Abstract
A laboratory colony of human body lice was experimentally infected by feeding on rabbits made artificially bacteremic with a green fluorescent protein-expressing Bartonella quintana. B. quintana was detected in the gut and feces until death but not in the eggs. The life span of the lice was not modified. The rabbit model should provide valuable clues to the role of lice in the transmission of B. quintana.
Bartonella quintana is a fastidious gram-negative bacterium that is regarded as a reemerging human pathogen (1) and is responsible for various human diseases (12). Although trench fever, the first clinical manifestation of B. quintana infection to be recognized (13), affected thousands of soldiers during World Wars I and II, medical interest in trench fever waned for almost 30 years because the disease was only rarely encountered. In the 1990s, B. quintana was identified as an agent of bacillary angiomatosis in AIDS patients (17), endocarditis (5, 16, 18), chronic bacteremia (3, 19), and chronic lymphadenopathy (15). These diseases are associated with homelessness or cramped, unhygienic circumstances, together with cold weather and the presence of body lice. The role of lice had been observed as early as 1920 (4). Various experiments have demonstrated that the disease could be induced in human volunteers (20) and Macacus rhesus monkeys (14) by injection of B. quintana and that the bacterium multiplied in the gut lumen of naturally (21) or intrarectally (8) infected lice without interfering with viability and was excreted in their feces (4, 8). However, despite growing interest in louse-transmitted diseases, there is no currently available experimental model to describe the relationship between B. quintana and the louse.
GFP-expressing B. quintana.
B. quintana strain Oklahoma (ATCC 49793) was obtained from the American Type Culture Collection (Rockville, Md.) and cultivated as previously described (10). Plasmid pJMBGFP, containing the B. bacilliformis flagellin promoter and a Rep origin of replication obtained from pBBR1-MCS2 (9) (Table 1), was provided by James M. Battisti (Division of Biological Sciences, University Montana, Missoula, Mont.). It was extracted and purified from Escherichia coli using a Midi Prep kit (Qiagen, Inc., Chatsworth, Calif.) and then adjusted to 0.5 μg/ml. Transformation procedures were as previously described (6). The transformed bacteria were cultivated on 5% sheep blood agar containing 25 μg of kanamycin sulfate per ml and were further verified as B. quintana by PCR amplification of the 16S-23S rDNA intergenic spacer (its) followed by sequencing (6). The presence of plasmid DNA was checked as previously described (6).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5 | F−(Φ80dlacZΔM15) Δ(lacZY AargF)U169 deoR recA1 endA1 hsdR17 (rK− mK+), supE44 λ-thi-1 gyrA96 relA1 λ-CH616 | Gibco-BRL (7) |
| B. quintana | ‘Oklahoma’ | |
| Plasmids | ||
| pBBR1-MCS2 | Kmr Mob Rep origin, Bartonella shuttle vector | Michael Kovach (7) |
| pFPV25 | Source of promoterless GFP (gfpmut3a) | Raphael Valdivia |
| PJMBGFP | PBBR1-MCS2 with a 970-bp HindIII fragment containing B. bacilliformis flagellin promoter (fla-pro) upstream of gfpmut3a | James M. Battitsti (2) |
Optimal transformation efficiencies were obtained at 6 ms for a field strength of 12.5 kV/cm and a plasmid DNA amount of 60 to 80 ng but were low, ranging from 3 × 10−5 to 7 × 10−5. Colonies of green fluorescent protein (GFP)-expressing B. quintana began to appear at aproximately 12 to 16 days following electroporation, which represents a growth lag time of approximately 8 to 12 days compared with the growth of untransformed bacteria on plate-to-plate passage. The colonies were typically smaller than those formed by untransformed B. quintana at the same stage of growth. When the colonies were subcultured on selective media, GFP expression was conserved after 15 passages, suggesting that plasmid maintenance was stable in the presence of antibiotic, but was lost after 5 passages in the absence of antibiotic. No growth was obtained on selective media with wild-type B. quintana prepared under the same conditions or B. quintana electroporated without DNA. Sequencing of PCR-products amplified from the its revealed 100% identity to known sequences of B. quintana, confirming that the transformants were B. quintana.
Experimental body louse infection.
