Abstract
Osteoclasts are bone-resorbing cells that play an essential role in bone remodeling. Defects in osteoclasts result in unbalanced bone remodeling and are linked to many bone diseases including osteoporosis, rheumatoid arthritis, primary bone cancer, and skeletal metastases. Receptor activator of NF-kappaB ligand (RANKL) is a classical inducer of osteoclast formation. In the presence of macrophage-colony-stimulating factor, RANKL and co-stimulatory signals synergistically regulate osteoclastogenesis. However, recent discoveries of alternative pathways for RANKL-independent osteoclastogenesis have led to a reassessment of the traditional mechanisms that regulate osteoclast formation. In this review, we provide an overview of signaling pathways and other regulatory elements governing osteoclastogenesis. We also identify how osteoclastogenesis is altered in pathological conditions and discuss therapeutic targets in osteoclasts for the treatment of skeletal diseases.
Keywords: Osteoclastogenesis, Osteoclasts, RANKL, RANK, MYC
Introduction
Bone remodeling is a complex process involving the constant replacement of old, mature bone with the formation of new bone. Thus, bone remodeling is tightly regulated by a balance between bone resorption by osteoclasts and bone formation by osteoblasts. In addition to its resorbing activity, osteoclasts directly and indirectly regulate osteoblast activity and survival. As a result, hyperactivated osteoclasts in many pathological conditions can exceed bone formation by diminishing the activity of osteoblasts, leading to the pathological bone loss present in osteoporosis, rheumatoid arthritis, primary bone cancer, and skeletal metastases.
Osteoclasts are multinuclear giant cells that are responsible for bone resorption and derived from myeloid lineage cells in response to receptor activator of NF-κB ligand (RANKL) [1–6]. In the presence of macrophage-colony stimulating factor (M-CSF), RANK, a signaling receptor for RANKL, is increased in myeloid precursor cells. RANKL signaling promotes the differentiation and fusion of pre-osteoclasts to become multinucleated mature osteoclasts, which tightly adhere in bone, release hydrogen ions, and acidify the interface between bone and osteoclasts, leading to bone resorption.
Recent advances in the understanding of osteoclastogenesis in physiological and pathological conditions provide a rational framework for developing targeted therapies to specifically inhibit or slow down the progressive bone destruction associated with bone disorders. This review summarizes the current understanding of the molecular mechanisms that regulate osteoclast formation and activity in physiological and pathological conditions.
RANKL–RANK signaling
Receptor activator of nuclear factor-κB (RANKL: TNFRSF11A, also known as TRANCE [7, 8], OPGL [9] and ODF [10]) has been identified as a new member of the superfamily of tumor necrosis factor-α (TNF-α). RANKL is expressed in many tissues such as skeletal muscle, skin, bone, brain, and lymphoid organs. The RANKL pathway plays an essential role in bone remodeling primarily through the regulation of osteoclast differentiation, and also in osteoclast-independent mechanisms through the mediation of T-cell–dendritic cell interactions in the immune system [11, 12], regulation of epithelial cell proliferation in mammary physiology and breast cancer [13], and control of the fever response in brain [14]. RANKL has three isoforms that are differentially expressed in a cell-type specific manner [15], although the functions of individual isoforms have not yet been clearly defined.
RANKL binds to its signaling receptor, receptor activator of nuclear factor-κB (RANK), which is expressed in many tissues and myeloid cells including osteoclast precursor cells and osteoclasts. The functions of RANK/RANKL have been clearly demonstrated by RANKL or RANK-deficient mice [11, 16, 17]. Both RANKL-deficient mice and RANK-deficient mice display bone abnormalities, such as severe osteopetrosis, a lack of mature osteoclasts, and a defect in tooth eruption. In addition, these mice exhibit defects in early differentiation of T and B lymphocytes and lack all lymph nodes, demonstrating the importance of RANKL and RANK in lymph node organogenesis and lymphoid development.
