Abstract
Affective disorders are a common psychological impairment. A major problem with respect to treatment is medication non-adherence. eHealth interventions are already widely used in the treatment of patients living with affective disorders. The aim of this systematic literature review is to obtain the current scientific evidence to eHealth as a tool to improve medication adherence in patients with affective disorders. A systematic search was performed across PubMed, Cochrane Library, Web of Science and PsycInfo. Studies in English and German published between 2007 and 2020 were included. The review followed the PRISMA guidelines and were performed with the CADIMA online tool. A total of 17 articles were included in this review. Eleven studies were randomized controlled trials, two were controlled clinical trials, and four had a pre-/post-design. Three different types of interventions could be identified: internet-based self-management programs (n=4), multi-faceted interventions addressing different dimensions of medication adherence (n=4), and single-faceted interventions (n=9) comprising four mobile interventions and five telehealth interventions. Eleven interventions addressed patients with (comorbid) depressions and six addressed patients with bipolar disorders. Six interventions showed a statistically significant positive effect on medication adherence. None of the studies showed a statistically significant negative effect. All interventions which had a statistically significant positive effect on medication adherence involved personal contacts between therapists and patients. All included eHealth interventions are at least as effective as control conditions and seems to be effective for patients with depression as well as with bipolar disorders. Personal contacts seem to improve the effectiveness of eHealth interventions. eHealth interventions are an effective way to improve medication adherence in patients with affective disorders. In rural or underserved regions, eHealth can supplement usual care interventions on medication adherence by expanding access. More analyses are needed in order to understand determinants for the effectiveness of eHealth interventions on medication adherence enhancement.
Keywords: affective disorder, eHealth, medication adherence, medication nonadherence, compliance, noncompliance
Plain Language Summary
People living with psychiatric disorders have, in some cases, problems with taking their medications as prescribed. Sometimes reminder apps on a smartphone or a video call with a psychiatrist, often comprised under the term telemedicine, would help those people to take their medications appropriate and to recover faster from psychiatric disorders or stay at least stable Our systematic literature review wants to find out how telemedicine helps people living with affective disorders to take their medications in an optimized way. This review includes 17 articles which met the inclusion criteria. 58% of the included studies showed that people living with psychiatric disorders benefit from telemedical offers. 42% of the studies showed no differences between telemedical support and usual care. This means that telemedicine is a good alternative for people with no or just limited access to usual care providers, eg, due to long distances or little money. From this we conclude that telemedicine is a useful tool for people living with psychiatric disorders. Some kind of personal contacts seems to be important. This may also correspond to the more recent and promising concept of blended care, in which face-to-face and telemedicine are intertwined.
Introduction
Overall, mental disorders are responsible for the loss of one quarter of all disability-adjusted life-years (DALYs) in Europe1 and contribute 7% of the overall global burden of diseases.2 Improving treatment adherence is paramount for an effective treatment of people living with mental disorder.3 While there are many psychotropic drugs available today that can help to cure mental disorders, non-adherence is a major issue for sufficient treatment of mental disorders.4,5
Non-adherence is associated with a number of negative consequences including relapse and worsening of symptoms, increased risk of rehospitalization, prolonged disability, increased suicides, increased co-morbid medical conditions and higher costs for healthcare utilization.4–7 50% of the patients with major depression and 44% of the patients with bipolar disorder are non-adherent to their medication schedules.5
Medication adherence can be defined as patient acceptance of recommended health behaviours8 and is seen as the extent to which a persons’ behaviour corresponds with agreed recommendations from a healthcare provider.3 This includes the initiation of the treatment, implementation of the dosing regimen, discontinuation and persistence of the medication.9 Therefore, medication adherence is understood as the extent to which a patient take his or her medication in accordance with the prescribed time and dose.10
Various methods can be used to measure (non)‐adherence. Subjective methods include determining rates of dispensing of repeat prescriptions, examining case‐note recordings, interviewing patients, obtaining collateral reports, and asking the attending physicians’ clinical judgement about the adherence of their patients. Interestingly, the latter is only 50% reliable.10 Patient self‐report is probably the most accurate subjective method.11 Within the subjective methods, indirect methods try to identify individuals at risk of non‐adherence. These methods mostly measure risk factors for adherence, like the subjects’ attitudes towards medication or their attitudes towards affective disorders and their treatment. Frequently used questionnaires include the Drug Attitude Inventory,12 the Lithium Attitudes Questionnaire,13 and Beliefs About Medication Questionnaire.14 Research studies show statistically significant correlations between measures of attitudes and objective measures of adherence.15
Objective methods of assessing adherence include counting the number of tablets, which patients were taking out of their pill boxes, monitoring of serum drug levels, the ratio of the plasma drug level and the administered dose (L/D ratio), analysis of urine for drugs or their metabolites and electronic event monitoring systems. Objective methods can be biased considering the time of medication’s ingestion and measurement of drug level, pharmacokinetic variability, expense or obtaining consent from a patient who may already be non‐adherent. The monitoring of antidepressant drug may show considerable inter-individual variation.16
A number of various individual as well as clinical confounders can potentially influence medication non-adherence. For example, social support improves medication adherence.17 However a longer duration of illness,18 comorbid substance abuse,19,20 polypharmacy and adverse effects favour medication non-adherence.21 The association between general intelligence and medication adherence in patients with schizophrenia and bipolar disorder is discussed.22 Moreover, some specific personality characteristics can mediate or complicate medication adherence. For example, it was found that conscientiousness is positively associated with medication adherence in chronic somatic disorders and depression.23,24 In summary, many factors in different disorders and depending on the specific treatment influence medication adherence.5,23
Telemedicine is a promising way to enhance medication adherence in patients with severe mental illness, to compensate for critical gaps in medical care,25 and to improve treatment access disparities (eg in rural areas).26 The WHO defines eHealth as the use of information and communication technologies (ICT) in support of health and health‐related fields. It encompasses multiple interventions, including telehealth, telemedicine, mobile health (mHealth), electronic medical or health records, big data, wearables, and even artificial intelligence.27
This review focuses on telemedicine in affective disorder in order to reduce disorder-related confounding, but also to include studies on eHealth applications in cross-diagnostic samples of patients. Affective disorders are part of the F-diagnosis of the ICD-10-CM Codes (mental, behavioural and neurodevelopmental disorders F01-F99). The ICD-10 codes for affective disorders, F30-F39, comprise mainly manic episode, bipolar disorder (BD), major depressive disorder as single episode, recurrent major depressive disorder (MDD), persistent affective disorders as well as unspecific affective disorders.28 According to recent prognoses, major depressive disorders (MDD) will rise to be one of the three top global disabling conditions alongside HIV/AIDS and ischemic heart disease by 2030.29
Our primary objective is to obtain evidence to eHealth as an effective tool to improve medication adherence in patients with affective disorders.
To operationalize this objective, the following research questions will be addressed:
Which different eHealth applications to improve medication adherence in patients with affective disorders can be found?
Which eHealth applications show positive results on medication adherence in patients with affective disorders?
Are there any differences in the results regarding the patient characteristics, like age, sex, severity of disease, diagnosis, duration of illness, specific drug?
Are there any differences in the results regarding the characteristics of the healthcare setting or the type of intervention?
Materials and Methods
Study Selection
Study Inclusion Criteria
To be eligible, articles had to meet the following criteria: Articles in English or German language and published in a peer-reviewed journal between 2007 and 2020; the study addresses patients with (comorbid) affective disorders (ICD-10 F30-F39).
The study evaluates an eHealth intervention. In order to differentiate between varying types of eHealth intervention, three main categories were built (Table 1) using an inductive-deductive content analysis approach.30,31 Outcome of the studies is the effect of eHealth interventions on adherence to psychiatric medications or other drugs prescribed, eg for the treatment of comorbid conditions; study protocols, reviews, and analyses of routine data were excluded from the analysis, because interventional studies were seen as most appropriate to answer the research questions of this review.
Table 1.
Different Categories of Intervention Included in This Review, Which Were Formed by Using a Deductive-Inductive Content Analysis
| Type of Intervention | Definition | |
|---|---|---|
| Single-faceted intervention (focuses on one dimension of medication adherence or consists of one component) | Mobile intervention | mHealth is a component of eHealth. It can be defined as medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants (PDAs), and other wireless devices. mHealth involves the use and capitalization on a mobile phone’s core utility of voice and short messaging service (SMS) as well as more complex functionalities and applications including general packet radio service (GPRS), third and fourth generation mobile telecommunications (3G and 4G systems), global positioning system (GPS), and Bluetooth technology.85 |
| Real-time Telehealth (via telephone or video consultations) | Remote delivery of health care to a patient through communication technology. Real-time (synchronous) telehealth is used to consult with, diagnose, and treat patients.86 | |
| Internet-based self-management programmes | Self-management interventions typically encompass information-based material and cognitive and/or behavioural strategies designed to increase participants’ knowledge, self-efficacy and use of self-management behaviours.87 | |
| Multi-faceted intervention | Multi-faceted interventions are interventions which address multiple components including pre-assessment of individual’s adherence level, counselling sessions, medication review, collaboration with physicians, and telephone or video calls.88 | |
Search Strategy
The literature search was conducted using the following databases: PubMed, Cochrane Library, Web of Science, PsycInfo.
The overall search term included the aspects “eHealth”, “affective disorders” and “medication adherence” and contains numerous terms as well as variations and synonyms (see Table 2). The search strategy was developed on the basis of the PICO scheme: population/participants (= patients with affective disorders), intervention (= eHealth), comparison (= not specified) and outcome (= improvement of medication adherence).32
Table 2.
