of OP prevalence in patients with DM based on age. B. The forest plot of OP prevalence in patients with DM based on gender. C. The forest plot of OP prevalence in patients with DM based on BMI. D. The forest plot of OP prevalence in patients with DM based on study quality. E. The forest plot of OP prevalence in patients with DM based on country. F. The forest plot of OP prevalence in patients with DM based on DM duration
Fig. 4.
a. Meta-regression: age at OP testing and OP prevalence in T2DM. y-Axis: % of T2DM study participants with OP. x-Axis: age in years when tested for OP (P = 0.354). b. Meta-regression: gender at OP testing and OP prevalence in T2DM. y-Axis: % of T2DM study participants with OP. x-Axis: % of female when tested for OP (P = 0.050). c. Meta-regression: published year and OP prevalence in T2DM. y-Axis: % of OP with T2DM. x-Axis: published year (P = 0.715). d. Meta-regression: sample size and OP prevalence in T2DM. y-Axis: sample size of studies. x-Axis: sample size (P = 0.086)
Gender. The prevalence of OP was higher in studies with a higher proportion of female participants (32.96% [95% CI 25.90-40.02%]) than in that with a lower proportion (23.01% [95% CI 16.09-29.92%]) (Fig. 3B). The meta-regression suggested a positive association between the OP prevalence and female proportion; however, the results were not statistically significant (P = 0.050, Fig. 4b).
BMI. The OP prevalence in obese patients with DM was higher (32.02% [95% CI 21.02-43.03%]) than that in non-obese (28.36% [95% CI 22.19-34.54%]) (Fig. 3C).
Quality of studies. The studies of high quality embrace slightly higher prevalence (27.83% [95% CI 20.98-34.67%]), compared to studies with moderate quality (27.00% [95% CI 22.05-31.95%]) (Fig. 3D).
Country. The prevalence of OP in diabetics in China (30.14% [95% CI 18.08-42.21%]) was higher than that in non-China (25.74% [95% CI 19.54-31.85%]) (Fig. 3E).
DM duration. There was a slightly higher OP prevalence in those who suffered diabetes for more than 10 years (31.23% [95% CI 21.78-40.68%]), compared with those less than 10 years (31.10% [95% CI 17.59-44.62%]) (Fig. 3F).
Other possible factors. The meta-regression indicated that there was no association between the prevalence of OP in patients with DM and the publishing year (P = 0.715; Fig. 4c), as well as the sample size (P = 0.086; Fig. 4d). Nevertheless, the results were not statistically significant.
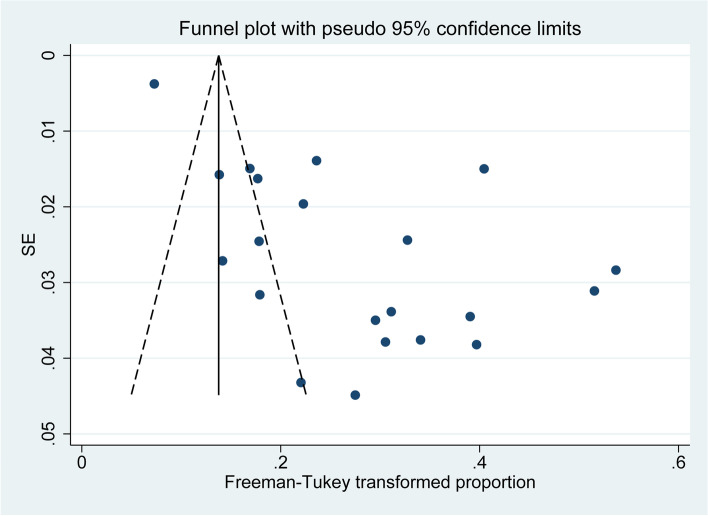
Additionally, a funnel plot was made, which indicated the existence of publication bias where larger studies seem more likely to be published if they show high OP prevalence for patients with DM (Fig. 5).
Fig. 5.
Funnel plot of included studies
Discussion
Based on the 21 analyzed articles, 11,603 individuals with DM, a OP prevalence of 27.67% (95% CI 21.37-33.98%) was observed in this meta-analysis. The current study provides direction for future studies on bone health in diabetics with many studies recommending population-based bone mineral density (BMD) studies in patients with DM.
Only a small number of studies published estimated the prevalence of OP in patients with T2DM worldwide, and a wide range reflects high inconsistency of the prevalence evaluation. This systematic review and meta-analysis found that the prevalence of OP in diabetics varies between 7.29 and 53.71%. The comparison groups in the different studies are not uniform and the primary outcomes not consistent, that makes it difficult to compare one another and arrive at valid conclusions.
