Abstract
Background:
Asthma is a frequent and potentially life-threatening disease that complicates many pregnancies. There are extensive data with regard to the diagnosis and treatment of asthma during pregnancy. Medical providers require an up-to-date summary of the critical aspects of asthma management during pregnancy.
Objective:
This review aimed to summarize the available data from clinical trials, cohort studies, expert opinions, and guideline recommendations with regard to asthma in pregnancy.
Methods:
A search through PubMed was conducted by using keywords previously mentioned and MeSH (Medical Subject Headings) terminology. Clinical trials, observational studies, expert opinions, guidelines, and other reviews were included. The quality of the studies was assessed, and data were extracted and summarized.
Results:
Asthma worsens in ∼40% of pregnant women, which can be associated with maternal and fetal complications. Physiologic changes in the respiratory, cardiovascular, and immune systems during pregnancy play a critical role in the manifestations of asthma. The diagnosis and the treatment of asthma are similar to that of patients who are not pregnant. Nonetheless, concern for fetal malformations, preterm birth, and low birth weight must be considered when managing pregnant patients with asthma. Importantly, cornerstones of the pharmacotherapy of asthma seem to be safe during pregnancy.
Conclusion:
Asthma in pregnancy is associated with adverse outcomes. Roadblocks to management include associated comorbidities, medication nonadherence, atopy, lack of education, and smoking habits. These need to be acknowledged and addressed for successful asthma management during pregnancy.
Keywords: Asthma, pregnancy, maternal, fetal, peripartum, management, congenital, birth, preterm, malformations
Asthma prevalence during pregnancy ranges from 3% to 6%.1 Among those pregnancies, 19% had severe asthma and 16% had poorly controlled asthma.1 Furthermore, asthma is one of the most common chronic diseases that complicate pregnancies. However, approximately a fourth of pregnant patients with asthma discontinue their medications due to negative beliefs about safety.2,3 Due to the extensive list of complications in pregnant patients with asthma and their fetuses, medical providers require an updated summary of key aspects in physiologic changes, diagnosis, and treatment. This review aimed to summarize the most recent data to assist the reader in the diagnosis and treatment of pregnant women with asthma. For this, we have gathered information from clinical trials, observational studies, expert opinions, guidelines, and other reviews. Institutional review board approval was not required.
RELEVANT PHYSIOLOGIC CHANGES DURING PREGNANCY
A myriad of cardiovascular changes occurs in response to increased metabolic demands from the mother and fetus to ensure proper uteroplacental circulation. In the first trimester, there is a diminished peripheral vascular resistance,4 with an increased cardiac output5 and heart rate.4 Also, oxygen and metabolic rate consumption increase by 20%.6 Moreover, the respiratory system also undergoes adaptations during pregnancy with significant anatomic and hormonal changes that affect pulmonary function parameters in the mother.7 As pregnancy progresses, there is an upward displacement of the diaphragm with an increased lower chest wall circumference and costal angle widening.8 As a consequence, expiratory reserve and residual volumes decrease, while tidal volume increases.9
In contrast, forced vital capacity and peak expiratory flow do not change.10 Interestingly, these changes are not associated with a significant deterioration of quality of life.11 In addition, the elevation of progesterone levels, especially at the end of the first trimester, induces hyperventilation and results in a decreased (partial pressure of carbon dioxide; PaCO2) with transient respiratory alkalosis.12 Due to these physiologic changes, 60%–70% of pregnant women can experience dyspnea during the first and second trimesters.12–14 Importantly, compared with pregnant women who are not asthmatic, lung function changes are more pronounced in pregnant women with asthma.
The immune system also changes during pregnancy. For instance, there is a predominant T-helper type 1 (Th1) response in the first trimester with a subsequent shift to a T-helper type 2 (Th2) response in the second and third trimesters.15,16 The recruitment of specialized immune cells occurs within the decidua on implantation, which mostly contains macrophages, natural killer cells, regulatory T cells (Treg), and dendritic cells, which creates a proinflammatory environment that favors trophoblastic invasion.17,18 After the first weeks of gestation, there are changes in B-cell populations, including a decrease in total B-cell numbers.18,19 Among the most significant changes, an increasing regulatory B cell population promotes immune tolerance to avoid fetal rejection.20 Furthermore, there is evidence that Tregs also promote anti-inflammatory conditions during the second trimester.21 Interestingly, other studies22–44 have shown multiple abnormalities in immune cell subgroups of women with asthma during pregnancy. There is an increased number of B cells, memory cells, plasmablasts,22 monocytes, and neutrophils compared with women who are not asthmatic.23 In addition, the pregnancy-induced increase in Tregs is decreased in asthmatic pregnancy, which may interfere with fetal development and tolerance.24
Hormonal changes during pregnancy also influence the cytokine milieu.25 In particular, the gradual increase of estrogen and progesterone at the end of the first trimester reduces tumor necrosis factor α production, interferon (IFN) γ expression, and natural killer cell activity, facilitating an anti-inflammatory environment.26 In pregnant women with asthma, an abnormally increased Th2 response is present.26 Notably, an observational study in pregnant women found higher levels of interleukin (IL) 4, IL-6, and IFN-γ.27 In addition, a statistically significant negative correlation has been reported between the levels of IL-4 and IFN-γ and maternal peak expiratory flow among pregnant women with asthma.28 Furthermore, asthma during pregnancy increases the circulating level of proinflammatory C5a, which is accompanied by impaired lung function and partly counteracted by the gestation-specific elevation of regulatory complement factor H level.29 Exhaled breath condensate pH is higher in healthy pregnant women compared with their counterparts with asthma, which suggests oxidative inflammation at play in pregnant women with asthma.30 Moreover, studies have also shown an increase in exhaled breath condensate pH during asthma exacerbations.31 The physiologic changes during pregnancy are summarized in Table 1.
Table 1.
