Abstract
Anti-melanoma differentiation-associated protein 5 (MDA5) antibody-positive dermatomyositis (MDA5-DM) is frequently complicated with rapidly progressive-interstitial lung disease (RP-ILD). The prognosis of MDA5-DM with RP-ILD is mostly poor despite intensive treatment with a combination of high-dose glucocorticoids and single conventional immunosuppressants. It was reported that the triple therapy (high-dose glucocorticoids, cyclophosphamide and tacrolimus) was more effective than a combination of high-dose glucocorticoids and stepwise addition of immunosuppressants. In addition, the efficacy of tofacitinib 10 mg/day for MDA5-DM with RP-ILD refractory to the triple therapy was suggested. However, the effect of those therapies was evaluated only in comparison to the historical control. Moreover, more importantly, there are still refractory patients even if treated with those therapies. In this case series, we report six MDA5-DM cases with RP-ILD in which the dose of tofacitinib was increased from 10 mg to 20 mg/day due to poor response to the triple therapy, followed by tofacitinib 10 mg/day. Four of six patients improved after dose escalation of tofacitinib, while two non-responders died. All six patients developed at least one infection including five cases of cytomegalovirus reactivation, one pulmonary aspergillosis, one herpes zoster and one herpes simplex keratitis. These cases suggest that the dose escalation of tofacitinib can be an option for MDA5-DM patients refractory to 10 mg/day of tofacitinib and other immunosuppressants although the risk of infection is a concern. The risk–benefit balance of the dose escalation of tofacitinib should be carefully assessed in each case.
Keywords: Dermatomyositis, Therapeutics, Cytokines
WHAT IS ALREADY KNOWN ON THIS TOPIC
There are still refractory patients with anti-melanoma differentiation-associated protein 5 antibody-positive dermatomyositis (MDA5-DM) with rapidly progressive-interstitial lung disease (RP-ILD) even if treated with triple therapy (high-dose glucocorticoids, cyclophosphamide and tacrolimus) and tofacitinib 10 mg/day.
WHAT THIS STUDY ADDS
We report the largest series of patients with MDA5-DM with RP-ILD treated with tofacitinib 20 mg/day due to inadequate response to previous treatment including triple therapy (high-dose glucocorticoids, cyclophosphamide and tacrolimus) and tofacitinib 10 mg/day.
Four of six patients improved after the dose escalation of tofacitinib.
Although all patients developed at least one infection, every infection was controllable.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Dose escalation of tofacitinib could be a treatment option for refractory MDA5-DM, considering a risk–benefit balance.
Introduction
Dermatomyositis is an idiopathic inflammatory myopathy that can affect the skin, muscles, joints and lungs. Clinical phenotypes of dermatomyositis differ according to myositis-specific antibodies. Anti-melanoma differentiation-associated protein 5 (MDA5) antibody-positive dermatomyositis (MDA5-DM) is characterised by typical skin lesions. Asian MDA5-DM patients often lack muscle symptoms and frequently present with rapidly progressive-interstitial lung disease (RP-ILD), while non-Asian MDA5-DM patients have more frequent myositis and less frequent RP-ILD than Asian MDA5-DM.1–4 The prognosis of MDA5-DM patients with RP-ILD is poorer than those without RP-ILD despite conventional immunosuppressive treatment.4 Tsuji et al reported that the early use of the triple therapy (high-dose glucocorticoids, cyclophosphamide and calcineurin inhibitor) was more effective than the combination of high-dose glucocorticoids and stepwise addition of immunosuppressants.5 Recently, the efficacy of tofacitinib 10 mg/day for MDA5-DM patients refractory to the triple therapy has also been reported.6–9 However, there are still refractory patients even if treated with those therapies.
Here, we report six cases of MDA5-DM Japanese patients who received an increased dose of tofacitinib of 20 mg/day because of poor response to the triple therapy and tofacitinib 10 mg/day. All surviving patients gave their informed consent before their inclusion in this report.
