Abstract
Purpose
The ENFORCE cohort is a national Danish prospective cohort of adults who received a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine as part of the Danish National SARS-CoV-2 vaccination programme. It was designed to investigate the long-term effectiveness, safety and durability of SARS-CoV-2 vaccines used in Denmark.
Participants
A total of 6943 adults scheduled to receive a SARS-CoV-2 vaccine in the Danish COVID-19 vaccination programme were enrolled in the study prior to their first vaccination. Participants will be followed for a total of 2 years with five predetermined follow-up visits and additional visits in relation to any booster vaccination. Serology measurements are performed after each study visit. T-cell immunity is evaluated at each study visit for a subgroup of 699 participants. Safety information is collected from participants at visits following each vaccination. Data on hospital admissions, diagnoses, deaths and SARS-CoV-2 PCR results are collected from national registries throughout the study period. The median age of participants was 64 years (IQR 53–75), 56.6% were women and 23% were individuals with an increased risk of a serious course of COVID-19. A total of 340 (4.9%) participants tested positive for SARS-CoV-2 spike IgG at baseline.
Findings to date
Results have been published on risk factors for humoral hyporesponsiveness and non-durable response to SARS-CoV-2 vaccination, the risk of breakthrough infections at different levels of SARS-CoV-2 spike IgG by viral variant and on the antibody neutralising capacity against different SARS-CoV-2 variants following primary and booster vaccinations.
Future plans
The ENFORCE cohort will continuously generate studies investigating immunological response, effectiveness, safety and durability of the SARS-CoV-2 vaccines.
Trial registration number
Keywords: COVID-19, Infection control, IMMUNOLOGY
Strengths and limitations of this study.
The ENFORCE study combines repeated detailed SARS-CoV-2 specific immunological measurements prior to, and throughout the course of SARS-CoV-2 vaccination, with register-based follow-up of safety data and microbiological test results.
The ENFORCE cohort includes a large proportion of elderly participants and participants with concomitant diseases.
The three vaccine groups display a high degree of variation in demographic factors and distribution across risk groups, due to the prioritisation of specific vaccines to risk groups during the primary roll out of the SARS-CoV-2 vaccination programme.
Introduction
On the emergence of SARS-CoV-2, the pathogen causing COVID-19, the development of effective vaccines quickly became of highest priority for decision-makers, researchers and pharmaceutical companies throughout the world. Subsequently, within 1 year of the emergence of the virus, the first reports from clinical phase 1–2 trials of SARS-CoV-2 vaccines in humans were published.1–5
The first SARS-CoV-2 vaccine approved for emergency use in Denmark was the Pfizer-BioNTech mRNA vaccine BNT162n2 (Pfizer, New York, USA; BioNTech SE, Mainz, Germany).6 It was used from the initiation of the Danish COVID-19 vaccination programme on 27 December 2020. In January 2021 both the Moderna mRNA-1273 vaccine (Moderna, Massachusetts, USA)7 and the AstraZeneca adenovirus-vectored ChAd0×1 nCoV-19 vaccine (AstraZeneca, Cambridge, UK)8 were approved for emergency use. The adenoviral vectored vaccine Ad26.COV2.S (Johnson & Johnson, New Jersey, USA)9 was used in a supplementary programme alongside the Danish National COVID-19 vaccination programme and only a small group of individuals received this vaccine in Denmark.
As of September 2022, more than 600 million cases and 6 million deaths due to COVID-19 have been reported to the WHO. Globally, over 12 billion vaccine doses against SARS-CoV-2 have been administered, however especially on the African continent, large parts of the population are still lacking their first SARS-CoV-2 vaccination.10 Conversely, in Denmark and similar countries, the fourth vaccination dose is being planned for selected parts of the population.11 Additionally, new Omicron specific and bivalent vaccines are being developed and introduced in vaccination programmes replacing the initial vaccines.12 13
The efficacy and safety of the SARS-CoV-2 vaccines were described in defined study populations and smaller cohorts as the vaccines were introduced,6–8 14–20 however, there is an ongoing need for knowledge on the long-term effectiveness and safety of the vaccines, the durability and magnitude of vaccine-induced immune response, protection against new emerging variants and the response to booster doses particularly in risk groups.
