Abstract
Background
Immune checkpoint inhibitors (ICIs) show a tremendous activity in microsatellite instability-high (MSI-H) metastatic colorectal cancer (mCRC), but a consistent fraction of patients does not respond. Prognostic/predictive markers are needed. Despite previous investigations in other tumor types, immune-related adverse events (irAEs) have not been well evaluated in patients with MSI-H cancers treated with ICIs.
Methods
We conducted an international cohort study at tertiary cancer centers collecting clinic-pathological features from 331 patients with MSI-H mCRC treated with ICIs. Of note, the irAEs were summarized using a ‘burden score’ constructed in a way that the same score value could be obtained by cumulating many low-grade irAEs or few high-grade irAEs; as a result, the lower the burden the better. Clearly, the irAE burden is not a baseline information, thus it was modeled as a time-dependent variable in univariable and multivariable Cox models.
Results
Among 331 patients, irAEs were reported in 144 (43.5%) patients. After a median follow-up time of 29.7 months, patients with higher burden of skin, endocrine and musculoskeletal irAEs (the latter two’s effect was confirmed at multivariable analysis) had longer overall survival (OS), as opposed to gastrointestinal, pneumonitis, neurological, liver, renal and other irAEs, which showed an harmful effect. Similar results were observed for progression-free survival (PFS). Based on the results retrieved from organ-specific irAEs, ‘aggregated’ burden scores were developed to distinguish ‘protective’ (endocrine and musculoskeletal) and ‘harmful’ (gastrointestinal, pneumonitis, neurological, hepatic) irAEs showing prognostic effects on OS and PFS.
Conclusions
Our results demonstrate that not all irAEs could exert a protective effect on oncologic outcome. An easy-to-use model for ICIs toxicity (burden score of protective and harmful irAEs) may be used as surrogate marker of response.
Keywords: Translational Medical Research, Tumor Biomarkers, Immunotherapy, Gastrointestinal Neoplasms
WHAT IS ALREADY KNOWN ON THIS TOPIC
Immune-related adverse events (irAEs) have been previously linked to different outcomes in response to immune checkpoint inhibitor (ICI). However, no existing data in literature explore the association of irAEs with survival outcomes in microsatellite instability metastatic colorectal cancer (MSI mCRC).
WHAT THIS STUDY ADDS
Through the analysis of a large international cohort of MSI mCRC patients (n=331), we showed that not all irAEs seem to exert a positive prognostic effect on overall survival/progression-free survival in this setting, but rather there are ‘protective’ irAEs and ‘harmful’ irAEs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides with a model to aggregate ICI toxicity (burden score of protective and harmful irAEs) that could represent a surrogate clinical marker of response to ICI in MSI mCRC patients.
Introduction
In a histology-agnostic fashion, Food and Drug Administration (FDA) has approved the use of immune checkpoint inhibitors (ICIs) in solid tumors bearing deficiency in mismatch repair (dMMR) and/or microsatellite instability-high (MSI-H).1–4 dMMR/MSI-H tumors are characterized by a hypermutated genome and a high load of immunogenic neoepitopes, capable to elicit robust lymphocytic infiltration and upregulation of immune checkpoint molecules, making them vulnerable to ICIs.5 6
Patients with dMMR/MSI-H metastatic colorectal cancer (mCRC) represents a small (5% of all cases) but unique subset and derive durable benefit from ICIs. Nevertheless, a significant fraction of patients does not respond, making predictive markers utterly needed. Among putative factors, potentially useful on-treatment clinical factors are the development of immune-related adverse events (irAEs).2 7–9
irAEs likely result from cross-reactivity of T cells due to antigens shared between tumor tissue and target organ, although both tumor-intrinsic and tumor-extrinsic factors, such as microbiome or pre-existing autoimmunity, contribute to their generation.10 Interestingly, ever-growing literature suggests that patients with irAEs may obtain greater clinical benefit from ICI therapy.11 12 The relationship between irAEs and efficacy is not entirely linear, depending on cancer type, as non-univocal data have been reported in melanoma, and on ICI subtype, since anti-CTLA-4 (cytotoxic T-lymphocyte antigen-4) monotherapy-induced AEs do not correlate with outcomes.13 14 irAEs characteristics, such as severity, site, whether multiple systems are affected at once, as well as the timing of onset, may also be important players.11 15–18 Notably, most of the available data correlating the occurrence of irAEs and cancer prognosis are retrospective and may be influenced by immortal time bias and, in particular, data on the role of irAEs specifically obtained in patients with dMMR/MSI-H mCRC are extremely scarce.19
Drawing from these considerations, we aimed to dissect irAEs occurring in dMMR/MSI-H mCRC patients treated with ICIs in terms of organ(s) affected and grade of severity, and to investigate the relationship of specific irAEs with the survival outcomes.
