ABSTRACT
In the 1960s, sperm cryopreservation was developed as a method to preserve fertility. Currently, techniques for the cryopreservation of human spermatozoa have been widely used in assisted reproduction. However, although sperm cryobiology has made notable achievements, the optimal method for the recovery of viable spermatozoa after cryopreservation remains elusive. Postthawing sperm quality can be affected by cryoprotectants, ice formation, storage conditions, and osmotic stress during the freezing process. This review discusses recent advances in different cryopreservation techniques, cryoprotectants, and freezing and thawing methods during cryopreservation and new indications for the use of cryopreserved spermatozoa.
Keywords: cryodamage, cryoprotectant, dry preservation, freezing and thawing, sperm cryopreservation
INTRODUCTION
The first sperm freezing technique record can be traced back to more than two hundred years ago. In 1776, an Italian priest, Spallanzani, attempted to store human spermatozoa by cooling in snow;1 however, his discovery did not attract people’ attention at that time. In the 19th century, Montegazza succeeded in freezing spermatozoa at 15°C and proposed the concept called a “human sperm bank” for the first time.2 However, most of the freeze-thawed spermatozoa died at that time, and techniques for the cryopreservation of human spermatozoa were difficult in practical application. Further scientific progress was made later when Hoagland and Pincus3 used rapid freezing to store human semen in liquid nitrogen (−196°C), achieving a maximum yield of recoverable motile human of 50%. Since then, human sperm cryopreservation techniques have been improved. In 1949, Polge et al.4 found that glycerol could protect sperm against the effects of low temperatures; thus, glycerol has remained the most important component of sperm cryoprotectant. In 1953, Sherman and Bunge5 mixed one part of absolute glycerol with nine parts of liquefied human semen and placed the mixture in an insulated box with dry ice. After quick thawing in a 37°C water bath, the semen could be preserved for a long time (about 3 months), with an average sperm survival of 67%. It was reported that the first offspring were generated successfully from cryopreserved spermatozoa in the following year.6 Today, human sperm cryopreservation is applied extensively in assisted reproduction technology (ART). However, despite multiple successes in the cryobiology of spermatozoa, research continues to identify the best way to recover viable cells after cryopreservation.
NEW CRYOPRESERVANTS FOR SLOW FREEZING AND VITRIFICATION
Sperm freezing is usually accompanied by many adverse consequences, such as extracellular and intracellular freezing injury and pervasive pressure. To avoid these harmful influences, cryoprotectants (CPAs) are added to biomaterials before freezing. There are two types of CPAs: those that can, and those that cannot, permeate cell membranes, both of which act by reducing the ice point of intracellular and extracellular water, thus protecting cells from cellular mechanical injury caused by ice crystals during freezing and recovery. At the same time, the composition of a CPA can result in a more reasonable osmotic pressure of fluid, protecting the spermatozoa from damage caused by severe changes in osmotic pressure during freezing and recovery.7
Original CPAs
The first highly effective CPA was glycerol.4 Following this, Lovelock and Bishop8 discovered that dimethyl sulfoxide (DMSO) penetrated cells faster than glycerol and required a much lower concentration to completely prevent freezing damage. DMSO and glycerol are membrane-permeant compounds, which are difficult to be removed from spermatozoa after thawing. In addition, glycerol, DMSO, and other membrane-permeant compounds might be toxic to spermatozoa.9,10 Therefore, currently, cryoprotectants are being developed that reduce or avoid the use of DMSO and other membrane-permeant compounds to reduce their adverse effects on the spermatozoa. Some high-molecular-weight CPAs cannot permeate cell membranes. Sugars were perhaps the first high-molecular-weight CPAs to be used as additives before freezing.11 Both mono- and di-saccharides have been used for the cryopreservation of spermatozoa; however, compared with monosaccharides, disaccharides such as trehalose and sucrose are used more extensively.
CPA additives
The mechanism by which freezing damages human spermatozoa is multifactorial, consisting of osmotic stress, oxidative stress, cold shock, intracellular ice crystal formation, and their combination. Additives with antioxidant properties can reduce the damage caused by cold shock and the effects of reactive oxygen species (ROS). Common additives include vitamin E, vitamin C, glutathione (GSH), melatonin (MLT), taurine, zinc/selenium, and nonenzymatic antioxidants such as L-carnitine. Enzymatic adjuncts include glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT). Herbal extracts, including genistein, rosemary, and rhodiola, have also been added. Notably, the combined use of different additives or ingredients might have detrimental effects on postthaw sperm quality.12 In recent years, some studies have reported that soybean lecithin,13,14 low-density lipoprotein,15 and other lipids could be used as additives to ameliorate ROS damage.
In addition, the addition of antifreeze proteins,16 magnetized extenders,17,18 light irradiation,19 and sublethal hydrostatic pressure stress20 treatment before freezing has also been demonstrated to benefit the cryopreservation of mammalian spermatozoa. However, few studies have applied these techniques to human sperm cryopreservation, and in future, attention should be paid to the safety and feasibility of their clinical application.
EFFECT OF TEMPERATURE IN THE FREEZING AND WARMING PROCESS
The speed of freezing and thawing of sperm is a significant element that influences the survival rate of thawed spermatozoa after cryopreservation; a high freezing and thawing speed can reduce the formation of ice crystal regions in and outside spermatozoa, the time of sperm exposure to osmotic pressure, and the damage to spermatozoa caused by the freezing and thawing process.
Slow or rapid freezing
Slow freezing uses the maximum cooling rate that will result in adequate osmotic dehydration to maintain the cell water’s chemical potential near an equilibrium with the chemical potential of the water in the partially frozen external medium.21 In 1970, Morris22 proposed a two-factor hypothesis in which cells that are cooled too quickly are killed by intracellular ice formation and those that are cooled too slowly die because of lengthy exposure to concentrated solutions that are produced during the progressive conversion of water to ice. The balance between these two forces results in graphs of survival vs cooling rate appearing as an inverted “U”. The slow freezing technique comprises gradually cooling of the cells in two or three steps within 2–4 h, which is now widely used for conventional sperm cryopreservation.23 However, this slow freezing is not ideal for cryopreservation of single or small numbers of spermatozoa. Currently, rapid freezing after adding CPAs comprising highly permeant chemicals is widely applied for the cryopreservation of single or small numbers of them. This method requires the carrier to be in direct contact for 8–10 min with nitrogen vapor and to be immersed in nitrogen liquid at the temperature of −196°C.9 However, the rapid freezing gives superior postthaw motility and survival rate than slow freezing.24,25 This method does not completely solve the problem of ice crystal formation and recrystallization, and it is very difficult to completely remove these low-molecular-weight chemicals from spermatozoa after thawing.