Cohort description
ENFORCE was designed as an open-labelled, non-randomised, parallel group, phase IV study enrolling adults residing in Denmark before their first SARS-CoV-2 vaccination offered through the Danish COVID-19 vaccination programme. The inclusion period ran from 13 February 2021 to 5 August 2021.
The study was planned by the ENFORCE Consortium and is being carried out as a collaborative effort with seven study sites in the cities Aalborg, Silkeborg, Aarhus, Odense, Roskilde, Hvidovre and Herlev covering all five Danish regions. A full list of members of the ENFORCE study group is provided as online supplemental material. The study was approved by the Danish Medicines Agency.
bmjopen-2022-069065supp001.pdf (60.7KB, pdf)
Study population
Participants had to be eligible for SARS-CoV-2 vaccination through the Danish COVID-19 vaccination programme, willing and able to provide informed consent and willing and able to comply with trial procedures. Potential participants would be excluded if they were below 18 years of age, belonged to a subgroup for which the SARS-CoV-2 vaccines were contraindicated or had received a previous SARS-CoV-2 vaccination.
Potential study participants were invited to join the ENFORCE study through a number of channels selected to ensure a high proportion of persons at increased risk of a serious course of SARS-CoV-2 infection, and healthcare workers (HCW) at risk of SARS-CoV-2 exposure. A letter of invitation was sent to the selected risk groups such as patients with cancer in active therapy, organ transplant recipients, patients with immunodeficiencies, patients in haemodialysis treatment and patients with certain rheumatological and pulmonary diseases. HCW were invited to participate via hospital information channels. The general population was invited to participate through a letter of invitation sent via the vaccination centres to adults with a scheduled appointment for vaccination in any of the study site cities.
Enrolment visits were performed by ENFORCE study personnel, either at vaccination centres located near study sites, or at hospital clinics. Participants could be enrolled from 14 days up to 30 min before their first dose of a SARS-CoV-2 vaccine. All participants gave written informed consent prior to any study procedures.
Data collection
At enrolment, baseline information was collected on age, sex, focused medical history, concomitant medication, vaccination priority group (defined by the Danish COVID-19 vaccination programme21), scheduled vaccination date and vaccine manufacturer (Pfizer-BioNTech, Moderna, AstraZeneca or Johnson & Johnson). Participants also provided blood samples for baseline SARS-CoV-2 serology. Persons at increased risk of a serious course of SARS-CoV-2 infection were classified as such, on the basis that they were referred to vaccination by a medical specialist tending to their disease. The group included patients with cancer in active treatment, patients with immunodeficiencies (acquired or inherent), organ transplant recipients, haemodialysis patients and some patients with haematological, pulmonary or rheumatological diseases. Individuals that were vaccinated because of an occupation as HCW were registered as such. Vaccine type was confirmed by cross referencing each individual’s unique civil registration (CPR) number with data from the Danish Vaccination Register (Statens Serum Institut, Copenhagen, Denmark).
Safety data is collected using a questionnaire (either online or physical) on solicited local and systemic reactions filled out by the participants at 1 and 2 weeks following each vaccination. Additionally, information regarding adverse events is collected until the visits following each vaccination. Data on specific concomitant diseases, newly diagnosed medical conditions, hospitalisations and death are collected from the Danish National Patient Register (Danish Health Data Authority, Copenhagen, Denmark).
Data on any SARS-CoV-2 PCR-tests or SARS-CoV-2 antibody measurements were extracted from the surveillance system Key Infectious Diseases System (Statens Serum Institut, Copenhagen, Denmark), whereas viral variant information is obtained through the Danish National Microbiology Database (Statens Serum Institut, Copenhagen, Denmark).
Follow-up
Study participants are followed with five predetermined clinical visits in addition to the baseline visit. The second study visit is scheduled at 0–5 days prior to the participants second SARS-CoV-2 vaccination, while the following visits are at 3, 6, 12 and 24 months after the first vaccination. Furthermore, study visits are scheduled 0–14 days before and 1 month after any SARS-CoV-2 booster vaccination. An outline of the study visit schedule is shown in figure 1.
