ABSTRACT
Silent information regulator 2-related enzyme 1 (SIRT1) is an aging-related protein activated with aging. Herein, we evaluated the role of SIRT1 in aging-related erectile dysfunction. The expression of SIRT1 was modulated in aged Sprague-Dawley rats following intragastric administration of resveratrol (Res; 5 mg kg−1), niacinamide (NAM; 500 mg kg−1) or Res (5 mg kg−1) + tadalafil (Tad; phosphodiesterase-5 [PDE5] inhibitor; 5 mg kg−1) for 8 weeks. Then, we determined erectile function by the ratio of intracavernosal pressure (ICP)/mean systemic arterial pressure (MAP). Cavernosal tissues were extracted to evaluate histological changes, cell apoptosis, nitric oxide (NO)/cyclic guanosine monophosphate (cGMP), the superoxide dismutase (SOD)/3,4-methylenedioxyamphetamine (MDA) level, and the expression of SIRT1, p53, and forkhead box O3 (FOXO3a) using immunohistochemistry, terminal deoxynucleotidyl transferase (TdT)-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick-end labeling (TUNEL), enzyme-linked immunosorbent assays, and western blot analysis. Compared with the control, Res treatment significantly improved erectile function, reflected by an increased content of smooth muscle and endothelium, NO/cGMP and SOD activity, and reduced cell apoptosis and MDA levels. The effect of Res was improved by adding Tad. In addition, the protein expression of SIRT1 was increased in the Res group, accompanied by decreased p53 and FOXO3a levels. In addition, inhibition of SIRT1 by NAM treatment resulted in adverse results compared with Res treatment. SIRT1 activation ameliorated aging-related erectile dysfunction, supporting the potential of SIRT1 as a target for erectile dysfunction treatment.
Keywords: apoptosis, erectile function, nitric oxide/cyclic guanosine monophosphate signaling, oxidative stress, SIRT1 expression
INTRODUCTION
Erectile dysfunction refers to a penile dysfunction characterized by the inability to maintain penile erection induced by sexual arousal. Aging is one of the primary factors responsible for erectile dysfunction. Erectile dysfunction is prevalent in the elderly, especially in men aged more than 60 years.1 The treatment of erectile dysfunction is dependent on etiology. Oral medication, such as phosphodiesterase-5 (PDE5) inhibitors (tadalafil [Tad]), is currently the first-line treatment for erectile dysfunction. However, most patients are unable to receive this treatment because it seriously affects their quality of life and results in various other health-related complications.
Further elucidation of the pathogenesis of erectile dysfunction in the elderly may facilitate therapeutic advancements. A previous study reported that apoptosis, oxidative stress, and endothelial function are dysregulated in erectile dysfunction. Silent information regulator 2-related enzyme 1 (SIRT1) is an aging-related gene and plays a key role in nitric oxide (NO) production and endothelial function.2 Previous evidence has shown that the overexpression of miRNA-200a plays a key role in aging-induced erectile dysfunction by inhibiting SIRT1.3 However, the effect of SIRT1 expression on regulating age-related erectile function has not been fully elucidated.
Resveratrol (Res) is a natural phytoalexin that exerts multiple biological functions, including anticancer, antidiabetic, and cardioprotective effects. Res, as the SIRT1 activator, has relieved erectile dysfunction in diabetic rat models.4 Nicotinamide (NAM) is a component of the vitamin B family and exhibits an anti-inflammatory effect in dermatological disorders.5 As a SIRT1 inhibitor, NAM is involved in cell viability, apoptosis, and aging.6,7
Herein, we modulated the expression of SIRT1 by Res and NAM in aged rats, investigated its effect on erectile function and histological changes, and compared these treatments with the controls. In addition, rats were treated with Res combined with Tad, and erectile function was examined. In the present study, we aimed to explore the role of SIRT1 expression and the effect of Res combined with Tad in the erectile function of aged rats.
MATERIALS AND METHODS
Animals
Twenty-month-old specific pathogen-free (SPF) Sprague-Dawley (SD) rats weighing 700–750 g were purchased from the Animal Center of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School (Nanjing, China). All rats were maintained in a humidified environment with 45%–65% humidity at 20°C–25°C within a 12-h light:12-h night cycle. The rats have free access to food and water. The animal study procedures complied with the guidelines for the protection and application of experimental animals and were approved by the ethical batch number for animal experiments of Nanjing Drum Tower Hospital (approval No. 2021AE02011).
