Abstract
Cytotoxic T-lymphocyte (CTL) activity developed against the major infected target cells of rickettsial infections, endothelial cells and macrophages. Spleen cells from mice immune to Rickettsia conorii exerted specific major histocompatibility complex (MHC) class I-matched CTL activity against R. conorii-infected SVEC-10 endothelial cells, with peak activity on day 10. Similarly, spleen cells from Rickettsia australis-immune mice exerted specific CTL activity against an R. australis-infected macrophage-like cell line. Gamma interferon (IFN-γ) gene knockout mice were more than 100-fold more susceptible to R. australis infection than wild-type C57BL/6 mice. MHC class I gene knockout mice were the most susceptible, more than 50,000-fold more susceptible to a lethal outcome of R. australis infection than wild-type C57BL/6 mice. These results indicate that CTL activity was more critical to recovery from rickettsial infection than were the effects of IFN-γ. The observation that perforin gene knockout mice were more than 100-fold more susceptible than wild-type C57BL/6 mice indicates that perforin-mediated activity accounts for a large component, but not all, of the CTL-mediated antirickettsial effect. CTL activity was expressed by immune CD8 T lymphocytes. Adoptive transfer of immune CD8 T lymphocytes from IFN-γ gene knockout mice into R. australis-infected IFN-γ gene knockout mice dramatically reduced the infectious rickettsial content in the organs, confirming that CD8 T lymphocytes provide immunity against rickettsiae besides that provided by the secretion of IFN-γ. CTLs appear to be crucial to recovery from rickettsial infection.
Rickettsial diseases, including the life-threatening infections caused by Rickettsia rickettsii, R. conorii, R. sibirica, R. australis, R. prowazekii, and R. typhi, are acute infectious diseases (3, 21, 24, 26, 29, 31, 32). Either patients control the growth and spread of the obligately intracellular bacteria, often with the assistance of timely administered bacteriostatic antimicrobial drugs, or they die. The immune mechanisms by which the host controls rickettsial infection are highly dependent on cellular immunity, with a critical role identified for T lymphocytes (6, 7, 12, 13, 16, 23). An important contribution to protective immunity involved in the control of rickettsial infection is provided by cytokines, especially gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). These cytokines act in concert to activate endothelial cells, the major target cells of rickettsial infections, as well as other minor target cells to kill intracellular organisms via a nitric oxide synthesis-dependent mechanism (5, 27, 28). The sources of these protective cytokines are hypothesized to be the T lymphocytes and macrophages that infiltrate the perivascular space surrounding the vessels with infected endothelium (7, 9).
Evaluation of the roles of the T-lymphocyte subsets revealed that CD8 T lymphocytes are crucial to the clearance of R. conorii in a disseminated endothelial target mouse model of infection (6, 7, 25). Although adoptive transfer of either CD4 or CD8 immune T lymphocytes controlled the infection and led to survival, depletion of only CD8 T lymphocytes altered the outcome of infection. CD4 and CD8 T lymphocytes are both potential rich sources of IFN-γ, which could have activated endothelial rickettsicidal activity and tipped the balance in favor of survival. However, depletion of CD4 cells had no observed effect on the course or outcome of infection. In contrast, CD8-depleted mice infected with an ordinarily sublethal dose of R. conorii remained persistently infected and ill, and a high proportion of these animals died of uncontrolled rickettsial infection and its consequent pathologic effects.
The presently reported investigations were undertaken to determine the mechanism(s) by which CD8 T lymphocytes control rickettsial infection. Because cytotoxic T-lymphocyte (CTL) activity had been observed among a population of immune spleen cells exposed to major histocompatibility complex class I (MHC-I)-matched fibroblasts (L-929 cells) infected with R. typhi, we hypothesized that rickettsia-infected endothelial cells, and to a lesser extent rickettsia-infected macrophages, are the targets of CTL-mediated clearance of the infection (20). The experiments reported here employed R. conorii- and R. australis-infected endothelial cells and macrophages as targets to assess CTL activity in vitro, endothelial cell target animal models, purified CD8 T-lymphocyte populations, MHC-I, IFN-γ, and perforin gene knockout mice, and adoptive transfer of CD8 T lymphocytes to evaluate the roles of CTL activity and cytokine production by CD8 T lymphocytes in immunity to spotted fever group (SFG) rickettsiae.
MATERIALS AND METHODS
Rickettsiae.
R. australis (Cutlack strain) was a gift from C. Pretzman (Department of Health Laboratory, Columbus, Ohio) and was passaged three times in Vero cells (ATCC CCL81; American Type Culture Collection, Manassas, Va.) and four times in embryonated chicken yolk sacs in our laboratory. The 10% yolk sac suspension stock contained 106 PFU per ml. R. conorii (Malish 7 strain), a human isolate from South Africa, was obtained from the American Type Culture Collection (ATCC VR-613). The 10% yolk sac suspension stock contained 4 × 106 PFU per ml.
Mice.