Search Terms
| Concept | Search Terms |
|---|---|
| Affective disorders | Mood, affective, maniac, bipolar, depressive |
| eHealth | ehealth, e-health, electronic health, mobile health, mhealth, m-health, telemedicine, digital health, digital-health, telecommunication technologies, tele health, tele-health, telehealth, tele care, tele-care, telecare, tele medical, tele-medical, telemedical, tele psychiatry, tele-psychiatry, telepsychiatry, phone call, phone-call, phonecall, medicalapps, medical apps, medical-apps, medical applications, medical-applications, webapp, web-app, web app, web application, web-application, webapplication, webportal, web-portal, web portal, internet monitoring, internet-monitoring, digital monitoring, digital-monitoring, internet delivered, internet-delivered, videoconference, video-conference, video conference, mobile intervention, mobile-intervention, mobileintervention |
| Medication adherence | Medication adherence, medication compliance, medication concordance, treatment adherence, treatment compliance, treatment concordance |
Study Screening Mode
The review was conducted with the help of CADIMA, an open access online tool that supports the conduction and reporting of systematic reviews and systematic maps.33
The screening for the inclusion criteria was conducted independently by three reviewers (ML, NP and LR) according to the PRISMA standard. Prior to the study selection, all reviewers performed a consistency check in order to increase interrater reliability. The consistency check finished after the kappa value between all reviewers reached 0.51.
Critical Appraisal
For the risk-of-bias analysis, we followed the Cochrane Collaboration Handbook for systematic reviews of Interventions with the revised Cochrane risk-of-bias tool for randomized trials (RoB 2).32 For the assessment of non-randomized interventional studies, we used the Risk of bias in non-randomized studies of interventions (ROBINS-I) assessment tool.34
Data Extraction
For each included article, a priori defined parameters were systematically extracted and presented in a results table. The extraction process was conducted with a structured data extraction worksheet. Equal sets of publications were assigned to each of the three reviewers for data extraction. Afterwards, the extracted data were double-checked by at least one other reviewer. Supplementary materials related to the included publications were considered in the data extraction process.
Analysis and Presentation of Results
The results of the included studies are presented in tables and charts. A systematic narrative synthesis35,36 was performed with information presented in the text and tables to summarize and explain the characteristics and findings of the included studies. For each included article, the information as described in “Data extraction strategy” has been systematically extracted and presented in a results table. After that, the results were compared and differences or similarities between the studies were highlighted. The research questions served as a framework for the narrative synthesis. Categories were formed by performing an deductive-inductive content analysis according to Gläser and Laudel.31
Results
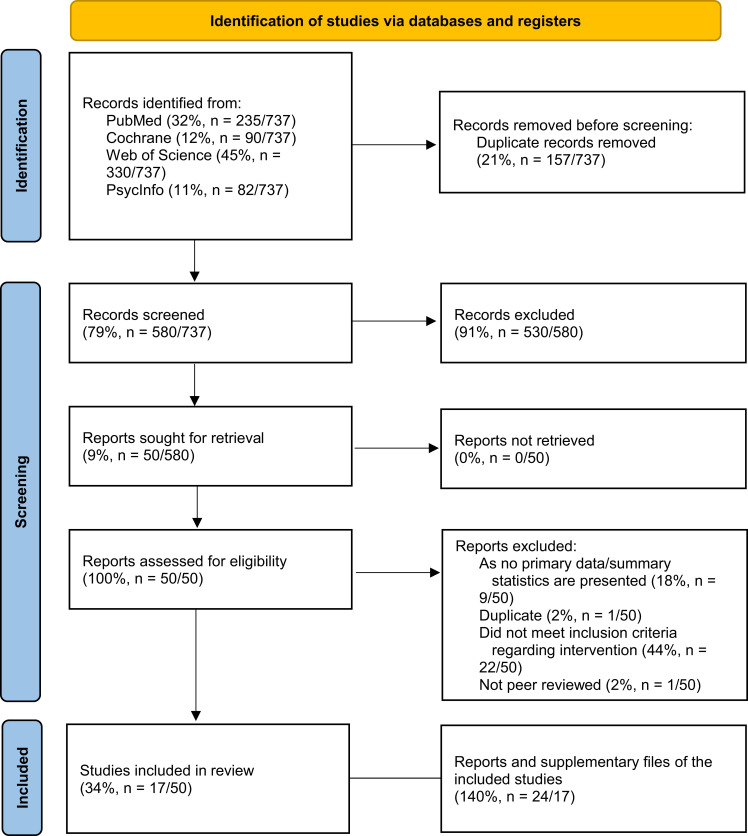
A total of 737 records were identified through database searching on October 23, 2020. After duplicate removal, 580 articles were included, of which 17 (3%, n=17/580) articles met all inclusion criteria (Figure 1). A list of the excluded articles is available from the authors on request.
Figure 1.
PRISMA 2020 flow diagram (A “study” includes a defined group of participants and one or more interventions and outcomes and might have multiple “reports”, that can be any document providing information).
The articles were published between 2007 and 2020. Thereof, 11 (65%, n=11/17) were randomized controlled trials, four had a pre-/post-design (24%, n=4/17), and two were non-randomized controlled clinical trials (12%, n=2/17). Table 3 summarizes the results of the studies’ data extraction. See Table 4 for a more detailed description of the interventions.
Table 3.
Results of the Data Extraction
| Reference | Type of Intervention* | Study Design | Trial Arms | Population (Intervention Group) | Setting / Provider Type | Personal Contact | Medication Assessed | Adherence Measure | Adherence Measure Outcome | Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Pauly et. al. 201548 | Multi-faceted intervention | CCT | Two: multi-dimensional and inter-sectoral program, TAU | Psychiatric patients with focus on depression and anxiety (n=269) | Stationary and Ambulatory setting | Yes | NI | MARS-5 | Three months follow-up: 1. CG: mean 22.47 (SD 2.99) 2. IG: mean 23.75 (SD 2.08). Adjusted effect of the intervention 1.33 points (95% CI 0.73–1.93) |
+ (significant) |
| Corden et al 201637 | Single-faceted intervention/mobile intervention | Pre-/post design | One: Mobile App, no CG | Patients with depression (n=11) | Ambulatory setting | No | Antidepressants | Pill counting | Baseline: 88.5% Follow-up: 73.0% (p=0.16). | - (not significant) |
| Schulze et al 201925 | Single-faceted intervention/ Telehealth | RCT | Two: Telephone intervention, TAU | Patients with Schizophrenia or BD (n=120) | Ambulatory setting | Yes | Antipsychotics | MARS-5-D | OR=4.11, 95% CI=1.47–11.45, p=0.007 | + (significant) |
| Gliddon et al 201949 | Internet-based self-management program | RCT | Three: Discussion forum only (Group 1), Discussion forum plus psychoeducational modules (Group 2) or plus psychoeducational modules and CBT-based interactive tools (Group 3) | Patients with Schizophrenia or BD (n=322) | No specific setting mentioned | Yes | NI | MARS-10 | Cohen´s effect size: 1) Comparison Group 2 and Group 1 (12 months follow-up): −0.21, p = 0.48 2) Comparison Group 3 and Group 1: −0.45, p= 0.21 |
- (not significant) |
| Himelhoch et al 201350 | Single-faceted intervention/ Telehealth | RCT | Two: Telephone intervention, F2F | HIV infected patients with depression (n=147) | Ambulatory setting | Yes | HAART | Pill counting | (adherence = pills counted/pills prescribed) IG: 0.83 ± 0.27 vs CG: 0.68 ± 0.21, t = 2.07; p = 0.04, effect size: 0.60 |
+ (significant) |
| Hammonds et al 201538 | Single-faceted intervention/ mobile intervention | RCT | Two: Mobile App, TAU | Patients with depression (n=57) | No specific setting mentioned | No | Antidepressants | Pill counting | X² (1, N=40) = 3.64, 95% CI [0.945, 12.966], p=0.057, ɸ=0.30, | + (not significant) |
| Cohen et al 202052 | Multi-faceted intervention | RCT | Two: Telehealth, TAU | Patients with diabetes and co-occurring depression (n=30) | No specific setting mentioned | Yes | Cardiovascular medications, antidepressants, adjunct antidepressants, diabetes medication and insulin, all medications combined | Pill counting | Follow-up mean difference between the groups (95% CI): 1. cardiovascular medications (10.3; 95% CI –10.0 to 30.6) 2. diabetes medications and insulin (8.9; 95% CI –20.0 to 37.4) 3. antidepressants (8.5; 95% CI –54.4 to 37.4) 4. adjunct antidepressants (15.3; 95% CI –40.4 to 71.1) 5. all medications combined (2.3; 95% CI –13.7 to 18.3) |
+ (not significant) |
| Choudhry et al 201739 | Single-faceted intervention/ telehealth | RCT | Four: Three Devices (one of them eHealth), TAU | Patients with depression (n=15,948) Patients with other chronic conditions (n= 37,532) | No specific setting mentioned | No | Antidepressants or medication for cardiovascular disease or other non-depression chronic condition | MPR | Digital timer cap vs control: OR (95% CI) = 0.97 (0.84–1.11) | - (not significant) |
| Aikens et al 201540 | Single-faceted intervention/ mobile intervention | Pre-/post design | Two: IVR with support person, IVR TAU | Patients with depressions (n=221) | No specific setting mentioned | Yes | NI | MGL Scale | IG: the odds of antidepressant medication adherence increased in IG 31% per week (AOR=1.31, 95% CI: 1.16–1.47, p<0.001) vs 11% for controls (AOR=1.11, 95% CI: 0.99–1.24, p=0.070) | Not applicable, because no comparison with CG |
| Gervasoni et al 201041 | Single-faceted intervention/ telehealth | CCT | Two: Telephone intervention, TAU | Patients with depressions (n=131) | Ambulatory setting | Yes | Psychotropic drugs | Paroxetine plasma level | Paroxetine plasma level (ng/mL) intervention: Med=24, Range=1–87 control: Med=24, Range=7–126; p=0.88 | 0 (no difference) |
| Fortney et al 200742 | Multi-faceted intervention | RCT | Two: Telemedicine, TAU | Patients with depressions (n=395) | Ambulatory setting | Yes | NI | Single item from questionnaire (number of days tablets were taken as prescribed) | IG vs CG, 12 months: OR=2.7, p=0.01 | + (significant) |
| Sajatovic et al 201545 | Multi-faceted intervention | Pre-/post design | One: Device, no CG | Patients with BD (n=5) | No specific setting mentioned | No | NI | MGL Scale | Mean scores on the Morisky scale improved from baseline to follow-up (3.20–3.60) | + (no further statistical details) |
| Lobban et al 201746 | Internet-based self-management program | RCT | Two: Online intervention, TAU | Patients with BD (n=109) | No specific setting mentioned | Yes | Mood stabilizer, other | MARS-10 | 48-week follow-up estimate: difference between beta estimates −0.356, CI 95% (−1.39 to 0.674), p=0.50 | - (not significant) |
| Moore et al 201551 | Single-faceted intervention/ mobile intervention | RCT | Two: Texting, Active control | HIV infected patients with BD (n=62) | No specific setting mentioned | Yes | Psychiatric medication (PSY), antiretroviral medications (ARV) | Pill counting | Mean adherence: ARV (IG 86.2% vs CG 84.8%; p=0.95, Cliff’s d = 0.01) and PSY (IG 78.9% vs CG 77.3%; p = 0.43, Cliff’s d = −0.13) Dosing time (Median): ARV: IG: 27.8 vs CG: 77.0 min from target time; p=0.02, Cliff’s d = 0.37; 16PSY: IG: 46.8 vs CG: 66.5, p=0.42, Cliff’s d = 0.14 |
ARV: + (not significant) PSY: + (not significant) (but improved dosing time for ARV (significant) |
| Salisbury et al 201643 | Internet-based self-management program | RCT | Two: Telehealth, TAU | Patients with depression (n=726) | Ambulatory setting | Yes | Antidepressants | MGL Scale | Adjusted difference in means after 12 months’ follow-up (CI 95%): –0.1 (–0.2 to 0.1); p= 0.511 | - (not significant) |
| Hungerbuehler et al 201644 | Single-faceted intervention/ telehealth | RCT | Two: Videoconferencing consultations, F2F | Patients with depression (n=107) | Ambulatory setting | Yes | Antidepressants | MGL Scale | Participants in the F2F group tended to be more adherent than participants in the videoconferencing group at 12 months (X2 = 2.864, p=0.07). | - (not significant) |