Vitrification
Vitrification refers to the suspension of cells in appropriately high concentrations of CPA mixtures, which, combined with sufficiently high rates of cooling and warming, prevent ice formation in the cells and the surrounding medium during cooling or warming. CPAs used in vitrifying cells are commonly the same type with those in slow or rapid freezing, but the concentrations are much higher. Vitrification of human spermatozoa has been applied in the clinic in recent years and has good application prospects. In 2002, Nawroth et al.26 reported human sperm vitrification, and successful pregnancies have been reported using intracytoplasmic sperm injection (ICSI)27 and intrauterine insemination (IUI)28 using spermatozoa that were cryopreserved via vitrification. A recent meta-analysis was performed to compare vitrification and conventional freezing methods for sperm cryopreservation.29 The study found that vitrification is better than traditional freezing methods for sperm preservation and progressive maneuverability; however, the DNA fragmentation index (DFI) of the two freezing methods was similar. Although the included studies used different freezing and thawing protocols, types of CPAs, and quality of spermatozoa, at the very least, vitrification is simpler and cheaper than conventional freezing methods.30
Warming
A rapid warming rate is another necessary condition for the high survival rate of frozen spermatozoa. A study reported that the survival of spermatozoa decreased when they were warmed at a suboptimal rate (10–100 times) after being frozen at an optimal rate (20°C–130°C per min). The authors suggested that this decrease in survival occurred because of the physical injury caused by ice recrystallization outside the cells during slow warming.31 Ultra-fast warming techniques, such as microwaving, infrared radiation (IR) laser pulse, and magneto-calorific techniques, have received more attention in recent years; however, there are no reports stating that they have been used for sperm thawing. It has been reported that using an IR laser pulse increased the survival of mouse oocytes32 and embryos,33 provided that after vitrification, an infrared pulse by a laser is applied to heat the specimen at an ultra-high rate of about 1 × 107°C per min, which is almost 100 times faster than conventional thawing methods. The exceedingly high rate seems to protect cells and embryos by preventing small ice crystal recrystallization formed during cooling. In summary, rapid freezing and thawing rates are required for successful sperm cryopreservation; however, the warming rate is more important.
SLOW FREEZING OR VITRIFICATION OF A SMALL NUMBER OF SPERMATOZOA
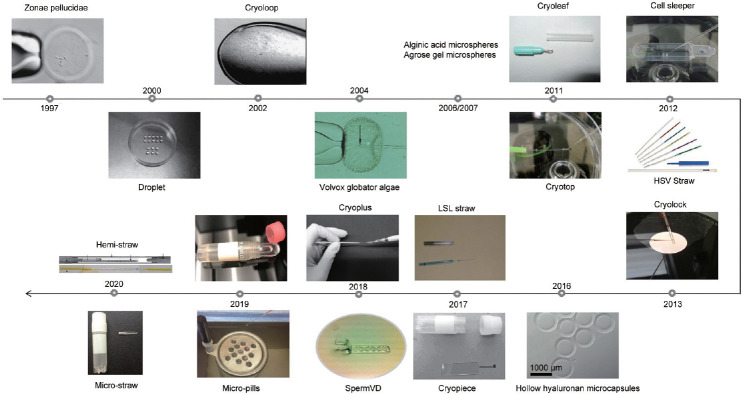
Cryopreservation of spermatozoa can preserve male fertility and help avoid lack of spermatozoa on woman’s oocyte-retrieval day. However, because of low number and lack of motility of spermatozoa, conventional sperm cryopreservation methods are unsuitable for their freezing. Sperm sample processing using conventional methods involves their dilution in a large volume, making recovery for ICSI difficult. Hence, there is a need to improve the existing cryopreservation techniques to improve the cryopreservation of these spermatozoa. To develop with a feasible clinical solution to such a difficulty, certain carriers and techniques have been investigated,34 e.g., to avoid the loss of a few spermatozoa during the freezing using multiple microquantities of equally divided spermatozoa, or even a small number of isolated spermatozoa (Figure 1).
Figure 1.
Different sperm vitrification devices developed since 1997.
Biological carriers
The zona pellucida was the first carrier developed for low number cryopreservation of spermatozoa. After the removal of cellular material from embryos or oocytes, the resulting empty zona pellucida can act as a suitable carrier to preserve a small number of spermatozoa.35 Using human and hamster zonae for sperm cryopreservation, scientists achieved a live birth for the first time in 1998.36 Empty zonae pellucidae are mainly derived from humans and mice, and there were no differences between the two when used for sperm cryopreservation.37 However, controversy surrounding the source of the zona pellucida has limited its widespread use in the clinic. Using the zona pellucida of mice to cryopreserve spermatozoa might introduce heterologous biological DNA. Other techniques have been developed using microencapsulation carriers, similar to empty zonae, as a means of cryopreserving small numbers of sperm. Microspheres were observed to have the same recovery rate as the empty zona pellucida and could be used for sperm cryopreservation in a few numbers. However, they have not been further used, because the algae Volvox globator38 are used to make these microspheres, and the new Food and Drug Administration (FDA) and European Organization directives stipulated that using algae (nonhuman tissue) to reserve human sperm in the clinical environment is not acceptable. The alginic acid in the microspheres39 leaves residues on the sperm surface, which affects sperm motility. In addition, agarose gel microspheres40,41,42 and hollow hyaluronan microcapsules43 have not been used in the clinical setting, and their safety remains to be discovered.