Case presentation
Six MDA5-DM patients were identified and were observed from March 2020 to August 2022. The mean age at diagnosis was 55.5 years old, and three of six cases were females (table 1 and online supplemental figure 1). All patients fulfilled Bohan and Peter’s criteria10 and/or Sontheimer’s criteria11 for clinically amyopathic dermatomyositis and presented with RP-ILD. Anti-MDA5 antibody titres at diagnosis were markedly elevated in all patients, and ferritin and Krebs von den Lungen-6 (KL-6) levels were also high in most cases. Before increasing the dose of tofacitinib, all patients received the triple therapy (high-dose glucocorticoids, cyclophosphamide and tacrolimus) and additional tofacitinib 10 mg/day. In addition, four of six patients underwent plasma exchange.
Table 1.
Patient’s characteristics at baseline and clinical course
| Case number | 1 | 2 | 3 | 4 | 5 | 6 |
| Age/Sex | 71/F | 44/M | 58/M | 47/M | 47/F | 66/F |
| Observation period | 48 days | 16 months | 43 days | 19 months | 12 months | 8 months |
| Clinical involvement at baseline* | ||||||
| Skin | + | + | + | + | + | + |
| Muscle | + | + | – | – | + | – |
| Joint | – | – | – | + | + | – |
| Lung (RP-ILD) | + | + | + | + | + | + |
| Laboratory data (baseline*/at the time of TOF dose-escalation/Last observation) | ||||||
| LDH (124–222 (U/L)) | 393/288/777 | 481/293/265 | 622/338/571 | 338/320/195 | 269/342/199 | 309/486/270 |
| CRP (0.00–0.14 (mg/dL)) | 0.19/5.32/4.03 | 2.09/0.03/0.02 | 6.05/3.91/3.28 | 0.64/0.05/0.01 | 1.61/0.01/0.01 | 0.75/0.32/0.01 |
| Lymphocyte (825–3870 (/μL)) | 186/134/105 | 680/529/1348 | 427/312/109 | 1145/117/570 | 266/93/231 | 1165/243/1411 |
| KL-6 (<500 (U/mL)) | 802/344/1370 | 416/564/526 | 1360/573/3242 | 685/762/399 | 434/1218/486 | 1045/1337/923 |
| Ferritin (12.0–60.0 (ng/dL)) | 1289/493/622 | 960/2959/134 | 1185/1592/1643 | 852/295/20 | 67/275/67 | 791/943/55 |
| MDA5 Ab (<32 (Index))† | 2050/300/70 | 2200/2200/31 | 3700/950/300 | 6650/900/5 | 3650/2700/21 | 3150/1350/30 |
| P/F ratio (mm Hg) | 350/220/64 | NA/160/NA | 76/89/44 | 384/341/414 | NA/NA/NA | NA/NA/NA |
| Oxygen demand | Room air/mechanical ventilation/mechanical ventilation | Nasal cannula/nasal high flow therapy/room air | Reservoir mask/mechanical ventilation/mechanical ventilation | Nasal cannula/nasal cannula/nasal cannula | Room air/room air/room air | Room air/room air/room air |
| No of infiltrated lung fields‡ | 4/6/6 | 5/5/0 | 6/6/6 | 6/6/0 | 5/5/0 | 4/4/0 |
| Treatment before escalation of the dose of TOF | GC, CY, TAC, TOF§, PE | GC, CY, TAC, TOF§, PE | GC, CY, TAC, TOF§, PE | GC, CY, TAC, TOF§, PE | GC, CY, TAC, TOF§ | GC, CY, TAC, TOF§ |
| Reasons for escalation of the dose of TOF | Worsening of dyspnoea, decreased P/F ratio, new GGO | Worsening of dyspnoea, decreased P/F ratio, new GGO | Worsening of dyspnoea, decreased P/F ratio, new GGO | Worsening of dyspnoea, decreased P/F ratio, new GGO | Worsening of dyspnoea and myalgia, elevated ferritin | Worsening of coughing and Gottron’s papule, elevated ferritin |