C3H/HeN (H-2k) mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) BALB/c (H-2d) and C57BL/6 (H-2b) mice and MHC-I, IFN-γ, and perforin gene knockout mice (with a C57BL/6 background) were all purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were 6 to 8 weeks old and male.
Determination of the relative resistance of wild-type C57BL/6 mice MHC-I gene knockout mice, IFN-γ gene knockout mice, and perforin gene knockout mice to R. australis infection.
Because wild-type C57BL/6 mice are highly resistant to R. conorii, R. australis was used as the SFG rickettsial agent to investigate the relative importance of IFN-γ and CTLs in IFN-γ gene knockout and MHC-I gene knockout mice, respectively. The C57BL/6 mouse-R. australis model is a valid model of human SFG rickettsiosis because it has disseminated endothelial infection. However, R. australis is more invasive than R. rickettsii in humans and than R. conorii in C3H/HeN mice. Mice (30 wild-type C57BL/6, 30 IFN-γ gene knockout, 33 MHC-I gene knockout, and 22 perforin gene knockout) were inoculated intravenously with different concentrations of R. australis and observed daily for illness and death.
CTL assays.
CTL assays were performed according to the standard method reported previously (2). Since the major target cell of rickettsial infection in humans and in the C3H/HeN mouse model is the endothelial cell, an R. conorii-infected MHC-matched endothelial cell line, SVEC-10 (H-2k), was chosen as the target cell in a CTL assay (17, 25). The SVEC-10 cell line was kindly provided by Michael Edidin (Johns Hopkins University, Baltimore, Md.). The effector cells were obtained from C3H/HeN or BALB/c mice that had been infected with a sublethal dose of R. conorii (500 PFU/mouse). The CTL studies were repeated more than three times. In some cases the effector cells were further purified with a mouse CD8 lymphocyte subset column kit (R&D Systems, Inc., Minneapolis, Minn.).
In the R. australis-infected BALB/c mouse model, because an H-2 matching endothelial cell line was not available, the J774A.1 mouse monocyte-macrophage cell line (ATCC TIB 67) was infected and used as target cells (4). The percentage of target cell lysis was calculated by the following formula: [(cpm of the experiment − cpm spontaneously released)/(cpm of the maximum release − cpm spontaneously released)] × 100, where cpm is the counts per minute.
Histopathology.
Necropsies were performed on animals representative of the C57BL/6 mouse model of R. australis infection sacrificed on day 6 on animals representative of the R. australis-infected MHC-I gene knockout model sacrificed on days 11 and 16, and on the IFN-γ gene knockout and perforin gene knockout mice on days 12 and 6, respectively. Samples of brain, liver, lung, spleen, kidney, and heart were fixed in a 4% neutral buffered solution of formaldehyde, embedded in paraffin, sectioned at a 4-μm thickness, and processed by staining with hematoxylin and eosin for evaluation of the histopathology and by immunohistology for detection of SFG rickettsiae as described previously (4).
Adoptive transfer of CD8 T cells to IFN-γ gene knockout mice.
A sublethal dose (35 PFU/mouse) of R. australis was inoculated into IFN-γ gene knockout mice. After 1 month the immune CD8 T lymphocytes were separated from a suspension of spleen cells from these mice with a mouse CD8 T-lymphocyte subset column by negative selection (R&D Systems, Inc.). The CD8 T cells were adjusted to 107 per 0.25 ml of phosphate-buffered saline. Control CD8 T cells were obtained from the spleens of nonimmune IFN-γ gene knockout C57BL/6 mice. On the same day, six IFN-γ gene knockout C57BL/6 mice were divided into three groups (n = 2 per group). All groups of mice were inoculated intravenously with 5 50% lethal doses (LD50) of R. australis suspended in 0.25 ml of sucrose phosphate glutamate buffer (0.128 M sucrose, 0.0038 M KH2PO4, 0.0072 M K2HPO4, 0.0049 M monosodium l-glutamic acid).
The first groups of two mice were subjected to adoptive transfer with 107 immune CD8 T lymphocytes from IFN-γ gene knockout mice intravenously along with 5 LD50 of R. australis (total inoculum, 0.5 ml). The second group was subjected to adoptive transfer with naïve IFN-γ gene knockout C57BL/6 CD8+ T cells and infected similarly with 5 LD50 of R. australis. The third group was inoculated only with 5 LD50 of R. australis. On day 6 all of the mice were sacrificed. Portions of the spleen, liver, and lungs were suspended in 5 ml of Eagle minimum essential medium containing 1% fetal bovine serum and serially diluted as an inoculum for titration of infectious rickettsial content by plaque assay as described previously (25). This experiment was repeated once.
Investigation of the role of apoptosis mediated by CD8 T lymphocytes adoptively transferred from immune or naïve mice into R. australis-infected animals.
C57BL/6 mice were infected with a lethal dose of R. australis and subjected to adoptive transfer with 5 × 107 immune or naïve column-purified CD8 T lymphocytes after inoculation. The animals were then sacrificed at 8, 18, and 24 h, and the livers were fixed in 10% formaldehyde, embedded in paraffin, and sectioned at a 5-μm thickness.
The terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling method was used to evaluate the liver for apoptosis. The tissue sections were deparaffinized in multiple changes of xylene. The tissue was then permeabilized by covering the entire specimen with a 20-μg/ml solution of proteinase K diluted in 10 mM Tris, pH 8, and incubated for 20 min at room temperature. The tissue sections were then washed in Tris-buffered saline (TBS) (20 mM Tris, 140 mM NaCl [pH 7.6]). Inactivation of endogenous peroxidases was accomplished by immersing the tissue sections in 3% hydrogen peroxide diluted in methanol for 5 min at room temperature. The glass slides were then placed in equilibration buffer (1 M sodium cacodylate, 0.15 M Tris, 1.5 mg of bovine serum albumin per ml, 3.75 mM CaCl2 [pH 6.6]) for 30 min. The tissue sections were then incubated with 60 μl of a solution containing TdT and a mixture of biotinylated deoxynucleoside triphosphates, according to the manufacturer's instructions (TdT-Fragel; Oncogene Research Products, Cambridge, Mass.) at 37°C for 90 min in a hybridization chamber (Hybri-Well; Sigma, St. Louis, Mo.). The enzymatic reaction was stopped by incubation with 0.5 M EDTA, pH 8, for 5 min at room temperature. The slides were then washed with TBS and immersed in blocking buffer (4% bovine serum albumin in phosphate-buffered saline) for 20 min followed by incubation with 100 μl of a solution containing peroxidase-streptavidin for 30 min at room temperature in a humidified chamber according to the manufacturer's instructions (Oncogene Research Products). The tissue sections were then washed in TBS and covered with a solution containing 3,3′-diaminobenzidine (0.7 mg/ml), hydrogen peroxide, and urea (0.6 mg/ml). The slides were then rinsed with distilled water and counterstained with Azure A for 10 s. Positive controls were generated by digesting the tissue sections with DNase I (10-μg/ml final concentration) in TBS containing 1 mM MgCl2 for 20 min. TdT was omitted in slides used as negative controls. A total of five noncontiguous areas, each containing 10 high-power fields (HPF), for a total of 50 HPF, in the liver sections were evaluated for apoptotic bodies. The results obtained from two animals at each time point were averaged and used for statistical analysis (Student t test, Stat-200; Biosoft, Ferguson, Mo.).
RESULTS
Increased susceptibility of MHC-I or IFN-γ gene knockout mice to R. australis infection compared to that of wild-type C57BL/6 mice.
The C57BL/6 strain is among the most highly resistant of mouse strains to rickettsial infection (1). The LD50 of R. australis for wild-type C57BL/6 mice was 3.6 × 104 PFU. In IFN-γ gene knockout mice, the LD50 of R. australis was 195 PFU, indicating that susceptibility of the IFN-γ gene knockout mice was more than 100-fold greater than that of wild-type C57BL/6 mice. The MHC-I gene knockout mice with the C57BL/6 background were even more highly susceptible to rickettsial infection. The LD50 was as little as 0.5 PFU per mouse (more than 50,000-fold greater than for wild-type C57BL/6 mice), as shown in Table 1. The MHC-I gene knockout mice infected with R. australis died between days 12 and 19 after rickettsial inoculation. All the ill mice uniformly showed severe subcutaneous edema and pleural and peritoneal effusions.
TABLE 1.
Median LD50 of R. australis in wild-type C57BL/6 and MHC-I, IFN-γ, and perforin gene knockout mice
| Strain of mice | Dilution of R. australis | No. of survivors/ total no. of mice | LD50 (PFU/mouse) |
|---|---|---|---|
| MHC-I KOa | 10−5 | 0/6 | 10−6.34 (0.5) |
| 10−6 | 4/10 | ||
| 10−7 | 8/10 | ||
| 10−8 | 7/7 | ||
| IFN-γ KO | 10−3 | 0/10 | 10−3.71 (195) |
| 10−4 | 7/10 | ||
| 10−5 | 10/10 | ||
| Perforin KO | 10−3 | 0/8 | 10−4.51 (31) |
| 10−4 | 1/7 | ||
| 10−5 | 5/6 | ||
| Wild-type C57BL/6 | 10−1 | 3/10 | 10−1.44 (3.6 × 104) |
| 10−2 | 8/10 | ||
| 10−3 | 10/10 |
KO, knockout.
Histopathology and immunohistologic observations of R. australis.
C57BL/6 mice infected intravenously with R. australis developed disseminated infection involving endothelial cells in the brain, the appropriate target since it is a critical target in human rickettsioses. There were also prominent infections in the lung, liver, testis, epididymus, adnexal fat, and kidney and milder infection in the spleen. There was only focal rickettsial infection extending to the peritoneal lining. The histopathologic lesions included vasculitis with venous thrombosis involving the testicular adnexal fat pad.
