Abstract
Reactivation of latent tuberculosis contributes significantly to the incidence of disease caused by Mycobacterium tuberculosis. The mechanisms involved in the containment of latent tuberculosis are poorly understood. Using the low-dose model of persistent murine tuberculosis in conjunction with MP6-XT22, a monoclonal antibody that functionally neutralizes tumor necrosis factor alpha (TNF-α), we examined the effects of TNF-α on the immunological response of the host in both persistent and reactivated tuberculous infections. The results confirm an essential role for TNF-α in the containment of persistent tuberculosis. TNF-α neutralization resulted in fatal reactivation of persistent tuberculosis characterized by a moderately increased tissue bacillary burden and severe pulmonic histopathological deterioration that was associated with changes indicative of squamous metaplasia and fluid accumulation in the alveolar space. Analysis of pulmonic gene and protein expression of mice in the low-dose model revealed that nitric oxide synthase was attenuated during MP6-XT22-induced reactivation, but was not totally suppressed. Interleukin-12p40 and gamma interferon gene expression in TNF-α-neutralized mice was similar to that in control mice. In contrast, interleukin-10 expression was augmented in the TNF-α-neutralized mice. In summary, results of this study suggest that TNF-α plays an essential role in preventing reactivation of persistent tuberculosis, modulates the pulmonic expression of specific immunologic factors, and limits the pathological response of the host.
Active tuberculosis arises in approximately 10% of infected individuals (51) and requires long-term antibiotic therapy to cure. It is generally accepted that in the majority of infected persons, a clinically asymptomatic latent persistent infection develops (51–53), and these latently infected individuals harbor dormant, yet viable, tubercle bacilli that are capable of reactivating to cause active disease. The physiological and biochemical states of the bacteria within dormant foci are unknown, making latent tuberculosis difficult to model experimentally. Epidemiological studies suggest that reactivation of latent tuberculous infection contributes significantly to the incidence of tuberculosis (3). Considering that one-third of the world's population is infected with the tubercle bacillus (55) and is, therefore, potentially at risk for developing active disease, understanding the mechanisms by which latent infection is established and by which reactivation occurs will improve the management, control, and prevention of tuberculosis.
Using experimental murine tuberculosis models of latency, we have previously shown that in vivo inhibition of the production of reactive nitrogen intermediates (RNI) by the nitric oxide synthase (NOS2) inhibitor aminoguanidine results in reactivation, which implicates toxic nitrogen oxides as effective antituberculous agents in the persistent phase of infection (21). These studies also reveal that tumor necrosis factor alpha (TNF-α) is expressed in Mycobacterium tuberculosis-infected tissues throughout the quiescent phase of infection (21), which suggests that this cytokine may contribute to the containment of chronic persistent tuberculosis. Acting synergistically with gamma interferon (IFN-γ), TNF-α is critical for the expression of RNI-mediated antimycobacterial activity via the induction of the inducible form of macrophage NOS2 (15). Mice that are functionally deficient in TNF-α develop fulminant acute tuberculous infection (5, 20). Relevant to latent tuberculosis, administration of a recombinant adenovirus expressing the 55-kDa TNF receptor to mice that are infected with M. tuberculosis 6 months earlier increases bacterial numbers and mouse mortality (1). Using a variant of the Cornell model for latency in which antimycobacterial drugs reduce bacterial numbers to undetectable levels (48), we have provided further evidence for a role for this cytokine in controlling latent tuberculosis. In that study, neutralization of TNF-α resulted in reactivation of the infection in a subset of mice, although technical difficulties inherent in the model precluded further mechanistic analysis (48).
The present study employs the low-dose model of persistent murine tuberculosis to examine the effects of TNF-α on the immunological response of the host during the persistent and reactivation phases of tuberculous infection. The model (21) shares some features of human latent tuberculosis: the host response is solely responsible for control of the initial infection, the bacteria are contained within granulomata, and the persistently infected mice remain clinically well for a prolonged period (at least 10 months), during which time the bacillary burden is stably maintained (21). The results of this study provide evidence that neutralization of TNF-α in mice with chronic persistent M. tuberculosis infection results in (i) disease recrudescence associated with moderately increased bacterial burden and 100% mortality; (ii) selectively altered levels of specific genes in the lungs—increased interleukin-10 and decreased NOS2 expression; and (iii) severe pulmonic infiltration of inflammatory cells. In sum, these data indicate that TNF-α exerts a variety of effects on the immune response of the host in persistent chronic tuberculosis, including those that influence the control of infection and the organization of granuloma, as well as those that modulate macrophage functions and limit pathology.
MATERIALS AND METHODS
Animals.
C57BL/6 strain female mice (Charles River, Rockland, Mass.) that were 8 to 10 weeks old were used in all experiments. Mice maintained in our biosafety-level-3 animal laboratories are routinely monitored for murine pathogens by means of serological and histopathological examinations. All animal protocols employed in this study have been approved by the institutional animal care and use committees.
Chemical and reagents.
All chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless noted otherwise. Middlebrook 7H9 liquid medium and 7H10 agar were purchased from Difco Laboratories (Detroit, Mich.). The MP6-XT22 rat anti-murine TNF-α hybridoma (DNAX, Inc., Palo Alto, Calif.), obtained through the American Type Culture Collection (Rockville, Md.), was used to prepare ascites (Harlan Bioproducts for Science, Indianapolis, Ind.). The ascites were subjected to sodium ammonium sulfate precipitation to obtain the murine TNF-α-specific immunoglobulin G (IgG) MP6-XT22. Normal rat IgG (Jackson Immuno Research Laboratories, West Grove, Pa.) was used as a control. Antibody specific for NOS2 was purchased from Transduction Lab (Cincinnati, Ohio). The keratin-specific antibody M14 was a gift from BAbCO (Richmond, Calif.).