Patients and methods
Study population
This was an ambispective, multicenter cohort study including consecutive patients with dMMR/MSI-H mCRC treated at 11 cancer centers with immune-checkpoint inhibitors (ICI), that is, an anti-PD-(L)1 (programmed death (ligand)1) ± an anti-CTLA-4 agent. Mismatch repair and/or MSI status was locally assessed through immunohistochemistry or multiplex PCR, respectively, as per international guidelines.20 IrAEs were defined as the first AE that occurred after ICI start considered to result from immunological dysfunction, by treating physicians. They were graded based on National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) V.5.0.
Statistical methods
The study endpoints were progression-free survival (PFS) and overall survival (OS). PFS was defined as the time interval between date of first ICI administration and first disease progression, relapse or death due to any cause, whichever occurred first. OS was defined as the time interval between date of first ICI administration and death due to any cause. Time was censored at the date of last follow-up (FU) for patients who were still alive for OS, and event-free for PFS.
We analyzed the first occurrence of irAEs, together with the corresponding grade according to the organ affected, and calculated a summary score of organ-specific irAEs burden. This ‘burden score’ consisted in a simplified version of the adverse event burden score proposed by Le-Rademacher et al,21 and was obtained by summing the grade of organ-specific irAEs. Therefore, higher burden score values correlate with higher grade and/or frequency of irAEs, and the same value of the score may be obtained by cumulating many low-grade irAEs or few high-grade irAEs. Univariable and multivariable Cox models were fitted to evaluate the association between the end-points and the organ-specific irAE burdens modeled as time-dependent variables. Several patients and disease characteristics were considered as adjustment factors in the multivariable models: sex, age, Eastern Cooperative Oncology Group Performance Status, primary tumor sidedness (left, right), primary tumor resection, mutational status (RAS mutation, BRAF mutation, RAS, and BRAF wild-type), presence of synchronous metastases, number of metastatic sites (1, >1), presence of bone metastases, lung metastases, peritoneal metastases, brain metastases, nodal metastases, prior adjuvant treatment, prior treatment(s) for metastatic disease, ICI regimen (anti-CTLA-4-based combination or anti-PD-(L)1 monotherapy). To prevent overfitting and obtain reliable estimates, the adjustment was operated by means of an end-point-specific score beforehand estimated as the linear predictor from a Cox model including all the above listed adjustment factors.
Statistical analyses were conducted using the SAS (version 9.2) and R software programs (http://www.r-project.org/).
Results
Characteristics of study population and description of irAEs
Data of 331 patients with dMMR/MSI-H mCRC receiving with ICIs were retrieved. Median FU time was 29.65 months (IQR, IQR 12.43–44.84). The main patients and disease characteristics are summarized in table 1.
Table 1.