Groups
A total of 68 male SD rats were divided into 4 groups (n=17 per group): control group, Res group, NAM group, and Res+Tad group. Rats in the Res group were administered Res intragastrically at a dose of 5 mg kg−1 daily (#34092, Sigma, Saint Louis, MO, USA). The dose of Res was adapted from our previous study and proved effective.8 Rats in the NAM group received daily intragastric administration of 500 mg kg−1 NAM (#72340, Sigma). The dose of NAM was adopted from previously published studies, which reported 300–1000 mg kg−1 to be effective.9,10 We chose daily administration of 500 mg kg−1 NAM in our research. Animals in the Res+Tad group received daily intragastric Res (5 mg kg−1) and Tad (5 mg kg−1; #11302020, Lilly, Indianapolis, IN, USA).11 The control rats were administered intragastric normal saline at the same dose. After being treated for 8 consecutive weeks, the rats were evaluated for erectile function. Subsequently, the rats were sacrificed, and penile tissues were isolated. The middle part of the penis (4 mm from the glans) was separated and embedded in an optimal cutting temperature compound (OCT; 4583, SAKURA, Torrance, CA, USA) for frozen section preparation. The remaining tissues were immediately stored at −80°C for further experimentation.
Erectile function evaluation
The erectile function of the rats was evaluated by the ratio of intracavernous pressure (ICP)/mean systemic arterial pressure (MAP) induced by cavernous nerve stimulation as previously described.12 Briefly, the rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (100 mg kg−1; SML1873, Sigma) and diazepam (50 mg kg−1; D0899 Diazepam, Sigma). The pelvic stellate ganglion and penile tissue were exposed, followed by puncture in the internal carotid artery and corpus cavernosum. The nerve was stimulated by a continuous square wave with a pulse width of 5 ms at a frequency of 20 Hz and voltage of 5 V for 60 consecutive times. Stimulation of the cavernous nerve on either side was performed 3 times. ICP and MAP were recorded, and the ICP/MAP ratio was calculated.
Terminal deoxynucleotidyl transferase (TdT)-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick-end labeling (TUNEL) assay
The sections of penile tissues (5-μm thickness) were fixed in 4% paraformaldehyde/0.01 mmol l−1 phosphate-buffered saline (PBS; p5368-10PAK, Sigma) at room temperature for 30 min to 60 min and blocked with 3% H2O2. After washing, the sections were incubated with 20 μl of labeling buffer containing terminal deoxynucleotidyl transferase (TdT; 1 μl) and DIG-d-UTP (1 μl) for 2 h at 37°C. Then, the samples were stained with diaminobenzidine (DAB; SNM475, Biolab, Beijing, China), followed by counterstaining with hematoxylin. The nuclei of apoptotic cells were stained brown, and positive staining was observed under a microscope.
Immunohistochemical staining
Tissue sections (5 μm) were washed and fixed in 4% paraformaldehyde at room temperature for 10 min. Sections were blocked with 3% goat serum, followed by incubation with primary antibodies against von Willebrand factor (vWF; 1:400; ab6994, Abcam, New York, NY, USA) and α-SMA (1:200; #19245, Cell Signaling Technology, Boston, MA, USA) and biotin-labeled secondary antibody under routine conditions. Subsequently, the slides were counterstained with hematoxylin. A BX51 microscope (Olympus, Tokyo, Japan) equipped with a camera (Nikon, Tokyo, Japan) was used to capture images. Image-Pro software (Media Cybernetics, New York, NY, USA) was used to analyze the proportion of smooth muscle area in the overall area of the picture.
Western blot
Protein extract from 50–100 mg cavernous tissue of the penis was obtained using radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Nantong, China) with protease inhibitor. The protein supernatant was isolated by centrifugation (14 400g; HITACHI, Hitachi City, Japan) at 4°C for 15 min, and the protein concentration was determined by bicinchoninic acid (BCA) assays (Beyotime). According to the quantitative results of the BCA assay, the total protein content was adjusted to 60 μg for each sample. Protein bands were separated by the sodium salt dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE) system and transferred to polyvinylidene fluoride (PVDF) membranes. For protein labeling, the primary antibodies against SIRT1 (ab110304, Abcam), p53 (ab26, Abcam), and forkhead box O3 (FOXO3a; ab154786, Abcam) were diluted at 1:1000, while anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab82445, Abcam) was diluted at 1:3000. Antibodies against rabbit IgG (H+L) and mouse IgG (H+L) (MedImmune, Gaithersburg, MD, USA) were used as secondary antibodies. The protein bands were observed using a chemiluminescence system and were analyzed by Quantity One software (Bio-Rad, Hercules, CA, USA).