Mycobacteria and infection and treatment of mice.
To prepare bacterial stock, M. tuberculosis strain Erdman (Trudeau Institute, Saranac Lake, N.Y.) was used to infect mice, and then bacteria were harvested from their lungs, expanded in 7H9 liquid medium, and stored in aliquots at −80°C (21). Mice were infected with 5 × 103 to 1 × 104 viable CFU of M. tuberculosis intravenously via the lateral tail veins (21). Beginning 6 to 8 months postinfection, neutralization of TNF-α was initiated by intraperitoneal (i.p.) injection of 0.5 mg of MP6-XT22 twice weekly for the duration of the experiment. Control animals received similar injections of rat IgG. The efficacy of MP6-XT22 in vivo was established by its ability to exacerbate an acute murine M. tuberculosis infection, with mycobacterial burdens in MP6-XT22-treated mice similar to those observed in TNFp55R−/− mice (48). RNase protection assay (RPA) analysis (see below) of pulmonic mRNA levels in MP6-XT22-treated, uninfected C57BL/6 strain mice (3.5-week treatment using the above described protocol) revealed that this antibody has no direct effects on the expression of various cytokines (data not shown). At various intervals after initiation of in vivo neutralization of TNF-α, the tissue bacillary load was quantified by plating serial dilutions of lung, liver, or spleen homogenates onto 7H10 agar as described previously (21). In parallel, mice of each experimental group were monitored for mortality. To avoid unnecessary suffering, all moribund animals expected to succumb to the infection within 2 to 3 days were euthanized and scored as dead.
Histopathological and immunohistochemical studies.
Tissue samples for histopathological studies were prepared as described previously (21). In brief, tissues were fixed in 10% buffered formalin followed by paraffin embedment. For histopathological studies, 5- to 6-μm sections were stained with hematoxylin and eosin (H&E). To examine the tissue bacillary load, tissues were stained for acid-fast bacilli using the Ziehl-Neelsen or Kinyoun method. Immunohistochemical detection of NOS2 and keratin was performed using an antigen retrieval protocol described previously (9). Briefly, 5- to 6-μm sections of formalin-fixed, paraffin-embedded tissues were allowed to react with the appropriate antibody at a dilution of 1:500. The avidin-biotin-peroxidase system (Vector Laboratories, Burlingame, Calif.) was used to detect target antigens. The terminal deoxynucleotidyl transferase-UTP-nicked-end-labeling (TUNEL)-based Apoptag kit (Intergen, New York, N.Y.) was used to locate apoptotic cells in formalin-fixed, paraffin-embedded tissues according to the manufacturer's protocol. The antidigoxigenin-peroxidase system was used to detect digoxigenin-labeled nucleotides transferred to the 3′-OH termini of fragmented DNA present in apoptotic cells.
RPA.
Determination of the levels of mRNA for the genes of interest at various time intervals after the initiation of TNF-α neutralization was performed using a multiprobe RNase assay system (Pharmingen, San Diego, Calif.). Lungs harvested from infected mice were snap-frozen in liquid nitrogen and stored at −80°C. Total RNA, extracted from lung tissue using Trizol reagent (Life Technology, Grand Island, N.Y.), was subjected to RPA according to manufacturer's instructions. Protected [32P]UTP-labeled probes were resolved on a 6% polyacrylamide gel and analyzed by autoradiography. Cytokine analysis was performed using custom-made probe sets specific for NOS2, IL-4, IL-12p40, TNF-α, IL-1α, IL-1β, IFN-γ, and IL-10. The expression of specific genes was quantified densitometrically (Image Quant, Molecular Dynamics, Sunnyvale, Calif.) relative to the abundance of housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or L32.
Statistical analysis.
Statistical significance was evaluated using the unpaired Student t test and InStat v2.03 (San Diego, Calif.). CFU were subjected to log transformation prior to statistical analysis.
RESULTS
Neutralization of TNF-α reactivates chronic persistent tuberculosis.
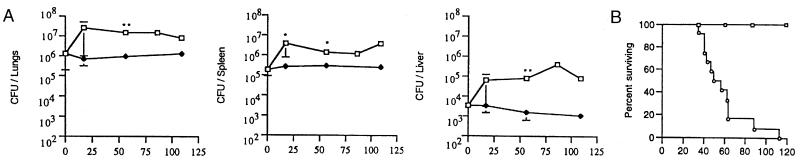
In the low-dose model of murine chronic persistent tuberculosis employed in this study, mice infected with ∼104 CFU of the virulent Erdman strain of M. tuberculosis maintain a stable tissue bacillary burden beginning 1 month postinfection, which continues for at least 9 months thereafter (21). TNF-α neutralization was initiated 6 months after infection with M. tuberculosis by administration of MP6-XT22 twice weekly at a dose of 0.5 mg intraperitoneally for the duration of the experiment. Control mice received normal rat IgG on the same schedule. TNF-α neutralization resulted in reactivation of the disease associated with an initial increase in tissue bacillary burden, which peaked at 20 days after MP6-XT22 treatment and did not increase appreciably thereafter, despite continued antibody administration (Fig. 1A). Bacterial numbers in the lungs of MP6-XT22-treated mice increased 10-fold compared to controls receiving rat IgG, reaching ∼107 CFU/organ, a level that is rarely fatal in immunocompetent C57BL/6 strain mice (21, 21, 41, 43; unpublished observations). Although bacterial numbers stabilized after 20 days of TNF-α neutralization, the mice succumbed, with a mean survival time of 59 ± 22 days after initiation of MP6-XT22 treatment (Fig. 1B). Increases in bacterial numbers were not observed in control mice over the course of the experiment, and these mice survived at least up to 6 months of rat IgG treatment (Fig. 1A and B). It was surprising that even moribund mice (those judged to be within 2 days of death) had bacterial loads in the same range as the peak CFU attained after 20 days of TNF-α neutralization. This finding suggested that the 10-fold increase in pulmonic bacterial burden might not be directly responsible for the enhanced mortality observed in the MP6-XT22-treated mice. This experiment was performed thrice, using mice infected 6 or 8.5 months prior to antibody treatment, with similar results (data not shown), although the mice infected for a longer period of time succumbed more quickly (mean survival time 30 ± 5 days) following TNF-α neutralization.