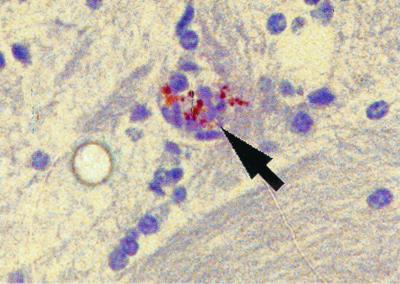
On day 16, in the R. australis-infected MHC-I gene knockout mice, persistent rickettsial infection, reflecting failure to control rickettsial growth and eliminate the infectious organisms, was observed in vascular foci in the brain (Fig. 1) and subarachnoid space, and extensive spread of the infection to the inflammatory exudate in the peritoneal and pleural cavities was also observed. Immunohistochemical studies also revealed a disseminated vasculocentric infection with many rickettsiae in the endothelia and perivascular cells of perforin gene knockout animals sacrificed on day 12 postinfection and in IFN-γ gene knockout mice sacrificed on day 6 postinfection. The rickettsiae were observed mainly in spleen, liver, and lung tissues, with lesser amounts in brain and kidney tissues. The lesions in the infected MHC-I, IFN-γ, and perforin gene knockout mice included mononuclear cell-rich vasculitis and perivasculitis, interstitial pneumonitis, hepatic granulomas, cell death of hepatocytes, mononuclear cell-rich peritonitis, and submesothelial inflammation.
FIG. 1.
Photomicrograph of an MHC-I gene knockout mouse with cerebral vascular endothelial infection with R. australis on day 16 after inoculation demonstrated by immunohistochemistry (anti-SFG rickettsial immunoperoxidase staining). Magnification, ×420.
Identification and kinetics of CTL activity of T lymphocytes of rickettsia-infected mice.
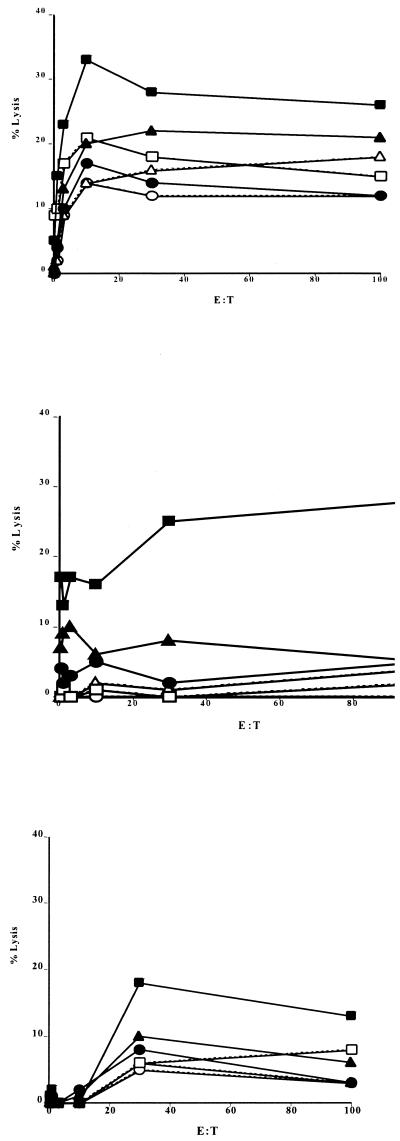
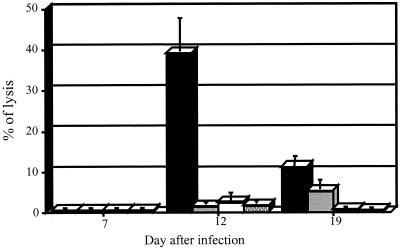
CTL activity was observed in both the C3H/HeN-R. conorii and the BALB/c-R. australis animal models of rickettsial infection. In the C3H/HeN mouse model, the CTL activity was detected on days 9 to 12 after R. conorii infection. At its peak on day 10, this CTL activity was detected only in rickettsia-infected, MHC-matched target cells, not in uninfected target cells or with MHC-unmatched immune effector cells (BALB/c mice immunized with R. conorii) (Fig. 2B). During the first week of infection, the cytotoxicity studies revealed a relatively high nonspecific reaction (Fig. 2A). The specific CTL activity had decreased significantly at 3 weeks after infection (Fig. 2C). In the R. australis-infected BALB/c mouse model, the highest specific CTL activity was observed on day 12 after rickettsial infection (Fig. 3). The results for CTL activity with purified CD8 T lymphocytes were similar to those with unpurified T lymphocytes.
FIG. 2.
CTL activity directed against endothelial cells on day 6 (top graph), day 10 (middle graph), and day 15 (bottom graph) after infection with R. conorii at effector-to-target cell (E:T) ratios of 3, 10, 30, and 100, measured as the percentage of lysis of target cells. Target-effector pairs are indicated as follows: ■, immune H-2k effector lymphocytes and R. conorii-infected H-2k endothelial cells; ▴, immune H-2d effector lymphocytes and R. conorii-infected H-2k endothelial cells; ●, naïve H-2k effector lymphocytes and R. conorii-infected H-2k endothelial cells; □, immune H-2d effector lymphocytes and uninfected endothelial cells; ▵, immune H-2k effector lymphocytes and uninfected endothelial cells; and ○, naïve H-2k effector lymphocytes and uninfected endothelial cells.
