Abstract
Trypanosoma cruzi, the agent of Chagas' disease, expresses trans-sialidase, a unique enzyme activity that enables the parasite to invade host cells by transferring sialyl residues from host glyconjugates to the parasite's surface acceptor molecules. The enzyme is also shed into the surrounding environment, causing apoptosis in cells from the immune system. During infections, an antibody response against the catalytic region of the trans-sialidase that is coincident with the control of the parasitemia and survival of the host is observed. This low-titer humoral response is characterized by its persistence for many years in benznidazole-treated patients. Here we analyzed the antigenic structure of the molecule by phage-displayed peptide combinatorial libraries and SPOT synthesis. Several epitopes were defined and located on the three-dimensional model of the enzyme. Unexpectedly, cross-reaction was found among several epitopes distributed in different locations displaying nonconsensus sequences. This finding was confirmed by the reactivity of three monoclonal antibodies able to recognize non-sequence-related peptides that together constitute the surface surrounding the catalytic site of the enzyme. The presence of cross-reacting epitopes within a single molecule suggests a mechanism developed to avoid a strong humoral response by displaying an undefined target to the immune system.
Chagas' disease is an endemic parasitosis that affects about 16 million people in the Americas. The causative agent of this disease is the protozoan parasite Trypanosoma cruzi. The parasite has a complex life cycle involving a hematophagous insect vector (kissing bugs) and a mammalian host. Metacyclic trypomastigote forms present in the feces of the insect invade the mammalian host through mucosae or microlesions in the skin. Metacyclic forms are unable to duplicate, but they invade host cells, where they transform into the amastigote replicative form. After several cycles of duplication they transform into the nonreplicative bloodstream trypomastigote stage, which disseminates the infection by invading other cells.
The adhesion and invasion of the host cell is a crucial step of the parasite life cycle. To interact with the mammalian host cell, the parasite requires sialylated glycoconjugates (14, 41, 51). However, T. cruzi is unable to synthesize sialic acids de novo (47). To obtain the sugar, the parasite expresses trans-sialidase (TcTS), an enzyme not present in mammals that is able to direct transfer of sialyl residues among macromolecules (24, 25, 52). Sialic acids are correlated not only with host cell interaction but also with protection against lysis mediated by either complement (55) or antibodies against alpha-galactosyl epitopes (42). TcTS is anchored to the membrane by glycosylphosphatidylinositol (2, 3) and is shed into the surrounding environment, being detected in blood from infected animals and human patients during the acute stage of the infection (17, 34). Recently, it was found that TcTS induces apoptosis in vivo in components of the immune system (36). The injection of TcTS is able to increase the virulence of a parasite inoculum (13) and recently it was shown that Leishmania major expressing heterologous TcTS displays increased virulence (12). Therefore, the molecule constitutes a virulence factor playing key roles in the life cycle of the parasite and in its interplay with the host.
TcTS, as expressed in the invasive trypomastigote stage, has two clearly defined regions: a globular amino terminus of about 640 amino acids containing the catalytic activity and a variable number of a repeated highly antigenic motif of 12 amino acids known as SAPA (24, 25). SAPA is located at the C terminus and allows the enzyme to remain in blood (6, 7). Early during the infection, a strong anti-SAPA humoral response is observed (1, 7, 34, 49). In a later stage, this is followed by the induction of antibodies directed to the catalytic region, some of them with enzyme-inhibitory characteristics (4, 7, 16, 33, 34, 37, 38, 43, 44). The inhibitory response is elicited only when the enzyme is in its native state, suggesting that discontinuous epitopes are involved (7). This humoral response seems to be related to survival of infection (16, 22) and usually appears simultaneously with control of the parasitemia by the host response (34). Antibodies directed to the catalytic region are still detected many years after successful benznidazole treatment of chagasic patients that leads to the absence of other T. cruzi-specific antibodies and parasitemia (33, 37).
An interesting approach to analyzing the antigenic properties of a molecule is provided by the phage-displayed combinatorial libraries technology (18, 21, 53). The screening of an unbiased large number of different peptides allows the identification of reactive sequences (mimotopes) that mimic either sequential or discontinuous (conformational) epitopes (15, 20, 39). Another approach successfully employed to identify sequential epitopes is SPOT synthesis technology (23). This technique is based on the low-cost generation of membrane-displayed peptides suitable for screening with the antibodies of interest.
By employing these techniques, an epitope mapping of the catalytic N-terminal domain of the enzyme was performed. Several B-cell epitopes were identified and characterized, including various cross-reacting epitopes that suggest a T. cruzi mechanism to evade the immune response.
MATERIALS AND METHODS
Peptide combinatorial library screening.
Two phage-displayed libraries of combinatorial nonapeptides expressed on the pVIII protein of filamentous phage (18), one of them flanked by cysteines (“constrained”) (39), were kindly provided by F. Felici (Istituto di Ricerche di Biologia Molecolare P. Angeletti, Rome, Italy). A TcTS affinity column was constructed by coupling purified recombinant enzyme without SAPA (8, 11) to N-hydroxysuccinimidyl-activated Sepharose (HiTrap, NHS activated; Amersham-Pharmacia Biotech, Uppsala, Sweden). Sera from T. cruzi (CAI and RA strains)-infected rabbits were taken 3 months after infection. Immunoglobulin G (IgG) obtained by protein A chromatography (Hi-Trap protein A; Amersham-Pharmacia Biotech) was applied to the TcTS affinity column, and after several washings with saline, specific antibodies were acid eluted. Libraries (2.6 × 1011 PFU) were diluted to 100 μl in phosphate-buffered saline (PBS) and preadsorbed for 2 h with protein A-Sepharose 4B (Amersham-Pharmacia Biotech) by adding 50 μl of a 50% suspension in PBS. Supernatants from four washings with 100 μl of PBS were pooled, and 5 μg of purified antibodies was added and allowed to react for 2 h in a final volume of 550 μl. A 50% protein A-Sepharose suspension in PBS (100 μl) was added and carefully rotated end over end for 2 h at room temperature. After 10 washings with 1 ml of PBS and one more with 0.15 M NaCl, beads were resuspended in 500 μl of glycine–HCl (concentration, 1 M; pH 2.5) for 30 min. Tris base (50 μl of a 1 M solution) was added, and the suspension was immediately used to infect 1 ml of competent Escherichia coli XL1-Blue cells (Stratagene, La Jolla, Calif.); then the techniques described in detail by Felici et al. (19) were carefully followed in the phage rescue and filter immunoscreening procedures. Filters were screened with TcTS affinity-purified immunoglobulins from T. cruzi-infected rabbits. As a secondary antibody, a horseradish peroxidase (HRP)-labeled goat immunoglobulin against rabbit IgG (Dako Corporation, Carpinteria, Calif.) was employed and revealed by chemiluminescence with SuperSignal (Pierce Chemical Co., Rockford, Ill.).
