Abstract
We present the first case of a patient with indolent polyarteritis nodosa who suffered severe exacerbations following significant emotional stressors. This report highlights the close relationship between emotions and autoimmune diseases mediated by the deleterious effects of stressors presumptively by skewing immunity from Type 1 to Type 2.
Keywords: psychology, autoimmunity, vasculitis, stressors
Introduction
Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that affects medium-sized and small muscular arteries, which most authors consider an autoimmune disorder. Cutaneous PAN (CPAN) is a variant that targets mainly the skin.1,2 A large body of evidence supports the relationship between stress and autoimmunity, suggesting that stressors may trigger or exacerbate autoimmune diseases.3–5
Throughout evolution, animals, including humans, have developed a critical survival mechanism that allows them to deal with short-lived external stressors such as the presence of predators; this acute stress response is adaptive and immunologically protective with a duration of minutes to hours. In contrast, exposure to a stressor for a more extended period, depending on the individual makeup of the subject,6 may lead to a non-adaptive response, significant disruption of the immune system, dysregulation of the circadian cortisol rhythm, and downregulation of the glucocorticoid receptors.6–10
This report highlights a case of smoldering CPAN in which emotional stress appears to have triggered a devastating disease exacerbation.
Case Description
A 54-year-old woman with a long-standing history of well-controlled psoriatic arthritis on adalimumab and methotrexate presented with a five-month duration of a slowly progressive blanching livedo reticularis that started on bilateral dorsal feet and toes and extended to the calves (Figure 1). Her blood pressure was normal, like the rest of her physical examination. She denied paresthesia, pain, constitutional or other symptoms. Aside from a slightly elevated sedimentation rate (32mm/h), additional pertinent laboratory evaluations, including complete blood cell, comprehensive metabolic panel, LDH, β-2 microglobulin, rheumatoid factor, C3, C4, CH50, anti-CCP, ANCA, ANA, anti-SSA, anti-SSB, anti-SM, anti-RNP, anti-dsDNA, anti-cardiolipins, anti-β-2 glycoprotein1, lupus anticoagulant, ANCA, anti-MPO, anti-PR3, hepatitis B, hepatitis C, HIV, serum and urine light chain, urine protein immunofixation and complete urinalysis, were negative or within normal limits. The echocardiogram and CT angiography of the chest, abdomen, and pelvis were unremarkable. An incisional full-thickness skin biopsy of the right calf that included an unaffected center and adjacent reticulated area revealed a leukocytoclastic and necrotizing medium and small muscular artery vasculitis (Figure 2). The results were relayed to the patient, who immediately began researching PAN on the internet, becoming intensely distressed. Over the next two days, she developed excruciating pain and dysesthesia of her legs and feet, prompting her admission to the hospital, where her physical examination was notable for purple, cool, and numbed second to fifth digits of the right foot as well as livedo racemosa throughout the bilateral lower extremities and pinnae. Blood pressure remained normal, and there was no evidence of renal or systemic involvement. She was initiated on 3-day intravenous (IV) pulses of methylprednisolone followed by oral prednisone, pentoxifylline, nifedipine, aspirin, topical nitroglycerin, and enoxaparin. During her 28-day hospital course, the lesions on her ears resolved. Still, she developed worsening necrosis of the affected toes of her right foot (Figure 3) and marked improvement in pain and dysesthesia. She was initiated on six bi-weekly IV pulses of cyclophosphamide, IV rituximab every six months, and oral prednisone tapering, which continued upon discharge. Over the next several months, her mummified digits autoamputated. Her PAN remained otherwise well controlled on rituximab, daily low-dose azathioprine, and weekly oral methotrexate with no evidence of systemic disease.
Figure 1.