Nonbiological carriers
For the cryopreservation of small numbers of spermatozoa, other carriers have been studied, such as straws,44,45 droplets,46,47,48 and ICSI or denuding pipettes.49,50 These methods have different drawbacks. Straws are a suitable carrier used for the cryopreservation of a few spermatozoa; however, a single selected spermatozoon is not easy to separate from the straw. Droplets of the mixture of cryoprotectant and spermatozoa have a high sperm recovery rate for the cryopreservation of a single or few numbers of spermatozoa; however, culture dishes with droplets cannot be sealed to make a closed system, which makes their handling and preservation in traditional refrigerators and liquid nitrogen tanks more difficult because of their size and shape. Using ICSI or denuding pipettes as carriers for the cryopreservation of a single spermatozoon is convenient and easy; however, their long-term storage is impractical because the pipette suction head is fragile and is easily broken. Therefore, certain techniques developed for embryo or oocyte cryopreservation have been adapted to cryopreserve small sperm numbers. For example, cryoloop48,51,52,53 and cryotop54,55,56, and some additional carriers have been developed as improvements of cryotop, such as cryolock,57 cryoleaf,58 and cryoplus.59 Although these techniques are ideal for the cryopreservation of single spermatozoon or small numbers of human spermatozoa, their major weakness is that they are open systems, in which the spermatozoa are in direct contact with liquid nitrogen in the vial, with the associated risk of cross-contamination.
Widely implicated carriers
A cell sleeper comprises a sealing system for cell cryopreservation, which is not difficult to use and is commercially feasible. The sample droplets of spermatozoa are put into the cell sleeper on its trays. Then, the tray is placed into the freezing tube, sealed it with a screw cap, and cooled in liquid nitrogen.56 In studies from Endo et al.55 and Coetzee et al.,60 eight clinical pregnancies were achieved using small numbers of human spermatozoa cryopreserved in cell sleepers. The cryopreservation and thawing techniques using the cell sleeper are simple, and after thawing, the spermatozoa’s recovery rate is comparatively high. However, the cell sleeper has a drawback in which the tray has a high edge, and during the constant raising and lowering of the ICSI pipettes during the process, there is an increased risk of pipette breakage. In contrast to the cell sleeper, in a cryopiece, the cell sleeper tray is replaced by a piece with a negligible height; therefore, the constant raising and lowering of the ICSI pipettes are not required during the process, making the clinical application of a cryopiece more convenient; three clinical pregnancies have been reported using this technique.61 A sperm vitrification device (VD) also substitutes a piece for the tray in the cell sleeper, which allows a single spermatozoon to be frozen in lower-volume droplets: 24 clinical pregnancies have been achieved with this carrier.62
PRESERVATION OF SPERMATOZOA IN THE EXPERIMENTAL STAGE
Dry preservation
At present, the best way to preserve spermatozoa is to store them at a low temperature. Dry storage is a good alternative that eliminates the demand for an ultra-low temperature, provides organizational adaptability for shipping, and reduces the costs of storage maintenance.63,64,65 The volume of spermatozoa is low, with a low water content; therefore, the DNA of spermatozoa is highly concentrated. If CPAs comprising trehalose are added to the spermatozoa after cooling, in low-pressure environments, the solid water in the spermatozoa can be released directly as a vapor, thereby achieving dry dehydration. So far, spermatozoa have been stored at 4°C for several years using an improved freeze-drying scheme, which allows carrying of freeze-dried spermatozoa to another place at the surrounding temperature. By adding sterile ultrapure water, spermatozoa have been rehydrated and then used for ICSI.66 Freeze-dried spermatozoa can be used for fertilization because the integrity of sperm DNA, centrosomes, and biological functions can be preserved during the whole process of dry rehydration. Nevertheless, dry preservation might injure the sperm membrane and mitochondria, resulting in few motile cells after rehydration. Therefore, the introduction of freeze-dried immobile spermatozoa into oocytes using ICSI is vital for success.67 Earlier studies of sperm freeze-drying reported good motility, including rehydration of chicken and bull spermatozoa with values of up to 50%;4 however, to date, studies have failed to reproduce this initial success.
At present, the technology of dry preservation of spermatozoa is more mature in animals, and mice, rats, rabbits, hamsters, and horses have produced offspring successfully using spermatozoa stored using this method.66 By contrast, maintaining freeze-dried spermatozoa at room temperature for long periods is unsatisfactory,68 leading to reduced fertility. It is reported that freeze-dried mouse sperm were successfully stored at 4°C for 3 years and fertile offspring from oocytes microinjected with these sperm were obtained.69 Several reports on freeze-drying of animal spermatozoa have been published; however, because of ethical limitations or other unpublished reasons, ICSI has not been performed using freeze-dried human spermatozoa.70,71 Studies on the dry preservation of human spermatozoa showed that the survival and maneuverability membrane of sperm were completely impaired after being freeze-dried;72 however, the chromatin structure of spermatozoa and the protoplast structure in the sperm head were retained.71
Dropping into aseptic nitrogen liquid
Spermatozoa are commonly cryopreserved in cryoprotectant-free vitrification, which involves direct plunging into liquid nitrogen. However, sperm cryopreservation via direct contact with commercial liquid nitrogen carries a potential risk of microorganism contamination. Hence, Isachenko et al.73 proposed aseptic technology, in which, during vitrification, the spermatozoa should be isolated from liquid nitrogen to avoid the cross-contamination caused by direct contact between sperm and liquid nitrogen. However, it is impossible to sterilize completely commercially-produced, pathogen-contaminated liquid nitrogen.
To avoid the potential risks of contamination during cryopreservation, and to produce sterile liquid air, and thus to reduce the potential for contamination, benchtop device (CLAir, FertileSafe, Nes Ziona, Israel) has been developed. The device produces sterile liquid air at −195.7°C, which is similar to the temperature of liquid nitrogen (−196°C). Meanwhile, mouse embryos and human oocytes have been vitrified successfully using sterile liquid air.74 In addition, as an alternative to nonsterile liquid nitrogen, submerging cells directly in clean liquid air (aseptic system) is a good alternative for vitrification. Recently, devices to produce clean liquid air have been successfully employed for spermatozoa vitrification,75,76 supporting the view that cryoprotectant-free vitrification achieved by dropping human spermatozoa directly into clean liquid air is a viable substitute for nonsterile liquid nitrogen immersion. This technique for cooling cells minimizes the risk of contamination by microbes. However, there are limited data related to the vitrification of human spermatozoa using clean liquid air. Therefore, further studies to assess whether clean liquid air is more effective than conventional liquid nitrogen to vitrify human spermatozoan are needed.