Patients and disease characteristics in the study population
| Characteristics | Study population (N=331) n (%) |
| Age (years) | |
| Median | 60 |
| IQR | (47-70) |
| Sex | |
| Female | 151 (45.6) |
| Male | 180 (54.4) |
| ECOG PS | |
| 0 | 167 (50.5) |
| ≥ 1 | 164 (49.5) |
| Primary tumor location | |
| Right colon | 222 (67.1) |
| Left colon/rectum | 109 (32.9) |
| Primary tumor resection | |
| Yes | 268 (81.0) |
| No | 63 (19.0) |
| RAS and BRAF mutational status | |
| RAS mutated | 103 (31.1) |
| BRAF mutated | 89 (26.9) |
| All wild-type | 127 (38.4) |
| Unknown | 12 (3.6) |
| Time to metastases | |
| Synchronous | 162 (48.9) |
| Metachronous | 169 (51.1) |
| Metastatic sites (N) | |
| 1 | 158 (47.7) |
| >1 | 173 (52.3) |
| Previous treatment for metastatic disease | |
| Yes | 260 (78.5) |
| No | 71 (21.5) |
| Choice of ICI regimen | |
| Anti-PD-(L)1 monotherapy | 239 (72.2) |
| Anti-PD-1 plus anti-CTLA-4 combo | 92 (27.8) |
CTLA-4, cytotoxic T-lymphocyte antigen 4; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; PD-(L)1, programmed death- (ligand)1.
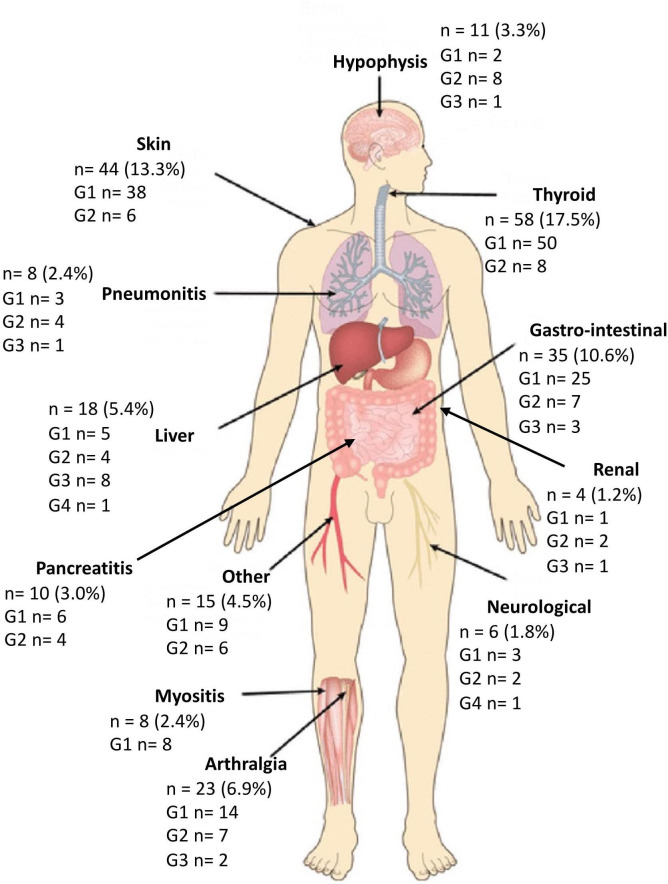
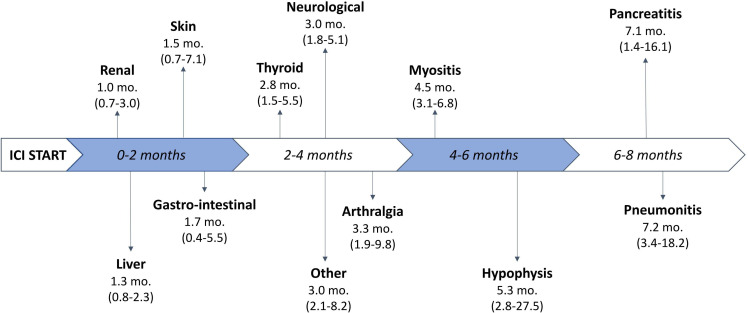
Most patients (78.5%) received at least one previous treatment line for advanced disease before ICI start. Eventually, 72.2% patients were treated with anti-PD-(L)1 monotherapy, while the remaining patients (27.8%) received anti-CTLA-4-based combination therapy. At censoring time for this study, a total of 122 patients (36.8%) experienced progressive disease (PD). Overall, 144 patients (43.5%) experienced at least one irAE (any grade and site). A detailed description of irAEs according to organ affected and grade of severity can be found in figure 1. Most common adverse effects were hypo- or hyper-thyroidism (n=58; 17.5%), skin toxicity (n=44; 13.3%) and gastrointestinal disorders (n=35; 10.6%). We grouped together less frequent irAEs, such as anemia, infusion reactions, xeropthalmia, under the category ‘other’. Most irAEs were reported as <G3. Of note, no lethal (G5) irAE was registered in our cohort. Among the patients who experienced at least 1 irAEs, toxicity was managed through