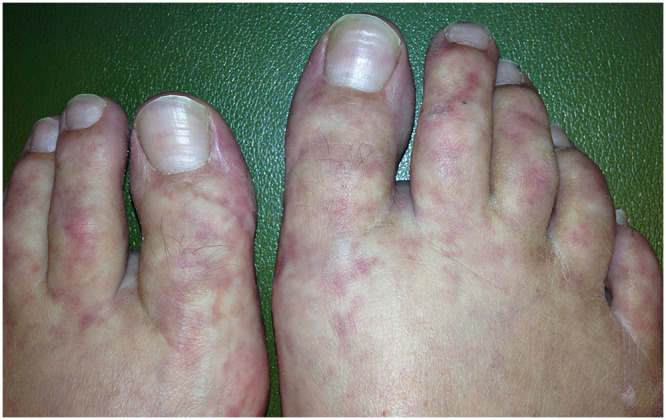
Initial presentation. Slowly progressive blanching livedo reticularis.
Figure 2.
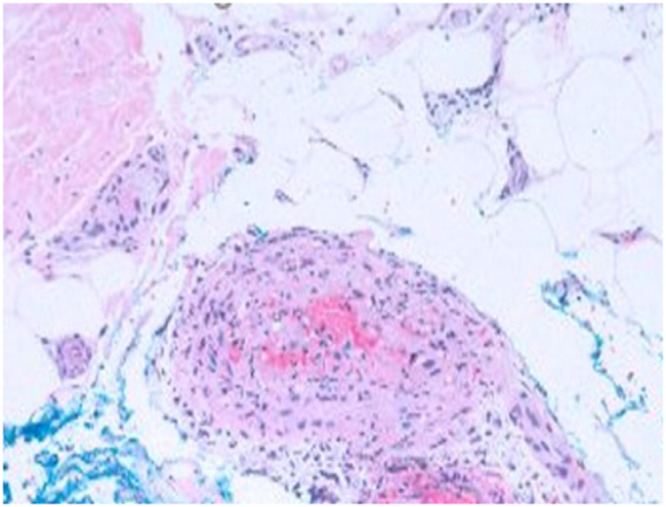
Hematoxylin-eosin (original magnification ×40). Histologic examination of an excisional biopsy revealed medium-sized vessel arteritis in the upper subcutis showing lymphocytes and few neutrophils within the vessel wall with incipient necrotizing features and plumped endothelium.
Figure 3.
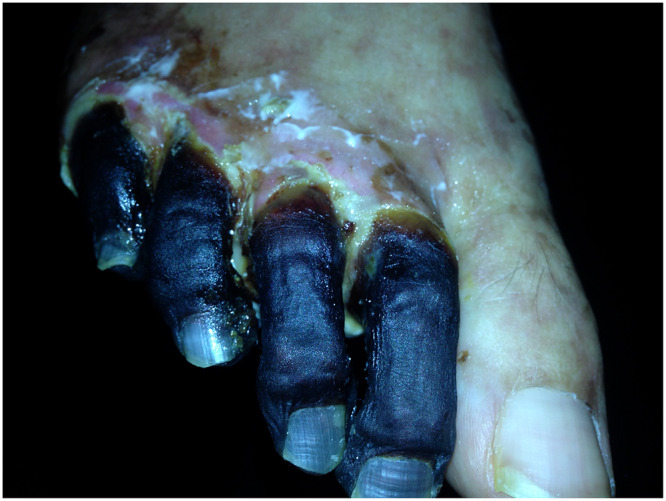
Necrotic toes.
Nineteen months following her hospital admission, she was presented with a new flare with painful livedo reticularis of the lower extremities. This happened in the context of family issues. As a result, prednisone was restarted, and the methotrexate dose was increased. As her lesions and symptoms persisted, she was readmitted, receiving IV pulses of methylprednisolone and cyclophosphamide and continued oral prednisone tapering. After several weeks of treatment, she eventually achieved remission and was re-transitioned to oral methotrexate and rituximab.
The patient’s disease remained well controlled for about 3.5 years. Around this time, she was involved in a motor vehicle accident resulting in splenic rupture, and her mother contracted COVID-19. She returned to the clinic with painful livedo reticularis and livedo racemosa of the second and third digits of the left foot, the first digit of the right foot (Figure 4), and the joint lines of the digits of the bilateral hands. She again received IV pulses of methylprednisolone, cyclophosphamide, oral tapering prednisone, nifedipine, and pentoxifylline. After two weeks, her symptoms and lesions began to improve, and she was transitioned to oral mycophenolate mofetil for maintenance therapy which she remained on. Twelve months later, her symptoms and lesions show near-complete resolution, with well-healed ulcers on her feet.