Immunization procedures.
Synthetic peptides were coupled to maleimide-activated keyhole limpet hemocyanin (KLH) and bovine serum albumin (BSA) (both from Pierce). Peptides were dissolved in a small amount of dimethylformamide (when necessary) and then brought to a concentration of 1.3 mg/ml with PBS and coupled with carrier proteins at a molar ratio of 3:1 (peptide/protein). BALB/c mice (90 days old) received 50 μg of peptide-KLH emulsified in Freund's complete adjuvant (Sigma Chemical Co., St. Louis, Mo.) intraperitoneally, followed by two doses of 10 μg in incomplete Freund's adjuvant (Sigma) at a 20-day interval. Animals were bled 10 days after the last booster. Rabbits were immunized following the same schedule but employing 500 μg in the first dose and 100 μg for boosters by the subcutaneous route. Reaction of sera against peptides was tested by using peptides coupled to BSA by either enzyme-linked immunosorbent assay (ELISA) or the dot spot technique.
Phages were purified by polyethylene glycol precipitation by following the procedures described in reference 19, and immunization was then performed by employing 1011 PFU emulsified in Freund's incomplete adjuvant (Sigma) as described in reference 26. Both mice and rabbits were used.
ELISA and dot spot assay.
We found that TcTS is inactivated when adsorbed to ELISA plates (Maxisorp; Nunc, Roskilde, Denmark). Purified enzyme was then coupled at 80 ng/well with a monoclonal antibody (MAb) against SAPA (35) when sera from rabbits were assayed or with rabbit IgG against SAPA when sera from mice were assayed. Secondary antibodies coupled to HRP were employed (either from Pierce or Dako). The assay was developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and read at 415 nm in a Benchmark microplate reader (Bio-Rad, Hercules, Calif.).
Dot spots were performed by spotting 1.3 μg of peptides coupled to BSA or 1010 PFU of purified phages in a 1-μl volume onto nitrocellulose membranes (Sartorious, Göttingen, Germany). Membranes were then blocked in 3% BSA in PBS and treated with sera diluted in the same solution for 1 h, and after three washings with PBS, a secondary antibody coupled to HRP (either from Pierce or Dako) was added and revealed by chemiluminescence with SuperSignal (Pierce).
Synthetic peptides.
Peptides synthesized by Fmoc (9-fluorenylmethoxycarbonyl) chemistry were ordered from Research Genetics (Huntsville, Ala.) and Alpha Diagnostic Intl. Inc. (San Antonio, Tex.). Sequences were as follows: peptide 1, CLNFKGRWLRDRL; peptide 2, CPLSLRSK; peptide 3, CRYETSNDNSLI; peptide 4, CYNSSRSYWT; peptide 5, CGQVSIGDENSA; peptide 1439, YSVDDGETWC; peptide 1440, YPVDRSTFWC; peptide 1441, WQPIYGSTPVTPTGSC; peptide 1442, PRVRSKPVVC; peptide 1443, TPADPAASSSERGC; and peptide 1445, NPIDATARSC. Peptides were analyzed by mass spectrometry, and purity was 80% or better.
Recombinant TcTS purification.
Recombinant TcTS was purified from E. coli XL1-Blue (Stratagene) transformed with either pTSHis1 (encoding TcTS without SAPA) (8) or pTS-3R (encoding TcTS with three SAPA repeats) (6), which was employed in ELISA procedures. Cultures were induced with isopropyl-β-d-thiogalactopyranoside (Sigma) as described previously (6, 8). Lysates were applied to chelating HiTrap columns (Amersham-Pharmacia Biotech) loaded with Ni2+ and eluted with a gradient of imidazole as described previously (6). Enzymatically active fractions were pooled, dialyzed against 20 mM Tris–20 mM NaCl (pH 8) and loaded onto a Mono Q column (Amersham-Pharmacia Biotech). Elution was performed with a linear gradient of 20 to 500 mM NaCl in the same buffer. The procedure yielded a single protein band in sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis in both cases.
Enzyme activity evaluation and inhibition assays.
TcTS was assayed for its ability to transfer the sialyl residue from alpha-2,3-sialyllactose (Sigma) to [d-glucose-1-14C]lactose (Amersham-Pharmacia Biotech) as previously described (33). TcTS inhibition assay (TIA) was performed by preincubating the enzyme with sera from immunized animals, adding the substrates, and quantifying the remnant enzymatic activity as described previously (33).
In competition assays, T. cruzi-infected rabbit TcTS-neutralizing sera were diluted 1/50 in PBS (this corresponds to the 1:2 dilution before the neutralizing titer). Then, phages (1010 PFU) or BSA-coupled peptides (2 μg) were added and TIA was performed. For depletion assays, BSA-coupled peptides (1.5 μg) or phages (1010 PFU) were adsorbed on ELISA plates, and then TcTS-neutralizing sera were added. After 1 h, sera were aspirated, loaded onto another antigen-coated well, and allowed to react for another hour. After a check to see that no further reaction by ELISA or dot spot was detected, depleted sera were used in the TIA procedure. For substrate competition, ELISA was performed with the TcTS linked with anti-SAPA antibodies as described above. After the test sera had been washed out, substrates were added (either 4-methylumbelliferyl-N-acetylneuraminic acid [0.2 mM], lactose [1 mM], sialyllactose [5 mM], or lactose plus sialyllactose [all from Sigma]), and after 10 min, plates were washed three times and developed with a secondary HRP-labeled antibody.