supportive treatment in 83 patients (57.6%), while steroid therapy was required in 51 (35.4%). In 10 patients (6.9%), irAEs led to drug cessation (3 due to gastrointestinal, 2 due to neurological, 2 due to renal, 2 due to hepatic irAEs, and 1 due to pneumonitis). Mean prednisone-equivalent dosage of steroid therapy was 62.8 mg/day (IQR 25–82.5). A table with the summary of the patients who required steroid treatment for specific irAE(s) can be found in online supplemental material. Median time to onset of toxicity (figure 2) was shortest for renal (1.0 months, IQR, 0.7–3.0), liver (1.3 months, IQR 0.8–2.3), skin (1.5 months, IQR 0.7–7.1), and gastrointestinal (1.7 months, IQR 0.4–5.5) irAEs. Other toxicities generally presenting within 4 months from ICI start date were thyroid, other, neurological irAEs and arthritis. Late immune-related toxicities included myositis, hypophysitis, pancreatitis, and pneumonitis. A visual representation of median onset timing of organ-specific irAEs can be found in figure 2.
Figure 1.
Summary of incidence and severity of immune-related adverse events recorded in the study population.
Figure 2.
Timeline with median onset-timing (in months, mo.) (IQR) of the different organ-specific irAEs. ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events.
jitc-2022-005493supp001.pdf (25.3KB, pdf)
Association of irAEs with survival outcomes
Higher burden of any irAEs was not significantly associated with OS (HR 0.89; 95% CI CI 0.53 to 1.48; p=0.582) nor with PFS (HR 1.27; 95% CI 0.82 to 1.96; p=0.068).
This led us to perform an in-depth analysis of association between survival outcomes and organ-specific irAEs burden. To build the organ-specific burden score, we grouped together myositis and arthralgia as musculoskeletal irAEs, thyroiditis and hypophysitis as endocrine irAEs, and colitis and pancreatitis as gastrointestinal irAEs.22
At univariable analysis (table 2), the higher burden of skin, endocrine, and musculoskeletal irAEs had a protective effect on OS, whereas higher burden of gastrointestinal, pneumonitis, neurological, liver, renal, and other irAEs had a detrimental effect on OS.
Table 2.