Given the pivotal role of RANK/RANKL signaling in osteoclast biology, downstream signaling pathways of RANK/RANKL have been extensively studied (Fig. 1). Since RANK lacks intrinsic kinases activity, RANK/RANKL interaction first recruits TNF receptor-associated factors (TRAF6) to a unique motif [Pro-X-Glu-X–X-(aromatic/acid residue)] in RANK [18], activates TRAF6, and initiates downstream canonical signaling pathways. RANK also interacts with other TRAF family proteins such as TRAF1, 2, 3, and 5. Of these proteins, TRAF6, a RING-dependent ubiquitin kinase, is indispensable for osteoclastogenesis. Given that TRAF6-deficient cells have few or no osteoclasts and TRAF6-deficient mice exhibit an osteopetrotic bone phenotype [19, 20], TRAF6 is considered the only TRAF protein required for RANKL-induced osteoclastogenesis. TRAF6 uses a scaffolding protein p62 to interact with PKC or an adaptor protein TGF-beta activated kinase 1 binding protein 2 (TAB 2) to interact with TGF-beta-activated kinase 1 (TAK1), leading to the activation of IκB kinase 1/2 (IKK1/2) [21]. TRAF6 complexes with TAK1 and MKK6 selectively activate p38 [22]. In addition to TRAF6, RANK has been shown to interact with many other proteins, including Gab2. Gab2 is an adaptor molecule that is phosphorylated by RANK signaling; phosphorylated Gab2 binds with RANK to control RANK signals [23]. RANK signaling activates p38, ERK, JNK, AKT, and NFκB (canonical and non-canonical NF-κB) [24] and induces key transcription factors such as c-FOS [25]. RANK and ITAM immunoreceptor co-stimulatory signaling pathways also synergistically induce the expression of nuclear factor of activated T cells c1 (NFATc1), a master regulator of osteoclastogenesis [26–28].
Fig. 1.
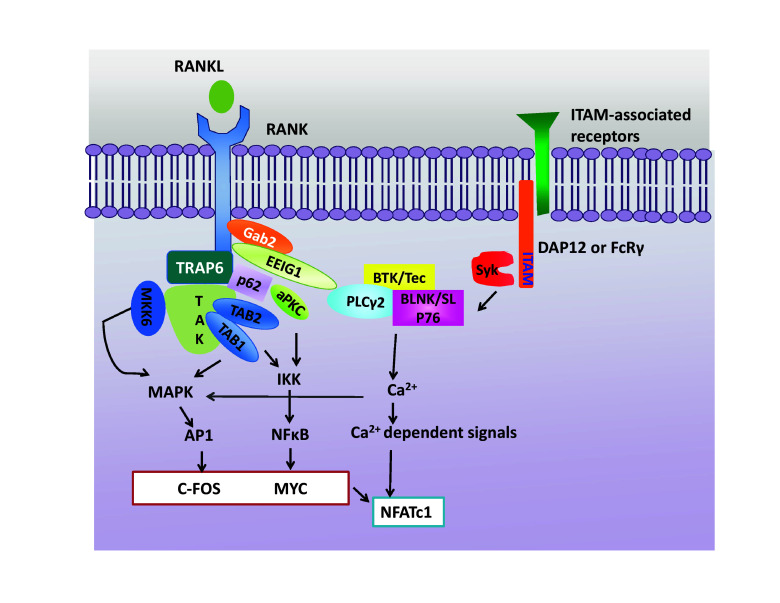
Signaling cascades on osteoclast differentiation. Osteoclastogenesis requires the activation of RANK signaling as well as ITAM-mediated co-stimulatory signals. RANK/RANKL interactions recruit TRAF6 and activate downstream signaling pathways; NF-κB pathways and MAPK pathways including ERK, JNK, and p38. NF-κB, AP-1 (c-FOS), and MYC are important downstream transcription factors for osteoclast formation and induce the expression of NFATc1, a master regulator of osteoclastogenesis. RANK signals also activate the ITAM receptor-mediated signaling pathway. Syk is recruited to phosphorylated ITAM adaptors (DAP12 or FcRγ), and then, Btk/Tec and BLNK/SLP-76 form a complex with PLCγ2. ITAM signals and RANK signals cooperatively induce Ca2+ oscillation, activate Ca2+-dependent signaling pathways, and synergistically induce NFATc1. RANK receptor activator of nuclear factor-κB, RANKL receptor activator of nuclear factor-κB ligand, ITAM immunoreceptor tyrosine activation motif, DAP12 DNAX-activating protein 12, FcRγ Fc receptor common γ subunit, TRAF6 TNF receptor-associated factors 6, NF-κB nuclear factor-κB, aPCK atypical protein kinase C, IKK IκB kinase, TAK1 TGFβ-activated kinase 1, Gab2 growth factor receptor-bound protein 2 (Grb2)-associated binder-2, Syk spleen tyrosine kinase, PLCγ2 phospholipase Cγ2, TAB TAK1-binding protein, MAPKs mitogen-activated protein kinases, JNK c-Jun N-terminal kinase, ERK extracellular signal-regulated kinase, AP-1 activator protein-1, NFATc1 nuclear factor of activated T-cell cytoplasmic 1
Immunoreceptor tyrosine-based activation motif signaling
Immunoreceptor tyrosine-based activation motif (ITAM)-mediated signals have been identified as co-stimulatory signals for RANKL-signaling pathways [29, 30]. ITAM-bearing adapter proteins such as the γ chain of Fc receptor (FcRγ) and DNAX-activating protein of 12 kDa (DAP12) are associated with ITAM-bearing receptors. DAP12 is associated with immune receptors such as triggering receptor expressed on myeloid cells-2 (TREM2), myeloid DAP12-associating lectin-1 (MDL-1), and signal regulatory protein β1 (SIRPβ1). FcRγ chains are associated with osteoclast-associated receptor (OSCAR), paired Ig-like receptor-A (PIR-A), and FcγRs. ITAM signaling activates protein kinase Syk, downstream PLCγ2, and a complex of Btk/Tec kinase and BLNK/SLP-76 [31]. This signaling induces calcium oscillation and then synergistically induces NFATc1 expression with RANK-signaling pathways.