FIG. 1.
TNF-α neutralization with the monoclonal antibody MP6-XT22 resulted in disease reactivation in strain C57BL/6 mice persistently infected with M. tuberculosis strain Erdman. C57BL/6 mice were infected with M. tuberculosis Erdman 6 months prior the initiation of TNF-α neutralization (open squares); controls received rat IgG (closed diamonds). The reactivation was associated with increased (A) bacillary burden in lungs, liver, and spleen, respectively (three to five animals were studied per group per time point; bars represent standard error) and (B) mortality rate (six mice were monitored in the rat IgG-treated group [open squares]). There were 12 mice in the MP6-XT22-treated group (open circles). Asterisks, P ≤ 0.05. This experiment was performed three times with similar results.
Tissue pathological response to TNF-α neutralization during the quiescent phase of tuberculous infection.
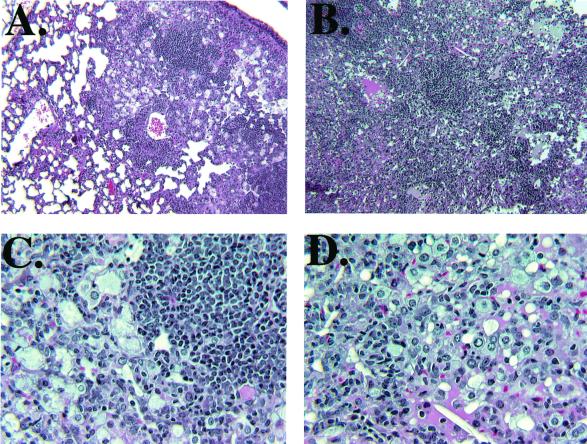
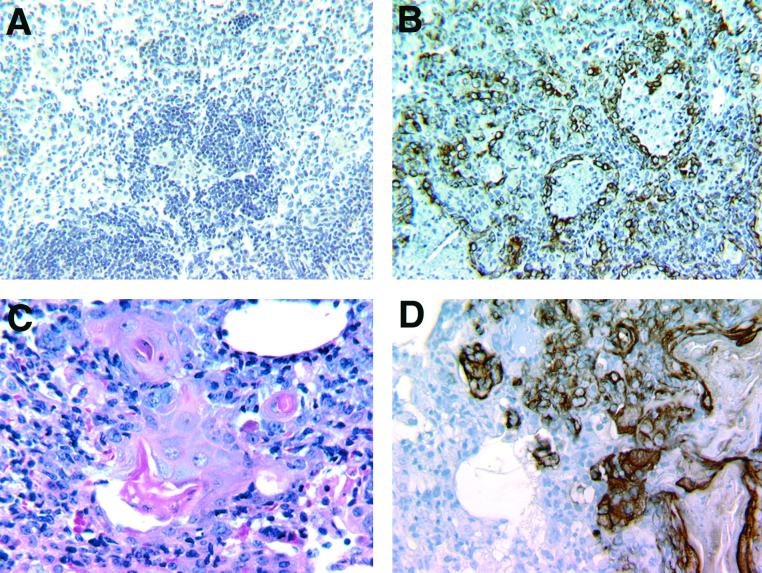
Examination of H&E-stained tissue sections of the lungs of MP6-XT22- and IgG-treated mice infected with M. tuberculosis revealed a remarkable difference in histopathology between the two groups (Fig. 2). In the IgG-treated controls, the pulmonic granulomatous response was characterized by well-demarcated conspicuous lymphoid aggregates (with a minor monocytic component) among areas of consolidation resulting from a diffuse interstitial infiltration of lymphocytes and histiocytic cells. The alveolar spaces were filled with foamy macrophages (Fig. 2A and C). The lymphoid aggregates were much less apparent in MP6-XT22-treated mice after 3 weeks of TNF-α neutralization (Fig. 2B and D) and, compared to the IgG-treated controls, there was a markedly enhanced and diffuse infiltration of mononuclear cells (compare Fig. 2A and B), suggesting defective recruitment and/or migration of inflammatory cells in the TNF-α-neutralized mice. In certain areas, a ground glass-appearing eosinophilic material suggestive of fluid accumulation was present in the alveolar space (Fig. 2B). Despite the remarkable degree of inflammation, pulmonary necrosis was not a prominent feature in these mice. The lungs of mice treated with MP6-XT22 displayed unusual focal areas of inflammation suggestive of the formation of tightly bridged squamous cells and/or multinucleated giant cells (Fig. 3C). These areas also contained eosinophilic amorphous materials (Fig. 3C). Examination of the lungs of mice treated with MP6-XT22 at 6 months postinfection revealed substantial keratin immunoreactivity (Fig. 3B), which was absent in control mice treated with rat IgG (Fig. 3A). The keratin-containing areas of inflammation were most conspicuous in mice whose MP6-XT22 treatment began 8.5 months postinfection (Fig. 3D). In this experiment, the lungs of IgG-treated control mice displayed a small degree of keratin immunoreactivity (data not shown). The presence of keratin is indicative of squamous metaplasia, a pathological response to chronic inflammatory processes (27), and it suggested enhanced tissue inflammation following TNF-α neutralization in mice persistently infected with M. tuberculosis. In contrast to the severe histopathological response observed in the lungs of TNF-α-neutralized mice, there was no apparent difference in the hepatic and splenic inflammatory reaction between the MP6-XT22- and IgG-treated mice (data not shown).