Summary of physiologic changes during pregnancy
DIAGNOSIS
Most pregnant women with asthma already have an established diagnosis before gestation. For those who present with respiratory symptoms during pregnancy and without a previous diagnosis of asthma, multiple conditions need to be considered. Importantly, 60% of pregnant women report shortness of breath due to the previously described changes in the pulmonary system.32 However, shortness of breath that impairs functionality and the association with other symptoms such as chest pain, cough, or wheezing warrant further workup. Medical conditions to consider include upper respiratory infections, gastroesophageal reflux disease, pulmonary embolism, pulmonary edema, and asthma.33
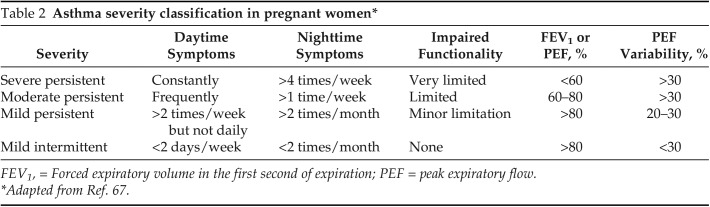
A clinical presentation typical of asthma increases the probability of this condition but is not confirmatory. Importantly, forced vital capacity and forced expiratory volume in the first second do not change during pregnancy.10 A confirmed parameter of expiratory flow limitation should be met with lung function testing and a bronchodilator test, as referenced in The Working Group on Asthma and Pregnancy Guidelines.34 In addition, asthma severity is classified according to the parameters defined by the National Asthma Education and Prevention Program Working Group on Asthma and Pregnancy as mild, moderate, moderate with additional therapy, and severe (Table 2). This classification considers daytime and nighttime symptoms plus spirometry values and implications for treatment options.35,36
Table 2.
Asthma severity classification in pregnant women*
FEV1, = Forced expiratory volume in the first second of expiration; PEF = peak expiratory flow.
Adapted from Ref. 67.
FOLLOW-UP
Asthma's course during pregnancy is highly variable. Retrospective and prospective studies have shown that asthma worsens in a third of patients, improves in a fourth of patients, and remains unchanged in a third of them, with similar disease courses in subsequent pregnancies.37 In addition, asthma severity during pregnancy is similar to the severity observed during the prepregnancy state when these patients continued to use their medications.38 Determinants of low-risk asthma exacerbation are clinically stable asthma, no history of exacerbations, and no necessity of treatment with controller medication because of mild disease.39
Evaluation of asthma control during pregnancy is critical, and it should be assessed by spirometry and validated questionnaires in prenatal visits.40 As described in the Global Initiative for Asthma (GINA) recommendations,41 an assessment of asthma symptom control could be made by questioning the frequency of asthma symptoms, the necessity of short-acting inhaled therapy, and the time of appearance of such symptoms. In addition, numerical questionnaires, e.g., the Asthma Control Test (QualityMetric Incorporated, Johnston), have been used and validated to assess asthma control in pregnant women.42,43
Other tools to assist asthma control evaluation during follow-up of pregnant patients are being studied. For instance, fractional exhaled nitric oxide (FeNO), was evaluated in a prospective study44 in which 111 women were randomly assigned to the FeNO group. An exacerbation rate was lower in the FeNO group than in the control group, with a number needed to treat of six. In the FeNO group, the quality of life was improved.44,45 As with nonpregnant adults with asthma, further studies are needed to evaluate FeNO-guided treatment.
MATERNAL AND FETAL OUTCOMES
Asthma has been associated with a wide variety of complications and adverse outcomes for mothers in all phases of gestation and among neonates, with a growing prevalence in recent years.46 As stated by Kwon et al.,47 higher numbers of pregnant women with asthma are driven by an increasing prevalence of asthma among younger pregnant women, likely as a consequence of lifestyle and urbanization changes.48,49 Some investigators postulate that complications among pregnant women are increasing due to increased obesity, consumption of tobacco products, and a higher prevalence of psychosocial issues.50 Some complications reported by observational studies include spontaneous abortion, antepartum and postpartum hemorrhage, placental abruption, gestational diabetes, cesarean section, placenta previa, premature rupture of membranes, preterm birth, a higher risk of a breech presentation, pulmonary embolism, and maternal intensive care unit admission.51–56
Furthermore, it seems that asthma severity influences the risk of complications because adverse outcomes are more prevalent in pregnant women with moderate-to-severe asthma.52 Pregnant women with asthma are also at an increased risk of experiencing transient hypertension of pregnancy, preeclampsia, or eclampsia.57 Notably, obesity and weight gain during pregnancy have also been associated with worse outcomes in pregnant patients with asthma, and this relationship seems to increase in a dose-dependent matter.58 Maternal asthma is also associated with an increased risk of multiple diseases in the offspring, including infectious, respiratory, cutaneous, and hematologic illnesses,59 and childhood asthma.60 A higher rate of congenital abnormalities and being small for gestational age have also been noted.46 In contrast, another study found no significant association between maternal asthma and birth weight, Apgar scores, or respiratory distress syndrome.61
TREATMENT
Management of asthma in pregnant patients includes education about the disease, inhaler technique, the importance of adherence independent of risk classification, and management of other associated comorbidities.62 It is essential to identify potential roadblocks to adequate asthma management in pregnant women (Fig. 1). A retrospective cohort study of 115,169 pregnant women with asthma recognized a tendency of these patients to decrease their asthma therapy during gestation with a subsequent increase in the rate of exacerbations.63 Furthermore, poor asthma control was observed in pregnant women with lower income, less education, younger age, and a smoking habit.64,65 Clinicians need to adequately assess concerns about asthma management and perceptions of disease course to ensure proper adherence.66
Figure 1.

Comorbidities and socioeconomical factors to consider when managing women with asthma during pregnancy.
