Abstract
Purpose:
The assumption that traversal of the cell nucleus by ionizing radiation is a prerequisite to induce genetic damage, or other important biological responses, has been challenged by studies showing that oxidative alterations extend beyond the irradiated cells and occur also in neighboring bystander cells. Cells and tissues outside the radiation field experience significant biochemical and phenotypic changes that are often similar to those observed in the irradiated cells and tissues. With relevance to the assessment of long-term health risks of occupational, environmental and clinical exposures, measurable genetic, epigenetic, and metabolic changes have been also detected in the progeny of bystander cells. How the oxidative damage spreads from the irradiated cells to their neighboring bystander cells has been under intense investigation. Following a brief summary of the trends in radiobiology leading to this paradigm shift in the field, we review key findings of bystander effects induced by low and high doses of various types of radiation that differ in their biophysical characteristics. While notable mechanistic insights continue to emerge, here the focus is on the many means of intercellular communication that mediate these effects, namely junctional channels, secreted molecules and extracellular vesicles, and immune pathways.
Conclusions:
The insights gained by studying radiation bystander effects are leading to a basic understanding of the intercellular communications that occur under mild and severe oxidative stress in both normal and cancerous tissues. Understanding the mechanisms underlying these communications will likely contribute to reducing the uncertainty of predicting adverse health effects following exposure to low dose/low fluence ionizing radiation, guide novel interventions that mitigate adverse out-of-field effects, and contribute to better outcomes of radiotherapeutic treatments of cancer. In this review, we highlight novel routes of intercellular communication for investigation, and raise the rationale for reconsidering classification of bystander responses, abscopal effects, and expression of genomic instability as non-targeted effects of radiation.
Keywords: out-of-field radiation effects, radiation protection, radiotherapy, radiation dose, linear energy transfer, radiation-induced abscopal effects
Introduction
A historical perspective
Since the discovery of X rays in 1895, physics has had a large impact on the advances in radiation biology and radiation oncology: the physical characteristics of the radiation and the size of the dose absorbed by irradiated cells and tissues determine the nature and magnitude of the biological response to the exposure. Using first generation X ray tubes, major radiobiological discoveries were made shortly after Roentgen’s discovery of X rays. In 1906, Bergonié and Tribondeau determined that rapidly proliferating cells are more radiosensitive than specialized cells (Bergonié and Tribondeau 2003), and in the early 1920’s, Regaud showed that fractionation of the radiation dose mitigates damage to normal tissue (Regaud and Ferroux 1927), a finding that has had an enduring impact on the mode of delivery of radiotherapy. In the decades that followed, advances in the development of techniques and instruments capable of detecting and quantifying subtle biochemical changes in specific cells within tissues greatly advanced the field of radiobiology. Simultaneously, novel radiation delivery equipment, advanced imaging techniques, and 3-dimensional treatment planning systems enhanced the efficacy of therapeutic treatments. The rapid pace of these technological developments and scientific discoveries enriched our basic knowledge of the molecular, cellular, and tissue responses to radiation exposure. However, even up to the 1990’s, it remained generally assumed that radiation traversal through the nucleus is necessary to produce genetic damage or an important biological response (Little 2003), although a prominent role for membranes had been advocated (Alper 1977; Petkau 1980). The assumption was mainly influenced by the target theory, which evolved during a period spanning the mid-1920’s to mid-1940’s to explain the adverse biological effects of ionizing radiation, mainly killing of unicellular organisms. The classical target theory was based on a physical model and assumed that cell killing is directly related to the amount of energy deposition (number of hits) in radiosensitive targets with biological function (Lea 1947). Through seminal work by Zirkle and co-workers, we came to understand that these targets are contained within the nucleus (Zirkle and Bloom 1953), which coincided with the discovery of the 3-dimensional structure of the DNA molecule. Notably, the early work of Zirkle and colleagues highlighted the importance of the biophysical characteristics (specifically linear energy transfer, LET) of the impacting radiation in inducing chromosomal damage (Zirkle and Tobias 1953). Follow-up work using sparsely (low LET) and densely (high LET) ionizing radiations showed that the DNA double strand break was the central lesion inducing cell death (reviewed in (Ward 1988)).
At the onset of the pioneering work of the radiation sciences founders (reviewed in (Bedford and Dewey 2002)), the concept of radiation absorbed dose and its importance in the biological response of irradiated cells was proposed by Gioacchino Failla (Failla 1921) to allow comparisons between irradiations at different laboratories. A 100 years ago, Failla observed that the same physical dose of the two radiation sources available at that time (radiation emitted from radium or 140 kV X rays) produced different levels of biological effects (Failla 1926). While radiation dose was originally conceived to measure the amount of energy deposited per unit volume, Failla suggested that “doses of radiation be reckoned according to the amount of energy absorbed by the radiated tissue”, or in other words the amount of energy deposited per unit mass. However, biological effects vary greatly whether the mass in consideration is cellular (on the order of nanogram) or nuclear (on the order of picogram), or in the case of a densely ionizing particle whether the diameter of the radiation track (core vs penumbra) (Goodhead and Nikjoo 1989) is considered. Yet, numerous observations made during the past century showed that the biological response is not solely affected by energy deposition in the irradiated cells or tissues. In a landmark paper, Hatsumi Nagasawa and John B. Little (Nagasawa and Little 1992) showed that the target for genetic damage following exposure to α particles extends significantly beyond the traversed cells and occurs in neighboring cells that do not experience energy deposition (i.e., bystander cells): recombination events leading to sister chromatid exchanges were increased in ~ 30% of cells in monolayer cultures exposed to α particles where less than 1 % of nuclei were estimated, by dosimetric calculations, to be traversed by a particle track (note that the range of δ rays produced by the 3.6 MeV α particles used in these studies is less than 0.1 μm and cross-irradiation is unlikely). These findings led to a paradigm shift in radiation biology, and implied that assessment of the collective response of a cell population to ionizing radiation is more informative of potential health outcomes than the responses of individual cells wherein the physical dose is deposited.
In the early work, the expression of bystander effects, measured by cytogenetic alterations and changes in gene expression, was thought to be independent of the dose absorbed by the irradiated cells and saturated at relatively low mean absorbed doses (Nagasawa and Little 1992). However, later studies have shown that the amount of dose absorbed by irradiated cells affects the extent of spread of the induced stress and the magnitude of biochemical changes in bystander cells (e.g., (Shao, Furusawa, et al. 2003; Persaud et al. 2005; Buonanno, de Toledo, and Azzam 2011; Buonanno et al. 2011; Gonon et al. 2013; Shao, Aoki, and Furusawa 2003; Mitchell et al. 2004; Maguire et al. 2005)). These observations led to a fundamental change in radiation biology and have been considered of importance in the assessment of health risks associated with radiation exposure, whether from environmental sources or during occupational activities and in clinical settings. Moreover, they advanced the thinking of exploiting the mechanisms underlying bystander effects for beneficial therapeutic outcomes, such as amplifying toxic effects among irradiated tumor cells (‘cohort effects’) (Autsavapromporn et al. 2011) and leading to regression of tumors at distant sites, a phenomenon known as ‘abscopal effect’ (Demaria et al. 2004). In the following, we review the field of bystander responses, and briefly that of genomic instability, two radiobiological aspects that have been impacted by pioneering work of Professor John B. Little.
Paradigm shift in radiation biology: evidence for out-of-field effects
Since the beginning of the twentieth century intriguing biological phenomena were reported describing biological changes in unirradiated cells and tissues present in irradiated living specimens. For example, as early as 1915, Murphy and Morton observed that tumors implanted in irradiated syngeneic animals shrank by 50% (Murphy and Morton 1915) (for historical review of early observations see (Mothersill et al. 2018)). Over subsequent decades, several studies showed that lymphocytes cultured in the presence of plasma from individuals exposed to radiation several years earlier, whether accidentally, during the A-bombing in Hiroshima and Nagasaki or as a result of the Chernobyl nuclear accident, underwent genetic damage (Goh and Sumner 1968; Hollowell and Littlefield 1968; Pant and Kamada 1977; Emerit et al. 1995). Notably, Brooks and co-workers showed in animal experiments performed in the 1970s that α particles emitted by plutonium dioxide (239PuO2) concentrated in a small fraction of the total liver cell population resulted in chromosomal damage in the majority of cells in the liver (Brooks, Retherford, and McClellan 1974). These early observations, together with the seminal work of Nagasawa and Little in the 1990s with α particles from an external plutonium source, cemented the challenge to the assumption that biological effects are limited to cells receiving energy deposition in their nuclei. Collectively, the observed effects have been classified as radiation-induced non-targeted effects and include bystander responses and the expression of genomic instability. However, as the field has evolved, the term ‘non-targeted effect’ is ripe for redefinition as it can be argued that a ‘bystander cell’ or the ‘progeny of irradiated or affected bystander cells’ are also targets of the radiation exposure. Hence, here we use the term ‘out-of-field’ effect.
Genomic instability, a hallmark of cancer (Hanahan and Weinberg 2011), has been the subject of extensive research in radiobiology (reviewed in (Kronenberg 1994; Baulch 2019)). Briefly, studies have demonstrated evidence for non-clonal mutations and chromosome aberrations appearing in the descendants of irradiated mammalian cells several generations of replication after exposure (e.g., (Pampfer and Streffer 1989; Little, Gorgojo, and Vetrovs 1990; Watson et al. 2001; Chang and Little 1992; Holmberg et al. 1995; Grosovsky et al. 1996; Harper, Lorimore, and Wright 1997; Little et al. 1997; Sudo et al. 2008). Notably, hematopoietic cells derived from murine stem cells exposed to moderate mean absorbed doses of 238Pu α particles (LET ~121 keV/μm) harbored a high frequency of non-clonal aberrations in their clonal descendants (Kadhim et al. 1992). Ensuing papers have also reported an enhanced rate of non-clonal mutations and other changes as a result of genome destabilization of the descendants of mammalian cells exposed to radiations with a wide range of high LET (386–13,600 keV/μm) (Sabatier, Dutrillaux, and Martin 1992) (reviewed in (Morgan 2003a, 2003b)), and generated evidence for factors that contribute to genomic stability (Wiese et al. 2002). This evidence was preceded by seminal observations made decades earlier. In 1949, Newcombe and Scott reported on the delayed appearance of radiation-induced mutants in bacteria and speculated that the delayed effect resulted from induced ‘gene instability causing an increase in the spontaneous mutation rate over a number of cell generations’ (Newcombe and Scott 1949), a phenomenon reminiscent of the induction, by mustard gas of chromosomal instabilities in Drosophila melanogaster (Auerbach 1947). In the 1970s, Terzaghi and Little (Terzaghi and Little 1976) also showed that expression of the transformed state of a single irradiated mammalian cell requires several cell replications, depending on the cell type. They suggested that exposure to radiation induces a genetic instability in irradiated cells, which enhances the rate of mutations and transformation in their progeny. Here, we highlight evidence for genomic instability in progeny of bystander cells, a response with significant implication in assessment of the health risks of exposure to radiation.
Bystander effects: in vitro evidence using broadbeam and microbeam irradiators, and internally incorporated radionuclides
The scientific interest in studying bystander effects was particularly stimulated by the concern about lung cancer caused by α particles emitted by environmental radon gas and its decay products. At exposures similar to those from indoor radon, the majority of cells in the bronchial epithelium would not be traversed by an α particle track, and those whose nuclei are in the path of the tracks are likely to experience a large energy deposition that can lead to their death (Barendsen 1994). Therefore, the study of bystander responses became an attractive approach to investigate biological changes, including neoplastic transformation, in non-irradiated cells adjoining α particle-irradiated cells (Sawant et al. 2001; Buonanno, de Toledo, and Azzam 2011).
Using α particle broadbeams, energetic helium ion microbeams, and X ray-, electron- and heavy ion broadbeams and microbeams, numerous studies rapidly confirmed Nagasawa and Little’s discoveries (Nagasawa and Little 1992). Using the text ‘radiation bystander’, a March 2022 PubMed search reveals over ~ 1600 papers describing such findings. A selection of key results describing in vitro manifestation of bystander effects is outlined in Table 1.
Table 1:
Key findings describing the bystander effect phenomenon in vitro
| Radiation type | Irradiation method | Main finding | References | |
|---|---|---|---|---|
| High LET | α particles | Broadbeam | Sister chromatid exchanges are induced in a greater fraction of cells than those whose nuclei are traversed by α particles. | (Nagasawa and Little 1992, Deshpande, Goodwin et al. 1996) |
| Broadbeam | Evidence that gap-junction intercellular communication is involved in transmission of detrimental signals to non-irradiated bystander cells. | (Azzam, de Toledo et al. 1998, Azzam, de Toledo et al. 2001, Zhou, Suzuki et al. 2001) | ||
| Broadbeam | Bystander effects are induced by factors secreted by irradiated cells. | (Lehnert, Goodwin et al. 1997) | ||
| Microbeam | Nuclear irradiation induces bystander effects in non-hit cells. | (Prise, Belyakov et al. 1998, Zhou, Randers-Pehrson et al. 2000) | ||
| Microbeam | Bystander effects were elicited by cytoplasmic irradiation. | (Shao, Folkard et al. 2004) | ||
| Microbeam | Cytoplasmic irradiation induces nuclear mutations. | (Zhou, Suzuki et al. 2001) | ||
| High LET other than α particles | Heavy ions | Microbeam | Micronuclei are induced in bystander cells after irradiation of a single cell within a cell monolayer with a single energetic 40Ar or 20Ne ion. | (Shao, Furusawa et al. 2003) |
| Microbeam & Broadbeam | Heavy ions reduce the clonogenic potential of bystander cells; the timescale of the response to heavy ions differed between irradiated and bystander cells. | (Hamada, Ni et al. 2008) | ||
| Moderate LET | Soft X rays | Microprobe | Bystander responses are detected in hamster fibroblasts when only a single cell is irradiated. | (Folkard, Schettino et al. 2001, Schettino, Folkard et al. 2002) |
| Low LET | γ rays | Broadbeam | Medium from γ-irradiated epithelial cells reduces the clonogenic survival of recipient unirradiated cells. | (Mothersill and Seymour 1997) |
| X rays | Microbeam | DNA foci are induced by a synchrotron X ray microbeam. | (Usami, Maeda et al. 2006) | |
| High energy electrons | Broadbeam | Medium from cells exposed to high-energy electrons reduces the clonogenic survival of recipient unirradiated cells. | (Gow, Seymour et al. 2010) | |
| Protons | Microbeam | Bystander effects are induced by proton microbeam. | (Frankenberg, Greif et al. 2006, Desai, Kobayashi et al. 2014) | |
Using a variety of mammalian cells maintained under confluent or sparse culture conditions and a multitude of biological endpoints, bystander effects were firmly shown to occur following exposure of a small fraction of cells in a culture to low fluences of α particles delivered from broadbeam irradiators. They were also observed in protocols involving co-culture experiments, and transfer of growth medium from cell cultures exposed to moderate and high doses of X and γ rays, or particulate radiations to unirradiated cultures. A few studies involving medium transfer have also shown that low doses of X and γ rays, similar to those encountered in clinical and occupational settings, induce cytotoxic and clastogenic effects (Mothersill and Seymour 1997; Lyng, Seymour, and Mothersill 2002; Yang, Asaad, and Held 2005).
