Abstract
Like most solid tumours, the microenvironment of epithelial-derived gastric adenocarcinoma (GAC) consists of a variety of stromal cell types, including fibroblasts, neuronal, endothelial and immune cells. In this article, we review the role of the immune microenvironment in the progression of chronic inflammation to GAC, primarily the immune microenvironment driven by the gram-negative bacterial species Helicobacter pylori (H. pylori). The infection-driven nature of most GACs has renewed awareness of the immune microenvironment and its effect on tumour development and progression. About 75–90% of GACs are associated with prior H. pylori infection and 5–10% with Epstein Barr Virus (EBV). Although 50% of the world’s population is infected with H. pylori, only 1–3% will progress to GAC, with progression the result of a combination of the H. pylori strain, host susceptibility and composition of the chronic inflammatory response. Other environmental risk factors include exposure to a high-salt diet and nitrates. Genetically, chromosome instability occurs in ~50% of GACs and 21% of GACs are microsatellite instability-high (MSI-H) tumours. Here, we review the timeline and pathogenesis of the events triggered by H. pylori that can create an immunosuppressive microenvironment by modulating the host's innate and adaptive immune responses, and subsequently favour GAC development.
Introduction
The focus of this Review is to explain how the chronic inflammatory microenvironment in the gastric mucosal epithelia during Helicobacter pylori (H. pylori) infection can stimulate intracellular signalling pathways that can lead to gastric adenocarcinoma (GAC). As with many solid tumours arising from epithelial cells, cells in the surrounding support structures have multiple roles in tumour growth, as initiators, promoters or enablers. These supporting cell types include stromal cells, neuronal cells, endothelial cells and a vast array of immune cells1. H. pylori infection has been shown to be associated with a sustained immune infiltrate (chronic gastritis) that can contribute to the development of gastric neoplasia. Therefore, adenocarcinoma of the stomach is now considered a cancer that can be initiated by infectious agents2,3. About 75–90% of GACs exhibit positive H. pylori serology4-7. The incidence of adenocarcinoma of the gastric cardia is increasing in the US and epidemiologically correlates with increases in the prevalence of obesity, gastrooesophageal reflux disease and oesophageal adenocarcinoma8,9. By contrast, non-cardia gastric cancers, which include GAC in the stomach body or antrum, show a stronger association with H. pylori infection, low socioeconomic status and minority ethnic groups10-12. Epstein-Barr virus (EBV) infection is associated with about 5–10% of cases of GAC7,13,14. Owing to these associations, some GACs should be preventable either at the level of public health interventions, bacterial eradication, vaccination or suppression of the immune response; however, the latter two approaches have not been overly successful3,15. Most individuals infected with H. pylori are asymptomatic15 and antibiotic treatment fails to eradicate the organism in at least 20% of patients due to the existence of resistant strains and a lack of patient compliance16. Only about 20% of infected individuals develop serious complications related to a combination of environmental factors (smoking and occupational tobacco exposure), dietary factors (high intake of smoked foods, high intake of foods high in nitrates and/or a high-salt diet) and host susceptibility (particularly single nucleotide polymorphisms (SNPs), iron deficiency and blood type O15,17-23. In particular, several cytokine variants affect an individual’s susceptibility to GAC24-26.
During the 1860’s, Rudolf Virchow proposed that cancer is the response to a “wound that will not heal”, and since then investigators have sought to understand the role of immune cells in various cancers27,28. The immune infiltrate accompanying an H. pylori infection has a prominent role in creating and shaping the tumour immune microenvironment (TIME) that promotes progression to GAC. Moreover, GAC has become a paradigm for inflammation-driven cancers21,29. This Review discusses how acute and chronic inflammatory infiltrates induced by H. pylori infection transform epithelial cell populations in the pathogenesis of GAC.
