Abstract
Afa/Dr diffusely adhering Escherichia coli strain IH11128 bacteria basolaterally entered polarized epithelial cells by a CD55- and CD66e-independent mechanism through interaction with the α5β1 integrin and a pathway involving caveolae and dynamic microtubules (MTs). IH11128 invasion within HeLa cells was dramatically decreased after the cells were treated with the cholesterol-extracting drug methyl-β-cyclodextrin or the caveola-disrupting drug filipin. Disassembly of the dynamically unstable MT network by the compound 201-F resulted in a total abolition of IH11128 entry. In apically infected polarized fully differentiated Caco-2/TC7 cells, no IH11128 entry was observed. The entry of bacteria into apically IH11128-infected fully differentiated Caco-2/TC7 cells was greatly enhanced by treating cells with Ca2+-free medium supplemented with EGTA, a procedure that disrupts intercellular junctions and thus exposes the basolateral surface to bacteria. Basally infected fully differentiated polarized Caco-2/TC7 cells grown on inverted inserts mounted in chamber culture showed a highly significant level of intracellular IH11128 bacteria compared with cells subjected to the apical route of infection. No expression of CD55 and CD66e, the receptors for the Afa/Dr adhesins, was found at the basolateral domains of these cells. Consistent with the hypothesis that a cell-to-cell adhesion molecule acts as a receptor for polarized IH11128 entry, an antibody blockade using anti-α5β1 integrin polyclonal antibody completely abolished bacterial entry. Experiments conducted with the laboratory strain E. coli K-12 EC901 carrying the recombinant plasmid pBJN406, which expresses Dr hemagglutinin, demonstrated that the dra operon is involved in polarized entry of IH11128 bacteria. Examined as a function of cell differentiation, the number of internalized bacteria decreased dramatically beyond cell confluency. Surviving intracellular IH11128 bacteria residing intracellularly had no effect on the functional differentiation of Caco-2/TC7 cells.
Two classes of enteroinvasive pathogens are those that enter host cells at a high level and those that invade cells at a low level. Among the six well-defined groups of enterovirulent Escherichia coli, only enteroinvasive E. coli (EIEC) cells develop high-level invasion of epithelial cells as a key virulence process (for a review, see reference 46). Similarities have been found among the invasion processes developed by EIEC and Shigella species (for a review, see reference 21). In contrast, an efficiently invasive E. coli strain isolated from a patient with Crohn's disease showed several differences in the mechanism of cell entry from that of EIEC (8). Cell entry at low levels of invasion by enterotoxinogenic E. coli (19), enterohemorrhagic E. coli (50), enteroaggregative E. coli (4), enteropathogenic E. coli (22), and Afa/Dr diffusely adhering E. coli (DAEC) (25, 26, 35, 63) has been reported.
Afa/Dr DAEC strains express adhesins of the Afa/Dr family which include the afimbrial adhesins AfaE-I (38) and AfaE-III (39) and the fimbrial adhesins Dr (49), Dr-II (58), and F1845 (7). Afa/Dr adhesins have similar genetic organizations, consisting of operons of at least five genes. Genes A to D, encoding accessory proteins, are highly conserved among the family members, whereas gene E, encoding the adhesin molecule itself, is more divergent. Afa/Dr adhesins mediate bacterial attachment onto target cells by binding to the complement regulatory glycosylphosphatidylinositol (GPI)-anchored protein decay accelerating factor (PDAF [also known as CD55]) (48). It has been recently reported that members of the Afa/Dr family of adhesins recognize, together with the CD55 molecule, another membrane-associated GPI-anchored protein, the carcinoembryonic antigen (DAF [also known as CD66e]) (29). Mobilization of CD55 and CD66e molecules around adhering Afa/Dr DAEC bacteria has been observed (27, 29). This adhesin-dependent mobilization of GPI-anchored proteins is apparently consistent with a bacterial host cell cross talk, since both CD55 and CD66e function as transducing molecules upon bacterial infection (30, 44, 57). For example, Afa/Dr DAEC infection in human intestinal cells is followed by cytoskeleton injury that results from an activation of a Ca2+-dependent signaling pathway (57), promoting brush border impairment (5) and alterations in intestinal functions (56). The Afa/Dr DAEC strain harboring Afa-III adhesin expresses protein AfaD which, like the AfaE protein, was exposed at the bacterial cell surface but, unlike AfaE, is able to detach from the surface of bacteria and to be internalized (25, 28). Pioneering investigation by Jouve et al. (35) demonstrated that the AfaD protein functions as an invasive factor in the cell entry of AfaE-III bacteria. Indeed, colloidal gold tagging of AfaE-III and AfaD proteins shows that AfaE-III-gold complexes only bind to the cell surface, whereas AfaD-gold complexes enter the cells. The role of AfaD in cell entry has been ascertained by the observation that endowing polycarbonate beads with AfaD protein results in cell entry of the beads (25). Entry within the epithelial cells of latex beads coated with Dr hemagglutinin belonging to the Afa/Dr family of adhesins has been further reported (63). Mobilization of cytoskeletal proteins has been observed, outlining the recombinant E. coli-expressing Dr hemagglutinin or fimbrial F1845 adhesin and Dr-coated polystyrene beads bound onto the cells (27). The mechanism of Dr+ E. coli cell entry within HeLa cells is microtubule (MT) dependent and microfilament (MF) independent (26). In Chinese hamster ovary (CHO) cell transfectant clones that stably express CD55 cDNA or various CD55 deletion constructs, Selvarangan et al. (63) observed that the short consensus repeat domain 3 (SCR3) and the GPI anchor of CD55 were critical for internalization to occur. The aim of the present study was to give further insight into the cell invasion process of Afa/Dr DAEC. For this purpose, we investigated the cell entry of Afa/Dr DAEC strain IH11128 into undifferentiated or differentiated nonintestinal and intestinal human epithelial cells lacking phagocytic properties.
MATERIALS AND METHODS
Reagents and antibodies.
4-Amino-antipyrine was obtained from Baker. All other reagents were obtained from Sigma-Aldrich (L'lsle d'Abeau Chesnes, France): aprotein, antipain, benzamidine, leupeptin, pepstatin A, phenylmethylsulfonyl fluoride, glucose oxidase type V, peroxidase type II, p-hydroxybenzoic acid, geneticin, taxol, nocodazole, cytochalasin D, filipin, and EGTA. 201-F was kindly provided by M. Guyot (Laboratoire de Chimie, Unité 401 associé au CNRS, Muséum National d'Histoire Naturelle, Paris, France). Fluorescein isothiocyanate (FITC)-phalloidin-labeling F-actin was from Molecular Probes Inc. (Eugene, Oreg.). Rat anti-human sucrase isomaltase (SI) monoclonal antibody (MAb) 8A9 was a generous gift from S. Maroux (ESA 6033 CNRS, Marseille, France). Native (clone DM1A) anti-α-tubulin was purchased from Sigma-Aldrich Chimie SARL (L'Isle d'Abeau Chesnes, France). The polyclonal anti-CD55 antibody and the MAb anti-CD55 SCR3 (1H4) were from D. M. Lublin (Washington University, St. Louis, Mo.). The polyclonal anti-CEA rabbit antibody was from Dako (Tebu, France). Polyclonal anti-α5β1 integrin was from Biovalley (Conches, France). Polyclonal anti-intercellular adhesion molecule 1 (ICAM-1) was from R & D Systems (Bington, United Kingdom). The rabbit immunoglobulin G (IgG), anti-Dr adhesin antibody was from B. Nowicki (University of Texas, Galveston). Anti-rat and anti-rabbit FITC- and tetramethyl rhodamine isothiocyanate-conjugated antibodies were from Institut Pasteur Productions (Paris, France).
Cell lines and culture.
Human cervix HeLa cells, stably transfected HeLa cells expressing CD66e (Hela-CD66e) or containing the expression vector alone (HeLa-SFFV.neo) were obtained from F. Grunert (Immunbiologisches Institut, Universität Freiburg, Freiburg, Germany) (71). The cells were cultured at 37°C in a 5% CO2–95% air atmosphere in RPMI 1640 with l-glutamine (Life Technologies, Cergy, France) supplemented with 10% heat-inactivated (30 min, 56°C) fetal calf serum (FCS) (Boehringer, Mannheim, Germany) and 500 μg of geneticin per ml, as previously described (29). Cells were used for infection assays at confluence, i.e., after 4 days.
INT407 cells (human embryonic intestine; ATCC CCL 6) were from a stock culture of the American Type Culture Collection (Rockville, Md.). Cells were cultured in Dulbecco modified Eagle's minimal essential medium (DMEM) (25 mM glucose) supplemented with 1% nonessential amino acids (Life Technologies) and 10% inactivated FCS at 37°C in a 10% CO2–90% air atmosphere as previously described (6, 57). Cells were used for infection assays at confluence, i.e., after 4 days.
The Caco-2/TC7 clone (10) established from the human colonic adenocarcinoma parental Caco-2 cell line (59) was used. Cells were routinely grown in DMEM (25 mM glucose) (Life Technologies) supplemented with 15% heat-inactivated FCS and 1% nonessential amino acids, as previously described (5, 6). For maintenance purposes, cells were passaged weekly using 0.02% trypsin in Ca2+/Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Maintenance of the cells was carried out at 37°C in a 10% CO2–90% air atmosphere. The culture medium was changed daily. Cells were used for infection assays as stated for each experiment.
Some experiments were performed using Caco-2/TC7 cells grown on inverted inserts mounted in chamber culture (Costar culture plate inserts, 3-μm pore size, 4.7 cm2, 3 × 104 cells per cm2), which delineates apical (luminal) and a basolateral (serosal) reservoirs. The cells were seeded onto inverted inserts and were allowed to attach overnight, after which the filters were placed upright in 12-well culture plates, thus orienting the basolateral side of the monolayer upward (20). The integrity of the confluent polarized monolayers was checked by measuring transepithelial membrane resistance with a volt-ohmmeter (Millicel ERS; Millipore). Transepithelial membrane resistance was calculated as Ω per cm2 by multiplying the measured electrical resistance with the surface area of the filter. Background reading of the free control filter was subtracted. Experiments and maintenance of the cells were carried out at 37°C in a 10% CO2–90% air atmosphere. The culture medium was changed daily. Cells were used for infection assays at late postconfluence (day 15 in culture).