Using both broadbeam and microbeam irradiators and various cell culture techniques, bystander effects were also shown to be elicited by low fluences of densely ionizing energetic heavy ions (Kobayashi et al. 2009), which is of concern for prolonged human deep space exploration missions (Shao, Furusawa, et al. 2003; Yang, Anzenberg, and Held 2007; Held 2009; Gonon et al. 2013; Autsavapromporn et al. 2015). Bystander effects were also detected in unirradiated cells co-cultured with cells exposed to high doses of external beams of densely or sparsely ionizing radiations (e.g., (Gerashchenko and Howell 2003; Yang, Anzenberg, and Held 2007)). Likewise, they were detected in experiments involving co-culture of cells labelled with short range β emitters (5-[125]iodo-2’-deoxyuridine, 3H-thymidine) with unlabeled cells. In these experiments the labelled cells imparted cytotoxic effects to adjoining non-labeled cells (Bishayee, Rao, and Howell 1999; Xue et al. 2002; Persaud et al. 2005).
In the initial experiments with α particle broadbeam irradiators, the concept of bystander responses provided a suitable explanation for the fact that the number of adversely affected cells greatly exceeded that expected on the basis of an effect due solely to traversal by radiation of an estimated small fraction of nuclei (Nagasawa and Little 1992; Deshpande et al. 1996; Azzam et al. 1998). The development of precision microbeam irradiators allowed to unequivocally identify the cellular organelle that is irradiated and quantifying biological changes in traversed cells and in the bystanders (Prise et al. 1998; Folkard et al. 2001; Randers-Pehrson et al. 2001). Refinements in microbeam technology now permit delivery of a specified number of particles to selected cell compartments with sub-micrometer precision. Mimicking original experiments using a polonium tipped needle positioned at different distances from cell nuclei (Munro 1970), microbeam irradiation has been employed to study the effect of either nuclear or cytoplasmic irradiation on biochemical changes in both the irradiated and bystander cells (Wu et al. 1999; Shao et al. 2004). Recent studies of cells exposed to microbeams of heavier ions (e.g., carbon, neon, and argon) have shown that, for any particle, the induced DNA-damaging bystander effect is dose-dependent and is facilitated by junctional intercellular communication (Suzuki and Tsuruoka 2004; Autsavapromporn et al. 2013). Using a staining technique to distinguish between irradiated and bystander cells in an exposed monolayer culture, Ponnaiya et al. (Ponnaiya et al. 2004) have shown that traversal of a cell by even a single energetic helium ion can induce micronucleus formation and cell cycle delays in bystander cells. Such results visually supported the initial work based on microdosimetric consideration that non-irradiated cells in the population contribute to the risk of exposure to low fluences of α particles. When exposed to low fluences of helium nuclei from microbeam, significantly more cells than those that were irradiated harbored genetic damage leading to enhanced mutations (Zhou et al. 2000) and neoplastic transformation (Sawant et al. 2001), implying that the risk of adverse health outcomes at these levels of exposure can be higher than expected (Brenner, Little, and Sachs 2001).
Overall, a multitude of in vitro studies in various cell types challenged the paradigm that radiation traversal through a cell nucleus is the only prerequisite to produce genetic damage or other biological changes. They indicate that bystander cells in the vicinity of irradiated cells or those incubated in growth media harvested from irradiated cultures may undergo measurable biochemical modifications that are often similar in nature to those induced in the irradiated cells.
In vivo evidence of bystander effects
In addition to observations in cell culture experiments, radiation-induced bystander effects (RIBE) were detected in tissue models and in partial body-irradiated animals. In tissue explants, or reconstituted 3-dimensional tissue models, α particle microbeam irradiation of a thin plane of cells through the tissue induced a variety of biological changes in bystander cells as a function of the distance away from the irradiated cell plane (Belyakov et al. 2002). Further, tissue culture media conditioned by placentae irradiated in utero induced DNA damage in neonatal bone marrow cells cultured ex vivo, supporting the two-hit model of childhood leukemogenesis through bystander signalling (Mansell et al. 2019). Out-of-field effects were also reported in tissues distant from the irradiated area (e.g., (Khan, Hill, and Van Dyk 1998; Kovalchuk et al. 2016; Chai et al. 2013)). Investigation of bystander effects in complex models not only contributed to a better understanding of the phenomenon but also in characterizing the extent of its propagation and its relevance in therapeutic treatments and assessment of health risks (Prise and O’Sullivan 2009; Azzam et al. 2013).
Early in situ investigations using a broadbeam irradiator and cell culture dishes with a solid-state nuclear track detector grafted to the cell growth surface, indicated that low fluence α particles induced the stress responsive protein CDKN1A (p21Waf1) within a 100 μm radius from a targeted cell (Gaillard et al. 2009). Similarly, in the simple living organism Caenorhabditis elegans (Sugimoto et al. 2006; Bertucci et al. 2009) a 1 μm diameter 3 MeV proton microbeam induced bystander stress responses up to ~150 μm away from the irradiated region of the worm. Comparable results were obtained in a mouse ear model with induction of γ-H2AX foci formation as endpoint (Buonanno, Randers-Pehrson, et al. 2015). Models of complex living organisms in which multicellular architecture and physiological condition are preserved have contributed to a better understanding of the various implications of bystander effects in vivo; they informed on the molecular pathways mediating the effects, and showed that redox modulated metabolic events as well as genetic and epigenetic changes occur in bystander tissues (Koturbash et al. 2008; Jain et al. 2011; Chai et al. 2013; Lorimore et al. 2001; Tamminga and Kovalchuk 2011). With significance to cancer risk, oncogenic effects were observed in the cerebellum of radiosensitive mice when only their lower body was X irradiated (Mancuso et al. 2008). Although the radiation-induced bystander/abscopal effect has been linked to deregulation of cellular energetics, oxidative stress, and inflammatory responses (Heeran, Berrigan, and O’Sullivan 2019; Azzam, de Toledo, and Little 2003b) intriguing mechanistic insights continue to emerge.
Mechanistic aspects
Several mechanisms have been shown to mediate RIBE with intercellular communications and oxidative metabolism being extensively investigated (reviewed in (Azzam, de Toledo, and Little 2003b; Spitz et al. 2004; Pouget, Georgakilas, and Ravanat 2018) (Figure 1 with focus on intercellular communications). Often, a crosstalk between the pathways mediating these mechanisms has been described (Little 2003). DNA repair and cell cycle checkpoints were also found to be involved (Huo, Nagasawa, and Little 2001). Moreover, inflammatory responses are emerging as critical pathways in mediating both beneficial and adverse abscopal effects. Here, we review mainly the role of direct and indirect modes of intercellular communications.
Figure 1. Schematic illustrating the propagation of radiation-induced bystander effects in vitro.
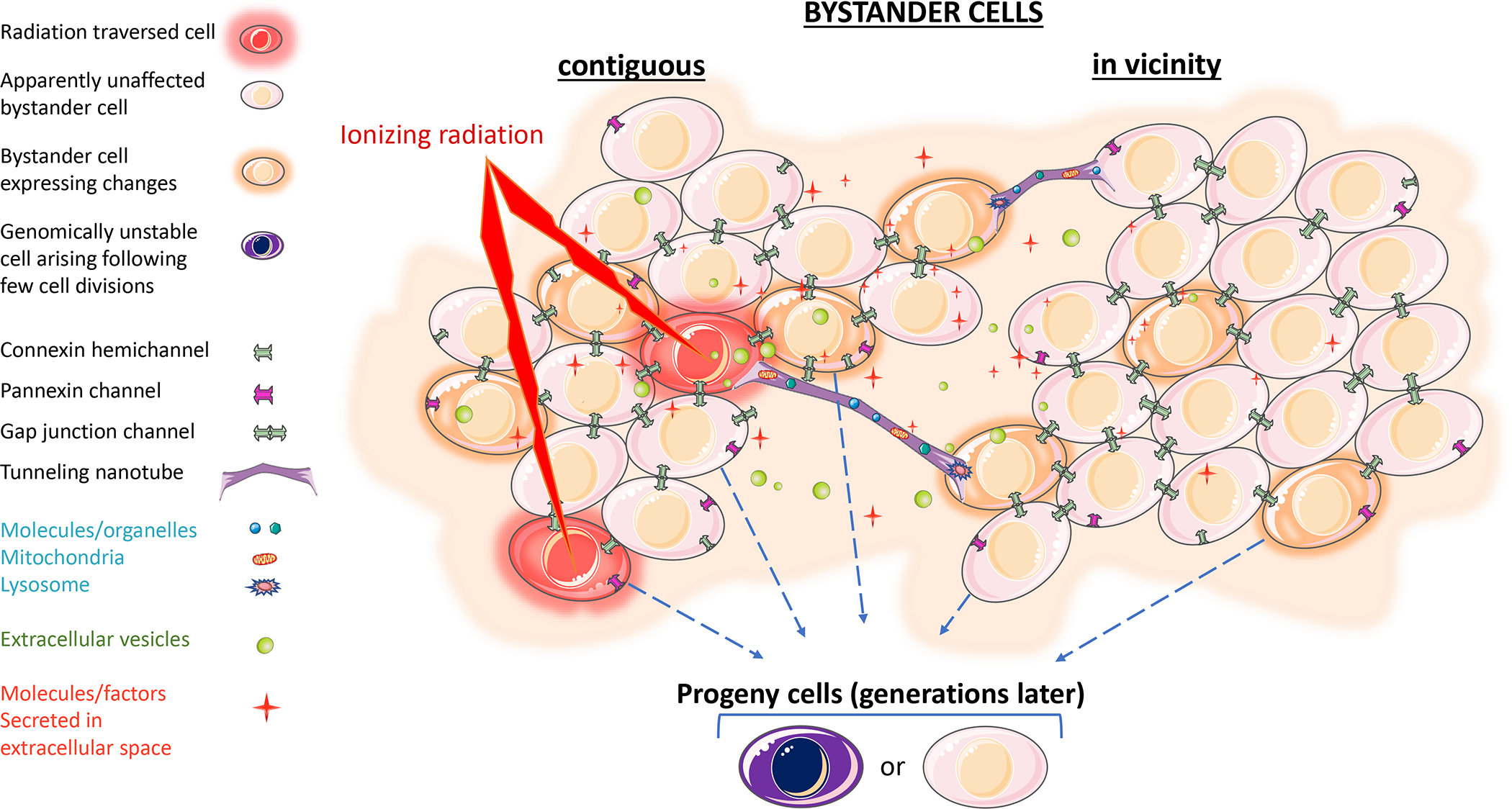
Cells traversed by ionizing radiation propagate molecular signals to non-irradiated cells in the population i) directly via gap junctional channels connecting contiguous bystander/irradiated cells, or tunneling nanotubes connecting cells situated up to 300 μm apart, and/or ii) indirectly via released factors, including soluble molecules and vesicles (contiguous, in vicinity, and distant bystander cells). The propagated signals may induce transient, persistent or delayed effects in the bystander cells. Biological changes may also occur in progeny of both bystander and surviving irradiated cells. The image elements are not to scale with each other. Images were modified using Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License https://smart.servier.com/.
As described in Figure 1, RIBE occur in cells that are contiguous to irradiated cells or in their vicinity, and in distant (abscopal) cells/organs (Figure 2). When bystander cells are adjacent to irradiated cells, transmissible factors may be exchanged intercellularly through junctional channels (e.g., gap junctions) and/or conduits (e.g., tunneling nanotubes, TNTs). On the other hand, vesicles, cytokines, and other molecules secreted by irradiated cells/tissues can lead to biochemical changes in bystander cells in their vicinity or in cells/tissues that are distant. These effects occur at lethal or sub-lethal doses of radiation, and when they occur in vivo may involve participation of the immune system (Rodriguez-Ruiz et al. 2018). When lethal doses of radiation are applied, different profiles of secreted factors result in bystander or abscopal effects. It is thought that the responses depend on the magnitude of injury and the induced mode of cell death and occur with or without involvement of the immune system (Heeran, Berrigan, and O’Sullivan 2019; Grass, Krishna, and Kim 2016). Hence, in purview of mechanistic aspects, inter- and extracellular modalities of communication are discussed below (Figure 2 with focus on various forms of in vivo intercellular communications: cell-to-cell contact, paracrine and non-paracrine signaling).
Figure 2. Schematic illustration of radiation-induced bystander effect (RIBE) and radiation-induced abscopal effect (RIAE) at the cellular, tissue and organism levels in the context of cancer radiotherapy.