Many studies have suggested a potential link between RANK-signaling pathways and ITAM-signaling pathways. Because Src family kinases phosphorylate ITAM receptors in immune cells, Src family kinases are viewed as candidate proteins that link RANK with ITAM signals. Notably, Src-deficient mice exhibit osteopetrotic phenotype with dysfunctional osteoclasts [32, 33]. Inhibiting Src suppresses M-CSF-induced DAP12 phosphorylation [34], while RANKL-induced DAP12 phosphorylation is diminished in Fyn-deficient cells [35]. However, in the absence of Fyn, RANK is still associated with FcγR/DAP12, suggesting the involvement of other factors linking RANK with ITAM-mediated signals in osteoclasts. RANKL has been shown to activate Btk/Tec, which is specifically involved in PLCγ2 activation and thus links RANK signals to ITAM signals [31]. Furthermore, PLCγ2 complexes with Gab2 [36], an interacting partner of RANKL-inducible early estrogen induced gene 1 (EEIG1) and Btk/Tec [37]. Therefore, RANK-bound Gab2 as well as EEIG1 and PLCγ2/Btk/Tec complex may be physical connectors between RANK signals and calcium signals (Fig. 1).
Although FcRγ-deficient mice have a normal bone mass, DAP12-deficient mice have increased bone mass with impaired osteoclastogenesis [29, 38, 39]. FcRγ/DAP12 double-deficient mice exhibit a severe osteopetrotic phenotype, due to attenuated activity of osteoclasts [29, 30]. In humans, inactivating mutations of either DAP12 or TREM2 are linked to a disorder known as Nasu–Hakola disease (NHD) or polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL). Correspondingly, human cells with functional mutations in DAP12 and TREM2 show inefficient and delayed differentiation of osteoclasts with a remarkably reduced bone resorption capability in vitro [40]. Downregulation of TREM2 expression by RNA interference in human cells and in the murine RAW264.7 results in defective osteoclastogenesis [41]. TREM2-mediated signals also play an important role in IL-10-mediated inhibition of osteoclastogenesis [42]. However, TREM2-deficient mice exhibit an osteoporotic phenotype and accelerated osteoclast differentiation through the suppression of M-CSF-mediated proliferation [43]. The role of TREM2 in osteoclastogenesis remains controversial.
ITAMs can generate inhibitory signals under certain circumstances, although ITAMs are typically associated with activating signals. This diversity of the ITAM-based signaling mechanism has been characterized in osteoclasts. For example, FcγRIIIA-deficient mice show low bone mass with an increased number of osteoclasts [44]. FcγRIIIA is an ITAM-associated receptor, but transduces an inhibitory signal for osteoclast differentiation. In addition, the activation of FcγRIIa (human-specific ITAM coupled FcγR) by IVIG suppresses osteoclastogenesis [45]. Under certain conditions, such as low calcium diet-induced bone loss, FcRγ/DAP12 double-deficient mice also show comparable bone loss with wild-type mice. Therefore, further studies will be needed to understand the context-dependent ITAM-mediated signals during osteoclastogenesis.
