Abstract
Cervical cancer is the commonest cancer affecting women worldwide. During the last decades, the incidence and mortality rates of cervical cancer have increased in China. This research aims to assess the overall and genotype-specific prevalence of the human papillomavirus (HPV) infection among Chinese women with normal cervix, considering age, and geographic location. We selected studies about HPV prevalence in women from Chinese in Mainland China with normal cervix and abnormal cervical lesions, published between January 1995 and December 2020. The HPV prevalence was analyzed using meta-analysis based on the following: cytological and histological diagnoses, regions, and ages. The overall HPV prevalence in 856,535 women was 14.3%, 95% confidence interval (CI) and it increased from 8.2% to 16.5% in studies published from 2006 to 2020. The prevalence of high-risk (HR) and low-risk (LR) HPV types was 11.3% and 2.7%, respectively. The commonest types of HPV in women from Mainland China were HPV 16 (2.6%), 52 (2.4%), 58 (1.7%), 18 (0.9%), and 33 (0.8%). According to the geographical analysis, the prevalence of different HPV genotypes varied by region, Central China had the highest overall HPV prevalence. HPV16 was the commonest type in all the regions except in South China and East China, where HPV52 was found to be common. Regarding diagnosis, the HPV infection led to cervical cancer diagnosis by cytology and histology with 90.1% and 91.5% rates, respectively. HPV16 and HPV18 were common types associated with cervical cancer diagnosed by cytology and histology. HPV 16, 58, 52, 18, and 33 were the commonest types found in women with normal cervixes from Mainland China. The prevalence of different HPV genotypes varied by age group and region.
Keywords: Genotype, human papillomavirus, Mainland China, prevalence
BBACKGROUND
Cervical cancer is the fourth most common cancer affecting women in worldwide. An estimated 604,000 new cervical cancer cases and 342,000 deaths were reported globally in 2020.[1] In China, there were up to 110,000 and 60,000 of new cases and deaths from cervical cancer, respectively, in 2020,[2] representing increases of approximately 3.5% and 23.0% relative to 2018.[3] The World Health Organization (WHO) launched the “Global Strategy for Accelerating the Elimination of Cervical Cancer as a Public Health Problem” on November 17, 2020, and 194 countries, which including China, committed to eliminating cervical cancer for the first time.[4,5] Therefore, the Chinese government has taken action to prevent cervical cancer by implementing a National Cervical Cancer Screening Programme in Rural Areas (NACCSPRA) and introducing the human papillomavirus (HPV) vaccine into the Chinese market.
The HPV infection is related to cervical cancer and other cancer types such as anal, vaginal, vulvar, penile, and oropharyngeal. Persistent infections caused by high-risk human papillomavirus (HR-HPV) types are a necessary factor for cervical cancer occurrence, specifically HPV16 and HPV18 − which have demonstrated to cause many cervical cancer cases.[6,7] However, 14 HR-HPV types are recognized as carcinogenic and associated with cervical cancer.[8,9] Further, low-risk human papillomavirus (LR-HPV) types cause benign hyper-proliferative lesions and fail to cause malignant carcinoma in the general population. Because LR-HPV has a lower impact as a carcinogenic agent, lesions are self-limiting and often cleared by the host immune system. Therefore, in some individuals, LR-HPV types produce asymptomatic infections only, and that is why research about LR-HPV types is yet to be prioritized. The distribution of HPV genotypes has regional variation, and the carcinogenicity and consequences of HPV infection are different for each subtype.[10]
Although many articles and systematic reviews have described the prevalence of HPV infection and the distribution of different HPV genotypes in patients with cervical cancer or cervical lesions in China, the information is still limited to specific regions of the country and it is yet to represent the statistics of the general population.[11,12,13,14] Furthermore, most of the information available about cervical cancer in China, lack updated data. The overall HPV prevalence range in Chinese women with normal cervix accounted to be 13.1 − 18.8% in previous meta-analyses done in 2007 to 2013. However, there was yet no analysis that focused on the geographical distribution of HPV subtypes in China. To date, few studies have discussed HPV infection in Chinese women with a normal cervix, especially using a pooled analysis of HPV type-distribution stratified at cervical disease stage by cytology and histology diagnosis.
This study aims to update the previous meta-analysis with data about HPV type-specific prevalence in Chinese women with a normal cervix and abnormal cervical lesions in Mainland China by various regions and ages. In addition, this study intends to evaluate whether the prevalence trends of type-specific HPV have changed during the last 10 years or not. Therefore, we performed a meta-analysis to estimate the geographical prevalence trends of HPV subtypes in the population from Mainland China. Finally, we compared HPV genotype distribution in women affected by different cervical precancerous lesions. We consider it critical to analyze the HPV prevalence and the HPV genotype distribution in women with normal and abnormal cervical lesions to implement future health policies regarding HPV screening and vaccination in China.
METHODS
Literature search strategy
Peer-reviewed research articles reporting HPV prevalence in Mainland China,[15] published from January 1, 1995 (when the HPV detection technology entered the Chinese market) to December 30, 2020, were included in this study only. The included articles were in English (PubMed database) and Chinese language (including CNKI/Wang Fang/other resources). We searched the literature using Mesh and/or index terms combinations: “Human Papilloma Virus (HPV)”, “Cervix neoplasms (epidemiology or virology or prevention and control)”, “epidemiology”, “prevalence”, “DNA probes (HPV)”, “polymerase chain reaction”, “women/female” and “population.” Additional references cited in the retrieved articles were evaluated for inclusion together with the review of abstract books from scientific meetings, the Cochrane library, and the Lancet database.
Inclusion/exclusion criteria
Inclusion criteria for the literature: 1) published SCI/SCIE or Chinese journals articles, 2) cervical HPV infection of women from Mainland China, 3) original research articles, 4) the population including: screening, check-up, community, census and migrant population, 5) sample size larger than 100, 6) use of polymerase chain reaction (PCR) based technology to detect human papillomavirus-deoxyribonucleic acid (HPV DNA); 7) the overall and type-specific HPV elicitation, were data are calculated correctly, 8) the detection of at least two different HPV types. The study had to qualify all inclusion criteria to be included.
Exclusion criteria for literature: 1) duplicate data, 2) reviews, letters, conference reports or degree thesis, 3) patient studies, pregnancy, hospitalized, and any diseases such as the human immunodeficiency virus (HIV) infection, cervical cancer, or other sexually transmitted pathogens, 3) articles about Hong Kong, Macao, and Taiwan; 4) incomplete data, 5) other studies such as questionnaire, investigation about HPV knowledge or mechanism of cervical cancer. The presence of any single exclusion criterion is sufficient to be excluded.
Ethical approval and inform consent
We received ethical approval from the Ethics Committee of the First Affiliated Hospital of Baotou Medical College in Inner Mongolia. In addition, we refused to seek patients' consent and/or form, because this study is a meta-analysis based on published studies.
Quality assessment
We assessed the study quality using the Agency for Healthcare Research and Quality (AHRQ) scale for cross-sectional studies published by the National Institutes of Health, with a total score of 11.[16] Two independent reviewers completed a blinded assessment of the quality of the included studies. If the literature quality score is greater than or equal to 5, then the article meets the inclusion criteria.
Data extraction
Two independent investigators extracted the data with discrepancies resolved by consensus. In situations where different opinions or inconsistencies occurred, they were resolved by a third party. For each eligible study, the following items were retrieved: 1) basic information: first author, journal name, year of publication, study period, study sample type (population-based, screening, check-up, community, and census), study design (derived from cross-sectional studies), sample size, and age. 2) Outcomes: amount, number of HPV-positive, type-specific HPV, and the overall prevalence of HPV infection, stratified by cervical disease grade, ages, and areas.
Study characteristics
We analyzed 29 subtypes and selected 17 commonest types of all HPV genotypes that were discovered. We divided the commonest types into HR-HPV: HPV16, 18, 31, 33, 45, 52, and 58; and LR-HPV: HPV 6, 11, and 42. From the available data, we synthesized the overall HPV and each HPV genotype prevalence as follows: (1) by seven geographical regions (North China, Northeast, East China, Central China, Southwest, Northwest, and South China); (2) nine age-groups (25 years and less, 25 − 30 years, 31 − 35 years, 36 − 40 years, 41 − 45 years, 46 − 50 years, 51 − 55 years, 56 − 60 years, and more than 61 years).