Cell infection.
The clinical isolate E. coli IH11128 harboring the Dr hemagglutinin (49) was grown at 37°C for 18 h on Luria broth. The laboratory strain E. coli K-12 EC901, carrying the recombinant plasmid pBJN406 that expresses Dr hemagglutinin (E. coli BN406), was grown on Luria broth supplemented with chloramphenicol.
The method used for Afa/Dr DAEC infection of cultured epithelial cells has been described previously (5, 6, 29). Briefly, cells were washed twice with sterile PBS. Infecting E. coli bacteria were suspended in the cell culture medium, and the cells were infected at a multiplicity of infection of 100 in the presence of 1% mannose to prevent type 1 fimbria-mediated binding. The plates were incubated at 37°C in 10% CO2–90% air for 3 h. The monolayers were then washed three times with sterile PBS. All assays were conducted in triplicate with three successive cell passages.
Quantification of E. coli cell association.
E. coli cell association was determined by quantitative determination of bacteria associated with the infected cell monolayers (5, 6). After infection, cells were washed twice with sterile PBS and lysed with sterilized H2O. Appropriate dilutions were plated on tryptic soy agar to determine the number of viable cell-associated bacteria by bacterial colony counts. Each cell association assay was conducted at least in triplicate with three successive cell passages. Results were expressed as CFU of cell-associated bacteria per milliliter.
Quantification of intracellular E. coli.
Internalization of E. coli was measured by quantitative determination of bacteria located within the infected cell monolayers by using the aminoglycoside antibiotic assay (13, 22). The concentration of gentamicin that reduced the bacterial count by 99.99% was determined in a preliminary experiment. After incubation, monolayers were washed twice with sterile PBS and afterwards were incubated for 1 h in a medium containing 75 μg of gentamicin per ml. Bacteria that adhered to the cells were rapidly killed, whereas those located within the cells were not. The monolayer was washed with PBS and lysed with sterilized H2O. Appropriate dilutions were plated on tryptic soy agar to determine the number of viable intracellular bacteria by bacterial colony counts. Results were expressed as the CFU of intracellular bacteria per milliliter, or as a percentage of the cell-associated bacteria.
For measurement of intracellular multiplication of IH11128 bacteria in Caco-2/TC7 cells as a function of the days in culture, cell monolayers at day 6 in culture were infected for 3 h as described above. Then the infected cells were incubated with gentamicin as described above to kill the extracellular adhering bacteria. The infected cells were subsequently cultured in the presence of cell culture medium containing gentamicin (50 μg/ml), and the medium was changed daily to prevent extracellular replication of remaining viable extracellular bacteria. The number of intracellular bacteria was determined as a function of the day postinfection at days 1, 3, 5, 6, and 9 after the gentamicin assay, corresponding to days 7, 9, 11, 13, and 15 in cell culture, respectively.
Antibody blockade assay.
Cells were preincubated for 1 h with polyclonal antibodies directed against CD55, ICAM-1, and α5β1 integrin diluted 1:20 to 1:50 in culture medium prior to infection. The cells were infected with IH11128 bacteria for 3 h at a concentration of 108 CFU/well. Both incubation with antibodies and infection were conducted at 37°C in a 5% CO2–90% air atmosphere.
Disruption of intercellular junctions.
Postconfluent, fully differentiated Caco-2/TC7 monolayers (day 15 in culture) were washed three times with Ca2+-free DMEM (GIBCO Laboratories, Paris, France) and treated with the same medium with no addition or with 0.1 mM EGTA 1 h prior to infection (22, 45).
Treatment of cells with MT-depolymerizing or lipid-modifying drugs.
To depolymerize the MT network, cells were cultured alone or with nocodazole 1 h prior to cell infection. To depolymerize the dynamic unstable MT network, cells were cultured alone or with 201-F for 1 h prior to cell infection (61). To freeze the MT network, cells were cultured alone or with taxol for 1 h prior to infection. To depolymerize the MF network, cells were cultured alone or with cytochalasin D for 1 h prior to infection.
To investigate the role of cholesterol- and glycosphingolipid-enriched microdomains, cells were incubated at 37°C in DMEM alone or with methyl-β-cyclodextrin (MBCD) for 1 h prior to infection (32). To investigate the role of caveolae, the cells were incubated at 37°C in DMEM alone or with filipin for 1 h prior to infection (62).
Drugs were diluted in culture medium before use. Stock solutions were made in ethanol (5 mM 201-F) or in dimethyl sulfoxide (10 mM nocodazole). The working concentration was 1 μg/ml for cytochalasin D, 10 μM for nocodazole, 25 μM for 201-F, 50 μM for taxol, 0.5 to 5 mM for MBCD, and 2.5 to 5 μg/ml for filipin. None of these treatments modified the IH11128 binding onto the cells (not shown) or affected cell viability as measured by lactate dehydrogenase release assay (control cells, 16 ± 5 U/liter; H2O-lysed cells, 2,995 ± 25 U/liter; cytochalasin D-treated cells, 18 ± 5 U/liter; nocodazole-treated cells, 19 ± 6 U/liter; 201-F-treated cells, 18 ± 5 U/liter; taxol-treated cells, 18 ± 7 U/liter; MBCD-treated cells, 17 ± 9 U/liter; filipin-treated cells, 17 ± 6 U/liter).
Immunofluorescence.
Cells were prepared on glass coverslips which were placed in 24-well tissue culture plates (Corning Glass Works, Corning, N.Y.). The brush border-associated hydrolase SI (EC 3.2.1.10-48) was stained by indirect immunofluorescence labeling as previously described (5, 6, 56). Immunolabeling was conducted without cell permeabilization in cells fixed with 3% paraformaldehyde for 15 min at room temperature, washed three times with PBS, and then treated with 50 mM NH4Cl for 10 min (for aldehyde function saturation). Cells were incubated with the anti-SI MAb for 45 min at room temperature. After three washes in PBS, incubation with the FITC-conjugated secondary antibody diluted 1:200 in 0.2% bovine serum albumin–PBS was performed for 45 min at room temperature. No fluorescent staining was observed when the primary antibody was omitted.
To visualize MFs (F-actin) (5, 57), coverslips were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min at room temperature before incubation with fluorescein-phalloidin for 45 min at 22°C. To visualize MTs (61), Triton X-100-permeabilized coverslips were incubated with specific primary anti-tubulin antibody (diluted 1:20 to 1:100 in 0.2% gelatin–PBS) for 45 min at room temperature, washed, and then incubated with the respective secondary FITC-conjugated antibody used at a dilution of 1:200 in 0.2% gelatin–PBS. No fluorescent staining was observed when primary antibody was omitted.
Double-fluorescence staining of membrane-associated and intracellular bacteria was conducted as previously described by Merien et al. (43). Briefly, IH11128-infected cells were first incubated with polyclonal anti-Dr antibody for 30 min at room temperature before being washed three times with PBS and fixed for 2 min in pure methanol. The slides were incubated for 30 min at room temperature with goat anti-rabbit IgG fluorescein F(ab′) fragment to stain extracellularly located bacteria. The coverslips were extensively washed with PBS and subjected to a second incubation with the polyclonal anti-Dr antibody for 30 min at room temperature. Then intracellularly located bacteria were stained for 30 min at room temperature with goat anti-rabbit IgG rhodamine F(ab′) fragment, and excess antibody was washed off with PBS. No fluorescent staining was observed when primary antibody was omitted.
The CD55 and CD66e molecules were stained by indirect immunofluorescence labeling. Immunolabeling was conducted in cells fixed with 3% paraformaldehyde for 15 min at room temperature, permeabilized with 0.2% Triton X-100 in PBS for 4 min at room temperature, washed three times with PBS, and then treated with 50 mM NH4Cl for 10 min (for aldehyde function saturation). Monolayers were incubated with each specific primary antibody described above for 45 min at room temperature. After three washes in PBS, incubation with an FITC-conjugated secondary antibody was performed for 45 min at room temperature. No fluorescent staining was observed when primary antibody was omitted.
Specimens were mounted in Vectashield (Citifluor Laboratories, Birmingham, United Kingdom). They were examined by conventional epifluorescence microscopy using a Leitz Aristoplan microscope (Leica, Heidelberg, Germany). Relative immunofluorescence intensity was measured by coupling the epifluorescence microscope with an image analyzer Visiolab 1000 (Biocom, Les Ulis, France). To visualize the distribution of CD55 and CD66e immunolabeling in polarized Caco-2/TC7 cells, confocal analysis was conducted with a confocal laser-scanning microscope (model TCS SP; Leica), using a 100× 4NA 0.1 PL APO 1.4-0.7 objective. Optical sectioning was used to collect 50 en face images 0.3 to 0.4 μm apart. Lateral views were obtained by integration of images gathered at a step position of 1 on the x and y axes by using the accompanying software and Microsoft Windows NT.
Photographic images were resized, organized, and labeled using Adobe Photoshop software (San Jose, Calif.). The printed images are representative of the original data. All photographs were taken on Kodak T-MAX 400 black and white film or on Kodak Electronic Imaging Paper (Eastman Kodak Co., Rochester, N.Y.).
Enzymatic assay.
Cells were washed in ice-cold PBS, scraped, suspended in PBS, and homogenized. SI activity was determined as previously described (56) by using a glucose oxidase-peroxidase reagent that contains 4-amino-antipyrine instead of o-dianisidine as the chromogen. Enzyme activity is expressed as milliunits of protein per milligram. One unit is defined as the activity that hydrolyzes 1 μmol of substrate/min at 37°C. Protein concentration was determined using the bicinchoninic acid assay (Pierce).
Statistics.
Data are expressed as means ± standard error of the mean (SEM). A typical experiment was conducted at least in triplicate in three successive passages of cells. The statistical significance was assessed by Student's t test.