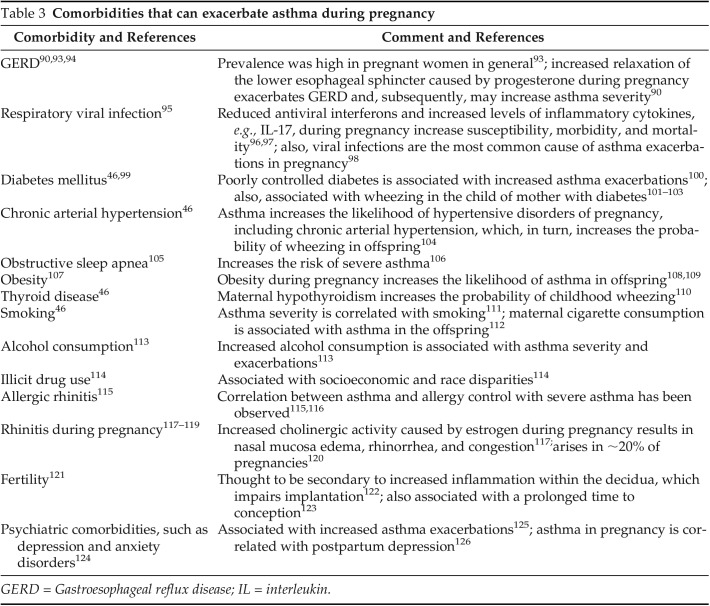
Pregnant women with asthma and with associated comorbidities, including atopy, rhinitis, and gastroesophageal reflux disease, require proper management to avoid poor asthma control (Table 3). For instance, atopy treatment needs lifestyle modifications and avoidance of common allergens, including pet dander, pollens, mold, house-dust mite, and cockroaches,67 to decrease the probability of asthma exacerbations.68 Allergen-specific immunotherapy may be continued if started before conception, but its initiation is contraindicated during pregnancy due to concerns of anaphylaxis.69
Table 3.
Comorbidities that can exacerbate asthma during pregnancy
GERD = Gastroesophageal reflux disease; IL = interleukin.
Multiple studies exhibited the association of appropriate asthma control and perception of the disease with multidisciplinary team involvement in the care of pregnant women with asthma.70 Interestingly, antenatal asthma management services reduce the risk of exacerbations, persistent uncontrolled asthma, and loss of disease control.58,71,72 In this regard, a randomized control trial that involved 60 pregnant women with asthma evaluated a multidisciplinary model of care for asthma management, including monitoring, education, and pharmacist-led intervention.73 This study demonstrated a decrease in the rate of asthma exacerbations and improvement in disease control among pregnant women.73
As mentioned in the GINA recommendations,41 asthma management should consider symptom control and risk reduction when prescribing medication. There is evidence of the importance of controlling asthma exacerbations in pregnant women to avoid substantial morbidity, mortality, and adverse fetal outcomes.74,75 As such, continuing inhaled therapy during pregnancy outweighs the risks of potential medication adverse effects.76,77 Medications for pregnant women with asthma include inhaled corticosteroids, leukotriene receptor antagonists, long-acting β2-agonists, short-acting β2-agonists, inhaled muscarinic antagonists, and, most recently, biologics.41 The therapeutic options according to asthma severity are summarized in Table 4.41
Table 4.
Medications for the management of asthma during pregnancy
ICS = Inhaled corticosteroid; LABA = long-acting β-2 agonist; LRA = leukotriene receptor antagonist; IgE = immunoglobulin E; IL = interleukin; SABA = short-acting β2-agonists.
Medication nonadherence is a critical problem when managing pregnant women with asthma. In a population-based control study78 that describes the use of asthma medications during pregnancy, the investigators described that 85% of women with asthma used albuterol, 46% used fluticasone, and 15% used montelukast. Importantly, 70% of women who used inhaled bronchodilators during the preconception period continued their use amid gestation,78 with other medications being more frequently discontinued.79 Limitations for adequate asthma management include medication safety concerns during pregnancy because women perceive a deleterious effect on the fetus as a reason to discontinue therapy. Importantly, evidence from multiple observational studies has not shown a statistically significant correlation between inhaled therapy and congenital heart defects,78 and cleft lip, stillbirth, neonatal hospitalization, respiratory distress syndrome, and neonatal sepsis.80 Notably, inhaled corticosteroids do not seem to affect fetal adrenal function.81
It is also important to note that there is a surge in novel therapies for asthma, including biologics, e.g., omalizumab.70 Notably, a prospective cohort study did not demonstrate an increased risk of congenital abnormalities in pregnant women treated with omalizumab.70 Nonetheless, because evidence is limited, current guidelines recommend continuing the use of omalizumab in pregnant women treated preconceptionally and not initiating it during pregnancy.82 Animal studies and case reports of patients with asthma who received anti–IL-5 biologics and dupilumab during pregnancy suggest that these biologics have a good safety profile.83–89 Further prospective studies are warranted to investigate the effects of asthma biologics during pregnancy.
Experimental and epidemiologic evidence has revealed increased reactive oxygen species production and inflammation during asthma in pregnancy. Introducing dietary antioxidants might decrease asthma severity,90 as demonstrated in some randomized controlled trials that used lycopene and β-carotene as supplements.91,92 However, interventional studies in pregnant women with asthma are needed to fully elucidate the benefits of antioxidants in this population.62
MANAGEMENT OF ACUTE EXACERBATIONS
Fifty percent of asthma exacerbations during pregnancy occur before 20 weeks of gestation 93 and are associated with adverse obstetric outcomes,94 including congenital malformations when severe exacerbations occur during the first trimester,95 low birth weight,93 preterm delivery,96 preeclampsia, and spontaneous abortion.97 Overall, treatment of asthma exacerbations during pregnancy is similar to patients who are not pregnant. First, exacerbation severity should be assessed by measuring expiratory airflow with a peak flow meter. Constant maternal and fetal monitoring must be ensured by using maternal oxygen saturation >95% and fetal heart rate testing; signs of impending respiratory failure must be routinely evaluated.