Measurement of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) content
The NO/cGMP pathway is the main pathway regulating penile erection. NO activates guanylate cyclase, which converts guanosine-5’-triphosphate (GTP) into cGMP. cGMP reduces intracellular calcium concentration, relaxes cavernous smooth muscle, increases penile blood flow, and induces erection. The levels of NO and cGMP reflect erectile function; thus, the contents of NO and cGMP in penile tissues were evaluated. Cavernous tissue of the penis (50 mg) was homogenized and centrifuged at 1000g for 15 min. The supernatant was collected for NO and cGMP analysis using an enzyme-linked immunosorbent assay (ELISA) kit (Servicebio, Wuhan, China) following the manufacturer’s instructions.
Measurement of superoxide dismutase (SOD) activity and 3,4-methylenedioxyamphetamine (MDA) content
A decrease in SOD activity and an increase in MDA content are crucial for aging and senile diseases. SOD activity and MDA content were used to evaluate oxidative stress.13 The cavernous tissues (80 mg) were homogenized in PBS. The supernatant was collected by centrifugation at 1000g and 4°C for 5 min. The activity of the oxidative stress markers SOD and MDA was determined by a commercial assay kit (Nanjing Jiancheng Company, Nanjing, China). A microplate reader (Molecular Devices, VERSAmax; Thermo Scientific, New York, NY, USA) was used for quantitative measurement.
Statistical analyses
The results are presented as the mean ± standard error of mean (s.e.m.) and were analyzed by SPSS 16.0 software (IBM, New York, NY, USA). Multigroup comparisons were performed by one-way analysis of variance (ANOVA), and two-group comparisons were carried out by Tukey’s test. P < 0.05 was considered statistically significant.
RESULTS
The effect of SIRT1 expression on erectile function in aged rats
After the rats were treated with Res (SIRT1 activator), NAM (SIRT1 inhibitor), and Tad (PDE5 inhibitor), the ICP/MAP ratio was determined to evaluate erectile function. As shown in Figure 1, the erectile function of the rats was significantly enhanced in the Res group but reduced in the NAM group (P < 0.05). Tad treatment significantly improved the effect of Res on erectile function (P < 0.05). Thus, SIRT1 activation improved the erectile function of aged rats.
Figure 1.
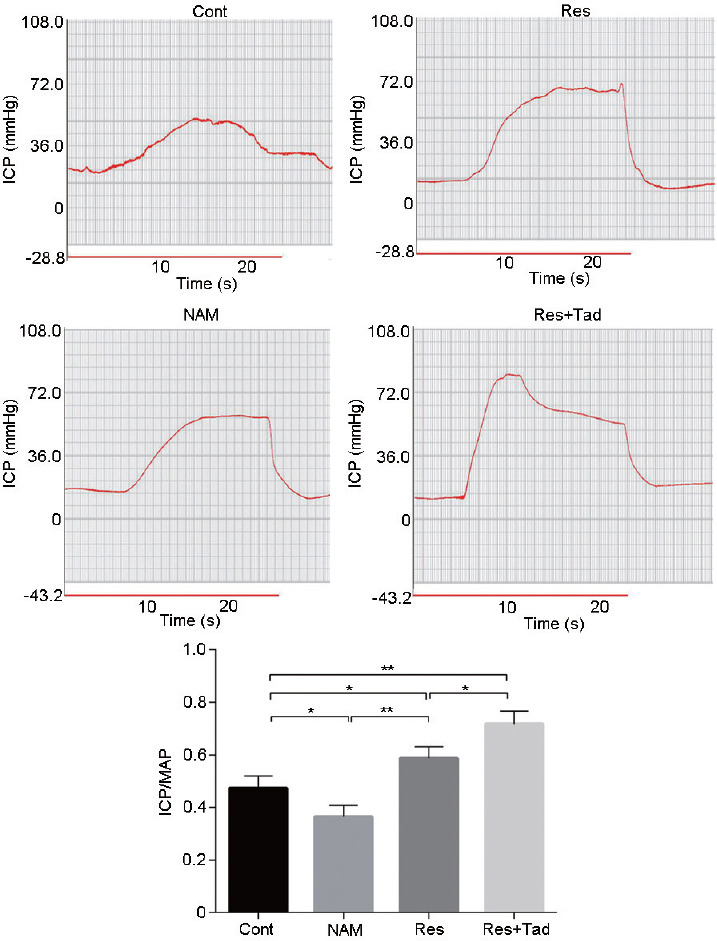