RESULTS
Entry of IH11128 bacteria into epithelial cells through a dynamic unstable MT-dependent pathway.
The invasion capacity of the Afa/Dr DAEC strain IH11128 was examined in a subset of either undifferentiated or differentiated nonintestinal or intestinal cells. Considering that Afa/Dr DAEC causes symptomatic urinary tract or intestinal infections, we chose human cervix HeLa cells, human embryonic undifferentiated intestinal INT407 cells, and human undifferentiated or fully differentiated intestinal Caco-2/TC7 cells, all of which express the CD55 molecule (6, 29) that functions as a receptor for Afa/Dr DAEC adhesins (48). Although these cell lines displayed different patterns of CD55 expression, we observed an identical level of cell-associated IH11128 bacteria after 3 h of infection, whatever the cell line examined (Table 1). In contrast, when examining the cell entry of IH11128 bacteria we observed that the level of intracellular IH11128 after 3 h of infection markedly differed among the cell lines examined (Table 1). We found that bacteria more efficiently invaded undifferentiated nonintestinal HeLa cells than undifferentiated intestinal INT407 and Caco-2/TC7 cells. When polarized fully differentiated Caco-2/TC7 cells were apically infected with IH11128 bacteria, the level of intracellular bacteria was dramatically lower than that of the infected undifferentiated Caco-2/TC7 cells.
TABLE 1.
Entry of IH11128 bacteria into undifferentiated or differentiated nonintestinal and intestinal epithelial cells expressing CD55a
| Cell | Expression amount and pattern of CD55b | Mean level of bacteria (CFU/ml) ± SEMc
|
|
|---|---|---|---|
| Cell associated | Intracellular | ||
| HeLa (N, U) | 1.35 ± 0.15; diffuse labeling | 1.81 × 107 ± 0.14 × 107 | 1.26 × 105 ± 0.14 × 105 (0.696) |
| INT407 (I, U) | 1.40 ± 0.24; punctate labeling | 2.77 × 107 ± 0.88 × 107 | 3.10 × 104 ± 0.87 × 104 (0.112) |
| Caco-2/TC7 cells at day 6 in culture (I, U) | 1.38 ± 0.18; punctate labeling | 2.10 × 107 ± 0.86 × 107 | 3.23 × 104 ± 0.74 × 104 (0.154) |
| Caco-2/TC7 cells at day 15 in culture (I, D) | 1.40 ± 0.18; punctate labeling with mosaic pattern | 2.35 × 107 ± 0.75 × 107 | 8.86 × 102 ± 0.67 × 102 (0.0037) |
Cells were infected with IH11128 bacteria for 3 h at a concentration of 108 CFU/ml. N, nonintestinal cells; I, intestinal cells; U, undifferentiated cells; D, differentiated cells.
Relative immunofluorescence intensity was measured with a conventional fluorescence microscope connected to a Visiolab 1000 image analyzer. Results are expressed as arbitrary units.
The levels of cell-associated bacteria were determined after the cells were lysed with H2O. The levels of intracellular bacteria were determined by gentamicin assay. The values in parentheses are the percentages of cell-associated bacteria that were also intracellular.
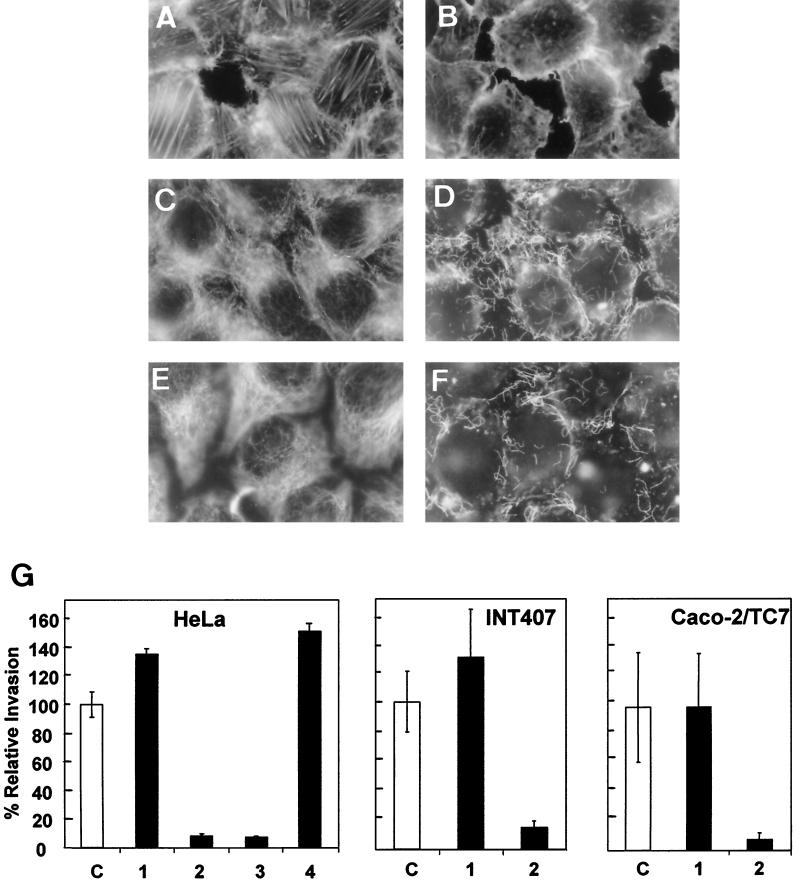
Goluszko et al. (26) have reported that the Dr-dependent entry of strain IH11128 into HeLa cells was MF independent and MT dependent. In order to examine whether or not this characteristic is cell line dependent, we conducted experiments with undifferentiated HeLa, INT407, and Caco-2/TC7 cells (Fig. 1). Treatment of the cells with the cytoskeleton-disrupting drug cytochalasin D followed by F-actin immunolabeling showed the typical F-actin disassembly (Fig. 1A and B). Treatment of the cells with the MT-disrupting drug nocodazole followed by MT immunolabeling showed the typical disruption of MTs (Fig. 1C and D). Treatment of the cells with the compound 201-F, which specifically disassembles dynamically unstable MTs, showed the typical disruption of dynamic MTs and that the stable MTs remained present (Fig. 1E and F). In parallel, we observed that the entry of IH11128 bacteria into undifferentiated HeLa, INT407, and Caco-2/TC7 cells was dramatically inhibited when the cells were treated with nocodazole, whereas treatment with cytochalasin D did not modify the bacterial invasion (Fig. 1G). In HeLa cells, 201-F treatment dramatically decreased IH11128 entry, whereas treatment with taxol did not modify the bacterial invasion.
FIG. 1.
Effect of MF and microtubules MT disorganization on entry of IH11128 bacteria into epithelial nonintestinal HeLa cells and undifferentiated human intestinal INT407 and Caco-2/TC7 cells. (A and B) Immunofluorescence labeling of F-actin; (C to F) immunofluorescence labeling of MTs. Shown are control untreated cells (A, C, and E), undifferentiated Caco-2/TC7 cells treated with cytochalasin D (1 μg/ml) for 1 h at 37°C before infection (B), undifferentiated Caco-2/TC7 cells treated with nocodazole (10 μg/ml) for 1 h at 37°C before infection (D), and HeLa cells treated with 201-F (25 μM) for 1 h at 37°C before infection (F). (G) Entry of IH11128 bacteria into HeLa, INT407, and undifferentiated Caco-2/TC7 cells. The cells were infected apically for 3 h at a concentration of 108 CFU/well at 37°C in a 5% CO2–90% air atmosphere. Shown are control untreated cells (C) and cells treated with taxol (50 μM) (1), nocodazole (10 μg/ml), (2) or 201-F (25 μM) (3). Student's t test showed a highly significant difference (P < 0.01) between control untreated infected cells and infected cells treated with nocodazole or 201-F. No significant difference was found between control untreated infected cells and infected cells treated with taxol.
Caveola-dependent entry of IH11128 bacteria into epithelial cells.
Results by Selvaragan et al. (63) have shown that MBCD treatment of CHO cells inhibited the cell entry of Dr-fimbriated E. coli, indicating an invasion process dependent on lipid rafts. Indeed, β-cyclodextrins are oligosaccharidic molecules which have a high affinity for sterols and are effective in rapidly extracting cholesterol from the plasma membrane of a number of cells. Moreover, it has been recently reported that caveolae, which are known to be associated with the lipid rafts, play a pivotal role in FimH-mediated internalization of E. coli (3, 42, 66). These results prompted us to examine whether or not caveolae played a role in IH11128 entry.
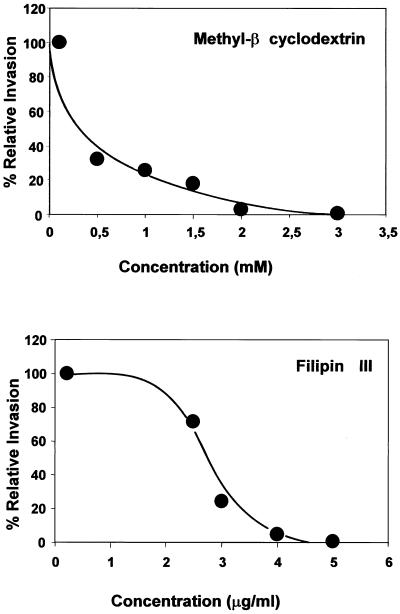
It has been previously reported that GPI-anchored proteins were extracted from the cell membrane by MBCD at a concentration of 20 mM (32), and we chose to use MBCD at a concentration that preserves the expression of CD55 (control cells, 1.35 ± 0.15 immunofluorescence intensity arbitrary units; MBCD-treated cells, 1.40 ± 0.28 immunofluorescence intensity arbitrary units) and does not affect IH11128 binding (control cells, 1.81 × 107 ± 0.14 × 107 CFU/ml; MBCD-treated cells, 1.77 × 107 ± 0.25 × 107 CFU/ml). As shown in Fig. 2, MBCD dose dependently inhibited the entry of IH11128 into HeLa cells.