Special consideration to physiologic changes in the acid-base balance during pregnancy is required when interpreting arterial blood gas results because an apparent normal partial pressure of carbon dioxide (PaCO2) may signify a more severe respiratory compromise.69 Medications should include supplemental oxygen titrated to maintain adequate saturation, short-acting β-agonists, inhaled muscarinic antagonists (e.g., ipratropium bromide), systemic glucocorticoids in oral or intravenous preparations, and adjunct therapies in poor response, including magnesium sulfate and terbutaline.67,69
PERIPARTUM MANAGEMENT OF ASTHMA
Pregnant patients with asthma have a higher incidence of labor induction with oxytocin and cesarean section rates.98 Furthermore, ∼10% of pregnant patients with asthma will have increased symptoms during labor, usually controlled with bronchodilators.99 During labor and delivery, asthma therapy should be continued and adequate hydration and analgesia must be provided to avoid complications.35 Generally, labor and delivery management medications are safe with a few exceptions; among them, the prostaglandin F2-α analogs (e.g., carboprost) cause bronchoconstriction in animal studies and are contraindicated in pregnant women with asthma.100 Also, the use of morphine and meperidine for pain control should be avoided due to the risk of inducing histamine release.67
CONCLUSION
Further studies are required to elucidate the pathologic mechanisms involved in pregnant women with asthma. Moreover, additional investigations of the variable behaviors of asthma during gestation and the determinants that influence asthma's severity during pregnancy are warranted. Future directions should also focus on determining the risks of adverse maternal and fetal outcomes associated with specific asthma medications. This is particularly important given the advent of novel biologics for the treatment of asthma.
Footnotes
This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases K08AI141765 grant and the Mayo Clinic Specialized Center of Research Excellence and Women's Health Research Center Career Enhancement Core Award U54AG044170 to S.E. Chiarella
The authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. Cohen JM, Bateman BT, Huybrechts KF, et al. Poorly controlled asthma during pregnancy remains common in the United States. J Allergy Clin Immunol Pract. 2019; 7:2672–2680.e10. [DOI] [PubMed] [Google Scholar]
- 2. Ceulemans M, Lupattelli A, Nordeng H, et al. Women's beliefs about medicines and adherence to pharmacotherapy in pregnancy: opportunities for community pharmacists. Curr Pharm Des. 2019; 25:469–482. [DOI] [PubMed] [Google Scholar]
- 3. Baarnes CB, Hansen AV, Ulrik CS. Enrolment in an asthma management program during pregnancy and adherence with inhaled corticosteroids: the ‘Management of Asthma during Pregnancy’ Program. Respiration. 2016; 92:9–15. [DOI] [PubMed] [Google Scholar]
- 4. Mahendru AA, Everett TR, Wilkinson IB, et al. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J Hypertens. 2014; 32:849–856. [DOI] [PubMed] [Google Scholar]
- 5. Bader RA, Bader ME, Rose DF, et al. Hemodynamics at rest and during exercise in normal pregnancy as studies by cardiac catheterization. J Clin Invest. 1955; 34:1524–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson-Piercy C, Waldron M, Moore-Gillon J. Respiratory disease in pregnancy. Br J Hosp Med. 1994; 51:398–401. [PubMed] [Google Scholar]
- 7. Pereira A, Krieger BP. Pulmonary complications of pregnancy. Clin Chest Med. 2004; 25:299–310. [DOI] [PubMed] [Google Scholar]
- 8. Crapo RO. Normal cardiopulmonary physiology during pregnancy. Clin Obstet Gynecol. 1996; 39:3–16. [DOI] [PubMed] [Google Scholar]
- 9. Vatti RR, Teuber SS. Asthma and pregnancy. Clin Rev Allergy Immunol. 2012; 43:45–56. [DOI] [PubMed] [Google Scholar]
- 10. Grindheim G, Toska K, Estensen M-E, et al. Changes in pulmonary function during pregnancy: a longitudinal cohort study. BJOG. 2012; 119:94–101. [DOI] [PubMed] [Google Scholar]
- 11. Zairina E, Abramson MJ, McDonald CF, et al. A prospective cohort study of pulmonary function during pregnancy in women with and without asthma. J Asthma. 2016; 53:155–163. [DOI] [PubMed] [Google Scholar]
- 12. Prowse CM, Gaensler EA. Respiratory and acid-base changes during pregnancy. Anesthesiology. 1965; 26:381–392. [DOI] [PubMed] [Google Scholar]
- 13. Tenholder MF, South-Paul JE. Dyspnea in pregnancy. Chest. 1989; 96:381–388. [DOI] [PubMed] [Google Scholar]
- 14. Simon PM, Schwartzstein RM, Weiss JW, et al. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990; 142:1009–1014. [DOI] [PubMed] [Google Scholar]
- 15. Aris A, Lambert F, Bessette P, et al. Maternal circulating interferon-gamma and interleukin-6 as biomarkers of Th1/Th2 immune status throughout pregnancy. J Obstet Gynaecol Res. 2008; 34:7–11. [DOI] [PubMed] [Google Scholar]
- 16. Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010; 63:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006; 12:1065–1074. [DOI] [PubMed] [Google Scholar]
- 18. Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988; 28:1599–1613. [DOI] [PubMed] [Google Scholar]
- 19. Dutta S, Sengupta P, Haque N. Reproductive immunomodulatory functions of B cells in pregnancy. Int Rev Immunol. 2020; 39:53–66. [DOI] [PubMed] [Google Scholar]
- 20. Guzman-Genuino RM, Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. 2017; 8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017; 17:469–482. [DOI] [PubMed] [Google Scholar]
- 22. Martins C, Lima J, Nunes G, et al. Pregnancy alters the circulating B cell compartment in atopic asthmatic women, and transitional B cells are positively associated with the development of allergy manifestations in their progeny. Am J Reprod Immunol. 2016; 76:465–474. [DOI] [PubMed] [Google Scholar]