We analyzed the prevalence of each HPV subtype in women with normal and abnormal cervical lesions diagnosed by cytology and histology. The normal and abnormal cervical lesions diagnosed by cytology were classified into four stages: negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial neoplasia (LSIL), high-grade squamous intraepithelial neoplasia, and atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion and cancer (≥HSIL). The normal and abnormal cervical lesions diagnosed by histology were classified into four stages: NILM, cervical intraepithelial neoplasia grade 1/2 (CIN1/2), cervical intraepithelial neoplasia grade, and cervical cancer (≥CIN3).
Statistical analysis
We conducted the meta-analysis to synthesize HPV prevalence using STATA12.0. Overall and type-specific HPV prevalence was estimated by a weight coefficient. We applied heterogeneity study to determine the difference between the data by a Chi-square test, which was analyzed by I2. In all analyses, we selected the random-effects model for the pooled analyses when the results obtained were highly heterogenous. The overall and type-specific HPV pooled prevalence was 95% CI and corresponding P values were recorded. The publication bias was evaluated by Egger's test.
RESULTS
Summary of eligible studies
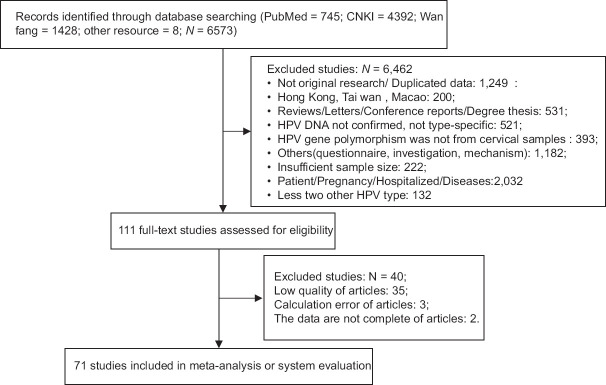
We retrieved 6573 citations and identified 111 relevant articles for full-text review. Data were obtained from 71 eligible studies that met the eligibility criteria,[17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87] including 45 Chinese studies and 26 English literature [See Figure 1].
Figure 1.
PRISMA flow diagram for identification of studies for meta-analysis
The prevalence of overall HPV and type-specific HPV
According to the analyzed literature, the overall HPV prevalence, HR-HPV, and LR-HPV types are 14.3, 11.3, and 2.7%, respectively, in 853,945 women with normal cervix from Mainland China. From the prevalence analysis of the 17 commonest HPV subtypes in women with a normal cervix living in Mainland China, we present the prevalent in descending order, such as: HPV 16, 52, 58, 18, 33, 51, 68, 39, 31, 56, 66, 6, 59, 11, 42, 35, and 45. The first most common HPV type was HPV 16 (2.6%), followed by 52 (2.4%), 58 (1.7%), 18 (0.9%), 33 (0.8%), and 51/39/68 (0.7%). The most common LR-HPV subtype was HPV6, with a prevalence of 0.5% (95%CI: 0.4, 0.5), followed by HPV 11, 0.4% (95%CI: 0.3, 0.4) [See Table 1]
Table 1.
Type-specific HPV prevalence and overall HPV prevalence in healthy women from mainland, stratified by geographical region
| HPV type | Amount prevalence | North China | Northeast | East China | Central China | Southwest | Northwest | South China |
|---|---|---|---|---|---|---|---|---|
| HPV ration | 14.3 (13.1, 15.5) | 14.5 (11.0,17.9) | 12.8 (8.6,17.0) | 15.6 (13.4,17.8) | 19.8 (15.4,24.2) | 13.3 (10.9,15.8) | 12.7 (6.3,19.0) | 12.1 (10.6,13.6) |
| HR HPV | 11.3 (10.5,12.2) | 10.8 (7.1,14.6) | 10.3 (7.5,13.1) | 11.7 (10.8,12.6) | 15.1 (11.6,18.7) | 9.2 (6.5,11.9) | 9.6 (6.3,13.0) | 11.6 (10.0,13.2) |
| LR HPV | 2.7 (2.3,3.2) | 2.3 (1.4,3.2) | 2.0 (1.1,2.9) | 2.9 (1.9,3.9) | 4.8 (2.9,6.7) | 3.1 (0.9,5.4) | 1.7 (1.1,2.3) | 2.5 (1.3,3.6) |
| HPV6/11 | 0.8 (0.6,0.9) | 0.6 (0.3,0.9) | 0.7 (0.4,0.9) | 0.9 (0.7,1.2) | 0.5 (0.1,0.9) | 0.5 (0.4,0.6) | 0.9 (0.3,1.5) | 0.8 (0.5,1.0) |
| HPV16 | 2.6 (2.4,2.9) | 4.1 (3.1,5.1) | 3.0 (1.8,4.3) | 2.2 (2.0,2.5) | 3.9 (2.5,5.4) | 2.7 (1.7,3.6) | 5.3 (3.9,6.6) | 1.8 (1.6,2.1) |
| HPV52 | 2.4 (2.1,2.7) | 1.5 (0.8,2.2) | 2.0 (1.6,2.4) | 2.8 (2.5,3.2) | 2.5 (1.4,3.7) | 2.1 (1.3,3.0) | 1.0 (0.8,1.3) | 2.7 (2.3,3.2) |
| HPV58 | 1.7 (1.6,1.9) | 2.1 (1.5,2.7) | 1.6 (1.0,2.2) | 1.8 (1.7,2.0) | 2.3 (1.8,2.8) | 1.6 (1.2,1.9) | 2.0 (0.8,3.2) | 1.2 (1.1,1.4) |
| HPV18 | 0.9 (0.8,1.0) | 1.0 (0.7,1.4) | 0.9 (0.7,1.1) | 0.9 (0.8,1.0) | 1.3 (0.7,2.0) | 0.7 (0.4,1.0) | 1.1 (0.2,2.1) | 0.8 (0.6,0.9) |
| HPV33 | 0.8 (0.7, 0.8) | 1.1 (0.9,1.3) | 0.8 (0.5,1.1) | 0.9 (0.8,1.0) | 1.0 (0.7,1.2) | 0.8 (0.5,1.0) | 0.5 (0.3,0.7) | 0.5 (0.4,0.6) |
| HPV51 | 0.7 (0.6, 0.8) | 0.7 (0.3,1.0) | 0.4 (0.1,0.7) | 0.7 (0.5,0.8) | 1.3 (0.4,2.3) | 0.6 (0.4,0.9) | 0.7 (0.1,1.3) | 0.7 (0.5,0.8) |
| HPV39 | 0.7 (0.6, 0.8) | 0.7 (0.3,1.0) | 0.9 (0.4,1.3) | 0.6 (0.4,0.7) | 0.7 (0.4,1.0) | 0.8 (0.6,1.0) | 1.0 (0.6,1.3) | 0.7 (0.5,0.8) |
| HPV68 | 0.7 (0.6,0.8) | 0.6 (0.4,0.7) | 0.6 (0.3,0.8) | 0.9 (0.7,1.1) | 0.0.8 (0.4,1.2) | 0. 6 (0.3,0.8) | 0.42 (0.22,0.6) | 0.5 (0.4,0.6) |
| HPV31 | 0.6 (0.5,0.7) | 0.7 (0.5,1.0) | 0.6 (0.3,0.9) | 0.7 (0.6,0.8) | 0.7 (0.4,1.0) | 0.5 (0.3,0.7) | 0.4 (0.2,0.5) | 0.4 (0.3,0.4) |
| HPV56 | 0.6 (0.5,0.6) | 0.7 (0.5,0.9) | 0.3 (0.1,0.5) | 0.5 (0.4,0.6) | 0.6 (0.2,0.9) | 0.8 (0.5,1.1) | 1.2 (1.1,1.3) | 0.4 (0.3,0.5) |