FIG. 2.
Inhibition of invasiveness of IH11128 bacteria within HeLa cells by the lipid-extracting drug methyl-β-cyclodextrin and the caveola-disrupting drug filipin. Monolayers were incubated with drugs diluted in culture medium for 1 h prior to infection. Cells were infected apically for 3 h at a concentration of 108 CFU/well at 37°C in a 5% CO2–90% air atmosphere. The infected cells were processed for determination of internalized bacteria as described in Materials and Methods. Results represent the means ± standard deviations of three experiments.
Filipin is a sterol-binding agent that disrupts caveolae and caveola-like structures (62). Treatment of HeLa cells with filipin did not modify the apical expression of CD55 (control cells, 1.35 ± 0.15 immunofluorescence intensity arbitrary units; filipin-treated cells, 1.44 ± 0.28 immunofluorescence intensity arbitrary units) or the IH11128 binding (control cells, 1.81 × 107 ± 0.14 × 107 CFU/ml; filipin-treated cells, 2.15 × 107 ± 0.15 × 107 CFU/ml) of CD55. As shown in Fig. 2, treatment of HeLa cells with filipin dose dependently inhibited IH11128 internalization. Taken together, these results indicated that IH11128 bacteria were internalized into epithelial cells after association with the plasma membrane invaginations, the so-called caveolae.
Cell confluency down-regulates entry of IH11128 bacteria into Caco-2/TC7 cells.
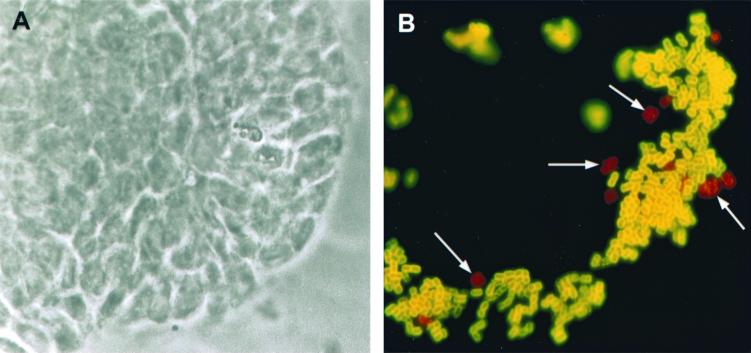
Caco-2/TC7 cells (10) as the parental Caco-2 cells (59) spontaneously differentiate in culture. After confluency, Caco-2 cells polarize with a particular organization of apical and basolateral domains (for a review, see reference 40). Organization of these polarized domains was through the control of the junctional complex, which is a highly developed structure which functions as a fence separating apical and basolateral domains, thereby segregating cell surface proteins and lipids into each domain (for a review, see reference 18). The observation that undifferentiated Caco-2/TC7 cells allowed entry of IH11128 bacteria while fully differentiated Caco-2/TC7 cells did not prompt us to investigate how this down-regulation of cell entry develops as a function of cell differentiation. The cell entry of IH11128 bacteria was examined as a function of the days in culture. Consistent with the fact that both undifferentiated and differentiated Caco-2 cells expressed the CD55 molecule (6), similar levels of cell-associated IH11128 bacteria were found, whatever the state of Caco-2/TC7 cell differentiation (Table 2). When examining cell entry of IH11128 bacteria as a function of days in culture, we found that the situation markedly differed from that of IH11128 cell association (Table 2). Indeed, the IH11128 bacteria invaded the proliferating cells, forming isolated cells (day 2 in culture) or clusters of 5 to 15 cells (day 4 in culture). When the cells were at confluency (day 6 in culture), IH11128 cell entry immediately decreased. When the cells were at late postconfluency (day 15 in culture), IH11128 bacteria did not significantly enter the cells. We used a double-fluorescence immunolabeling method (43) to visualize the extracellular and intracellular IH11128 bacteria in proliferating and postconfluent Caco-2/TC7 cells in the same microscopic field (Fig. 3). In isolated proliferating Caco-2/TC7 cells (Fig. 3A), highly concentrated extracellular IH11128 bacteria were seen in the periphery of the cell forming large clusters of bacteria. Intracellular bacteria were found at the periphery of the bacterial clusters of extracellular bacteria (Fig. 3B). In postconfluent Caco-2/TC7 cells in which IH11128 adhered diffusely, no intracellular bacteria were found (data not shown).
TABLE 2.
Entry of IH11128 bacteria into Caco-2/TC7 cells as a function of the day in culturea
| Day in culture | Levels of bacteria in CFU/ml ± SEM
|
Expression ofb:
|
Cell developmentc | ||
|---|---|---|---|---|---|
| Cell associated | Intracellular | SI | CD55 | ||
| 2 | 1.20 × 102 ± 0.26 × 102 | 7.64 × 102 ± 2.89 × 102 (0.64) | 0 | 1.05 | Isolated cells |
| 4 | 4.25 × 106 ± 0.43 × 106 | 1.02 × 104 ± 0.46 × 104 (0.24) | 0 | 1.18 | Clusters of cells |
| 6 | 2.10 × 107 ± 0.34 × 107 | 3.23 × 104 ± 0.55 × 104 (0.15) | 0.80 | 1.15 | Confluency |
| 15 | 2.55 × 107 ± 0.66 × 107 | 9.45 × 102 ± 0.67 × 102 (0.0037)* | 2.65 | 1.41 | Postconfluency |
At each day in culture, cells were infected with IH11128 bacteria for 3 h at a multiplicity of infection of 100, and the level of cell-associated bacteria was determined. The level of intracellular bacteria was determined by gentamicin assay. The values in parentheses are the percentages of cell-associated bacteria that were also intracellular. An asterisk represents a P value of <0.01 compared with intracellular bacteria at days 4 and 6 in culture, respectively (Student's t test).
The relative immunofluorescence intensities (arbitrary units) of the differentiation marker SI and the GPI-anchored protein CD55 were measured by conventional epifluorescence microscopy coupled with an image analyzer.
The cells were examined by light microscopy to determine the evolution of the cells in culture from cells organized in clusters to cells in monolayer. Days 2 and 4, cells proliferate; day 6, cell differentiation commences; day 15, cell differentiation is achieved and the cells are fully differentiated.
FIG. 3.
Dual immunofluorescence labeling of IH11128 bacteria in infected Caco-2/TC7 cells and visualization of extracellular and internalized bacteria with FITC or tetramethyl rhodamine isothiocyanate immunolabeling, respectively. Caco-2/TC7 cells at 3 days in culture were infected with IH11128 bacteria apically at 37°C in a 10% CO2–90% air atmosphere for 3 h. After extensive washes to remove the nonadhering bacteria, cells were processed for dual immunofluorescence labeling as described in Materials and Methods. (A) Phase-contrast microscopy showing the cell cluster (3 days in culture). (B) Green extracellular bacteria and red intracellular bacteria (arrows) were seen at the periphery of the cell cluster in which proliferative cells were localized. Magnification, ×100.
It has been previously reported that Caco-2 cells at late postconfluency whose intercellular junctions had been disrupted by Ca2+ depletion associated with EGTA treatment in order to render the basolateral surface accessible become infected by enteroinvasive pathogens that normally do not enter by the apical domain of differentiated cells (23, 45). When Ca2+ depletion associated with EGTA treatment was conducted with cells forming large clusters of 6 to 10 cells (day 4 in culture), the pattern of peripheral invasion found with untreated cells was abolished and replaced by an increase in invading bacteria randomly distributed over the entire surface of the cells but preferentially localized at the cell-cell junctions (not shown). An identical experiment was conducted with fully differentiated Caco-2/TC7 cells cultured in inserts mounted in chamber culture (Table 3). Disruption of cell-cell junctions in fully differentiated Caco-2/TC7 cells forming a monolayer was assessed by measurement of transepithelial resistance in cells cultured in Transwell filters (control cells, 784 ± 10 Ω per cm2; cells treated with low-Ca2+ medium plus EGTA, 195 ± 15 Ω per cm2). In this experimental condition, the apical cell association of IH11128 bacteria was not modified compared with the untreated control cells, whereas in contrast, a highly significant 50-fold increase in cell-associated IH11128 bacteria that were also intracellular was observed.
TABLE 3.
Polarized entry of Dr+ bacteria into fully differentiated Caco-2/TC7 cellsa
| Cell and infection typeb | Mean level of bacteria (CFU/ml) ± SEMd
|
|
|---|---|---|
| Cell-associated | Intracellular | |
| Apically IH11128-infected Caco-2/TC7 cells | 2.45 × 107 ± 0.55 × 107 | 9.60 × 102 ± 0.47 × 102 (0.0039) |
| Basally IH11128-infected Caco-2/TC7 cells | 6.88 × 106 ± 0.59 × 106 | 7.39 × 104 ± 3.39 × 104 (1.07)* |
| Apically IH11128-infected Caco-2/TC7 cells after opening of tight junctionsc | 2.40 × 107 ± 0.42 × 107 | 4.33 × 104 ± 0.65 × 104 (0.18)* |
| Apically IH11128-infected Caco-2/TC7 cells after opening of tight junctions in the presence of nocodazolec | ND | 1.75 × 103 ± 1.33 × 103** |
| Apically E. coli BN406-infected Caco-2/TC7 cells | 1.90 × 107 ± 0.66 × 107 | 9.13 × 102 ± 1.60 × 102 (0.0048) |
| Apically E. coli BN406-infected Caco-2/TC7 cells after opening of tight junctionsc | ND | 3.40 × 104 ± 0.56 × 104* |
| Apically E. coli BN406-infected Caco-2/TC7 cells after opening of tight junctions in the presence of polyclonal α5β1 integrinc | ND | 2.61 × 103 ± 1.3 × 103** |
Experimental conditions were as for experiments described in Table 1.
Fully differentiated Caco-2/TC7 cells (day 15 in culture) were grown on inserts mounted in chamber culture. For basal infection, the cells were cultured on inverted inserts mounted in chamber culture.
Fully differentiated cells were treated for 1 h prior to infection with Ca2+-free DMEM and EGTA (0.1 mM).