- 23. Osei-Kumah A, Wark PAB, Smith R, et al. Asthma during pregnancy alters immune cell profile and airway epithelial chemokine release. Inflamm Res. 2010; 59:349–358. [DOI] [PubMed] [Google Scholar]
- 24. Bohács A, Cseh A, Stenczer B, et al. Effector and regulatory lymphocytes in asthmatic pregnant women. Am J Reprod Immunol. 2010; 64:393–401. [DOI] [PubMed] [Google Scholar]
- 25. Piccinni M-P, Raghupathy R, Saito S, et al. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. 2021; 12:717808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldaçara RP, Silva I. Association between asthma and female sex hormones. Sao Paulo Med J. 2017; 135:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osei-Kumah A, Ammit AJ, Smith R, et al. Inflammatory mediator release in normal bronchial smooth muscle cells is altered by pregnant maternal and fetal plasma independent of asthma. Placenta. 2006; 27:847–852. [DOI] [PubMed] [Google Scholar]
- 28. Tamási L, Bohács A, Pállinger E, et al. Increased interferon-gamma- and interleukin-4-synthesizing subsets of circulating T lymphocytes in pregnant asthmatics. Clin Exp Allergy. 2005; 35:1197–1203. [DOI] [PubMed] [Google Scholar]
- 29. Bohács A, Bikov A, Ivancsó I, et al. Relationship of circulating C5a and complement factor H levels with disease control in pregnant women with asthma. Respir Care. 2016; 61:502–509. [DOI] [PubMed] [Google Scholar]
- 30. Eszes N, Bikov A, Lázár Z, et al. Changes in exhaled breath condensate pH in healthy and asthmatic pregnant women. Acta Obstet Gynecol Scand. 2013; 92:591–597. [DOI] [PubMed] [Google Scholar]
- 31. Caffarelli C, Dascola CP, Peroni D, et al. Airway acidification in childhood asthma exacerbations. Allergy Asthma Proc. 2014; 35:51–56. [DOI] [PubMed] [Google Scholar]
- 32. Jensen D, Webb KA, Davies GA, et al. Mechanisms of activity-related breathlessness in healthy human pregnancy. Eur J Appl Physiol. 2009; 106:253–265. [DOI] [PubMed] [Google Scholar]
- 33. Bidad K, Heidarnazhad H, Pourpak Z, et al. Frequency of asthma as the cause of dyspnea in pregnancy. Int J Gynaecol Obstet. 2010; 111:140–143. [DOI] [PubMed] [Google Scholar]
- 34. Stone RG, McDonald M, Elnazir B. Global Initiative for Asthma 2019 Guidelines: new changes to the treatment of mild asthmatics 12 years and older. Ir Med J. 2020; 113:69. [PubMed] [Google Scholar]
- 35. Dombrowski MP, Schatz M, ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 90, February 2008: asthma in pregnancy. Obstet Gynecol. 2008; 111(pt 1):457–464. [DOI] [PubMed] [Google Scholar]
- 36. Luskin AT. An overview of the recommendations of the Working Group on Asthma and Pregnancy. National Asthma Education and Prevention Program. J Allergy Clin Immunol. 1999; 103 (pt 1):S350–S353. [DOI] [PubMed] [Google Scholar]
- 37. Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988; 81:509–517. [PubMed] [Google Scholar]
- 38. Belanger K, Hellenbrand ME, Holford TR, et al. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010; 115:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ali Z, Nilas L, Ulrik CS. Determinants of low risk of asthma exacerbation during pregnancy. Clin Exp Allergy. 2018; 48:23–28. [DOI] [PubMed] [Google Scholar]
- 40. Amaral L, Martins C, Coimbra A. Use of the Control of Allergic Rhinitis and Asthma Test and pulmonary function tests to assess asthma control in pregnancy. Aust N Z J Obstet Gynaecol. 2018; 58:86–90. [DOI] [PubMed] [Google Scholar]
- 41. Global Strategy for Asthma Management and Prevention. 2020. Available online at www.ginasthma.org; accessed January 12, 2022.
- 42. Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006; 117:549–556. [DOI] [PubMed] [Google Scholar]
- 43. Palmsten K, Schatz M, Chan PH, et al. Validation of the Pregnancy Asthma Control Test. J Allergy Clin Immunol Pract. 2016; 4:310–315.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011; 378:983–990. [DOI] [PubMed] [Google Scholar]
- 45. Tamási L, Bohács A, Bikov A, et al. Exhaled nitric oxide in pregnant healthy and asthmatic women. J Asthma. 2009; 46:786–791. [DOI] [PubMed] [Google Scholar]
- 46. Baghlaf H, Spence AR, Czuzoj-Shulman N, et al. Pregnancy outcomes among women with asthma. J Matern Fetal Neonatal Med. 2019; 32:1325–1331. [DOI] [PubMed] [Google Scholar]
- 47. Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003; 13:317–324. [DOI] [PubMed] [Google Scholar]
- 48. Lundback B, Backman H, Lotvall J, et al. Is asthma prevalence still increasing? Expert Rev Respir Med. 2016; 10:39–51. [DOI] [PubMed] [Google Scholar]
- 49. Backman H, Raisanen P, Hedman L, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016—results from three population surveys. Clin Exp Allergy. 2017; 47:1426–1435. [DOI] [PubMed] [Google Scholar]
- 50. Carter RM, Symons Downs D, Bascom R, et al. The moderating influence of asthma diagnosis on biobehavioral health characteristics of women of reproductive age. Matern Child Health J. 2012; 16:448–455. [DOI] [PubMed] [Google Scholar]
- 51. Blais L, Kettani F-Z, Forget A. Relationship between maternal asthma, its severity and control and abortion. Hum Reprod. 2013; 28:908–915. [DOI] [PubMed] [Google Scholar]
- 52. Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2014; 27:934–942. [DOI] [PubMed] [Google Scholar]
- 53. Mendola P, Laughon SK, Männistö TI, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol. 2013; 208:127.e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Firoozi F, Lemiere C, Beauchesne M-F, et al. Impact of maternal asthma on perinatal outcomes: a two-stage sampling cohort study. Eur J Epidemiol. 2012; 27:205–214. [DOI] [PubMed] [Google Scholar]