SPOT synthesis of peptides.
The procedures described by Frank and Overwin (23) were carefully followed under the direct supervision of R. Frank and A. Hollnagel in the context of a course held in Argentina. The membranes were blocked with blocking buffer (Genosys Biotechnologies, The Woodlands, Tex.). Then, sera from T. cruzi-infected rabbits or mice or from chronically chagasic patients were employed at a 1/50 dilution. Secondary antibodies coupled to alkaline phosphatase (Dako) were employed at a 1/2,000 dilution, and the reaction was developed with 5-bromo-4-chloro-3-indolylphosphate–3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (BCIP-MTT; both from Sigma) (23).
MAbs.
BALB/c mice were immunized with recombinant TcTS adsorbed onto alumin (7). Three doses of 20 μg were given at 20-day intervals intraperitoneally. The best responders were killed, and splenocytes were obtained. Fusion with SP2/0 cells and other procedures were as described in reference 27.
Screening of TcTS-specific antibody secreting clones was performed by ELISA as described above.
Molecular modeling of TcTS.
The three-dimensional (3D) model of the catalytic domain (residues 1 to 640) of the TcTS has been obtained by standard homology modeling techniques using the crystal structure of the sialidase from Trypanosoma rangeli (TrSA; 70% of identical amino acids) determined at a 2.2-Å resolution (9). There are no insertions or deletions between the two enzyme sequences, except for a single amino acid deletion at position 22 of TrSA, in a solvent-exposed loop. Therefore, modeling of TcTS was carried out by manually introducing the individual amino acid substitutions into the TrSA framework and assuming, for each mutated residue, the most frequent side chain rotamer consistent (as defined by the program O [28]) with the local stereochemistry. A final energy minimization was carried out with program XPLOR (5) in order to optimize the overall stereochemistry of the model. The deduced TcTS model is validated by the high degree of amino acid identities (70%) between the two enzymes sequences, the absence of insertions or deletions between the two sequences, and the conservative character of all internal and most exposed amino acid substitutions.
RESULTS
Identification of mimotopes by screening of peptide combinatorial libraries.
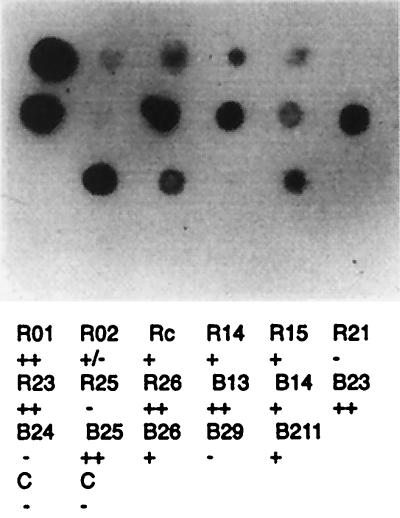
Two phage-displayed peptide combinatorial libraries containing 9-amino-acid-long random peptides were employed (18, 39). One of these libraries encodes peptides flanked by cysteines, which allows the generation and retention of spatial structures through a disulfide bridge (39). To identify phage-displayed mimotopes, affinity-purified TcTS-specific antibodies were obtained from T. cruzi-infected rabbits. The screening yielded several phages, and their reactivities with a serum obtained from a T. cruzi-infected rabbit are displayed in Fig. 1. Sera from 11 rabbits infected with several T. cruzi stocks reacted with the phages bearing mimotopes at various degrees, supporting the idea that the epitopes actually represent the antigenic structure of the enzyme. When sera from eight chagasic patients were tested, six recognized B13, three recognized B25, two recognized B211, two recognized R14, and all recognized R23. Meanwhile, the B26 and R15 epitopes were also recognized by normal human sera. Sera from rabbits and mice immunized with different purified phage preparations were found to be reactive with TcTS by ELISA, lending further support to the hypothesis that the phage-displayed peptides were mimicking epitopes present in the TcTS.
FIG. 1.
Reactivity of cloned phages bearing different mimotopes with a serum from a T. cruzi-infected rabbit diluted 1/100. TcTS affinity-purified antibodies were obtained from sera of T. cruzi-infected rabbits and used to screen the combinatorial libraries constructed with (clones named with “R”) or without (clones named with “B”) cysteine flanking amino acid residues. Reactivity is depicted with plus and minus signs to correspond to the nomenclature in Table 1. C, control (phage without insert). Adobe Photoshop version 4.0 and Adobe Illustrator version 8.0 for Macintosh were employed.
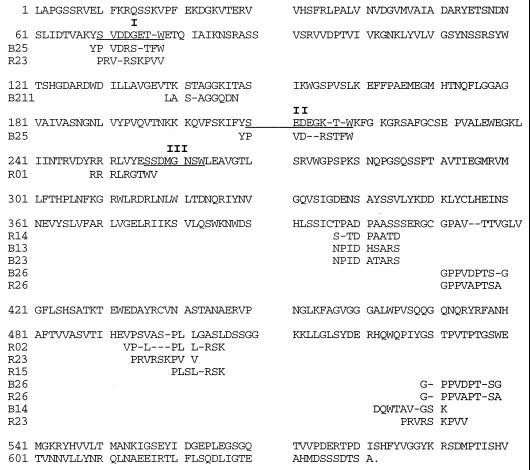
The deduced amino acid sequences of these mimotopes are shown in Fig. 2, tentatively aligned with sequence segments from TcTS. As expected, matches were partial, as is frequently found with this approach. Sequences showing a similarity of about 30% (paired test) were considered to putatively ascribe them to matched sequences. Under these conditions, various mimotopes could be assigned to more than one putative location (Fig. 2). Some regions of the protein (in particular, the sequence segments corresponding to amino acid positions 397 to 405 and 523 to 538) seem to be highlighted in connection with the immune system, since more than one mimotope could be assigned to them. The overall assignment of mimotopes to the TcTS sequence is in agreement with the 3D structure model of the enzyme (Fig. 3a). For example, the two regions with several peptide matches correspond to highly exposed loops of TcTS which are easily accessible for antibody binding: the loop connecting the two structural domains of the enzyme (segment 397–405) and a highly flexible loop of the lectin-like domain (segment 523–538). Indeed, both loops were only partially visible in the crystal structure of TrSA (9) due to their intrinsic flexibility and their high degree of solvent exposure. Other mimotopes could be assigned to the characteristic Asp boxes of microbial sialidases (50), which also correspond to solvent-exposed loops on the opposite face of the molecule with respect to the active site (Fig. 3a). Interestingly, no convincing match could be found in the neighborhood of the active site. Phage R15 might be putatively assigned to a loop segment which is close to the catalytic cleft (positions 305 to 316), but this assignment requires the introduction of an internal gap which is not consistent with the conformation of the corresponding peptide loop in the 3D structure model of TcTS.