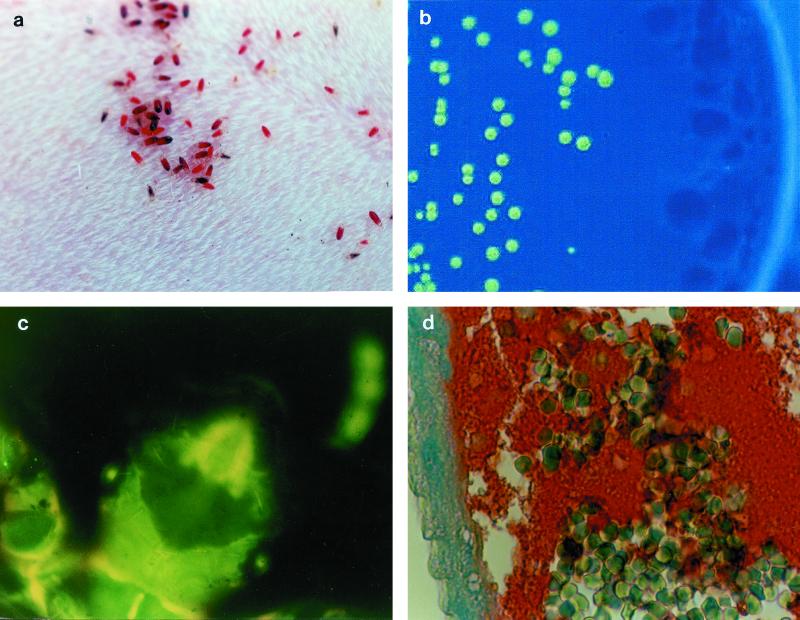
Body lice (Pediculus humanus corporis, strain Orlando) were kindly provided by D. Richard-Lenoble (Laboratoire de Parasitologie, Faculté de Médecine, Tours, France). A colony of lice was established and nourished daily on the shaved abdomen of specific-pathogen-free (SPF) New Zealand White rabbits (Fig. 1a). The lice were shown to be free from B. quintana by periodic its PCR amplification of samples from their gut and feces. One SPF rabbit, designated R1, was first injected intravenously with 2.5 ml of a solution of 10 mg of kanamycin (Sigma, St. Louis, Mo.) per ml, and 15 min later was injected with 20 ml of a suspension of 106 CFU of pJMBGFP-expressing B. quintana per ml in saline, before 800 15-day-old lice, half of which were female, were allowed to feed on its abdomen. The lice were then kept at 30°C and 70% humidity. The day of infection was referred to as day 1. On the following days, lice were allowed to feed daily on a second SPF rabbit designated R2. Before the lice were allowed to feed each day, R2 was injected intravenously each day with 2.5 ml of 10 mg of kanamycin sulfate (Sigma) per ml. To detect B. quintana, its amplification by PCR and culture on kanamycin-containing agar for up to 60 days at 37°C under a 5% CO2 atmosphere were performed on blood drawn from the infected rabbit after the lice had fed then every day for 5 days, and then once a week for 2 months. Similar tests were performed on 15 lice harvested after feeding on the infected rabbit, 15 lice harvested daily for 1 week and twice a week after that until no lice from the first generation remained, and, when present, eggs, larvae, and feces. Blood samples drawn from the infected rabbit (R1) were positive for B. quintana by culture and PCR on days 1 and 2, with 2 × 104/ml and 7 × 102 CFU/ml, respectively, but were negative on day 3 and at all later times. On follow-up, the rabbit did not present with any abnormal symptoms but developed an anti-B. quintana immunoglobulin G response by day 15, with a titer of 1:400 as determined by indirect immunofluorescence. Blood drawn from rabbit R2 was negative by both culture and PCR, and they did not seroconvert. GFP-expressing B. quintana was grown and PCR amplified from all groups of lice. Between 7 and 20 CFU of Bartonella (mean ± standard deviation = 16.3 ± 2.9) was obtained from each louse (Fig. 1b). The number of CFU did not decrease over time. B. quintana was also grown and PCR amplified from fecal samples harvested throughout the experiment. Qualitative but not quantitative analysis of the colonies grown from infected feces was performed. Only fluorescent colonies grew. The GFP marker was therefore very useful for directly identifying B. quintana colonies (Fig. 1B). None of the uninfected control lice were positive by culture or PCR analysis. The average daily number of eggs from the infected group was 682.8 ± 335.1 and was not significantly different from the 705.2 ± 357.9 observed in the control group (P = 0.51). No growth or PCR amplification of B. quintana was obtained from the 300 eggs and 100 larvae tested. Unfortunately, we observed that body lice were autofluorescent, and therefore detection of GFP-expressing Bartonella was not possible (Fig. 1c).
FIG. 1.
Experimental model of body louse infection by GFP-expressing B. quintana. (a) Pediculus humanus humanus feeding on a rabbit; (b) fluorescent colony isolated on selective agar from an infected louse 10 days after the initial infection; (c) autofluorescence of body lice; (d) immunohistological detection of B. quintana. Note the numerous erythrocytes and the clusters of bartonellae in the intestine lumen (red clumps) and against the intestine wall (blue). Streptavidin-biotin-peroxidase method, polyclonal rabbit anti-B. quintana used at a dilution of 1:400, hemalun counterstain. Magnification, ×660.
For histological examination, five lice collected on each of days 0, 3, 5, 7, and then once a week were fixed in 10% formalin overnight, paraffin embedded, and cut to 5-μm thickness. Hematoxylin-eosin stain was used to visualize the gut system, and Warthin-Starry stain was used to detect bartonellae. Immunochemistry using anti-B. quintana polyclonal antibodies (11) was performed on 5-μm-thick formalin-fixed and paraffin-embedded gut sections. For each section, a negative control was prepared using gut sections from uninfected lice. The gut system was easy to recognize by its thin wall and large lumen. Bartonella were identified as dense clusters of bacteria in the intestinal lumen. Masses of bacteria occupied an extracellular location (Fig. 1d).