Figure 1.
Schematic overview of the study visit schedule. Syringe icons signify SARS-CoV-2 vaccinations.
Serology measurements
At each study visit serum and plasma samples are collected from all participants for measuring specific SARS-CoV-2 serology. SARS-CoV-2 spike IgG is measured on serum samples using a Wantai ELISA based assay (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China) performed at Statens Serum Institut, Copenhagen, Denmark. Additionally, a Multiantigen Serology Assay (Meso Scale Diagnostics, Maryland, USA) quantifying spike receptor binding domain, full spike and nucleocapsid directed IgG and ACE-2 competition is performed on plasma samples at the Research Laboratory at the Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark. Additional plasma and serum samples for future research analyses are collected and stored at the Danish National Biobank at Statens Serum Institut.
Cellular immune response
A group consisting of 699 participants evenly distributed across vaccine and age groups were enrolled in a substudy evaluating SARS-CoV-2 spike specific T-cell immunity using peripheral blood mononuclear cells. For this subgroup additional blood samples, including RNA stabilising PAXgene tubes, are collected at each visit. Analyses of T-cell immunity are performed at the Research Laboratory at the Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark.
Vaccine groups
The first participant included in this study received the AstraZeneca adenovirus vectored vaccine on 13 February 2021. However, the use of the AstraZeneca vaccine in the Danish COVID-19 vaccination programme was stopped on 11 March 2021, and therefore only 474 participants received this vaccine. A small group of 25 participants received the adenovirus vectored vaccine manufactured by Johnson & Johnson. In total 499 participants received an adenovirus vectored vaccine at their first vaccination and are included in the study as the adenovirus vector/messenger RNA (mRNA) group.
Inclusion in the Pfizer-BioNTech group started on 16 February 2021. This group was temporarily paused from mid-April to mid-May 2021 when the initial target of 2500 participants was reached, but was subsequently resumed in order to enrol more participants in younger age groups and concluded at 3824 participants.
Inclusion in the Moderna group started on 24 February 2021, but was slow-paced in the beginning due to limited vaccine deliveries, but finalised at 2620 participants. Enrolment progression during the inclusion period for each vaccine group is shown in figure 2.
Figure 2.
Cumulative number of participants enrolled over the inclusion period shown by vaccine type.
The timing of additional doses of any of the COVID-19 vaccines was determined by the schedule outlined in the Danish National COVID-19 vaccination programme at the time of vaccination. The duration between first and second dose varied between 3 and 12 weeks, depending on the vaccine and the calendar time.
Demographics
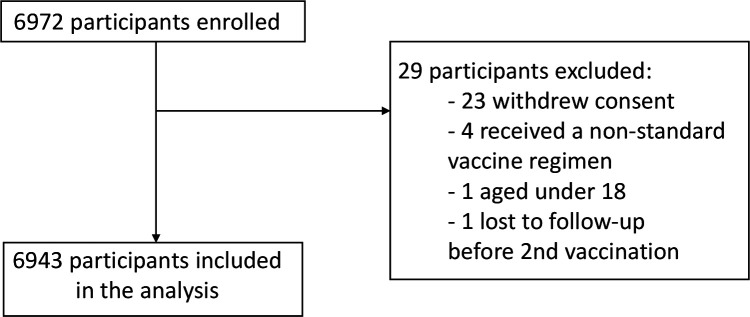
A total of 6972 participants were enrolled in the study and 6943 participants (99.6%) are included in the analysis. The most common reason for exclusion was withdrawal of consent (n=23) and receiving a non-standard vaccine regimen (n=4). Reasons for exclusion are shown in figure 3.
Figure 3.
Flowchart of reasons for exclusion from the study.