Cox proportional hazards regression models for OS and PFS in the entire study population according to organ-specific irAEs
| Organ-specific irAEs | OS | PFS | ||||||
| Univariable model | Multivariable model | Univariable model | Multivariable model | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Gastrointestinal | 1.15 (0.80 to 1.65) | 0.438 | 1.33 (0.87 to 2.03) | 0.189 | 1.27 (0.94 to 1.72) | 0.113 | 1.30 (0.92 to 1.84) | 0.141 |
| Skin | 0.84 (0.44 to 1.61) | 0.601 | 1.16 (0.64 to 2.11) | 0.628 | 1.49 (0.96 to 2.31) | 0.078 | 1.81 (1.18 to 2.27) | 0.006 |
| Endocrine | 0.46 (0.24 to 0.88) | 0.019 | 0.43 (0.21 to 0.89) | 0.023 | 0.78 (0.51 to 1.17) | 0.223 | 0.76 (0.50 to 1.16) | 0.207 |
| Pneumonitis | 1.92 (0.97 to 3.84) | 0.063 | 1.41 (0.73 to 2.72) | 0.304 | 1.78 (0.88 to 3.60) | 0.108 | 1.50 (0.76 to 2.97) | 0.241 |
| Neurological | 1.39 (0.87 to 2.22) | 0.163 | 1.64 (1.03 to 2.60) | 0.036 | 1.28 (0.80 to 2.04) | 0.296 | 1.63 (1.04 to 2.56) | 0.032 |
| Musculoskeletal | 0.29 (0.08 to 1.08) | 0.066 | 0.22 (0.06 to 0.88) | 0.032 | 0.79 (0.44 to 1.44) | 0.449 | 0.56 (0.28 to 1.11) | 0.098 |
| Liver | 1.38 (1.02 to 1.86) | 0.034 | 1.53 (1.11 to 2.10) | 0.009 | 1.38 (1.06 to 1.80) | 0.018 | 1.52 (1.15 to 2.01) | 0.004 |
| Renal | 1.20 (0.56 to 2.54) | 0.641 | 1.19 (0.52 to 2.70) | 0.683 | 1.02 (0.48 to 2.15) | 0.960 | 1.02 (0.45 to 2.30) | 0.958 |
| Other | 1.25 (0.62 to 2.54) | 0.537 | 1.15 (0.57 to 2.32) | 0.701 | 1.42 (0.77 to 2.60) | 0.258 | 1.45 (0.79 to 2.66) | 0.225 |
P values <0.05 are highlighted in bold
irAEs, immune-related adverse events; OS, overall survival; PFS, progression-free survival.
At multivariable analysis (table 2), adjusting for patients’ and disease characteristics, higher burden of endocrine and musculoskeletal irAEs had a protective effect on OS, whereas higher burden of skin, gastrointestinal, pneumonitis, neurological, hepatic, renal, and other irAEs had a detrimental effect on OS.
Based on the above results, we built ‘aggregated’ burden scores of protective (endocrine and musculoskeletal) and harmful (gastrointestinal, pneumonitis, neurological, liver) irAEs. This aggregation was based on the prognostic effect of organ-specific burden scores at multivariable analysis, and determined by HRs direction (<1 protective, >1 harmful), HR magnitude (<0.83 for protective and >1.2 for harmful events) and/or p value (<20%). The aggregated burden scores showed very strong prognostic effect at multivariable analysis (protective burden score HR 0.36; 95% CI 0.19 to 0.66; p=0.001; harmful burden score HR 1.46; 95% CI 1.19 to 1.79; p<0.001).
At univariable analysis (table 2), higher burden of endocrine and musculoskeletal irAEs had a protective effect on PFS, whereas higher burden of gastrointestinal, skin, pneumonitis, neurological, hepatic, renal and other irAEs had a detrimental effect on PFS.
At multivariable analysis (table 2), higher burden of endocrine and musculoskeletal irAEs had a protective effect on PFS, whereas higher burden of gastrointestinal, skin, pneumonitis, neurological, hepatic, renal and other irAEs had a detrimental effect on PFS. As for OS, we built ‘aggregated’ burden scores of protective (endocrine and musculoskeletal) and harmful (gastrointestinal, skin, pneumonitis, neurological, liver) irAEs. The adjusted model including these aggregated burden scores showed a stronger effect of protective (HR 0.68; 95% CI 0.47 to 0.98; p=0.04) than harmful (HR 1.49; 95% CI 1.26 to 1.76; p<0.001) burden score.
Finally, we assessed whether a different management of irAEs (ie, either supportive therapy alone or steroid therapy or ICI discontinuation) could result in significantly different outcomes (online supplemental material 2). Median PFS was not reached for patients who discontinued ICI, 71.3 months for those treated with supportive therapy alone and 38.9 months for those who required steroids as well, however, these were not statistically significant (p=0.53). Instead, mOS was not reached for all three groups of patients (p=0.49). Notably, no significant differences in terms of PFS and OS were observed in these three subgroups of patients.
jitc-2022-005493supp002.pdf (77.4KB, pdf)
Discussion
This multicenter cohort study is the largest study evaluating the association between irAEs and survival outcomes in a large international dataset of over 300 dMMR/MSI-H mCRC patients treated with ICIs. Our results show that different organ-specific irAEs may selectively exert ‘protective’ or ‘harmful’ effects.