Erectile function evaluation after SIRT1 expression modulation by Res, NAM, and Res+Tad. Erectile function was evaluated by the ICP/MAP ratio. Res treatment significantly elevated erectile function in aged rats, while NAM treatment decreased erectile function. *P < 0.05, **P < 0.01. Cont: control group, the control rats were administered intragastric normal saline; NAM: niacinamide group, rats in the NAM group were administered niacinamide intragastrically; Res: resveratrol group, rats in the Res group were administered resveratrol intragastrically; Res+Tad: resveratrol and tadalafil group, rats in the Res+Tad group received daily intragastric resveratrol and tadalafil. ICP/MAP: intracavernosal pressure/mean systemic arterial pressure; SIRT1: silent information regulator 2-related enzyme 1.
The effect of SIRT1 on histological changes of rat corpus cavernosum
To determine the histological changes in the corpus cavernosum mediated by the overexpression or inhibition of SIRT1, we quantitatively analyzed smooth muscle and endothelial contents by immunohistochemical staining of α-SMA and vWF. Compared with those of the rats treated with normal saline, the smooth muscle and endothelium were significantly elevated in the cavernosum of rats after 8 weeks of treatment with Res (P < 0.05). Notably, this increase was reinforced by combined treatment with Tad. Conversely, the smooth muscle and endothelial content decreased significantly in the NAM treatment group (both P < 0.05; Figure 2a and 2b). Moreover, Res administration relieved cell apoptosis in the cavernosum, and the effect was enhanced by combining Res with Tad (P < 0.05). Compared with those of the control group, apoptotic cells were increased in the NAM group (P < 0.05; Figure 2c). These results indicated that the activation of SIRT1 ameliorated erectile dysfunction and induced histological changes in the corpus cavernosum.
Figure 2.
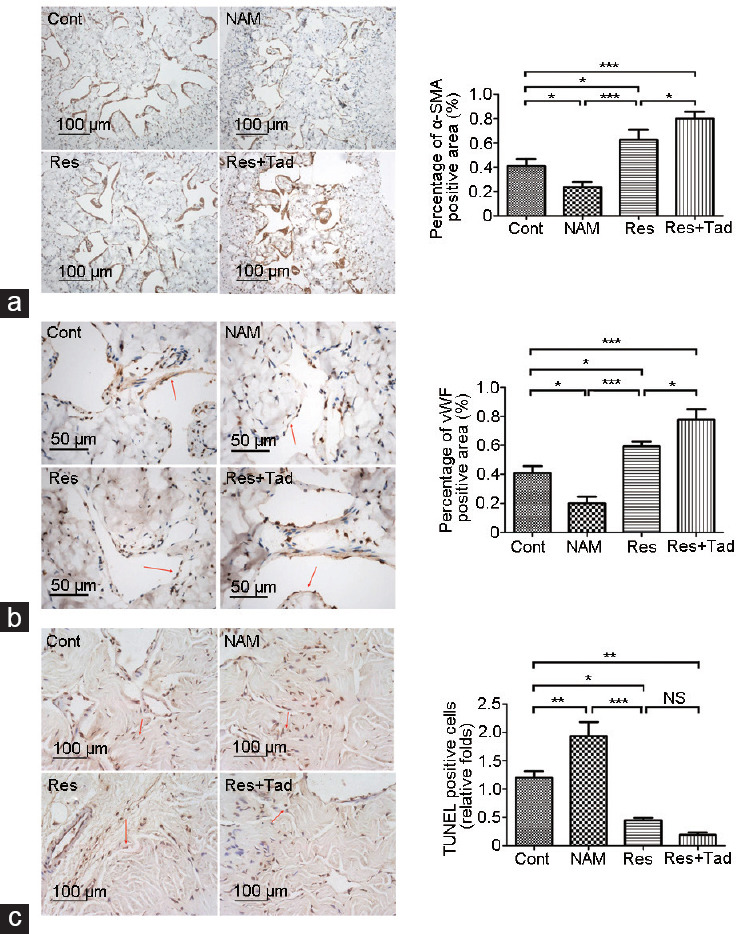
Histological changes in the rat corpus cavernosum mediated by SIRT1. Immunohistochemical staining was performed to evaluate (a) penile vascular smooth muscle (α-SMA positive) and (b) endothelium (vWF positive), and (c) TUNEL staining was used to evaluate apoptotic cells. The content of penile vascular smooth muscle and endothelium was significantly increased, while the apoptotic cells were reduced in the Res group. The opposite results were found with NAM treatment. The red arrow indicates positive staining. *P < 0.05, **P < 0.01, and ***P < 0.001. Cont: control group, the control rats were administered intragastric normal saline; NAM: niacinamide group, rats in the NAM group were administered niacinamide intragastrically; Res: resveratrol group, rats in the Res group were administered resveratrol intragastrically; Res+Tad: resveratrol and tadalafil group, rats in the Res+Tad group received daily intragastric resveratrol and tadalafil. NS: no significant difference; SIRT1: silent information regulator 2-related enzyme 1; TUNEL: TdT-mediated dUTP nick-end labeling; vWF: von Willebrand factor.