FIG. 2.
Histopathologic studies (H&E stain) of lung tissues from MP6-XT22-treated C57BL/6 mice persistently infected with M. tuberculosis Erdman. Compared to IgG-treated controls (panels A and C), TNF-α-neutralized mice (panels B and D) exhibited marked histopathological deterioration associated with disorganization of granulomata, diffuse infiltration of inflammatory cells, and changes suggestive of fluid accumulation in the alveolar space. Original magnification: ×100 (panels A and B); ×400 (panels C and D).
FIG. 3.
Keratin immunoreactivity in the lungs of mice with MP6-XT22-induced reactivation tuberculosis. In mice with reactivation tuberculosis following TNF-α neutralization, areas of tightly bridged squamous cells and/or multinucleated giant cells were observed in the lungs (panel C). These areas contained eosinophilic material that reacted immunohistochemically to a keratin-specific antibody, a finding indicative of squamous metaplasia (panel D). The sections shown are representative of tissues from mice with reactivating tuberculosis after receiving 40 days of MP6-XT22 treatment beginning at 8.5 months postinfection. Keratin immunoreactivity was also observed in areas of lungs with similar histopathologic changes in mice with disease recrudescence following TNF-α neutralization (duration of treatment, 56 days) initiated at 6 months postinfection (B). Immunohistochemical studies revealed the absence of keratin in the lungs of nonreactivating control mice treated with normal rat IgG (duration of treatment, 56 days) beginning 6 months postinfection (A). Tissue sections presented in C were H&E stained. Sections shown in A, B, and D were counterstained with hematoxylin.
TNF-α neutralization during chronic persistent tuberculosis attenuates the expression of NOS2 in the lungs.
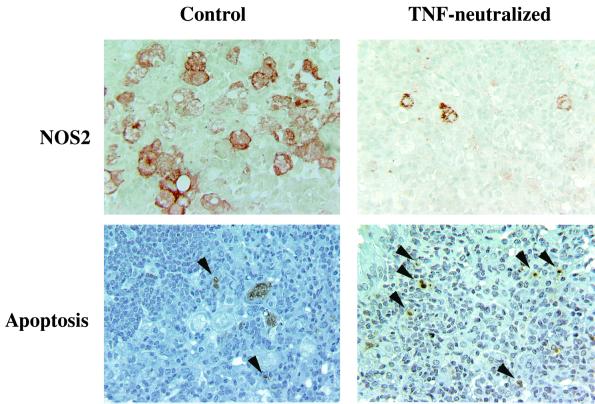
MP6-XT22-induced reactivation of tuberculous infection might be secondary to deficient production of RNIs. TNF-α is a key factor that, in conjunction with IFN-γ, activates the macrophage RNI-generating antimycobacterial pathway (15). Immunohistochemical studies revealed that the expression of NOS2 protein was attenuated in the TNF-α-neutralized mice, compared to NOS2 expression in animals receiving nonspecific rat IgG (Fig. 4). NOS2 immunoreactivity in the IgG-treated, M. tuberculosis-infected mice was detected in large cellular aggregates, primarily in epithelioid and foamy macrophages, while that in TNF-α-neutralized animals was distributed diffusely, with much of the reactivity localized to a single or a small number of cells. NOS2-positive cells in the MP6-XT22-treated mice were smaller and more compact, lacking a foamy appearance, which suggests a less-activated phenotype. The scattered distribution of NOS2-positive cells reinforced the observation that neutralization of TNF-α in the chronic persistent phase of tuberculous infection resulted in disorganization of granulomata and diffuse, nontargeted infiltration of inflammatory cells. RPA confirmed a decrease in the level of NOS2 mRNA in the MP6-XT22-treated mice (Fig. 5), which was apparent as early as 14 days after initiation of the neutralization regimen. These data suggest that attenuated NOS2 expression contributed, at least in part, to reactivation of infection in TNF-α-neutralized mice.
FIG. 4.
Immunohistochemical and TUNEL analysis of lungs after TNF-α neutralization in mice with persistent tuberculosis. Mice were treated with rat IgG (left panels) or MP6-XT22 (right panels) beginning at 6 months postinfection. Immunohistochemical staining using anti-NOS2 antibodies demonstrated decreased but not absent expression of NOS2 in the lungs of MP6-XT22-treated mice compared to control animals receiving rat IgG. TUNEL staining of lung tissue showed that TNF-α neutralization increased apoptotic activity (arrows) compared with rat IgG treatment. Sections are representative of tissues obtained from mice 59 days after initiation of antibody treatment. Original magnification, ×400.
FIG. 5.
Analysis of NOS2 gene expression by RPA using total lung RNA of TNF-α-neutralized mice with persistent tuberculosis. NOS2 gene expression is reported as a ratio to GAPDH expression. Compared to control mice treated with rat IgG (closed diamonds), the expression of NOS2 mRNA in TNF-α-neutralized animals (open squares) was attenuated. Each point is the mean of the results obtained from three to five mice, and bars represent the standard error. Asterisks, P ≤ 0.05. Similar results were obtained in two additional TNF-α-neutralization experiments.
Effects of TNF-α neutralization on cytokine expression in the lungs of mice persistently infected with M. tuberculosis: increased levels of IL-10.