In this study, although some minor differences in OP prevalence were identified in subgroups, the prevalence of OP in diabetics could not be influenced significantly by age, gender (female proportion), BMI, country, published year, and sample size. This is notable given that the OP is more prevalent in certain populations, such as obese patients [36], postmenopausal women or older men [37, 38]. For instance, data from National Health and Nutrition Examination Survey (NHANES) from 2005 to 2010 highlighted that 16.2% of adults aged 65 and over had OP at the lumbar spine or femur neck. The age-adjusted prevalence of osteoporosis at either skeletal site was higher among women (24.8%) compared to men (5.6%). In the United States, the unadjusted prevalence was higher among adults aged 80 and over (25.7%) than for adults aged 65 to 79 (12.8%) [39]. Age and gender differences could lead to obvious distinction in the prevalence of OP.
Although we also found that the OP prevalence in diabetics was higher in postmenopausal women, the elderly, obesity group, the effect was not significant. Several confounders could account for this contradiction. Bone mineral density (BMD) is one of the factors that must not be ignored in studies on OP. For the majority of individuals with OP, BMD T-score < − 2.5 SD or less was chosen as the diagnostic criteria produced by the World Health Organization (WHO) (Table 1). The diagnostic criteria provided a tool that could be used in epidemiological studies to quantify the prevalence of OP and confirmed the importance of low BMD in the pathogenesis of fragility fractures. Whereas, the utility of BMD as a clinical indicator of OP is limited, as BMD is only one of a lot of important risk factors for fracture, and the majority of vulnerability fractures occur in groups with BMD values above this threshold [40]. These suggested that BMD could not be a faultless clinical indicator of OP and osteoporotic fractures. Subsequently, BMD varies by skeletal site. In our included studies, Viegas M and colleagues [20] reported the prevalence of OP was 30.4% at lumbar spine (LS) and 9.5% at femoral neck (FN). Furthermore, based on NHANFS (2005-2014) data, significant trends (quadratic or linear) were observed for the femur neck (mean T-score and OP in both sexes; low bone mass in women) but not for the lumbar spine. The trend in femur neck status was somewhat U-shaped, with prevalence being most consistently significantly higher (by 1.1-6.6 percentage points) in 2013-2014 than in 2007-2008. FN trend was unchanged even after adjusting for variations in BMI, smoking, milk intake, and physician’s diagnosis of OP between surveys. In 2013-2014, the percent of older adults with OP was 6% at the femur neck, 8% at the lumbar spine, and 11% at either site [41].
The current study showed that the prevalence of OP in individuals with T2DM cannot be significantly affected by BMI. The aforementioned studies revealed the complex relation between BMI and BMD. Al-Homood et al. [23] showed that BMI protects against a decrease in BMD, such a finding has been claimed by Chen et al. in their study among elderly type 2 diabetic men [42]. Nonetheless, Doğan, A and his teammates [43] confirmed BMD values increased as BMI values increased and the effect of a high BMI on femoral neck and L2-L4 BMD among older men and women, but the effect of age was not shown above 75 years of age. In addition, glycemic control, use of oral agents, individual’s lifestyles, the method used for measurements and the study design were not considered in this study.
Research quality can part explain the significant heterogeneity. In sub-analyses, there was no difference between moderate-quality studies (P = 0.333). Another one explanation for the significant variation in reported OP prevalence may be publication bias. The small and inconsistent sample size in selected studies was one limitation in drawing stable conclusions with regard to OP occurrence in patients with DM, further prospective and high quality studies are warranted on a larger scale, which includes more potentially confounding factors.
Conclusions
This systematic review and meta-analysis revealed that OP is a common comorbidity in diabetics. We found a 27.67% prevalence of OP in patients with DM worldwide. This finding highlights the case for action to implement the control of OP in diabetic patients. Such efforts include the improvement of access to laboratory testing, training of professionals for OP management, and facilitation of access to comprehensive therapy for OP and T2DM.
Supplementary Information
Additional file 1. The detailed retrieval strategy in PubMed, Embase, Cochrane Library, Medline and CBM databases.
Acknowledgments
An Hui Medical University for carrying out the initial search for relevant research papers.
Authors’ contributions
Conceptualization, Q Zhang and X. Y Liu; methodology, X. Y Liu; software, X. Y Liu and L Liu; validation, F. H Chen, X. Y Liu and Q Zhang; literatures research, X. Y Liu, F. H Chen; studies selection and data extraction, X. Y Liu, F. H Chen and L Liu; quality assessment, X. Y Liu and F. H Chen; data analysis, X. Y Liu; writing-original draft preparation, X. Y Liu; writing-review and editing, L Liu and X. Y Liu; supervision and funding acquisition, Q Zhang; project administration, Q Zhang. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China, grant number: 81970703.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueying Liu, Email: m18721956172@163.com.
Fuhua Chen, Email: cfh1016@163.com.