| HPV66 | 0.5 (0.4, 0.6) | 0.8 (0.7,1.0) | 0.3 (0.1,0.5) | 0.5 (0.4,0.6) | 1.0 (0.3,1.6) | 0.4 (0.3,0.5) | 0.5 (0.2,0.9) | 0.4 (0.3,0.5) |
| HPV6 | 0.43 (0.35,0.50) | 0.29 (0.11,0.46) | 0.35 (0.20,0.50) | 0.45 (0.31,0.60) | 0.36 (0.09,0.62) | 0.28 (0.12,0.44) | 0.61 (0.23,0.99) | 0.48 (0.32,0.65) |
| HPV59 | 0.36 (0.31,0.40) | 0.37 (0.25,0.49) | 0.27 (0.10,0.44) | 0.38 (0.31,0.45). | 0.61 (0.24,0.98) | 0.28 (0.14,0.42) | 0.65 (0.01,1.28) | 0.29 (0.22,0.35) |
| HPV11 | 0.34 (0.28, 0.40) | 0.34 (0.17,0.52) | 0.24 (0.06,0.41) | 0.45 (0.30,0.60) | 0.51 (0.17,0.84) | 0.26 (0.13,0.39) | 0.36 (0.15,0.57) | 0.25 (0.17,0.34) |
| HPV42 | 0.27 (0.22,0.32) | 0.22 (0.10,0.34) | 0.69 (0.01,1.48) | 0.34 (0.25,0.44) | 0.21 (0.04,0.39) | 0.21 (0.06,0.36) | 0.29 (0.24,0.35) | 0.24 (0.09,0.39) |
| HPV35 | 0.26 (0.22,0.31) | 0.22 (0.06,0.38) | 0.19 (0.09,0.28) | 0.30 (0.21,0.40) | 0.36 (0.12,0.60) | 0.13 (0.06,0.20) | 0.91 (0.31,1.51) | 0.23 (0.17,0.29) |
| HPV45 | 0.24 (0.20,0.28) | 0.18 (0.12,0.24) | 0.15 (0.09,0.22) | 0.23 (0.16,0.30) | 0.27 (0.15,0.39) | 0.19 (0.08,0.30) | 0.19 (0.14,0.23) | 0.32 (0.21,0.43) |
CI: confidence interval; HR-HPV: high-risk human papillomavirus; LR-HPV: low-risk human papillomavirus; East China: including Shan dong, Jiangsu, Anhui, Jiangxi, Zhejiang, Fujian and Shanghai; Central China: including Henan, Hubei and Hunan; North China: including Beijing, Tianjin, Hebei, Shanxi, and Inner Mongolia; Northwest China: including Ningxia, Xinjiang, Qinghai, Shanxi, and Gansu; Southwest China: Including Sichuan, Yunnan, Guizhou, Tibet, and Chongqing; Northeast China: including Liaoning, Jilin, and Heilongjiang; South China: Guangdong, Guangxi, and Hainan
The prevalence of overall HPV and type-specific HPV stratified by regions
The results showed that the prevalence of overall HPV and type-specific HPV varied by geographical region. The prevalence of overall HPV varied from 12.1% in South China to 19.8% in Central China. East China has the second-highest overall HPV prevalence (15.6%) and HR-HPV prevalence (11.7%) after Central China. Similarly, the Southwest showed the second highest prevalence of LR-HPV after Central China. According to the results, South China has the lowest rate of overall HPV prevalence (12.1%). While in Northwest and East China, the prevalence of LR-HPV was 0.9% in both areas. Regarding specific HPV types, HPV16 was the commonest type in all the regions except in South China and East China, where HPV52 was the most common type. The LR-HPV, such as HPV6 and HPV11, were common types in the seven analyzed regions [See Table 1].
Age-specific prevalence
We stratified HPV prevalence by age. The curve of prevalence for overall HPV and HR-HPV healthy women showed two peaks. One was observed at aged ≤25 years, declining to a plateau around middle age. The second peak was observed between 41 and 45 years in overall HPV and HR-HPV with a prevalence of 14.3 and 12.2%, respectively. We observed a decline to a plateau after ≥46 years of age. However, the prevalence of LR-HPV had no significant trends variations with age [See Figure 2].
Figure 2.
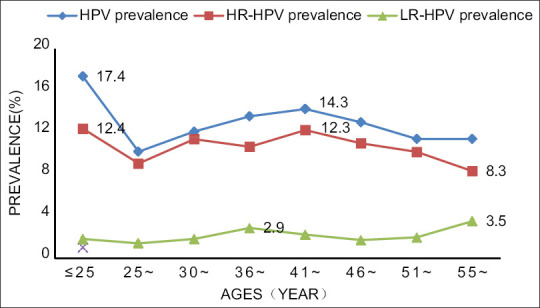
Type-specific HPV prevalence and HPV prevalence in healthy women from mainland, stratified by ages
The prevalence of overall HPV and type-specific HPV stratified by cervical disease grade of histology diagnosis
Women with cervical lesions and cervical cancer showed high significant of HPV prevalence than women with normal cervical histology. The HPV prevalence was 10.5% (8.4% to 12.7%) for the normal group, 73.9% (65.8% to 80.8%) for CIN1, 89.5% (84.3% to 94.4%) for CIN2, and 91.5% (80.3% to 96.8%) for CIN3+. In descending order of HPV prevalence, the ten commonest HR-HPV types were: HPV 16, 52, 58, 18, 39, 33, 68, and 31. The LR-HPV 11 and 6 were the most common types in women with a normal cervical histological diagnosis. In women with CIN1, the most common HR-HPV types were: 16, 52, 33, 58, 18, 68, 31, and 39. The commonest LR-HPV types are 6 and 11. The often-detected types were HPV 16, 58, 52, 33, 31, 18, 35, 51, 68, and 39 in women with CIN2. While, HPV 16 (62.0%) and 18 (15.8%) were the common types in women with ≥CIN3, which led to cervical cancer and increased with the grade of cervical disease diagnosis by histology. HPV58, 52, and 31 prevalence rates failed to show variations when the cervical histological grades were analyzed. Except for HPV16, 18, 58, 52, and 31, the prevalence of all other HPV types ranged from 0.6 to 3.7% in women with ≥CIN3, from 1.2 to 7.7% in CIN2, from 1.5 to 5.4% in women with CIN1, and from 0.2 to 0.7% in women with normal histological results [See Table 2].
Table 2.