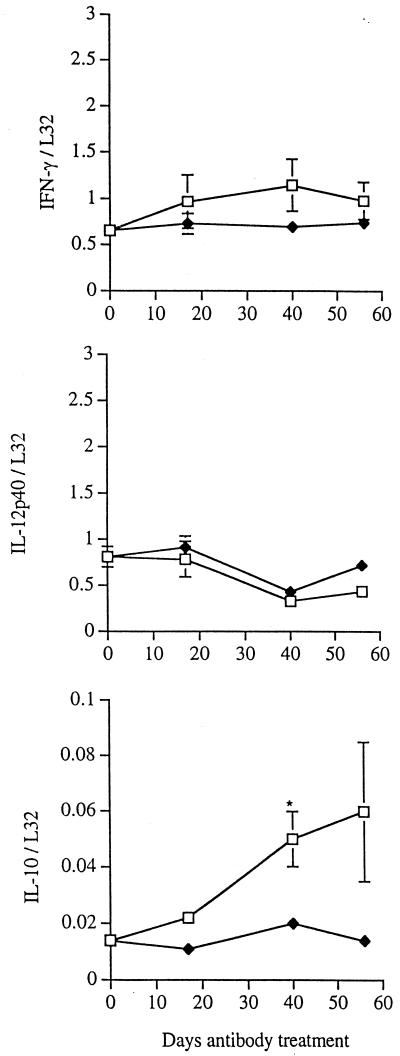
We examined the expression of IFN-γ and IL-12 in the lungs of the control and anti-TNF-α monoclonal antibody-treated mice, because of their roles in activating the innate immune response and in engendering protective immunity by promoting the development of TH1 T cells (6, 32, 38, 56). Both cytokines are essential in control of M. tuberculosis infection (11, 12, 19). In addition, down-regulation of IL-12 has been implicated in mediating the antiinflammatory effect of TNF-α in a Corynebacterium parvum model (26). IL-12p40 and IFN-γ mRNA levels in MP6-XT22-treated mice were not significantly different than those in mice receiving nonspecific rat IgG (Fig. 6). The level of IL-10 transcription was investigated because of the ability of this cytokine to down-regulate macrophage functions (7, 14) including those essential for antimycobacterial activity (18, 39). The expression of IL-10 mRNA was enhanced in MP6-XT22-treated mice throughout the reactivation phase (Fig. 6).
FIG. 6.
RPA analysis of cytokine gene expression in lungs of mice with chronic persistent M. tuberculosis infection. Six months after infection with M. tuberculosis, mice were injected with either rat IgG (closed diamonds) or MP6-XT22 monoclonal anti-TNF-α antibody (open squares). Total RNA from lungs was prepared at initiation of antibody treatment (day 0) and at the indicated times therafter. Three to five mice were analyzed at each time point. The autoradiographs were digitized on a flatbed scanner, and gene expression is reported as a ratio to L32 housekeeping genes; combined data for all mice at each time point are shown for the genes for IFN-γ, IL-12, and IL-10. Bars represent the standard error; asterisks, P ≤ 0.05. Similar results were obtained in two additional TNF-α-neutralization experiments.
Effects of TNF-α neutralization on apoptotic activity in the lungs of mice with persistent tuberculosis.
Emerging evidence indicates that apoptosis plays an important role in host defense (36, 42) and immunopathology (25, 31, 34) during tuberculous infection. Directly relevant to this study, it has been reported that both TNF-α and IL-10 can regulate apoptosis in mycobacterial infection. These cytokines have been reported to exert both pro- and anti-apoptotic activities in both in vitro and in vivo mycobacterial experimental models (4, 16, 28, 29, 37, 45). Apoptotic activity in the lungs of TNF-α-neutralized mice with chronic persistent tuberculosis, assessed using the TUNEL assay, was enhanced and increased progressively compared to that observed in rat IgG-treated controls (Fig. 4 and Table 1). By 56 days postneutralization, the number of apoptotic cells observed in the lungs of TNF-α-neutralized mice was threefold higher than that in controls (Table 1). Examination by light microscopy revealed that cells displaying the morphology of lymphocytes and macrophages both underwent apoptosis.
TABLE 1.
Apoptotic activity in the absence of TNF-α in persistent tuberculosisa
| Dayb | No. of apoptotic cellsc (mean ± SD) after treatment with:
|
|
|---|---|---|
| MP6-XT22 | Control IgG | |
| 0 | 20 ± 13 | 20 ± 13 |
| 28 | 18 ± 4 | 15 ± 3 |
| 56 | 48 ± 6d | 18 ± 8 |
Apoptotic activity was measured in lungs of mice persistently infected with M. tuberculosis for 6 months, followed by in vivo neutralization of TNF-α with the monoclonal anti-TNF-α MP6-XT22. Similarly infected control mice received rat IgG. Paraffin-embedded lung sections were stained for apoptosis using the TUNEL assay. The number of apoptotic cells in the lungs of mice prior to antibody treatment was 20 ± 13.
Number of days after initiation of antibody treatment.
For each group of three to four mice, 20 fields were examined at ×100 magnification.
P = 0.033.
DISCUSSION
Using persistent M. tuberculosis infection in mice as a model for latent tuberculosis (21), this study has provided evidence that TNF-α is essential for the prevention of disease recrudescence. Specifically, neutralization of TNF-α by administering the monoclonal antibody MP6-XT22 to mice with persistent tuberculosis resulted in fatal reactivation of the disease, characterized by moderately increased tissue bacterial burden and severe histopathological deterioration, changes indicative of squamous metaplasia and fluid accumulation in the alveolar space, as well as altered expression of IL-10 and NOS2.