Lei Liu, Email: liuleilife@163.com.
Qiu Zhang, Email: zhangqiu@ahmu.edu.cn.
References
- 1.Consensus development conference Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-E. [DOI] [PubMed] [Google Scholar]
- 2.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Xu J. Association between muscle mass, bone mineral density and osteoporosis in type 2 diabetes. J Diabetes Investig. 2022;13(2):351–358. doi: 10.1111/jdi.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiu S, Chhetri JK, Sun L, Mu Z, Wang L. Association of serum prealbumin with risk of osteoporosis in older adults with type 2 diabetes mellitus: a cross-sectional study. Ther Adv Chronic Dis. 2019;10:2040622319857361. doi: 10.1177/2040622319857361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si Y, Wang C, Guo Y, Xu G, Ma Y. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: a systematic review and Meta-analysis. Iran J Public Health. 2019;48(7):1203–1214. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, Cui L, Zhao N, Lin Q, Chen L, et al. Prevalence of osteoporosis and fracture in China: the China osteoporosis prevalence study. JAMA Netw Open. 2021;4(8):e2121106. doi: 10.1001/jamanetworkopen.2021.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Noubiap JJ, Bigna JJ, Nansseu JR, Nyaga UF, Balti EV, Echouffo-Tcheugui JB, Kengne AP. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(9):e998–e1007. doi: 10.1016/S2214-109X(18)30275-4. [DOI] [PubMed] [Google Scholar]
- 10.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56(3):247–253. [PubMed] [Google Scholar]
- 13.He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y, Song A, Heymsfield SB, Zhang S, Zhu S. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring) 2015;23(3):684–691. doi: 10.1002/oby.20995. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE, Shorr RI, Nevitt MC, Black DM, Cauley JA, et al. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005;20(4):596–603. doi: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Chattopadhyay K, Xu S, Chen Y, Xu M, Li L, et al. Prevalence of comorbidities and their associated factors in patients with type 2 diabetes at a tertiary care department in Ningbo, China: a cross-sectional study. BMJ Open. 2021;11(1):e040532. [DOI] [PMC free article] [PubMed]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luis Román DA, Aller R, Perez Castrillon JL, De Luis J, Gonzalez Sagrado M, Izaola O, Romero E, Martín Escudero JC, Herreros V. Effects of dietary intake and life style on bone density in patients with diabetes mellitus type 2. Ann Nutr Metab. 2004;48(3):141–145. doi: 10.1159/000078376. [DOI] [PubMed] [Google Scholar]
- 18.Afshinnia F, Chacko S, Zahedi T. Association of lower serum cholesterol levels with higher risk of osteoporosis in type 2 diabetes. Endocr Pract. 2007;13(6):620–628. doi: 10.4158/EP.13.6.620. [DOI] [PubMed] [Google Scholar]
- 19.Yaturu S, Humphrey S, Landry C, Jain SK. Decreased bone mineral density in men with metabolic syndrome alone and with type 2 diabetes. Med Sci Monit. 2009;15(1):CR5–CR9. [PubMed] [Google Scholar]
- 20.Viegas M, Costa C, Lopes A, Griz L, Medeiro MA, Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complicat. 2011;25(4):216–221. doi: 10.1016/j.jdiacomp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Karimifar M, Pasha MAP, Salari A, Zamani A, Salesi M, Motaghi P. Evaluation of bone loss in diabetic postmenopausal women. J Res Med Sci. 2012;17(11):1033–1038. [PMC free article] [PubMed] [Google Scholar]
- 22.Jianbo L, Zhang H, Yan L, Xie M, Mei Y, Jiawei C. Homocysteine, an additional factor, is linked to osteoporosis in postmenopausal women with type 2 diabetes. J Bone Miner Metab. 2014;32(6):718–724. doi: 10.1007/s00774-013-0548-4. [DOI] [PubMed] [Google Scholar]
- 23.Al-Homood IA, Sheshah I, Mohammed AGA, Gasim GI. The prevalence and risk factors of osteoporosis among a Saudi female diabetic population. Open Access Maced J Med Sci. 2017;5(2):177–181. doi: 10.3889/oamjms.2017.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Song J, Yang S, Meng S, Lv X, Yue J, Mina A, Puchi B, Geng Y, Yang L. Bone mass loss is associated with systolic blood pressure in postmenopausal women with type 2 diabetes in Tibet: a retrospective cross-sectional study. Osteoporos Int. 2017;28(5):1693–1698. doi: 10.1007/s00198-017-3930-6. [DOI] [PubMed] [Google Scholar]