The expression of SIRT1, p53, and FOXO3a in penile tissues of aged rats
Western blot analysis was used to evaluate the expression levels of SIRT1, p53, and FOXO3a in cavernous tissues. The results revealed that SIRT1 protein was significantly accumulated in the rats treated with Res for 8 weeks compared with the control rats treated with normal saline (both P < 0.05). However, p53 and FOXO3a levels were significantly downregulated in the Res group compared with the control group (all P < 0.05). In contrast, the expression of SIRT1 was dramatically reduced in the rats treated with NAM, paralleled by the increased expression of p53 and FOXO3a (P < 0.05). The increase in SIRT1 expression induced by Res was intense after cotreatment with Tad, while the expression of p53 and FOXO3a in the Res group was significantly weakened by Tad treatment (all P < 0.05; Figure 3).
Figure 3.
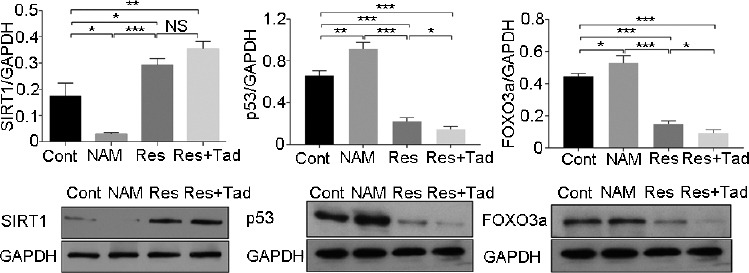
The expressions of SIRT1, p53, and FOXO3a in penile tissues. The expression of (a) SIRT1, (b) p53, and (c) FOXO3a in penile tissues was detected by western blot analysis. SIRT1 expression was significantly elevated in the Res treatment group, while FOXO3a and p53 expression declined. The expression trend was enhanced by Tad addition. In contrast, NAM treatment resulted in the opposite expression profile. *P < 0.05, **P < 0.01, and ***P < 0.001. Cont: control group, the control rats were administered intragastric normal saline; NAM: niacinamide group, rats in the NAM group were administered niacinamide intragastrically; Res: resveratrol group, rats in the Res group were administered resveratrol intragastrically; Res+Tad: resveratrol and tadalafil group, rats in the Res+Tad group received daily intragastric resveratrol and tadalafil. NS: no significant difference; SIRT1: silent information regulator 2-related enzyme 1; FOXO3a: forkhead box O3; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Effect of SIRT1 on NO and cGMP production
The NO and cGMP levels are closely associated with erectile function, which was determined in cavernosal tissues by ELISAs. As shown in Figure 4, the levels of NO and cGMP were significantly decreased in the NAM-treated rats but pronouncedly elevated in the rats treated with Res compared with the controls (all P < 0.05). The levels of NO and cGMP were further elevated in the Res+Tad group compared with the Res group (both P < 0.05). In summary, these results indicate that SIRT1 activation preserved erectile function in aged rats.