Figure 4.
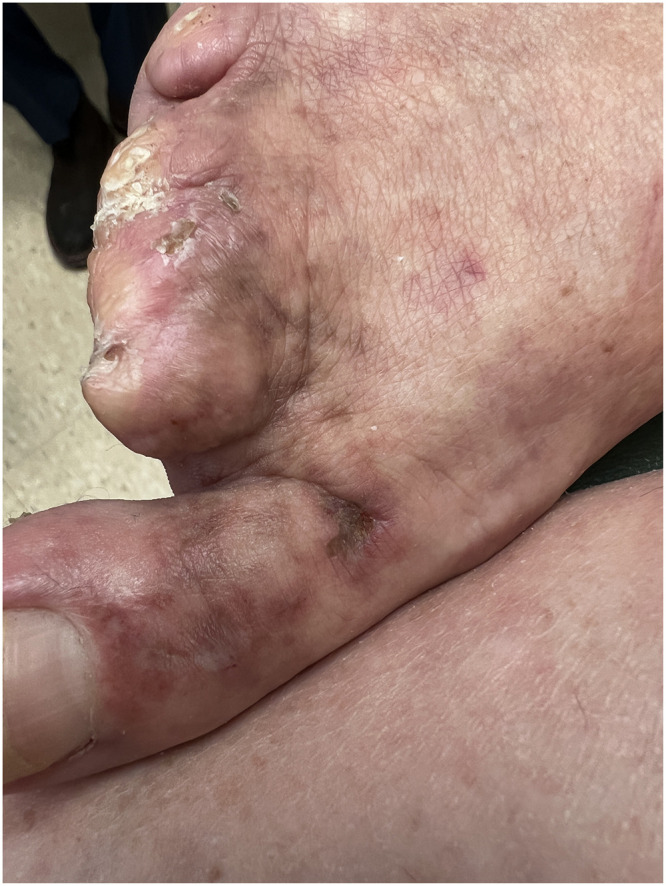
Livedo racemosa of the first toe. Note auto-amputated second to fifth toes of the right foot.
Discussion
The skin lesions of cPAN most often present with tender subcutaneous and deep dermal nodules, livedo reticularis, and much less commonly livedo racemosa, retiform purpura, ulcerations, digital ischemia, and digital infarcts. Virtually all cPAN cases involve the lower extremities, with most patients having relatively indolent yet chronic relapsing and remitting course.1,2
This case’s salient features lie in the severity of the cPAN and the sequence of stressors followed by disease exacerbations. Although this is the first report of stress-exacerbated cPAN, numerous studies have shown the close relationship between stressful life events and the flaring of various autoimmune diseases such as Graves’ Disease, rheumatoid arthritis, Sjogren’s Syndrome, multiple sclerosis, and ANCA vasculitis.4,5 An interesting aspect of our patient lies in the fact that she presented two diseases (psoriasis and cPAN) found at opposite poles of the TH1/TH2 immune response. This can be explained by numerous reports documenting the development of autoimmune diseases, particularly vasculitis, following treatments with anti-TNF agents, as seen in this patient.11–16
Underlying this phenomenon, stressors mediate the switch from Type 1 to Type 2 humoral immune response by activating the HPA-axis and the sympathetic nervous system (SNS). For example, catecholamines were shown to stimulate IL4 and IL-10 production by immune cells and inhibit TNF-α and IL-12 production. Similarly, glucocorticoids promote the Type 2 cytokines IL-4, IL-10, and IL-13 and reduce the production of the Type 1 cytokines IFN-γ and TNF-α.17,18 More importantly, nor-epinephrine (NE) secreted by peripheral nerves of the SNS can reach high tissue concentrations during stress and have profound effects on immune cells carrying β-2 adreno-receptors, resulting in a shift in the ratio of TH1/TH2.19 This favors the secretion of TH2-type cytokines and the dominance of humoral immunity, which triggers the exacerbation of autoimmune diseases20 as presumptively seen in our patient, albeit serum levels of cytokines and stress hormones were not assessed.