Values in parentheses are the percentages of cell-associated bacteria that were intracellular. An asterisk represents a P value of <0.01 compared with apically infected cells, and a double asterisk represents a P value of <0.01 compared with apically infected cells after opening of tight junctions (Student's t test). ND, not determined.
Cell entry of virus through the basal domain of polarized fully differentiated Caco-2 cells has been recently demonstrated using cells cultured on the lower side of a porous filter mounted in chamber culture (20). In order to examine whether or not IH11128 bacteria use a basolateral membrane-associated component to enter cells, Caco-2/TC7 cells grown on inverted inserts (pore size, 3 μm) mounted in chamber culture were infected through the basal domain. As shown in Table 3, the level of cell-associated intracellular IH11128 bacteria observed after a basal cell infection was 270-fold higher than that observed after an apical infection.
As indicated above and as previously reported (26), IH11128 entry into undifferentiated epithelial cells is MT dependent. In order to investigate whether or not the polarized entry of IH11128 bacteria into fully differentiated Caco-2/TC7 cells is MT dependent, an additional experiment was conducted. Fully differentiated cells in which disruption of cell-cell junctions had been promoted were treated with the MT-disrupting drug nocodazole and were apically infected (Table 3). Nocodazole treatment resulted in a complete inhibition of bacterial entry, demonstrating that polarized IH11128 entry is MT dependent.
Taken together, these results demonstrated that, following confluency, a membrane-associated molecule acting as a receptor for MT-dependent IH11128 entry and expressed over the cell surface of proliferating cells redistributed at the basolateral cell domain, thereby down-regulating entry of IH11128 bacteria into human polarized intestinal cells as a function of cell differentiation.
Are CD55 and CD66e, the receptors for Afa/Dr DAEC adhesins, involved in polarized entry of IH11128 bacteria?
It has been previously documented that the GPI-anchored proteins CD55 (48) and CD66e (29) act as receptors for the Afa/Dr DAEC adhesins. Moreover, it has been recently reported that CD66e plays a pivotal role in Neisseria cell entry following signaling (30, 44, 71). To demonstrate whether or not CD66e plays a role in IH11128 cell entry, we examined the bacterial internalization in HeLa cells that do or do not express the CD66e molecule. For this purpose, stably transfected HeLa cells expressing CD66e (HeLa-CD66e) or cells containing the expression vector alone (HeLa-SFFV.neo) were used (71). Results showed that these cell lines were equally infected by IH11128 bacteria (cell-associated levels of bacteria for HeLa- SFFV.neo and HeLa-CD66e cells were 2.20 × 107 ± 0.43 × 107 and 1.67 × 107 ± 0.23 × 107 CFU/ml, respectively). No significant increase in the level of intracellular IH11128 bacteria was observed in the HeLa-CD66e cells (3.66 × 104 ± 1.45 × 104 CFU/ml) compared to HeLa-SFFV.neo cells (4.57 × 104 ± 1.33 × 104 CFU/ml). This result indicated that CD66e probably does not play a role in IH11128 cell entry.
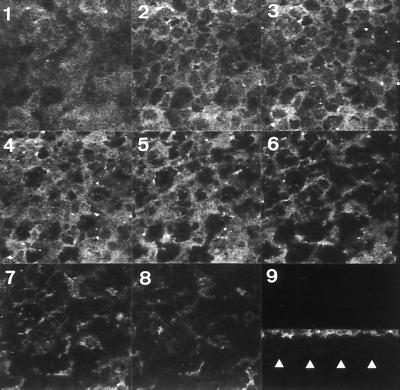
In a CHO cell transfectant clone that stably expressed CD55 cDNA, Selvarangan et al. (63) observed that the membrane-associated receptor for Afa/Dr adhesins, i.e., the CD55 molecule, was critical for IH11128 internalization. The fact that IH11128 bacteria enter the fully differentiated Caco-2/TC7 cells by a basolateral route prompted us to examine how CD55 is distributed in these cells. For this purpose, CD55 expression was examined by indirect immunofluorescence labeling and confocal scanning electron microscopy analysis. Specific antibodies were applied to fixed and permeabilized cells, thus allowing us to detect proteins present within the apical and basolateral cell membranes. Examination of immunolabeling in Caco-2/TC7 cells by confocal scanning electron microscopy analysis showed that CD55 GPI-anchored protein is distributed in a homogeneous band, localized at the apical surface of the cells, whereas no immunolabeling was found in the basolateral domain (Fig. 4). An identical distribution has been found for the CD66e molecule (not shown). This distribution was consistent with the reported selective insertion of GPI-anchored proteins in the brush border membrane of polarized epithelial cells of the small intestine (25). The observation that IH11128 bacteria could enter through the basolateral domain of Caco-2/TC7 cells, in which no expression of CD55 and CD66e was found, indicates that a basolateral domain-associated molecule functions as a receptor for IH11128 cell entry.
FIG. 4.
Confocal laser scanning electron microscopy analysis of CD55 immunofluorescence labeling in fully differentiated human intestinal Caco-2/TC7 cells. Paraformaldehyde-fixed and Triton X-100-permeabilized cells were washed and processed for indirect immunofluorescence labeling of CD55 as described in Materials and Methods. Cells were examined using a confocal laser scanning electron microscope. The samples were analyzed by serial optical horizontal sectioning, starting at the apical domain of the cells and following to the basal domain (one section every 0.30 μm). Micrographs 1 to 8 are en face micrographs of the immunolocalization of CD55 (horizontal x and y optical sections obtained at apical and subapical domains). Note that CD55 distribution starts on section 1, that the majority of CD55 labeling is distributed on sections 2 to 5 (apical domain), and that afterwards CD55 labeling rapidly disappears (subapical domain). Micrograph 9 shows lateral views (vertical x and z optical section) of CD55 distribution obtained by integration of images gathered at a step position of 1 on the x and y axes (collection of 50 en face images). CD55 labeling is restricted at the apical domain in a homogeneous band, whereas no CD55 labeling was found at the basolateral domain. Arrowheads indicate the basal domain. Identical distribution was found for the CD66e molecule (not shown).
Recognition of cell adhesion molecule α5β1 integrin allows IH11128 entry.
The results presented above strongly suggested that IH11128 entry into epithelial cells was in a direct relationship with the recognition of a cell-to-cell adhesion molecule. Two cell-to-cell adhesion molecules had our attention due to their known involvement in microbial cell entry. The first cell-to-cell adhesion molecule we examined was ICAM-1, which associates with the CD55 molecule to allow cell entry of the human enterovirus coxsackievirus A21 (64). The second type of cell-to-cell adhesion molecule we investigated was the integrins. Indeed, coxsackieviruses B1, B3, and B5 that bind to CD55 require integrins as secondary or accessory receptors to enter the cells (1). Among the integrins known to be involved in microbial cell entry, we focused our study on the β1 integrin, since Jouve et al. (35) observed that a zipper-like mechanism is involved in the cell entry of Afa/Dr DAEC strain Afa-III and since β1 integrin is involved in the invasin-dependent zipper mechanism that allows cell entry of Yersinia spp. (33).
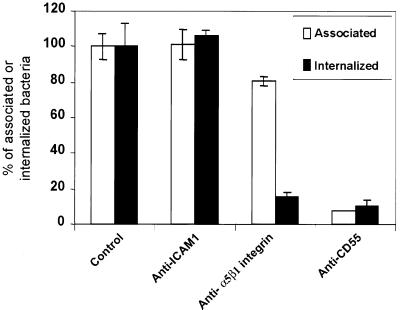
As a cellular model, we chose the HeLa cells, which as observed earlier, were better invaded by IH11128. As previously reported, both ICAM-1 (41) and integrins (54) were expressed at the cell surface of HeLa cells and trigger the cell entry of microbial pathogens. As shown in Fig. 5, infection of HeLa cells by IH11128 bacteria in the presence of a MAb directed against ICAM-1 did not modify the cell association or cell entry of IH11128 bacteria. In contrast, the presence of a MAb directed against the α5β1 integrin resulted in a decrease in cell association and in a complete inhibition of bacterial entry. Goluszko et al. have previously reported (26) that an anti-SCR3 MAb CD55 dramatically reduced internalization of Dr+ recombinant E. coli BN406 into HeLa cells. Here, we found that a polyclonal anti-CD55 antibody very significantly decreased the level of internalized IH11128 bacteria as the result of a corresponding decrease in adhesion.
FIG. 5.
Inhibition of invasiveness of IH11128 bacteria within HeLa cells by polyclonal antibodies directed against ICAM-1, α5β1 integrin, and CD55. Monolayers were incubated with antibodies diluted 1:20 to 1:50 in culture medium for 1 h prior to infection. The cells were infected apically for 3 h at a concentration of 108 CFU/well. Both incubation with antibodies and infection were conducted at 37°C in a 5% CO2–90% air atmosphere. The infected cells were processed for determination of cell-associated and internalized bacteria as described in Materials and Methods. Results represent the means ± standard deviations of three experiments. Student's t test showed a highly significant difference (P < 0.01) for internalized bacteria between control and anti-α5β1 integrin antibody-treated cells. No significant difference was observed for internalized bacteria between control and anti-ICAM1 antibody-treated cells. Note that the highly significant decrease in internalized bacteria in the presence of anti-DAF antibody results from a highly significant decrease in cell association.
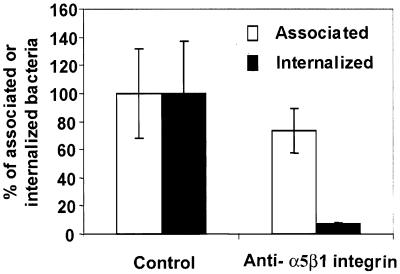
In order to investigate whether or not polarized entry of IH11128 bacteria into fully differentiated Caco-2/TC7 cells is dependent on the recognition of the α5β1 integrin as a basolateral receptor, we conducted the following experiment. Fully differentiated cells in which disruption of cell-cell junctions had been promoted to expose the basolateral domain as described above were apically infected in the presence of polyclonal anti-α5β1 integrin (Table 3). The antibody treatment resulted in a complete inhibition of bacterial entry, demonstrating that polarized IH11128 entry is α5β1 integrin dependent.