Negative-feedback mechanisms
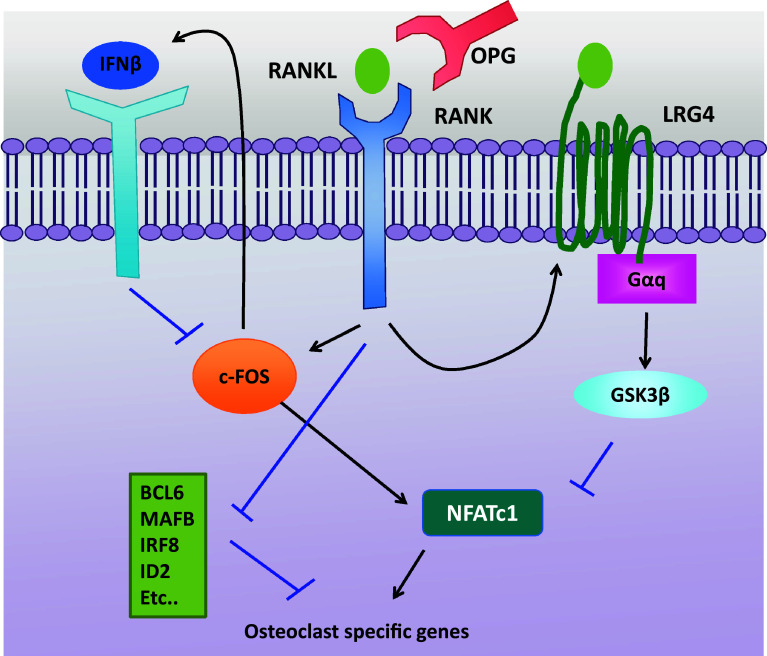
As the unbalanced regulation between osteoclasts and osteoblasts results in the development of pathogenesis bone diseases, osteoclast differentiation and bone resorption must be stringently regulated to avert potentially harmful consequences. Therefore, it is essential that negative regulation acts at multiple levels in the RANKL-signaling cascade and that negative-feedback mechanisms are elicited to orchestrate positive and negative regulations in osteoclasts (Fig. 2). The interaction between RANKL and RANK is controlled by ostoprotegerin (OPG, TNFRSF11B), a decoy receptor for RANKL [46]. OPG competes with RANKL to bind to RANK. OPG-deficient mice develop osteoporosis with an increased osteoclastogenesis [47] and OPG transgenic mice exhibit osteopetrosis [46]. The alteration of the ratio of RANKL/OPG in vivo affects osteoclast formation and activity, supporting the essential role of the RANK/RANKL/OPG system in fine-tuning osteoclast differentiation and bone remodeling.
Fig. 2.
Negative-feedback regulation of osteoclastogenesis. Osteoclastogenesis is regulated in multiple levels. OPG is a soluble decoy receptor for RANKL and competes with RANK for binding RANKL. The RANK/RANKL/OPG system has an essential regulatory role in osteoclast biology. LRG4 is a new receptor for RANKL and maintains the balance of RANKL-mediated activation by competing with RANK and suppressing the activation of NFATc1 via Gαq–GSK3β signaling pathway. RANK/RANKL interactions induce IFNβ via c-FOS. Then, IFNβ binds to its receptors and transduces negative signals to suppress the expression of c-FOS. RANK signals also downregulate the negative regulators of osteoclastogenesis such as BCL6, MAFB, IRF8, and ID2 to counteract NFATc1-mediated induction of osteoclast-specific genes. RANK receptor activator of nuclear factor-κB, RANKL receptor activator of nuclear factor-κB ligand, OPG osteoprotegerin, NFATc1 nuclear factor of activated T-cell cytoplasmic 1, LRG4 leucine-rich repeat-containing G-protein-coupled receptor 4, GSK3 glycogen synthase kinase 3, IFNβ interferon-beta, BCL6 B-cell lymphoma 6, MafB V-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B, IRF8 interferon regulatory factor-8, ID2 inhibitors of differentiation 2
RANKL-signaling pathways induce several negative regulators to control excessive activation. A well-known example of a negative-feedback pathway is IFNβ [48]. RANKL induces IFNβ, which subsequently suppresses RANKL-induced expression of c-FOS. In addition to RANK, RANKL interacts with another receptor, LRG4 (leucine-rich repeat-containing G-protein-coupled receptor 4, also known as GPR48) [49]. The binding of LRG4 to RANKL suppresses NFATc1 activation via Gαq and GSK3-β-signaling pathway. LRG4 expression is also induced by RANKL signaling and thus LRG4 functions as a negative-feedback loop. RANKL-signaling pathways are negatively regulated by interaction with other types of cells. IFNγ, one of the T-cell-produced cytokines, suppresses osteoclastogenesis by inducing TRAF6 degradation [50] and/or downregulating RANK expression [51]. SEMA3A, secreted from osteoblastic cells, suppresses osteoclastogenesis by sequestering TREM2–DAP12-induced ITAM signals [52]. In addition, RANKL signaling suppresses negative regulators to lower the threshold for osteoclast differentiation. Several transcription factors such as BCL6, IRF8, MAFB, ID2, and EOS belong to this category [53]. For proper homeostatic function of osteoclasts, multiple levels of regulation occur during osteoclastogenesis.