NEW METHODS TO EVALUATE THE EFFECT OF FREEZING AND THAWING PROCESSES ON SPERM FUNCTION
The freezing and thawing of human spermatozoa is the most important part of male fertility technology. However, the mechanism by which the spermatozoa are damaged during cryopreservation, deep low temperature storage, and thawing has not been fully clarified. Currently, there is no uniform, recognized, and standard freezing and thawing method and procedure. Hence, different cryoprotectants, different storage conditions, and different freezing and thawing methods make the effect of spermatozoa cryopreservation uncertain, which not only affects the successfully implementation of reproductive technology but more importantly, also makes it unclear whether cryopreservation and freezing and thawing-related damage to sperm will affect the offspring’s health.
Flow cytometry and cryomicroscopes
Solution effect injury and ice crystal formation are the main mechanisms affecting sperm survival.7 Oxidative stress damage, changes in sperm morphology, and functional damage, such as alterations to enzyme activities, sperm membrane structural changes, and decreased DNA integrity, also affect sperm survival.77 The evaluation of oxidative stress, DNA fragmentation, and capacitation can be assessed by flow cytometry. The damage caused by cryopreservation is reflected in a reduction in the motility of spermatozoa.78 Furthermore, several studies have used flow cytometry to focus on the relationship between semen and cryodamage and revealed adverse effects of cryopreservation on morphology, DNA damage, and fertilizing capacity.79,80,81 A recent study reported that the proportion of human spermatozoa that were considered normal, as evaluated by using transmission electron microscopy, declined significantly after thawing, especially with regard to altered morphology of the plasmalemma, acrosomes, and tails.82 In addition, ice crystal formation could be observed in a cryomicroscope, which allows a sample to be cooled, frozen, and thawed on the microscope stage. This permits continual observation of spermatozoa throughout the protocol, including crystallization and recrystallization events. As early as in 1996, Mohammad et al.83 reported the direct assessment of human sperm cryopreservation using a cryomicroscope together with computer-aided sperm analysis.
The contribution of Omics
Changes in the proteome, transcriptome, and epigenetics of sperm before and after thawing have been reported. Some studies have found that the levels of certain proteins relating to sperm motility, sperm metabolism, sperm flagellum structure, and acrosomal integrity increased or decreased significantly after thawing.84,85 Meanwhile, genes related to fertility potential, such as the genome regions comprising small nucleolar RNA, C/D Box 116 (SNORD116)/Prader–Willi and Angelman (PWSAS), and ubiquitin protein ligase E3A (UBE3A), which are related to Angelman and Prader–Willi syndromes, were most severely damaged after thawing.86 In addition, freezing and thawing can partially change the transcription of sperm mRNA and inhibit the interaction between mRNAs and proteins, thus affecting the early development of embryos.87,88 Finally, in animal experiments, many epigenetic changes, such as sperm histone modification and methylation modification, have been observed after thawing.77 However, no difference was found in DNA methylation before and after thawing of human spermatozoa.
NEW INDICATIONS FOR THE USE OF CRYOPRESERVED SPERMATOZOA
Recently, there has been a wide expansion of the clinical indications for sperm cryopreservation, which has resulted in more patient groups becoming eligible for sperm freezing.
Use in viral sexually transmitted or inflammatory diseases
In sexually transmitted disease-discordant couples, semen washing is a safe reproductive strategy by which an infected male can induce pregnancy. Viral-positive spermatozoa could be removed through semen washing, and the vital-negative sperms were available for human-assisted reproduction.89 Hence, more patients with viral sexually transmitted diseases are eligible for sperm freezing. To date, semen and spermatozoa have been detected to contain 27 species of virus.90 Notably, at ultra-low temperatures and in appropriate protein concentrations, most viruses remain viable if stored dried. There is a vanishingly small risk of cross-contamination between samples stored in liquid nitrogen; however, the risk is finite; thus, all reasonable precautions should be taken to decrease the likelihood of its occurrence. Therefore, to prevent direct contact of viral-positive sperm with liquid nitrogen during cryopreservation, it might be advisable to use a separate liquid nitrogen container to quarantine spermatozoa derived from men with an infectious viral sexually transmitted disease. Meanwhile, for nonmalignant conditions, including diseases with inflammatory or pro-inflammatory components, before therapy, sperm freezing might also be performed. However, there is a paucity of data evaluating the clinical outcomes following sperm freezing for these conditions.
Social preservation of fertility in men over 45 years of age
Typically, women in their late 30s experience reduced fertility; however, advanced paternal age is also considered to be associated with reproductive changes. A recent systematic review and meta analysis showed that, with the increase of paternal age, the value of semen parameters decreased significantly. This suggests that male age-related reproductive decline should be considered as a potential contributor to negative pregnancy outcomes and the deleterious effects on offspring health.91 Increasing paternal age is also linked to neurocognitive disorders92 and is negatively associated with numerical chromosomal abnormalities.93 However, the literature related to the effect of paternal age on the success of medically assisted reproduction is limited and inconclusive; therefore, no upper limit of paternal reproductive potential has been set. Some studies considered age thresholds of <45 years for good sperm concentration and motility.94,95 Hence, sperm freezing might be performed at a younger age, and attention should be paid to the effect of paternal age on achieving a live birth.
Soldiers in a war zone, postmortem
What should happen to cryopreserved sperm after the death of the donor is subject to little regulation. A study revealed that 81.1% of men leave their cryopreserved spermatozoa to their partners and 18.9% wish their samples to be discarded after their death. The original reason for sperm cryopreservation has a major influence on men’s decisions concerning their cryopreserved spermatozoa.96 The reason that most men leave their cryopreserved spermatozoa to their partner might be the inherent desire for progeny, which is deeply seated in human neurobiology.97 However, there are limited data related to which factors affect men’s decisions regarding the postmortem collection and use of reproductive tissue and posthumous reproduction. Therefore, it is imperative to determine these influencing factors, because such information could influence legislation and institutional policies, thereby helping to resolve possible ethical dilemmas and legal conflicts and in the future.