Type-specific HPV prevalence and HPV prevalence of tissue-based detection in healthy women from mainland, stratified by cervical disease grade
| HPV type | NILM | CIN1 | CIN2 | ≥CIN3 |
|---|---|---|---|---|
| HPV ration | 10.5 (8.4,12.7) | 73.9 (65.8,80.8) | 89.5 (84.3,94.4) | 91.5 (80.3,96.8) |
| HPV6/11 | 0.4 (0.2,0.6) | 3.7 (2.0,5.4) | 2.0 (0.8,3.2) | 3.5 (1.5,5.4) |
| HPV16 | 2.4 (1.8,3.1) | 25.0 (16.1,33.9) | 49.9 (28.8,70.9) | 62.0 (46.5,77.6) |
| HPV18 | 0.7 (0.5,1.0) | 7.7 (5.5,9.8) | 6.9 (2.2,11.5) | 15.8 (5.2,20.4) |
| HPV58 | 1.4 (0.9,1.8) | 13.5 (10.3,16.7) | 16.1 (9.74,22.4) | 12.3 (8.1,16.5) |
| HPV52 | 2.3 (1.1,3.6) | 19.3 (12.4,26.1) | 13.9 (6.6,21.3) | 10.2 (5.0,15.4) |
| HPV31 | 0.5 (0.3,0.8) | 4.7 (1.8,7.6) | 7.7 (2.4,13.0) | 6.4 (1.5,11.4) |
| HPV33 | 0.6 (0.4,0.8) | 17.0 (15.6,18.3) | 11.1 (6.9,15.3) | 3.7 (2.2,5.3) |
| HPV68 | 0.5 (0.3,0.6) | 5.4 (4.1,6.6) | 3.9 (2.5,5.4) | 3.6 (2.0,5.1) |
| HPV35 | 0.3 (0.1,0.5) | 3.6 (1.2,3.0) | 6.6 (3.9,9.4) | 2.1 (0.4,3.8) |
| HPV6 | 0.1 (0.1,0.2) | 2.4 (1.08,3.7) | 1.7 (0.1,3.2) | 2.1 (0.9,3.4) |
| HPV59 | 0.3 (0.2,0.4) | 2.8 (1.6,3.9) | 1.6 (0.5,2.8) | 1.8 (0.7,3.0) |
| HPV66 | 0.4 (0.27,0.7) | 4.1 (1.5,6.7) | 3.4 (1.9,4.9) | 1.7 (0.5,2.8) |
| HPV39 | 0.7 (0.4,1.0) | 4.7 (2.8,6.6) | 3.5 (2.1,4.8) | 1.3 (0.3,2.3) |
| HPV51 | 0.6 (0.3,0.9) | 4.3 (3.2,5.4) | 4.5 (2.2,6.9) | 1.6 (0.4,4.0) |
| HPV45 | 0.2 (0.1,0.4) | 1.5 (0.8,2.3) | 3.1 (1.2,4.9) | 0.8 (0.0,1.6) |
| HPV56 | 0.5 (03,0.7) | 3.1 (2.1,4.0) | 3.3 (2.0,4.7) | 0.6 (0.1,1.3) |
| HPV11 | 0.2 (0.1,0.3) | 1.8 (0.7,3.7) | 1.2 (0.2,2.2) | 0.6 (0.1,1.2) |
CIN2=cervical intraepithelial neoplasia grade 2; CIN1=cervical intraepithelial neoplasia grade 1; ≥CIN3=cervical intraepithelial neoplasia grade 3and cancer; NILM: No intraepithelial lesion or malignant cells
The prevalence of overall HPV and type-specific HPV stratified by cervical disease grade of cytology diagnosis
The overall HPV and the type-specific HPV prevalence were stratified by the cervical disease grade of cytology diagnosis. In the ≥HSIL, LSIL, ASCUS, and NILM, the overall HPV prevalence was 90.1, 73.6, 42.2, and 12.5%, respectively. In descending order of HPV prevalence, the five common HPV types in ≥HSIL were: HPV 16, 18, 52, 58, and 59. The frequently detected HPV types were HPV 16, 52, 58, 51, and 33 in LSIL and HPV16, 52, 58, 18, and 66 in women with ASCUS. However, the common types in women with normal cervical cytology were: HPV 52, 16, 58, 18, and 51. The prevalence of HPV16, 18, 58, 31 and 59, increased by cervical lesion degree, however, the prevalence of HPV33, 56, 35, 66, 68, 52, 45, 51, 39, 11, and 6 increased from NILM to LSIL, followed by a decrease in ≥HSIL. In addition, HPV16 was the predominant type in women with ASC-US and other cytological diseases, whose prevalence ranged from 3.3 % to 54.5%. HPV52 was the second most prevalent type in cervix diseases diagnosed by cytology (NILM; 4.1%, ASCUS; 11.4%, LSIL; 14.1%, HSIL; 13.5%). HPV18 was the third common type in ≥HSIL, with a prevalence of 12.3% [See Table 3].
Table 3.
Type-specific HPV prevalence and overall prevalence of HPV cell -based detection in healthy women from mainland, stratified by cervical disease grade
| HPV type | NILM | ASCUS | LSIL | ≥HSIL |
|---|---|---|---|---|
| HPV ration | 12.5 (8.9,16.1) | 42.2 (21.5,63.0) | 73.6 (59.9,87.2) | 90.1 (83.6,96.7) |
| HR-HPV | 11.9 (9.8,14.1) | 28.3 (7.9,48.8) | 64.7 (41.8,87.7) | 69.4 (24.2,90.7) |
| LR-HPV | 2.6 (0.8,4.4) | 1.4 (0.0,2.8) | 8.3 (1.7,15.1) | 7.2 (0.8,13.6) |
| HPV6/11 | 2.1 (1.2,3.0) | 4.5 (3.2,5.8) | 5.4 (2.5,8.2) | 3.4 (1.4,5.3) |
| HPV16 | 3.3 (2.0,4.6) | 12.2 (5.9,18.5) | 14.5 (9.5,19.5) | 54.5 (19.2,60.1) |
| HPV52 | 4.1 (2.6,5.5) | 11.4 (5.5,17.3) | 14.1 (7.2,21.0) | 13.5 (5.9,15.5) |
| HPV18 | 1.2 (0.7,1.8) | 4.6 (1.5,7.7) | 6.4 (4.7,8.1) | 12.3 (3.2,18.9) |
| HPV58 | 2.1 (1.3,2.8) | 7.1 (2.7,11.4) | 10.8 (5.1,16.4) | 9.3 (2.8,13.8) |
| HPV59 | 0.5 (0.2,0.7) | 1.2 (0.3,2.2) | 2.3 (1.4,3.3) | 6.9 (2.5,11.3) |
| HPV33 | 0.8 (0.5,1.1) | 2.5 (1.7,3.2) | 8.0 (3.9,12.0) | 6.2 (4.6, 9.4) |
| HPV51 | 1.1 (0.7,1.6) | 3.9 (1.3,6.6) | 9.0 (6.5,11.5) | 6.0 (0.7,7.2) |
| HPV31 | 0.7 (0.4,1.0) | 3.3 (1.6,5.0) | 3.5 (2.0,5.0) | 4.6 (1.5, 4.7) |
| HPV56 | 0.6 (0.4,0.9) | 1.9 (1.1,2.8) | 6.8 (3.0,10.6) | 3.9 (1.3,6.6) |
| HPV45 | 0.7 (0.3,1.1) | 1.9 (0.3,3.6) | 3.7 (0.8,6.6) | 3.5 (1.9,5.1) |
| HPV42 | 0.4 (0.1,0.6) | 0.7 (0.1,1.4) | - | 3.0 (0.1,15.8) |
| HPV39 | 1.0 (0.6,1.5) | 3.5 (1.1,5.9) | 6.2 (4.2,8.2) | 2.7 (1.2,4.1) |
| HPV66 | 1.1 (0.6,1.6) | 4.0 (2.3,5.6) | 7.6 (5.8,9.3) | 2.4 (0.6,4.2) |
| HPV11 | 0.6 (0.2,0.9) | 1.2 (0.5,1.9) | 2.2 (1.2,3.2) | 2.1 (0.7,4.9) |
| HPV35 | 0.5 (0.2,0.7) | 1.3 (0.4,2.2) | 2.7 (0.4,5.1) | 1.6 (0.3,2.8) |
| HPV6 | 0.3 (0.1,0.5) | 3.2 (2.1,4.3) | 4.4 (3.0,5.8) | 1.6 (0.4,2.8) |
| HPV68 | 0.9 (0.6,1.3) | 2.2 (1.5,2.9) | 4.2 (1.8,6.6) | 1.2 (0.2,2.3) |
NILM: No intraepithelial lesion or malignant cells; ASCUS: Atypical squamous cells of undermined significance; LSIL: Low-grade squamous intraepithelial lesion;≥HSIL: High-grade squamous intraepithelial lesion and cancer. HR-HPV:high-risk human papillomavirus,LR-HPV:-risk human papillomavirus
DISCUSSION
HPV is one of the sexually transmitted infections worldwide, especially the persistent HR-HPV infection that causes cervical cancer. This study provides up-to-date evidence to propel potential HPV vaccination and cancer screening programs for women in Mainland China. First, compared with previously published systematic reviews, we have added new data about the prevalence and distribution of HPV genotypes among women with a normal cervix over nearly 10 years. Second, our results show the prevalence and distribution of HPV subtypes using the gold-standard method for cervical cancer screening in China, histology diagnosis. The distribution of HPV subtypes reported in cytology is different from those in histology assessment. Third, we further analyzed the prevalence and distribution of HPV subtypes in different geographical regions of China and other factors influencing HPV prevalence, such as age trends, in this study, which have been rarely analyzed in previous studies.