| Levin et al 201947 | Internet-based self-management program | Pre-/post design | One: Texting, no CG | Patients with BD (n=38) | Ambulatory setting | Yes | Bipolar medications, antihypertensives | Tablets Routine Questionnaire (TRQ) and pill bottle openings (eCAP) | Antihypertensive and bipolar drug adherence improvement was significant between screen and baseline, screen and V1, and screen and V2. 1. Past-Week-TRQ: 1.1 Hypertensives: χ2 (3) = 34.55, p< 0.001, W= 0.30 1.2 Bipolar medications: χ2 (3) = 18.97, p< 0.001, W= 0.17 2. Past-Month-TRQ: 2.1 Hypertensives: χ2 (3) = 35.39, p< 0.001, W= 0.31 2.2 Bipolar medications: χ2 (3) = 26.17, p< 0.001, W= 0.23 3. eCAP (Hypertensives): 3.1 Past-Week: χ2 (2) = 0.23, p= 0.89 3.2 Past-Month: χ2 (2) = 0.07, p= 0.97 |
+ (significant) |
Notes: The column “effect” shows the direction of the results reported regarding medication adherence, with the assessment of significance followed the information given in the reports (‚+‘Positive effect, ‚0‘No effect, ‚-‘Negative effect). *See Table 4 for a detailed description of the intervention.
Abbreviations: AOR, adjusted odds ratio; BD, bipolar disorder; CCT, controlled clinical trial; CG, control group; CI, confidence interval; F2F, face to face; HAART, highly active antiretroviral therapy; IG, intervention group; IVR, interactive voice response; MARS-10, medication adherence rating scale by Thompson et al89 MARS-5, medication adherence report scale by Horne;90 MARS-5-D, medication adherence report scale by Horne (German edition);90 MGL Scale, Morisky/Green/Levine medication adherence scale;91 MPR, medication possession ratio; NI, no information.
Table 4.
Detailed Description of the Interventions of the Studies Included in This Review
| Reference | Description of Intervention |
|---|---|
| Pauly et. al. 201548 | Multi-dimensional and inter-sectoral program that includes: Medication management with focus on simplification of therapy regimen; Counseling of patients during the hospital stay with verbal and written information and a medication plan at discharge; Phone calls 1.5 weeks and six weeks after discharge. Description of side effects in the information leaflets is adjusted for every patient. |
| Corden et al 201637 | A digital support system that intends to enhance antidepressant treatment processes and outcomes: Participants receive weekly alerts to read a unique didactic lesson and answer a set of questions to ensure knowledge about antidepressants and impart information. In the absence of a detectable medication adherence event within ten minutes of the patient’s dose time, users receive reminder notifications via android pop-ups. |
| Schulze et al 201925 | Proactive, regular telephone calls every second week for six months from three specially trained nurses in order to increase medication adherence. The telephone calls consist of standardized and individualized parts. If medication nonadherence or intolerable side effects are reported, participants are advised to see a physician. There is the option to receive individual text messages referring to a specific topic raised in the telephone calls. |
| Gliddon et al 201949 | Online self-guided intervention that includes educational modules, interactive tools, and discussion forums: Discussion forum plus psychoeducational modules (Group 2); Discussion forum, psychoeducational modules, plus cognitive behavioural therapy (CBT)-based interactive tools (Group 3). All participants have access to a peer discussion forum for the duration of the study period; with one forum per randomization arm. Before, discussion posts are screened by a moderator to going “live” for participants. There are five psychoeducational modules delivered bi-weekly followed by four booster modules delivered at three, six, nine, and twelve months. The CBT-based tools are delivered in conjunction with the initial five modules, but could only be accessed by Group 3. |
| Himelhoch et al 201350 | 11-session manualized telephone CBT intervention: One initial evaluation session, five sessions of behavioural activation and five sessions of cognitive restructuring delivered over a 14-week period. Participants are given a study workbook and the name and telephone number of their therapist. The therapist contacts the participant to set up the first session. |
| Hammonds et al 201538 | A medication reminder app is installed to the participant’s smartphones and prescribed information regarding dosing are entered. The use of the reminder app is instructed and the understanding of the app’s functions are demonstrated. Participants are required to use the medication reminder app to indicate when they have taken their medication by responding to the message received. |
| Cohen et al 202052 | Pharmacist-led telehealth: One-on-one in-person visit divided into two parts. The first part lasts approximately 45 minutes and replicates the usual care visit. For the second part of the visit, patients are asked to bring in all of their medications, including non-prescription and alternative medications. The telehealth research pharmacist answers questions and concerns. All subsequent interactions between the pharmacist and the patient are completed via telehealth equipment and phone contacts. The patient’s primary care physician is alerted to all medication changes. |
| Choudhry et al 201739 | Patients in the intervention arms receive a free device in a mail along with an information card explaining how to use the device as well as contact information to obtain additional information during the trial. Patients randomized to the pill bottle strip with toggles arm or the digital timer cap arm receive one device for each targeted medication. Patients randomized to the standard pillbox arm received one device to use for all of their medications. |
| Aikens et al 201553 | Patients receive weekly IVR calls: The system makes up to nine call attempts at patient-selected day/time combinations. The program is provided to all patients for at least six months. Assessed are patients’ symptoms of depression, adherence and functional impairment. Clinician alerts can be triggered. After each completed call, patients’ designated support persons are emailed a summarized report with detailed suggestions on strategies for supporting the patient’s selfmanagement. |
| Gervasoni et al 201041 | Patients receive a brief high-intensity structured telephone intervention: Patients are contacted by phone on three occasions during the first two weeks by an experienced research nurse. Each contact included a brief structured assessment of current depressive symptoms, current use of antidepressant medication and antidepressant side-effects, and motivational support for study adherence. If a problem is reported, the nurse contacts the psychiatrist. |
| Fortney et al 200742 | Stepped-care model of depression treatment: Treatment intensity is increased for patients failing to respond to lower levels of care by involving a greater number of intervention personnel with increasing mental health expertise. Patients receive the intervention until a 6-month continuation phase is complete or for 12 months of acute phase treatment. |
| Sajatovic et al 201545 | The DialogMeds-BD (BD = Bipolar Disorder) components include: an automated pill cap with remote monitoring sensor, a multimedia adherence enhancement program and a treatment incentive program for motivating patients to remain adherent and to improve treatment-related knowledge and skills. System data are intended for viewing by clinicians in real-time. |
| Lobban et al 201746 | ERPonline (ERP = Enhanced Relapse Prevention) is a free-to-access, web-based, self-management resource. The aim is to help people develop a coherent working model of their mood changes, recognize and manage triggers and early warning signs, and develop coping strategies to manage these effectively. Each module includes information, suggested strategies, and case examples. Users interact with the site to input personal information relevant to their own triggers, early warning signs, and coping strategies. It is possible to involve a close friend or relative as supporter. |
| Moore et al 201551 | Two-way, text messaging system that includes: Face-to-face psychoeducation that lasts approximately 30 minutes and personalized daily text message as medication reminders and mood assessments. Separate messages are sent for each medication at each dosing time and are automated by a central server. After five days of failure to respond to text messages, the study coordinator calls the participant. |
| Salisbury et al 201643 | Integrated telehealth service that cover regular telephone calls from a health adviser who is supported by an interactive software. The advisers support participants in addressing their own health goals and direct them to relevant online resources. After an initial assessment and a goal-setting telephone call, the advisers call each participant on six occasions over four months, and make up to three more calls at two month intervals to provide reinforcement and to detect relapse. The advisers send regular progress reports to participants’ general practitioner and copy them to participants. In cases of inadequate treatment response, the advisers contact participants’ general practitioners. |
| Hungerbuehler et al 201644 | Participants perform monthly home-based consultations with their psychiatrists using live interactive videoconferencing: There are in-person consultation at the beginning of the study, after six months and after twelve months. In between those consultations, the participants in the intervention group underwent five home-based video consultations once a month. The medications for these patients are delivered to the patient’s home. |
| Levin et al 201947 | After a face-to-face, individually psychoeducation program, an automatic SMS intervention was administered. Month one: participants receive one daily text message with psychoeducational or motivational content and a daily question assessing mood. Month two: specific medication reminders, contextual cues and immediate reinforcement are added and daily mood rating continued. After three consecutive days of unacknowledged messages, the automated system sends an outreach message. The research assistant has real-time access to participants’ response logs to identify technical problems. |
Characteristics of the Study Populations
The included studies addressed patients with diagnoses which belong to affective disorders according to the ICD-10 catalogue (F30-F39), like: patients with depression,37–44 patients with BD,45–47 psychiatric patients with depression and anxiety,48 and patients with schizophrenia or BD.25,49 Studies were also included, if they addressed affective disorders as comorbidity, for example, HIV infected patients with depression50 or BD,51 and patients with diabetes and co-occurring depression.52 The number of included patients varies between five (feasibility study)45 and 15,94839 (Table 3).