| Start of triple therapy* | Day 1 | Day 1 | Day 1 | Day 1 | Day 1 | Day 1 |
| Start of TOF 10 mg/day* | Day 14 | Day 6 | Day 10 | Day 34 | Day 11 | Day 19 |
| TOF escalation* | Day 34 | Day 15 | Day 17 | Day 63 | Day 35 | Day 45 |
| Clinical outcome | Dead (day 48, Respiratory failure due to RP-ILD) | Improved (dyspnoea, oxygen demanding, CT findings were improved) | Dead (Day 43, Respiratory failure due to RP-ILD) | Improved (dyspnoea, oxygen demanding, CT findings were improved) | Improved (dyspnoea, myalgia, ferritin were improved) | Improved (coughing, Gottron’s papule, ferritin were improved) |
| Discontinuation/reduction of TOF after improvement | NA | None | NA | Discontinued 16 months after escalation (due to infection) | None | Reduced to 10 mg/day 177 days after escalation (due to infection) |
| Infections | CMV reactivation | CMV reactivation, Herpes zoster | CMV reactivation | Herpes simplex keratitis | CMV reactivation (twice) | CMV reactivation (twice), Pulmonary aspergilloma |
*Baseline was defined as the date of starting immunosuppressive treatments for MDA5-DM (=day 1).
†Anti-MDA5 antibody was measured by the ELISA.
‡Numbers of infiltrated lung fields were scored as the extent of lung lesions. See the study of Kurasawa et al for detailed information.6
§The dose of TOF was 10 mg/day before escalation in all cases.
CMV, cytomegalovirus; CY, cyclophosphamide; F, female; GC, glucocorticoids; GGO, ground-grass opacity; KL-6, Krebs von den Lungen-6; M, male; MDA5, melanoma differentiation-associated protein 5; NA, not applicable; PE, plasma exchange; P/F ratio, PaO2/FiO2 ratio; RP-ILD, rapidly progressive interstitial lung disease; TAC, tacrolimus; TOF, tofacitinib.
rmdopen-2022-002795supp001.pdf (118.7KB, pdf)
The reasons for increasing the dose of tofacitinib to 20 mg/day were worsening respiratory involvement in five cases and deteriorating skin lesions combined with worsening cough and laboratory parameters in one case.
Clinical conditions and laboratory findings were improved in four cases after the escalation of the dose of tofacitinib, while two cases under mechanical ventilation at the time of the escalation died of worsening RP-ILD. All patients were administrated either cotrimoxazole or atovaquone for pneumocystis pneumonia prophylaxis. Cytomegalovirus reactivation was defined as the positivity of the cytomegalovirus antigenemia assay with or without clinical symptoms. Cytomegalovirus reactivation occurred in five cases, and two of five cases experienced the second episodes of cytomegalovirus reactivation after recovery from the first reactivation. Pulmonary aspergillosis and herpes zoster developed in one case each. One patient discontinued tofacitinib 16 months after the escalation of the dose of tofacitinib because of herpes simplex keratitis, and another patient reduced tofacitinib to 10 mg/day 5 months after the escalation because of pulmonary aspergillosis. We intended to taper and discontinue glucocorticoids at first, tacrolimus next and finally tofacitinib. None of the surviving cases relapsed during the observation period.