These results are particularly relevant in light of the unmet need of developing prognostic and predictive factors to improve clinical stratification of patients with dMMR/MSI-H mCRC treated with ICI.2 23 24 Indeed, despite the introduction of ICI has dramatically improved the prognosis of these patients, with some of them achieving long-term benefit, there is yet a relevant proportion of patients displaying primary or acquired resistance.7 23 25–27
So far, no clear data have been specifically provided for patients with dMMR/MSI-H mCRC. Studies investigating the association between irAEs and outcomes of patients treated with ICI focused only on other types of ICI-sensitive cancers and were not conclusive. There is indeed conflicting evidence regarding the prognostic impact of irAEs, depending on timing of onset, severity or organ(s) affected, and ICI regimen used, for example, anti-PD-(L)1 monotherapy, anti-CTLA-4 agents alone or anti-CTLA-4-based combination therapy.11–14 17 18 Furthermore, the immortal time bias may have influenced earlier data on correlation between irAE and ICI efficacy: patients with toxicities may appear to have longer survivals simply because they must survive long enough to experience them, while patients dying shortly after ICI start do not have enough time to develop irAEs, eventually falsely inflating their positive prognostic effect.28 29 Current evidence overall suggests that the occurrence of irAEs as a whole should not be categorized as exclusively protective or detrimental and that immortal time bias has to be considered.
Based on these premises, we first dissected irAEs occurring in our population in terms of grade and site of origin, and then we built organ-specific irAEs burden scores modeling them as time-dependent variables to minimize the potential risk of survivor bias.
After adjusting for patients’ and disease characteristics at multivariable analyses, the only events that appeared to have a protective effect on OS were endocrine and musculoskeletal irAEs, while all the others (such as skin, gastrointestinal, pneumonitis) all had a detrimental effect on both OS and PFS.
The prognostic relevance of protective and harmful irAEs emerged more clearer in the analysis of ‘aggregated’ burden scores, that showed very strong prognostic effect for both at multivariable analysis for OS and a stronger effect of protective than harmful burden score at multivariable analysis for PFS.
Our results are in part in line with the previously published data regarding the positive association of endocrine and rheumatological (including arthralgia and arthritis) toxicities with ICI efficacy.30–33 On the contrary, the association of cutaneous irAEs with a negative effect on OS and PFS was not expected, since the opposite observation was previously made.15 34–36 We should consider that most of these previous results were shown in different disease setting (ie, advanced melanoma), where different specific cutaneous toxicities may arise. Specifically, the skin toxicity that was strongly associated to high response rates is vitiligo, but this specific skin irAE was rather uncommon in our population (which presented mainly with rash).37 38 Similar to cutaneous irAEs, gastrointestinal irAEs have been previously associated with better outcomes, while they demonstrated to be harmful in our dataset.36 This discordance could be attributed, again, to different settings: our dataset was made up of advanced CRC patients with accompanying gastrointestinal morbidity, and this could potentially blur this association and diminish ICI effects.
However, development of irAEs and the reason why different organ-specific irAEs are related to different outcomes remain unsolved questions and a controversial topic. A leading hypothesis for irAEs generation is tissue cross-reactivity but differences in immunogenomics may play as well a role.39–41 Further studies are needed to elucidate biological mechanisms behind organ-specific irAEs and to potentially dissociate pharmacological effects of ICIs from their unwanted toxicities.
We must acknowledge that our study has some limitations, first represented by the retrospective and heterogeneous nature of our study cohort. Furthermore, it could be argued that higher grade organ toxicities, that is, hepatic and renal irAEs, are more often associated with serious adverse events and could be life-threatening themselves or compromise subsequent treatments, thus worsening prognosis. However, there was no lethal irAE (G5) in our cohort and most irAEs were graded as <3. Additionally, more serious toxicities could require steroid or immunosuppressive therapy, potentially dampening the efficacy of ICIs.42–44 However, the use of steroid therapy was equally distributed in both subgroups identified by ‘harmful’ and ‘protective’ irAEs. Notably, we were also able to show that PFS and OS were not worsened by the use of steroid therapy or by the permanent discontinuation of ICIs because of irAEs.