Ten of the studies targeted participants aged 18 years and older,25,37–39,43–46,48,51 two studies targeted participants aged ≥21 years.47,49 Three studies had as additional inclusion criteria the access to internet,44,46,49 two the access to a landline phone,50,52 one study to telephone/internet/email,43 and one to a smartphone.38
Characteristics of Intervention and Comparison
Ten studies (59%, n=10/17) were controlled with treatment as usual,25,38–43,46,48,52 two (12%, n=2/17) with face to face therapy44,50 and one (6%, n=1/17) with a peer support control group.49 Three studies (17%, n=3/17) had no control group37,45,47 and one (6%, n=1/17) study had an active control51 where participants received psychoeducation and mood inquiries, but did not receive medication reminders.
Four studies (23%, n=4/17) comprise a multi-faceted intervention.42,43,45,48,52 Nine studies (54%, n=9/17) evaluated single-faceted interventions, whereof four studies (44%, n=4/9) examined mobile interventions,37,38,40,51 and five studies (56%, n=5/9) evaluated real-time telehealth interventions including telephone interventions,25,41,50 telemedicine,39 and video consultation.44 Finally, four (23%, n=4/17) internet-based self-management programs43,46,47,49 could be identified.
Eight studies (47%, n=8/17) were conducted in an ambulatory,25,37,41–44,47,50 one (6%, n=1/17) in a combined stationary and ambulatory setting48 and eight studies (47%, n=8/17) did not mention any specific setting.38–40,45,46,49,51,52
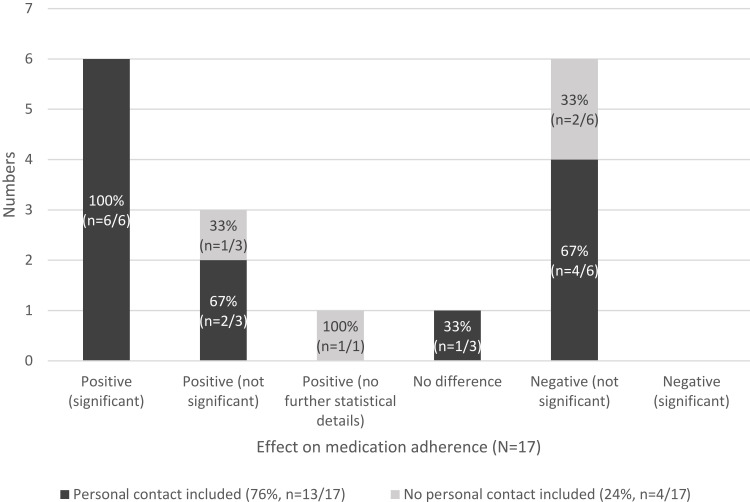
The majority of interventions (76.5%, n=13/17) included personal contact.25,40–44,46–52 In this review, personal contact is understood as a communicative interaction between the participants and therapists, the study personnel or peers. One intervention included an informal personal contact, defined as contact from a private setting,40 while twelve interventions had a formal personal contact, defined as contact by healthcare provider or study assistant25,41–44,46–52
Regarding the intervention, one study (6%, n=1/17) had an observation period less than one month,45 six studies (35%, n=6/17) between one and six months,37,38,47,48,50,51 three studies (18%, n=3/17) between six months and one year,25,46,52 six studies (35%, n=6/17) had an observation period of one year39,40,42–44,49 and one study (6%, n=1/17) provided no information about the observation period.41
Outcome of the Studies
Five studies (29%, n=5/17) reported medication adherence to antidepressants.37–39,43,44 Antidepressants in combination with diabetes medication was reported once52 (6%, n=1/17) as well as antipsychotics25 (6%, n=1/17), highly active antiretroviral therapy50 (6%, n=1/17), psychotropic drugs41 (6%, n=1/17), mood stabilizer46 (6%, n=1/17), bipolar medications and antihypertensives47 (6%, n=1/17), and psychiatric medication for patients with BD and antiretroviral medications for patients with HIV51 (6%, n=1/17). Five studies (29%, n=5/17) did not specify the medication of the included patients.40,42,45,48,49
Nine studies (53%, n=9/17) conducted a subjective method by using a questionnaire to measure medication adherence. Thereof, MGL Scale was used four times40,43–45 (44%, n=4/9), MARS-525,48 (22%, n=2/9) and MARS-1046,49 (22%, n=2/9) two times. Single-items from a questionnaire42 were used once (12%, n=1/9). An objective measurement method was used seven times (41%, n=7/17). Thereof, pill counting37,38,50–52 was used five times (72%, n=5/7), and medication possession ratio39 (14%, n=1/7) and paroxetine plasma level41 (14%, n=1/7) were used once. One study (6%, n=1/17) used both a subjective and an objective method by a tablet’s routine questionnaire as a self-report measure of the percentage of days with missed doses of a given medication in the past month, and by pill bottle openings.47
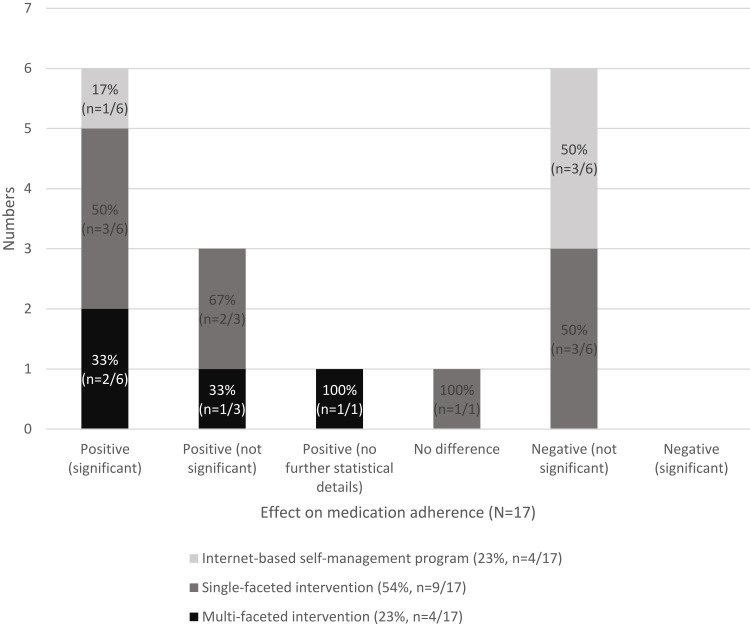
The majority (59%, n=10/17) of the interventions25,38,42,45,47,48,50–52 had a positive effect on medication adherence in patients with affective disorders. Six studies25,42,47,48,50,51 (35%, n=6/17) showed a statistically significant positive effect on medication adherence. The other studies showed no effect41 (6%, n=1/17) or not significant negative effects37,39,43,44,46,49 (35%, n=6/17). One study (6%, n=1/17) showed a significant positive effect on medication adherence in the longitudinal analysis, but not in comparison to the control group.53 None of the studies reported a statistically significant negative effect.