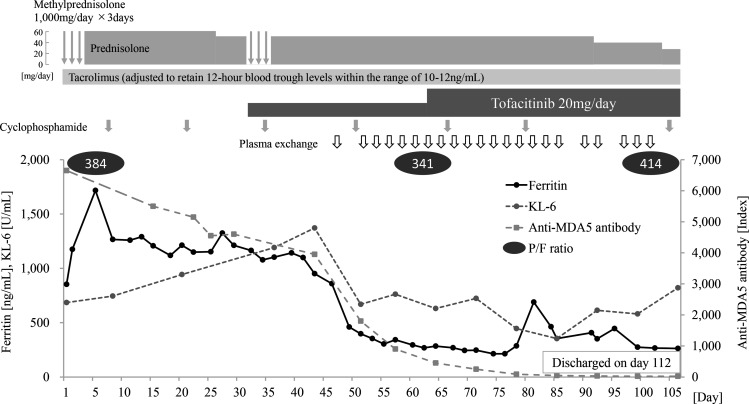
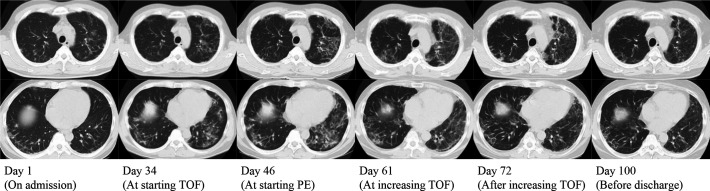
A clinical course of the representative case (case #4) is shown in figures 1 and 2. A 47-year-old man with no pre-existing medical conditions visited a local physician for a low-grade fever, cough and skin rash on his fingertips and eyelids. Chest X-ray showed trivial ground glass opacity at the base of the bilateral lungs. Dermatomyositis was suspected due to a distinctive skin rash, but watchful waiting was chosen because of the absence of muscle symptoms and normal myogenic enzyme levels. Two months later, his respiratory condition worsened rapidly, then he was referred to our hospital and urgently hospitalised the same day. He was diagnosed with clinically amyopathic dermatomyositis and associated interstitial lung disease based on the presence of specific skin lesions including Heliotrope’s rash, Gottron’s and inverse Gottron’s sign, and RP-ILD. His anti-MDA5 antibody was found to be positive later, and his diagnosis of MDA5-DM was confirmed. Although he was immediately treated with methylprednisolone pulse therapy, followed by the triple therapy (high-dose prednisolone, cyclophosphamide and tacrolimus), his respiratory condition still worsened. He commenced tofacitinib 10 mg/day in combination with another course of methylprednisolone pulse on day 34 and plasma exchange on day 46. His serum ferritin and KL-6 levels apparently decreased by exchanging his plasma, although proteins contained in plasma are generally removed by plasma exchange and decrease regardless of actual disease improvement. Chest CT findings on day 61 in bilateral lower lobes appeared to be slightly improved compared with the ones on day 46. However, despite those therapies, new ground-glass opacity (GGO) was still emerging in the bilateral upper lobes, accompanied by worsening dyspnoea and PaO2/FiO2 ratio. Therefore, we judged that the disease activity as a whole was uncontrolled and decided to increase the dose of tofacitinib from 10 mg/day to 20 mg/day on day 63. After the tofacitinib dose escalation, his respiratory status gradually improved, and GGO markedly decreased. The dose of glucocorticoids was successfully tapered, and he was discharged on day 112. Tofacitinib was discontinued due to herpes simplex keratitis at 16 months. During the 19-month observation period, this case had neither relapse nor severe infection.
Figure 1.
Clinical course of case #4. P/F ratio, PaO2/FiO2 ratio, mm Hg; KL-6, Krebs von den Lungen-6; MDA5, melanoma differentiation-associated protein 5.
Figure 2.
Changes in chest CT findings of case #4. PE, plasma exchange; TOF, tofacitinib.