Gender reassignment
Transgender refers to individuals who identify as a gender that does not match their biologically-assigned sex. Currently, the ratio of male-to-female reassignment (“trans-women”) to female-to-male reassignment (“trans-men”) is approximately 3:1. Estrogen therapy is used to treat trans-women, with or without bilateral orchiectomy, resulting in temporary or permanent infertility. However, transgender individuals often wish to conceive in the future, with the aid of appropriate fertility treatment. Therefore, the ethics committee of the American Society of Reproductive Medicine (ASRM) as recommended that all individuals undergoing gender reassignment should be offered the option of gamete freezing.98 Thus, human sperm banks could offer sperm cryopreservation to individuals before gender transition.
CONCLUSIONS
The cryopreservation of spermatozoa is a significant method of procreation management in ART. Although cryopreserved spermatozoa have been used effectively in ART for decades, the search continues for methods to optimize the recovery of viable spermatozoa after cryopreservation. Meanwhile, a long-term study on the offspring gained by cryopreserved spermatozoa should be performed to evaluate completely the biological security of the procedure. Furthermore, emerging technologies, such as spermatogonial stem cells and testicular tissue, have the potential to preserve the fertility of prepubescent boys in the future.
AUTHOR CONTRIBUTIONS
WBZ, ZL, and CH conceived and designed the study. CH, YLT, and JLH drafted the manuscript. WJZ, ZHH, and XFL revised the drafts. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 82001634), and the China Postdoctoral Science Foundation (No. 2019M661521).
REFERENCES
- 1.Talwar P. Update on sperm banking. In: Seli E, Agarwal A, editors. Fertility Preservation. New York: Springer; 2012. pp. 289–302. [Google Scholar]
- 2.Royere D, Barthelemy C, Hamamah S, Lansac J. Cryopreservation of spermatozoa:a 1996 review. Hum Reprod Update. 1996;2:553–9. doi: 10.1093/humupd/2.6.553. [DOI] [PubMed] [Google Scholar]
- 3.Hoagland H, Pincus G. Revival of mammalian sperm after immersion in liquid nitrogen. J Gen Physiol. 1942;25:337–44. doi: 10.1085/jgp.25.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 5.Sherman JK, Bunge RG. Observations on preservation of human spermatozoa at low temperatures. Proc Soc Exp Biol Med. 1953;82:686–8. doi: 10.3181/00379727-82-20219. [DOI] [PubMed] [Google Scholar]
- 6.Bunge RG, Keettel WC, Sherman JK. Clinical use of frozen semen:report of four cases. Fertil Steril. 1954;5:520–9. doi: 10.1016/s0015-0282(16)31802-7. [DOI] [PubMed] [Google Scholar]
- 7.Rajan R, Matsumura K. Development and application of cryoprotectants. Adv Exp Med Biol. 2018;1081:339–54. doi: 10.1007/978-981-13-1244-1_18. [DOI] [PubMed] [Google Scholar]
- 8.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–5. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 9.Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation:update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2012;2012:854837. doi: 10.1155/2012/854837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katkov II, Katkova N, Critser JK, Mazur P. Mouse spermatozoa in high concentrations of glycerol:chemical toxicity vs. osmotic shock at normal and reduced oxygen concentrations. Cryobiology. 1998;37:325–38. doi: 10.1006/cryo.1998.2128. [DOI] [PubMed] [Google Scholar]
- 11.Rajan R, Matsumura K. Development and application of cryoprotectants. In: Iwaya-Inoue M, Sakurai M, Uemura M, editors. Survival Strategies in Extreme Cold and Desiccation. Singapore: Springer Nature Singapore Pte. Ltd; 2018. pp. 339–54. [DOI] [PubMed] [Google Scholar]
- 12.Zhandi M, Sharafi M. Negative effect of combined cysteine and glutathione in soy lecithin-based extender on post-thawed ram spermatozoa. Cell Tissue Bank. 2015;16:443–8. doi: 10.1007/s10561-014-9488-z. [DOI] [PubMed] [Google Scholar]
- 13.Sicchieri F, Silva AB, Santana VP, Vasconcelos MA, Ferriani RA, et al. Phosphatidylcholine and L-acetyl-carnitine-based freezing medium can replace egg yolk and preserves human sperm function. Transl Androl Urol. 2021;10:397–407. doi: 10.21037/tau-20-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, He M, Wu C, Zhang S, Dai J, et al. Beneficial influence of soybean lecithin nanoparticles on rooster frozen-thawed semen quality and fertility. Animals (Basel) 2021;11:1769. doi: 10.3390/ani11061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snoeck PP, Moura LC, Silva MC, Machado-Neves M, Melo MI, et al. Effect of storage conditions on the LDL effectiveness in ovine sperm cryopreservation. Cryobiology. 2017;75:88–90. doi: 10.1016/j.cryobiol.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Robles V, Valcarce DG, Riesco MF. The use of antifreeze proteins in the cryopreservation of gametes and embryos. Biomolecules. 2019;9:181. doi: 10.3390/biom9050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Park CK. Effect of magnetized extender on sperm membrane integrity and development of oocytes in vitro fertilized with liquid storage boar semen. Anim Reprod Sc. 2015;154:86–94. doi: 10.1016/j.anireprosci.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Park CK. Antioxidative effects of magnetized extender containing bovine serum albumin on sperm oxidative stress during long-term liquid preservation of boar semen. Biochem Biophys Res Commun. 2015;464:467–72. doi: 10.1016/j.bbrc.2015.06.159. [DOI] [PubMed] [Google Scholar]
- 19.Yeste M, Castillo-Martin M, Bonet S, Rodriguez-Gil JE. Impact of light irradiation on preservation and function of mammalian spermatozoa. Anim Reprod Sci. 2018;194:19–32. doi: 10.1016/j.anireprosci.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Pribenszky C, Vajta G. Cells under pressure:how sublethal hydrostatic pressure stress treatment increases gametes’and embryos’performance. Reprod Fertil Dev. 2011;23:48–55. doi: 10.1071/RD10231. [DOI] [PubMed] [Google Scholar]