Overall, the median age was 64 years (IQR 53–75 years), 3929 (56.6%) participants were women, 590 (8.5%) were HCW and 1599 (23%) were classified as having an increased risk of a serious course of COVID-19 due to concomitant diseases. Overall, 3.4% (n=239) of the cohort had a positive SARS-CoV-2 PCR test prior to enrolment in the study. Participants in the adenoviral vector/mRNA group (N=499) were generally younger with a median age of 45 years (IQR 31–55 years) and 411 (82.4%) were women, while 453 (90.8%) were HCW. A higher proportion in the adenoviral vector/mRNA group (n=47, 9.4%) had a positive SARS-CoV-2 PCR prior to baseline compared with the other vaccine groups. The Pfizer-BioNTech group (N=3824) had a larger proportion of participants with an increased risk of a serious course of infection with SARS-CoV-2 (n=1437, 37.6%) and the participants were generally older, with a median age of 71 years (IQR 55–78 years), relative to the other vaccine groups. The demographic distribution overall and within the vaccine groups is summarised in table 1.
Table 1.
Participant demographics at study enrolment by vaccine type. Participant characteristics at baseline are presented overall and compared across vaccine groups, using either the χ2 test for categorical variables or Kruskal-Wallis test for continuous variables.
| Vaccine type | P value | ||||
| Total (N=6943) |
Pfizer-BioNTech (N=3824) |
Moderna (N=2620) |
Adenoviral Vector/mRNA (N=499) |
||
| Age at enrolment (median, IQR) | 64 (53–75) | 71 (55–78) | 61 (54–69) | 45 (31–55) | . |
| Age group (N, %) | <0.0001 | ||||
| 18–25 | 159 (2.3) | 59 (1.5) | 64 (2.4) | 36 (7.2) | . |
| 25–39 | 384 (5.5) | 152 (4.0) | 62 (2.4) | 170 (34.1) | . |
| 40–64 | 3191 (46.0) | 1326 (34.7) | 1577 (60.2) | 288 (57.7) | . |
| 65–79 | 2313 (33.3) | 1557 (40.7) | 752 (28.7) | 4 (0.8) | . |
| ≥80 | 896 (12.9) | 730 (19.1) | 165 (6.3) | 1 (0.2) | . |
| Sex (N, %) | <0.0001 | ||||
| Male | 3014 (43.4) | 1841 (48.1) | 1085 (41.4) | 88 (17.6) | . |
| Female | 3929 (56.6) | 1983 (51.9) | 1535 (58.6) | 411 (82.4) | . |
| Risk group (N,%) | <0.0001 | ||||
| Individuals at increased risk | 1599 (23.0) | 1437 (37.6) | 153 (5.8) | 9 (1.8) | . |
| Healthcare workers | 590 (8.5) | 109 (2.9) | 28 (1.1) | 453 (90.8) | . |
| General population | 4754 (68.5) | 2278 (59.6) | 2439 (93.1) | 37 (7.4) | . |
| Ever SARS-CoV-2 PCR positive | <0.0001 | ||||
| Never | 6704 (96.6) | 3718 (97.2) | 2534 (96.7) | 452 (90.6) | . |
| Positive ≤6 months | 180 (2.6) | 82 (2.1) | 56 (2.1) | 42 (8.4) | . |
| Positive >6 months | 59 (0.8) | 24 (0.6) | 30 (1.1) | 5 (1.0) | . |
Baseline Wantai serology results
A total of 6889 participants (99.2% of the analysed population) had a conclusive result of the baseline Wantai analysis measuring SARS-CoV-2 spike IgG. There were five participants (0.07%) with an inconclusive result of the Wantai analysis, and 49 participants (0.71%) with missing data, these participants were excluded from the Wantai results presented in figure 4. Of the participants with a conclusive result 340 participants (4.9%) had a positive result, indicating previous infection with SARS-CoV-2. The proportion of positive participants was 17/157 (10.8%) among the youngest participants aged 18–25 years, while it was 26/893 (2.9%) among those over the age of 80 years. Serology results are summarised in figure 4.
Figure 4.
Percentage of population testing positive and negative for SARS-CoV-2 IgG by means of the Wantai analysis at enrolment, shown for age groups and overall.
Participant and public involvement
Public reports on the preliminary study results are shared on the ENFORCE website and a public seminar was held, where study results were presented. Participants in ENFORCE receive regular updates on the study progress and results, including results of their own antibody measurements.