All multi-faceted interventions had a positive effect (n=4, 100% of all interventions of this type), but the results of two studies were not significant. The effective interventions include medication management,48,52 treatment planning,45 or second opinions by a healthcare professional42 (Figure 2).
Figure 2.
Effects on medication adherence reported in the included studies in relation to different types of intervention (n=17).
Single-faceted mobile, phone, or video interventions showed mostly a positive effect on medication adherence (55%, n=5/9), which was often statistically significant (60%, n=3/5). Effective interventions in this category include remote psychiatric therapy via phone,50 regular phone calls to assess, for example, mood and medication issues,25 or reminders via text messages.51 Interventions in this group without a significant better result for the intervention compared to the control group or no differences between the groups were medication reminder notifications,37 pill bottles with a digital timer cap,39 monitoring phone calls,41 reminders via mobile app,38,40 or video consultations.44
One of the internet-based self-management interventions (25%, n=1/4) showed a positive, statistically significant effect on medication adherence. This intervention47 consisted of providing psychoeducational and a medication adherence building program via text-messages. The self-management interventions without a statistically significant effect (75%, n=3/4) provided online group discussion forums without or with additional psychoeducational materials to a different extent,49 an online psychoeducational information in several modules for building coping strategies,46 or psychoeducational training in combination with monitoring phone calls.43
All interventions which showed statistically significant positive effects on medication adherence also included personal contact (100%, n=6/6) (Figure 3).
Figure 3.
Effects on medication adherence reported by the included studies subdivided by interventions with and without personal contact including (n=17).
The interventions that have shown statistically significant effects were addressing equally, (comorbid) BD25,47,51 (50%, n=3) and (comorbid) depressions42,48,50 (50%, n=3).
Risk of Bias in the Included Studies
The risk of Bias32,34 was assessed for each included article. The risk-of-bias analyses were conducted in order to assess the risk of bias in the findings of the studies. The results are not suitable to assess the methodologic quality of studies. Six of the included RCTs (54%, n=6/11) had a “low” risk of bias25,39,42,43,49,50 and five (46%, n=5/11) showed “some concerns”38,44,46,51,52 (Table 5). Considering the non-randomized studies, three studies (50%, n=3/6) had a “moderate” risk of bias,40,45,48 two (33%, n=2/6) had a “critical” risk of bias,37,47 and one (17%, n=1/6) possessed a “serious” risk of bias41 (Table 6). In RCTs, unblinded participants and/or study assessors were the main reasons for a risk of deviation from the intended interventions. As with many telemedical, psychologic intervention, it is not always possible to blind participants and study employees to the intervention received by the participants.
Table 5.
Results of the Risk of Bias Analysis of Randomized Interventional Studies (RCTs) According to the RoB 2 Tool
| Publication | Randomization Process (eg Imbalanced Prognostic Factors Between Intervention and Control Group and/or Controlling for Confounder is Missing) | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall Risk-of-Bias |
|---|---|---|---|---|---|---|
| Schulze et al, 201925 | Low | Low | Low | Low | Low | Low |
| Gliddon et al, 201949 | Low | Low | Low | Low | Low | Low |
| Himelhoch et al, 201350 | Low | Low | Low | Low | Low | Low |
| Hammonds et al, 201538 | Low | Some concerns | Low | Low | Low | Some concerns |
| Cohen et al, 202052 | Some Concerns | Low | Low | Low | Low | Some concerns |
| Choudhry et al, 201739 | Low | Low | Low | Low | Low | Low |
| Fortney et al, 200742 | Low | Low | Low | Low | Low | Low |
| Lobban et al, 201746 | Low | Some concerns | Low | Some concerns | Low | Some concerns |
| Moore et al, 201551 | Some Concerns | Some concerns | Low | Low | Low | Some concerns |
| Salisbury et al, 201643 | Low | Low | Low | Low | Low | Low |
| Hungerbuehler et al, 201644 | Low | Some concerns | Low | Low | Low | Some concerns |
Table 6.
Results of the Risk of Bias Analysis of Non-Randomized Interventional Studies According to the ROBINS 1 Tool
| Publication | Bias Due to Confounding | Bias in Selection of Participants into the Study | Missing Outcome Data | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Risk-of-Bias |
|---|---|---|---|---|---|---|---|---|
| Pauly et al, 201548 | Low | Low | Moderate | Low | Low | Moderate | Moderate | Moderate |
| Corden et al, 201637 | Critical | Low | Low | Low | Low | Moderate | Low | Critical |
| Aikens et al, 201553 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Gervasoni et al, 201041 | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Sajatovic et al, 201545 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Levin et al, 201947 | Critical | Low | Low | Low | Low | Moderate | Low | Critical |
Considering the studies which showed a significant positive effect on medication adherence, three (50%, n=3/6) had an overall low risk of bias, one had some concerns (17%, n=1/6), one (17%, n=1/6) had a moderate risk of bias, and one (17%, n=1/6) had a critical risk of bias.
The judgements in the column randomization process in Table 5 and bias due to confounding in Table 6 considered the question whether the included studies have adequately considered the potential confounders for medication adherence during the randomization process and the analysis. As Table 5 shows, most of the randomized studies (82%, n=9/11) had no concerns on this issue, two studies (18%, n=2/11) had some concerns. However, considering the non-randomized studies, one study (16%, n=1/6) had a low risk of bias due to confounding and five studies (84%, n=5/6) had at least a moderate risk of bias.
The risk-of-bias analysis is helpful in order to appraise which studies had the highest potential to produce statistically significant results regarding the effectiveness of eHealth interventions for improving medication adherence in patients with affective disorders. Especially, feasibility studies with only a small number of participants or just descriptive results are often rated as having a high risk of bias, even though they might be performed at a high level of methodological quality. Despite this fact, feasibility studies are indispensable for developing and identifying new interventions which have the potential to improve mental health care.
Discussion
The primary aim of this systematic review was to analyse, whether eHealth applications can improve medication adherence in patients with affective disorders. Furthermore, we sought to categorize the different medication enhancement interventions that can be found and to identify which of these interventions show good results. Differences in the results due to patient characteristics, like age, sex, severity of disease, diagnosis, specific drug, and duration of illness were also considered. In addition, differences in the addressed healthcare settings and the type of intervention were considered.
EHealth applications for improving medication adherence in patients with affective disorders range widely from electronic provision of psychoeducational training material, which we categorized as internet-based self-management applications, over monitoring phone calls at regular intervals, which we summarized under the term single-faceted interventions, up to multi-faceted interventions programmes addressing multiple dimensions of medication adherence. The majority of the included studies evaluated single-faceted interventions (52%, n=9/17). Four studies each assessed multifaceted interventions (24%, n=4/17) and internet-based self-management programs (24%, n=4/17). It can be seen that multifaceted interventions and internet-based self-management programs are understudied in comparison with single-faceted interventions. Even if most of the included studies had a randomized, controlled design, many of them were limited due to a small number of participants and short-term follow-up. Long-term investigations on eHealth interventions in cross-sectoral settings evaluating the sustainability of positive effects on medication adherence for a broad population of affected people are lacking.
Most of the interventions addressed persons with affective disorders exclusively, some interventions targeted at persons who have other (chronic) morbidities as well, like diabetes or HIV. Six (35%, n=6/17) interventions addressed patients with (at least comorbid) BD while 11 (65%, n=11/17) interventions addressed people living with depression. One reason for the smaller number of interventions addressing BD than depression may be that the lifetime prevalence of BD (2.4%54) is relatively low compared to the lifetime prevalence of depression (10.8%55). Another reason may be that interventional studies on patients with BD are challenging to conduct due to the fact that the ability to remember and perform tasks often is impaired in patients with BD.56 However, out of the included studies which has shown significantly positive effects on medication adherence (35%, n=6/17), the same number of interventions addressed patients with depression (50%, n=3/6) and patients with BD (50%, n=3/6).