In conclusion, through a joint effort between multiple international institutions, we went further into understanding the prognostic impact of irAEs and developed an easy-to-use model to aggregate ICI toxicity (burden score of protective and harmful irAEs) that may be used as surrogate marker of response to ICI in dMMR/MSI-H mCRC patients. Further analyses of completed and ongoing randomized controlled trials are required to validate our findings and to properly integrate organ-specific irAEs as prognostic indicators in clinical practice.
Footnotes
Twitter: @nasca_vincenzo
Contributors: Conception and design: VN, RM and FP. Acquisition of data: FC, SL, MN, MEE, MF, PJ, ATS, MS, EF, LS, CC, JR, MA, GM, RI and MJO. Analysis and interpretation of data: VN, FB, RM and FP. Manuscript drafting: VN, FB, RM and FP. Manuscript revision: FC, SL, MN, MEE, MF, PJ, ATS, MS, EF, LS, CC, JR, MA, GM, RI and MJO. Final approval: VN, FB, FC, SL, MN, MEE, MF, PJ, ATS, MS, EF, LS, CC, JR, MA, GM, RI, MJO, RM and FP. Author responsible for the overall content as the guarantor: FP.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LS: Speakers’ and consultant’s fees from MSD, Astra-Zeneca, Servier, Bayer, Merck, Amgen, Pierre-FabreMJO: Consulting fees from Pfizer, Merck, Glaxosmithkline, 3D Medicine, Nouscom, Roche. Research fees from Roche, Takeda, Merck, BMS, Astra-Zeneca, Nouscom. FP: Honoraria: Servier, Bayer, AstraZeneca/MedImmune, Lilly, MSD Oncology, Amgen, Pierre-Fabre, Merck-Serono, BMS. Consulting or Advisory Role: Merck-Serono, Amgen, Servier, MSD Oncology, Organon. Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Incyte. All other authors declare no specific conflicts of interest(s).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of participating centers (INT 117/15). Participants gave informed consent to participate in the study before taking part.
References
- 1.Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med 2017;377:1409–12. 10.1056/NEJMp1709968 [DOI] [PubMed] [Google Scholar]
- 2.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43–51. 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maby P, Tougeron D, Hamieh M, et al. Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res 2015;75:3446–55. 10.1158/0008-5472.CAN-14-3051 [DOI] [PubMed] [Google Scholar]
- 7.Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite Instability-High/Mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020;38:11–19. 10.1200/JCO.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurjao C, Liu D, Hofree M, et al. Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol Res 2019;7:1230–6. 10.1158/2326-6066.CIR-18-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard E, Verschoor CP, Ma GW, et al. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front Immunol 2020;11:369. 10.3389/fimmu.2020.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Sidlow R, Hellmann MD. Immune-Related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 11.Hussaini S, Chehade R, Boldt RG, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev 2021;92:102134. 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 12.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519–27. 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering cancer center. J Clin Oncol 2015;33:3193–8. 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ascierto PA, Simeone E, Sileni VC, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med 2014;12:116. 10.1186/1479-5876-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Y, Xie W, Huang H, et al. Association of immune related adverse events with efficacy of immune checkpoint inhibitors and overall survival in cancers: a systemic review and meta-analysis. Front Oncol 2021;11:633032. 10.3389/fonc.2021.633032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulati N, Donnelly D, Qian Y, et al. Revisiting the association between skin toxicity and better response in advanced cancer patients treated with immune checkpoint inhibitors. J Transl Med 2020;18:430. 10.1186/s12967-020-02612-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiehchen D, Naqash AR, Espinoza M, et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology 2022;11:2017162. 10.1080/2162402X.2021.2017162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol 2020;6:1952–6. 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das S, Ciombor KK, Haraldsdottir S, et al. Immune-Related adverse events and immune checkpoint inhibitor efficacy in patients with gastrointestinal cancer with food and drug Administration-approved indications for immunotherapy. Oncologist 2020;25:669–79. 