Discussion
MDA5-DM is a rare autoimmune disease, and there is no randomised controlled trial regarding MDA5-DM due to its rarity and high mortality. Thus, there is no standard treatment for MDA5-DM at the moment, but some therapeutic strategies have been suggested. In a single-arm prospective study reported by Tsuji et al, patients who received the triple therapy including high-dose glucocorticoids, cyclophosphamide and calcineurin inhibitors showed a better survival rate than the plural historical controls who received stepwise addition of immunosuppressants.5 Currently, the triple therapy is widely used as first-line therapy for MDA5-DM in Japan. On the other hand, Kurasawa et al reported that four of five patients with MDA5-DM refractory to previous therapy including triple therapy improved by administration of tofacitinib 10 mg/day.6 Chen et al reported in their prospective study that the survival rate was better in patients treated with tofacitinib 10 mg/day than in the historical controls without tofacitinib.12 Regarding tofacitinib 10 mg/day, several case reports suggest its efficacy for MDA5-DM.7–9 13 However, even with additional tofacitinib 10 mg/day, there are still refractory cases with worsening RP-ILD. A new option for treatment is required.
Previous clinical trials suggested the dose-dependent efficacy of tofacitinib in patients with ulcerative colitis14 and rheumatoid arthritis.15 Based on the result demonstrating a higher remission rate in the 20 mg/day group than in the 10 mg/day group, 20 mg/day of tofacitinib was approved for ulcerative colitis. To the best of our knowledge, this is the first case series of MDA5-DM patients who were refractory to 10 mg/day of tofacitinib and other immunosuppressants and treated with tofacitinib 20 mg/day apart from a single-case report by Ohmura et al.16 The case reported by Ohmura et al was a 55-year-old man who had MDA5-DM with RP-ILD. After he did not respond to the triple therapy followed by tofacitinib 10 mg/day, dose-escalation of tofacitinib to 20 mg/day was decided and improved his disease. In our cases, four of six patients also improved clinically after tofacitinib dose escalation from 10 mg/day to 20 mg/day, which was consistent with previous data showing dose-dependent efficacy in ulcerative colitis and rheumatoid arthritis.14 15
Our experience suggests that 20 mg/day of tofacitinib could be a useful treatment option for MDA5-DM patients with poor response to intensive therapies including 10 mg/day of tofacitinib. On the other hand, attention should be given to an increased infection risk when using 20 mg/day of tofacitinib. While a similar safety profile of tofacitinib 20 mg/day compared with 10 mg/day was reported in patients with ulcerative colitis,14 17 tofacitinib showed a dose-dependent risk of total mortality, venous thromboembolism and infection in rheumatoid arthritis.18 19 As a result, tofacitinib 20 mg/day was approved for ulcerative colitis but not for rheumatoid arthritis. In addition to the above, concomitant use of high-dose glucocorticoids and multiple immunosuppressants is assumed when using 20 mg/day of tofacitinib for MDA5-DM patients. Indeed, five of our six cases developed cytomegalovirus infection including two cases of recurrent infection. Of the six patients, one herpes simplex keratitis, one pulmonary aspergillosis and one herpes zoster also occurred. All infections observed in our cohort responded well to treatments, and no cases developed serious outcomes.
Additionally, more caution is warranted regarding drug interactions with CYP3A4 inhibitors like tacrolimus and cyclosporine when the dose of tofacitinib is escalated. The trough levels of those immunosuppressants should be monitored to avoid side effects, especially when administered with 20 mg/day of tofacitinib.
Several studies have reported poor prognostic factors in MDA5-DM.4 20 In this case series, all six patients had multiple risk factors (RP-ILD, lymphopenia, elevated ferritin). Thus, our experience should imply that the dose escalation of tofacitinib was effective for refractory MDA5-DM patients even with multiple risk factors. On the other hand, two fatal cases were already under mechanical ventilation at the time of tofacitinib dose escalation. It might be difficult to save a case whose pulmonary involvement has already severely progressed, even with any intensive therapies including tofacitinib 20 mg/day.
Many limitations exist due to the nature of the case series; however, our experience suggests that dose escalation of tofacitinib could be an option for refractory MDA5-DM patients to 10 mg/day of tofacitinib and other intensive therapies like the triple therapy. An increased risk of infection is a concern, and a risk–benefit balance of the dose escalation should be carefully assessed in each case.
Acknowledgments
We thank all the staff for taking care of patients who were enrolled in this study.