Figure 4.
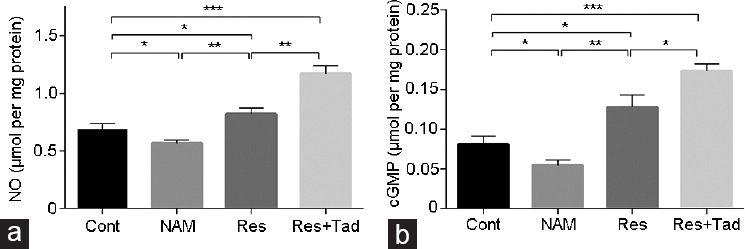
NO and cGMP productions were determined in cavernosal tissues by ELISAs. The (a) NO and (b) cGMP levels were detected by ELISAs. The levels of NO and cGMP were remarkably decreased in the NAM-treated rats but markedly elevated in the rats treated with Res compared with the control. *P < 0.05, **P < 0.01, and ***P < 0.001. Cont: control group, the control rats were administered intragastric normal saline; NAM: niacinamide group, rats in the NAM group were administered niacinamide intragastrically; Res: resveratrol group, rats in the Res group were administered resveratrol intragastrically; Res+Tad: resveratrol and tadalafil group, rats in the Res+Tad group received daily intragastric resveratrol and tadalafil. NO: nitrogen monoxide; ELISA: enzyme-linked immunosorbent assay; cGMP: cyclic guanosine monophosphate.
Effect of SIRT1 on oxidative stress in aged rats
To evaluate the effect of SIRT1 activation on oxidative stress, we determined the SOD activity and MDA concentration by a commercial assay kit. A marked decline in SOD activity and an increase in MDA levels were observed in the rats in the NAM group compared with the controls (both P < 0.05). Res treatment increased SOD activity and reduced MDA concentration compared to those of the control group rats (both P < 0.05). The effect of Res combined with Tad on elevating SOD activity and reducing MDA concentration was more promising than that of Res treatment alone (Figure 5). Thus, SIRT1 activation inhibited oxidative stress in aged rats.
Figure 5.
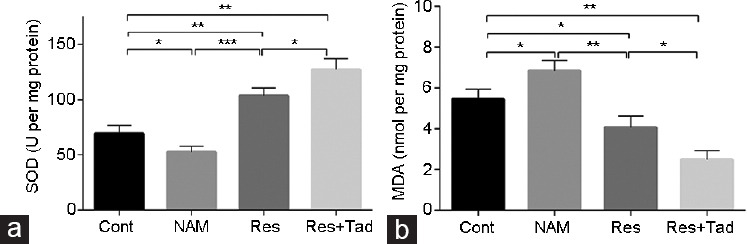
SOD activity and MDA concentration were determined by a commercial assay kit. (a) SOD activity and (b) MDA concentration were quantitatively assessed. Compared with those of the control, a marked decline in SOD activity and an increase in MDA levels were observed in the NAM group. Res treatment significantly increased SOD activity and reduced MDA concentration. *P < 0.05, **P < 0.01, and ***P < 0.001. Cont: control group, the control rats were administered intragastric normal saline; NAM: niacinamide group, rats in the NAM group were administered niacinamide intragastrically; Res: resveratrol group, rats in the Res group were administered resveratrol intragastrically; Res+Tad: resveratrol and tadalafil group, rats in the Res+Tad group received daily intragastric resveratrol and tadalafil. SOD: superoxide dismutase; MDA: 3,4-methylenedioxyamphetamine.
DISCUSSION
In this study, we used Res and NAM to explore the role of SIRT1 in erectile dysfunction in aged rats and further investigated the combined effect of Res and Tad on erectile dysfunction. Our results showed that the activation of SIRT1 prevented the progression of erectile dysfunction. Res and Tad showed a synergistic effect in inhibiting erectile dysfunction in aged rats.
Res is a type of natural polyphenol present in grapes and various berries.14 This molecule has various therapeutic effects, including anti-inflammatory, anticancer, and cardioprotective effects. A previous study reported that Res showed antiaging effects through phosphoinositide 3-kinase (PI3K)- and forkhead transcription factors of class O (FOXO)-related pathways.15 Emerging evidence has determined the positive effect of Res by mediating SIRT1 activation to extend lifespan and prevent aging-related diseases, such as diabetes and cardiovascular and cerebrovascular diseases.16 A previous study suggested that Res preserved erectile function in diabetic rat models.8 Erectile dysfunction is an aging-related disorder, and the effect of SIRT1 activation on aging-associated erectile dysfunction has not been fully elucidated.