Type-specific HPV prevalence and HPV prevalence
We found that the overall prevalence of HPV was 14.3% in women with normal cervix in Mainland China, similar to other findings in the Chinese population (13.1 − 18.8%), and higher compared to the values presented in the International Agency for Research on Cancer (IARC) previous survey − which showed a prevalence of 11.7% of women worldwide with cervical HPV.[10,15,88] The HPV subtype confirmation tests have different sensitivities that might explain the disparity of HPV prevalence in China. However, the HPV prevalence included single and multiple infections, though, it may lead to a higher prevalence in some areas. But compared with previous published meta-analyses in China, due to cervical cancer screening programs and the administration of the HPV vaccine, the HPV prevalence has decreased.[15] In our study, the prevalence of HR-HPV and LR-HPV types was 11.3% and 2.7%, respectively. According to previously mentioned information, it is necessary to promote cervical cancer vaccination and cervical cancer screening programs for women with normal cervix. Consistent with previous epidemiological studies,[89,90] our analysis suggested that HPV16 is the main HR-HPV type, followed by 52, 58, and 18 in women with normal cervix in Mainland China − which differs from the IARC survey findings.[91] The prevalence of all dominant HPV types (HPV16, 18, 52, and 58) was higher than other subtypes reported in a study that analyzed HPV prevalence in five continents.[10] The HPV16 (2.6%) was not only the most prevalent type, but it also showed the highest relative contribution to cervical cancer compared with other types. The second prevalent HPV type in Mainland China was HPV52 accounts for 2.4% prevalence, but other HR-HPV types' prevalence ranges from 0.2 to 1.7%. Our findings show the most common LR-HPV type is HPV6 with a prevalence of 0.4%, followed by HPV11, which shows a prevalence of 0.3%.
Type-specific HPV prevalence and overall HPV prevalence, stratified by regions
Worldwide, the incidence of each common HPV type in Chinese female populations varies by region.[92] Our results confirmed that the variation also occurs across different Chinese territories. Central China accounted for the highest overall HPV prevalence-HR-HPV and LR-HPV 9.8, 15.1, and 4.8%, respectively, in healthy women from the Chinese mainland − which is consistent with a higher cervical cancer mortality in that region. This result is consistent with the findings of Ma XM et al.[14] Compared with other regions, East China and South China have less variability in HPV prevalence, though the data available in these regions are larger than that in other areas. East China has the second-highest overall HPV prevalence and LR-HPV prevalence, which accounted for 15.6 and 2.9%, respectively. However, women from South China have the lowest HPV prevalence, which demonstrates the vaccination access women from this region (South China) receive from neighboring cities such as Hong Kong and Macau − where the HPV vaccine has been used for over a decade. That might be one of the reasons why the HPV52 prevalence was higher than HPV16 in East China and South China.[19] This evidence suggests that geographical differences in HPV prevalence may be related to accessibility to vaccines, but further investigations are required to confirm this statement. Consistent with previous epidemiological studies,[78,88] our study shows that HPV16, 52, 58, and 18 were the most common types in all regions, which might be related to HPV variants, habits, and hygienic conditions.
Age-specific prevalence
In this study, we confirm that the overall HPV prevalence and HR-HPV prevalence have two peaks, aged ≤25 years and aged 41~ years in healthy Chinese women. However, the results are not consistent with previous articles reporting that the overall HPV prevalence and HR-HPV prevalence formed a “U-shaped curve” at aged 30~ years and aged 41~years in Chinese and Asian women.[92,93] It may be that the age-specific phenomenon observed at aged ≤25 years in healthy Chinese women of Mainland China is compatible with underlying causes. First, the finding showed that the average age of the first sexual intercourse of girls was 17-year-old, so it may be the first-time transient HPV infection and insufficient adaptive immune responses up to peak at the age of 18 − 24 years. Second, Chinese women may have changed their attitudes toward sexual intercourse. The three reasons to explain the second peak at aged 41~ years: studies showed that the women and/or their husbands have extramarital relationships with more than two sexual partners at middle age[94,95]; the time at which women got cervical cancer screening, so, it could be the reflection of population-specific period and/or cohort effects; and women aged 41~ years have HPV persistent and co-infection. The prevalence of LR-HPV had no significant trends or variations with age because it is rarely detected by HPV testing.
The prevalence of overall HPV and type-specific HPV stratified by cervical disease grade of histology diagnosis
Women with abnormal cervical lesions and cervical cancer had high HPV prevalence than women with normal cervical histology 10.5% for the NILM, 73.9% for CIN1, 89.5% for CIN2, and 91.5% for ≥CIN3, which was higher than the findings reported in a similar systematic review where 87%, and 67.1% in ≥HSIL and LSIL, respectively, were observed.[9,15] It may be related to HPV detection methods, especially the sensitivity of different PCR protocols used in the included studies. Though the type-specific HPV prevalence was varied in different cervical lesion grades, the main common LR-HPV types were HPV 16, 52, 58, and 18. However, these results are consistent with reports from 2019 that demonstrated that HPV 18, 16, 58, and 33 were the most prevalent HPV types in different cervical lesion grades.[91] The change might be related to an increase in cervical cancer screening programs, prevention, and pathogenicity.[96] The present study shows overall HPV, HPV16, and HPV18 prevalence increases with the grade of cervical lesion, which is different from the other HPV subtypes that failed to show the same trend. HPV16 (from 2.4 to 62.0%) and HPV18 (from 0.7 to 15.8%) were the commonest types in cervical histological grades, consistent with previously reported findings. The HPV18 was the second most prevalent type in women with ≥CIN3, compared to the worldwide data report of 2017.[96] According to Huang, S., et al.,[97] HPV 58 and 52 were the third and fourth most prevalent among women with ≥CIN3 in China. Therefore, the prevalence of HPV6 in cervical cancer was higher than other cervical lesion degrees, which may suggest an alternative to promoting HPV vaccination.
Type-specific HPV prevalence and overall prevalence HPV, stratified by cervical lesion degrees cytology diagnosis
Our study shows an overall HPV prevalence of 90.1, 73.6, 42.2, and 12.5% in the ≥HSIL, LSIL, ASCUS, and NILM, respectively, which is higher compared with a previous systematic review that showed 87% and 67.1% in ≥HSIL and LSIL.[15] The previously mentioned values were lower than the HPV prevalence worldwide.[98] The type-specific HPV prevalence varied in different cervical disease grades detected by cytology, where the commonest HPV types were HPV16, 52, 18, and 58. These results demonstrated that the main HPV types are different to those described in worldwide reports about the Chinese population,[98] which may be due to the overtime change of HPV types, especially in cancer screening in China and the lack of cervical cytology diagnosis. HPV genotypes vary in different cervical stages by cervical cytology diagnosis. The prevalence of HPV16, 18, 58, and 31 was increased by ASCUS and more advanced disease grades. The increase demonstrated strong carcinogenicity in ≥HSIL and other types that may accelerate the carcinogenic process. HPV16 was the most predominant type in cervical disease, except for normal cytological diagnosis, which ranged from 3.3 to 54.5%, which was different from histological due to the lack of cytology diagnosis in cervical cancer screening. HPV18 was the second most common type in ≥HSIL, which has been proven to be the main HPV type reported in many articles that have studied the Chinese population.[15] This is a target to be achieved in cervical cancer prevention by 2013–2020 through the Global Action Plan for “call to action ending cervical cancer. “
Study strengths and limitations
This study has some limitations. First, the included studies lack information about the occupations and ethnic groups of the analyzed healthy populations, which may be unequal in every province; thus, we ignored some critical points in our analysis leading to weak homogeneity that might affect the prevalence of HPV. Second, the prevalence of specific types of HPV included in multiple and single infections was counted in this analysis to contribute to a higher positive − which could have potentially increased the attributable fraction of HPV and specific types of HPV in intraepithelial cervical cancer cases, especially in HPV16 or other HR-HPV types. Third, the type-specific HPV prevalence in the analyzed studies had variation in histopathology and cytology diagnoses. But due to limited resources, we are unable to identify the real association between HPV and the range of cervical diseases detected by cytology or histology.
Disclosure
This meta-analysis demonstrated that HPV16, 52, 58, 18, and 33 are the most common HPV types in women from Mainland China. The HPV infection is widely prevalent in the Chinese mainland population, such as those in Central China. The overall HPV and HR-HPV prevalence in healthy women showed a peak at two different ages, under 25 years and between 41 and 45 years of age. Regarding histology and cytology analysis, women with cervical lesions and cervical cancer showed a significantly higher HPV prevalence than women with normal results. In conclusion, this study helps to update the available data about HPV in Mainland China and further provides important information that might be useful for implementing and guiding new policies in China and/or other developing environments worldwide.