Footnotes
Contributors: All authors have contributed to the submitted article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This manuscript is a retrospective case series that does not require ethics committee approval at our institution. Participants gave informed consent to participate in the study before taking part.
References
- 1.Nombel A, Fabien N, Coutant F. Dermatomyositis with Anti-MDA5 antibodies: Bioclinical features, pathogenesis and emerging therapies. Front Immunol 2021;12:773352. 10.3389/fimmu.2021.773352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res 2013;65:1316–24. 10.1002/acr.21985 [DOI] [PubMed] [Google Scholar]
- 3.Cavagna L, Meloni F, Meyer A, et al. Clinical spectrum time course in non-Asian patients positive for anti-MDA5 antibodies. Clin Exp Rheumatol 2022;40:274–83. 10.55563/clinexprheumatol/di1083 [DOI] [PubMed] [Google Scholar]
- 4.Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology 2020;95:e70–8. 10.1212/WNL.0000000000009727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol 2020;72:488–98. 10.1002/art.41105 [DOI] [PubMed] [Google Scholar]
- 6.Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology 2018;57:2114–9. 10.1093/rheumatology/key188 [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Ikeda K, Kageyama T, et al. Successful treatment for refractory interstitial lung disease and pneumomediastinum with multidisciplinary therapy including tofacitinib in a patient with Anti-MDA5 antibody-positive dermatomyositis. J Clin Rheumatol 2019. 10.1097/RHU.0000000000000984 [DOI] [PubMed] [Google Scholar]
- 8.Hama S, Akiyama M, Higashida-Konishi M, et al. Successful treatment with tofacitinib for relapse of rapidly progressive interstitial lung disease in anti-melanoma differentiation-associated gene 5 antibody-positive clinically Amyopathic dermatomyositis. Mod Rheumatol Case Rep 2022. doi: 10.1093/mrcr/rxac049. [Epub ahead of print: 10 Jun 2022]. [DOI] [PubMed] [Google Scholar]
- 9.Hosokawa Y, Oiwa H. A case of refractory interstitial lung disease in anti-MDA5-positive dermatomyositis that improved after switching to tofacitinib. J Clin Rheumatol 2021;27:S661–2. 10.1097/RHU.0000000000001645 [DOI] [PubMed] [Google Scholar]
- 10.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med Overseas Ed 1975;292:403–7. 10.1056/NEJM197502202920807 [DOI] [PubMed] [Google Scholar]
- 11.Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol 1999;11:475–82. 10.1097/00002281-199911000-00005 [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis-associated interstitial lung disease. N Engl J Med 2019;381:291–3. 10.1056/NEJMc1900045 [DOI] [PubMed] [Google Scholar]
- 13.Luo M, Chen L, He H, et al. Treatment of MDA5-positive dermatomyositis complicated by gangrenous cholecystitis with tofacitinib. Eur J Med Res 2022;27:68. 10.1186/s40001-022-00693-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012;367:616–24. 10.1056/NEJMoa1112168 [DOI] [PubMed] [Google Scholar]
- 15.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 16.Ohmura S-I, Yamabe T, Naniwa T. Successful dose escalation of tofacitinib for refractory dermatomyositis and interstitial lung disease with anti-melanoma differentiation-associated gene 5 antibodies. Mod Rheumatol Case Rep 2021;5:76–81. 10.1080/24725625.2020.1816674 [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022;55:464–78. 10.1111/apt.16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 19.Balanescu A-R, Citera G, Pascual-Ramos V, et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled oral surveillance trial. Ann Rheum Dis 2022;81:annrheumdis-2022-222405. 10.1136/ard-2022-222405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Liu Y, Cheng L, et al. Roles of biomarkers in anti-MDA5-positive dermatomyositis, associated interstitial lung disease, and rapidly progressive interstitial lung disease. J Clin Lab Anal 2022;36:e24726. 10.1002/jcla.24726 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002795supp001.pdf (118.7KB, pdf)