All eHealth-based multi-faceted interventions (100%, n=4/4) were effective in order to improve medication adherence in patients with affective disorders. However, the statistical evidence for the positive effect of this type of intervention often lacks (50%, n=2/4). Many of the single-faceted interventions (56%, n=5/9) showed also positive effects, but only a few with statistical significance (33%, n=3/9). The fact that multi-faceted interventions are relatively more often effective than single-faceted interventions is in line with another review.57 Nieuwlaat et al58 hypothesise in a review on interventions for enhancing medication adherence that multi-faceted interventions are superior in comparison to single-faceted interventions in terms of improvement of medication adherence, but that studies on multi-faceted intervention often do not apply a factorial design, which would allow to identify the most effective components with statistical significance. However, clinical trials effectively testing single-faceted interventions or certain components of eHealth interventions for medication adherence enhancement need rigorous control conditions. For example, Moore et al used a control group that, like the intervention group, received text messages in which the mood was asked. The intervention group received, in addition, individualized medication reminders. As a result of the positive effect of the control condition on medication adherence, both groups showed high medication adherence rates compared with other studies at the end of the trial, but Moore et al could not find a significant difference between intervention and control group.51
One internet-based, psychoeducational self-management programme (25%, n=1/4) showed a statistically significant positive effect on medication adherence and three interventions of this type (75%, n=3/4) tended to show negative effects, but are statistically not significant. A reason for this may be that evaluation of self-management programmes is more difficult than that of other types of intervention, because controlling of people using self-management programmes in private settings might be more challenging, or crossover biases might occur. Another reason might be that patients do less continuously use interventions or do not consequently perform given tasks when they are responsible for coping with their mental disorders.59
Another review on eHealth and mHealth interventions seeking to enhance medication adherence in patients with serious mental illness also showed ambiguous results regarding effectiveness, even though feasibility and acceptability of eHealth interventions could be confirmed.60
Nonetheless, none of the included studies showed statistically significant negative effects on medication adherence. Thus, it can be assumed that eHealth interventions are at least as effective as usual care in terms of improving medication adherence in patients with affective disorders.44 However, given the same effectiveness, eHealth interventions can have additional benefits for the healthcare provision in certain settings like in rural or underserved regions.42,49,50 Additionally, benefits usually related to eHealth interventions are, for example, improved cost-effectiveness,61 access26 especially for minorities and people who fear stigmatization of mental health care,62,63 and maybe reduced hospitalizations.64
It is noteworthy that all interventions which have shown a significant effect on medication adherence included personal contacts. For example, it has been shown that provider-guided interventions are more effective than their self-guided counterparts.25,39,48,49,52,53 Provider-guided interventions are also more sustainable.39 There seems to be no difference in terms of effectiveness between nurse-led and pharmacist-led eHealth interventions.52 However, studies comparing different interventions or components in terms of their cost-effectiveness are lacking. Also, other studies on the effectiveness of eHealth interventions pointed out that the personal contact with a healthcare professional or non-professional person can support medication adherence.65,66 For example, patients are concerned that without personal contact advices might be performed wrongly or they might lose their motivation for the intervention.67 Choi68 showed that informal social support and eHealth can synergistically promote healthy behaviour. Moreover, telehealth group therapy seems to produce outcomes similar to in-person group therapy.44,45,69 However, there are no studies, yet, that are designed to compare personal guided to fully automated eHealth interventions.70
Other studies71,72 suggest that rates of nonadherence in patients with affective disorders are comparable to other patients with long-term conditions. One included study51 showed that daily text message reminders for HIV infected patients with affective disorders had neither a significant effect on their adherence to psychiatric medication nor to antiretroviral medication. However, due to the intervention, patients took their antiretroviral medication closer to the prescribed dosing time which mean the prescribed time of taking the medication. Such an effect was not found regarding the psychiatric medication. The study suggests that adherence enhancement interventions can work differently for individual drug regimens and might affect various aspects of medication adherence, like dosing time.
The investigation of medication adherence inheres some potential biases. Interventions that cannot be blinded inhere potential performance bias. If medication adherence is measured via self-report, there might be a social bias. People might tend to answer socially desirable with the result that medication adherence might be overestimated.73–77 Moreover, it is challenging to assess medication adherence in a control group without likewise affecting the medication adherence in this group due to the Hawthorne-effect. People might change their behaviour as a response to observation and assessment.78 Hence, it might be difficult, even for randomized controlled studies, to show significant effects on medication adherence.79
Moreover, medication non-adherence is a multifactorial issue. Potential confounding factors are, for example, beliefs about stigmatization,80 polypharmacy,23 social support,17 specific drug prescribed, and specific disorder.5,23 Against this background, the included studies are often limited, because they do not report all of the potentially relevant confounding factors. For example, none of the included studies reported the specific drugs which the participants were taking. However, as Keyloun et al have shown for patients with MDD, different classes of medications have distinct side effect profiles and toxicities, and therefore may affect the medication adherence.81
Most of the studies in this review (53%, n=9/17) assessed medication adherence by using subjective means like the MARS questionnaire. Even if objective means are often preferred, using subjective means is not seen as a weakness of the included studies, because former studies on non‐adherence with mood stabilizers showed that self‐report of missing prescribed medication is highly comparable with independently assessed adherence, eg by measuring serum lithium levels in patients with severe mental disorders and prescribed lithium therapy.82,83 Furthermore, objective methods can also be biased considering the time of ingestion of medication and measurement of drug level, pharmacokinetic variability, expense and obtaining consent from a patient who may already be non‐adherent. For example, the monitoring of plasma concentrations of antidepressant drugs show considerable inter-individual variation.16 Some indirect methods try to identify individuals at risk of non‐adherence. Research studies show statistically significant correlations between measures of attitudes and objective measures of adherence.15 However, none of the included studies used indirect measures which might be more cost-effective and less invasive than direct measures.
This review planned to analyse if there were any differences in the interventions’ effect on medication adherence related to the study setting, ethnicity of the patients, secondary diagnoses, length of illness, or total number of drugs prescribed. Since the required information was often omitted or reported differently between the studies, we could not draw conclusions on these parameters.
Another important aspect of telemedicine is security. Only one of the included studies (6%, n=1/17) reported that they used Skype for videoconferencing and that the encryption quality meet the Advanced Encryption Standard specified by the US National Institute of Standard Technology. However, security in telemedicine comprises more than transmission reliability. For example, telemedicine must also demonstrably ensure and document the authenticity of the involved participants. Moreover, the safety and health of the patients must also be guaranteed, even if a telemedical service is timely not available.84
This review included studies between 2007 and 2020 (when the phases of study selection and analysis began). Another search on PubMed were performed on 2022–11-17 by using the original search term and applicating the inclusion criteria in order to check for new publications on the research question that were published recently. Otherwise, none of the results met the inclusion criteria, so that there seems to be no recent publications within the scope of this review. Finally, none of the included studies considered barriers and facilitators for the implementation of eHealth interventions in real-world settings.
Strengths and Limitations
A strength of the review is that it is not restricted to a certain setting, medication or study design, which allows to present detailed contextual information of the different eHealth interventions addressing patients with affective disorders. This review includes a considerable number of RCTs, which allows it to estimate the effect of eHealth intervention on medication adherence well.
Moreover, this review followed the PRISMA guideline for reporting systematic reviews.33 The processes of searching, study selection, critical appraisal, and data extraction were performed in a standardized way by using the online tool CADIMA.33 The study selection, critical appraisal, and the data extraction were conducted by three researchers in parallel. Before that, the interrater reliability of all reviewers was tested until the kappa value indicated that the agreement between all reviewers was acceptable.
For the interpretation if an effect was statistically significant or not, we depended on the estimations of the publications. Often the significance level used was not given, so that the statistical evidence could possibly vary between the different studies. Moreover, one study45 reported a positive effect, but gave no further details on the statistical estimations regarding the medication adherence. Another study reported a statistically significant positive effect on medication adherence, even though this study did not compare the follow-up estimates between the intervention and the control group, and only compared changes in the outcome measure between baseline and follow-up for each trial arm. One study,48 that shows a statistically significant effect on medication adherence, included patients with affective disorders as well as patients with anxiety disorders, but provided no subgroup analyses for each group. That is why the results of this study are not completely comparable with the results of the other studies in this review.
Moreover, this review included all studies which addressed patients with affective disorders despite the fact that there may be differences in the medication adherence between patients with depression and patients with bipolar disorders, for example.23
Multi-faceted interventions including eHealth and internet-based self-management tools showed less often significant effects than single-faceted interventions. However, it must be considered that the former categories included fewer studies than the latter ones. The interventions considered are very different with respect to the dimensions of medication adherence addressed and the setting, which limits the generalizability of the results.
Conclusion
In conclusion, some of the regarded eHealth interventions (35%, n=6) showed statistically significant effects on medication adherence and none showed a statistically negative effect. For this reason, eHealth intervention on improving medication adherence in patients with affective disorder seems to be at least as good as usual care approaches which makes eHealth interventions attractive for serving people who live in underserved regions, where usual care is not always available or the availability is limited due to financial or geographical restraints. In some cases, eHealth interventions seem to be even more effective than usual care interventions in order to enhance medication adherence in patients with affective disorders. That is why, effective eHealth interventions should be promoted and integrated into pharmacological therapy of affective disorders in order to reduce negative effects of medication non-adherence. Moreover, all studies which showed statistically significant results included also personal contact. More research is needed to evaluate the role of personal contact or of the comparatively new concepts of blended care as a promoting factor in eHealth interventions for improving medication adherence. In addition, the cost-effectiveness of different types of intervention as well as certain components needs to be assessed in order to give appropriate recommendations for reconsidering healthcare policies. This also includes that more research on the users’ acceptance as well as implementation barriers and facilitators is needed.
A wide range of different eHealth interventions for improving medication adherence in patients with affective disorders exists. Comparison of eHealth interventions for enhancing medication adherence in patients with affective disorder is difficult due to the heterogeneity of the studies in terms of addressed diagnoses, specific drug regimen, intervention strategy, length of trial and intended primary outcomes. Patients with BD seems to be underrepresented in comparison to patients with depression in the research on eHealth interventions for enhancing medication adherence.
Funding Statement
This review was performed without any sources of funding.
Ethics Approval and Informed Consent
Not applicable because this is a review.
Consent for Publication
The authors declare that all details can be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Maren Leiz and Nils Pfeuffer are co-first authors for this study. The authors declare no conflicts of interest in this work.
References
- 1.Andlin-Sobocki P, Jönsson B, Wittchen H-U, Olesen J. Cost of disorders of the brain in Europe. Eur J Neurol. 2005;12(s1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x [DOI] [PubMed] [Google Scholar]
- 2.Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10. doi: 10.1007/s11920-019-0997-0 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Defining Adherence. Adherence to long-term therapies: Evidence for action; 2003.