Histopathological changes were the most striking feature associated with MP6-XT22-induced reactivation. Results of an immunohistochemical study using a polyclonal mouse anti-rat IgG to detect MP6-XT22 in the lungs of TNF-α-neutralized mice strongly suggests that the histopathological changes observed were not secondary to the deposition of immune complex: rat IgG-specific immunoreactivity was not detected in these animals (data not shown). The inability of the MP6-XT22-treated mice to maintain the granulomatous lymphoid aggregates despite an apparent increase in cellular infiltrate suggests that TNF-α participates in the recruitment and specific trafficking of relevant immune cells to infected foci. This observation reinforces the notion that the tuberculous granuloma is a dynamic structure and that its maintenance in an organized form may require substantial cellular turnover (2, 13, 44). A common link to the unfavorable disease outcome associated with TNF-α deficiency in acute tuberculosis (5, 20) as well as chronic persistent and reactivation tuberculosis appears to be the lack of an organized granulomatous response. These results suggest that TNF-α is critical in (i) influencing the trafficking of immune cells to the appropriate infectious foci, thereby promoting the formation of well-organized granulomata capable of controlling disease progression; and (ii) maintaining the structural integrity of the tuberculous granuloma in latent tuberculosis. The ability of TNF-α to affect the expression of adhesion molecules (10, 54) and chemokines and their receptors (24, 30, 40, 47) is a possible explanation for the nonfocused infiltration of leukocytes that was observed.
Although generally considered to be an inflammatory cytokine, TNF-α has been shown to be capable of exerting an antiinflammatory effect in vivo. TNF-α−/− mice infected with C. parvum developed a delayed but intense tissue inflammatory response associated with high mortality (26). Exogenous TNF-α ameliorated this pathological response and reversed mortality. In this study, attenuation of TNF-α-dependent antiinflammatory mechanisms may have contributed to the severe histopathological deterioration observed in the lungs of TNF-α-neutralized mice with chronic persistent tuberculosis. In this regard, the accumulation of keratin, a sign of squamous metaplasia associated with chronic inflammatory processes in the lungs (27), as well as the apparent fluid accumulation in the alveolar space suggestive of tissue damage, may be by-products of excessive inflammation following TNF-α neutralization. The regulatory mechanism for the expression of keratin in these M. tuberculosis-infected mice is unclear. However, our results suggest that the level of keratin immunoreactivity correlates with the chronicity of the inflammatory process and/or TNF-α neutralization. In studies of reactivation tuberculosis using different immunocompromising strategies in the same murine model (e.g., NOS2 inhibition [21] and CD4 depletion [49]) in which the level of TNF-α expression in the mice with reactivation tuberculosis was comparable to those of control animals, pathology distinct from that described here was observed. In particular, the disorganization of granulomata, diffuse infiltration of cells, and the prominence of keratin in the lungs were not hallmarks of reactivation in these other models. It was surprising, in the Cornell model (48), that severe pulmonic inflammatory infiltrate could be detected in a subset of mice with MP6-XT22-induced reactivation despite a bacillary load in the lungs that is over 3 log lower than that in mice with disease recrudescence in the low-dose model. The bacillary load in the Cornell model was ∼5 × 103 CFU/organ and in the low-dose model it was ∼107 CFU/organ (data not shown). Together, these observations strongly suggest that TNF-α deficiency in chronic persistent tuberculosis contributes significantly to the severe histopathologic response to M. tuberculosis regardless of the level of tissue bacillary burden. In the C. parvum study (26), the antiinflammatory effects of TNF-α were attributed to IL-12 inhibition. In this study, the overall expression of IL-12p40 was comparable among MP6-XT22- and rat IgG-treated mice, although actual levels of the bioactive heterodimer IL-12p70 were not assayed. In a Mycobacterium avium murine model, TNFRp55−/− mice experienced severe pathology characterized by diffuse granulomatous lesions and necrosis that was independent of bacterial burden in the organs (17). Since it is generally accepted that TNF-α contributes significantly to the immunopathology associated with tuberculous infection (46), the mechanisms underlying the role of TNF-α in modulating the inflammatory state of the lungs in chronic persistent tuberculosis deserve further study.
Since NOS2 plays an important role in mediating antimycobacterial functions in both acute and persistent murine tuberculosis (reviewed in reference 8), attenuation of the expression of NOS2 in the lungs of TNF-α-neutralized, persistently infected mice could have contributed to increased bacterial load and disease recrudescence. However, unlike the progressive increase in bacillary burden following inhibition of NOS2 in persistently infected mice (21), the rise in bacterial numbers in the TNF-α-neutralized animals was transient, reaching its peak early after initiation of the MP6-XT22 treatment. The ability of the MP6-XT22-treated mice to maintain their tissue bacillary burden for a prolonged period of time after the initial rise could be due to the presence of NOS2, albeit attenuated, and/or the existence of as yet undefined RNI-independent antimycobacterial mechanisms that require TNF-α. The precise mechanism underlying this observation remains to be determined.
In general, IL-10 expression in the persistently infected control mice was minimally detectable by RPA. An increase in expression of IL-10 in TNF-α-neutralized mice is noteworthy. In other studies on tuberculous reactivation in which bacterial growth increased to a higher level in response to compromised immunity than that described in this study, enhanced expression of IL-10 was not observed (49). Thus, the relatively high levels of IL-10 in the MP6-XT22-treated mice cannot be attributed simply to an increased bacterial burden and instead may be the direct or indirect result of TNF-α neutralization. IL-10 is generally considered to be an antiinflammatory cytokine (7, 14) and thus may be expressed in response to the specific severe pathology that results from TNF-α neutralization. The effects of IL-10 on the pathophysiology of tuberculous infection are complex. Suppressive effects of this cytokine on antimicrobial functions of macrophages have been described (7, 14, 18, 39). In vitro studies examining the interactions between human peripheral mononuclear cells and the tubercle bacilli have shown that IL-10 has the ability to down-regulate M. tuberculosis-induced expression of the T-cell costimulatory molecule CTLA-4 (23), as well as the production of IL-12 (22, 23). Transgenic mice overexpressing IL-10 were less capable of clearing infection with an avirulent mycobacterial strain, BCG (39), suggesting that this cytokine may impair elimination of mycobacteria from macrophages and thus contribute to the establishment of a persistent infection. However, progression of acute tuberculosis in IL-10−/− mice was similar to that observed in wild-type mice (41). In the context of the migration of inflammatory cells, there is in vitro evidence that in infectious and inflammatory processes, IL-10 can regulate the expression of chemokines and chemokine receptors (35, 50). Therefore, the increased expression of IL-10 in the lungs of TNF-α-neutralized mice could potentially impact disease progression by attenuating macrophage antimycobacterial activity and modulating the histopathological response of the host by regulating the expression of inflammatory cytokines, chemokines, and chemokine receptors.