FIG. 3.
CTL activity directed against J774A.1 macrophage-like
cells on days 7, 12, and 19 after infection with R.
australis at a multiplicity of infection of 1:1 and an
effector-to-target cell ratio of 100, measured as the percentage of
lysis of target cells. MHC-I-matched target-effector pairs are
indicated as follows: ■, R. australis-infected
macrophage-like cells and immune lymphocytes;
 , R
australis-infected macrophage-like cells and
naïve lymphocytes; □, uninfected macrophage-like cells
and immune lymphocytes; ▩, uninfected macrophage-like cells and
naïve lymphocytes.
, R
australis-infected macrophage-like cells and
naïve lymphocytes; □, uninfected macrophage-like cells
and immune lymphocytes; ▩, uninfected macrophage-like cells and
naïve lymphocytes.
CTL activity of CD8 T cells plays an important role in the control of the rickettsial content in R. australis-infected IFN-γ gene knockout mice.
CD8 T lymphocytes have two major functions: CTL activity and production of cytokines, such as IFN-γ. It has been established that IFN-γ plays an important role in controlling rickettsial infection (5, 27, 28). This experiment was designed to determine whether the remarkable susceptibility of MHC-I gene knockout mice was due to a lower level of IFN-γ production or a lack of CTL activity. To study the role of CTL activity in conferring immunity against rickettsial infection, we chose IFN-γ gene knockout mice to exclude the possibility of immune function induced by IFN-γ. The adoptively transferred immune or normal CD8 T cells were also derived from IFN-γ gene knockout mice. All three groups of mice became ill on day 5 whether they were subjected to adoptive transfer with immune or nonimmune CD8 T lymphocytes (107/mouse) or were not subjected to adoptive transfer with any cells after inoculation with 5 LD50 of R. australis. On day 6, one of the mice in the non-adoptive-transfer group was moribund. As shown in Table 2, mice that were subjected to transfer with immune CD8 T lymphocytes from IFN-γ gene knockout mice had dramatically lower rickettsial infectivity titers than the mice given normal CD8 T lymphocytes or the group that received no cells.
TABLE 2.
Infectious contenta of R. australis in IFN-γ gene knockout mice subjected to adoptive transfer with immune or nonimmune CD8 T lymphocytes
| Group no. | Adoptive transfer | 103 PFU of R.
australis/g of tissue from the:
|
||
|---|---|---|---|---|
| Spleen | Lungs | Liver | ||
| 1 | Immune CD8b | 29.6 ± 1.5 | 17.2 ± 3.9 | 19 ± 0.9 |
| 2 | Naïve CD8 | 4,814 ± 758 | 5,449 ± 282 | 2,837 ± 400 |
| 3 | No transfer | 2,816 ± 189 | 2,778 ± 379 | 1,996 ± 163 |
Mean of two repeated experiments, ± standard deviation.
Immune IFN-γ gene knockout CD8 T-lymphocyte recipients' organs contained significantly fewer R. australis PFU than the corresponding organs of recipients of naïve IFN-γ gene knockout CD8 T lymphocytes (P < 0.05) or those of mice with no transfer (P < 0.05).
Role of perforin in antirickettsial CTL activity.
Among the perforin gene knockout mice, the LD50 of R. australis was 31 PFU. Thus, although these mice were much more susceptible to R. australis than immunocompetent C57BL/6 mice, they were not as susceptible as the MHC-I gene knockout mice. It can be concluded that perforin is an effector of antirickettsial activity in this model but that it does not account for all of the CTL activity directed against R. australis-infected cells.
Investigation of the role of apoptosis mediated by CD8 T lymphocytes adoptively transferred from immune or naïve mice into R. australis-infected animals.
The number of apoptotic cells in the liver 8 h after infection with R. australis and adoptive transfer of naïve and immune CD8 T cells was significantly different between the two groups (0.6 apoptotic cell/50 HPF in the naïve CD8 recipients versus 23.6 apoptotic cells/50 HPF in the immune CD8 recipients, P < 0.001). The number of apoptotic cells decreased at 18 and 24 h but the differences between the groups receiving nonimmune and immune CD8 T lymphocytes were still statistically significant (3.6 versus 10.4 apoptotic cells/50 HPF, respectively, at 18 h, P < 0.002; 4.6 versus 7.8 apoptotic cells/50 HPF, respectively, at 24 h, P < 0.017).
DISCUSSION
This report established for the first time that rickettsia-infected endothelial cells and macrophages are targets of CTL activity. The pioneering work of Rollwagen and colleagues had previously demonstrated the existence of T-lymphocyte-mediated CTL activity against MHC-I-matched, R. typhi-infected fibroblasts (20). In addition, the present report strongly suggested for the first time that CTL activity is a more critical function of CD8 T lymphocytes in the clearance of rickettsiae than the production of IFN-γ is. It is important to consider that CD4 and NK cells can secrete IFN-γ, possibly replacing the function of IFN-γ production. However, only CD8 T lymphocytes provide MHC-I-restricted CTL activity.