Role of dra operon in polarized IH11128 entry.
The above observation prompted us to examine the role of the dra gene cluster in IH11128 entry. For this purpose experiments were conducted using the laboratory strain E. coli K-12 EC901, carrying the recombinant plasmid pBJN406 that expresses Dr hemagglutinin (E. coli BN406). We examined entry of E. coli BN406 into fully differentiated Caco-2/TC7 cells. As shown in Table 3, when the cells were apically infected with the BN406 bacteria a small amount of intracellular BN406 bacteria was observed. In cells whose intercellular junctions had been disrupted by Ca2+ depletion associated with EGTA treatment, a highly significant 40-fold increase in internalized E. coli BN406 bacteria was observed. Moreover, in these cells the polarized entry of BN406 bacteria was very significantly inhibited when the cells were treated with nocodazole.
Considering the above observed role played by the cell-to-cell adhesion molecule, with α5β1 integrin acting as a receptor for polarized IH11128 entry, we examined whether or not the polyclonal antibody directed against α5β1 integrin inhibits the entry of E. coli BN406. A highly significant decrease in entry was observed when HeLa cells were infected with E. coli BN406 in the presence of polyclonal antibody directed against α5β1 integrin (Fig. 6). An identical result was obtained with fully differentiated Caco-2/TC7 cells whose intercellular junctions had been opened. Indeed, apical infection by E. coli BN406 in the presence of polyclonal antibody directed against α5β1 integrin resulted in a complete blockading of bacterial entry (Table 3).
FIG. 6.
Entry of laboratory strain E. coli K-12 EC901 carrying the recombinant plasmid pBJN406, which expresses Dr hemagglutinin, within HeLa cells. Experimental conditions were as for experiments shown in Fig. 5. Results represent the means ± standard deviations of three experiments. Student's t test showed a highly significant difference (P < 0.01) for internalized bacteria between control and anti-α5β1 integrin antibody-treated cells.
Altogether, these results indicated that the dra operon plays a role in the polarized entry of IH11128 bacteria.
Intracellular lifestyle of IH11128 bacteria within Caco-2/TC7 cells.
The observation above that IH11128 bacteria could invade undifferentiated intestinal Caco-2/TC7 cells, which then further differentiate to reach confluency, leads to the issues of the fate of these intracellular bacteria and how the functionality of the infected cells evolves when they differentiate. We examined how the level of intracellular IH11128 bacteria evolves after undifferentiated infected Caco-2/TC7 cells were subsequently cultured over a long time period postinfection. For this purpose, the Caco-2/TC7 cells at day 6 in culture were infected by IH11128 bacteria for 3 h as described above. Then the infected cells were incubated with gentamicin to kill the extracellular adhering bacteria. During the subculture of infected cells following gentamicin assay, the medium was replaced by tissue culture medium containing antibiotic to prevent further replication of extracellular bacteria. Viable intracellular bacteria were determined at 1, 3, 5, 7, and 9 days postinfection, corresponding to days 7, 9, 11, 13, and 15 in cell culture, respectively. As shown in Table 4, no multiplication of intracellular IH11128 bacteria was found. In contrast, the number of viable bacteria residing intracellularly decreased regularly postinfection. However, a stable level of intracellular bacteria appeared at day 5 postinfection and thereafter.
TABLE 4.
Survival of IH11128 bacteria residing within Caco-2/TC7 cells as a function of days in culture postinfectiona
| Day in culture | Viable intracellular bacteriab |
|---|---|
| 6 | 3.23 × 104 ± 0.55 × 104 (100) |
| 7 | 2.34 × 104 ± 0.35 × 104 (72) |
| 9 | 1.77 × 104 ± 0.30 × 104 (55)* |
| 11 | 1.20 × 104 ± 0.25 × 104 (37)* |
| 13 | 1.14 × 104 ± 0.18 × 104 (35)* |
| 15 | 1.24 × 104 ± 0.15 × 104 (38)* |
The cells at day 6 in culture were infected with IH11128 bacteria for 3 h at a concentration of 108 CFU/ml. The infected cells were washed three times with PBS and then incubated with gentamicin (75 μg/ml) for 1 h. The infected cells were subsequently cultured in the presence of cell culture medium containing gentamicin (50 μg/ml), and the medium was changed daily to prevent extracellular replication of the remaining viable extracellular bacteria. The number of intracellular bacteria was determined as a function of the day postinfection at days 1, 3, 5, 6, and 9, corresponding to days 7, 9, 11, 13, and 15 in cell culture, respectively.
Results are expressed as mean numbers of CFU/ml ± SEM. The values in parentheses are the percentages of remaining viable intracellular bacteria compared to bacteria that invaded the cells at day 6 in culture (100%). An asterisk represents a P value of <0.01 compared with intracellular bacteria at day 6 in culture.
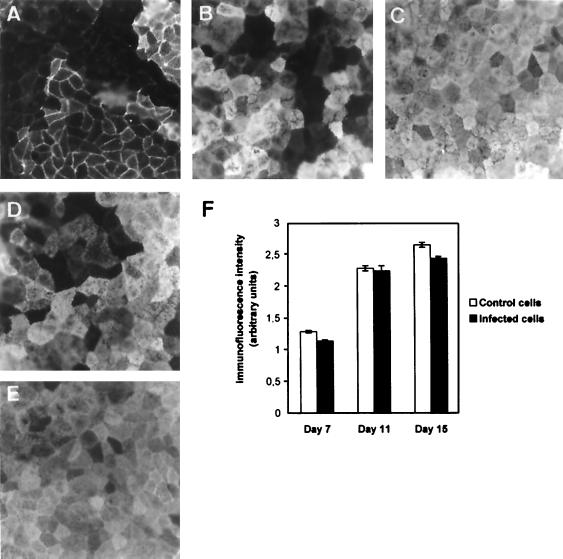
Of interest is the observation that a stable and significant level of viable intracellular IH11128 bacteria remained present during the period of culture required for complete differentiation of Caco-2/TC7 cells, which thus acquired the functionality of mature enterocytes of the small intestine (for a review, see reference 40). To examine how the functionality of the IH11128-infected Caco-2/TC7 cells evolved during this period of culture, we chose to examine the expression of the brush border-associated differentiation marker hydrolase SI. Expression of SI was measured by immunolabeling cells with a specific antibody. As shown in Fig. 7, the number of SI-positive cells in the control increased as a function of the days in culture. Once the cells reached confluency, the number of cells expressing SI increased (Fig. 7A and B), and at subconfluency (Fig. 7C), 98% ± 3% of the cells displayed the typical mosaic pattern characteristic of SI expression in fully differentiated intestinal cells (6, 10, 37, 56). In the IH11128-infected cells observed at days 9 and 15 in culture (days 3 and 9 postinfection) (Fig. 7D and E), the cells displayed the same SI mosaic pattern as that observed in uninfected cells (Fig. 7B and C, respectively). This result demonstrates that the expression of SI in cells which contained intracellular, viable IH11128 bacteria develops normally, as in uninfected cells (Fig. 7F). To ascertain that SI was functional, SI enzyme activity was measured. No difference was found between uninfected control cells and IH11128-infected cells at day 15 in culture (day 9 postinfection) (134 ± 17 and 129 ± 14 mU of protein per mg, respectively).
FIG. 7.
Immunofluorescence labeling of SI in infected undifferentiated human intestinal Caco-2/TC7 cells subsequently cultured postinfection for observation of cell differentiation. Confluent undifferentiated cells at day 6 in culture were infected with IH11128 bacteria for 3 h at a concentration of 108 CFU/ml. The cells were then washed three times with sterile PBS and subjected to gentamicin (100 μg/ml) to kill the extracellular bacteria. Following gentamicin assay, the infected cells were further subcultured in the presence of tissue culture medium containing gentamicin (50 μg/ml). At the days indicated, cells were processed for indirect immunofluorescence labeling of SI with specific antibody as described in Materials and Methods. Expression of SI was observed at days 7, 9, 11, and 15 in culture, corresponding to days 1, 3, 5, and 9 postinfection, respectively. (A to C) Evolution of the SI expression in control uninfected cells cultured in the presence of gentamicin (50 μg/ml) at days 7, 9, and 15, respectively. (D and E) Cells infected with IH11128 bacteria at day 9 (D) and day 15 (E) in culture. Magnification for panels A to E, ×40. (F) The relative immunofluorescence intensity of SI was measured by conventional epifluorescence microscopy coupled with an image analyzer. No change in immunofluorescence intensity was observed between uninfected control cells and IH11128-infected cells, whatever the day in culture examined.
DISCUSSION
Previous reports (5, 6, 25–29, 35–37, 56, 57, 63) have demonstrated that Afa/Dr DAEC cells are strong colonizers of epithelial cells. This efficiency results from a high expression of the adhesin receptors, i.e., the GPI-anchored CD55 and CD66e molecules, at the surface of the epithelial cells. The results presented here show that the Afa/Dr DAEC are poorly invasive bacteria compared to EIEC and Salmonella spp. (for a review, see reference 21). Indeed, its appears that only a subpopulation of the adhering bacteria enters the cells. To enter undifferentiated epithelial cells, adhering Afa/Dr DAEC cells promote plasma membrane projections that engulf the adhering bacteria (15, 25–28). It has been previously established that Afa/Dr adhesins bind to SCR 2 and SCR3 of the GPI-anchored receptor, CD55 (48). SCR3 of CD55 is essential in CD55 functions (16), including signaling (47). Selvarangan et al. (63) recently observed that a MAb blocking SCR3 and the adhesin-dependent interaction of Dr+ E. coli with CD55 inhibited cell entry by bacteria, whereas a MAb blocking SCR2 did not. They hypothesized that the sole binding onto SCR3 of CD55 triggers cell entry by the bacteria. The results presented here are in favor of another mechanism for promoting the entry of Afa/Dr DAEC into epithelial cells, independently of Afa/Dr adhesin recognition of the GPI-anchored proteins CD55 and CD66e as receptors. However, we cannot exclude the fact that Afa/Dr DAEC may use another mechanism to enter unpolarized undifferentiated epithelial cells such as the CD55-dependent mechanism recently reported by Selvarangan et al. in CHO cells (63).