At the organism level (A), when a tumor (for example lung tumor here) is irradiated, two scenarios may arise. In the first, an effect may be propagated from the irradiated to unirradiated tumor or normal regions (RIBE). In the second situation, irradiation of the tumor may induce systemic changes affecting distant organs (e.g., brain and liver as described here) or metastatic tumor sites. The effect would not be only unidirectional (i.e., from irradiated to unirradiated organs/tissues), but also bidirectional (as illustrated by the arrows), and the magnitude of the effects may vary depending on several factors (magnitude of radiation damage, redox environment, type of tumor/normal tissue, etc.). At the tissue level (B), induced effects may be transmitted from irradiated tumor tissue to unirradiated tumor or normal tissue regions. At the cellular level (C), irradiated cells transmit the bystander signals to contiguous cells directly through gap junctions, or to distantly located cells through tunneling nanotubes (TNTs), which may involve transfer of cell organelles (like mitochondria, lysosomes, ions/molecules) to bystander cells. Irradiated cells may also secrete soluble factors (e.g., cytokines/chemokines, ions, ATP), and extracellular vesicles among others, which are transferred to distant locations through circulating blood. Secretion of soluble factors affecting close or distant cells may involve pannexin channels. The RIAE at distant organs or metastatic sites may occur with or without involvement of the immune system. These effects may manifest early or may be expressed in the progeny of bystander or irradiated cells through mechanisms of genomic instability. The image elements are not to scale with each other. Images were modified using Servier Medical Art by Servier which is licensed under a Creative Commons Attribution 3.0 Unported License https://smart.servier.com/.
A. Intercellular communications
A.1. Gap junctions:
Under normal physiological conditions, gap junctional intercellular communication (GJIC) is required for maintenance of tissue homeostasis. It is also a major pathway for cell-to-cell transmission of the toxic effects of radiation and chemotherapeutic agents (reviewed in (Aasen et al. 2019)). Its role has been studied at the onset to understand the means of propagation of radiation-induced stressful effects under both in vitro and in vivo conditions. Gap junctions are integral membrane channels composed of diverse isoforms of connexin (Goodenough, Goliger, and Paul 1996). They link the cytoplasm of contiguous cells through the exchange of small molecules (<1 kDa), such as atomic ions (K+ and Ca2+), second messengers (e.g., cAMP, cGMP, IP3), glutathione, glucose and other molecules that permeate through their aqueous pores. In the process, they couple contiguous cells not only metabolically but also electrically (Harris 2018). A gap junction is composed of connexin (Cx) proteins, with Cx43 being the most abundant and well-studied form in the Cx family (21 isoforms in humans). Notably, different Cxs form channels with distinctive selectivity for molecular permeants (e.g., second messengers (Niessen et al. 2000; Harris 2007)) leading to transmission of either damage or rescue signals between irradiated cells and their neighboring bystanders (de Toledo et al. 2017; Zhao et al. 2014). The phosphorylation of Cx proteins governing their gating and trafficking properties is a post-translational modification that was found to be exquisitely sensitive to low fluence α particle irradiation (Azzam, de Toledo, and Little 2003a). Under stress conditions, gap junctions facilitate rapid intercellular communication of shared biochemical signals, which contributes to attenuating dilution or degradation effects that may occur when these signals are secreted extracellularly.
Initial evidence for the involvement of gap junctions in RIBE was generated after exposure of cell cultures to low fluences of broadbeam α particles. In studies involving confluent, density inhibited human diploid fibroblasts, a higher fraction of cells than predicted upregulated the level of stress inducible CDKN1A (p21Waf1). The effect was abrogated by treatment with the gap junction inhibitor lindane (Azzam et al. 1998). Direct evidence of the involvement of Cx43 was subsequently generated through genetic approaches revealing DNA damage and other stress responses in a higher fraction of cells than the nuclei traversed by α particles. The effect was observed only in CX43 wild-type and gap junction-competent cells (Azzam, de Toledo, and Little 2001). The role of gap junctions in transmitting lethal and mutagenic effects to bystander cells was studied in a variety of other cell types exposed to low fluences of high LET particulate radiations (Zhou et al. 2001; Suzuki and Tsuruoka 2004; Suzuki, Yasuda, and Kitamura 2020) or photon radiation (Hoorelbeke et al. 2020). Together, the studies showed that radiation-induced elevation of reactive oxygen species (ROS) generation is associated with gap junctional permeation of signaling molecules between irradiated and bystander cells. For example, due to its size and charge, Ca2+ easily permeates through gap junctions (Harris 2018), and previous studies have described the contribution of Ca2+ to bystander effects (Lyng et al. 2006).
The role of GJIC in propagating radiation induced effects to distant bystander sites has been also reported in vivo. The oncogenic bystander effect in the shielded cerebellum of radiosensitive Patched-1 (Ptch1) heterozygous (Ptch1+/−) mice exposed to X rays shown by Mancuso et al. (Mancuso et al. 2008) was mitigated after treatment with 12-O-tetradecanoylphorbol-13-acetate, a potent protein kinase C activator and cell-to-cell communication inhibitor. Although numerous studies have addressed the role of connexin phosphorylation in assembly/disassembly, degradation, and gating of the channels they form (Lampe and Lau 2004), the role of Cx phosphorylation in RIBE effects warrants further investigation. Interestingly, in α particle-irradiated non-tumorigenic HepG2 cells, activation of Src kinase leading to a Cx43 phosphorylation (Tyr265) responsible for RIBE was regulated by ROS-mediated autophagy (Yang et al. 2018). On the other hand, exposure of human A549 lung cancer cell cultures to a low fluence of α particles resulted in enhanced proliferation and a greater tumor volume upon implantation in SCID mice. The effect was also associated with increased phosphorylation of Cx43, but a decrease in GJIC (Rajan and Pandey 2021). Studies examining phosphorylation changes in specific amino acids of Cx proteins in irradiated cells and whether these changes enable or inhibit functional junctional communication would shed light on the underlying biochemical events.
It has been proposed that in addition to extracellular release, long-lived reactive oxygen and nitrogen species permeate among cells through gap junctions (Upham and Trosko 2009). Notably, membrane bound NAD(P)H oxidase, which has been implicated in α particle-induced adverse bystander effects (Narayanan, Goodwin, and Lehnert 1997; Azzam et al. 2002), is located in cholesterol-rich domains of the plasma membrane where junctional channels are also localized. In addition, recent reports have shown that gap junctions are permeable to microRNAs (miRNAs) (Xu et al. 2015; Hong et al. 2015). The latter are ~22 nucleotide long single-stranded non-coding RNAs that form linear molecules with a diameter of ~1 nm in the order of gap junction channel pore size. Their highly efficient transfer between irradiated and bystander cells (Zong et al. 2016; Peng et al. 2019) would control the expression of target genes, leading to significant phenotypic and behavioral changes. There is evidence that different Cx channels display differential permeability to miRNAs (Cx43 > Cx26/30 > Cx26 > Cx31 > Cx30) (Zong et al. 2016). Whereas Cx mutations and gap junction blockers eliminate miRNA transfer, gap junction enhancers and overexpression of Cx increase it (Zong et al. 2016). Therefore, gap junction mediated transfer of miRNA may play a crucial role in the mechanism(s) underlying RIBE and is a significant avenue for further investigation, particularly in the search for mitigators of adverse effects and in treatment of gap junction competent tumors.
A.2. Bidirectional bystander effects and the role of communication channels
Contrary to canonical unidirectional bystander signals propagated from irradiated cells, bidirectional transmission of signals has been observed under various conditions, showing that transmission of signals from bystander to irradiated cells may attenuate or enhance the stress induced in irradiated cells, at least transiently. Notably, a rescue effect leading to attenuation of DNA damage in proton-irradiated cancer cells was detected only when the cells were co-cultured with normal bystander cells, and not with other cancer cells (Desai et al. 2014). The communication of protective bystander effects has been also reported after exposure to other radiation types (photons, α particles) (Yu 2019) but with limited mechanistic insights. Compensation for cyclic adenosine monophosphate (cAMP) levels in irradiated cells by bystander cells was suggested to contribute to the rescue mechanism (He et al. 2014). Other studies have shown that rescue effects by bystander cells involve nitric oxide (•NO) and the NF-κB pathway (Yu 2019), as well as autophagy (Kong, Cheng, and Yu 2018).
The role of the selective permeability of junctional channels in propagating damaging or protective effect was also investigated in studies of long-term effects in progeny of bystander cells. Whereas rescue effects were communicated at early times through junctional channels consisting of Cx32 (Autsavapromporn et al. 2013), several generation of replication later, the progeny of bystander cells that communicated with irradiated cells through Cx32 channels expressed elevated levels of spontaneous DNA damage (de Toledo et al. 2017). In contrast when irradiated and bystander cells communicated through junctions consisting of Cx26 or Cx43, the harmful effects detected in bystander cells within hours after co-culture decayed in their progeny, perhaps as a result of death or permanent proliferative arrest of the affected bystander cells (de Toledo et al. 2017). These results suggest that intercellular communications play different roles under stress conditions, and permeability of the junctional channels contribute to both early effects in bystander cells and delayed effects in their progeny. The permeability is likely impacted by the redox state of the cell and radiation-induced post-translational modifications of the connexins forming the channel (Zhao et al. 2014). Clearly, identification of the modified amino acids (phosphorylated, nitrosylated, ubiquitinilated) sites and the responsible enzymes, together with the communicated molecules, may help development of countermeasures to harmful effects and may enhance the efficacy of radiotherapeutic treatments.
A.3. Pannexin channels and tunneling nanotubes
Like Cx hemichannels, pannexin (Panx) channels are rapidly activated following exposure to stressful agents and mediate the controlled release of small signaling molecules such as ATP, which has been associated with expression of radiation bystander/abscopal effects (Ohshima et al. 2012). Along with recruiting inflammatory cells to the site of insult, extracellular ATP activates cellular receptors, such as P2 receptors, in an autocrine and paracrine fashion to activate caspases (Aguirre et al. 2013), thus triggering death of out-of-field cells. While Panxs have no sequence homology to Cxs, both proteins have similar membrane topology (Penuela, Gehi, and Laird 2013) and both are regulated by redox potential, thus examining their role in bystander effects is warranted.
Tunneling nanotubes (TNTs, also referred to as tumor microtubules in tumors) are intercellular bridges that form in vitro and in vivo. TNTs act as conduits with variable diameter (~ 50–800 nm) connecting cells situated up to 300 μm apart. They enable the bidirectional transfer of proteins, RNAs, organelles, pathogens etc. (Rustom et al. 2004). They are formed during normal developmental processes in immune cells, are present among tumor cells, and between tumor and immune cells residing in their micro-environment (Patheja et al. 2015; Patheja and Sahu 2017). They have been reported to cause chemo-resistance and promote metastasis of cancer cells (reviewed in (Ariazi et al. 2017)). Interestingly, TNTs possess gap junctional components (e,g., Cx43) and enable the transfer of electrical signals between remote cells in a manner dependent on the presence of connexons interposed at the membrane interface between TNT and the connected cell (Wang et al. 2010), which leads to speculate that these two intercellular communication systems have evolved to complement each other in multicellular organisms. Even though numerous publications have described the role of TNTs in cancer and normal cells, only one recent study reports about their effect in the response to irradiation. In this study, faster and greater TNT network formation was observed in α particle-irradiated human glioblastoma cells (Matejka and Reindl 2020). Since TNTs provide an efficient conduit for the transfer of molecules/organelles among distant cells, investigating their role in communication of RIBE would further enhance our understanding of the underlying mechanisms and range of propagation of the effect.
B. Extracellular vesicles
Under normal physiological conditions and during stress, cells release a variety of extracellular vesicles (EVs). Whereas these EVs were initially considered as cell debris, with advances in characterizing their content (miRNA, mRNA, DNA, non-coding RNA, proteins, lipids, mitochondria, etc.) and their association with pathological conditions, they have emerged as key modalities for intercellular communication (Devhare and Ray 2018; Malloci et al. 2019). Depending on their origin, EVs are classified as exosomes, microvesicles (or microparticles), apoptotic bodies, large oncosomes, and microplasts. While exosomes (50–130 nm) are created from an involution of endosomes, microvesicles are larger in size (100–1000 nm) and are produced by direct outward budding of the plasma membrane. EVs released by apoptotic cells (apoptotic bodies) are larger (100–5000 nm), and oncosomes are even bigger (1–10 μm).
The ability to deliver their varied content to recipient cells makes EVs ideal candidates for an active role in radiation bystander and abscopal effects. However among the EVs, it is primarily exosomes that have been studied in the context of RIBE (reviewed in (Al-Mayah et al. 2015)). Both the magnitude of release and the composition of exosomes’ cargo are altered following irradiation (Jelonek, Widlak, and Pietrowska 2016; Colangelo and Azzam 2020), which renders them efficient ‘extracellular messengers’ able to modulate signalling pathways that control survival and physiological functions in recipient cells. In vivo, exosomes are stable and are continuously produced in the human body. Analyses of their content revealed increases in the levels of proteins involved in important functions, including ROS metabolism, DNA repair, chromatin packaging, protein folding, and metastasis, among others (Abramowicz et al. 2019; Colangelo and Azzam 2020). Together, the studies provided insight into the mechanism underlying the responses to irradiation (increased killing, invasiveness of tumor cells, regression of metastatic foci, etc.), and informed on how the tumor microenvironment modulates radiosensitivity (He et al. 2021).