The specific CTL activity of immune T lymphocytes, particularly the CD8 subset, derived from two different animal models of SFG rickettsial infection was directed against R. conorii-infected, MHC-I-matched endothelial cells and against R. australis-infected, MHC-I-matched macrophages. These cell types are those which are infected in human rickettsioses and in these mouse models. That they are susceptible to CTL activity is an important consideration implying that MHC-I molecules are not significantly downregulated during rickettsial infection and that particular rickettsial epitopes are recognized by CD8 T lymphocytes in the context of MHC-I molecules. Indeed, MHC-I molecules are not downregulated in R. conorii-infected SVEC-10 mouse endothelial cells as assayed by flow cytometry (M. Diaz, J. P. Olano, and D. H. Walker, unpublished data). CTL activity is a potential mechanism of elimination of rickettsia-infected cells. Consistent with this hypothesis is the persistence of R. conorii infection in mice depleted of CD8 T lymphocytes (7). If the effect of CTLs on infected cells is induction of apoptosis, the rickettsia-containing apoptotic bodies could hypothetically be phagocytosed rapidly by adjacent cells (e.g., perivascular macrophages) and degraded in their phagolysosomes.
The demonstration that apoptosis is significantly greater in the livers of R. australis-infected mice subjected to adoptive transfer with immune CD8 T lymphocytes than in infected mice subjected to adoptive transfer with naïve CD8 T lymphocytes suggests that infected cells are eliminated by apoptosis associated with CTL activity. Further evidence supporting a role for apoptosis induced by CD8 T lymphocytes was derived from investigation of tissues performed in a previously reported study involving depletion of CD8 T lymphocytes (7). R. conorii-infected C3H/HeN mice depleted of CD8 T lymphocytes had significantly less apoptosis than infected, sham-depleted animals in the livers (4.8 apoptotic cells/50 HPF in the CD8-depleted animals versus 28.6 apoptotic cells/50 HPF in the sham-depleted animals, P < 0.001) and in the lungs (7.4 apoptotic cells/50 HPF versus 14.6 apoptotic cells/50 HPF, respectively; P = 0.001) on day 5 of infection (J. P. Olano, D. H. Walker, and H.-M. Feng, unpublished data).
Moreover, the kinetics of the CTL activity paralleled rickettsial clearance in the well-studied R. conorii-C3H/HeN mouse model with the demonstration of CTLs and evidence for an effect on rickettsial growth on day 6, peak of CTL activity and clearance of R. conorii on day 10, and downregulation of CTLs after rickettsiae are no longer present on day 15 (25). The C57BL/6-R. australis model showed a somewhat prolonged peak of the CTL activity, correlating with greater perivascular invasiveness of R. australis and the consequently longer period required for clearance.
The studies with MHC-I and IFN-γ gene knockout mice revealed that CD8 T lymphocytes contribute to protective immunity to SFG rickettsiae by both CTL activity and production of IFN-γ. It is remarkable that mice with the C57BL/6 background associated with resistance to rickettsial infection appeared to suffer a fatal course of infection when inoculated with a dose that approximated a single PFU of rickettsiae in the absence of MHC-I molecules. Moreover, adoptive transfer of R. australis-immune CD8 T lymphocytes incapable of producing IFN-γ into IFN-γ gene knockout mice provided protective immunity as demonstrated by marked reduction in rickettsial content in the lungs, liver, and spleen, most likely due to a CTL-dependent mechanism. The importance of IFN-γ in immunity to rickettsiae is emphasized by the observation that the IFN-γ gene knockout mice with the C57BL/6 background were more than 100 times more susceptible to a lethal outcome of R. australis infection than wild-type C57BL/6 mice. Mice can survive rickettsial infection without IFN-γ, but they cannot survive without MHC-I.
In other studies, CD8 T-lymphocyte clones directed against R. conorii were established (H.-M. Feng and D. H. Walker, unpublished data). Among 15 R. conorii-reactive CD8 T-lymphocyte clones that were evaluated for their ability to confer protection via adoptive transfer, 5 clones protected mice from a lethal rickettsial infection. Four of the protective clones produced a high concentration (ranging from 3,495 ± 79 pg/ml [mean ± standard deviation] to more than 4,000 pg/ml) of IFN-γ when stimulated by R. conorii antigen. Thus, even though CTL activity is of greater importance in immunity to rickettsiae, the fact that CD8 T lymphocytes contribute to immunity also via secretion of IFN-γ is confirmed by the demonstration that the ability to produce IFN-γ when stimulated by a single epitope of R. conorii can provide a decisive edge to immunologically naïve mice with an intact immune system. It is unfortunate that during the period when the CD8 clones were available the CTL assay was not established in our laboratory.