Abbreviations
SPSS = Statistical Package for Social Science, ASMR = age-standardized mortality rate, HPV = Human papillomavirus, ≥HSIL = High-grade squamous intraepithelial lesion or worse, LSIL = Low-grade squamous intraepithelial lesion, ASCUS = Atypical squamous cell of undetermined significance, CIN1 = Cervical intraepithelial neoplasia grade 1, CIN2 = Cervical intraepithelial neoplasia grade 2, ≥CIN3 = Cervical intraepithelial neoplasia grade 3 or worse, PCR = Polymerase chain reaction.
Availability of data and materials
Data within this manuscript were presented in part as Excel. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Yan Qin Yu, Shi Lan Fu, JinQi Hao,Maria J. G. Mendez, Bangura Mohamed S, Fang Hui Zhao, and You Lin Qiao participated to design the study, performed data analysis, visualization, validation the whole work, and prepared the manuscript. Yan Qin Yu and Shi Lan Fu took part in data collection, supervision and software, and other resources. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics approval and patient consent were not required because this study is a meta-analysis based on published studies.
Consent for publication
All authors have given their consent to publishing this work.
Financial support and sponsorship
The study was funded by Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (No. 2021-I2M-1-004) and Foundation of Baotou Medical College (No. BYJJ-YF 201723).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Daehee Kang for partial support of this work.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Song B, Ding C, Chen W, Sun H, Zhang M, Chen W. Incidence and mortality of cervical cancer in China, 2013. Chin J Cancer Res. 2017;29:471–76. doi: 10.21147/j.issn.1000-9604.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Xia C, Ding C, Zheng R, Zhang S, Zeng H, Wang J, et al. Trends in geographical disparities for cervical cancer mortality in China from 1973 to 2013: A subnational spatio-temporal study. Chin J Cancer Res. 2017;29:487–95. doi: 10.21147/j.issn.1000-9604.2017.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Zheng R, Li X, Shan H, Wu Q, Wang Y, Chen W. Trends of incidence rate and age at diagnosis for cervical cancer in China, from 2000 to 2014. Chin J Cancer Res. 2017;29:477–86. doi: 10.21147/j.issn.1000-9604.2017.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausen zur H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 7.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–99. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 9.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 10.Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 11.Xu HH, Wang K, Feng XJ, Dong SS, Lin A, Zheng LZ, et al. Prevalence of human papillomavirus genotypes and relative risk of cervical cancer in China: A systematic review and meta-analysis. Oncotarget. 2018;9:15386–97. doi: 10.18632/oncotarget.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Molijn A, Enqi W, Zhang X, Jenkins D, Yu X, et al. The variable clinicopathological categories and role of human papillomavirus in cervical adenocarcinoma: A hospital based nation-wide multi-center retrospective study across China. Int J Cancer. 2016;139:2687–97. doi: 10.1002/ijc.30401. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y-Q, Fu S-L, Xu H-F, Wei M-N, Li Z-F, Zhao F-H, et al. Meta-analysis of cervical papillomavirus infection among women in Gansu. Chinese Maternity Child Health. 2017;32:2811–3. [Google Scholar]
- 14.Ma X, Wang Q, Ong JJ, Fairley CK, Su S, Peng P, et al. Prevalence of human papillomavirus by geographical regions, sexual orientation and HIV status in China: A systematic review and meta-analysis. Sex Transm Infect. 2018;94:434–42. doi: 10.1136/sextrans-2017-053412. [DOI] [PubMed] [Google Scholar]
- 15.Bao YP, Li N, Smith JS, Qiao YL. Human papillomavirus type-distribution in the cervix of Chinese women: A meta-analysis. Int J STD AIDS. 2008;19:106–11. doi: 10.1258/ijsa.2007.007113. [DOI] [PubMed] [Google Scholar]
- 16.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7:7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao R, Zhang WY, Wu MH, Zhang SW, Pan J, Zhu L, et al. Human papillomavirus infection in Beijing, People's Republic of China: A population-based study. Br J Cancer. 2009;101:1635–40. doi: 10.1038/sj.bjc.6605351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhao F, Hu S, Chen W, Chen F, Cui J, et al. Multi-center cross-sectional study on type-specific human papillomavirus infection among Chinese women. Chinese. 2015:1351–6. [PubMed] [Google Scholar]
- 19.Jing L, Zhong X, Huang W, Liu Y, Wang M, Miao Z, et al. HPV genotypes and associated cervical cytological abnormalities in women from the Pearl River Delta region of Guangdong province, China: A cross-sectional study? BMC Infect Dis. 2014;14:388. doi: 10.1186/1471-2334-14-388. doi: 10.1186/1471-2334-14-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Xie LX, Qing ZR, Li LJ, Luo ZY, Lin M, et al. Epidemiologic characterization of human papillomavirus infection in rural Chaozhou, eastern Guangdong Province of China. PLoS One. 2012;7:e32149. doi: 10.1371/journal.pone.0032149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu RF, Dai M, Qiao YL, Clifford GM, Liu ZH, Arslan A, et al. Human papillomavirus infection in women in Shenzhen City, People's Republic of China, a population typical of recent Chinese urbanization. Int J Cancer. 2007;121:1306–11. doi: 10.1002/ijc.22726. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Liu L, Guo C, Liu Z, Nie S. Prevalence of human papillomavirus infection and genotyping for population-based cervical screening in developed regions in China. Oncotarget. 2016;7:62411–24. doi: 10.18632/oncotarget.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Derong, Tang Li, Luo Zhenyun, Wu Ruiping, Han Liwei. Current situation of HPV infection and cervical cancer screening in rural women of childbearing age in Lechang City. Chinese Journal of Reproductive Health. 2015;26:39–41. [Google Scholar]
- 24.Zhimin L, Xiping L, Lingzhi M, Xiaoli S, Bing L, Xiaozhuang Z. Analysis of cervical human papillomavirus infection and genotype in 13750 women in Chaozhou City, Guangdong Province. Chinese Journal of Obstetrics & Gynecology and Pediatrics (Electronic Edition) 2012;8:357–60. [Google Scholar]
- 25.Wang Mingzhen, Miao Yun, Zhang Ling, Lin Lin. Investigation on infection status and risk factors of HPV in women in Yantian District of Shenzhen. Chinese journal of woman and child health research. 2012;23:302–4. [Google Scholar]
- 26.Wei F, Yin K, Wu X, Lan J, Huang S, Sheng W, et al. Human papillomavirus prevalence and associated factors in women and men in south China: A population-based study. Emerg Microbes Infect. 2016;5:e119. doi: 10.1038/emi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Wang Y, Peng M, She Q, Xiang Q, Chen Q, et al. Prevalence and type distribution of high-risk human papillomavirus infections among women in Wu Feng County, China. Arch Gynecol Obstet. 2012;286:695–9. doi: 10.1007/s00404-012-2344-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhao FH, Zhu FC, Chen W, Li J, Hu YM, Hong Y, et al. Baseline prevalence and type distribution of human papillomavirus in healthy Chinese women aged 18-25 years enrolled in a clinical trial. Int J Cancer. 2014;135:2604–11. doi: 10.1002/ijc.28896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YP, Wang JX, Sui Q, Chen MH, Qian JY, He Y, et al. Analysis of cervical cancer screening in Wuxi from 2013 to 2014. Chin J Prev Med. 2016;50:451–4. doi: 10.3760/cma.j.issn.0253-9624.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Xiaoling X, Longyu L, Baohua Y, Xiaoxia L, Ling L. Cross sectional study on high risk human papillomavirus infection in rural women in Jing'an County, Jiangxi Province. Clinical oncology in China. 2013;(18):1102–5. [Google Scholar]