- 55. Kemppainen M, Lahesmaa-Korpinen A-M, Kauppi P, et al. Maternal asthma is associated with increased risk of perinatal mortality. PLoS One. 2018; 13:e0197593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vaezi A, Haghighi L, Beigmohammadi F, et al. Maternal asthma, pregnancy, delivery and birth outcomes: a retrospective cohort study. Iran J Allergy Asthma Immunol. 2017; 16:92–98. [PubMed] [Google Scholar]
- 57. Wang M, He W, Li M, et al. Maternal asthma and the risk of hypertensive disorders of pregnancy: a systematic review and meta-analysis of cohort studies. Hypertens Pregnancy. 2020; 39:12–24. [DOI] [PubMed] [Google Scholar]
- 58. Ali Z, Nilas L, Ulrik CS. Excessive gestational weight gain in first trimester is a risk factor for exacerbation of asthma during pregnancy: a prospective study of 1283 pregnancies. J Allergy Clin Immunol. 2018; 141:761–767. [DOI] [PubMed] [Google Scholar]
- 59. Tegethoff M, Olsen J, Schaffner E, et al. Asthma during pregnancy and clinical outcomes in offspring: a national cohort study. Pediatrics. 2013; 132:483–491. [DOI] [PubMed] [Google Scholar]
- 60. Venter C, Palumbo MP, Sauder KA, et al. Incidence and timing of offspring asthma, wheeze, allergic rhinitis, atopic dermatitis, and food allergy and association with maternal history of asthma and allergic rhinitis. World Allergy Organ J. 2021; 14:100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fazel N, Kundi M, Jensen-Jarolim E, et al. Prospective cohort study of pregnancy complications and birth outcomes in women with asthma. Arch Gynecol Obstet. 2018; 298:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murphy VE. Managing asthma in pregnancy. Breathe (Sheff). 2015; 11:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koo S-M, Kim Y, Park C, et al. Effect of pregnancy on quantitative medication use and relation to exacerbations in asthma. Biomed Res Int. 2017; 2017:8276190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Louik C, Schatz M, Hernández-Díaz S, et al. Asthma in pregnancy and its pharmacologic treatment. Ann Allergy Asthma Immunol. 2010; 105:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zetstra-van der Woude PA, Vroegop JS, Bos HJ, et al. A population analysis of prescriptions for asthma medications during pregnancy. J Allergy Clin Immunol. 2013; 131:711–717. [DOI] [PubMed] [Google Scholar]
- 66. Lim AS, Stewart K, Abramson MJ, et al. Asthma during pregnancy: the experiences, concerns and views of pregnant women with asthma. J Asthma. 2012; 49:474–479. [DOI] [PubMed] [Google Scholar]
- 67. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005; 115:34–46. [DOI] [PubMed] [Google Scholar]
- 68. Sande AK, Torkildsen EA, Sande RK, et al. Maternal allergy as an isolated risk factor for early-onset preeclampsia: an epidemiological study. J Reprod Immunol. 2018; 127:43–47. [DOI] [PubMed] [Google Scholar]
- 69. Bonham CA, Patterson KC, Strek ME. Asthma outcomes and management during pregnancy. Chest. 2018; 153:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mihălţan FD, Antoniu SA, Ulmeanu R. Asthma and pregnancy: therapeutic challenges. Arch Gynecol Obstet. 2014; 290:621–627. [DOI] [PubMed] [Google Scholar]
- 71. Grzeskowiak LE, Smith B, Roy A, et al. An observational study of the impact of an antenatal asthma management service on asthma control during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2016; 197:48–53. [DOI] [PubMed] [Google Scholar]
- 72. Ibrahim WH, Rasul F, Ahmad M, et al. Asthma knowledge, care, and outcome during pregnancy: the QAKCOP study. Chron Respir Dis. 2019; 16:1479972318767719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lim AS, Stewart K, Abramson MJ, et al. Multidisciplinary Approach to Management of Maternal Asthma (MAMMA): a randomized controlled trial. Chest. 2014; 145:1046–1054. [DOI] [PubMed] [Google Scholar]
- 74. Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011; 118:1314–1323. [DOI] [PubMed] [Google Scholar]
- 75. Rejnö G, Lundholm C, Larsson K, et al. Adverse pregnancy outcomes in asthmatic women: a population-based family design study. J Allergy Clin Immunol Pract. 2018; 6:916–922.e6. [DOI] [PubMed] [Google Scholar]
- 76. Schatz M, Dombrowski MP, Wise R, et al. The relationship of asthma-specific quality of life during pregnancy to subsequent asthma and perinatal morbidity. J Asthma. 2010; 47:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shaked E, Wainstock T, Sheiner E, et al. Maternal asthma: pregnancy course and outcome. J Matern Fetal Neonatal Med. 2019; 32:103–108. [DOI] [PubMed] [Google Scholar]
- 78. Van Zutphen AR, Bell EM, Browne ML, et al. Maternal asthma medication use during pregnancy and risk of congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2015; 103:951–961. [DOI] [PubMed] [Google Scholar]
- 79. Chambers K. Asthma education and outcomes for women of childbearing age. Case Manager. 2003; 14:58–61. [DOI] [PubMed] [Google Scholar]
- 80. Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG. 2013; 120:812–822. [DOI] [PubMed] [Google Scholar]
- 81. Hodyl NA, Stark MJ, Osei-Kumah A, et al. Fetal glucocorticoid-regulated pathways are not affected by inhaled corticosteroid use for asthma during pregnancy. Am J Respir Crit Care Med. 2011; 183:716–722. [DOI] [PubMed] [Google Scholar]
- 82. Namazy JA, Blais L, Andrews EB, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol. 2020; 145:528–536.e1. [DOI] [PubMed] [Google Scholar]
- 83. Kage P, Simon J-C, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021; 48:E484–E485. [DOI] [PubMed] [Google Scholar]
- 84. Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021; 13:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020; 6:1051–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022; 35:e15237. [DOI] [PubMed] [Google Scholar]