Yu et al.8 suggested that Res restored erectile function in rats with diabetes, reflected by an increased ratio of ICP/MAP. Our data showed that Res administration significantly increased the ICP/MAP ratio in aged rats with accumulation of SIRT1, compared with those of the controls.
A previous study reported that oxidative stress induced by aging plays a pathological role in erectile dysfunction, facilitating NO production and smooth muscle cell apoptosis.17 NO is produced by endothelial and nervous tissues. High levels of NO can release cGMP, promoting smooth muscle relaxation. The loss of NO production is one of the major characteristics that confer aging-related erectile dysfunction. The deregulation of NO/cGMP signaling, oxidative stress, and nervous impairment contribute to the pathogenesis of erectile dysfunction.
The SIRT1 protein, as a deacetylase, modulates pathways responding to stress.18 The SIRT1 protein deacetylase activity mediates caloric intake, which is closely associated with lifespan and blood pressure. A previous study revealed that SIRT1 plays a critical role in endothelium-dependent vascular vasodilation by regulating endothelial NO.19 A previous study reported that radiotherapy-induced erectile dysfunction, which was rescued by Res treatment, is reflected by increased NO, cGMP, and SIRT1 levels.20 This study found that Res administration increased α-SMA-positive smooth muscle cells and vWF-positive endothelial cells in cavernosal tissues. Compared with those of the controls, the contents of NO and cGMP increased in the Res group. These results suggested that the increased expression of SIRT1 modulated by Res improved the endothelial function of the penis. Moreover, NAM, an inhibitor of benzamide acetylation substrates, competitively inhibits SIRT1.21 To further determine the relationship between SIRT1 and erectile dysfunction, we inhibited the expression of SIRT1 in rats by NAM treatment. Our results showed that SIRT1 inhibition markedly accelerated erectile dysfunction, verifying the key role of SIRT1 in aging-related erectile dysfunction.
In addition, Tab, a PDE5 inhibitor, is widely used for erectile dysfunction treatment. PDE5 inhibitors function by preventing cGMP breakdown, which improves the relaxation of smooth muscle.22 In this study, Res combined with Tad significantly elevated the NO and cGMP levels and improved erectile function, indicating that Res and Tad exhibit a synergistic therapeutic effect on erectile dysfunction.
In addition, SOD is a key antioxidant enzyme in the body, and its level reflects the ability to scavenge free radicals. MDA is produced by lipid peroxidation and is a marker of tissue damage. To measure oxidative stress in the corpus cavernosum, we determined the activity of SOD and MDA levels. Our data suggested that SOD activity was significantly increased, while the MDA level was significantly decreased by Res treatment, which is in good agreement with previous reports.8,20 In addition, the number of apoptotic cells in cavernosal tissues was decreased in the Res group, which was consistent with the reduced caspase-3 level induced by Res.8 Therefore, Res prevented oxidative stress-induced damage in cavernosal tissues.
Furthermore, apoptosis and oxidative stress-related proteins (p53 and FOXO3a) were evaluated after SIRT1 modulation. p53 and FOXO3a are substrates of SIRT1 and are deacetylated by SIRT1, which underlies the stress response.23 As previously described, the elevated SIRT1 activity induced by Res reduced p53 acetylation and inhibited cell apoptosis.24 p53 was inactivated following oxidative stress-induced DNA damage by deacetylation of SIRT1.25 In addition, FOXO showed a protective role against oxidative stress through SIRT1-mediated deacetylation.26 In the present study, SIRT1 activity mediated by Res treatment dramatically decreased oxidative stress-induced tissue damage and apoptosis, accompanied by increased p53 and FOXO3a expression. Thus, SIRT1 could relieve oxidative damage and apoptosis through p53/FOXO3a signaling.
In conclusion, we found that modulating SIRT1 expression by Res and NAM in aged rats indicated the important role of SIRT1 in aging-related erectile dysfunction. Res combined with a PDE5 inhibitor synergistically restored erectile function in aged rats. SIRT1 activity plays a protective role in erectile function in aged rats by reducing oxidative stress-induced damage through the p53 and FOXO3a pathways. SIRT1 may be a promising candidate target for aging-induced erectile dysfunction.