- 31.Dai M, Bao YP, Li N, Clifford GM, Vaccarella S, Snijders PJ, et al. Human papillomavirus infection in Shanxi Province, People's Republic of China: A population-based study. Br J Cancer. 2006;95:96–101. doi: 10.1038/sj.bjc.6603208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu XW, Zhang XW, Wang L, Li F, Xu J. Status of human papillomavirus infection in the rural female population in Northwestern China: An observational study. J Low Genit Tract Dis. 2013;17:17–22. doi: 10.1097/LGT.0b013e31825707ab. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Shi TY, Ren Y, Lu H, Wei ZH, Hou WJ, et al. Risk factors for human papillomavirus infection in Shanghai suburbs: A population-based study with 10,000 women. J Clin Virol. 2013;58:144–8. doi: 10.1016/j.jcv.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Yan L, Yong S, Mei Q. Analysis of screening results of cervical human papillomavirus infection among women aged 40 ~ 64 in a community in Shanghai. Health education and health promotion. 2016;11:231–2. [Google Scholar]
- 35.Li LK, Dai M, Clifford GM, Yao WQ, Arslan A, Li N, et al. Human papillomavirus infection in Shenyang City, People's Republic of China: A population-based study. Br J Cancer. 2006;95:1593–7. doi: 10.1038/sj.bjc.6603450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Wallin KL, Duan M, Gharizadeh B, Zheng B, Qu P. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. 2015;87:1966–72. doi: 10.1002/jmv.24248. [DOI] [PubMed] [Google Scholar]
- 37.Jin Q, Shen K, Li H, Zhou XR, Huang HF, Leng JH. Age-specific prevalence of human papillomavirus by grade of cervical cytology in Tibetan women. Chin Med J (Engl) 2010;123:2004–11. [PubMed] [Google Scholar]
- 38.Sun LL, Jin Q, Li H, Zhou XR, Song ZQ, Cheng XM, et al. Population-based study on the prevalence of and risk factors for human papillomavirus infection in Qujing of Yunnan province, Southwest China? Virol J. 2012;9:153. doi: 10.1186/1743-422X-9-153. doi: 10.1186/1743-422X-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baloch Z, Yuan T, Wang B, Tai W, Feng Y, Liu Y, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in north-western Yunnan, China. J Med Virol. 2016;88:532–40. doi: 10.1002/jmv.24352. [DOI] [PubMed] [Google Scholar]
- 40.Hong H, He TF, Ni HX, Zhang S, Xu GZ. Prevalence and genotype distribution of HPV infection among women in Ningbo, China. Int J Gynaecol Obstet. 2015;131:96–9. doi: 10.1016/j.ijgo.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 41.Ye J, Cheng X, Chen X, Ye F, Lü W, Xie X. Prevalence and risk profile of cervical Human papillomavirus infection in Zhejiang Province, southeast China: A population-based study? Virol J. 2010;7:66. doi: 10.1186/1743-422X-7-66. doi: 10.1186/1743-422X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qing H, Qiner M, Yaxian F, Qiong H. Prevalence and Risk Factor of Human Papillomavirus Infection in Xiao shan District, Hangzhou City, Zhejiang Province. Journal of Oncology. 2010;16(12):965–7. [Google Scholar]
- 43.Xiuhua H, Xianghong Y, Lina S, shuqun C, Weiguo LV, Xing X. HPV infection and genotype distribution in women in Cixi area Zhejiang Journal of Preventive Medicine. 2010;22(6):66–8. [Google Scholar]
- 44.Jicai C, Xiaoshan Y, Shaowu X, Ren C, Huiying W. Epidemiological investigation on human papillomavirus nucleic acid genotyping in townswomen of Shantou city. Lab Med Clin. 2012;9:1681–3. [Google Scholar]
- 45.Yuanhong T, Lipeng J, Weihuang H, Ya L, Huilian Y, Hongyun A, et al. Analysis of 3852 women with cervical infection of human papilloma virus and its genotypes distribution in Yang jiang. Chinese Journal of Family Planning. 2015;(11):748–751,754. [Google Scholar]
- 46.Liu M, He Z, Zhang C, Liu F, Liu Y, Li J, et al. Transmission of genital human papillomavirus infection in couples: A population-based cohort study in rural China? Sci Rep. 2015;5:10986. doi: 10.1038/srep10986. doi: 10.1038/srep10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuanlai W, Jiong L, Yu D, Rongjie Q, Yankun L. Study on human papillomavirus infection among different age women in Wan yuan Road Community. Chinese Journal of modern medicine. 2012;14:21–23. [Google Scholar]
- 48.Yu J, Qing L, Jian L, Yulin Z. Epidemiological investigation and analysis of human papillomavirus infection in Southern Fujian. Modern Preventive Medicine. 2010;37:110–111+118. [Google Scholar]
- 49.Ting G, Long Z, Yanfang X, Juan Y, Yan J, Jun S. Analysis of application of HPV genotyping testing in cervical cancer screening. Progress in Modern Biomedicine. 2016;(6):544–7. [Google Scholar]
- 50.Huasheng Z, Dinan Z, Jianmin L, Min L, Duan CL, Muchang Z. Investigation and subtype distribution of HPV infection in 2663 normal women. Contemporary Medicine. 2011;17:20–21+109. [Google Scholar]
- 51.Danling Z, Jian M, Huixia L, Fuxiang X, Lijie P. Analysis of HPV infection and related factors in 7588 women with physical examination. Chinese Journal of Maternal and Child Health. 2016;7:25–27+35. [Google Scholar]
- 52.Jinlong H, Lizhen H, Weiyi X, Xi Q. Analysis of HPV subtype infection in 4 037 healthy women in Haikou area. Chin J MicrobiolImmunol. 2016;36:918–23. [Google Scholar]
- 53.Yang L, Li N, Guo L-W, Li Q, Cui H, Dai M. Prevalence of human papilloma virus and analysis of it's risk factors in Daqing city, Heilongjiang province in 2010. Chin J Prev Med. 2013:118–23. [PubMed] [Google Scholar]
- 54.Zhao Q, Chen Z, Li Z, Zhu X, Liu Y. Human papillomavirus infection and its risk factors among women receiving health check-up in Changsha area. Int J Clin Exp Med. 2016;9:8550–6. [Google Scholar]
- 55.Xueli L, Xiangui Z, Hongying L. Analysis of human papillomavirus infection characteristics among women of Yichang area. Chinese Journal of Cancer Prevention and Treatment. 2015;(16):1261–65. [Google Scholar]
- 56.Xue H, Lin X, Li T, Yan X, Guo K, Zhang Y. Prevalence and genotype distribution of human papillomavirus infection in asymptomatic women in Liaoning province, China. J Med Virol. 2015;87:1248–53. doi: 10.1002/jmv.24029. [DOI] [PubMed] [Google Scholar]
- 57.Xinxing Z, Xuejun L, Changchun Y, Hongqi Y, Hui Z. Epidemiology investigation on human papillomavirus subtypes for the exploitation and introduction of vaccine. Modern Preventive Medicine. 2013;40:4425–7. [Google Scholar]
- 58.Lingyun H, Yawen W, AI F, Xuewen Y. Survey and analysis of HPV infection in cervical cells of 1 650 local married women living in Xi'an city. Chin J Woman Child Health Res. 2012:738–41. [Google Scholar]
- 59.Yuping L, Taimin S, Ping S, Jun C, Jing T. Human papillomavirus infection in uterine cervix and subtype analysis among health examination women in Chengdu. Sichuan Medical Journal. 2018;39:435–7. [Google Scholar]
- 60.Haiyan H, Zhaomei X, Xiaodan Y, Xianqian Q. Cervical papillomavirus infection status and genotype distribution in Tian Tai County. Chin J Hygiene Inspect. 2014;24:3275–8. [Google Scholar]
- 61.Hu HX. Infection status of high-risk HPV in healthy populationHealth Management. 2021;(23):123–4. [Google Scholar]
- 62.Qiu YC. Epidemiological survey of cervical infection of human papillomavirus among women in Shaoxing, Zhejiang. Disease Surveillance. 2013;(9):743–7. [Google Scholar]
- 63.Zhong R, Wang M. Analysis of Human Papilloma Virus Infection State and Genotypes of 2240 Clients in Centre of Physical Examination. Medical Information. 2015;(32):19–20. [Google Scholar]
- 64.Ping Z, Wu Z, Mo S. Human papillomavirus infection and distribution in female physical examination population in Wenzhou. Chinese Journal of hygiene testing. 2015;25(19):3353–5. [Google Scholar]