With respect to bystander signaling, exosomes purified from growth media of irradiated cells or sera of irradiated mice were found to contain mitochondrial DNA that increased γ-H2AX and 53BP1 foci in recipient cells (Ariyoshi et al. 2019). In other studies, exosomes shed from irradiated glioma cells decreased proliferation of bystander neural stem cells, and when injected in the hippocampi of mice, caused inhibition of neurogenesis and cognitive impairment (Yang et al. 2021). Furthermore, exosomes derived from the organs of whole or partial body irradiated mice led to changes in viability, DNA damage, and redox changes in recipient mouse embryonic fibroblasts (Tuncay Cagatay et al. 2020). Even though exosomes harbour a varied cargo, it is their role as miRNA carrier that has been mainly studied in RIBE. Significantly, exosomes released from irradiated human pancreatic cancer cells showed higher internalization than exosomes from non-irradiated cells, and resulted in miR-6823-5p mediated inhibition of antioxidant defense and increase of DNA damage in bystander cells (Nakaoka et al. 2021). In other studies, exosomes containing miR-1246, when secreted from γ-irradiated human bronchial epithelial cells affected the efficiency of non-homologous end joining (NHEJ) pathway of DNA repair in bystander cells (Mo et al. 2018). Moreover, γ-irradiation of mouse brain caused the release of exosomal miR-7 triggering autophagy in shielded lung (Cai et al. 2017) by mechanisms likely involving disruption of the blood-brain-barrier (reviewed in (Gao et al. 2021)). Exosomal miR-7-5p from γ-irradiated epithelial bronchial cells enhanced autophagy in recipient cells through EGFR/Akt/mTOR signaling (Song et al. 2016).
In addition to exosomes, it was found that microparticles derived from irradiated tumor cells control tumor growth through ferroptosis and immune reprogramming by repolarizing pro-tumoral M2 macrophages to anti-tumoral M1 macrophages (Wan et al. 2020). A limited literature exists about other EVs (microvesicles, microplasts, large oncosomes) in the cellular response to radiation, and to our knowledge, their role in the mechanism of bystander/abscopal effects has not been reported. In sum, the various EVs and their content constitute promising biomarkers of radiation exposure and provide new avenues for therapeutic interventions, including the modulation of amount and function of candidate molecules through selective enrichment or inactivation.
C. Secreted molecules
Several studies examined alterations in the nature and level of factors secreted by cultured cells exposed to radiation (Liu et al. 2006; Balduzzi et al. 2010), including proteomic profiling of supernatant from γ-irradiated cells revealing increased levels of certain proteins (e.g., annexin A1, actin (cytoplasmic 1/2)), but decreased levels of others (e.g., annexin A2, cofilin (Desai et al. 2016). Analyses of the secretome of X-irradiated reconstituted 3-dimensional skin tissue model showed that release of certain proteins (e.g., carboxypeptidase E and ubiquitin carboxyl-terminal hydrolase isozyme L1) is independent of the exposure dose, but activation of others (e.g., the proteasome activator complex subunit 2 protein) is dose-dependent (Zhang et al. 2014). In other studies, peroxidase was increased when a small proportion of low dose X-irradiated cells were co-cultured with a large fraction of bystander cells (Abdelrazzak et al. 2016). Notably, it is the retrospective analysis of cytokines and chemokines in the sera of patients receiving radiotherapy that alerted on the role of inflammatory/immune responses in beneficial abscopal effects (Ohba et al. 1998).
Among the secreted extracellular factors/molecules, cytokines and chemokines orchestrate the interactions among epithelial, mesenchymal and immune cells under physiological and stress conditions. Radiation dose, dose rate, and type, as well as the origin of the irradiated tissue govern the nature and magnitude of cytokines release. Characterization of analytes in the secretome of human cancer cell lines (HT1080, U373MG, HT29, A549 and MCF-7) under normal culture conditions and after acute single bolus or fractionated doses of γ radiation revealed the presence of cytokines/growth factors, namely TNF-α, IL-1β, PDGF-AA, TGF-β1, fractalkine, IL-8, VEGF and GCSF. A dose-dependent increase in the levels of most of these molecules was observed, with fold changes being lower after fractionated compared to acute single bolus doses, and A549 showing the largest changes. Functionally, clonogenic survival of bystander A549 cells treated with medium harvested from irradiated cells was decreased (Desai, Kumar et al. 2013). More recently, fractionation of an 8 Gy X ray dose delivered to the thoracic region of mice was shown to induce DNA damage and apoptosis in distant bone marrow cells, which was attributed to an increase in systemic TNF-α and serum amyloid A (Song et al. 2021). In other studies with high LET radiations, secreted IL-33 and prostaglandin-2 (PGE-2) activated the NF-kappa B and MAPK pathways through autocrine and paracrine types of communication (Ivanov et al. 2010), and IL-6 and IL-8 enhanced generation of ROS/RNS in bystander cells (Mariotti et al. 2012).
Melanoma differentiation associated gene-7 (mda-7), a member of the expanding interleukin (IL)-10 gene family named mda-7/IL-24, exhibits selective killing and radio-sensitization effects in cancer cells and not in normal cells, rendering it unique in its function. MDA-7/IL-24 binds to its receptor dimers (IL-20R1 and IL-20R2)/(IL-22R1 and IL-20R2), which in turn activates JAK/STAT3 downstream signaling to control proliferation and differentiation of target cells (Menezes et al. 2014). Mda-7/IL-24 secreted by irradiated normal cells was cytotoxic to bystander tumor cells with functional IL-20/IL-22 receptors and those overexpressing anti-apoptotic bcl-2/blc-xL (Su et al. 2005); such results encourage characterizing the role of this protein in abscopal effects.
Factors such as nitric oxide are also initiators/mediators of intercellular signalling. Irradiation stimulates inducible nitric oxide synthases, thereby generating large amounts of •NO, which upon reacting with O2•− forms highly reactive peroxynitrite anion capable of attacking a wide range of cellular targets (Azzam, Jay-Gerin, and Pain 2012). Being hydrophobic in nature, •NO is not dependent on gap junctions for transmission to bystander cells as it can diffuse through the plasma membrane. •NO release can cause post-translation modifications of target proteins through S-nitrosylation and tyrosine nitration (Yakovlev 2015), events that greatly impact the function of antioxidants such as thioredoxin and perroxiredin (Wu et al. 2010). •NO mediated bystander signaling in high LET irradiated cell cultures was shown to involve downstream Cox-2 and Nrf-2 pathways (Tomita et al. 2015; Han et al. 2010) with increase in nitrile concentrations in human lung fibroblasts (Yokota et al. 2015). Notably, microbeam irradiation with energetic helium ions showed that inhibition of inducible nitric oxide synthase prevents the increase in DNA damage in bystander cells (Shao, Folkard, and Prise 2008).
The release of damage associated molecular patterns (DAMPs) from dying irradiated cells is a prominent feature of immunogenic cell death (ICD), which was reported to cause in few instances regression/tumor control at distant sites (Golden and Apetoh 2015). In the process, the released DAMPs trigger the engulfment of released tumor antigens by dendritic cells, thereby improving antigen presentation to cytotoxic immune cells whose migration and function is facilitated by secreted cytokines (Golden et al. 2014). Clearly, thorough elucidation of the underlying mechanism may be exploited in controlling the growth of micrometastases and tumors at distant sites (Reynders et al. 2015). Within this framework, HMGB1 released from irradiated breast cancer cells was shown to promote bystander macrophages to secrete TNF-α through TLR-4 pathway responsible for inhibition of proliferation and migration of bystander cancer cells (Zhu et al. 2021).
Relevance of radiation-induced bystander effects
Radiation protection
Residential radon:
The manifestation of adverse bystander effects in cell populations exposed to low fluences of high LET radiation such as α particles imply an impact on the assessment of cancer and other health risks (Figure 3). Because health effects at low level radiation exposures are uncertain and require large human cohort size to achieve statistical significance, mechanistic laboratory studies are essential to understanding biological effects and characterizing the extent of health risks. Currently, assessment of lung cancer risk from exposure to environmental radon (e.g., in poorly ventilated homes and workplace) is based on extrapolation of data from studies of uranium mine workers who are typically exposed to inhaled radon at rather higher doses while simultaneously inhaling/ingesting uranium ore dust (Brenner and Sachs 2003, 2002). However, during exposure in residential situations, the majority of cells in the bronchial epithelium are not traversed by an α particle track from radon gas and its decay products. Based on experimental studies, these cells may show deleterious bystander responses. In addition, the progeny of such bystander cells may be prone to increased genomic instability (de Toledo et al. 2017; Hu et al. 2012).
Figure 3: Relevance of radiation-induced bystander effects (RIBE).

RIBE are relevant for radiation protection and therapeutic applications. The manifestation of RIBE during environmental exposures such as residential radon or occupational exposures (e.g., mining, energy generation, clinical work, space exploration) may contribute to biologically based dose response models that seek to reduce the uncertainty in estimating health risks associated with low doses/low fluence exposures. With regard to radiotherapy, out-of-field/abscopal effects may contribute to improve outcomes, but may also contribute to emergence of degenerative conditions and second cancers.
Space exploration:
Quantification of health risks from occupational radiation exposures also concerns space exploration. During mission, only parts of the astronaut body are irradiated at any one time (Cucinotta, Nikjoo, and Goodhead 1998), and radiation traversals are separated in both tissue location and time (Held 2009). It has been estimated that during transit beyond low Earth orbit, every cell nucleus within an astronaut’s body is traversed, on average, by an energetic proton every few days, by a helium nucleus once every few weeks, and by heavier high-charge particles (HZE) every few months (Blakely 2000; Cucinotta, Nikjoo, and Goodhead 1998) while the rest of the cells would be bystanders. Besides highly localized energy deposition, HZE particles give rise to secondary radiation along the track due to heavy ion fragments and energetic electrons (i.e., δ rays). Whereas the radial spread of dose due to secondary heavy ion fragments extends up to 10–20 μm (Gonon et al. 2013), the range of δ rays can extend up to several cell diameters (Metting et al. 1988; Cucinotta, Nikjoo, and Goodhead 1998). In cell cultures exposed to doses wherein a small fraction of the cells are targeted with HZE particles, markers of damage were increased in more cells than expected based on the number of cells traversed by HZE particles (Gonon et al. 2013). Using an insert co-culture system that allowed investigating HZE-particle-induced bystander effects in the absence of δ rays and secondary fragmentation products, bystander effects were shown to persist in progeny of bystander cells many generations after co-culture with HZE particle-irradiated cells (Buonanno et al. 2011), and depended on the type of junctional channels that connected the irradiated donor cells with the bystander cells (de Toledo et al. 2017). The propagation of such detrimental effects was not observed in progeny of bystander cells present in cultures where less than 1% of the cells were exposed to microbeam protons, suggesting LET-dependence (Autsavapromporn et al. 2015). The possibility of increased risk of carcinogenesis caused by exposure to space radiation during prolonged space travel has been considered a limiting factor for human exploratory missions (National Research Council 2008). Work in mouse embryo fibroblasts has supported this premise by showing increased frequency of spontaneous neoplastic transformation in progeny of bystander cells from cultures exposed to densely ionizing radiations, including HZE or α particles (Buonanno, de Toledo, and Azzam 2011). Notably, gap junctional communication contributed to mediating the effect. Hence, whether in the case of residential radon or space exploration, studies of bystander effects have the potential to inform the available limited epidemiological surveys.
Normal tissue damage and second cancer formation:
The development of genomic instability in progeny of bystander cells (Hu et al. 2012; de Toledo et al. 2017), in particular the work showing that these progeny are at a greater risk of developing spontaneous neoplastic transformation (Buonanno, de Toledo, and Azzam 2011) is a fitting explanation for the emergence of degenerative conditions and second cancers at sites distant from the irradiated primary tumor, in particular in survivors of childhood cancer (Friedman et al. 2010). In this context, there is need for studies of bystander effects in stem cells: when harmed, tissue homeostasis may be perturbed as they differentiate, which would lead to the development of pathologic conditions and impaired response to therapy. Thorough characterization of the intercellular communications and the signaling pathways they affect would enhance understanding the basis of secondary effects of radiotherapy, and guiding novel interventions that mitigate adverse out-of-field delayed outcomes.
Out-of-field effects and enhancement of radiotherapy benefits
Evidence for the relevance of bystander effects in therapeutic treatments with radionuclides was generated when tumor cells labeled with 5-[125I]iodo-2-deoxyuridine (DNA bound I-125 emits electrons with subcellular range) were mixed with non-labeled tumor cells and administered subcutaneously into nude mice. Compared to control, growth of the tumor formed by the cell mixture was significantly slowed (Xue et al. 2002). Similar results were obtained with studies involving γ rays where slow growth of the formed tumor was associated with increased senescence and poor angiogenesis (Desai et al. 2016). With relevance to treatment of cancer disseminated to bone, studies with a mouse model of metastatic breast cancer have shown that the bone seeking α particle–emitting radiopharmaceutical radium-223 dichloride delayed, in a bystander manner, the growth of tumor cells disseminated to the marrow (Leung et al. 2020; Canter et al. 2021). These studies highlight the role of bystander effects in enhancing the effectiveness of targeted radionuclide therapy where uptake of radioactivity is often non-uniform.
In the past two decades there has been a marked interest in harnessing the power of the intercellular communications mediating abscopal responses for beneficial outcomes. Compared to bystander effects that are mostly facilitated by intercellular communications involving cell-to- cell contact and signaling pathways triggered by secreted factors, abscopal effects promoting regression of distant unirradiated tumors are thought to be mostly immune-mediated (Demaria et al. 2004). In the process, the irradiated tumor becomes an in situ vaccine whereby DNA fragments released by irradiated cells activate the cGAS/STING [cyclic GMP-AMP synthase (cGAS)/stimulator of Interferon genes (STING)] pathway, which triggers release of a variety of inflammatory cytokines and chemokines that help recruit innate and adaptive immune cells to the tumor (Constanzo et al. 2021). Immature dendritic cells become active and mature, and cytotoxic CD8+ T lymphocytes get activated (Golden et al. 2014).