New versus old
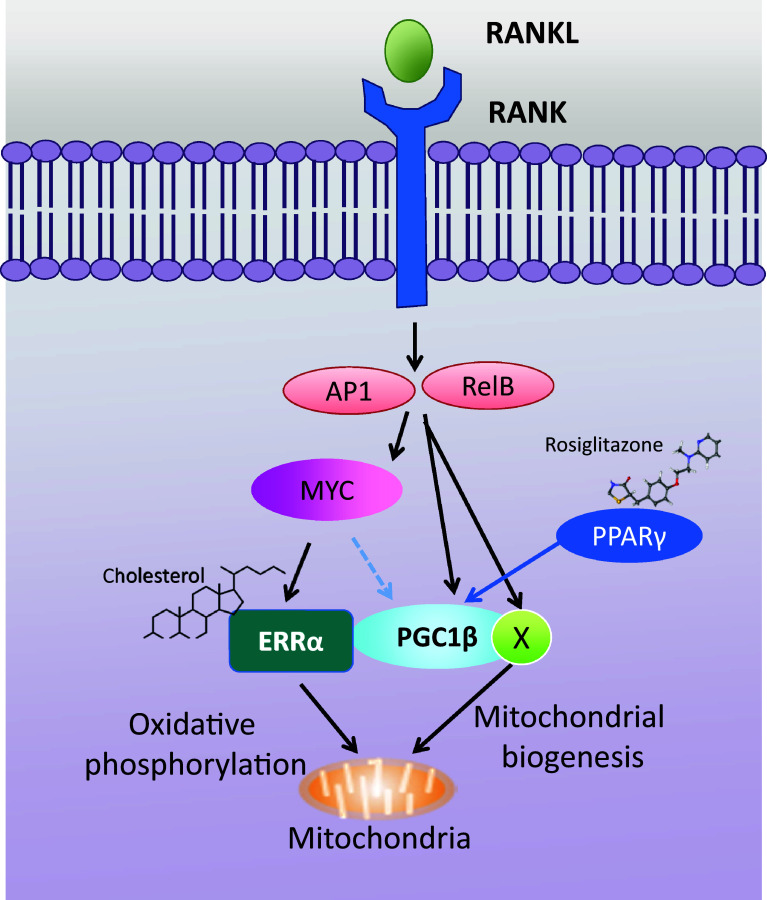
During osteoclastogenesis, pre-osteoclast cells fuse with each other to become multinucleated cells that resorb bone. These steps in osteoclast differentiation require increased energy demand [54–57], and thereby, cells undergo metabolic adaptation. The important function of metabolic reprogramming in osteoclast differentiation has been increasingly appreciated during the past several years and the molecules/factors that drive metabolic reprogramming during osteoclast differentiation have recently been uncovered. Several factors related to mitochondrial biogenesis and functions, such as peroxisome proliferator-activated receptor-gamma coactivator 1β (PGC1β), peroxisome proliferator-activated receptor γ (PPARγ), and estrogen-related receptor α (ERRα), play a fundamental role in osteoclast differentiation and function. Such factors govern key metabolic processes by transcriptionally regulating distinct metabolic genes during osteoclast differentiation as a part of bone remodeling [58–61]. Recent studies have illuminated the key upstream regulator in metabolic reprogramming in osteoclasts by showing that MYC [62]- and DNMT3A [63]-mediated regulation of oxidative respiration provides potential links between RANK signaling and metabolic reprogramming during osteoclastogenesis (Fig. 3). Furthermore, osteoclast-specific MYC deficient mice exhibit increased bone mass due to defective osteoclast development and MYC deficiency protects mice from osteoporosis-induced bone loss [62]. Targeting the pathways associated with metabolic reprogramming shows beneficial effects on pathological bone loss in a preclinical model of osteoporosis. Therefore, a deeper understanding of metabolic regulation in osteoclasts offers broader translational potential for the treatment of human bone disorders.
Fig. 3.
MYC–ERRα axis in osteoclast differentiation. RANKL stimulation activates downstream signaling molecules including c-Jun (AP1) and RelB (a signaling mediator in a non-canonical NF-κB pathway), and induces MYC expression. MYC stimulates the expression of ERRα and NFATc1 (shown in Fig. 1). ERRα induces genes for mitochondrial oxidative phosphorylation and the MYC–ERRα axis plays an important role in mitochondrial oxidative phosphorylation during osteoclast differentiation. The expression of PGC1β and other factors (X) is directly regulated by RelB and c-Jun and controls mitochondrial biogenesis. PGC1β also regulates the expression of c-FOS, a key factor of osteoclastogenesis. In addition, ERRα and PGC1β can form complexes in osteoclasts which have transcriptional activity. Cholesterol is a newly identified ligand for ERRα that enhances transcriptional activity of ERRα. In this vein, cholesterol-mediated enhancement of osteoclastogenesis is significantly reduced in ERRα-deficient mice. Although the role of PPARγ in in vivo bone resorption is controversial, rosiglitazone, a PPARγ agonist, increases bone loss and skeletal fragility by suppressing bone formation and enhancing bone resorption. PGC1β-deficient mice are resistant to rosiglitazone-induced bone loss. ERRα estrogen receptor-related alpha, PGC1β peroxisome proliferation-activated receptor-gamma coactivator 1b, PPARγ peroxisome proliferation-activated receptor-gamma
RANKL-independent osteoclastogenesis
While studies on RANKL-independent osteoclastogenesis have been reported, several have failed to demonstrate the independent generation of osteoclasts in RANK or RANKL-deficient backgrounds. For example, lysyl oxidase (LOX) was originally identified as a RANKL-independent stimulator of osteoclastogenesis [64]. However, a subsequent study revealed that LOX enhances osteoclastogenesis by amplifying RANKL signaling, but fails to induce osteoclast differentiation in RANK or RANKL-deficient cells [65].