Gastric adenocarcinoma histopathology
In 1975, Pelayo Correa published the astute observation that intestinal type GAC was associated with an inflammatory process in the stomach30. This publication occurred ~10 years prior to the seminal report that showed that a particular bacterial species, H. pylori (previously known as Campylobacter pylori), colonizes the stomach and initiates an inflammatory response or gastritis 31,32. Approximately 50% of the world’s population is infected with H. pylori; about 20% of those infected develop chronic gastritis and/or more serious complications such as duodenal or gastric ulcers20,33 and approximately 1–3% of those infected subsequently develop GAC5 (Fig. 1). In 2014, researchers performed a comprehensive molecular evaluation of 295 human GACs and divided them into four molecular subtypes—tumours with chromosome instability (50%), tumours positive for EBV (9%), microsatellite instability-high (MSI-H) tumours (21%) and genomically stable tumours (20%) 34. These four subtypes exhibit distinct molecular signatures that correlate with methylation status, EBV DNA and the presence of mismatch repair gene mutations, or show no genome perturbations (genomically stable and microsatellite stable tumours). In this study, activated signalling pathways were only inferred from the genes mutated as concurrent gene expression profiling was not performed34. An association with H. pylori could not be accurately assessed owing to the absence of serology and because H. pylori DNA was only sporadically detected in the GACs. Understanding the genomic changes that occur in GAC is useful but does not provide complete insight into the stepwise sequence through which multiple pathways lead to gastric epithelial cell transformation, nor does it provide information on the TIME.
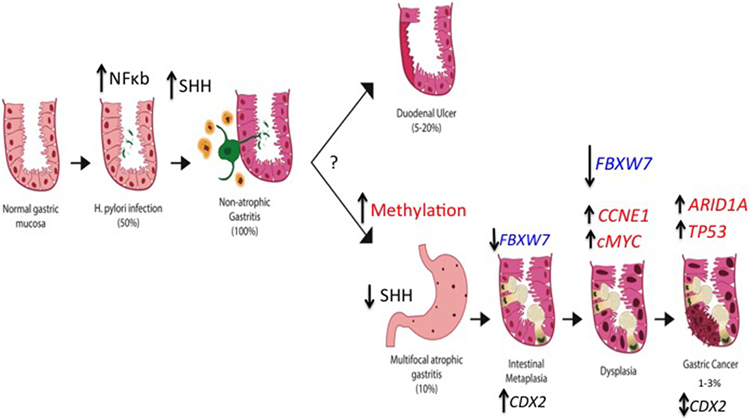
Figure 1 ∣. Timeline of complications from Helicobacter pylori infection.
A schematic of the timeline from H. pylori infection to the development of gastric ulcers versus gastric atrophy, intestinal metaplasia, dysplasia and gastric cancer. Key genomic changes were identified by profiling of human gastric intestinal metaplasia and cancer123,260. Vertical arrows indicate direction of the change; for example, FBXW7 mutations identified in intestinal metaplasia and more numerous in the WD40 domain are represented by larger arrow. Bidirectional arrow indicates increase or decrease. Infection of the gastric mucosa initiates acute inflammatory responses most of which result in an increase in NFκB. Injured parietal cells release Shh within 2 days of the infection and Shh can be detected in the circulation, which subsequently recruits myeloid cells to the stomach210. Why some individuals do not develop atrophy and instead develop duodenal ulcers owing to the hyperacidity is unknown, but RNA and DNA profiling suggests that hypermethylation, loss of parietal cells and a LOF in the ubiquitin ligase FBXW7 are early steps that lead to the pathway from intestinal metaplasia to GAC123.
In 2020, analysis of the TIME was performed on a subset of TCGA cancers using RNA sequencing and showed that the chromosomally unstable tumours could be subdivided into T-cell-rich ‘hot’ gastroesophageal cancers (GEAs) and T-cell-poor ‘cold’ GEAs. The cold chromosomally unstable GEAs showed enrichment of cMYC and amplification of the cyclin E1 gene (CCNE1) compared with ‘hot GEAs35. In a separate study, an immunohistochemical analysis of immune subsets—including tumour-infiltrating lymphocytes (TILs) and tumour-associated macrophages (TAMs)—was performed in 43 GACs classified according to the four TCGA subtypes36. EBV-positive (n=6) and MSI-H (n=11) GACs showed the highest number of immune cells (‘hot’ tumours) and highest expression of checkpoint inhibitors while intestinal (a surrogate for chromosomal instability; n=14) and diffuse (a surrogate for genomically stable; n=12) cancers showed the least number of immune infiltrates (‘cold’ tumours). In the latter two categories, H. pylori infection was documented in only three of 25 patients. However, this retrospective analysis was based on immunohistochemistry (IHC), not correlation with H. pylori serology. Thus, whether immune-poor GACs (70–75% of GACs in this study) represent the initiation of GAC pathogenesis by mechanisms other than H. pylori, EBV or MSI-H, is unclear. Nevertheless, the findings underscore the need to quantify and characterize the TIME as a function of the cancer subtype to identify which cancers are most responsive to treatment.