The use of two different animal models was required by the desire to study rickettsial infection in gene knockout mice that were available only with the C57BL/6 background, which are highly resistant to R. conorii infection. The R. australis-C57BL/6 mouse model resembled the R. australis-BALB/c mouse model, which was the original endothelial target mouse model of rickettsial infection (4). In C57BL/6 mice, endothelial cells were the primary target; then highly invasive infection followed. The model allowed more definitive conclusions regarding the roles of MHC-I and IFN-γ in immunity to rickettsiae.
This work extends the knowledge of the role of CTL in intracellular infection. The CTL was originally described for lymphocytic choriomeningitis virus and has been noted for its importance mainly for viral infections. Studies of CTLs in bacterial infections have focused primarily on facultatively intracellular organisms that parasitize macrophages, such as Listeria monocytogenes and Mycobacterium tuberculosis (8, 11, 14, 30). For obligately intracellular bacteria and bacterial infections with target cells other than macrophages, few investigations have been reported.
Because of its obligate intracellular parasitism principally of epithelial cells, infection with Chlamydia is of particular interest for comparison with rickettsial infection principally of endothelial cells. Somewhat surprisingly, the T-lymphocyte subset of greater importance for chlamydiae is different from that for rickettsiae. Adoptive transfer of a CD4 cell-enriched T-cell line provided more effective immunity than a CD8 T-cell line in clearing Chlamydia trachomatis from the genital tracts of persistently infected nude mice (18). In the same model, one of two CD8 T-lymphocyte clones provided the ability to clear the chlamydial infection. The protective clone was directed against a genus-specific antigen and produced TNF-α and IFN-γ (10). In a pulmonary infection model of C. trachomatis (mouse pneumonitis strain), depletion of CD4 T lymphocytes produced a greater effect, with higher levels of chlamydial growth and mortality, than did depletion of CD8 T lymphocytes (15). The importance of CD8 T lymphocytes was more apparent in MHC-I gene knockout mice, in which chlamydial growth and mortality were increased, but survivors had resolved the infection by day 34. Our results for rickettsial infection differ from these chlamydial models in that immune clearance of rickettsial infection was more dependent on CD8 T lymphocytes, and there is evidence that CTL activity is a critical factor.
Currently, our framework of knowledge of immunity to rickettsiae includes major contributions of cellular immunity, particularly nitric oxide-dependent killing of rickettsiae in endothelial cells activated by IFN-γ and TNF-α followed by the elimination of the remaining rickettsia-infected cells by CTL activity. From the results of infection of perforin gene knockout mice, it can be concluded that perforin secretion was one of the mechanisms of elimination of R. conorii-infected cells by CTLs, but it was not the only mechanism since the LD50 for MHC-I gene knockout mice was only 0.5 PFU of R. australis, nearly 2 orders of magnitude lower than the LD50 of R. australis in the perforin gene knockout mice. As of yet, the roles of Fas-Fas ligand or granulysin in antirickettsial CTL activity have not been determined. It should be noted that all of the mice studied contained CD4 T lymphocytes, B cells capable of antibody production, and innate immunity including NK cells and complement and that these components very likely contributed to antirickettsial immunity although less critically than CD8 T lymphocytes. Indeed, SCID-C57BL/6 mice, which lack CD4 and CD8 T lymphocytes and antibody produced by B lymphocytes, are even more susceptible to R. australis than MHC-I gene knockout mice (H. M. Feng and D. H. Walker, unpublished data).
The discovery that endothelial cells infected with rickettsiae are a target of CTL activity that seems necessary for the host's survival raises other questions related to the role of endothelial cells as antigen-presenting cells in the development of the immune response, the identification of CD8 T-lymphocyte epitopes of Rickettsia species, and the potential contribution of CTLs to the pathogenesis of rickettsial diseases.
ACKNOWLEDGMENTS
We thank Josie Ramirez-Kim, Kelly Cassity, and Kathleen Fagan for excellent assistance in the preparation of the manuscript, Thomas Bednarek for assistance with preparation of the illustrations, and Gui-Min He for statistical analyses.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI 21242).