MFs have been demonstrated to be involved in bacterial entry into epithelial cells by a triggered phagocytosis via macropinosomes (for reviews, see references 21 and 34). Moreover, it has been recently reported that internalization into host cells of several bacterial pathogens requires MFs and MTs together (52). A few bacterial pathogens have been found to specifically use the MT-dependent internalization pathway alone (for a review, see reference 52). For example, several Campylobacter jejuni strains in human intestinal embryonic INT407 cells initiate an MT-dependent endocytic process, which results in their uptake into endosomal vacuoles (51). To enter into McCoy cells, Chlamydia trachomatis utilizes the MT network (12). The cell entry process of Afa/Dr DAEC IH11128 is the third example of a process of bacterial internalization which involves an MF-independent and MT-dependent mechanism. There are two distinct classes of MTs in epithelial cells, the stable MTs and the dynamic unstable MTs (14). They are possibly involved in specialized intracellular transport steps of vesicular carriers and participate, in consequence, in their selective delivery into specific membranous domains of the cells. Functional specialization may be in relation to the specific association with the MT-associated molecular motors, as kinesin or dynein were involved in basolateral or apical transport, respectively. In order to gain new insights into the MT-dependent mechanism by which IH11128 bacteria enter epithelial cells, we have reexamined this phenomenon by the use of a new tool recently used with WIF-B-polarized hepatic cells to investigate the functionality of MTs (61). The compound, 201-F, a magnesium salt of the sesquiterpene-quinone ilimaquinone isolated from the marine sponge Smenospongia sp., specifically disrupts the highly dynamic MTs without affecting the function of stable MTs. Using 201-F, we observed here for the first time that the entry of IH11128 bacteria is dependent on dynamically unstable MTs. Two models of MT-dependent internalization of bacteria have been recently proposed (for a review, see reference 52). Discrimination between these two models is based on experiments conducted with taxol, a molecule which freezes the MT network. Indeed, it has been demonstrated that taxol blocks the MT-dependent, molecular motor-independent pathway, whereas it does not block the MT-dependent, molecular motor-dependent pathway. Interestingly, experiments conducted with taxol indicated that IH11128 bacteria use an MT-dependent pathway involving a molecular motor molecule, since taxol failed to block IH11128 entry. Both MT-dependent C. jejuni (31) and C. trachomatis (12) entry into epithelial cells involved the MT-associated molecular motor dynein.
It has been observed that MT-dependent entry of C. jejuni 81–176 into INT407 cells involves coated-pit formation, since entry was abolished by g-strophantin or monodansylcadaverine treatment (51). Our results demonstrate for the first time that IH11128 bacteria enter epithelial cells by a mechanism involving caveolae. Along with the lipid rafts (9), these cholesterol-glycosphingolipid-rich microdomains have recently attracted much attention. Caveolae or caveola-like domains are known to be enriched in cholesterol, glycolipids, and GPI-anchored proteins (for a review, see reference 2). They are associated with cellular functions that include potocytosis, non-clathrin-dependent transcytosis, endocytosis, calcium regulation, and signal transduction (for reviews, see references 2 and 65). Interestingly, it has been reported that caveolae could serve as concentration devices, allowing internalization of toxins and bacteria. For example, the FimH-mediated internalization of E. coli involves the GPI-anchored CD48 receptor and lipid-rich microdomains containing caveolin (3, 42, 66). Caveolae were found to be involved in the uptake of respiratory syncytial virus antigen by dendritic cells (73). Observations that Afa/Dr DAEC IH11128 bacteria utilize caveolae to enter epithelial cells indicate that the process of IH11128 entry could follow a specific signalling. However, it remains to be determined what the signalling pathway associated with caveolae (which is activated by IH11128 bacteria) is. Moreover, caveola-dependent endocytosis has been found to be dependent on the actin cytoskeleton (17, 55). Our results, showing that internalization of IH11128 bacteria involving caveolae is dependent on the dynamic unstable MTs, is of interest, since it represents the first example revealing that a caveola-dependent process is MT dependent.
When we examined the process of strain IH11128 entry into Caco-2/TC7 as a function of cell differentiation, our results showed that entry of the bacterium was down-regulated when the cells reached confluency and were differentiated. This cell entry process resembles several characteristics previously observed for Yersinia spp. (13), enteropathogenic E. coli (22), and Listeria monocytogenes (23) entry into cultured human intestinal Caco-2 cells. Indeed, invasion by these pathogens occurs in proliferating undifferentiated cells, confluency of the cells promotes a decrease in cell entry, and no invasion occurs when the cells are at late postconfluency. This phenomenon is in close relation to the transition process from undifferentiated to differentiated cells, in which the pivotal change in cell organization is the establishment of polarization which delineates specific cell domains (for reviews, see references 18 and 40). It has been previously observed that cell-to-cell adhesion molecule α1β1, α5β1, and α6β1 integrins were found to accumulate as small aggregates in proliferating cells for spreading (68). Moreover, in Madin-Darby canine kidney (53) and Caco-2 cells (69), integrins redistributed during cell culture to localize at the cell-to-cell contact and at the basal domain of the cells when they reached late confluency. The invasion process of host cells by Yersinia spp. (33), Streptococcus pyogenes (54), and Staphylococcus aureus (67) required the cell-to-cell adhesion molecule integrins. A zipper mechanism allows the invasin-dependent entry of Yersinia spp. (33, 34). Uropathogenic E. coli expressing the type 1 pilus adhesin FimH internalized by a zipper-like mechanism involves activation of phosphoinositide 3-kinase and host protein tyrosine phosphorylation, accompanied by host cytoskeleton-associated protein reorganization (42). Interestingly, Jouve et al. (35) have previously observed that cell entry by Afa/Dr DAEC strain Afa-III involves a zipper-like mechanism. Moreover, it was of interest that several of the bacterial species inducing MT-dependent internalization mechanisms in host cells used the β1 integrins as internalization receptors (for a review, see reference 52). The α5β1 integrin has been found to be highly expressed at the surface of proliferating epithelial cells, such as undifferentiated Caco-2 cells (13, 69). Consistent with this, we identified the α5β1 integrin as the cell-cell adhesion molecule that plays a pivotal role in the entry of IH11128 into epithelial cells. However, our results show that only a subpopulation of the adhering bacteria enters epithelial proliferating cells. The question is how can we reconcile this paradoxical situation? One hypothesis is that a particular conformation of the caveolae within the cell membrane that allows signal transduction could be required to allow IH11128 cell entry. Indeed, the caveola-associated signaling proteins display a particular insertion into the host cell membrane (for a review, see reference 2). It has been observed that these proteins can move into the membrane to concentrate within lipid-enriched platforms and/or microdomains. Certain β1 integrins have been shown to interact with caveolin (for a review, see reference 60), the main structural component of caveolae. For example, association of the GPI-anchored urokinase receptor (u-PAR [also known as CD87]) with β1 integrins into caveolae has been reported to organize within kinase-rich lipid domains, promoting efficient integrin-mediated signaling (11, 72). Whether IH11128 cell entry required a situation in which caveolae associated with α5β1 integrin remains to be examined.
The results presented here indicated that the dra gene plays a role in the polarized entry of IH11128 bacteria. However, the bacterial factor expressed by the Afa/Dr DAEC strain IH11128, which recognizes the α5β1 integrin, remains to be identified. The AfaD protein in the Afa/Dr DAEC strain expressing the Afa-III adhesin functions as an invasin without recognizing CD55 as a receptor (25, 35). It remains to be determined whether or not the DraD protein, an analog of AfaD, functions as an invasin.
Epidemiological studies demonstrated that Afa/Dr DAEC strains are present in pregnant women with urinary tract infections and in infants with diarrhea (for a review, see reference 46). Cell entry by Afa/Dr DAEC could benefit pathogens, since it represent a strategy to evade host defenses and to avoid antibiotic treatments, thus allowing the pathogens to persist within the epithelium. Observation that a subpopulation of adhering Afa/Dr DAEC bacteria remains viable within polarized epithelial cells has clinical significance, since the bacterium forms a reservoir sufficient to reinfect the host cells, leading to persistent or chronic infections. For uropathogenic Afa/Dr DAEC, it has been observed that a twofold-increased risk of a second episode of urinary tract infection exists in patients in whom the first episode is due to E. coli-expressing Afa/Dr adhesins. Our results bring a putatively interesting insight into the mechanisms of chronic and persistent infections by Afa/Dr DAEC. Indeed, considering that the flow of urine in the urinary tract eliminates adhering pathogens, the entrance of bacteria into uroepithelial cells may allow these bacteria to evade this efficient physiological mechanism of clearance. In infants with protracted diarrhea, 50% of the bacterial isolates shared Afa/Dr adhesins. The results reported here indicate that the presence of intracellular viable IH11128 bacteria is not detrimental to the development of the functionality of cultured human intestinal cells. However, the observation of entry into the undifferentiated cells could be important in terms of the causes of persistent diarrhea. Indeed, in physiological situations cell renewal along the crypt-villus axis involves the release of differentiated cells following the development of an apoptotic process (for a review, see reference 40). Extrapolation of our results to these physiological situations leads us to tentatively speculate that at the end of the differentiation process, exfoliation of cells containing intracellular viable bacteria would allow the release of infecting bacteria into the lumen. Moreover, our results raise the question of the entry of diarrheagenic Afa/Dr DAEC within cells apically expressing the integrins and lining the intestinal epithelium. Such cells are the M cells previously shown to be involved in invasion by enteropathogens (70).
ACKNOWLEDGMENTS
We thank G. Delrue (INSERM SC6) for his skills in producing the art drawings. We thank M. Métioui for critical reading of the manuscript.