Three major histologic cancer subtypes arise from the gastric epithelium—intestinal, diffuse and mixed35. Pelayo Correa described a cascade of events (the Correa cascade or paradigm) that precedes GAC based upon epidemiological observations30. The Correa cascade was ultimately incorporated into the Sydney Classification system, which describes sequential events in histological terms: chronic inflammation proceeds to intestinal-type GAC through distinguishable lesions consisting of atrophic gastritis and intestinal metaplasia37 (Fig. 1). Nevertheless, H. pylori infection is not as strongly correlated with diffuse GAC as with intestinal type GAC38,39. However, the evidence is mixed, with some studies reporting that up to 80% of diffuse GAC cases were positive for H. pylori40,41. H. pylori infection increases the likelihood that patients with MSI-H will develop GAC42,43. A 2020 study suggests that different cag pathogenicity island (cagPAI) genes, such as CagA and the vacuolating cytotoxin (VacA) gene (CagA+ and VacA+) affect which GAC histologic subtypes (diffuse versus intestinal) develop44. COX-2, DAPK and CDH1 have been shown to be the most highly mutated genes during infection by CagA+ H. pylori45. Thus, the predisposing factors driving specific GAC subtypes are not clearly defined.
Diffuse-type GACs
Hereditary diffuse gastric cancers (HDGC) develop in young adults and comprise 1–3% of GACs46,47. Affected individuals frequently carry loss of function (LOF) mutations in the genes encoding tumour suppressors cadherin 1 (CDH1) or catenin α1 (CTNN1A), a gain of function (GOF) mutation in the locus encoding transforming protein RhoA (RHOA; a downstream GTPase signalling component of the actin cytoskeleton), or have a fusion gene CLDN18-ARHGAP46-50. Disruption of CDH1 and loss of cell junction protein expression renders the gastric mucosa more susceptible to transformation, cancer invasion and metastasis21. HDGC is inherited in an autosomal dominant pattern, is highly penetrant and predominates in young women, especially in Asia51,52. CDH1 mutations are also associated with lobular breast cancer in women53 and with colon cancer in women and men54. Despite a strong association of H. pylori with sporadic diffuse-type and intestinal-type GAC, the organism is rarely detected in total gastrectomy specimens prophylactically removed from asymptomatic patients with HDGC55,56.
Sporadic diffuse GACs make up ~20–30% of GAC cases in Western countries57. However, gastric atrophy and intestinal metaplasia are not prominent histological features of diffuse GACs58. Thus, commitment to tumour pathogenesis is less obvious in the absence of precursor lesions than with intestinal type GACs, with hypermethylation causing LOF or GOF in tumour suppressor or proto-oncogenes controlling tight junction proteins such as CDH1 and CTNN1A46,57. A transgenic model of diffuse GAC in which the most common oncogenic variant (Y42C) of the RhoA locus was conditionally activated together with inactivation of Cdh1 mimicked features of diffuse GAC such as lung, liver and peritoneal metastasis48. In particular, the RhoAY42C mutant shows impaired GTP hydrolysis and exhibits enhanced interaction with its effector kinase, ROCK. The subsequent stimulation of actin stress fibres and cytoskeleton rearrangements activate focal adhesion kinase (FAK) that modulates downstream pro-proliferative pathways phosphoinositide-3 kinase (PI3K)–AKT, β-catenin and the Hippo signalling pathway through YAP–TAZ48. In the absence of Helicobacter infection in this diffuse GAC mouse model, loss of the Cdh1 tumour suppressor gene uncovered the effects of oncogenic RhoAY42C, suggesting that FAK might be a relevant therapeutic target.