AUTHOR CONTRIBUTIONS
The study was conceived and designed by ZPX; experiments were performed by WY, JW, BW, YX, and QQG; data were analyzed and paper was written by WY and JW; the manuscript was revised by YTD and ZPX. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81170563).
REFERENCES
- 1.Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res. 2003;15:63–71. doi: 10.1038/sj.ijir.3900949. [DOI] [PubMed] [Google Scholar]
- 2.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 3.Pan F, Qiu XF, Yu W, Zhang QP, Chen Q, et al. MicroRNA-200a is up-regulated in aged rats with erectile dysfunction and could attenuate endothelial function via SIRT1 inhibition. Asian J Androl. 2016;18:74–9. doi: 10.4103/1008-682X.154991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuhara S, Tsujimura A, Okuda H, Yamamoto K, Takao T, et al. Vardenafil and resveratrol synergistically enhance the nitric oxide/cyclic guanosine monophosphate pathway in corpus cavernosal smooth muscle cells and its therapeutic potential for erectile dysfunction in the streptozotocin-induced diabetic rat:preliminary findings. J Sex Med. 2011;8:1061–71. doi: 10.1111/j.1743-6109.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- 5.Niren NM. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions:a review. Cutis. 2006;77:11–6. [PubMed] [Google Scholar]
- 6.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol Lett. 2013;6:600–4. doi: 10.3892/ol.2013.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast Sir2 and human SIRT1. J Biol Chem. 2002;277:45099–107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 8.Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15:646–51. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunimoto R, Jimbow K, Tanimura A, Sato M, Horimoto K, et al. SIRT1 regulates lamellipodium extension and migration of melanoma cells. J Invest Dermatol. 2014;134:1693–700. doi: 10.1038/jid.2014.50. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Wan T, Ye M, Qiu Y, Pei L, et al. Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1α/mitochondrial biosynthesis pathway. Redox Biol. 2018;17:89–98. doi: 10.1016/j.redox.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Li XX, Lin HC, Qiu XF, Gao J, et al. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J Androl. 2012;14:616–20. doi: 10.1038/aja.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Lin H, Wang Y, Yu W, Chen Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Wu ZZ, Cheng YL, Lin W, Qu C. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression. Eur Rev Med Pharmacol Sci. 2019;23:378–88. doi: 10.26355/eurrev_201901_16786. [DOI] [PubMed] [Google Scholar]
- 14.Jasiński M, Jasińska L, Ogrodowczyk M. Resveratrol in prostate diseases–a short review. Cent European J Urol. 2013;66:144–9. doi: 10.5173/ceju.2013.02.art8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1:effects on aging and age-related diseases. Nutr Rev. 2008;66:591–6. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrini MG, Gonzalez-Cadavid NF, Rajfer J. Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl Androl Urol. 2017;6:20–7. doi: 10.21037/tau.2016.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamakuchi M. MicroRNA regulation of SIRT1. Front Physiol. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sener TE, Tavukcu HH, Atasoy BM, Cevik O, Kaya OT, et al. Resveratrol treatment may preserve the erectile function after radiotherapy by restoring antioxidant defence mechanisms, SIRT1 and NOS protein expressions. Int J Impot Res. 2018;30:179–88. doi: 10.1038/s41443-018-0042-6. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Imai K, Nakagawa H, Miyata N. 2-anilinobenzamides as SIRT inhibitors. Chem Med Chem. 2006;1:1059–62. doi: 10.1002/cmdc.200600162. [DOI] [PubMed] [Google Scholar]
- 22.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Wang D, Zhao Y, Tu B, Zheng Z, et al. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1) Proc Natl Acad Sci U S A. 2011;108:1925–30. doi: 10.1073/pnas.1019619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu RY, Xu XW, Deng YZ, Ma ZX, Li XR, et al. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem Biophys Res Commun. 2019;514:401–6. doi: 10.1016/j.bbrc.2019.04.165. [DOI] [PubMed] [Google Scholar]
- 25.Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, et al. Sirtuin 1 and sirtuin 3:physiological modulators of metabolism. Physiol Rev. 2012;92:1479–514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