- 87. Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022; 47:960–961. [DOI] [PubMed] [Google Scholar]
- 88. Riquelme-Mc Loughlin C, Mascaro JM, Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021; 46:1578–1579. [DOI] [PubMed] [Google Scholar]
- 89. Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022; 18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Grieger JA, Wood LG, Clifton VL. Improving asthma during pregnancy with dietary antioxidants: the current evidence. Nutrients. 2013; 5:3212–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wood LG, Garg ML, Powell H, et al. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008; 42:94–102. [DOI] [PubMed] [Google Scholar]
- 92. Neuman I, Nahum H, Ben-Amotz A. Prevention of exercise-induced asthma by a natural isomer mixture of beta-carotene. Ann Allergy Asthma Immunol. 1999; 82:549–553. [DOI] [PubMed] [Google Scholar]
- 93. Grzeskowiak LE, Smith B, Roy A, et al. Patterns, predictors and outcomes of asthma control and exacerbations during pregnancy: a prospective cohort study. ERJ Open Res. 2016; 2:00054-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ali Z, Hansen AV, Ulrik CS. Exacerbations of asthma during pregnancy: impact on pregnancy complications and outcome. J Obstet Gynaecol. 2016; 36:455–461. [DOI] [PubMed] [Google Scholar]
- 95. Blais L, Kettani F-Z, Forget A, et al. Asthma exacerbations during the first trimester of pregnancy and congenital malformations: revisiting the association in a large representative cohort. Thorax. 2015; 70:647–652. [DOI] [PubMed] [Google Scholar]
- 96. Namazy JA, Murphy VE, Powell H, et al. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J. 2013; 41:1082–1090. [DOI] [PubMed] [Google Scholar]
- 97. Bokern MP, Robijn AL, Jensen ME, et al. Factors associated with asthma exacerbations during pregnancy. J Allergy Clin Immunol Pract. 2021; 9:4343–4352.e4. [DOI] [PubMed] [Google Scholar]
- 98. Minerbi-Codish I, Fraser D, Avnun L, et al. Influence of asthma in pregnancy on labor and the newborn. Respiration. 1998; 65:130–135. [DOI] [PubMed] [Google Scholar]
- 99. Gluck JC. The change of asthma course during pregnancy. Clin Rev Allergy Immunol. 2004; 26:171–180. [DOI] [PubMed] [Google Scholar]
- 100. Kawikova I, Barnes PJ, Takahashi T, et al. 8-Epi-PGF2 alpha, a novel noncyclooxygenase-derived prostaglandin, constricts airways in vitro. Am J Respir Crit Care Med. 1996; 153:590–596. [DOI] [PubMed] [Google Scholar]
- 101. Clifton VL, Vanderlelie J, Perkins AV. Increased anti-oxidant enzyme activity and biological oxidation in placentae of pregnancies complicated by maternal asthma. Placenta. 2005; 26:773–779. [DOI] [PubMed] [Google Scholar]
- 102. Bidad K, Heidarnazhad H, Pourpak Z, et al. Gastroesophagial reflux disease and asthma in pregnant women with dyspnea. Iran J Allergy Asthma Immunol. 2014; 13:104–109. [PubMed] [Google Scholar]
- 103. Trigueros JA, Plaza V, Dominguez-Ortega J, et al. Asthma, comorbidities, and aggravating circumstances: the GEMA-FORUM II Task Force. J Investig Allergol Clin Immunol. 2020; 30:140–143. [DOI] [PubMed] [Google Scholar]
- 104. Forbes RL, Gibson PG, Murphy VE, et al. Impaired type I and III interferon response to rhinovirus infection during pregnancy and asthma. Thorax. 2012; 67:209–214. [DOI] [PubMed] [Google Scholar]
- 105. Vanders RL, Gibson PG, Wark PA, et al. Alterations in inflammatory, antiviral and regulatory cytokine responses in peripheral blood mononuclear cells from pregnant women with asthma. Respirology. 2013; 18:827–833. [DOI] [PubMed] [Google Scholar]
- 106. Murphy VE, Powell H, Wark PAB, et al. A prospective study of respiratory viral infection in pregnant women with and without asthma. Chest. 2013; 144:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Murphy VE, Mattes J, Powell H, et al. Respiratory viral infections in pregnant women with asthma are associated with wheezing in the first 12 months of life. Pediatr Allergy Immunol. 2014; 25:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baribeau V, Beauchesne M-F, Rey E, et al. The use of asthma controller medications during pregnancy and the risk of gestational diabetes. J Allergy Clin Immunol. 2016; 138:1732–1733.e6. [DOI] [PubMed] [Google Scholar]
- 109. Cardet JC, Bulkhi AA, Lockey RF. Nonrespiratory comorbidities in asthma. J Allergy Clin Immunol Pract. 2021; 9:3887–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Adgent MA, Gebretsadik T, Reedus J, et al. Gestational diabetes and childhood asthma in a racially diverse US pregnancy cohort. Pediatr Allergy Immunol. 2021; 32:1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Azad MB, Moyce BL, Guillemette L, et al. Diabetes in pregnancy and lung health in offspring: developmental origins of respiratory disease. Paediatr Respir Rev. 2017; 21:19–26. [DOI] [PubMed] [Google Scholar]
- 112. Azad MB, Becker AB, Kozyrskyj AL. Association of maternal diabetes and child asthma. Pediatr Pulmonol. 2013; 48:545–552. [DOI] [PubMed] [Google Scholar]
- 113. Wilmink FA, den Dekker HT, de Jongste JC, et al. Maternal blood pressure and hypertensive disorders during pregnancy and childhood respiratory morbidity: the Generation R Study. Eur Respir J. 2018; 52:1800378. [DOI] [PubMed] [Google Scholar]
- 114. Williams MA, Gelaye B, Qiu C, et al. S. Habitual snoring and asthma comorbidity among pregnant women. J Asthma. 2011; 48:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bourjeily G, Barbara N, Larson L, et al. Clinical manifestations of obstructive sleep apnoea in pregnancy: more than snoring and witnessed apnoeas. J Obstet Gynaecol. 2012; 32:434–438. [DOI] [PubMed] [Google Scholar]
- 116. Murphy VE, Jensen ME, Powell H, et al. Influence of maternal body mass index and macrophage activation on asthma exacerbations in pregnancy. J Allergy Clin Immunol Pract. 2017; 5:981–987.e1. [DOI] [PubMed] [Google Scholar]