Galectins are prominent glycoproteins that contribute to the peritoneal metastasis of diffuse cancers59. Plasma membrane glycosylation is frequently expressed aberrantly in GACs and can also contribute to heightened invasiveness21,60,61. For example, the overexpression of Galectin-3, a β-galactosidase specific binding protein, localizes to the cytoplasm of normal gastric mucosal cells, where it is expressed in both the cytoplasm and nucleus of GAC cells62. When expressed in the epithelial cell compartment, Galectin-3 increases cell motility63. Galactin-9 is a ligand for T-cell immunoglobulin and TIM-3 (also known as hepatitis A virus cellular receptor 2), encoded by the HAVCR2 gene and expressed on almost all immune cells as an immune checkpoint receptor21. TIM-3 expression is significantly upregulated on peripheral blood monocytes from patients with GAC64. Importantly, the TIM-3/Gal-9 axis might have a critical role in T-cell exhaustion within the GAC microenvironment65. Therefore glycan-binding proteins might be useful prognostic markers for patients with GAC63,66.
Diffuse GAC also correlates with H. pylori-mediated methylation of CpG islands in the CDH1 gene and other promoters40,67-69. Genomic suppression of CDH1 is often observed in the CpG island hypermethylator phenotype (CIMP) GAC subtypes, as observed with patients with EBV or Lynch syndrome in which hypermethylation or inherited mutations in the loci encoding mismatch repair proteins leads to high microsatellite instability (MSI-H) 70,71. By contrast, APC or CTNN1B mutations correlate with low microsatellite instability (MSI-L) and microsatellite stable (MSS) intestinal-type gastric cancers of the antrum72-74. MSI-H cancers, including some GACs, display a higher mutational burden than non-MSI-H cancers and create important neoantigens that are subsequently recognized by the immune system75. As such, these MSI-H tumours exhibit higher densities of immune infiltrates and are more susceptible to immune checkpoint blockade than non-MSI-H 76. MSI-H GACs typically account for only 10–20% of MSI-H tumours34,77-79, which might explain the overall poor response rate for GACs treated with the current immune checkpoint inhibitors76,78-81.
EBV-positive GACs
EBV-positive GACs are typically of the diffuse-type and are located in the gastric body and cardia rather than the antrum82,83. EBV infection creates a CIMP genomic environment of hypermethylation. The hypermethylation in EBV-driven cancers is non-random, with DNA repair genes being a frequent target, suggesting some genomic mutational overlap with MSI-H GACs 82. Normal gastric tissue displays a promoter CpG island methylation level of 1–2% 34,84, whereas EBV-positive GACs display a CpG island methylation frequency of 19% and MSI-H GACs show a CpG island methylation frequency of 10%34. EBV-driven GACs also exhibit a greater degree of immune infiltration and increased expression of immune checkpoint proteins (for example, programmed cell death protein 1 (PD-1; also known as CD279)36, CTLA-4 (also known as CD152)) compared with non-EBV cancers and thus show a better response to immune checkpoint inhibitors85. One study suggested that 15–38% of all GACs are EBV-positive, depending on the number of mismatch repair loci evaluated84,86. The EBV latent membrane proteins (LMP1 and LMP2A) induce methylation of the tumour suppressor PTEN promoter by increasing STAT3-mediated expression of DNA methyltransferase1 (DNMT1)87,88. This dual-specificity phosphatase inhibits PI3K–AKT signalling and in many cancers is suppressed through epigenetic silencing89. CDX2, KLF4 and TFF1 are representative pro-intestinal differentiation genes that are epigenetically silenced in EBV-driven cancers82. A review of EBV-positive GACs indicates that EBV-mediated hypermethylation does not occur without pre-existing conditions such as chronic atrophic gastritis, intestinal metaplasia or dysplasia 82, suggesting that coinfection or sequential infection with H. pylori might have occurred82,90, as reported for late-stage or metastatic GAC tumours90,91.