FIG. 2.
Comparison of deduced sequences of the TcTS and the mimotopes identified. Underlined sequences correspond to the three Asp box (SXDXGXTW) motifs of microbial sialidases (50). Dashes are employed for better alignment. Numbering on the left indicates amino acid positions. Another mimotope with no ascribed sequence homology was RC (AKALNAYF).
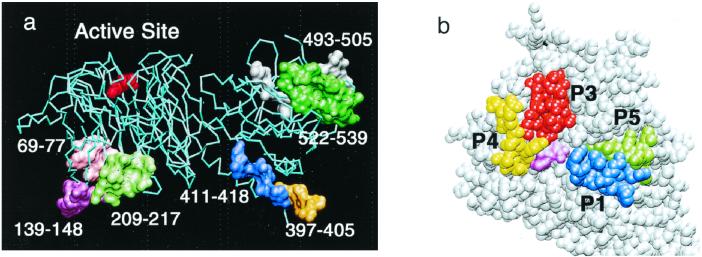
FIG. 3.
(a) Overall view of the 3D structure model of TcTS showing the distribution of putative epitopes as identified by screening a phage library of peptides. A sialic acid residue placed in the catalytic site is indicated in red. (b) Close view of the TcTS active-site cleft showing selected peptide sequences for SPOT epitope analysis. Peptide 1 is shown in blue, peptide 3 is in red, peptide 4 is in yellow, and peptide 5 is in green. A sialic acid residue placed in the catalytic site is in light purple.
The assigned putative location of the epitopes was assayed for five phages (B13, B23, B25, R14, and R23). Sera from phage-immunized mice were tested by dot spot assay against synthetic peptides modeled on the TcTS sequence. The positions 69 to 77 (peptide 1439), 397 to 410 (peptide 1443), and 524 to 538 (peptide 1441) were tested. Sera against phages B13, B23, and R14 reacted with peptide 1443, and sera against phages B25 and R23 reacted with peptide 1439 (not shown). These results confirm the previous putative locations of phages. Unexpectedly, all these sera were also reactive with peptide 1441. These results support the possibility that the sequences encoded by the phages were in fact mimicking sequences found in the enzyme and suggest cross-reactivity of the protein epitope associated with peptide 1441 (see below).
Mapping of epitopes in the vicinity of the catalytic site of the TS shows evidence of cross-reactive epitopes inside and around the catalytic site.
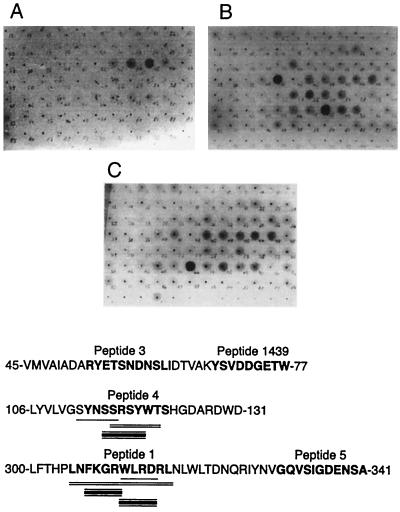
Although the identified epitopes seem to be distributed through most of the molecular surface of TcTS, no convincing epitope close to the catalytic cleft could be identified. Therefore, we decided to further analyze this region by SPOT synthesis of peptides (23) derived from three solvent-exposed protein loops located near or inside the catalytic site, as deduced from the 3D model of the enzyme. Regions selected correspond to positions 45 to 77, 106 to 131, and 300 to 341 (Fig. 3b). A membrane containing hexapeptides constructed by moving one position at a time on the sequence of the three selected regions was tested with sera obtained either from experimentally T. cruzi-infected rabbits and mice or from chronically chagasic human patients. As shown in Fig. 4, sera from the three species reacted with hexapeptides located in similar regions. The reactions of human antibodies were distributed among several hexapeptides, overlapping epitopes detected by mouse and rabbit sera and therefore suggesting that more than a single determinant was included in that region.
FIG. 4.
Epitopes located in the catalytic site as defined by reaction with peptides obtained by SPOT synthesis. Each spot contains a hexapeptide constructed by moving one amino acid residue at a time. Epitopes identified with sera from several T. cruzi-infected hosts are underlined with single (mouse), double (human), or triple (rabbit) lines. Numbering indicates amino acid positions. Reactions were carried out with sera from chronically chagasic patients (A), experimentally T. cruzi-infected rabbits (B), and experimentally infected mice (C). The peptides highlighted in Fig. 3 are in bold. Adobe Photoshop version 4.0 and Adobe Illustrator version 8.0 for Macintosh were employed.