By building on pioneering work showing the power of the host’s immune capability in radiocurability (Stone, Peters, and Milas 1979), research on the abscopal effect is translating in promising proof-of-principle clinical trials (Golden et al. 2015). Whether the mechanisms underlying the abscopal effect can be also combined with PD-1/PD-L1 checkpoint inhibitors is attractive to investigate. Likewise, elucidating how cytosolic DNA is transported from irradiated to bystander cells, and whether charged particles are more effective than photons at inducing clinical abscopal effects would be enlightening.
In addition to abscopal effects, immune system activation is assumed to contribute to the benefits of microbeam/spatially fractionated radiotherapy (Kanagavelu et al. 2014). In this modality, cells receiving very large doses are contiguous to cells receiving lower doses. The latter mimic bystander cells and are thought to receive signals from neighboring cells in the high dose region (Desai et al. 2014; Dilmanian et al. 2007; Asur et al. 2012). Remarkably, while the volume of tissue that is irradiated in this modality is reduced, yet tumor regression is observed with reduced normal tissue toxicity and the spared healthy tissue acting as center of regeneration ((Prezado et al. 2018) and reviewed in (Yan et al. 2020)). Several in vitro and in vivo studies (Suchowerska et al. 2005; Asur et al. 2012; Butterworth et al. 2011) have implicated radiation-induced abscopal effects as potential mediators of biological responses following advanced radiotherapy modalities such as fractionated radiation therapy (GRID) and intensity modulated radiotherapy (IMRT) (Asur et al., 2015; reviewed in Griffin et al. 2020). In sum, while the contribution of out-of-field effects to the balance between tumor eradication and normal tissue complications in the context of radiotherapeutic regimens remains ripe for further investigations (Suit et al. 2007; Newhauser and Durante 2011; Durante, Brenner, and Formenti 2016), examination of the intercellular communication mediated by immune responses promises to result in translational clinical applications.
Conclusions and future directions
Though research in the past three decades has revealed how out-of-field radiation effects contribute to adverse outcomes, more recent work is investigating how the underlying mechanisms can be harnessed to help achieve beneficial effects. In either case, the investigations have shown that both direct and indirect modes of intercellular communications, with and without involvement of the immune system, are pivotal in mediating these effects. These intercellular communications were found to be exquisitely sensitive to redox modulation (Azzam et al. 2002; Narayanan, Goodwin, and Lehnert 1997), indicating the participation of ROS regulated pathways with significant support from work involving the role of perturbations in oxidative metabolism whether through disruption of mitochondrial function (Zhou et al. 2008) or treatment with antioxidant enzymes (Azzam et al. 2002; Lehnert, Goodwin, and Deshpande 1997). Elucidating the sequence of the early molecular signaling events underlying the propagation of oxidative stress and induction of DNA damage in bystander cells would enhance our understating of the phenomenon, in particular when the irradiated cells are targeted with either high or low LET radiations. Since the landmark report of Nagasawa and Little (Nagasawa and Little 1992), there has been ample evidence indicating that in presence of functional gap junctions between affected cells, the induced oxidative stress is rapidly amplified (Zhao et al. 2014). Furthermore, the progeny of those cells, and those adjacent but not directly damaged, exhibit propagated DNA damage and elevated ROS even though they are many generations removed from the radiation exposure. On the other hand, signaling events propagated from cells exposed to low dose/low fluence radiation were also shown to protect bystander cells, at least transiently, against oxidative damage from normal metabolism or exposure to a challenge dose of radiation (Portess et al. 2007; Rajan and Pandey 2021; Buonanno, De Toledo, et al. 2015). Follow up studies in progeny cells have informed on whether early harmful effects decay and whether early apparent protective effects are followed by an effect on intracellular metabolic redox reactions that contribute to genomic instability. Although abundant evidence described adverse abscopal effects (Mancuso et al. 2008; Mancuso et al. 2011; Chai et al. 2013; Koturbash et al. 2008; Jain et al. 2011), proteomic studies in affected abscopal organs revealed modulation of pathways leading to increased stress, and also increased defense against the induced oxidative stress (Jain et al. 2011). Therefore, the net effect likely involves a role for the tissue microenvironment and immune reprogramming (Ochoa de Olza et al. 2020).
Studies of radiation-induced bystander responses are yet another instance where the radiation sciences have contributed to basic knowledge. They have led to new understanding of the intercellular communications that occur under mild and severe radiation stress, in both normal and cancerous tissues. They have highlighted the critical role that the plasma membrane exercises as initially emphasized by Tikvah Alper (Alper 1977). It is in the plasma membrane where connexin and pannexin channels, cyclooxygenase-2, sphingomyelinase, NAD(P)H oxidase, cytokine and chemokine receptors, and key kinases that regulate gating of communicating channels are localized, with many residing in its cholesterol rich domains. Therefore, studies of lipid mediators and changes in cholesterol levels in the plasma membrane would inform not only on the microenvironment of communicating channels, but also on events associated with inflammatory responses (Laiakis et al. 2014). Together the findings promise to translate in enhancing the efficacy of cancer treatments, whether by radiation or other treatment modalities. Enhancement of intercellular communication by a mild treatment with one modality (e.g., gap junctional communication by mild hyperthermia (Azzam, de Toledo, and Little 2003b), may increase the efficacy of radiotherapy through bystander/cohort effects. Conversely, elucidation of the intercellular communications between normal bystander cells and tumor cells treated with novel techniques such as SBRT (Stereotactic Body Radiation Therapy), SFRT (Spatially Fractionated Radiation Therapy) or hadron therapy (Tubin et al. 2020) may inform on adverse effects that may develop long after the treatment. Knowledge of these communications and how they are regulated will likely refine the delivery of treatments and advise on measures that mitigate harm. The growing interest within the Radiation Science community to elucidate the mechanisms by which FLASH radiotherapy (i.e., irradiation at ultra-high dose rate) achieves tumor control while sparing healthy surrounding organs, may shed light on the relationship between dose-rate and the manifestation of bystander/abscopal effects when compared with outcomes observed following conventional treatments. Long term follow up studies in experimental animals will inform on whether delayed adverse out-of-field emerge following FLASH radiotherapy, which is currently unknown.
To reiterate, rapid advances in the past three decades in detecting and quantifying biological changes strongly show that when specific cells or tissues are traversed by ionizing radiation, a significant proportion of non-irradiated cells and tissues adjacent to the irradiated zone, or distant from it, experience measurable biological changes that are transient, persistent, or whose expression emerge at later time. Important biological changes may also manifest in their progeny. Hence, bystander and abscopal responses, as well as the expression of genomic instability in progeny of surviving bystander or irradiated cells cannot be considered as non-targeted effects. The cells and tissues that were not themselves irradiated are also targets of the radiation exposure and experience biochemical changes that are often similar to the cells traversed by radiation. Therefore, classification of these responses as non-targeted effects may be inappropriate. Here, it is proposed that these effects be classified as out-of-field effects of ionizing radiation.
While further work is needed to elucidate the role of the redox environment and sensitivity of the irradiated tissue in propagation of bystander/abscopal effects, studies of the modulating effect of age and sex of the organism would be enlightening. The use of human organoids will help compare findings obtained in animal models. Together, the results will contribute to generation of biologically based dose response models in which out-of-field effects will help reduce the uncertainly in predicting the risk of adverse health outcomes following low dose/low fluence environmental or occupational radiation exposures (NCRP 2020) and enhance understanding of secondary outcomes of radiotherapy (Friedman et al. 2010).
Supplementary Material
Table 2:
Major intercellular routes and signaling events in the mechanisms of radiation-induced bystander effects
Acknowledgements
We thank Sonia M. de Toledo for her instrumental participation in our research on the radiation bystander effect. We also thank Roger W. Howell and members of the Division of Radiation Research at Rutgers New Jersey Medical School for the many helpful and instrumental discussions. EA thanks Walter Schimmerling for insightful conversation on classification of bystander effects. This work received support from the National Institutes of Health (Grants CA92262 and CA049062), the National Aeronautics and Space Administration (grants NNJ06HD91G and NNX15AD62G), the US department of Energy Low Dose Radiation Research program (DE-FG02-02ER63447), the New Jersey Commission on Cancer Research (Grant No. 02-1081-CCR-S2), and the Federal Nuclear Science and Technology program at Canadian Nuclear Laboratories (project FST-51320.50.19.06).
While best effort was made to include all the relevant references, the authors express regret if a significant reference had not been included in this review.
Footnotes
Disclosure of interest
The authors report no conflict of interest
References
- Aasen T, Leithe E, Graham SV, Kameritsch P, Mayan MD, Mesnil M, Pogoda K, Tabernero A. 2019. Connexins in cancer: bridging the gap to the clinic. Oncogene, 38: 4429–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelrazzak AB, Pottgießer SJ, Hill MA, O’Neill P, Bauer G. 2016. Enhancement of Peroxidase Release from Non-Malignant and Malignant Cells through Low-Dose Irradiation with Different Radiation Quality, Radiat Res, 185: 199–213. [DOI] [PubMed] [Google Scholar]
- Abramowicz A, Wojakowska A, Marczak L, Lysek-Gladysinska M, Smolarz M, Story MD, Polanska J, Widlak P, Pietrowska M. 2019. Ionizing radiation affects the composition of the proteome of extracellular vesicles released by head-and-neck cancer cells in vitro, J Radiat Res, 60: 289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Shoji KF, Saez JC, Henriquez M, Quest AF. 2013. FasL-triggered death of Jurkat cells requires caspase 8-induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7), J Cell Physiol, 228: 485–93. [DOI] [PubMed] [Google Scholar]
- Al-Mayah AH, Bright S, Chapman K, Irons S, Luo P, Carter D, Goodwin E, Kadhim M. 2015. The non-targeted effects of radiation are perpetuated by exosomes, Mutat Res, 772: 38–45. [DOI] [PubMed] [Google Scholar]
- Al-Mayah AH,Irons SL, Pink RC, Carter DR, Kadhim M. 2012. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation, Radiat Res, 177: 539–45. [DOI] [PubMed] [Google Scholar]
- Alper T 1977. The role of membrane damage in radiation-induced cell death, Adv Exp Med Biol, 84: 139–65. [DOI] [PubMed] [Google Scholar]
- Ariazi J, Benowitz A, De Biasi V, Den Boer ML, Cherqui S, Cui H, Douillet N, Eugenin EA, Favre D, Goodman S, Gousset K, Hanein D, Israel DI, Kimura S, Kirkpatrick RB, Kuhn N, Jeong C, Lou E, Mailliard R, Maio S, Okafo G, Osswald M, Pasquier J, Polak R, Pradel G, de Rooij B, Schaeffer P, Skeberdis VA, Smith IF, Tanveer A, Volkmann N, Wu Z, Zurzolo C. 2017. Tunneling Nanotubes and Gap Junctions-Their Role in Long-Range Intercellular Communication during Development, Health, and Disease Conditions, Front Mol Neurosci, 10: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi K, Miura T, Kasai K, Fujishima Y, Nakata A, Yoshida M. 2019. Radiation-Induced Bystander Effect is Mediated by Mitochondrial DNA in Exosome-Like Vesicles, Sci Rep, 9: 9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asur RS, Sharma S, Chang CW, Penagaricano J, Kommuru IM, Moros EG, Corry PM, Griffin RJ. 2012. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells, Radiat Res, 177: 751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asur RS, Sharma S, Chang CW, Penagaricano J, Kommuru IM, Moros EG, Corry PM, Griffin RJ. Spatially fractionated radiation induces cytotoxicity and changes in gene expression in bystander and radiation adjacent murine carcinoma cells. Radiat Res. 2012. Jun;177(6):751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asur R, Butterworth KT, Penagaricano JA, Prise KM, Griffin RJ. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015. Jan 1;356(1):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach C 1947. The induction by mustard gas of chromosomal instabilities in Drosophila melanogaster, Proc R Soc Edinb Biol, 62: 307–20. [PubMed] [Google Scholar]
- Autsavapromporn N, De Toledo SM, Buonanno M, Jay-Gerin JP, Harris AL, Azzam EI. 2011. Intercellular communication amplifies stressful effects in high-charge, high-energy (HZE) particle-irradiated human cells, J Radiat Res, 52: 408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, Plante I, Liu C, Konishi T, Usami N, Funayama T, Azzam EI, Murakami T, Suzuki M. 2015. Genetic changes in progeny of bystander human fibroblasts after microbeam irradiation with X-rays, protons or carbon ions: the relevance to cancer risk, Int J Radiat Biol, 91: 62–70. [DOI] [PubMed] [Google Scholar]