Evidence that both TNF-α and IL-10 can be pro- or antiapoptotic in various mycobacteria infection models suggest that the in vivo regulation of apoptosis in a tuberculous host is complex (4, 16, 28, 29, 37, 45). The mechanisms for and the significance of the enhanced apoptotic activity observed in this study in the lungs of TNF-α-neutralized, persistently infected mice remain to be determined. It is possible that this phenomenon is the result of the 10-fold increase in bacillary burden associated with MP6-XT22-induced reactivation. However, increased levels of apoptosis were not detected in a CD4 T-cell-depletion reactivation model (49) in which pulmonic bacterial numbers in mice with disease recrudescence were 100-fold greater than those in controls (108/lung) (data not shown). Alternatively, enhanced apoptotic activity in the lungs could simply be due to the increased infiltration of inflammatory cells. Our data do not allow evaluation of this possibility since precise enumeration of the number of cells in the lungs could not be performed using fixed tissues.
TNF-α has been the focus of intense investigation in the context of both host defense mechanisms against M. tuberculosis and the immunopathology (46) associated with tuberculous infection. Results of this study have provided evidence confirming the previously reported role of TNF-α in the control of persistent tuberculosis (1, 48). It is interesting that TNF-α neutralization induced a severe histopathological response to M. tuberculosis—conceivably modulated by altered levels of TNF-α and IL-10 and possibly other as yet unidentified factors—that does not correlate well with the level of tissue bacillary load. Direct examination of the impact of IL-10 on persistent tuberculosis and the relevance of enhanced apoptosis in MP6-XT22-treated mice, as well as characterization of the mechanisms by which TNF-α affects granuloma formation, inflammatory cell trafficking, and inflammation, will likely enhance our understanding of tuberculous latency and reactivation. Finally, a case of disseminated tuberculosis has been described in a patient receiving TNF-α-neutralizing antibodies for the treatment of rheumatoid arthritis (33). If causality is demonstrated, this case suggests that TNF-α is important in host defense in human tuberculosis and may validate the use of various murine models for the study of antimycobacterial immune mechanisms in humans.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant ROI 36990 (J.C. and J.L.F.).
We are grateful to Heather Joseph for preparation of antibodies and technical assistance, to Simon Watkins for use of the University of Pittsburgh Center for Biologic Imaging, and to Edwin Klein for assistance with histologic analysis. We thank DNAX for providing access to the hybridoma-producing MP6-XT22 monoclonal antibody. We also thank the members of the Flynn and Chan laboratories for helpful discussions.
V. P. Mohan and C. A. Scanga contributed equally to this study.
REFERENCES
- 1.Adams L B, Mason C M, Kolls J K, Scollard D, Krahenbuhl J L, Nelson S. Exacerbation of acute and chronic murine tuberculosis by adminstration of a tumor necrosis factor receptor-expressing adenovirus. J Infect Dis. 1995;171:400–405. doi: 10.1093/infdis/171.2.400. [DOI] [PubMed] [Google Scholar]
- 2.Ando M, Dannenberg A M, Shima K. Macrophage accumulation, division, maturation and digestive and microbiocidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972;109:8–19. [PubMed] [Google Scholar]
- 3.Anonymous. The use of preventive therapy for tubercuous infection in the United States: recommendations of the Advisory Committee for Elimination of Tuberculosis. Morb Mortal Wkly Rep. 1990;39:9. [PubMed] [Google Scholar]
- 4.Balcewicz-Sablinska M K, Keane J, Kornfeld H, Remold H G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J Immunol. 1998;161:2636–2641. [PubMed] [Google Scholar]
- 5.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 6.Biron C A, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Flynn J. Nitric oxide in Mycobacterium tuberculosis infection. In: Fang F, editor. Nitric oxide and infection. New York, N.Y: Plenum Publishing Corp.; 1999. pp. 281–310. [Google Scholar]
- 9.Chan J, Tanaka K E, Tsang M S, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom B R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci USA. 1996;93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins T, Read M A, Neish A S, Whitley M Z, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 11.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper A M, Dalton D K, Stewart T A, Griffen J P, Russell D G, Orme I M. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2248. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cree I A, Nurbhai S, Milne G, Beck J S. Cell death in granulomata: the role of apoptosis. J Clin Pathol. 1987;40:1314–1319. doi: 10.1136/jcp.40.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries J E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–541. doi: 10.3109/07853899509002465. [DOI] [PubMed] [Google Scholar]
- 15.Ding A H, Nathan C, Stuehr D. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 16.Durrbaum-Landmann I, Gercken J, Flad H D, Ernst M. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect Immun. 1996;64:5384–5389. doi: 10.1128/iai.64.12.5384-5389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlers S, Benin J, Kutsch S, Endres R, Rietschel E T, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flesch I E, Hess J H, Oswald I P, Kaufmann S H E. Growth inhibition of Mycobacterium bovis by IFN-γ stimulated macrophages: regulation by endogenous tumor necrosis factor-α and IL-10. Int Immunol. 1994;6:693. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 19.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against M. tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 21.Flynn J L, Scanga C A, Tanaka K E, Chan J. Effects of aminoguanidine on latent murine tuberculosis. J Immunol. 1998;160:1796–1803. [PubMed] [Google Scholar]