REFERENCES
- 1.Anderson G W, Jr, Osterman J V. Host defenses in experimental rickettsialpox: genetics of natural resistance to infection. Infect Immun. 1980;28:132–136. doi: 10.1128/iai.28.1.132-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner K T, Engers H D, Grottini J C. The 51Cr-release assay as used for the quantitative measurement of cell-mediated cytolysis in vitro. In: Bloom B R, David J R, editors. In vitro methods in cell-mediated and tumor immunity. New York, N.Y: Academic Press; 1976. p. 423. [Google Scholar]
- 3.Dumler J S, Taylor J P, Walker D H. Clinical and laboratory features of murine typhus in South Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 4.Feng H-M, Wen J, Walker D H. Rickettsia australisinfection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol. 1993;142:1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 5.Feng H-M, Popov V L, Walker D H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun. 1994;62:1952–1960. doi: 10.1128/iai.62.5.1952-1960.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng H-M, Popov V L, Billings A, Yuoh G, Walker D H. Role of T-lymphocyte subsets in immunity of SFG rickettsiae. In: Kazár J, Toman R, editors. Rickettsiae and rickettsial diseases. Bratislava, Slovakia: Slovak Academy of Sciences; 1996. pp. 255–260. [Google Scholar]
- 7.Feng H-M, Popov V L, Yuoh G, Walker D H. Role of T-lymphocyte subsets in immunity to spotted fever group rickettsiae. J Immunol. 1997;158:5314–5320. [PubMed] [Google Scholar]
- 8.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class-I restricted T cells are required for resistance to Mycobacterium tuberculosisinfection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrero-Herrero J I, Walker D H, Ruiz-Beltran R. Immunohistochemical evaluation of the cellular immune response to Rickettsia conorii in taches noires. J Infect Dis. 1987;155:802–805. doi: 10.1093/infdis/155.4.802. [DOI] [PubMed] [Google Scholar]
- 10.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+T cells in antichlamydial immunity defined by chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman S H E, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovisBCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 12.Kenyon R H, Pedersen C E., Jr Immune responses to Rickettsia akariinfection in congenitally athymic nude mice. Infect Immun. 1980;28:310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokorin I N, Kabanova E A, Shirokova E M, Abrosimova E G, Rybkina N N, Pushkareva V I. Role of T-lymphocytes in Rickettsia conoriiinfection. Acta Virol. 1982;26:91–97. [PubMed] [Google Scholar]
- 14.Ladel C H, Flesch I E A, Arnoldi J, Kaufmann S H E. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T-cell responses on Listeria monocytogenesinfection. J Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- 15.Magee D M, Williams D M, Smith J G, Bleicker C A, Grubbs B G, Schachter J, Rank R G. Role of CD8 T cells in primary Chlamydiainfection. Infect Immun. 1995;63:516–521. doi: 10.1128/iai.63.2.516-521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montenegro N R, Walker D H, Hegarty B C. Infection of genetically immunodeficient mice with Rickettsia conorii. Acta Virol. 1984;28:508–514. [PubMed] [Google Scholar]
- 17.O'Connell K A, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- 18.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts A D, Ordway D J, Orme I M. Listeria monocytogenesinfection in β2 microglobulin-deficient mice. Infect Immun. 1993;61:1113–1116. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollwagen F M, Dasch G A, Jerrells T R. Mechanisms of immunity to rickettsial infection: characterization of a cytotoxic effector cell. J Immunol. 1986;136:1418–1421. [PubMed] [Google Scholar]
- 21.Sexton D J, King G, Dwyer B. Fatal Queensland tick typhus. J Infect Dis. 1990;162:779–780. doi: 10.1093/infdis/162.3.779. [DOI] [PubMed] [Google Scholar]
- 22.Starnbach M N, Bevan M J. Cells infected with Yersiniapresent an epitope to class I MHC-restricted CTL. J Immunol. 1994;153:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 23.Walker D H, Henderson F W. Effect of immunosuppression on Rickettsia rickettsiiinfection in guinea pigs. Infect Immun. 1978;20:221–227. doi: 10.1128/iai.20.1.221-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker D H, Gear J H S. Correlation of the distribution of Rickettsia conorii, microscopic lesions, and clinical features in South African tick bite fever. Am J Trop Med Hyg. 1985;34:361–371. doi: 10.4269/ajtmh.1985.34.361. [DOI] [PubMed] [Google Scholar]
- 25.Walker D H, Popov V L, Wen J, Feng H-M. Rickettsia conoriiinfection of C3H/HeN mice. A model of endothelial-target rickettsiosis. Lab Investig. 1994;70:358–368. [PubMed] [Google Scholar]
- 26.Walker D H. Rocky Mountain spotted fever: a seasonal alert. Clin Infect Dis. 1995;20:1111–1117. doi: 10.1093/clinids/20.5.1111. [DOI] [PubMed] [Google Scholar]
- 27.Walker D H, Popov V L, Crocquet-Valdes P A, Welsh C J R, Feng H-M. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab Investig. 1997;76:129–138. [PubMed] [Google Scholar]
- 28.Walker D H, Crocquet-Valdes P, Feng H-M. Intracellular anti-rickettsial mechanisms of chemokine- and cytokine-activated human macrophages and hepatocytes. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. Paris, France: Elsevier; 1999. pp. 189–194. [Google Scholar]
- 29.Walker D H, Raoult D. Rickettsia rickettsii and other spotted fever group rickettsiae (Rocky Mountain spotted fever and other spotted fevers) In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2035–2042. [Google Scholar]
- 30.White D, Harty J T. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenesby a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J Immunol. 1998;160:898–905. [PubMed] [Google Scholar]
- 31.Wolbach S B. Studies on Rocky Mountain spotted fever. J Med Res. 1919;41:1–70. [PMC free article] [PubMed] [Google Scholar]
- 32.Wolbach S B, Todd J L, Palfrey F W. Pathology of typhus in man. In: Wolbach S B, Todd J L, Palfrey F W, editors. The etiology and pathology of typhus. Cambridge, Mass: League Red Cross Societies at the Harvard University Press; 1922. pp. 152–221. [Google Scholar]