- Autsavapromporn N, Suzuki M, Funayama T, Usami N, Plante I, Yokota Y, Mutou Y, Ikeda H, Kobayashi K, Kobayashi Y, Uchihori Y, Hei TH, Azzam EI, Murakami T. 2013. Gap junction communication and the propagation of bystander effects induced by microbeam irradiation in human fibroblast cultures: the impact of radiation quality, Radiat Res, 180: 367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Harris AL, Ivanov V, Zhou S, Amundson S, Lieberman H, Hei TH. 2013. The Ionizing Radiation-Induced Bystander Effect: Evidence, Mechanism and Significance, Pathobiology of Cancer Regimen-Related Toxicities: 42–68. [Google Scholar]
- Azzam EI, de Toledo SM, Gooding T, Little JB. 1998. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles, Radiat Res, 150: 497–504. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2001. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells, Proc Natl Acad Sci U S A, 98: 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2003a. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses, Cancer Res, 63: 7128–35. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. 2003b. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect, Oncogene, 22: 7050–7. [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Spitz DR, Little JB. 2002. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures, Cancer Res, 62: 5436–42. [PubMed] [Google Scholar]
- Azzam EI, Jay-Gerin JP, Pain D. 2012. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury, Cancer Lett, 327: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi M, Sapora O, Matteucci A, Paradisi S. 2010. Modulation of the bystander effects induced by soluble factors in HaCaT cells by different exposure strategies, Radiat Res, 173: 779–88. [DOI] [PubMed] [Google Scholar]
- Barendsen GW. 1994. The relationships between RBE and LET for different types of lethal damage in mammalian cells: biophysical and molecular mechanisms, Radiat Res, 139: 257–70. [PubMed] [Google Scholar]
- Baulch JE. 2019. Radiation-induced genomic instability, epigenetic mechanisms and the mitochondria: a dysfunctional ménage a trois?, Int J Radiat Biol, 95: 516–25. [DOI] [PubMed] [Google Scholar]
- Bedford JS, Dewey WC. 2002. Radiation Research Society. 1952–2002. Historical and current highlights in radiation biology: has anything important been learned by irradiating cells?, Radiat Res, 158: 251–91. [DOI] [PubMed] [Google Scholar]
- Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. 2002. Bystander-induced apoptosis and premature differentiation in primary urothelial explants after charged particle microbeam irradiation, Radiat Prot Dosimetry, 99: 249–51. [DOI] [PubMed] [Google Scholar]
- Bergonié J, Tribondeau L. 2003. Interpretation of some results from radiotherapy and an attempt to determine a rational treatment technique. 1906, Yale J Biol Med, 76: 181–2. [PMC free article] [PubMed] [Google Scholar]
- Bertucci A, Pocock RD, Randers-Pehrson G, Brenner DJ. 2009. Microbeam irradiation of the C. elegans nematode, J Radiat Res, 50 Suppl A: A49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee A, Rao DV, Howell RW. 1999. Evidence for pronounced bystander effects caused by nonuniform distributions of radioactivity using a novel three-dimensional tissue culture model, Radiat Res, 152: 88–97. [PMC free article] [PubMed] [Google Scholar]
- Blakely EA. 2000. Biological effects of cosmic radiation: deterministic and stochastic, Health Phys, 79: 495–506. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Little JB, Sachs RK. 2001. The bystander effect in radiation oncogenesis: II. A quantitative model, Radiat Res, 155: 402–8. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Sachs RK. 2002. Do low dose-rate bystander effects influence domestic radon risks?’ Int J Radiat Biol, 78: 593–604. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Sachs RK. 2003. Domestic radon risks may be dominated by bystander effects--but the risks are unlikely to be greater than we thought, Health Phys, 85: 103–8. [DOI] [PubMed] [Google Scholar]
- Brooks AL, Retherford JC, McClellan RO. 1974. Effect of 239PuO2 particle number and size on the frequency and distribution of chromosome aberrations in the liver of the Chinese hamster, Radiat Res, 59: 693–709. [PubMed] [Google Scholar]
- Buonanno M, de Toledo SM, Azzam EI. 2011. Increased frequency of spontaneous neoplastic transformation in progeny of bystander cells from cultures exposed to densely ionizing radiation, PLoS One, 6: e21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M, de Toledo SM, Howell RW, Azzam EI. 2015. Low-dose energetic protons induce adaptive and bystander effects that protect human cells against DNA damage caused by a subsequent exposure to energetic iron ions, J Radiat Res, 56: 502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M, de Toledo SM, Pain D, Azzam EI. 2011. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress, Radiat Res, 175: 405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M, Randers-Pehrson G, Smilenov LB, Kleiman NJ, Young E, Ponnayia B, Brenner DJ. 2015. A Mouse Ear Model for Bystander Studies Induced by Microbeam Irradiation, Radiat Res, 184: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdak-Rothkamm S, Rothkamm K, Prise KM. 2008. ATM acts downstream of ATR in the DNA damage response signaling of bystander cells Cancer Res, 68: 7059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth KT, McGarry CK, Trainor C, O’Sullivan JM, Hounsell AR, Prise KM. Out-of-field cell survival following exposure to intensity-modulated radiation fields. Int J Radiat Oncol Biol Phys. 2011. Apr 1;79(5):1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Shi GS, Cheng HY, Zeng YN, Li G, Zhang M, Song M, Zhou PK, Tian Y, Cui FM, Chen Q. 2017. Exosomal miR-7 Mediates Bystander Autophagy in Lung after Focal Brain Irradiation in Mice, Int J Biol Sci, 13: 1287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter BS, Leung CN, Fritton JC, Bäck T, Rajon D, Azzam EI, Howell RW. 2021. Radium-223-Induced Bystander Effects Cause DNA Damage and Apoptosis in Disseminated Tumor Cells in Bone Marrow, Mol Cancer Res, 19: 1739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Calaf GM, Zhou H, Ghandhi SA, Elliston CD, Wen G, Nohmi T, Amundson SA, Hei TK. 2013. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice, Br J Cancer, 108: 91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Little JB. 1992. Persistently elevated frequency of spontaneous mutations in progeny of CHO clones surviving X-irradiation: association with delayed reproductive death phenotype, Mutat Res, 270: 191–9. [DOI] [PubMed] [Google Scholar]
- Colangelo NW, Azzam EI. 2020. Extracellular vesicles originating from glioblastoma cells increase metalloproteinase release by astrocytes: the role of CD147 (EMMPRIN) and ionizing radiation, Cell Commun Signal, 18: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanzo J, Faget J, Ursino C, Badie C, Pouget JP. 2021. Radiation-Induced Immunity and Toxicities: The Versatility of the cGAS-STING Pathway, Front Immunol, 12: 680503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Nikjoo H, Goodhead DT. 1998. The effects of delta rays on the number of particle-track traversals per cell in laboratory and space exposures, Radiat Res, 150: 115–9. [PubMed] [Google Scholar]
- de Toledo SM, Buonanno M, Harris AL, Azzam EI. 2017. Genomic instability induced in distant progeny of bystander cells depends on the connexins expressed in the irradiated cells, Int J Radiat Biol, 93: 1182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. 2004. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated, Int J Radiat Oncol Biol Phys, 58: 862–70. [DOI] [PubMed] [Google Scholar]
- Desai S, Kobayashi A, Konishi T, Oikawa M, Pandey BN. 2014. Damaging and protective bystander cross-talk between human lung cancer and normal cells after proton microbeam irradiation, Mutat Res, 763–764: 39–44. [DOI] [PubMed] [Google Scholar]
- Desai S, Kumar A, Laskar S, Pandey BN. 2013. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells, Cytokine, 61: 54–62. [DOI] [PubMed] [Google Scholar]
- Desai S, Srambikkal N, Yadav HD, Shetake N, Balla MM, Kumar A, Ray P, Ghosh A, Pandey BN. 2016. Molecular Understanding of Growth Inhibitory Effect from Irradiated to Bystander Tumor Cells in Mouse Fibrosarcoma Tumor Model, PLoS One, 11: e0161662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE. 1996. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: evidence for an extranuclear target, Radiat Res, 145: 260–7. [PubMed] [Google Scholar]
- Devhare PB, Ray RB. 2018. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis, Mol Aspects Med, 60: 115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilmanian FA, Qu Y, Feinendegen LE, Pena LA, Bacarian T, Henn FA, Kalef-Ezra J, Liu S, Zhong Z, McDonald JW. 2007. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: is a bystander effect involved?, Exp Hematol, 35: 69–77. [DOI] [PubMed] [Google Scholar]
- Durante M, Brenner DJ, Formenti SC. 2016. Does Heavy Ion Therapy Work Through the Immune System?, Int J Radiat Oncol Biol Phys, 96: 934–36. [DOI] [PubMed] [Google Scholar]
- Emerit I, Oganesian N, Sarkisian T, Arutyunyan R, Pogosian A, Asrian K, Levy A, Cernjavski L. 1995. Clastogenic factors in the plasma of Chernobyl accident recovery workers: anticlastogenic effect of Ginkgo biloba extract, Radiat Res, 144: 198–205. [PubMed] [Google Scholar]
- Failla G 1921. Dosage in radium therapy, Am J Roentgenol 8 674–85. [Google Scholar]
- Failla G 1926. The question of a biologic unit of radiation, Acta Radiologica 6: 413–39. [Google Scholar]
- Folkard M, Schettino G, Vojnovic B, Gilchrist S, Michette AG, Pfauntsch SJ, Prise KM, Michael BD. 2001. A focused ultrasoft x-ray microbeam for targeting cells individually with submicrometer accuracy, Radiat Res, 156: 796–804. [DOI] [PubMed] [Google Scholar]
- Frankenberg D, Greif KD, Giesen U. 2006. Radiation response of primary human skin fibroblasts and their bystander cells after exposure to counted particles at low and high LET, Int J Radiat Biol, 82: 59–67. [DOI] [PubMed] [Google Scholar]
- Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP. 2010. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study, J Natl Cancer Inst, 102: 1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard S, Pusset D, de Toledo SM, Fromm M, Azzam EI. 2009. Propagation distance of the alpha-particle-induced bystander effect: the role of nuclear traversal and gap junction communication, Radiat Res, 171: 513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ma H, Lv C, Lan F, Wang Y, Deng Y. 2021. Exosomes and exosomal microRNA in non-targeted radiation bystander and abscopal effects in the central nervous system, Cancer Lett, 499: 73–84. [DOI] [PubMed] [Google Scholar]
- Gerashchenko BI, Howell RW. 2003. Cell proximity is a prerequisite for the proliferative response of bystander cells co-cultured with cells irradiated with gamma-rays, Cytometry A, 56: 71–80. [DOI] [PubMed] [Google Scholar]
- Goh K, Sumner H. 1968. Breaks in normal human chromosomes: are they induced by a transferable substance in the plasma of persons exposed to total-body irradiation?, Radiat Res, 35: 171–81. [PubMed] [Google Scholar]
- Golden EB, Apetoh L. 2015. Radiotherapy and immunogenic cell death, Semin Radiat Oncol, 25: 11–7. [DOI] [PubMed] [Google Scholar]
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, Demaria S, Formenti SC. 2015. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial, Lancet Oncol, 16: 795–803. [DOI] [PubMed] [Google Scholar]
- Golden EB, Frances D, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. 2014. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death, Oncoimmunology, 3: e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon G, Groetz JE, de Toledo SM, Howell RW, Fromm M, Azzam EI. 2013. Nontargeted stressful effects in normal human fibroblast cultures exposed to low fluences of high charge, high energy (HZE) particles: kinetics of biologic responses and significance of secondary radiations, Radiat Res, 179: 444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. 1996. Connexins, connexons, and intercellular communication, Annu Rev Biochem, 65: 475–502. [DOI] [PubMed] [Google Scholar]
- Goodhead DT, Nikjoo H. 1989. Track structure analysis of ultrasoft X-rays compared to high- and low-LET radiations, Int J Radiat Biol, 55: 513–29. [DOI] [PubMed] [Google Scholar]
- Gow MD, Seymour CB, Ryan LA, Mothersill CE. 2010. Induction of bystander response in human glioma cells using high-energy electrons: a role for TGF-beta1, Radiat Res, 173: 769–78. [DOI] [PubMed] [Google Scholar]
- Grass GD, Krishna N, Kim S. 2016. The immune mechanisms of abscopal effect in radiation therapy, Curr Probl Cancer, 40: 10–24. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Prise KM, McMahon SJ, Zhang X, Penagaricano J, Butterworth KT. History and current perspectives on the biological effects of high-dose spatial fractionation and high dose-rate approaches: GRID, Microbeam & FLASH radiotherapy. Br J Radiol. 2020. Sep 1;93(1113):20200217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosovsky AJ, Parks KK, Giver CR, Nelson SL. 1996. Clonal analysis of delayed karyotypic abnormalities and gene mutations in radiation-induced genetic instability, Mol Cell Biol, 16: 6252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada N, Ni M, Funayama T, Sakashita T, Kobayashi Y. 2008. Temporally distinct response of irradiated normal human fibroblasts and their bystander cells to energetic heavy ions, Mutat Res, 639: 35–44. [DOI] [PubMed] [Google Scholar]
- Han W, Chen S, Yu KN, Wu L. 2010. Nitric oxide mediated DNA double strand breaks induced in proliferating bystander cells after alpha-particle irradiation, Mutat Res, 684: 81–9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation, Cell, 144: 646–74. [DOI] [PubMed] [Google Scholar]
- Harper K, Lorimore SA, Wright EG. 1997. Delayed appearance of radiation-induced mutations at the Hprt locus in murine hemopoietic cells, Exp Hematol, 25: 263–9. [PubMed] [Google Scholar]
- Harris AL. 2007. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol, 94:120–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. 2018. Electrical coupling and its channels, J Gen Physiol, 150: 1606–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Li L, Wang L, Meng W, Hao Y, Zhu G. Exosome-mediated cellular crosstalk within the tumor microenvironment upon irradiation. Cancer Biol Med. 2021;18(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Dong C, Xie Y, Li J, Yuan D, Bai Y, Shao C. 2014. Reciprocal bystander effect between α-irradiated macrophage and hepatocyte is mediated by cAMP through a membrane signaling pathway, Mutat Res, 763–764: 1–9. [DOI] [PubMed] [Google Scholar]