- 4.Lacro JP, Dunn LB, Dolder CR, Jeste DV, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):15489. doi: 10.4088/JCP.v63n1007 [DOI] [PubMed] [Google Scholar]
- 5.Semahegn A, Torpey K, Manu A, Assefa N, Tesfaye G, Ankomah A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: a systematic review and meta-analysis. Syst Rev. 2020;9(1):1–18. doi: 10.1186/s13643-020-1274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindström E, Bingefors K. Patient compliance with drug therapy in schizophrenia. Pharmacoeconomics. 2000;18(2):105–124. doi: 10.2165/00019053-200018020-00002 [DOI] [PubMed] [Google Scholar]
- 7.Ho SC, Chong HY, Chaiyakunapruk N, Tangiisuran B, Jacob SA. Clinical and economic impact of non-adherence to antidepressants in major depressive disorder: a systematic review. J Affect Disord. 2016;193:1–10. doi: 10.1016/j.jad.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 8.Wright EC. Non-compliance--or how many aunts has Matilda? Lancet. 1993;342(8876):909–913. doi: 10.1016/0140-6736(93)91951-H [DOI] [PubMed] [Google Scholar]
- 9.Vrijens B, de Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):15. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JR, Evans CE, Haynes RB, Tugwell P. Predicting compliance with a regimen of digoxin therapy in family practice. Can Med Assoc J. 1980;123(2):119–122. [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. The rational clinical examination. Is this patient taking the treatment as prescribed? JAMA. 1993;269(21):2779–2781. doi: 10.1001/jama.1993.03500210079036 [DOI] [PubMed] [Google Scholar]
- 12.Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177–183. doi: 10.1017/S0033291700050182 [DOI] [PubMed] [Google Scholar]
- 13.Harvey NS. The development and descriptive use of the lithium attitudes questionnaire. J Affect Disord. 1991;22(4):211–219. doi: 10.1016/0165-0327(91)90067-3 [DOI] [PubMed] [Google Scholar]
- 14.Horne R, Weinman J. The Beliefs About Medication Questionnaire: a new measure for assessing lay beliefs about medicines. BPS. 1995;1995:1. [Google Scholar]
- 15.Harvey NS, Summerton AM, Forrest AR, Peet M. Compliance, the LAQ, and a new laboratory method of measuring RBC lithium. J Clin Psychiatry. 1990;51(3):126–127. [PubMed] [Google Scholar]
- 16.Altamura AC, Mauri M. Plasma concentrations, information and therapy adherence during long-term treatment with antidepressants. Br J Clin Pharmacol. 1985;20(6):714–716. doi: 10.1111/j.1365-2125.1985.tb05137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes a meta-analysis. Med Care. 2002;794–811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 18.Oller-Canet S, Fernández-San Martín MI, García-Lecina R, et al. Do depressed patients comply with treatments prescribed?: a cross-sectional study of adherence to the antidepressant treatment. Actas Esp Psiquiatr. 2011;39(5):288–293. [PubMed] [Google Scholar]
- 19.Åkerblad A-C, Bengtsson F, Holgersson M, von Knorring L, Ekselius L. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:379. doi: 10.2147/ppa.s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajatovic M, Levin J, Fuentes-Casiano E, Cassidy KA, Tatsuoka C, Jenkins JH. Illness experience and reasons for nonadherence among individuals with bipolar disorder who are poorly adherent with medication. Compr Psychiatry. 2011;52(3):280–287. doi: 10.1016/j.comppsych.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Human Psychopharmacol. 2008;23(2):95–105. doi: 10.1002/hup.908 [DOI] [PubMed] [Google Scholar]
- 22.Jónsdóttir H, Opjordsmoen S, Birkenaes A, et al. Predictors of medication adherence in patients with schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2013;127(1):23–33. doi: 10.1111/j.1600-0447.2012.01911.x [DOI] [PubMed] [Google Scholar]
- 23.Kirchner S-K, Lauseker M, Adorjan K, et al. Medication adherence in a cross-diagnostic sample of patients from the affective-to-psychotic spectrum: results from the PsyCourse study. Front Psychiatry. 2022;12:713060. doi: 10.3389/fpsyt.2021.713060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axelsson M, Brink E, Lundgren J, Lötvall J. The influence of personality traits on reported adherence to medication in individuals with chronic disease: an epidemiological study in West Sweden. PLoS One. 2011;6(3):e18241. doi: 10.1371/journal.pone.0018241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze LN, Stentzel U, Leipert J, et al. Improving medication adherence with telemedicine for adults with severe mental illness. Psychiatric Service. 2019;70(3):225–228. doi: 10.1176/appi.ps.201800286 [DOI] [PubMed] [Google Scholar]
- 26.Dennis CL, Grigoriadis S, Zupancic J, Kiss A, Ravitz P. Telephone-based nurse-delivered interpersonal psychotherapy for postpartum depression: nationwide randomised controlled trial. Br J Psychiatry. 2020;216(4):189–196. doi: 10.1192/bjp.2019.275 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Using e-health and information technology to improve health. Available from: https://www.who.int/westernpacific/activities/using-e-health-and-information-technology-to-improve-health. Accessed January 28, 2022.
- 28.Kapitel V; (DIMDI) DIfMDuI. Psychische und Verhaltensstörungen (F00-F99) ICD-10-GM Version 2018; 2017. Available from: https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2018/block-f30-f39.htm. Accessed June 23, 2020.
- 29.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5(1):80–92. doi: 10.1177/160940690600500107 [DOI] [Google Scholar]
- 31.Gläser J, Laudel G. Expert Interviews and Qualitative Content Analysis: As Instruments for Reconstructive Investigations. Springer; 2009. [Google Scholar]
- 32.Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0. Cochrane; 2019. Available from: www.training.cochrane.org/handbook. Accessed December 19, 2022.
- 33.Kohl C, McIntosh EJ, Unger S, et al. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environ Evid. 2018;7(8):17. [Google Scholar]
- 34.Sterne J, Higgins J, Elbers R, Reeves B; And the development group for ROBINS-I. Risk of bias in non-randomized studies of interventions (ROBINS-I): detailed guidance; 2016; Available from: http://www.riskofbias.info. Accessed February 11, 2021.
- 35.Prictor M, Hill S. Cochrane Consumers and Communication Review Group: leading the field on health communication evidence. J Evid Based Med. 2013;6:5. [DOI] [PubMed] [Google Scholar]
- 36.Ryan R. Cochrane consumers and communication review group: data synthesis and analysis; 2013.
- 37.Corden ME, Koucky EM, Brenner C, et al. MedLink: a mobile intervention to improve medication adherence and processes of care for treatment of depression in general medicine. Digital Health. 2016;2:2055207616663069. doi: 10.1177/2055207616663069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammonds T, Rickert K, Goldstein C, et al. Adherence to antidepressant medications: a randomized controlled trial of medication reminding in college students. J Am Coll Health. 2015;63(3):204–208. doi: 10.1080/07448481.2014.975716 [DOI] [PubMed] [Google Scholar]
- 39.Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017;177(5):624–631. doi: 10.1001/jamainternmed.2016.9627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aikens JE, Trivedi R, Heapy A, Pfeiffer PN, Piette JD. Potential impact of incorporating a patient-selected support person into mhealth for depression. J Gen Intern Med. 2015;30(6):797–803. doi: 10.1007/s11606-015-3208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gervasoni N, Legendre-Simon P, Aubry JM, Gex-Fabry M, Bertschy G, Bondolfi G. Early telephone intervention for psychiatric outpatients starting antidepressant treatment. Nord J Psychiatry. 2010;64(4):265–267. doi: 10.3109/08039480903528641 [DOI] [PubMed] [Google Scholar]
- 42.Fortney JC, Pyne JM, Edlund MJ, et al. A randomized trial of telemedicine-based collaborative care for depression. J Gen Intern Med. 2007;22(8):1086–1093. doi: 10.1007/s11606-007-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salisbury C, O’Cathain A, Edwards L, et al. Effectiveness of an integrated telehealth service for patients with depression: a pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry. 2016;3(6):515–525. doi: 10.1016/S2215-0366(16)00083-3 [DOI] [PubMed] [Google Scholar]
- 44.Hungerbuehler I, Valiengo L, Loch AA, Rössler W, Gattaz WF. Home-based psychiatric outpatient care through videoconferencing for depression: a randomized controlled follow-up trial. JMIR Mental Health. 2016;3(3):e36. doi: 10.2196/mental.5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sajatovic M, Davis MS, Cassidy KA, Nestor J, Sams J, Fuentes-Casiano E. A technology-enabled adherence enhancement system for people with bipolar disorder: results from a feasibility and patient acceptance analysis. Patient Prefer Adherence. 2015;9:753–758. doi: 10.2147/PPA.S81724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lobban F, Dodd AL, Sawczuk AP, et al. Assessing feasibility and acceptability of web-based enhanced relapse prevention for bipolar disorder (erponline): a randomized controlled trial. J Med Internet Res. 2017;19(3):e85. doi: 10.2196/jmir.7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin JB, Sajatovic M, Rahman M, et al. Outcomes of psychoeducation and a text messaging adherence intervention among individuals with hypertension and bipolar disorder. Psychiatr Serv. 2019;70(7):608–612. doi: 10.1176/appi.ps.201800482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauly A, Wolf C, Mayr A, Lenz B, Kornhuber J, Friedland K. Effect of a multi-dimensional and inter-sectoral intervention on the adherence of psychiatric patients. PLoS One. 2015;10(10). doi: 10.1371/journal.pone.0139302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gliddon E, Cosgrove V, Berk L, et al. A randomized controlled trial of MoodSwings 2.0: an internet-based self-management program for bipolar disorder. Bipolar Disord. 2019;21(1):28–39. doi: 10.1111/bdi.12669 [DOI] [PubMed] [Google Scholar]