- 21.Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research:importance, impact, status, and future directions. Biol Reprod. 2008;78:2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- 22.Morris GJ. The cryopreservation of Chlorella. 1. Interactions of rate of cooling, protective additive and warming rate. Arch Microbiol. 1976;107:57–62. doi: 10.1007/BF00427867. [DOI] [PubMed] [Google Scholar]
- 23.Tao Y, Sanger E, Saewu A, Leveille MC. Human sperm vitrification:the state of the art. Reprod Biol Endocrinol. 2020;18:17. doi: 10.1186/s12958-020-00580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vutyavanich T, Piromlertamorn W, Nunta S. Rapid freezing versus slow programmable freezing of human spermatozoa. Fertil Steril. 2010;93:1921–8. doi: 10.1016/j.fertnstert.2008.04.076. [DOI] [PubMed] [Google Scholar]
- 25.Riva NS, Ruhlmann C, Iaizzo RS, Marcial Lopez CA, Martinez AG. Comparative analysis between slow freezing and ultra-rapid freezing for human sperm cryopreservation. JBRA Assist Reprod. 2018;22:331–7. doi: 10.5935/1518-0557.20180060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawroth F, Isachenko V, Dessole S, Rahimi G, Farina M, et al. Vitrification of human spermatozoa without cryoprotectants. Cryo Letters. 2002;23:93–102. [PubMed] [Google Scholar]
- 27.Isachenko V, Isachenko E, Petrunkina AM, Sanchez R. Human spermatozoa vitrified in the absence of permeable cryoprotectants:birth of two healthy babies. Reprod Fertil Dev. 2012;24:323–6. doi: 10.1071/RD11061. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez R, Isachenko V, Petrunkina AM, Risopatron J, Schulz M, et al. Live birth after intrauterine insemination with spermatozoa from an oligoasthenozoospermic patient vitrified without permeable cryoprotectants. J Androl. 2012;33:559–62. doi: 10.2164/jandrol.111.014274. [DOI] [PubMed] [Google Scholar]
- 29.Li YX, Zhou L, Lv MQ, Ge P, Liu YC, et al. Vitrification and conventional freezing methods in sperm cryopreservation:a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2019;233:84–92. doi: 10.1016/j.ejogrb.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Ali Mohamed MS. Slow cryopreservation is not superior to vitrification in human spermatozoa;an experimental controlled study. Iran J Reprod Med. 2015;13:633–44. [PMC free article] [PubMed] [Google Scholar]
- 31.Koshimoto C, Mazur P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology. 2002;45:49–59. doi: 10.1016/s0011-2240(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 32.Jin B, Kleinhans FW, Mazur P. Survivals of mouse oocytes approach 100% after vitrification in 3-fold diluted media and ultra-rapid warming by an IR laser pulse. Cryobiology. 2014;68:419–30. doi: 10.1016/j.cryobiol.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin B, Mazur P. High survival of mouse oocytes/embryos after vitrification without permeating cryoprotectants followed by ultra-rapid warming with an IR laser pulse. Sci Rep. 2015;5:9271. doi: 10.1038/srep09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C, Gan RX, Hu JL, Liu F, Hong Y, et al. Clinical benefit for cryopreservation of single human spermatozoa for ICSI:a systematic review and meta-analysis. Andrology. 2022;10:82–91. doi: 10.1111/andr.13091. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J, Garrisi GJ, Congedo-Ferrara TA, Kieck KA, Schimmel TW, et al. Cryopreservation of single human spermatozoa. Hum Reprod. 1997;12:994–1001. doi: 10.1093/humrep/12.5.994. [DOI] [PubMed] [Google Scholar]
- 36.Walmsley R, Cohen J, Ferrara-Congedo T, Reing A, Garrisi J. The first births and ongoing pregnancies associated with sperm cryopreservation within evacuated egg zonae. Hum Reprod. 1998;13(Suppl 4):61–70. doi: 10.1093/humrep/13.suppl_4.61. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh Y, Tsai H, Chang C, Lo H. Cryopreservation of human spermatozoa within human or mouse empty zona pellucidae. Fertil Steril. 2000;73:694–8. doi: 10.1016/s0015-0282(99)00612-3. [DOI] [PubMed] [Google Scholar]
- 38.Just A, Gruber I, Wober M, Lahodny J, Obruca A, et al. Novel method for the cryopreservation of testicular sperm and ejaculated spermatozoa from patients with severe oligospermia:a pilot study. Fertil Steril. 2004;82:445–7. doi: 10.1016/j.fertnstert.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 39.Herrler A, Eisner S, Bach V, Weissenborn U, Beier HM. Cryopreservation of spermatozoa in alginic acid capsules. Fertil Steril. 2006;85:208–13. doi: 10.1016/j.fertnstert.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 40.Isaev D, Zaletov S, Zaeva V, Zakharova E, Shafei R, et al. Artificial microcontainers for cryopreservation of solitary spermatozoa. Hum Reprod. 2007;22:i154–5. [Google Scholar]
- 41.Araki Y, Yao T, Asayama Y, Matsuhisa A, Araki Y. Single human sperm cryopreservation method using hollow-core agarose capsules. Fertil Steril. 2015;104:1004–9. doi: 10.1016/j.fertnstert.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 42.Hatakeyama S, Tokuoka S, Abe H, Araki Y, Araki Y. Cryopreservation of very low numbers of spermatozoa from male patients undergoing infertility treatment using agarose capsules. Hum Cell. 2017;30:201–8. doi: 10.1007/s13577-017-0166-x. [DOI] [PubMed] [Google Scholar]
- 43.Tomita K, Sakai S, Khanmohammadi M, Yamochi T, Hashimoto S, et al. Cryopreservation of a small number of human sperm using enzymatically fabricated, hollow hyaluronan microcapsules handled by conventional ICSI procedures. J Assist Reprod Genet. 2016;33:501–11. doi: 10.1007/s10815-016-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai N, Goldberg J, Austin C, Sabanegh E, Falcone T. Cryopreservation of individually selected sperm:methodology and case report of a clinical pregnancy. J Assist Reprod Genet. 2012;29:375–9. doi: 10.1007/s10815-012-9733-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuznyetsov V, Moskovtsev SI, Crowe M, Lulat AG, Librach CL. Vitrification of a small number of spermatozoa in normozoospermic and severely oligozoospermic samples. Syst Biol Reprod Med. 2015;61:13–7. doi: 10.3109/19396368.2014.987855. [DOI] [PubMed] [Google Scholar]