- Heeran AB, Berrigan HP, O’Sullivan J. 2019. The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer, Radiat Res, 192: 668–79. [DOI] [PubMed] [Google Scholar]
- Held KD. 2009. Effects of low fluences of radiations found in space on cellular systems, Int J Radiat Biol, 85: 379–90. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Littlefield LG. 1968. Chromosome damage induced by plasma of x-rayed patients: an indirect effect of x-ray, Proc Soc Exp Biol Med, 129: 240–4. [DOI] [PubMed] [Google Scholar]
- Holmberg K, Meijer AE, Auer G, Lambert BO. 1995. Delayed chromosomal instability in human T-lymphocyte clones exposed to ionizing radiation, Int J Radiat Biol, 68: 245–55. [DOI] [PubMed] [Google Scholar]
- Hong X, Sin WC, Harris AL, Naus CC. 2015. Gap junctions modulate glioma invasion by direct transfer of microRNA, Oncotarget, 6: 15566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorelbeke D, Decrock E, De Smet M, De Bock M, Descamps B, Van Haver V, Delvaeye T, Krysko DV, Vanhove C, Bultynck G, Leybaert L. 2020. Cx43 channels and signaling via IP, Cell Death Dis, 11: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Grabham P, Nie J, Balajee AS, Zhou H, Hei TK, Geard CR. 2012. Intrachromosomal changes and genomic instability in site-specific microbeam-irradiated and bystander human-hamster hybrid cells, Radiat Res, 177: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Xu S, Yao B, Hong M, Wu X, Pei H, Chang L, Ding N, Gao X, Ye C, Wang J, Hei TK, Zhou G. 2014. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode, RNA Biol, 11: 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Nagasawa H, Little JB. 2001. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations, Radiat Res, 156: 521–5. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Zhou NH, Ghandhi SA, Karasic TB, Yaghoubian B, Amundson SA, Hei TK. 2010. Radiation-induced bystander signaling pathways in human fibroblasts: a role for interleukin-33 in the signal transmission, Cell Signal, 22: 1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE, Svensson R. 2000. Factors underlying the cell growth-related bystander responses to alpha particles, Cancer Res, 60: 1290–8. [PubMed] [Google Scholar]
- Jain MR, Li ML, Chen W, Liu T, de Toledo SM, Pandey BN, Li H, Rabin BM, Azzam EI. 2011. In vivo space radiation-induced non-targeted responses: late effects on molecular signaling in mitochondria, Curr Mol Pharmacol, 4: 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jella KK, Rani S, O’Driscoll L, McClean B, Byrne HJ, Lyng FM. 2014. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells, Radiat Res, 181: 138–45. [DOI] [PubMed] [Google Scholar]
- Jelonek K, Widlak P, Pietrowska M. 2016. The Influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication, Protein Pept Lett, 23: 656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesenko T, Bosnjak M, Markelc B, Sersa G, Znidar K, Heller L, Cemazar M. 2020. Radiation Induced Upregulation of DNA Sensing Pathways is Cell-Type Dependent and Can Mediate the Off-Target Effects, Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. 1992. Transmission of chromosomal instability after plutonium alpha-particle irradiation, Nature, 355: 738–40. [DOI] [PubMed] [Google Scholar]
- Kanagavelu S, Gupta S, Wu X, Philip S, Wattenberg MM, Hodge JW, Couto MD, Chung KD, Ahmed MM. 2014. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation, Radiat Res, 182: 149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Hill RP, Van Dyk J. 1998. Partial volume rat lung irradiation: an evaluation of early DNA damage, Int J Radiat Oncol Biol Phys, 40: 467–76. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Funayama T, Hamada N, Sakashita T, Konishi T, Imaseki H, Yasuda K, Hatashita M, Takagi K, Hatori S, Suzuki K, Yamauchi M, Yamashita S, Tomita M, Maeda M, Kobayashi K, Usami N, Wu L. 2009. Microbeam irradiation facilities for radiobiology in Japan and China, J Radiat Res, 50 Suppl A: A29–47. [DOI] [PubMed] [Google Scholar]
- Kong EY, Cheng SH, Yu KN. 2018. Induction of autophagy and interleukin 6 secretion in bystander cells: metabolic cooperation for radiation-induced rescue effect?, J Radiat Res, 59: 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I, Loree J, Kutanzi K, Koganow C, Pogribny I, Kovalchuk O. 2008. In vivo bystander effect: cranial X-irradiation leads to elevated DNA damage, altered cellular proliferation and apoptosis, and increased p53 levels in shielded spleen, Int J Radiat Oncol Biol Phys, 70: 554–62. [DOI] [PubMed] [Google Scholar]
- Kovalchuk A, Mychasiuk R, Muhammad A, Hossain S, Ilnytskyy S, Ghose A, Kirkby C, Ghasroddashti E, Kovalchuk O, Kolb B. 2016. Liver irradiation causes distal bystander effects in the rat brain and affects animal behaviour, Oncotarget, 7: 4385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg A 1994. Radiation-induced genomic instability, Int J Radiat Biol, 66: 603–9. [DOI] [PubMed] [Google Scholar]
- Laiakis EC, Strassburg K, Bogumil R, Lai S, Vreeken RJ, Hankemeier T, Langridge J, Plumb RS, Fornace AJ Jr, Astarita G. 2014. Metabolic phenotyping reveals a lipid mediator response to ionizing radiation, J Proteome Res, 13: 4143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. 2004. The effects of connexin phosphorylation on gap junctional communication, Int J Biochem Cell Biol, 36: 1171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea DE. 1947. “Actions of radiations of living cells.” In, 402. Cambridge Cambridgeshire New York: University Press The Macmillan Company. [Google Scholar]
- Lehnert BE, Goodwin EH, Deshpande A. 1997. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells, Cancer Res, 57: 2164–71. [PubMed] [Google Scholar]
- Leung CN, Canter BS, Rajon D, Bäck TA, Fritton JC, Azzam EI, Howell RW. 2020. Dose-Dependent Growth Delay of Breast Cancer Xenografts in the Bone Marrow of Mice Treated with, J Nucl Med, 61: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JB. 2003. Genomic instability and bystander effects: a historical perspective, Oncogene, 22: 6978–87. [DOI] [PubMed] [Google Scholar]
- Little JB, Gorgojo L, Vetrovs H. 1990. Delayed appearance of lethal and specific gene mutations in irradiated mammalian cells, Int J Radiat Oncol Biol Phys, 19: 1425–9. [DOI] [PubMed] [Google Scholar]
- Little JB, Nagasawa H, Li GC, Chen DJ. 2003. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations, Radiat Res, 159: 262–7. [DOI] [PubMed] [Google Scholar]
- Little JB, Nagasawa H, Pfenning T, Vetrovs H. 1997. Radiation-induced genomic instability: delayed mutagenic and cytogenetic effects of X rays and alpha particles, Radiat Res, 148: 299–307. [PubMed] [Google Scholar]
- Liu Z, Mothersill CE, McNeill FE, Lyng FM, Byun SH, Seymour CB, Prestwich WV. 2006. A dose threshold for a medium transfer bystander effect for a human skin cell line, Radiat Res, 166: 19–23. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. 2001. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects?, Oncogene, 20: 7085–95. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Maguire P, McClean B, Seymour CB, Mothersill CE. 2006. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects, Radiat Res, 165: 400–9. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill CE. 2002. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability?, Radiat Res, 157: 365–70. [DOI] [PubMed] [Google Scholar]
- Maguire P, Mothersill CE, Seymour CB, Lyng FM. 2005. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes, Radiat Res, 163: 384–90. [DOI] [PubMed] [Google Scholar]
- Malloci M, Perdomo L, Veerasamy M, Andriantsitohaina R, Simard G, Martínez MC. 2019. Extracellular Vesicles: Mechanisms in Human Health and Disease, Antioxid Redox Signal, 30: 813–56. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pasquali E, Leonardi S, Rebessi S, Tanori M, Giardullo P, Borra F, Pazzaglia S, Naus CC, Di Majo V, Saran A. 2011. Role of connexin43 and ATP in long-range bystander radiation damage and oncogenesis in vivo, Oncogene, 30: 4601–8. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, Pazzaglia S, Toni MP, Pimpinella M, Covelli V, Saran A. 2008. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum, Proc Natl Acad Sci U S A, 105: 12445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell E, Zareian N, Malouf C, Kapeni C, Brown N, Badie C, Baird D, Lane J, Ottersbach K, Blair A, Case CP. 2019. DNA damage signalling from the placenta to foetal blood as a potential mechanism for childhood leukaemia initiation, Sci Rep, 9: 4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti LG, Bertolotti A, Ranza E, Babini G, Ottolenghi A. 2012. Investigation of the mechanisms underpinning IL-6 cytokine release in bystander responses: the roles of radiation dose, radiation quality and specific ROS/RNS scavengers, Int J Radiat Biol, 88: 751–62. [DOI] [PubMed] [Google Scholar]
- Matejka N, Reindl J. 2020. Influence of α-Particle Radiation on Intercellular Communication Networks of Tunneling Nanotubes in U87 Glioblastoma Cells, Frontiers in Oncology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, Dent P, Wang XY, Sarkar D, Fisher PB. 2014. MDA-7/IL-24: multifunctional cancer killing cytokine, Adv Exp Med Biol, 818: 127–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meşe G, Richard G, White TW. 2007. Gap junctions: basic structure and function, J Invest Dermatol, 127: 2516–24. [DOI] [PubMed] [Google Scholar]
- Metting NF, Rossi HH, Braby LA, Kliauga PJ, Howard J, Zaider M, Schimmerling W, Wong M, Rapkin M. 1988. Microdosimetry near the trajectory of high-energy heavy ions, Radiat Res, 116: 183–95. [PubMed] [Google Scholar]
- Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ. 2004. The bystander response in C3H 10T1/2 cells: the influence of cell-to-cell contact, Radiat Res, 161: 397–401. [DOI] [PubMed] [Google Scholar]
- Mo LJ, Song M, Huang QH, Guan H, Liu XD, Xie DF, Huang B, Huang RX, Zhou PK. 2018. Exosome-packaged miR-1246 contributes to bystander DNA damage by targeting LIG4, Br J Cancer, 119: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WF. 2003a. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro, Radiat Res, 159: 567–80. [DOI] [PubMed] [Google Scholar]
- Morgan WF. 2003b. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects, Radiat Res, 159: 581–96. [DOI] [PubMed] [Google Scholar]
- Mothersill CE, Rusin A, Fernandez-Palomo C, Seymour CB. 2018. History of bystander effects research 1905-present; what is in a name?, Int J Radiat Biol, 94: 696–707. [DOI] [PubMed] [Google Scholar]
- Mothersill CE, Seymour CB. 1997. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells, Int J Radiat Biol, 71: 421–7. [DOI] [PubMed] [Google Scholar]
- Munro TR. 1970. The relative radiosensitivity of the nucleus and cytoplasm of Chinese hamster fibroblasts, Radiat Res, 42: 451–70. [PubMed] [Google Scholar]
- Murphy JB, Morton JJ. 1915. The effect of Roentgen rays on the rate of growth of spontaneous tumors in mice, J Exp Med, 22: 800–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Huo L, Little JB. 2003. Increased bystander mutagenic effect in DNA double-strand break repair-deficient mammalian cells, Int J Radiat Biol, 79: 35–41. [PubMed] [Google Scholar]
- Nagasawa H, Little JB. 1992. Induction of sister chromatid exchanges by extremely low doses of alpha-particles, Cancer Res, 52: 6394–6. [PubMed] [Google Scholar]
- Nakaoka A, Nakahana M, Inubushi S, Akasaka H, Salah M, Fujita Y, Kubota H, Hassan M, Nishikawa R, Mukumoto N, Ishihara T, Miyawaki D, Sasayama T, Sasaki R. 2021. Exosome-mediated radiosensitizing effect on neighboring cancer cells via increase in intracellular levels of reactive oxygen species, Oncol Rep, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan PK Goodwin EH, Lehnert BE. 1997. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells, Cancer Res, 57: 3963–71. [PubMed] [Google Scholar]
- National Research Council. 2008. “Managing space radiation risk in the new era of space exploration.” In National Research Council (U.S.). Committee on the Evaluation of Radiation Shielding for Space Exploration, xiii, 117 p. Washington, D.C.: National Academies Press. [Google Scholar]
- NCRP. 2020. “Approaches for integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment : recommendations of the National Council on Radiation Protection and Measurements.” In NCRP report no 186, 1 online resource. Bethesda, MD.: National Council on Radiation Protection and Measurements,. [Google Scholar]
- Newcombe HB, Scott GW. 1949. Factors Responsible for the Delayed Appearance of Radiation-Induced Mutants in Escherichia Coli, Genetics, 34: 475–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhauser WD, Durante M. 2011. Assessing the risk of second malignancies after modern radiotherapy, Nat Rev Cancer, 11: 438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K. 2000. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate, J Cell Sci, 113 ( Pt 8): 1365–72. [DOI] [PubMed] [Google Scholar]
- Ochoa de Olza M, Bourhis J, Irving M, Coukos G, Herrera FG. 2020. High versus low dose irradiation for tumor immune reprogramming, Curr Opin Biotechnol, 65: 268–83. [DOI] [PubMed] [Google Scholar]
- Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. 1998. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis, Gut, 43: 575–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Tsukimoto M, Harada H, Kojima S. 2012. Involvement of connexin43 hemichannel in ATP release after γ-irradiation, J Radiat Res, 53: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampfer S, Streffer C. 1989. Increased chromosome aberration levels in cells from mouse fetuses after zygote X-irradiation, Int J Radiat Biol, 55: 85–92. [DOI] [PubMed] [Google Scholar]
- Pant GS, Kamada N. 1977. Chromosome aberrations in normal leukocytes induced by the plasma of exposed individuals, Hiroshima J Med Sci, 26: 149–54. [PubMed] [Google Scholar]
- Patheja P, Dasgupta R, Dube A, Ahlawat S, Verma RS, Gupta PK. 2015. The use of optical trap and microbeam to investigate the mechanical and transport characteristics of tunneling nanotubes in tumor spheroids, J Biophotonics, 8: 694–704. [DOI] [PubMed] [Google Scholar]