- 25.Abdulameer SA, Sahib MN, Sulaiman SAS. The prevalence of osteopenia and osteoporosis among Malaysian type 2 diabetic patients using quantitative ultrasound densitometer. Open Rheumatol J. 2018;12:50–64. doi: 10.2174/1874312901812010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanazawa I, Takeno A, Tanaka KI, Yamane Y, Sugimoto T. Osteoporosis and vertebral fracture are associated with deterioration of activities of daily living and quality of life in patients with type 2 diabetes mellitus. J Bone Miner Metab. 2019;37(3):503–511. doi: 10.1007/s00774-018-0948-6. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Wang Y, Chen F, Wang J, Wang D. Assessment of risk factors for fractures in patients with type 2 diabetes over 60 years old: a cross-sectional study from Northeast China. J Diabetes Res. 2020;2020:1508258. doi: 10.1155/2020/1508258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zhao Z, Wang L, Fu Z, Ji L, Wu X. The prevalence of osteoporosis tested by quantitative computed tomography in patients with different glucose tolerances. J Clin Endocrinol Metab. 2020;105(1):dgz036. [DOI] [PubMed]
- 29.Qiu M, Zhai S, Liu D. DPP4 activities are associated with osteopenia/osteoporosis and fracture risk in newly diagnosed type 2 diabetes. Int J Endocrinol. 2020;2020:8874272. [DOI] [PMC free article] [PubMed]
- 30.Fang L, Zhong S, Ma D, Li C, Hao Y, Gao Y, et al. A cross-sectional study: an assessment of low muscle mass and osteoporosis in type 2 diabetes mellitus patients with a high glycated hemoglobin level. Ther Adv Chronic Dis. 2021;12:20406223211026762. [DOI] [PMC free article] [PubMed]
- 31.Ghodsi M, Keshtkar AA, Razi F, Mohammad Amoli M, Nasli-Esfahani E, Zarrabi F, Khashayar P, Khajavi A, Larijani B, Mohajeri-Tehrani MR. Association of vitamin D receptor gene polymorphism with the occurrence of low bone density, osteopenia, and osteoporosis in patients with type 2 diabetes. J Diabetes Metab Disord. 2021;20(2):1375–1383. doi: 10.1007/s40200-021-00871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Niu X, Si Q, Song Q, Jin M, Zhou R, Sun Y, Li J, Wang Q. Plasma periostin as a biomarker of osteoporosis in postmenopausal women with type 2 diabetes. J Bone Miner Metab. 2021;39(4):631–638. doi: 10.1007/s00774-020-01200-3. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Xiang S, Jiang X, Wang L, Wang K, Hua F. Relationship of bone status with serum uric acid and bilirubin in men with type 2 diabetes: a cross-sectional study. Med Sci Monit. 2021;27:e930410. [DOI] [PMC free article] [PubMed]
- 34.Xu X, Zhang M, Fei Z, Sheng H, Qu S, Cui R. Calcification of lower extremity arteries is related to the presence of osteoporosis in postmenopausal women with type 2 diabetes mellitus: a cross-sectional observational study. Osteoporos Int. 2021;32(6):1185–1193. doi: 10.1007/s00198-020-05775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leidig-Bruckner G, Grobholz S, Bruckner T, Scheidt-Nave C, Nawroth P, Schneider JG. Prevalence and determinants of osteoporosis in patients with type 1 and type 2 diabetes mellitus. BMC Endocr Disord. 2014;14:33. doi: 10.1186/1472-6823-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi W, Jiang Y, Liu W, Chi Y, Jiajue R, Pang Q, Wang O, Li M, Xing X, Yu W, et al. Bone microarchitecture in obese postmenopausal Chinese women: the Chinese vertebral osteoporosis study (ChiVOS) Front Endocrinol (Lausanne) 2022;13:891413. doi: 10.3389/fendo.2022.891413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NIH consensus development panel on osteoporosis prevention D, and therapy Osteoporosis prevention, diagnosis, and therapy. Jama. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 38.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anne C. Looker PDSMF, Ph.D., Division of Health and Nutrition Examination Surveys: Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005–2010. 2015. Available online https://www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf.
- 40.Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164(10):1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Wu Q. Decreasing trend of bone mineral density in US multiethnic population: analysis of continuous NHANES 2005-2014. Osteoporos Int. 2018;29(11):2437–2446. doi: 10.1007/s00198-018-4648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen HL, Deng LL, Li JF. Prevalence of osteoporosis and its associated factors among older men with type 2 diabetes. Int J Endocrinol. 2013;2013:285729. doi: 10.1155/2013/285729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doğan A, Nakipoğlu-Yüzer GF, Yıldızgören MT, Ozgirgin N. Is age or the body mass index (BMI) more determinant of the bone mineral density (BMD) in geriatric women and men? Arch Gerontol Geriatr. 2010;51(3):338–341. doi: 10.1016/j.archger.2010.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The detailed retrieval strategy in PubMed, Embase, Cochrane Library, Medline and CBM databases.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.