For further investigation, four peptides (peptides 1, 3, 4, and 5) were chosen because of their exposure to the solvent and because together they constitute the surface surrounding the catalytic site (Fig. 3b). The sequences of these peptides are shown in bold in Fig. 4. Peptides 1 and 4 were previously detected in the SPOT assays. Peptide 2 corresponds to the mimotope expressed by phage R15 that could match a region covered by peptide 1, near the catalytic site. As shown in Table 1, rabbit sera against peptides 2 to 4 were able to react with nonrelated peptides located in different regions of the enzyme, in particular with peptide 1441.
TABLE 1.
Reactivity of sera against synthetic peptides modelled on defined epitopesa
| Immunizing peptide | Reactivity with testing peptide
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1439 | 1441 | 1443 | 1 | 2 | 3 | 4 | 5 | |
| Mouse sera | ||||||||
| 1439 | ++ | − | − | − | − | − | ± | − |
| 1441 | + | ++ | − | + | + | + | + | − |
| 1443 | + | + | ++ | − | ++ | − | ++ | − |
| 1445 (B23) | ± | ± | − | − | ++ | ++ | ++ | ++ |
| 1440 (B25) | ++ | ++ | ++ | ± | ++ | ++ | ++ | ++ |
| 1442 (R23) | ± | − | − | − | ± | − | − | − |
| Rabbit sera | ||||||||
| 1 | − | − | − | ++ | − | − | − | − |
| 2 (R15) | ++ | ++ | + | − | ++ | + | + | + |
| 3 | ± | ++ | ± | − | − | ++ | − | − |
| 4 | ± | ++ | ++ | − | + | − | ++ | − |
| 5 | − | − | − | − | − | − | − | ++ |
Mice and rabbits were immunized with peptides coupled to KLH. Antisera diluted 1/200 were tested by dot spot assay against peptides coupled to BSA. No reaction was found with sera from nonimmunized animals. All antipeptide sera were reactive with the enzyme either in dot spot assays or ELISAs. Peptides were modelled on reactive sequences of the enzyme or on phage-displayed mimotopes (shown in parentheses). For peptide sequences and locations, see the text and Fig. 2. For reactivity symbols, see Fig. 1.
Mouse sera against synthetic peptides derived from phages B23, B25, and R23 or peptides 1439, 1441, and 1443 were also tested. Table 1 shows that only the antisera against peptide 1439 and the R23-derived peptide 1442 were unable to detect other peptides. All the antipeptide sera were reactive with the TcTS by either ELISA or dot spot assays (data not shown). Taken together, these results strongly suggest that the enzyme bears several cross-reactive epitopes.
MAbs against TcTS provide further evidence of epitope cross-reactivity.
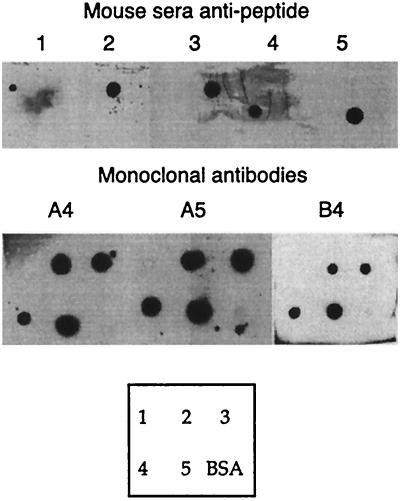
MAbs against the enzyme were derived from mice immunized with recombinant TcTS. Three of these MAbs (A4, A5, and B4) recognized peptides 2 to 5 by dot spots (Fig. 5); meanwhile, no reaction with any other peptide was found (data not shown). In spite of the cross-reaction observed with sera from rabbits (Table 1), no cross-reaction among these peptides was observed when sera from mice immunized with peptides 1 to 5 were assayed, excluding trivial explanations for the MAb reactivity, such as peptide contamination (Fig. 5). These results support the view that TcTS has the antigenic ability to induce cross-reactive antibodies capable of recognizing otherwise apparently unrelated sequences within the molecule.
FIG. 5.
TcTS immunization induces cross-reacting antibodies. The upper and lower blots show reactivity of sera from mice immunized with peptides 1 to 5 coupled to KLH and reactivity of mouse MAbs obtained after immunization of mice with TcTS, respectively. The box at the bottom shows the distribution of peptides 1 to 5 coupled to BSA on filters. Adobe Photoshop version 4.0 and Adobe Illustrator version 8.0 for Macintosh were employed.
Sera against the identified epitopes were unable to inhibit the enzyme.
In order to investigate whether antibodies against the identified epitopes are able to inhibit enzyme activity, we performed TIAs (34). Sera were obtained from mice and rabbits immunized either with purified phages bearing the mimotopes, with peptides derived from the enzyme sequence, or with peptides derived from the deduced sequence of phages B23, B25, R15, and R23 (peptides 1445, 1440, 2, and 1442, respectively). When undiluted sera were used, none resulted in more than 40% inhibition, and they were therefore considered nonneutralizing. In another attempt to determine if these epitopes were related to the induction of inhibitory antibodies, peptides and phages were used to deplete sera obtained from T. cruzi-infected rabbits of the specific neutralizing antibodies. Again, no alteration in the neutralizing activity of the infection-derived sera was found. Similar results were obtained when peptides or phages were added to the sera before the TIA. The possibility that soluble substrates were able to displace the antibody from the reaction site was also tested, but they were unable to dissociate the antibody-TcTS complex in ELISA (not shown).
Since antibodies which neutralize the catalytic activity do exist in sera from infected animals and humans (4, 16, 33, 34, 37, 38, 43, 44), it might be suggested that inhibitory antibodies target different regions in the molecule from the cross-reactive epitopes identified here. Alternatively, two or more epitopes should be simultaneously recognized to obtain the neutralization effect. Several combinations of antimimotope sera were used to test this alternative, but no enzyme inactivation was found (data not shown).
DISCUSSION
In the infective stage of T. cruzi, two clearly defined regions compose the TcTS, a highly antigenic repetitive C terminus known as SAPA and a globular catalytic N terminus (25). With either infection or immunization, a weak humoral response against the catalytic region is observed that includes a persistent inhibitory effect on the enzymatic activity that was related to survival of infection (16, 22). In this study, an analysis of the antigenic structure of the catalytic region of the enzyme was performed. Several epitopes located in different regions were detected close to or inside the catalytic site. The locations identified are exposed on the surface of the enzyme and are therefore easily accessible to the antibodies. In fact, these epitopes were reactive with sera obtained from T. cruzi infections, thus confirming their antigenicity.