Findings to date
We assessed levels of SARS-CoV-2 spike IgG and Spike-ACE2-receptor-blocking antibodies at visits up to 6 months after the participants’ first SARS-CoV-2 vaccination and identified risk factors for humoral hyporesponsiveness and lack of durability of the vaccine response. We found that male sex, level of comorbidity and vaccine type were all associated with hyporesponsiveness and non-durability of both antibodies assessed.22 After the emergence of the Omicron variant, we conducted a study comparing the risk of breakthrough infection at different levels of SARS-CoV-2 spike IgG stratified by viral variant. We found an inverse association between increasing level of spike IgG and risk of breakthrough infection for the Delta variant, while there was no association between antibody levels and risk of breakthrough infection with the Omicron variant.23 Recently, a study was published comparing the vaccine-induced antibody response and neutralising capacity towards a range of SARS-CoV-2 variants after primary vaccination and first booster dose. Here it was found that the neutralising capacity was reduced for each of the emergent variants tested as compared with wild type, and most pronounced for the Omicron variant. However, it was also seen that neutralising capacity increased significantly after the admission of a booster dose.24
Strengths and limitations
The primary strength of the ENFORCE study is that it combines repeated measurements of SARS-CoV-2 specific humoral and cellular immunological response throughout the course of the SARS-CoV-2 vaccination, with register-based follow-up of safety data and microbiological test results. This design allows the ENFORCE cohort to contribute with significant knowledge of the interplay between humoral and cellular immune response to SARS-CoV-2 vaccination and new emerging SARS-CoV-2 variants, hybrid immunity and long-term safety of SARS-CoV-2 vaccination.
The ENFORCE cohort has high proportions of participants over the age of 65 years and of participants with significant comorbidities. These groups are of particular interest in the context of vaccine effectiveness and durability as they have an increased risk of developing a serious course of COVID-19, and vaccine programmes are often designed to protect these individuals.
The three vaccine groups display a large degree of variation in demographic factors and distribution across risk groups, which may be explained by the availability and prioritising of specific vaccines to specific groups at different time points. The AstraZeneca vaccine was specifically prioritised for HCW before it was removed from the vaccination programme, and therefore the adenovirus vectored/mRNA group includes higher proportions of HCW, women and younger age groups. The Pfizer-BioNTech vaccine was used to a large extent for the elderly population and individuals at increased risk of a serious course of disease, as it was the vaccine mainly available in the first months of the vaccination programme. The between-group differences complicates the comparison of vaccine groups as age, sex and health status may be confounding factors. A randomised design would have eliminated this limitation, but due to vaccine shortage in the early stage of the vaccination programme and vaccine prioritisation for risk groups it was unfeasible to randomise participants to vaccine groups. When conducting comparative analyses of the vaccine groups this limitation will be mitigated by adjusting for confounding factors.
The use of registry data comprises a risk of misclassification due to incorrect registration. However, the large size of the ENFORCE cohort reduces the risk of misclassification affecting results significantly.
At enrolment the number of individuals with missing data was low (<1%). As the study progresses this number will vary depending on the question being addressed. At this stage we do not plan to perform imputation on missing data, instead the characteristics of those excluded due to missing values will be compared with those included in analysis and any differences described.
Future plans
Studies are planned or ongoing investigating the T-cell immune response of the cohort at different time points relative to primary vaccination and boosters, the significance of hybrid immunity, morbidity and mortality compared with those of a historic control group and the correlation of local and systemic reactions to vaccination with antibody response. Data collection will proceed until July 2023.
Collaboration
The ENFORCE study encourages scientific collaboration. While the study is ongoing data may be made available to scientists on approval of an application sent to the ENFORCE Scientific Steering Committee and further approval by relevant authorities. Applications for data must be sent to enforce.rigshospitalet@regionh.dk. Detailed information about data access may be found here: https://chip.dk/Research/Studies/ENFORCE/Study-Governance.
Supplementary Material
Acknowledgments
The ENFORCE study group members all contributed substantially to the study.