- 22.Fulton S A, Cross J V, Toossi Z T, Boom W H. Regulation of interleukin-12 by interleukin-10, transforming growth factor-b, tumor necrosis factor-a, and interferon-g in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–1114. doi: 10.1086/515698. [DOI] [PubMed] [Google Scholar]
- 23.Gong J-H, Zhang M, Modlin R L, Linsley P S, Iyer D, Lin Y, Barnes P F. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S K, Lysko P G, Pillarisetti K, Ohlstein E, Stadel J M. Chemokine receptors in human endothelial cells: functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch C S, Toossi Z, Vanham G, Johnson J L, Peters P, Okwera A, Mugerwa R, Mugyenyi P, Ellner J J. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–953. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 26.Hodge-Dufour J, Marino M W, Horton M R, Jungbluth A, Durdick M D, Strieter R M, Noble P W, Hunter C A, Pure E. Inhibition of interferon γ induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston W W. Cytologic correlations. In: Daily D H, Hammer S P, editors. Pulmonary pathology. New York, N.Y: Springer-Verlag; 1988. pp. 1029–1094. [Google Scholar]
- 28.Keane J, Balcewicz-Sablinska M K, Remold H G, Chupp G L, Meek B B, Fenton M J, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer L, Estaquier J, Brandt E, Ameisen J C, Locht C. Mycobacterium bovis bacillus Calmette Guérin infection prevents apoptosis of resting human monocytes. J Immunol. 1997;27:2450–2456. doi: 10.1002/eji.1830270945. [DOI] [PubMed] [Google Scholar]
- 30.Lane B R, Markovitz D M, Woodford N L, Rochford R, Strieter R M, Coffey M J. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- 31.Li B, Bassiri H, Rossman M D, Kramer P, Eyuboglu A F, Torres M, Sada E, Imir T, Carding S R. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–1567. [PubMed] [Google Scholar]
- 32.Locksley R M. Interleukin-12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maini R, St. Clair E W, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldman M, Lipsky P. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 34.Manfredi A A, Heltai S, Rovere P, Sciorati C, Paolucci C, Galati G, Rugarli C, Vaiani R, Clementi E, Ferrarini M. Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of γδ T cells to cause apoptosis. Eur J Immunol. 1998;28:1798–1806. doi: 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Marfaing-Koka A, Maravic M, Humbert M, Galanaud P, Emilie D. Contrasting effects of IL-4, IL-10 and corticosteroids on RANTES production by human monocytes. Int Immunol. 1996;8:1587–1594. doi: 10.1093/intimm/8.10.1587. [DOI] [PubMed] [Google Scholar]
- 36.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis but not necrosis of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira A L, Tsenova-Berkova L, Wang J, Laochumroonvorapong P, Freeman S, Freedman V H, Kaplan G. Effect of cytokine modulation by thalidomide on the granomatous response in murine tuberculosis. Tuber Lung Dis. 1997;78:47–55. doi: 10.1016/s0962-8479(97)90015-0. [DOI] [PubMed] [Google Scholar]
- 38.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 39.Murray P J, Yang L, Onufryk C, Tepper R I, Young R A. T cell-derived IL-10 antagonizes macrophage function in mycobacteria infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 40.Ngo V N, Korner H, Gunn M D, Schmidt K N, Riminton D S, Cooper M D, Browning J L, Sedgwick J D, Cyster J G. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.North R J. Mice incapable of making IL-4 and IL-10 display normal resistance in infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–58. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oddo M, Renno T, Attinger A, Bakker T, MacDonald H R, Meylan P R A. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- 43.Orme I M. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 44.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 45.Rojas M, Olivier M, Gros P, Barrera L F, Garcia L F. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–6131. [PubMed] [Google Scholar]
- 46.Rook G A, Stanford J L. The Koch phenomenon and the immunopathology of tuberculosis. Curr Top Microbiol Immunol. 1996;215:239–262. doi: 10.1007/978-3-642-80166-2_11. [DOI] [PubMed] [Google Scholar]
- 47.Saeki H, Moore A M, Brown M J, Hwang S T. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2372–2375. [PubMed] [Google Scholar]
- 48.Scanga C A, Mohan V P, Joseph H, Yu K, Chan J, Flynn J. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanga C A, Mohan V P, Yu K, Joseph H, Tanaka K E, Chan J, Flynn J L. Depletion of CD4+ T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells T, Biswas P, Vicenzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stead W W. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Resp Dis. 1967;95:729–745. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 52.Stead W W. The pathogenesis of pulmonary tuberculosis among older persons. Am Rev Respir Dis. 1965;91:811–818. doi: 10.1164/arrd.1965.91.6.811. [DOI] [PubMed] [Google Scholar]
- 53.Stead W W, Kerby G R, Schleuter D P, Jordahl C W. The clinical spectrum of primary tuberculosis in adults: confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med. 1968;68:731–745. doi: 10.7326/0003-4819-68-4-731. [DOI] [PubMed] [Google Scholar]
- 54.Stratowa C, Audette M. Transcriptional regulation of the human intercellular adhesion molecule-1 gene: a short overview. Immunobiology. 1995;193:293–304. doi: 10.1016/S0171-2985(11)80558-9. [DOI] [PubMed] [Google Scholar]
- 55.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 56.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]