- 46.Gil-Salom M, Romero J, Rubio C, Ruiz A, Remohí J, et al. Intracytoplasmic sperm injection with cryopreserved testicular spermatozoa. Mol Cell Endocrinol. 2000;169:15–9. doi: 10.1016/s0303-7207(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 47.Bouamama N, Briot P, Testart J. Comparison of two methods of cryoconservation of sperm when in very small numbers. Gynecol Obstet Fertil. 2003;31:132–5. doi: 10.1016/s1297-9589(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 48.Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, et al. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod Biomed Online. 2005;10:350–4. doi: 10.1016/s1472-6483(10)61795-6. [DOI] [PubMed] [Google Scholar]
- 49.Gvakharia M, Adamson G. A method of successful cryopreservation of small numbers of human spermatozoa. Fertil Steril. 2001;76:S101. [Google Scholar]
- 50.Sohn J, Jun S, Park L, Kim E, Chung T, et al. Comparison of recovery and viability of sperm in ICSI pipette after ultra rapid freezing or slow freezing. Fertil Steril. 2003;80:128. [Google Scholar]
- 51.Nawroth F, Isachenko V, Dessole S, Rahimi G, Farina M, et al. Vitrification of human spermatozoa without cryoprotectants. Cryo Letters. 2002;23:93–102. [PubMed] [Google Scholar]
- 52.Desai N, Culler C, Goldfarb J. Cryopreservation of single sperm from epididymal and testicular samples on cryoloops:preliminary case report. Fertil Steril. 2004;82:S264–5. [Google Scholar]
- 53.Desai NN, Blackmon H, Goldfarb J. Single sperm cryopreservation on cryoloops:an alternative to hamster zona for freezing individual spermatozoa. Reprod Biomed Online. 2004;9:47–53. doi: 10.1016/s1472-6483(10)62109-8. [DOI] [PubMed] [Google Scholar]
- 54.Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, et al. Single spermatozoon freezing using Cryotop. J Mamm Ova Res. 2011;28:47–52. [Google Scholar]
- 55.Endo Y, Fujii Y, Kurotsuchi S, Motoyama H, Funahashi H. Successful delivery derived from vitrified-warmed spermatozoa from a patient with nonobstructive azoospermia. Fertil Steril. 2012;98:1423–7. doi: 10.1016/j.fertnstert.2012.07.1128. [DOI] [PubMed] [Google Scholar]
- 56.Endo Y, Fujii Y, Shintani K, Seo M, Motoyama H, et al. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online. 2012;24:301–7. doi: 10.1016/j.rbmo.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 57.Stein A, Shufaro Y, Hadar S, Fisch B, Pinkas H. Successful use of the Cryolock device for cryopreservation of scarce human ejaculate and testicular spermatozoa. Andrology. 2015;3:220–4. doi: 10.1111/andr.12007. [DOI] [PubMed] [Google Scholar]
- 58.Peng QP, Cao SF, Lyu QF, Xue SG, Jin W, et al. A novel method for cryopreservation of individual human spermatozoa. In Vitro Cell Dev Biol Anim. 2011;47:565–72. doi: 10.1007/s11626-011-9428-1. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Wu Z, Hu Y, Wang Y, Tan Y, et al. An adapted carrier for the cryopreservation of human testicular spermatozoa. Reprod Biomed Online. 2018;37:590–9. doi: 10.1016/j.rbmo.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Coetzee K, Ozgur K, Berkkanoglu M, Bulut H, Isikli A. Reliable single sperm cryopreservation in cell sleepers for azoospermia management. Andrologia. 2016;48:203–10. doi: 10.1111/and.12434. [DOI] [PubMed] [Google Scholar]
- 61.Sun J, Chen W, Zhou L, Hu J, Li Z, et al. Successful delivery derived from cryopreserved rare human spermatozoa with novel cryopiece. Andrology. 2017;5:832–7. doi: 10.1111/andr.12380. [DOI] [PubMed] [Google Scholar]
- 62.Berkovitz A, Miller N, Silberman M, Belenky M, Itsykson P. A novel solution for freezing small numbers of spermatozoa using a sperm vitrification device. Hum Reprod. 2018;33:1975–83. doi: 10.1093/humrep/dey304. [DOI] [PubMed] [Google Scholar]
- 63.Patrick J, Comizzoli P, Elliott G. Dry preservation of spermatozoa:considerations for different species. Biopreserv Biobank. 2017;15:158–68. doi: 10.1089/bio.2016.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anzalone DA, Palazzese L, Iuso D, Martino G, Loi P. Freeze-dried spermatozoa:an alternative biobanking option for endangered species. Anim Reprod Sci. 2018;190:85–93. doi: 10.1016/j.anireprosci.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Saragusty J, Loi P. Exploring dry storage as an alternative biobanking strategy inspired by nature. Theriogenology. 2019;126:17–27. doi: 10.1016/j.theriogenology.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 66.Keskintepe L, Eroglu A. Preservation of mammalian sperm by freeze-drying. Methods Mol Biol. 2021;2180:721–30. doi: 10.1007/978-1-0716-0783-1_39. [DOI] [PubMed] [Google Scholar]
- 67.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol. 1998;16:639–41. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- 68.Klooster KL, Burruel VR, Meyers SA. Loss of fertilization potential of desiccated rhesus macaque spermatozoa following prolonged storage. Cryobiology. 2011;62:161–6. doi: 10.1016/j.cryobiol.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Kaneko T, Serikawa T. Long-term preservation of freeze-dried mouse spermatozoa. Cryobiology. 2012;64:211–4. doi: 10.1016/j.cryobiol.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Kusakabe H, Yanagimachi R, Kamiguchi Y. Mouse and human spermatozoa can be freeze-dried without damaging their chromosomes. Hum Reprod. 2008;23:233–9. doi: 10.1093/humrep/dem252. [DOI] [PubMed] [Google Scholar]
- 71.Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, et al. DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil Steril. 2012;97:1067–73.e1. doi: 10.1016/j.fertnstert.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Bossi RL, Cabral M, Oliveira M, Lopes S, Hurtado R, et al. Ultrastructural analysis of lyophilized human spermatozoa. JBRA Assist Reprod. 2021;25:473–9. doi: 10.5935/1518-0557.20210028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isachenko V, Rahimi G, Mallmann P, Sanchez R, Isachenko E. Technologies of cryoprotectant-free vitrification of human spermatozoa:asepticity as criterion of effectiveness. Andrology. 2017;5:1055–63. doi: 10.1111/andr.12414. [DOI] [PubMed] [Google Scholar]