Inflammation, which characterizes several pathological conditions, has been associated with increased recruitment of osteoclasts and enhanced bone erosion [66]. While inflammation indirectly promotes bone loss by increasing osteoclastogenic factors such as M-CSF [67] and RANKL [68], it is reported that inflammatory cytokines directly drive osteoclastogenesis independent of RANKL. Tumor necrosis factor (TNF)-α is one of the major inflammatory cytokines and synergizes RANKL-induced osteoclastogenesis. In addition, TNF-α alone can induce osteoclastogenesis independent of RANKL/RANK signals [69, 70]. However, whether an independent role for TNF-α in osteoclast differentiation exists remains controversial, as the continuous presence of OPG has been shown to interfere with in vitro TNF-α-induced osteoclastogenesis [71]. Recent studies reveal that both the combination of TNF-α with TGFβ [72] and the combination of TNF with IL-6 [73, 74] promote osteoclast differentiation independent of the RANK–RANKL system. TNF/IL-6-induced osteoclastogenesis is dependent on NFATc1, DAP12, and the IL6 receptor, but is independent of RANK [74]. Furthermore, reduced but substantial bone erosion in inflamed joints of the K/BXN serum-transfer arthritis model has been observed in inducible RANK-deficient mice [74]. These results suggest that inflammatory mediators alone can induce osteoclast differentiation and inflammatory bone erosion.
Despite the emerging role of RANKL-independent osteoclastogenesis, controlling the RANKL–RANK pathways has been shown to have prominent effects on blocking bone erosion in inflammatory arthritis. In human clinical trials, denosumab treatment has been shown to efficiently suppress systemic and articular bone erosion in patients with RA [75, 76]. Bone erosion and osteoclastogenesis in mice carrying RANKL-deficient fibroblasts are also significantly diminished in inflamed joints of both collagen antibody-induced arthritis and the collagen-induced arthritis model [68], supporting the importance of the RANKL–RANK axis for bone erosion in RA. Better understanding of the role of inflammatory cytokines in bone erosion and osteoclastogenesis in pathological conditions such as RA can provide valuable insights into the understanding of the pathogenesis of pathological bone erosion, and speaks to the additional benefit of cytokine blockers such as TNF blockers for inflammatory arthritis by directly targeting osteoclastogenesis.
Pathological bone erosion
Progressive bone destruction contributes significantly to fractures, disabilities, and pain. Therefore, there is considerable interest in establishing a better understanding of the pathologic mechanisms involved in the process and in developing therapies that can arrest its events. With pathological conditions affecting the differentiation, size, number, and activity of osteoclasts, osteoclasts have received substantial attention as a potential target for pathological bone resorption.
Bone erosion in pathological conditions results from excessive local bone resorption as well as poor bone formation, and is one of the clinical features of RA [66]. Analysis of animal models of inflammatory arthritis has been used to identify the molecular and cellular mechanisms underlying RA pathogenesis. The chronic inflammation that characterizes RA has thus been shown to be involved in the recruitment, differentiation, and activation of osteoclasts and promotes focal bone erosions in patients with RA. Thus, to optimize anti-resorptive therapy for patients with RA, the pathophysiology of osteoclast-driven bone loss in inflammatory conditions will need to be elucidated.