- 117. Miethe S, Karsonova A, Karaulov A, et al. Obesity and asthma. J Allergy Clin Immunol. 2020; 146:685–693. [DOI] [PubMed] [Google Scholar]
- 118. Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017; 5:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liu X, Andersen SL, Olsen J, et al. Maternal hypothyroidism in the perinatal period and childhood asthma in the offspring. Allergy. 2018; 73:932–939. [DOI] [PubMed] [Google Scholar]
- 120. Murphy VE, Clifton VL, Gibson PG. The effect of cigarette smoking on asthma control during exacerbations in pregnant women. Thorax. 2010; 65:739–744. [DOI] [PubMed] [Google Scholar]
- 121. Toppila-Salmi S, Luukkainen AT, Xu B, et al. Maternal smoking during pregnancy affects adult onset of asthma in offspring: a follow up from birth to age 46 years. Eur Respir J. 2020; 55:1901857. [DOI] [PubMed] [Google Scholar]
- 122. Almeida MLD, Santana PA, Guimaraes AM, et al. Asthma and pregnancy: repercussions for neonates. J Bras Pneumol. 2010; 36:293–300. [DOI] [PubMed] [Google Scholar]
- 123. Aly H, Nada A, Ahmad T, et al. Maternal asthma, race and low birth weight deliveries. Early Hum Dev. 2011; 87:457–460. [DOI] [PubMed] [Google Scholar]
- 124. Namazy J, Schatz M. The treatment of allergic respiratory disease during pregnancy. J Investig Allergol Clin Immunol. 2016; 26:1–7; quiz 2p following 7. [PubMed] [Google Scholar]
- 125. Turkeltaub PC, Cheon J, Friedmann E, et al. The influence of asthma and/or hay fever on pregnancy: data from the 1995 National Survey of Family Growth. J Allergy Clin Immunol Pract. 2017; 5:1679–1690. [DOI] [PubMed] [Google Scholar]
- 126. Lekas MD. Rhinitis during pregnancy and rhinitis medicamentosa. Otolaryngol Head Neck Surg. 1992; 107(pt 2):845–848, discussion 9. [DOI] [PubMed] [Google Scholar]
- 127. Schatz M, Zeiger RS. Diagnosis and management of rhinitis during pregnancy. Allergy Proc. 1988; 9:545–554. [DOI] [PubMed] [Google Scholar]
- 128. Blaiss MS, Food and Drug Administration (U.S.); ACAAI-ACOG (American College of Allergy, Asthma, and Immunology and American College of Obstetricians and Gynecologists), Acaai A. Management of rhinitis and asthma in pregnancy. Ann Allergy Asthma Immunol. 2003; 90(suppl 3):16–22. [DOI] [PubMed] [Google Scholar]
- 129. Mabry RL. Rhinitis of pregnancy. South Med J. 1986; 79:965–971. [DOI] [PubMed] [Google Scholar]
- 130. Gade EJ, Thomsen SF, Lindenberg S, et al. Asthma affects time to pregnancy and fertility: a register-based twin study. Eur Respir J. 2014; 43:1077–1085. [DOI] [PubMed] [Google Scholar]
- 131. Grzeskowiak LE, Smithers LG, Grieger JA, et al. Asthma treatment impacts time to pregnancy: evidence from the international SCOPE study. Eur Respir J. 2018; 51:1702035. [DOI] [PubMed] [Google Scholar]
- 132. Vejen Hansen A, Ali Z, Malchau SS, et al. Fertility treatment among women with asthma: a case-control study of 3689 women with live births. Eur Respir J. 2019; 53:1800597. [DOI] [PubMed] [Google Scholar]
- 133. Powell H, McCaffery K, Murphy VE, et al. Psychosocial variables are related to future exacerbation risk and perinatal outcomes in pregnant women with asthma. J Asthma. 2013; 50:383–389. [DOI] [PubMed] [Google Scholar]
- 134. Grzeskowiak LE, Smith B, Roy A, et al. Impact of a history of maternal depression and anxiety on asthma control during pregnancy. J Asthma. 2017; 54:706–713. [DOI] [PubMed] [Google Scholar]
- 135. Blais L, Salah Ahmed SI, Beauchesne M-F, et al. Risk of postpartum depression among women with asthma. J Allergy Clin Immunol Pract. 2019; 7:925–933.e2. [DOI] [PubMed] [Google Scholar]
- 136. Namazy JA, Schatz M. Pharmacotherapy options to treat asthma during pregnancy. Expert Opin Pharmacother. 2015; 16:1783–1791. [DOI] [PubMed] [Google Scholar]
- 137. Middleton PG, Gade EJ, Aguilera C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J. 2020; 55:1901208. [DOI] [PubMed] [Google Scholar]
- 138. Blais L, Beauchesne M-F, Lemiere C, et al. High doses of inhaled corticosteroids during the first trimester of pregnancy and congenital malformations. J Allergy Clin Immunol. 2009; 124:1229–1234.e4. [DOI] [PubMed] [Google Scholar]
- 139. Schatz M, Dombrowski MP, Wise R, et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004; 113:1040–1045. [DOI] [PubMed] [Google Scholar]
- 140. Eltonsy S, Forget A, Blais L. Beta2-agonists use during pregnancy and the risk of congenital malformations. Birth Defects Res A Clin Mol Teratol. 2011; 91:937–947. [DOI] [PubMed] [Google Scholar]
- 141. Bakhireva LN, Jones KL, Schatz M, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007; 119:618–625. [DOI] [PubMed] [Google Scholar]
- 142. Rocklin RE. Asthma, asthma medications and their effects on maternal/fetal outcomes during pregnancy. Reprod Toxicol. 2011; 32:189–197. [DOI] [PubMed] [Google Scholar]
- 143. Sarkar M, Koren G, Kalra S, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol. 2009; 65:1259–1264. [DOI] [PubMed] [Google Scholar]
- 144. Schatz M, Krishnan JA, Chambers C. Implications of changes in U.S. Food and Drug Administration Prescribing Information Regarding the Safety and Use of Asthma Biologics During Pregnancy. Ann Am Thorac Soc. 2018; 15:1131–1136. [DOI] [PubMed] [Google Scholar]
- 145. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017; 9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Benralizumab (Fasenra) for severe eosinophilic asthma. JAMA. 2018; 319:1501–1502. [DOI] [PubMed] [Google Scholar]
- 147. Kasuya A, Kitano S, Hoshino T, et al. Successful control of severe eosinophilic granulomatosis with polyangiitis in a pregnancy and perinatal period: a use of mepolizumab. J Dermatol. 2019; 46:e309–e311. [DOI] [PubMed] [Google Scholar]