J. Guignot is supported by a doctoral fellowship from the Ministère de I'Education Nationale, de la Recherche et de la Technologie (MENRT). L. Plançon is a recipient of an ATER fellowship (Paris XI University) from MENRT. A. L. Servin is supported for this work by a grant from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (PRFMMIP—MENRT). C. Le Bouguenec is supported by a grant from the PRFMMIP—MENRT.
REFERENCES
- 1.Agrez M V, Shafren D R, Gu X, Cox K, Sheppard D, Barry R D. Integrin alpha v beta 6 enhances coxsackievirus B1 lytic infection of human colon cancer cells. Virology. 1997;8:71–77. doi: 10.1006/viro.1997.8831. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R G W. The caveola membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 3.Baorto D M, Gao Z, Malaviya R, Dustin M L, van de Merwe A, Lublin D M, Abraham S N. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin P, Federman M, Wanke C A. Characterization of an invasive phenotype associated with enteroaggregative Escherichia coli. Infect Immun. 1995;63:3417–3421. doi: 10.1128/iai.63.9.3417-3421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernet-Camard M-F, Coconnier M-H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1918–1928. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernet-Camard M-F, Coconnier M-H, Hudault S, Servin A L. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38:248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudeau J, Glasser A-L, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499–4509. doi: 10.1128/iai.67.9.4499-4509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown D A, London E. Functions of lipids rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 10.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Zweibaum A, Rousset M. Differential expression of sucrase isomaltase in clones isolated from early and late passages of the cell line Caco-2 evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 11.Chapman H A, Wei Y, Simon D I, Walz D A. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemostasis. 1999;82:291–297. [PubMed] [Google Scholar]
- 12.Clausen J D, Christiansen G, Holst H U, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- 13.Coconnier M-H, Bernet M F, Servin A L. How intestinal epithelial cell differentiation inhibits the cell-entry of Yersinia pseudotuberculosis in colon carcinoma Caco-2 cell line in culture. Differentiation. 1994;58:87–94. doi: 10.1046/j.1432-0436.1994.5810087.x. [DOI] [PubMed] [Google Scholar]
- 14.Cole N B, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- 15.Cookson S T, Nataro J P. Characterization of Hep-2 cell projection formation induced by diffusely adherent Escherichia coli. Microb Pathog. 1996;21:421–434. doi: 10.1006/mpat.1996.0073. [DOI] [PubMed] [Google Scholar]
- 16.Coyne K E, Hall S E, Thompson E S, Acre M A, Kinoshita T, Fujita T, Anstee D J, Rosse W, Lublin D M. Mapping epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906–2913. [PubMed] [Google Scholar]
- 17.Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denker B M, Nigam S K. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 19.Elsinghorst E A, Kopecko D J. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esclatine A, Lemullois M, Servin A L, Quéro A-M, Geniteau-Legendre M. Human cytomegalovirus infects Caco-2 intestinal cells basolaterally regardless of the differentiation state. J Virol. 2000;74:513–517. doi: 10.1128/jvi.74.1.513-517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microb Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabastou J M, Kernéis S, Bernet-Camard M F, Barbat A, Coconnier M H, Kaper J B, Servin A L. Two stages of enteropathogenic Escherichia coli intestinal pathogenicity are up- and down-regulated by the epithelial cell differentiation. Differentiation. 1995;59:127–134. doi: 10.1046/j.1432-0436.1995.5920127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaillard J-L, Finlay B. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1997;64:1299–1308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia M, Mirre C, Quaroni A, Reggio H, Le Bivic A L. GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J Cell Sci. 1993;104:1281–1290. doi: 10.1242/jcs.104.4.1281. [DOI] [PubMed] [Google Scholar]
- 25.Garcia M I, Gounon P, Courcoux P, Labigne A, Le Bouguenec C. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol Microbiol. 1996;19:683–693. doi: 10.1046/j.1365-2958.1996.394935.x. [DOI] [PubMed] [Google Scholar]
- 26.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki B J. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 27.Goluszko P, Selvarangan R, Popov V, Pham T, Wen J W, Singhal J. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect Immun. 1999;67:3989–3997. doi: 10.1128/iai.67.8.3989-3997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gounon P, Jouve M, Le Bouguenec C. Immunocytochemistry of the AfaE adhesin and AfaD invasin produced by pathogenic Escherichia coli strains during interaction of the bacteria with HeLa cells by high resolution scanning electron microscopy. Microbes Infect. 2000;4:1–7. doi: 10.1016/s1286-4579(00)00331-2. [DOI] [PubMed] [Google Scholar]
- 29.Guignot J, Peiffer I, Bernet-Camard M-F, Lublin D M, Carnoy C, Moseley S L, Servin A L. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect Immun. 2000;68:3554–3563. doi: 10.1128/iai.68.6.3554-3563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauck C R, Meyer T F, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L, Kopecko D J. Campylobacter jejuni 81–176 associated with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IIangumaran S, Hoessli D C. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isberg R R, Leong J M. Multiple beta1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 34.Isberg R R, Van Nhieu G T. Two mammalian cell internalization strategies used by pathogenic bacteria. Annu Rev Genet. 1994;28:395–422. doi: 10.1146/annurev.ge.28.120194.002143. [DOI] [PubMed] [Google Scholar]
- 35.Jouve M, Garcia M I, Courcoux P, Labigne A, Gounon P, Le Bouguenec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kernéis S, Bernet M F, Gabastou J M, Coconnier M H, Nowicki B J, Servin A L. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol Lett. 1994;119:27–32. doi: 10.1111/j.1574-6968.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 37.Kernéis S, Bilge S, Fourel V, Chauvière G, Coconnier M H, Servin A L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli (DAEC) during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991;59:4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labigne-Roussel A, Schmidt M A, Waltz W, Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985;162:1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Bouguenec C, Garcia M I, Ouin V, Desperrier J M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louvard D, Kedinger M, Hauri H P. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- 41.Marlovits T C, Abrahamsberg C, Blaas D. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J Virol. 1998;72:10246–10250. doi: 10.1128/jvi.72.12.10246-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez J J, Mulvey M A, Schilling J D, Pinkner J S, Hultgren S J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merien F, Baranton G, Perolat P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect Immun. 1997;65:729–738. doi: 10.1128/iai.65.2.729-738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merz A J, So M. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect Immun. 1997;65:4341–4349. doi: 10.1128/iai.65.10.4341-4349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:403–503. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson-Weller A, Wang C. Structure and function of decay-accelerating factor CD55. J Lab Clin Med. 1994;123:485–491. [PubMed] [Google Scholar]
- 48.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oelschlaeger T A, Barrett T J, Kopecko D J. Some structures and processes of human epithelial cells involved in uptake of enterohemoragic Escherichia coli O157:H7 strains. Infect Immun. 1994;62:5142–5150. doi: 10.1128/iai.62.11.5142-5150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanism triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oelschlaeger T A, Kopecko D J. Microtubule dependent invasion pathways of bacteria. In: Oelschlaeger T A, Hacker J H, editors. Subcellular biochemistry, bacterial invasion into eukaryotic cells. Vol. 33. New York, N.Y: Kluwer Academic/Plenum Publishers; 2000. pp. 3–19. [DOI] [PubMed] [Google Scholar]
- 53.Ojakian G K, Schwimmer R. Regulation of epithelial cell surface polarity reversal by β1 integrins. J Cell Sci. 1994;107:561–576. [PubMed] [Google Scholar]
- 54.Ozeri V, Rosenshine I, Mosher D F, Fassler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 55.Parton R G, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peiffer I, Guignot J, Barbat A, Carnoy C, Moseley S L, Nowicki B J, Servin A L, Bernet-Camard M F. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect Immun. 2000;68:5979–5990. doi: 10.1128/iai.68.10.5979-5990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peiffer I, Servin A L, Bernet-Camard M-F. Piracy of decay-accelerating factor (DAF-CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;66:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin D M, Nowicki B J. Dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 60.Portner J C, Hogg N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- 61.Poüs C, Chabin K, Drechou A, Barbot L, Phung-Koskas T, Settegrana C, Bourguet-Kondracki M L, Maurice M, Cassio D, Guyot M, Durand G. Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells. J Cell Biol. 1998;142:153–165. doi: 10.1083/jcb.142.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnitzer J E, Oh P, Pinney E, Allard J. Filipin-sensitive caveola mediated transport in endothelium: reduced transcytosis, scavenger endocytosis and capillary permeability of selected macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selvarangan R, Goluszko P, Popov V, Singhal J, Pham T, Lublin D M, Nowicki S, Nowicki B. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect Immun. 2000;68:1391–1399. doi: 10.1128/iai.68.3.1391-1399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafren D R, Dorathy D J, Ingham R C, Burnd G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaul P W, Anderson R G. Role of plasmalemmal caveolin in signal transduction. Am J Physiol. 1998;275:843–851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 66.Shin J-S, Gao Z, Abraham S N. Involvement of cellular caveolae in bacterial entry into mast cells. Nature. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- 67.Sinha B, François P P, Nüße O, Foti M, Hartford O M, Vaudaux P, Forster T J, Lew D P, Herrmann M, Krause K-H. Fibronectin-binding protein acts as a Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 68.Tawil N, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vachon P H, Durand J, Beaulieu J-F. Basement membrane formation and re-distribution of the β1 integrins in a human intestinal co-culture system. Anat Rec. 1993;236:567–576. doi: 10.1002/ar.1092350409. [DOI] [PubMed] [Google Scholar]
- 70.Vazquez-Torres A, Fang F C. Cellular routes of invasion by enteropathogens. Curr Opin Microbiol. 2000;3:54–59. doi: 10.1016/s1369-5274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- 71.Virji M, Evans D, Hadfield A, Grunert F, Teixeira A M, Watt S M. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34:538–551. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- 72.Wei Y, Yang X, Liu Q, Wilkins J A, Chapman H A. A role for caveolin and urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285–1294. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werling D, Hope J C, Chaplin P, Collins R A, Taylor G, Howard C J. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–58. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]