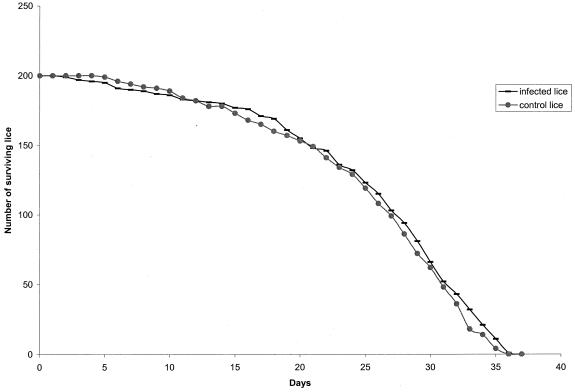
To determine whether B. quintana would influence louse mortality, 200 infected lice, half of which were female, were fed daily on rabbit R2 and were compared with 200 control lice, half of which were female, that fed only on an SPF rabbit (R3). The number of dead lice in both groups was compared using a Kaplan-Meier life table (GB-STAT version 6.5; Dynamic Systems Inc., Silver Springs, Md.), and the equality of survival between the two populations was estimated using the generalized Wilcoxon test. No significant difference in the mortality rate was observed between infected and control lice (P = 0.49) (Fig. 2).
FIG. 2.
Comparison of the survival rate over time of 200 lice infected with B. quintana and 200 control lice. Day 0, day of infection.
In conclusion, we report the first experimental animal model of body louse infection by using a laboratory colony of lice feeding on a rabbit with a GFP-expressing B. quintana bacteremia. The lice maintained an asymptomatic extracellular infection inside their gut during their entire life but did not transmit B. quintana to the next generation, supporting the role of body lice as vectors, but not reservoirs, for B. quintana. This rabbit model of human body louse infection may be valuable for other louse-transmitted pathogens.
Acknowledgments
We thank James M. Battisti, Michael Kovacs, and Raphael Valdivia for kindly providing plasmids.
REFERENCES
- 1.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battisti J M, Minnick M F. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Environ Microbiol. 1999;65:3441–3448. doi: 10.1128/aem.65.8.3441-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouqui P, La Scola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 4.Byam W, Lloyd L L. Trench fever: Its epidemiology and endemiology. R Soc Med Proc. 1920;13:1–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 6.Fournier P E, Minnick M, Raoult D. Transformation of Bartonella quintana to green fluorescent protein expression by electroporation. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millenium. Marseilles, France: Elsevier; 1999. pp. 38–42. [Google Scholar]
- 7.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Vinson J W. Fine structure of rickettsia quintana cultivated in vitro and in the louse. J Bacteriol. 1965;89:481–495. doi: 10.1128/jb.89.2.481-495.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovach M E, Elzer P H, Hill D S, Robertson S T, Farris M A, Roop R M I, Peterson K M. Four different derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 10.La Scola B, Raoult D. Culture of Bartonella quintana and Bartonella henselae from human samples: a 5-year experience (1993 to 1998) J Clin Microbiol. 1999;37:1899–1905. doi: 10.1128/jcm.37.6.1899-1905.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Z, Raoult D. Species-specific monoclonal antibodies for rapid identification of Bartonella quintana. Clin Diagn Lab Immunol. 2000;7:40–44. doi: 10.1128/cdli.7.1.21-24.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/cmr.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNee J W, Renshaw A, Brunt E H. “Trench fever”: a relapsing fever occurring with the British forces in France. Br Med J. 1916;12:225–234. [Google Scholar]
- 14.Mooser H, Weyer F. Experimental infection of Macacus rhesus with Rickettsia quintana (trench fever) Proc Soc Exp Biol Med. 1953;83:699–701. doi: 10.3181/00379727-83-20464. [DOI] [PubMed] [Google Scholar]
- 15.Raoult D, Drancourt M, Carta A, Gastaut J A. Bartonella (Rochalimaea) quintana isolation in patient with chronic adenopathy, lymphopenia, and a cat. Lancet. 1994;343:977. doi: 10.1016/s0140-6736(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 16.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton AM. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 18.Spach D H, Kanter A S, Daniels N A, Nowowiejski D J, Larson A M, Schmidt R A, Swaminathan B, Brenner D J. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis. 1995;20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 19.Spach D H, Kanter A S, Dougherty M J, Larson A M, Coyle M B, Brenner D J, Swaminathan B, Matar G M, Welch D F, Root R K, Stamm W E. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 20.Vinson J W, Varela G, Molina-Pasquel C. Trench fever. III. Induction of clinical disease in volunteers inoculated with Rickettsia quintana propagated on blood agar. Am J Trop Med Hyg. 1969;18:713–722. [PubMed] [Google Scholar]
- 21.Weigl R. Further studies on rickettsia rochalimae. J Trop Med Hyg. 1924;27:14–15. [Google Scholar]