- 74.Arav A, Natan Y, Levi-Setti PE, Menduni F, Patrizio P. New methods for cooling and storing oocytes and embryos in a clean environment of -196°C. Reprod Biomed Online. 2016;33:71–8. doi: 10.1016/j.rbmo.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Wang M, Isachenko E, Todorov P, Rahimi G, Mallmann P, et al. Aseptic technology for cryoprotectant-free vitrification of human spermatozoa by direct dropping into clean liquid air:apoptosis, necrosis, motility, and viability. Biomed Res Int. 2020;2020:2934315. doi: 10.1155/2020/2934315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaz-Jimenez M, Wang M, Wang W, Isachenko E, Rahimi G, et al. Cryo-banking of human spermatozoa by aseptic cryoprotectants-free vitrification in liquid air:positive effect of elevated warming temperature. Cell Tissue Bank. 2022;23:17–29. doi: 10.1007/s10561-021-09904-0. [DOI] [PubMed] [Google Scholar]
- 77.Hezavehei M, Sharafi M, Kouchesfahani HM, Henkel R, Agarwal A, et al. Sperm cryopreservation:a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37:327–39. doi: 10.1016/j.rbmo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 78.Nijs M, Creemers E, Cox A, Janssen M, Vanheusden E, et al. Influence of freeze-thawing on hyaluronic acid binding of human spermatozoa. Reprod Biomed Online. 2009;19:202–6. doi: 10.1016/s1472-6483(10)60073-9. [DOI] [PubMed] [Google Scholar]
- 79.Raad G, Lteif L, Lahoud R, Azoury J, Azoury J, et al. Cryopreservation media differentially affect sperm motility, morphology and DNA integrity. Andrology. 2018;6:836–45. doi: 10.1111/andr.12531. [DOI] [PubMed] [Google Scholar]
- 80.Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos:principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21:209–27. doi: 10.1093/humupd/dmu063. [DOI] [PubMed] [Google Scholar]
- 81.Rarani FZ, Golshan-Iranpour F, Dashti GR. Correlation between sperm motility and sperm chromatin/DNA damage before and after cryopreservation and the effect of folic acid and nicotinic acid on post-thaw sperm quality in normozoospermic men. Cell Tissue Bank. 2019;20:367–78. doi: 10.1007/s10561-019-09775-6. [DOI] [PubMed] [Google Scholar]
- 82.Ezzati M, Shanehbandi D, Hamdi K, Rahbar S, Pashaiasl M. Influence of cryopreservation on structure and function of mammalian spermatozoa:an overview. Cell Tissue Bank. 2020;21:1–15. doi: 10.1007/s10561-019-09797-0. [DOI] [PubMed] [Google Scholar]
- 83.Mohammad SN, Barratt CL, Cooke ID, Moore HD. Direct assessment of cryopreservation of human spermatozoa using a cryomicroscope and computer-aided sperm analysis. Hum Reprod. 1996;11:2687–92. doi: 10.1093/oxfordjournals.humrep.a019192. [DOI] [PubMed] [Google Scholar]
- 84.Wang S, Wang W, Xu Y, Tang M, Fang J, et al. Proteomic characteristics of human sperm cryopreservation. Proteomics. 2014;14:298–310. doi: 10.1002/pmic.201300225. [DOI] [PubMed] [Google Scholar]
- 85.Bogle OA, Kumar K, Attardo-Parrinello C, Lewis SE, Estanyol JM, et al. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology. 2017;5:10–22. doi: 10.1111/andr.12279. [DOI] [PubMed] [Google Scholar]
- 86.Valcarce DG, Carton-Garcia F, Riesco MF, Herraez MP, Robles V. Analysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo development. Andrology. 2013;1:723–30. doi: 10.1111/j.2047-2927.2013.00116.x. [DOI] [PubMed] [Google Scholar]
- 87.Klaver R, Bleiziffer A, Redmann K, Mallidis C, Kliesch S, et al. Routine cryopreservation of spermatozoa is safe–evidence from the DNA methylation pattern of nine spermatozoa genes. J Assist Reprod Genet. 2012;29:943–50. doi: 10.1007/s10815-012-9813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valcarce DG, Carton-Garcia F, Herraez MP, Robles V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology. 2013;67:84–90. doi: 10.1016/j.cryobiol.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Zafer M, Horvath H, Mmeje O, van der Poel S, Semprini AE, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples:a systematic review and meta-analysis. Fertil Steril. 2016;105:645–55.e2. doi: 10.1016/j.fertnstert.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salam AP, Horby PW. The breadth of viruses in human semen. Emerg Infect Dis. 2017;23:1922–4. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality:a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Wiener-Megnazi Z, Auslender R, Dirnfeld M. Advanced paternal age and reproductive outcome. Asian J Androl. 2012;14:69–76. doi: 10.1038/aja.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin RH, Rademaker AW. The effect of age on the frequency of sperm chromosomal abnormalities in normal men. Am J Hum Genet. 1987;41:484–92. [PMC free article] [PubMed] [Google Scholar]
- 94.Pasqualotto FF, Sobreiro BP, Hallak J, Pasqualotto EB, Lucon AM. Sperm concentration and normal sperm morphology decrease and follicle-stimulating hormone level increases with age. BJU Int. 2005;96:1087–91. doi: 10.1111/j.1464-410X.2005.05806.x. [DOI] [PubMed] [Google Scholar]
- 95.Alio AP, Salihu HM, McIntosh C, August EM, Weldeselasse H, et al. The effect of paternal age on fetal birth outcomes. Am J Mens Health. 2012;6:427–35. doi: 10.1177/1557988312440718. [DOI] [PubMed] [Google Scholar]
- 96.Blachman-Braun R, Best JC, Wyant WA, Ramos L, Ibrahim E, et al. Factors influencing postmortem disposition of cryopreserved sperm in men undergoing fertility preservation. F S Rep. 2020;1:21–4. doi: 10.1016/j.xfre.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feldman R. The neurobiology of human attachments. Trends Cogn Sci. 2017;21:80–99. doi: 10.1016/j.tics.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender and nonbinary persons:an Ethics Committee opinion. Fertil Steril. 2021;115:874–8. doi: 10.1016/j.fertnstert.2021.01.049. [DOI] [PubMed] [Google Scholar]