Moreover, inherited genetic bone diseases provide insight into the mechanisms of osteoclastogeneis. Paget’s disease and familial expansile osteolysis (FEO) are rare, autosomal dominant conditions in which bone remodeling is enhanced and characteristic osteolytic lesions are present in long bone. In particular, Paget’s disease is a focal bone disorder characterized by dysregulated osteoclast differentiation and activity. Mutation in exon 1 of RANK, which results in a constitutively active form, has been identified in Paget’s disease bone (PDB). These results affirm the importance of RANKL/RANK signaling in osteoclasts. In addition, other functional mutations have been identified in PDB. rs7528153 polymorphism in VAV3, Rho GEF (guanine-nucleotide exchange factors), has been shown to be associated with PDB [77]. Although the role of VAV3 rs7528153 in osteoclastogenesis is unclear, the significance of the function of VAV3 rs7528153 in osteoclasts can be reasoned. Indeed, VAV3-deficient mice display increased bone density and thickness and VAV3 plays an important role in co-stimulatory signals during osteoclast differentiation, and during the integrin signaling and cytoskeletal organization of mature osteoclasts [78]. Osteopetrosis is a rare genetic bone disorder caused by functionally defective osteoclasts. Mutations in TCIRG1 (encode an osteoclast-specific α3 vacuolar proton pump) [79] and CLCN7 (encode an osteoclast chloride channel) [80] explain nearly 70% of all patients with autosomal recessive osteopetrosis. Mutations in TCIRG1 and CLCN7 lead to the formation of defective osteoclasts that have impaired resorptive activity and impaired ruffled boarder which is induced by dysfunctional endosomal and lysosomal vesicle trafficking [81, 82]. Therefore, discovery of the specific mutations that cause defects in osteoclasts offers new potential candidates for controlling osteoclast formation and activity.
Closing remarks
Osteoclasts have received considerable attention as a potential target for pathological bone resorption, and many therapeutic interventions targeting osteoclasts have been developed. Directly targeting RANK–RANKL interaction, such as with denosumab, a human antibody against RANKL, is a currently used therapeutic treatment of osteoclast-mediated bone loss [83]. However, to develop optimal treatments and recovery for damaged bone in skeletal disorders, a number of aspects should be considered going forward. First, efforts to target osteoclasts should consider the influence of the pathological environment, such as individual genetic background and environmental influence. The effect of different pathological status on osteoclast differentiation and activity needs to be studied to comprehend and treat multifactorial bone disorder. Second, osteoclast-specific therapy, which allows for the avoidance of the side effects that limit current therapies, has great potential as preferred forms of treatment. In addition to the essential role of RANKL signals in osteoclastogenesis, the RANK–RANKL network is involved in a wide range of biological processes. Targeting the RANK–RANKL axis may cause adverse side effects [84]. Therefore, potential molecules/pathways that play a role in osteoclasts but not in other cell types must be identified. Third, there is a need to develop a dual target therapy that simultaneously suppresses bone erosion and promotes bone repair. In many pathological conditions, after diseases are controlled and further bone erosions are prevented, repair of existing bone erosions is rarely observed. Bone stays as weak, and with a high risk of fracture, even after treatment for bone erosion. Thus, treatment promoting regeneration and bone repair should be considered together with anti-resorptive therapies. In summary, a deeper understanding of the mechanisms involved in physiological and pathological osteoclastogenesis will be helpful in identifying new targets and developing potential therapeutic strategies of osteoclast-mediated diseases.
Acknowledgements
This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award numbers R01 AR069562 and AR073156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Hancox NM. The osteoclast. Biol Rev Camb Philos Soc. 1949;24:448–471. doi: 10.1111/j.1469-185X.1949.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 5.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 6.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 9.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann MF, Wong BR, Josien R, Steinman RM, Oxenius A, Choi Y. TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation. J Exp Med. 1999;189:1025–1031. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/S0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 14.Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, et al. Central control of fever and female body temperature by RANKL/RANK. Nature. 2009;462:505–509. doi: 10.1038/nature08596. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda T, Kasai M, Utsuyama M, Hirokawa K. Determination of three isoforms of the receptor activator of nuclear factor-kappaB ligand and their differential expression in bone and thymus. Endocrinology. 2001;142:1419–1426. doi: 10.1210/endo.142.4.8070. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- 19.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4:353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, et al. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB 2, and TRAF6. Mol Cell Biol. 2002;22:992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H, Ryu J, Ha J, Chang EJ, Kim HJ, Kim HM, et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13:1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 23.Wada T, Nakashima T, Oliveira-dos-Santos AJ, Gasser J, Hara H, Schett G, et al. The molecular scaffold Gab2 is a crucial component of RANK signaling and osteoclastogenesis. Nat Med. 2005;11:394–399. doi: 10.1038/nm1203. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL–RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 25.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 27.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 28.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 32.Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 34.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Kim DK, Kim AR, Mun SH, Lee SK, Kim JH, et al. Fyn positively regulates the activation of DAP12 and FcRgamma-mediated costimulatory signals by RANKL during osteoclastogenesis. Cell Signal. 2012;24:1306–1314. doi: 10.1016/j.cellsig.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi HK, Kang HR, Jung E, Kim TE, Lin JJ, Lee SY. Early estrogen-induced gene 1, a novel RANK signaling component, is essential for osteoclastogenesis. Cell Res. 2013;23:524–536. doi: 10.1038/cr.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Immel D, Xi CX, Bierhaus A, Feng X, Mei L, et al. Regulation of osteoclast function and bone mass by RAGE. J Exp Med. 2006;203:1067–1080. doi: 10.1084/jem.20051947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 42.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, et al. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, et al. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188:2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Negishi-Koga T, Gober HJ, Sumiya E, Komatsu N, Okamoto K, Sawa S, et al. Immune complexes regulate bone metabolism through FcRgamma signalling. Nat Commun. 2015;6:6637. doi: 10.1038/ncomms7637. [DOI] [PubMed] [Google Scholar]
- 45.Lee MJ, Lim E, Mun S, Bae S, Murata K, Ivashkiv LB, et al. Intravenous immunoglobulin (IVIG) attenuates TNF-induced pathologic bone resorption and suppresses osteoclastogenesis by inducing A20 expression. J Cell Physiol. 2016;231:449–458. doi: 10.1002/jcp.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 47.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 49.Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22:539–546. doi: 10.1038/nm.4076. [DOI] [PubMed] [Google Scholar]
- 50.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 51.Ji JD, Park-Min KH, Shen Z, Fajardo RJ, Goldring SR, McHugh KP, et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J Immunol. 2009;183:7223–7233. doi: 10.4049/jimmunol.0900072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 53.Ivashkiv LB, Zhao B, Park-Min KH, Takami M. Feedback inhibition of osteoclastogenesis during inflammation by IL-10, M-CSF receptor shedding, and induction of IRF8. Ann N Y Acad Sci. 2011;1237:88–94. doi: 10.1111/j.1749-6632.2011.06217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikeda K, Takeshita S. The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem. 2016;159:1–8. doi: 10.1093/jb/mvv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, et al. Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res. 2013;28:2392–2399. doi: 10.1002/jbmr.1976. [DOI] [PubMed] [Google Scholar]
- 56.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng R, Faccio R, Novack DV. Alternative NF-kappaB regulates RANKL-induced osteoclast differentiation and mitochondrial biogenesis via independent mechanisms. J Bone Miner Res. 2015;30(12):2287–2299. doi: 10.1002/jbmr.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 59.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, et al. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan Y. PPARgamma in bone homeostasis. Trends Endocrinol Metab. 2010;21:722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, et al. Ligand activation of ERRalpha by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23(3):479–491. doi: 10.1016/j.cmet.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bae S, Lee MJ, Mun SH, Giannopoulou EG, Yong-Gonzalez V, Cross JR, et al. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRalpha. J Clin Invest. 2017;127:2555–2568. doi: 10.1172/JCI89935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med. 2015;21:281–287. doi: 10.1038/nm.3774. [DOI] [PubMed] [Google Scholar]
- 64.Cox TR, Rumney RMH, Schoof EM, Perryman L, Hoye AM, Agrawal A, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Tsukasaki M, Hamada K, Okamoto K, Nagashima K, Terashima A, Komatsu N, et al. LOX fails to substitute for RANKL in osteoclastogenesis. J Bone Miner Res. 2017;32:434–439. doi: 10.1002/jbmr.2990. [DOI] [PubMed] [Google Scholar]
- 66.Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8:656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115:3418–3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis. 2016;75:1187–1195. doi: 10.1136/annrheumdis-2014-207137. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 71.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE–RANK–TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, et al. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66:121–129. doi: 10.1002/art.38218. [DOI] [PubMed] [Google Scholar]
- 74.O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 2016;68:2889–2900. doi: 10.1002/art.39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 76.Chiu YG, Ritchlin CT. Denosumab: targeting the RANKL pathway to treat rheumatoid arthritis. Expert Opin Biol Ther. 2017;17:119–128. doi: 10.1080/14712598.2017.1263614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usategui-Martin R, Calero-Paniagua I, Garcia-Aparicio J, Corral-Gudino L, Del Pino Montes J, Gonzalez Sarmiento R. VAV3 gene polymorphism is associated with Paget’s disease of bone. Genet Test Mol Biomark. 2016;20:335–337. doi: 10.1089/gtmb.2015.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 79.Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savarirayan R, et al. TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects, and in vitro rescue by U1 snRNA. Hum Mutat. 2004;24:225–235. doi: 10.1002/humu.20076. [DOI] [PubMed] [Google Scholar]
- 80.Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinun M, et al. Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res. 2003;18:1740–1747. doi: 10.1359/jbmr.2003.18.10.1740. [DOI] [PubMed] [Google Scholar]
- 81.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9:522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- 82.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2009;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 84.Toulis KA, Anastasilakis AD. Increased risk of serious infections in women with osteopenia or osteoporosis treated with denosumab. Osteoporos Int. 2010;21:1963–1964. doi: 10.1007/s00198-009-1145-1. [DOI] [PubMed] [Google Scholar]