The presence of a network of cross-reactive epitopes within the molecule can be envisaged from the findings reported here. In this sense it is worth noting that when mice were immunized with peptides 1 to 5, no cross-reaction among these peptides was observed, but MAbs obtained after TcTS immunization recognized peptides 2 to 5, supporting the idea that the cross-reactive antibodies might require the whole TcTS molecule to be induced. An example of cross-reacting antibodies induced against apparently unrelated sequences can be found in the hepatitis C virus protein E2 (48, 54). Through the combinatorial libraries technique, many examples of antibody reactivity with apparently unrelated peptide sequences have been identified (32). Recently, epitope cross-reactivity versus polyspecificity was molecularly defined through a human immunodeficiency virus-specific MAb (31, 32) able to react with both sequence-related and non-sequence-related determinants. Pathogens frequently evade the immune response by exposing closely related variant epitopes. In some cases, such as infections with hepatitis C virus (48, 54) and Plasmodium falciparum (45), antigens alter exposed regions. In T. cruzi, mucins containing closely similar epitopes are simultaneously exposed (46). It seems that in the cTS, the related epitopes are displayed simultaneously in the same molecule. This characteristic might have been acquired by the cTS during evolution, to provide an elusive target to the immune system.
We could not assign the neutralizing humoral response to any given epitope. A neutralizing antibody was assigned by other authors to a peptide designed on a misread DNA sequence of the TcTS (13), a result that we failed to reproduce (data not shown). The persistent low-titer neutralizing humoral response could be the result of different antibodies binding simultaneously to several epitopes throughout the molecule. Alternatively, it could be the consequence of antibodies directed to regions with low antigenicity. The TcTS is included in a superfamily of surface antigens (10, 25) that are expressed simultaneously in the parasite (29, 30). These proteins express similar but different sequences that might further contribute to the simultaneous presence of B-cell-related epitopes during the infection, as was recently demonstrated for T-cell epitopes (40). The parasite has evolved to evade the immune system and has acquired several abilities to disturb the host immune response. The strategy of developing cross-reactive epitopes inserted in a single critical protein that is also included in a superfamily of antigens may be another feature developed by the parasite to disturb the immune response.
ACKNOWLEDGMENTS
This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the Fundación Antorchas of Argentina and the World Bank/UNDP/WHO Special Program for Research and Training in Tropical Diseases (TDR). T.A.P. and P.A. are Fellows and M.S.L. and O.C. are Researchers for CONICET. J.M. is a Fellow of the Universidad Nacional de San Martín.
We are indebted to F. Felici (Istituto di Ricerche di Biologia Molecolare P. Angeletti, Rome, Italy) for kindly providing the phage-displayed peptide combinatorial libraries and to R. Frank and A. Hollnagel (Federal Research Center for Biotechnology and Technical University, Braunschweig, Germany) for SPOT synthesis advice. The critical reading of the manuscript by A. C. C. Frasch is also appreciated.
REFERENCES
- 1.Affranchino J L, Ibañez C F, Luquetti A O, Rassi A, Reyes M B, Macina R A, Aslund L, Pettersson U, Frasch A C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989;34:221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- 2.Agusti R, Couto A S, Campetella O, Frasch A C, de Lederkremer R M. Structure of the glycosylphosphatidylinositol-anchor of the trans-sialidase from Trypanosoma cruzi metacyclic trypomastigote forms. Mol Biochem Parasitol. 1998;97:123–131. doi: 10.1016/s0166-6851(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 3.Agusti R, Couto A S, Campetella O E, Frasch A C, de Lederkremer R M. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology. 1997;7:731–735. doi: 10.1093/glycob/7.6.731. [DOI] [PubMed] [Google Scholar]
- 4.Alcantara-Neves N M, Pontes-de-Carvalho L C. Circulating trans-sialidase activity and trans-sialidase-inhibiting antibodies in Trypanosoma cruzi-infected mice. Parasitol Res. 1995;81:560–564. doi: 10.1007/BF00932022. [DOI] [PubMed] [Google Scholar]
- 5.Brunger A T, Kuriyan J, Karplus M. Crystallographic R-factor refinement by molecular dynamics. Science. 1987;235:458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- 6.Buscaglia C A, Alfonso J, Campetella O, Frasch A C. Tandem amino acid repeats from Trypanosoma cruzi shed antigens increase the half-life of proteins in blood. Blood. 1999;93:2025–2032. [PubMed] [Google Scholar]
- 7.Buscaglia C A, Campetella O, Leguizamón M S, Frasch A C. The repetitive domain of Trypanosoma cruzi trans-sialidase enhances the immune response against the catalytic domain. J Infect Dis. 1998;177:431–436. doi: 10.1086/514199. [DOI] [PubMed] [Google Scholar]
- 8.Buschiazzo A, Frasch A C C, Campetella O. Medium scale production and purification to homogeneity of a recombinant trans-sialidase from Trypanosoma cruzi. Cell Mol Biol (Noisy-le-Grand) 1996;42:703–710. [PubMed] [Google Scholar]
- 9.Buschiazzo A, Tavares G A, Campetella O, Spinelli S, Cremona M L, Paris G, Amaya M F, Frasch A C, Alzari P M. Structural basis of sialyltransferase activity in trypanosomal sialidases. EMBO J. 2000;19:16–24. doi: 10.1093/emboj/19.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campetella O, Sánchez D O, Cazzulo J J, Frasch A C C. A superfamily of Trypanosoma cruzi surface antigens. Parasitol Today. 1992;8:378–381. doi: 10.1016/0169-4758(92)90175-2. [DOI] [PubMed] [Google Scholar]