- Patheja P, Sahu K. 2017. Macrophage conditioned medium induced cellular network formation in MCF-7 cells through enhanced tunneling nanotube formation and tunneling nanotube mediated release of viable cytoplasmic fragments, Exp Cell Res, 355: 182–93. [DOI] [PubMed] [Google Scholar]
- Peng Y, Wang X, Guo Y, Peng F, Zheng N, He B, Ge H, Tao L, WangQ. 2019. Pattern of cell-to-cell transfer of microRNA by gap junction and its effect on the proliferation of glioma cells, Cancer Sci, 110: 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Gehi R, Laird. 2013. DW. The biochemistry and function of pannexin channels, Biochim Biophys Acta, 1828: 15–22. [DOI] [PubMed] [Google Scholar]
- Persaud R, Zhou H, Baker SE, Hei TK, Hall EJ. 2005. Assessment of low linear energy transfer radiation-induced bystander mutagenesis in a three-dimensional culture model, Cancer Res, 65: 9876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau A 1980. Radiation carcinogenesis from a membrane perspective, Acta Physiol Scand Suppl, 492: 81–90. [PubMed] [Google Scholar]
- Ponnaiya B, Jenkins-Baker G, Brenner DJ, Hall EJ, Randers-Pehrson G, Geard CR. 2004. Biological responses in known bystander cells relative to known microbeam-irradiated cells, Radiat Res, 162: 426–32. [DOI] [PubMed] [Google Scholar]
- Portess DI, Bauer G, Hill MA, O’Neill P. 2007. Low-dose irradiation of nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis, Cancer Res, 67: 1246–53. [DOI] [PubMed] [Google Scholar]
- Pouget JP, Georgakilas AG, Ravanat JL. 2018. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis, Antioxid Redox Signal, 29: 1447–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezado Y, Jouvion G, Patriarca A, Nauraye C, Guardiola C, Juchaux M, Lamirault C, Labiod D, Jourdain L, Sebrie C, Dendale R, Gonzalez W, Pouzoulet F. 2018. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas, Sci Rep, 8: 16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, Belyakov OV, Folkard M, Michael BD. 1998. Studies of bystander effects in human fibroblasts using a charged particle microbeam, Int J Radiat Biol, 74: 793–8. [DOI] [PubMed] [Google Scholar]
- Prise KM, O’Sullivan JM. 2009. Radiation-induced bystander signalling in cancer therapy, Nat Rev Cancer, 9: 351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan V, Pandey BN. 2021. Cytoproliferative effect of low dose alpha radiation in human lung cancer cells is associated with connexin 43, caveolin-1, and survivin pathway, Int J Radiat Biol, 97: 356–66. [DOI] [PubMed] [Google Scholar]
- Randers-Pehrson G, Geard CR, Johnson G, Elliston CD, Brenner DJ. 2001. The Columbia University single-ion microbeam, Radiat Res, 156: 210–4. [DOI] [PubMed] [Google Scholar]
- Regaud C, Ferroux R. 1927. Discordance des effects de rayons X, d’une part dans le testicile, par le peau, d’autre parts dans le fractionment de la dose, Compt Rend Soc Biol 97: 431–4. [Google Scholar]
- Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. 2015. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant, Cancer Treat Rev, 41: 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. 2018. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect, Trends Immunol, 39: 644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. 2004. Nanotubular highways for intercellular organelle transport, Science, 303: 1007–10. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Dutrillaux B, Martin MB. 1992. Chromosomal instability, Nature, 357: 548. [DOI] [PubMed] [Google Scholar]
- Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. 2001. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells, Radiat Res, 155: 397–401. [DOI] [PubMed] [Google Scholar]
- Schettino G, Folkard M, Prise KM, Vojnovic B, Michael BD. 2002. Upgrading of the Gray Laboratory soft X ray microprobe and V79 survival measurements following irradiation of one or all cells with a CK X ray beam of different size, Radiat Prot Dosimetry, 99: 287–8. [DOI] [PubMed] [Google Scholar]
- Shao C, Aoki M, Furusawa Y. 2003. Bystander effect on cell growth stimulation in neoplastic HSGc cells induced by heavy-ion irradiation, Radiat Environ Biophys, 42: 183–7. [DOI] [PubMed] [Google Scholar]
- Shao C, Folkard M, Michael BD, Prise KM. 2004. Targeted cytoplasmic irradiation induces bystander responses, Proc Natl Acad Sci U S A, 101: 13495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Folkard M, Prise KM. 2008. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells, Oncogene, 27: 434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Kobayashi Y, Funayama T, Wada S. 2003. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: a mechanistic study, FASEB J, 17: 1422–7. [DOI] [PubMed] [Google Scholar]
- Shao C, Stewart V, Folkard M, Michael BD, Prise KM. 2003. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells, Cancer Res, 63: 8437–42. [PubMed] [Google Scholar]
- Song M, Wang Y, Shang ZF, Liu XD, Xie DF, Wang Q, Guan H, Zhou PK. 2016. Bystander autophagy mediated by radiation-induced exosomal miR-7–5p in non-targeted human bronchial epithelial cells, Sci Rep, 6: 30165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hu S, Zhang J, Zhu L, Zhao X, Chen Q, Bai Y, Pan Y, Shao C. 2021. Fractionated Irradiation of Right Thorax Induces Abscopal Damage on Bone Marrow Cells via TNF-α and SAA, Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR, Azzam EI, Li JJ, Gius D. 2004. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology, Cancer and Metastasis Rev, 23: 311–22. [DOI] [PubMed] [Google Scholar]
- Stone HB, Peters LJ, Milas L. 1979. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma, J Natl Cancer Inst, 63: 1229–35. [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. 2005. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells, Oncogene, 24: 7552–66. [DOI] [PubMed] [Google Scholar]
- Suchowerska N, Ebert MA, Zhang M, Jackson M. In vitro response of tumour cells to non-uniform irradiation. Phys Med Biol. 2005. Jul 7;50(13):3041–51. [DOI] [PubMed] [Google Scholar]
- Sudo H, Garbe J, Stampfer MR, Barcellos-Hoff MH, Kronenberg A. 2008. Karyotypic instability and centrosome aberrations in the progeny of finite life-span human mammary epithelial cells exposed to sparsely or densely ionizing radiation, Radiat Res, 170: 23–32. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Dazai K, Sakashita T, Funayama T, Wada S, Hamada N, Kakizaki T, Kobayashi Y, Higashitani A. 2006. Cell cycle arrest and apoptosis in Caenorhabditis elegans germline cells following heavy-ion microbeam irradiation, Int J Radiat Biol, 82: 31–8. [DOI] [PubMed] [Google Scholar]
- Sui H, Goldberg S, Niemierko A, Ancukiewicz M, Hall E, Goitein M, Wong W, Paganetti H. 2007. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects, Radiat Res, 167: 12–42. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Tsuruoka C. 2004. Heavy charged particles produce a bystander effect via cell-cell junctions, Biol Sci Space, 18: 241–6. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yasuda N, Kitamura H. 2020. Lethal and mutagenic bystander effects in human fibroblast cell cultures subjected to low-energy-carbon ions, Int J Radiat Biol, 96: 179–86. [DOI] [PubMed] [Google Scholar]
- Tamminga J, Kovalchuk O. 2011. Role of DNA damage and epigenetic DNA methylation changes in radiation-induced genomic instability and bystander effects in germline in vivo, Curr Mol Pharmacol, 4: 115–25. [DOI] [PubMed] [Google Scholar]
- Terzaghi M, Little JB. 1976. X-radiation-induced transformation in a C3H mouse embryo-derived cell line, Cancer Res, 36: 1367–74. [PubMed] [Google Scholar]
- Tomita M, Matsumoto H, Funayama T, Yokota Y, Otsuka K, Maeda M, Kobayashi Y. 2015. Nitric oxide-mediated bystander signal transduction induced by heavy-ion microbeam irradiation, Life Sci Space Res (Amst), 6: 36–43. [DOI] [PubMed] [Google Scholar]
- Tubin S, Yan W, Mourad WF, Fossati P, Khan MK. 2020. The future of radiation-induced abscopal response: beyond conventional radiotherapy approaches, Future Oncol, 16: 1137–51. [DOI] [PubMed] [Google Scholar]
- Tuncay Cagatay S, Mayah A, Mancuso M, Giardullo P, Pazzaglia S, Saran A, Daniel A, Traynor D, Meade AD, Lyng F, Tapio S, Kadhim M. 2020. Phenotypic and Functional Characteristics of Exosomes Derived from Irradiated Mouse Organs and Their Role in the Mechanisms Driving Non-Targeted Effects, Int J Mol Sci, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham BL, Trosko JE. 2009. Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression, Antioxid Redox Signal, 11: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami N, Maeda M, Eguchi-Kasai K, Maezawa H, Kobayashi K. 2006. Radiation-induced gamma-H2AX in mammalian cells irradiated with a synchrotron X-ray microbeam, Radiat Prot Dosimetry, 122: 307–9. [DOI] [PubMed] [Google Scholar]
- Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J, Huang J, He Q, Wu B, Zhang Z, Jiang K, Hu D, Wu G, Lovell JF, Jin H, Yang K. 2020. Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming, Sci Adv, 6: eaay9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. 2010. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels, Proc Natl Acad Sci U S A, 107: 17194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JF. 1988. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability, Prog Nucleic Acid Res Mol Biol, 35: 95–125. [DOI] [PubMed] [Google Scholar]
- Watson GE, Pocock DA, Papworth D, Lorimore SA, Wright EG. 2001. In vivo chromosomal instability and transmissible aberrations in the progeny of haemopoietic stem cells induced by high- and low-LET radiations, Int J Radiat Biol, 77: 409–17. [DOI] [PubMed] [Google Scholar]
- Wiese C, Pierce AJ, Gauny SS, Jasin M, Kronenberg A. 2002. Gene conversion is strongly induced in human cells by double-strand breaks and is modulated by the expression of BCL-x(L), Cancer Res, 62: 1279–83. [PubMed] [Google Scholar]
- Wu C, Liu T, Chen W, Oka S, Fu C, Jain MR, Parrott AM, Baykal AT, Sadoshima J, Li H. 2010. Redox regulatory mechanism of transnitrosylation by thioredoxin, Mol Cell Proteomics, 9: 2262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z, Hei TK. 1999. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells, Proc Natl Acad Sci U S A, 96: 4959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Wang J, Ding N, Hu W, Zhang X, Wang B, Hua J, Wei W, Zhu Q. 2015. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect, RNA Biol, 12: 1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Butler NJ, Makrigiorgos, Adelstein SJ, Kassis AI. 2002. Bystander effect produced by radiolabeled tumor cells in vivo, Proc Natl Acad Sci U S A, 99: 13765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev VA. 2015. Role of nitric oxide in the radiation-induced bystander effect, Redox Biol, 6: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Khan MK, Wu X, Simone CB 2nd, Fan J, Gressen E, Zhang X, Limoli CL, Bahig H, Tubin S, Mourad WF. 2020. Spatially fractionated radiation therapy: History, present and the future, Clin Transl Radiat Oncol, 20: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Anzenberg V, Held KD. 2007. The time dependence of bystander responses induced by iron-ion radiation in normal human skin fibroblasts, Radiat Res, 168: 292–8. [DOI] [PubMed] [Google Scholar]
- Yang H, Asaad N, Held KD. 2005. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts, Oncogene, 24: 2096–103. [DOI] [PubMed] [Google Scholar]
- Yang X, Ma L, Ye Z, Shi W, Zhang L, Wang J, Yang H. 2021. Radiation-induced bystander effects may contribute to radiation-induced cognitive impairment, Int J Radiat Biol, 97: 329–40. [DOI] [PubMed] [Google Scholar]
- Yang X, Xu S, Su Y, Chen B, Yuan H, Xu A, Wu L. 2018. Autophagy-Src Regulates Connexin43-Mediated Gap Junction Intercellular Communication in Irradiated HepG2 Cells, Radiat Res, 190: 494–503. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Funayama T, Mutou-Yoshihara Y, Ikeda H, Kobayashi Y. 2015. The bystander cell-killing effect mediated by nitric oxide in normal human fibroblasts varies with irradiation dose but not with radiation quality, Int J Radiat Biol, 91: 383–8. [DOI] [PubMed] [Google Scholar]
- Yu KN. 2019. Radiation-induced rescue effect, J Radiat Res, 60: 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Matzke M, Schepmoes AA, Moore RJ, Webb-Robertson BJ, Hu Z, Monroe ME, Qian WJ, Smith RD, Morgan WF. 2014. High and low doses of ionizing radiation induce different secretome profiles in a human skin model, PLoS One, 9: e92332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, de Toledo SM, Hu G, Hei TK, Azzam EI. 2014. Connexins and cyclooxygenase-2 crosstalk in the expression of radiation-induced bystander effects, Br J Cancer, 111: 125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. 2005. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway, Proc Natl Acad Sci U S A, 102: 14641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. 2008. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects, Cancer Res, 68: 2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. 2000. Induction of a bystander mutagenic effect of alpha particles in mammalian cells, Proc Natl Acad Sci U S A, 97: 2099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Chen G, Trosko JE, Waldren CA, Hei TK. 2001. Radiation risk to low fluences of alpha particles may be greater than we thought, Proc Natl Acad Sci U S A, 98: 14410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Zhou H, Leloup C, Marino SA, Geard CR, Hei TK, Lieberman HB. 2005. Differential impact of mouse Rad9 deletion on ionizing radiation-induced bystander effects, Radiat Res, 164: 655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Hu S, Chen Q, Zhang H, Fu J, Zhou Y, Bai Y, Pan Y, Shao C. 2021. Macrophage contributes to radiation-induced anti-tumor abscopal effect on transplanted breast cancer by HMGB1/TNF-α signaling factors, Int J Biol Sci, 17: 926–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkle RE, Bloom W. 1953. Irradiation of parts of individual cells, Science, 117: 487–93. [DOI] [PubMed] [Google Scholar]
- Zirkle RE, Tobias CA. 1953. Effects of ploidy and linear energy transfer on radiobiological survival curves, Arch Biochem Biophys, 47: 282–306. [DOI] [PubMed] [Google Scholar]
- Zong L, Zhu Y, Liang R, Zhao HB. 2016. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication, Sci Rep, 6: 19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