- 50.Himelhoch S, Medoff D, Maxfield J, et al. Telephone based cognitive behavioral therapy targeting major depression among urban dwelling, low income people living with HIV/AIDS: results of a randomized controlled trial. AIDS Behav. 2013;17(8):2756–2764. doi: 10.1007/s10461-013-0465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore DJ, Poquette A, Casaletto KB, et al. Individualized Texting for Adherence Building (iTAB): improving antiretroviral dose timing among HIV-infected persons with co-occurring bipolar disorder. AIDS Behav. 2015;19(3):459–471. doi: 10.1007/s10461-014-0971-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen LB, Taveira TH, Wu WC, Pirraglia PA. Pharmacist-led telehealth disease management program for patients with diabetes and depression. J Telemed Telecare. 2020;26(5):294–302. doi: 10.1177/1357633X18822575 [DOI] [PubMed] [Google Scholar]
- 53.Aikens JE, Rosland AM, Piette JD. Improvements in illness self-management and psychological distress associated with telemonitoring support for adults with diabetes. Prim Care Diabetes. 2015;9(2):127–134. doi: 10.1016/j.pcd.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merikangas KR, Jin R, J-P H, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-21243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levin JB, Krivenko A, Howland M, Schlachet R, Sajatovic M. Medication adherence in patients with bipolar disorder: a comprehensive review. CNS Drugs. 2016;30(9):819–835. doi: 10.1007/s40263-016-0368-x [DOI] [PubMed] [Google Scholar]
- 57.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2008(2):5. [DOI] [PubMed] [Google Scholar]
- 58.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;2014(11). doi: 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pelletier L, Shanmugasegaram S, Patten SB, Demers A. Self-management of mood and/or anxiety disorders through physical activity/exercise. Health Promot Chronic Dis Prev Can. 2017;37(5):149–159. doi: 10.24095/hpcdp.37.5.03 [DOI] [PubMed] [Google Scholar]
- 60.Naslund JA, Marsch LA, McHugo GJ, Bartels SJ. Emerging mHealth and eHealth interventions for serious mental illness: a review of the literature. J Mental Health. 2015;24(5):321–332. doi: 10.3109/09638237.2015.1019054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lokkerbol J, Adema D, Cuijpers P, et al. Improving the cost-effectiveness of a healthcare system for depressive disorders by implementing telemedicine: a health economic modeling study. Am J Geriatr Psychiatry. 2014;22(3):253–262. doi: 10.1016/j.jagp.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeung A, Martinson MA, Baer L, et al. The effectiveness of telepsychiatry-based culturally sensitive collaborative treatment for depressed Chinese American immigrants: a randomized controlled trial. J Clin Psychiatry. 2016;77(8):20755. doi: 10.4088/JCP.15m09952 [DOI] [PubMed] [Google Scholar]
- 63.Chen JA, Chung W-J, Young SK, et al. COVID-19 and telepsychiatry: early outpatient experiences and implications for the future. Gen Hosp Psychiatry. 2020;66:89–95. doi: 10.1016/j.genhosppsych.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faurholt-Jepsen M, Frost M, Martiny K, et al. Reducing the rate and duration of Re-ADMISsions among patients with unipolar disorder and bipolar disorder using smartphone-based monitoring and treatment – the RADMIS trials: study protocol for two randomized controlled trials. Trials. 2017;18(1):277. doi: 10.1186/s13063-017-2015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraft M, van den Berg N, Kraft K, et al. Development of a telemedical monitoring concept for the care of malnourished geriatric home-dwelling patients: a pilot study. Maturitas. 2012;72:126–131. doi: 10.1016/j.maturitas.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 66.van den Berg N, Schumann M, Kraft K, Hoffmann W. Telemedicine and telecare for older patients—A systematic review. Maturitas. 2012;2012(73 (2):94–114. doi: 10.1016/j.maturitas.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 67.Firet L, Teunissen D, Verhoeks C, Lagro-Janssen A. Expectations regarding eHealth among women with stress urinary incontinence. Int Urogynecol J. 2019;30(11):1955–1963. doi: 10.1007/s00192-018-3849-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi M. Association of eHealth use, literacy, informational social support, and health-promoting behaviors: mediation of health self-efficacy. Int J Environ Res Public Health. 2020;17(21):7890. doi: 10.3390/ijerph17217890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gentry MT, Lapid MI, Clark MM, Rummans TA. Evidence for telehealth group-based treatment: a systematic review. J Telemed Telecare. 2019;25(6):327–342. doi: 10.1177/1357633X18775855 [DOI] [PubMed] [Google Scholar]
- 70.Aardoom JJ, Loheide-Niesmann L, Ossebaard HC, Riper H. Effectiveness of eHealth interventions in improving treatment adherence for adults with obstructive sleep apnea: meta-analytic review. J Med Internet Res. 2020;22(2):e16972. doi: 10.2196/16972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatric Service. 1998;49(2):196–201. doi: 10.1176/ps.49.2.196 [DOI] [PubMed] [Google Scholar]
- 72.Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47–56. doi: 10.1007/s00127-009-0041-5 [DOI] [PubMed] [Google Scholar]
- 73.Brueton VC, Tierney J, Stenning S, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev. 2013;2013(12):Mr000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kemper CJ, Beierlein C, Bensch D, Kovaleva A, Rammstedt B. A short scale for measuring the gamma factor of socially desirable response behavior: the short scale Social Desirability-Gamma (KSE-G). GESIS-Working Papers. 2012;25:1–27. [Google Scholar]
- 75.Loughnan SA, Joubert AE, Grierson A, Andrews G, Newby JM. Internet-delivered psychological interventions for clinical anxiety and depression in perinatal women: a systematic review and meta-analysis. Arch Womens Ment Health. 2019;22(6):737–750. doi: 10.1007/s00737-019-00961-9 [DOI] [PubMed] [Google Scholar]
- 76.Paulhus DL, Braun HI, Jackson DN, Wiley DE. Socially desirable responding: the evolution of a construct. In: The Role of Constructs in Psychological and Educational Measurement. Routledge; 2002;49459. [Google Scholar]
- 77.Szymczyńska P. Retention of Patients with Schizophrenia in Complex Intervention Trials: Patterns, Issues, and Practices. Queen Mary University of London; 2018. [Google Scholar]
- 78.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351:h4672. doi: 10.1136/bmj.h4672 [DOI] [PubMed] [Google Scholar]
- 79.Hughes DA. Medication adherence—Key considerations for clinical pharmacologists. Br J Clin Pharmacol. 2020;86(4):628. doi: 10.1111/bcp.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Friedman SJ, Meyers BS. Stigma as a barrier to recovery: perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatric Service. 2001;52(12):1615–1620. doi: 10.1176/appi.ps.52.12.1615 [DOI] [PubMed] [Google Scholar]
- 81.Keyloun KR, Hansen RN, Hepp Z, Gillard P, Thase ME, Devine EB. Adherence and persistence across antidepressant therapeutic classes: a retrospective claims analysis among insured US patients with major depressive disorder (MDD). CNS Drugs. 2017;31(5):421–432. doi: 10.1007/s40263-017-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott J. Predicting medication non-adherence in severe affective disorders. Acta neuropsychiatrica. 2000;12(3):128–130. doi: 10.1017/S0924270800035584 [DOI] [PubMed] [Google Scholar]
- 83.Harvey NS, Peet M. Lithium maintenance: 2 effects of personality and attitude on health information acquisition and compliance. Br J Psychiatry. 2018;158(2):200–204. [DOI] [PubMed] [Google Scholar]
- 84.Martinengo L, Stona A-C, Griva K, et al. Self-guided cognitive behavioral therapy apps for depression: systematic assessment of features, functionality, and congruence with evidence. J Med Internet Res. 2021;23(7):e27619. doi: 10.2196/27619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kay M, Santos J, Takane M. mHealth: new horizons for health through mobile technologies. World Health Organiz. 2011;64(7):66–71. [Google Scholar]
- 86.Marcoux RM, Vogenberg FR. Telehealth: applications from a legal and regulatory perspective. PT. 2016;41(9):567. [PMC free article] [PubMed] [Google Scholar]
- 87.Stinson J, Wilson R, Gill N, Yamada J, Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. J Pediatr Psychol. 2008;34(5):495–510. doi: 10.1093/jpepsy/jsn115 [DOI] [PubMed] [Google Scholar]
- 88.Elnaem MH, Rosley NFF, Alhifany AA, Elrggal ME, Cheema E. Impact of pharmacist-led interventions on medication adherence and clinical outcomes in patients with hypertension and hyperlipidemia: a scoping review of published literature. J Multidiscip Healthc. 2020;13:635–645. doi: 10.2147/JMDH.S257273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (Mars) for the psychoses. Schizophr Res. 2000;42(3):241–247. doi: 10.1016/S0920-9964(99)00130-9 [DOI] [PubMed] [Google Scholar]
- 90.Mahler C, Hermann K, Horne R, et al. Assessing reported adherence to pharmacological treatment recommendations. Translation and evaluation of the Medication Adherence Report Scale (Mars) in Germany. J Eval Clin Pract. 2010;16(3):574–579. doi: 10.1111/j.1365-2753.2009.01169.x [DOI] [PubMed] [Google Scholar]
- 91.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007 [DOI] [PubMed] [Google Scholar]