- 65.Yanmei L, Xingjing X, Xuemin S. Study on human papillomavirus census of healthy women in Dong Ying area of Shandong. Med Lab Sci Clin. 2013:34–8. [Google Scholar]
- 66.Mijit F, Ablimit T, Abduxkur G, Abliz G. Distribution of human papillomavirus (HPV) genotypes detected by routine Pap smear in Uyghur-Muslim women from Karasay Township Hotan (Xinjiang, China) J Med Virol. 2015;87:1960–5. doi: 10.1002/jmv.24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dongdong X, Yuexin H, Hongyu J. Investigation on mental health status of high-risk human papillomavirus infection and analysis of influencing factors [J] Chinese Journal of Family Planning. 2022;30:1491–5. [Google Scholar]
- 68.Liang DM, Han CY, Wu YH. Investigation of high-risk HPV infection in community and psychological counseling for the infectors. Chinese Journal of Modern Nursing. 2017;23:3531–3. [Google Scholar]
- 69.Wu B. Statistical analysis of cervical HPV subtype infection among rural women in Xiang Zhou area [J] Medical information. 2017;30:98–100. [Google Scholar]
- 70.Jingxian C, Min Y, Xun H. HPV infection and genotype analysis of 13798 female patients in a hospital in Chengdu from 2014 to 2018 [J] Modern Preventive Medicine. 2021;48:542–54558. [Google Scholar]
- 71.Ren Z. HPV infection status and subtype distribution in 2070 healthy women in Mian Yang city. Int J Lab Med. 2018;39:1389–91. [Google Scholar]
- 72.Xiaohong Z, Liangfeng C, Donghong L, Xiaohui LV, Xiuqin L, Jia L, et al. Epidemiological status of cervical HPV infection in women in Shaanxi province. Chin J Woman Child Health Res. 2017;28:1589–92. [Google Scholar]
- 73.Zhao Q, Pan J, Zhu J, Zhao L, Wang L, Wang K, et al. Analysis of the distribution of various types of HPV and their influencing factors among women with normal cervical cytology in the Shanghai area. Chin J Health Manag. 2017;11:504–9. [Google Scholar]
- 74.Juan C, Linke Y, Xianning D, Yu T, Kai X. Characteristic analysis of human papillomavirus infection among female physical examination population in Wenzhou area from 2015 to 2017 [J] Rural Chinese Medicine. 2018;25:59–60. [Google Scholar]
- 75.Zhao HY, Hao GH, Liu Q, Ma XX, Zhou P. Prevalence of human papillomavirus (HPV) infection and its related factors in the mining area of Huabei oil field. Chinese Journal of Nosocomiology. 2017;27(11):2577–9,2606. [Google Scholar]
- 76.Wang Y, Chen WY, Luo Y. Investigation on high-risk human papillomavirus infection in 580 physical examination women. J Tai Shan Med Coll. 2018;39:760–2. [Google Scholar]
- 77.Wang YY, Tang C, Liu ZH. Risk factors of female HPV infection in Shenzhen. China Trop Med. 2018;18:1083–7. [Google Scholar]
- 78.Di JL, Luo XM, Wu JL, Song B, Ma L. Population-based study on infection and genotype distribution of high-risk human among women in rural areas of China, 2014. Chin J Prev Med. 2017;51:325–31. doi: 10.3760/cma.j.issn.0253-9624.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Lian Y, Xiaojun L, Yuanxin Y, Yi Z, Xuemei W, Binwu Y. Analysis of HPV infection in 8944 healthy women in West China Hospital of Sichuan University. Chin J Evid Based Med. 2017;17:634–9. [Google Scholar]
- 80.Pengchao F, Xiaotong L, Mingyue L, Lu C, Lifen W, Xiaohong G. Infection characteristics of human papillomavirus in healthy women. Chin J Pract Gynecol Obstet. 2018;34:304–7. [Google Scholar]
- 81.Liu ZH, Lin W, Wang YY, Wu B, Yuan SX, Yao JL, et al. Risk stratification of type-specific human papillomavirus for cervical precancers: Evidence from across-sectional study in Shenzhen. Chin J Oncol. 2018;40:757–63. doi: 10.3760/cma.j.issn.0253-3766.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 82.Jiang MY, Feng RM, Wang L, Li TY, Zhang AA, Cui JF, et al. Performance of combined liquid-based cytology and HPV nucleic acid test for detecting cervical precancer among women attending screening. Chinese Journal of Oncology. 2018;40(10):750–6. doi: 10.3760/cma.j.issn.0253-3766.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Zhong T-Y, Zhou J-C, Hu R, Fan X-N, Xie X-Y, Liu Z-X, Lin M, et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province China. Infect Public Health. 2017;10:783–8. doi: 10.1016/j.jiph.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Zhou XH, Shi YF, Wang LJ, Liu M, Li F. Distribution characteristics of human papillomavirus infection: A study based on data from physical examination. Asian Pac J Cancer Prev. 2017;18:1875–9. doi: 10.22034/APJCP.2017.18.7.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Ji Y, Li J, Dong H, Zhu B, Zhou Y, et al. Prevalence of human papillomavirus infection in women in the Autonomous Region of Inner Mongolia: A population-based study of a Chinese ethnic minority Med Virol. 2018;90:148–56. doi: 10.1002/jmv.24888. [DOI] [PubMed] [Google Scholar]
- 86.Zhao XL, Hu SY, Zhang Q, Feng LDRM, Han R, Zhao FH. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017;28:e30. doi: 10.3802/jgo.2017.28.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li N, Hang D, Yang L, Feng X, Lyu Z, Xie S, et al. Persistence of type-specific human papillomavirus infection among Daqing City women in China with normal cytology: A pilot prospective study. Oncotarget. 2017;8:81455–61. doi: 10.18632/oncotarget.20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao F-H, Lewkowitz AK, Hu S-Y, Chen F, Li L-Y, Zhang Q-M, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: A pooled analysis of 17 population-based studies. Int J Cancer. 2012;131:2929–38. doi: 10.1002/ijc.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang R, Guo X-L, Wisman GBA, Schuuring E, Wang W-F, Zeng Z-Y, Zhu H, Wu S-W. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. 2015;15:257. doi: 10.1186/s12879-015-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J, Huang R, Schmidt JE, Qiao YL. Epidemiological features of human papillomavirus (HPV) infection among women living in Mainland China. Asian Pac J Cancer Prev. 2013;14:4015–23. doi: 10.7314/apjcp.2013.14.7.4015. [DOI] [PubMed] [Google Scholar]
- 91.Bruni L, Albero G, Serrano B, Mena M, Muñoz J, Bosch FX, de Sanjose S. Human Papillomavirus and Related Diseases in China. Barcelona: ICO/IARC Information Centreon HPV and Cancer. 2019 [Google Scholar]
- 92.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 93.Kang LN, Castle PE, Zhao FH, Jeronimo J, Chen F, Bansil P, et al. A prospective study of age trends of high-risk human papillomavirus infection in rural China. BMC Infect Dis. 2014;14:96. doi: 10.1186/1471-2334-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Franceschi S, Herrero R, Clifford GM, Snijders PJF, Arslan A, Anh PTH, et al. Variations in the age specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677–84. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 95.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjosé S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 96.Dong L, Hu SY, Zhang Q, Feng RM, Zhang L, Zhao XL, et al. Changes in genotype prevalence of human papillomavirus over 10-year follow-up of a cervical cancer screening cohort. Chin J Epidemiol. 2017;38:20–5. doi: 10.3760/cma.j.issn.0254-6450.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 97.Huang S, Afonina I, Miller BA, Beckmann AM. Human papillomavirus type 52 and 58 are prevalent in cervical cancers from Chinese women. Int J Cancer. 1997;70:408–11. doi: 10.1002/(sici)1097-0215(19970207)70:4<408::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 98.Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40:602–8. doi: 10.1080/01443615.2019.1634030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data within this manuscript were presented in part as Excel. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.