- 11.Campetella O E, Uttaro A D, Parodi A J, Frasch A C. A recombinant Trypanosoma cruzi trans-sialidase lacking the amino acid repeats retains the enzymatic activity. Mol Biochem Parasitol. 1994;64:337–340. doi: 10.1016/0166-6851(94)00036-0. [DOI] [PubMed] [Google Scholar]
- 12.Carrillo B M, Gao W, Herrera M, Alroy J, Moore J B, Becerly S M, Pereira M A. Heterologous expression of Trypanosoma cruzi trans-sialidase in Leishmania major enhances virulence. Infect Immun. 2000;68:2728–2734. doi: 10.1128/iai.68.5.2728-2734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuenkova M, Pereira M E. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas' disease. J Exp Med. 1995;181:1693–1703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciavaglia M D C, de Carvalho T U, de Souza W. Interaction of Trypanosoma cruzi with cells with altered glycosylation patterns. Biochem Biophys Res Commun. 1993;193:718–721. doi: 10.1006/bbrc.1993.1684. [DOI] [PubMed] [Google Scholar]
- 15.Cortese R, Felici F, Galfre G, Luzzago A, Monaci P, Nicosia A. Epitope discovery using peptide libraries displayed on phage. Trends Biotechnol. 1994;12:262–267. doi: 10.1016/0167-7799(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 16.Costa F, Franchin G, Pereira-Chioccola V L, Ribeirao M, Schenkman S, Rodrigues M M. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine. 1998;16:768–774. doi: 10.1016/s0264-410x(97)00277-6. [DOI] [PubMed] [Google Scholar]
- 17.de Titto E H, Araujo F G. Serum neuraminidase activity and hematological alterations in acute human Chagas' disease. Clin Immunol Immunopathol. 1988;46:157–161. doi: 10.1016/0090-1229(88)90016-5. [DOI] [PubMed] [Google Scholar]
- 18.Felici F, Castagnoli L, Musacchio A, Jappelli R, Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol. 1991;222:301–310. doi: 10.1016/0022-2836(91)90213-p. [DOI] [PubMed] [Google Scholar]
- 19.Felici F, Galfre G, Luzzago A, Monaci P, Nicosia A, Cortese R. Phage-displayed peptides as tools for characterization of human sera. Methods Enzymol. 1996;267:116–129. doi: 10.1016/s0076-6879(96)67009-8. [DOI] [PubMed] [Google Scholar]
- 20.Felici F, Luzzago A, Folgori A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides. II. Selection of clones recognized by a protective monoclonal antibody against the Bordetella pertussis toxin from phage peptide libraries. Gene. 1993;128:21–27. doi: 10.1016/0378-1119(93)90148-v. [DOI] [PubMed] [Google Scholar]
- 21.Felici F, Luzzago A, Monaci P, Nicosia A, Sollazzo M, Traboni C. Peptide and protein display on the surface of filamentous bacteriophage. Biotechnol Annu Rev. 1995;1:149–183. doi: 10.1016/s1387-2656(08)70051-6. [DOI] [PubMed] [Google Scholar]
- 22.Franchin G, Pereira-Chioccola V L, Schenkman S, Rodrigues M M. Passive transfer of a monoclonal antibody specific for a sialic acid-dependent epitope on the surface of Trypanosoma cruzi trypomastigotes reduces infection in mice. Infect Immun. 1997;65:2548–2554. doi: 10.1128/iai.65.7.2548-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank R, Overwin H. SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Methods Mol Biol. 1996;66:149–169. doi: 10.1385/0-89603-375-9:149. [DOI] [PubMed] [Google Scholar]
- 24.Frasch A C. Trans-sialidase, SAPA amino acid repeats and the relationship between Trypanosoma cruzi and the mammalian host. Parasitology. 1994;108:S37–S44. doi: 10.1017/s0031182000075703. [DOI] [PubMed] [Google Scholar]
- 25.Frasch A C C. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol Today. 2000;16:282–286. doi: 10.1016/s0169-4758(00)01698-7. [DOI] [PubMed] [Google Scholar]
- 26.Galfre G, Monaci P, Nicosia A, Luzzago A, Felici F, Cortese R. Immunization with phage-displayed mimotopes. Methods Enzymol. 1996;267:109–115. doi: 10.1016/s0076-6879(96)67008-6. [DOI] [PubMed] [Google Scholar]
- 27.Goding J W. Monoclonal antibodies: principles and practice. San Diego, Calif: Academic Press; 1993. [Google Scholar]
- 28.Jones T A, Zou J Y, Cowan S W, Kjeldgaard M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 29.Kahn S, Colbert T G, Wallace J C, Hoagland N A, Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci USA. 1991;88:4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn S, Nguyen D, Norsen J, Wleklinski M, Granston T, Kahn M. Trypanosoma cruzi: monoclonal antibodies to the surface glycoprotein superfamily differentiate subsets of the 85-kDa surface glycoproteins and confirm simultaneous expression of variant 85-kDa surface glycoproteins. Exp Parasitol. 1999;92:48–56. doi: 10.1006/expr.1998.4394. [DOI] [PubMed] [Google Scholar]
- 31.Keitel T, Kramer A, Wessner H, Scholz C, Schneider-Mergener J, Höhne W. Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity. Cell. 1997;91:811–820. doi: 10.1016/s0092-8674(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 32.Kramer A, Keitel T, Winkler K, Stöcklein W, Höhne W, Schneider-Mergener J. Molecular basis for the binding promiscuity of an anti-p24 (HIV-1) monoclonal antibody. Cell. 1997;91:799–809. doi: 10.1016/s0092-8674(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 33.Leguizamón S M, Campetella O, Russomando G, Almiron M, Guillen I, González Cappa S M, Frasch A C. Antibodies inhibiting Trypanosoma cruzi trans-sialidase activity in sera from human infections. J Infect Dis. 1994;170:1570–1574. doi: 10.1093/infdis/170.6.1570. [DOI] [PubMed] [Google Scholar]