Footnotes
Contributors: JL, LØ, MT, OSS, NBS and JR contributed to the conception and design of the study. ISJ, HN, TB, LW, LSK, MBI, KF, IKH, KKI, BT, FDL, LL, SOL and NBS contributed to the acquisition of the data. AKT, MLJ and JR performed data curation. JR performed the statistical analyses. NBS wrote the first draft. All authors revised the manuscript critically and accepted the final version. LØ and JL act as guarantors.
Funding: Funding for this work was granted by the Danish Ministry of Health (legal deeds 150 28/1 2021 and 263 3/6 2021).
Competing interests: NBS declares to have served as investigator on studies sponsored by Pfizer, Gilead, Bavarian and Sanofi Pasteur. HN declares receiving a grant from Novo Nordisk Foundation and to have been on advisory boards for MSD and GSK. TB declares receipt of unrestricted research or travel grants from GSK, Pfizer, Gilead Sciences, MSD; and being principal investigator on trials conducted by Boehringer Ingelheim, Roche, Novartis, Kancera, Pfizer, MSD and Gilead; Board member on Pentabase, and advisory board member for MSD, Gilead, Pfizer, GSK, Janssen and AstraZeneca; consulting fees from GSK and Pfizer; receiving donation of study drug from Eli Lilly; and receiving honorarium for lectures from GSK, Pfizer, Gilead Sciences, Boehringer Ingelheim, AbbVie and AstraZeneca.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be made available to researchers upon approval of an application to the scientific steering committee of the ENFORCE study. More information about data access may be found at https://chip.dk/Research/Studies/ENFORCE/Study-Governance.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Ethical Committees of The Central Denmark Region. Participants gave informed consent to participate in the study before taking part.
References
- 1. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467–78. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 2020;383:1920–31. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an Rad26 and RAD5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020;396:887–97. 10.1016/S0140-6736(20)31866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu F-C, Li Y-H, Guan X-H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020;395:1845–54. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020;586:589–93. 10.1038/s41586-020-2639-4 [DOI] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–93. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med 2021;384:1824–35. 10.1056/NEJMoa2034201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Organization WH . WHO coronavirus (COVID-19) dashboard, 2022. Available: https://covid19.who.int/ [Accessed 13 Sep 2022].
- 11. Danish Health Authority . Vaccination fall/winter 2022-2023. Available: https://www.sst.dk/en/English/Corona-eng/Vaccination-against-covid-19/Vaccination-fall-and-winter-2022-2023
- 12. Chalkias SH, Vrbicky C;, Walsh K;. R. a bivalent Omicron-containing booster vaccine against Covid-19. MedRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FDA . COVID-19 bivalent vaccine boosters. Available: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-bivalent-vaccine-boosters [Accessed 13 Sep 2022].
- 14. Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021;398:121–30. 10.1016/S0140-6736(21)01420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021;11:136. 10.1038/s41408-021-00528-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021;8:e474–85. 10.1016/S2352-3018(21)00103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol 2021;14:81. 10.1186/s13045-021-01090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and Reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation 2021;105:2119–23. 10.1097/TP.0000000000003824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strauss AT, Hallett AM, Boyarsky BJ, et al. Antibody response to severe acute respiratory Syndrome-Coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl 2021;27:1852–6. 10.1002/lt.26273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danish Health Authority . Vaccination calendar. Available: https://www.sst.dk/en/English/Corona-eng/Vaccination-against-COVID-19/When-will-you-get-vaccinated
- 22. Søgaard OS, Reekie J, Johansen IS, et al. Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish nationwide enforce study. Clin Microbiol Infect 2022;28:1126–33. 10.1016/j.cmi.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stærke NB, Reekie J, Nielsen H, et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with delta or omicron variant breakthrough infections. Nat Commun 2022;13:4466. 10.1038/s41467-022-32254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hvidt AK, Baerends EAM, Søgaard OS, et al. Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front Med 2022;9:994160. 10.3389/fmed.2022.994160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069065supp001.pdf (60.7KB, pdf)
Data Availability Statement
Data may be made available to researchers upon approval of an application to the scientific steering committee of the ENFORCE study. More information about data access may be found at https://chip.dk/Research/Studies/ENFORCE/Study-Governance.