10.1634/theoncologist.2019-0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 21.Le-Rademacher JG, Hillman S, Storrick E, et al. Adverse event burden Score-A versatile summary measure for cancer clinical trials. Cancers 2020;12:3251. 10.3390/cancers12113251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esfahani K, Meti N, Miller WH, et al. Adverse events associated with immune checkpoint inhibitor treatment for cancer. CMAJ 2019;191:E40–6. 10.1503/cmaj.180870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.André T, Shiu K-K, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High advanced colorectal cancer. N Engl J Med 2020;383:2207–18. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 24.Pietrantonio F, Lonardi S, Corti F, et al. Nomogram to predict the outcomes of patients with microsatellite instability-high metastatic colorectal cancer receiving immune checkpoint inhibitors. J Immunother Cancer 2021;9:e003370. 10.1136/jitc-2021-003370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth MT, Das S. Pembrolizumab in unresectable or metastatic MSI-high colorectal cancer: safety and efficacy. Expert Rev Anticancer Ther 2021;21:229–38. 10.1080/14737140.2021.1851201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fucà G, Cohen R, Lonardi S, et al. Ascites and resistance to immune checkpoint inhibition in dMMR/MSI-H metastatic colorectal and gastric cancers. J Immunother Cancer 2022;10:e004001. 10.1136/jitc-2021-004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fucà G, Corti F, Ambrosini M, et al. Prognostic impact of early tumor shrinkage and depth of response in patients with microsatellite instability-high metastatic colorectal cancer receiving immune checkpoint inhibitors. J Immunother Cancer 2021;9:e002501. 10.1136/jitc-2021-002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street S, Chute D, Strohbehn I, et al. The positive effect of immune checkpoint inhibitor-induced thyroiditis on overall survival accounting for immortal time bias: a retrospective cohort study of 6596 patients. Ann Oncol 2021;32:1050–1. 10.1016/j.annonc.2021.05.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dall'Olio FG, Rizzo A, Mollica V, et al. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy 2021;13:257–70. 10.2217/imt-2020-0179 [DOI] [PubMed] [Google Scholar]
- 30.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583–9. 10.1093/annonc/mdw640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujisawa Y, Yoshino K, Otsuka A, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci 2018;89:60–6. 10.1016/j.jdermsci.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Gomes-Lima CJ, Kwagyan J, King F, et al. A comprehensive meta-analysis of endocrine immune-related adverse events of immune checkpoint inhibitors and outcomes in head and neck cancer and lung cancer. JCO 2019;37:e14096. 10.1200/JCO.2019.37.15_suppl.e14096 [DOI] [Google Scholar]
- 33.Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]
- 34.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015;151:1206–12. 10.1001/jamadermatol.2015.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quach HT, Dewan AK, Davis EJ, et al. Association of Anti-Programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol 2019;5:906–8. 10.1001/jamaoncol.2019.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong L, Wu Q, Chen F, et al. Immune-related adverse events: promising predictors for efficacy of immune checkpoint inhibitors. Cancer Immunol Immunother 2021;70:2559–76. 10.1007/s00262-020-02803-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773–81. 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol 2017;44:117–22. 10.1111/1346-8138.13520 [DOI] [PubMed] [Google Scholar]
- 39.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berner F, Bomze D, Diem S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol 2019;5:1043–7. 10.1001/jamaoncol.2019.0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan Z, Di Nucci F, Kwan A, et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci U S A 2020;117:12288–94. 10.1073/pnas.1922867117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 43.Garant A, Guilbault C, Ekmekjian T, et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol 2017;120:86–92. 10.1016/j.critrevonc.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 44.Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 2018;6:51. 10.1186/s40425-018-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005493supp001.pdf (25.3KB, pdf)
jitc-2022-005493supp002.pdf (77.4KB, pdf)
Data Availability Statement
Data are available on reasonable request.