- 34.Leguizamón S M, Campetella O E, González Cappa S M, Frasch A C. Mice infected with Trypanosoma cruzi produce antibodies against the enzymatic domain of trans-sialidase that inhibit its activity. Infect Immun. 1994;62:3441–3446. doi: 10.1128/iai.62.8.3441-3446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leguizamón S M, Campetella O E, Reyes M B, Ibañez C F, Basombrío M A, Rincón J, Örn A, Frasch A C. Bloodstream Trypanosoma cruzi parasites from mice simultaneously express antigens that are markers of acute and chronic human Chagas disease. Parasitology. 1991;102:379–385. doi: 10.1017/s0031182000064337. [DOI] [PubMed] [Google Scholar]
- 36.Leguizamón S M, Mocetti E, García Rivello H, Argibay P, Campetella O. Trans-sialidase from Trypanosoma cruzi induces apoptosis in cells from the immune system in vivo. J Infect Dis. 1999;180:1398–1402. doi: 10.1086/315001. [DOI] [PubMed] [Google Scholar]
- 37.Leguizamón S M, Russomando G, Luquetti A, Rassi A, Almirón M, González-Cappa S M, Frasch A C, Campetella O. Long-lasting antibodies detected by a trans-sialidase inhibition assay of sera from parasite-free, serologically cured chagasic patients. J Infect Dis. 1997;175:1272–1275. doi: 10.1086/593697. [DOI] [PubMed] [Google Scholar]
- 38.Leguizamón S M, Russomando G, Rojas de Arias A, Samudio M, Cabral M, González-Cappa S M, Frasch A C, Campetella O. Use of trans-sialidase inhibition assay in a population serologically negative for Trypanosoma cruzi but at a high risk of infection. Clin Diagn Lab Immunol. 1998;5:254–255. doi: 10.1128/cdli.5.2.254-255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luzzago A, Felici F, Tramontano A, Pessi A, Cortese R. Mimicking of discontinuous epitopes by phage-displayed peptides. I. Epitope mapping of human H ferritin using a phage library of constrained peptides. Gene. 1993;128:51–57. doi: 10.1016/0378-1119(93)90152-s. [DOI] [PubMed] [Google Scholar]
- 40.Millar A E, Wleklinski-Lee M, Kahn S J. The surface protein superfamily of Trypanosoma cruzi stimulates a polarized Th1 response that becomes anergic. J Immunol. 1999;162:6092–6099. [PubMed] [Google Scholar]
- 41.Ming M, Chuenkova M, Ortega-Barria E, Pereira M E. Mediation of Trypanosoma cruzi invasion by sialic acid on the host cell and trans-sialidase on the trypanosome. Mol Biochem Parasitol. 1993;59:243–252. doi: 10.1016/0166-6851(93)90222-j. [DOI] [PubMed] [Google Scholar]
- 42.Pereira-Chioccola V L, Acosta-Serrano A, Correia De Almeida I, Ferguson M A, Souto-Padron T, Rodrigues M M, Travassos L R, Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-α-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 43.Pereira-Chioccola V L, Costa F, Ribeirao M, Soares I S, Arena F, Schenkman S, Rodrigues M M. Comparison of antibody and protective immune responses against Trypanosoma cruzi infection elicited by immunization with a parasite antigen delivered as naked DNA or recombinant protein. Parasite Immunol. 1999;21:103–110. doi: 10.1046/j.1365-3024.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 44.Pereira-Chioccola V L, Schenkman S, Kloetzel J K. Sera from chronic Chagasic patients and rodents infected with Trypanosoma cruzi inhibit trans-sialidase by recognizing its amino-terminal and catalytic domain. Infect Immun. 1994;62:2973–2978. doi: 10.1128/iai.62.7.2973-2978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plebanski M, Hill A V S. The immunology of malaria infection. Curr Opin Immunol. 2000;12:437–441. doi: 10.1016/s0952-7915(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 46.Pollevick G D, Di Noia J M, Salto M L, Lima C, Leguizamon M S, de Lederkremer R M, Frasch A C. Trypanosoma cruzi surface mucins with exposed variant epitopes. J Biol Chem. 2000;275:27671–27680. doi: 10.1074/jbc.M000253200. [DOI] [PubMed] [Google Scholar]
- 47.Previato J O, Andrade A F, Pessolani M C, Mendonca Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985;16:85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- 48.Puntoriero G, Meola A, Lahm A, Zucchelli S, Ercole B B, Tafi R, Pezzanera M, Mondelli M U, Cortese R, Tramontano A, Galfre G, Nicosia A. Towards a solution for hepatitis C virus hypervariability: mimotopes of the hypervariable region 1 can induce antibodies cross-reacting with a large number of viral variants. EMBO J. 1998;17:3521–3533. doi: 10.1093/emboj/17.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes B M, Lorca M, Muñoz P, Frasch A C C. Fetal IgG specificities against Trypanosoma cruzi antigens in infected newborns. Proc Natl Acad Sci USA. 1990;87:2846–2850. doi: 10.1073/pnas.87.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roggentin P, Rothe B, Kaper J B, Galen J, Lawrisuk L, Vimr E R, Schauer R. Conserved sequences in bacterial and viral neuraminidases. Glycoconjugate J. 1989;6:349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- 51.Schenkman S, Eichinger D. Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol Today. 1993;9:218–225. doi: 10.1016/0169-4758(93)90017-a. [DOI] [PubMed] [Google Scholar]
- 52.Schenkman S, Eichinger D, Pereira M E A, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 53.Scott J K, Smith G P. Searching for epitope ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 54.Shang D, Zhai W, Allain J P. Broadly cross-reactive, high affinity antibody to hypervariable region 1 of the hepatitis C virus in rabbits. Virology. 1999;258:396–405. doi: 10.1006/viro.1999.9730. [DOI] [PubMed] [Google Scholar]
- 55.Tomlinson S, Pontes de Carvalho L C, Vandekerckhove F, Nussenzweig V. Role of sialic acid in the resistance of Trypanosoma cruzi trypomastigotes to complement. J Immunol. 1994;153:3141–3147. [PubMed] [Google Scholar]