Interestingly, all the components of the HPA axis are reproduced in the skin; it is the only peripheral organ that mirrors the central HPA axis, leading to the notion that stressors can induce the production of CNS peptides and finally produce cutaneous glucocorticosteroids as well as induce the secretion of immunomodulatory cytokines by keratinocytes.21
Conclusion
Our report suggests that stress and flares of autoimmunity are closely intertwined and call for a biopsychosocial approach to patient care. Just as we prescribe medical therapies to achieve disease remission so should we provide resources to address our patients’ psychological health to ameliorate the stress responses that can trigger a relapse.22
Ethics and Consent Statements
Appropriate informed consent to publish this case report including all the images has been obtained from the patient. The Institutional Review Board of the University of Rochester does not review case reports.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stone JH, Nousari HC. ”Essential” cutaneous vasculitis: what every rheumatologist should know about vasculitis of the skin. Curr Opin Rheumatol. 2001;13(1):23–34. doi: 10.1097/00002281-200101000-00005 [DOI] [PubMed] [Google Scholar]
- 2.De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15(6):564–570. doi: 10.1016/j.autrev.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 3.Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319(23):2388–2400. doi: 10.1001/jama.2018.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharif K, Watad A, Coplan L, et al. The role of stress in the mosaic of autoimmunity: an overlooked association. Autoimmun Rev. 2018;17(10):967–983. doi: 10.1016/j.autrev.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Golemati CV, Mavragani CP, Lionaki S, Karaiskos D, Moutsopoulos HM. Stress and disease onset in antineutrophil cytoplasmic antibody-associated vasculitis. Front Psychiatry. 2017;8:286. doi: 10.3389/fpsyt.2017.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- 7.Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi: 10.1016/j.psyneuen.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2–3):193–210. doi: 10.1007/s12026-014-8517-0 [DOI] [PubMed] [Google Scholar]
- 9.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306. doi: 10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- 10.Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125(9):1417–1431. doi: 10.1002/cncr.31943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva Cendon Duran C, da Paz AS, Barreto santiago M. Vasculitis induced by biological agents used in rheumatology practice: a systematic review. Arch Rheumatol. 2022;37(2):300–310. doi: 10.46497/ArchRheumatol.2022.9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Casals M, Brito-Zeron P, Munoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine. 2007;86(4):242–251. doi: 10.1097/MD.0b013e3181441a68 [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Casals M, Brito-Zeron P, Soto MJ, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol. 2008;22(5):847–861. doi: 10.1016/j.berh.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 14.Reitblat T, Reitblat O. Appearance of ANCA - associated vasculitis under Tumor necrosis factor-alpha inhibitors treatment. Am J Case Rep. 2013;14:80–82. doi: 10.12659/AJCR.883841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-alpha inhibitors. Mayo Clin Proc. 2012;87(8):739–745. doi: 10.1016/j.mayocp.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anandacoomarasamy A, Kannangara S, Barnsley L. Cutaneous vasculitis associated with infliximab in the treatment of rheumatoid arthritis. Intern Med J. 2005;35(10):638–640. doi: 10.1111/j.1445-5994.2005.00899.x [DOI] [PubMed] [Google Scholar]
- 17.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156(7):2406–2412. [PubMed] [Google Scholar]
- 18.Kim BJ, Jones HP. Epinephrine-primed murine bone marrow-derived dendritic cells facilitate production of IL-17A and IL-4 but not IFN-gamma by CD4+ T cells. Brain Behav Immun. 2010;24(7):1126–1136. doi: 10.1016/j.bbi.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 20.Bellocchi C, Carandina A, Montinaro B, et al. The interplay between autonomic nervous system and inflammation across systemic autoimmune diseases. Int J Mol Sci. 2022;23(5):2449. doi: 10.3390/ijms23052449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pondeljak N, Lugovic-Mihic L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther. 2020;42(5):757–770. doi: 10.1016/j.clinthera.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Stress Triggers Disease Flares in Patients With Vasculitis. Study shows psychological health important to controlling wegener’s granulomatosis; 2011. Available from: https://www.hss.edu/newsroom_stress-triggers-vasculitis-flares.asp. Accessed December 28, 2022.