H. pylori and gastric pathogenesis
H. pylori-induced pathogenesis is largely mediated by the virulence factors VacA and cytotoxin-associated gene A (CagA)17,92. Injection of these factors into epithelial cells via the type IV secretory system (T4SS) activates a number of pro-tumorigenic pathways that propels the gastric mucosa towards an intestinal stem-like state, for example through activation of WNT–β-catenin signalling, which in turn induces expression of pro-intestinal differentiation factors CDX1, KLF5 and LGR592,93. Mutations in Wnt signalling components, including gain of function (GOF) mutations, loss of function (LOF) mutations, mRNA activation and epigenetic alterations, which collectively are present in about 30% of GACs94,95. Other studies indicate that H. pylori virulence factors initiate the transition from atrophy to intestinal metaplasia through cytokine-mediated activation of NFκb and subsequent induction of the intestinal differentiation factors CDX1 and CDX296. In the progression to intestinal-type GAC, H. pylori reduces CDX1 promoter methylation level at the histologic stages of gastritis and intestinal metaplasia, reaching its lowest level of methylation in dysplasia, and then rising again in gastric cancer. NFκb ultimately binds to the unmethylated CDX1 promoter to initiate gastric mucosal transdifferentiation to an intestinal phenotype. However, once dysplasia segues into adenocarcinoma, CDX1 promoter methylation rises again96,97. H. pylori CagA subverts both phosphorylation-independent pathways (cMET, CDH1 and WNT–β-catenin) and phosphorylation-dependent kinase signalling pathways (EGFR and gp130–STAT3), in which both pathways can activate NFκB leading to changes in epithelial cell motility, the cell cycle and release of pro-inflammatory mediators98. In addition, H. pylori lipopolysaccharide (LPS) is recognized by toll-like receptor (TLR)4, a pathogen recognition receptor (PRR), which induces pro-inflammatory cytokine expression and type 1 interferons through formation of the inflammasome and activation of transcription factors NFκB, AP1 and interferon response factors (IRFs)99. Recognition of unmethylated CpG DNA motifs by endocytic TLR9 is another important mechanism through which H. pylori activates epithelial signal transduction cascades via the release of chemokines such as IL-8100,101. TLR9 mediates both anti-inflammatory and pro-inflammatory responses depending on the gastric microenvironment102. During the acute inflammatory phase, TLR9 activation by H. pylori bacterial DNA suppresses the immune response, which facilitates bacterial colonization103. In addition, LPS, peptidoglycans, flagellin (TLR5) and other non-protein molecules that enter the epithelial cell via the T4SS also activate TLR9104.
The major TLRs associated with GAC are TLR2, TLR3, TLR4, TLR5, TLR7 and TLR9105-107. Four intracellular, endocytic TLRs (TLR3, TLR7, TLR8 and TLR9)108 are known to be capable of responding to H. pylori RNA or DNA, but TLR3, TLR7 and TLR8 have been infrequently studied109. Activation of TLR3 requires double-stranded RNA species characteristic of specific viruses110. However, TLR7 and TLR8 recognize single-stranded RNA species and thus can detect H. pylori RNA in a MyD88-dependent and TRIF-dependent manner102. The cytoplasmic retinoic acid inducible gene (RIG-1; also known as DDX58), but not the melanoma differentiation associated antigen 5 (MDA5; also known as IFIH1) also recognizes bacterial RNA but its activation of type 1 interferons is independent of MyD88 and TRIF111. Antiviral innate immune response receptor RIG-I is a non-TLR pathogen recognition RNA helicase receptor that recognizes 5-triphosphate RNA (PPP-RNA), a specific feature of viral and prokaryotic RNAs111. The 5’ end of bacterial and viral RNA is not capped making it a genuine RIG-I ligand112,113. Although H. pylori initiates a cacophony of signalling cascades in both epithelial cells and antigen presenting cells (APCs), the combination of H. pylori strain and host susceptibility seems to ultimately determine which pro-inflammatory or anti-inflammatory responses predominate.
TLR9 and DAMP signalling
TLR9 receptors have been best characterized on APCs such as dendritic cells (DCs), macrophages and B cells101,114. Plasmacytoid dendritic cells (pDCs) are the major source of secreted type I interferons (IFNα and IFNβ) upon activation of endocytic TLR7 and/or TLR9 by viral or unmethylated self-derived DNA115,116. However, in contrast to TLR9 activation in epithelial cells, H. pylori DNA in pDCs is immunosuppressive as H. pylori DNA fails to stimulate secretion of type1 interferons and IL-12 in mouse or human bone marrow-derived dendritic cells (BMDCs) and human pDCs117. We reported that the rise in tissue levels of IFNα produced by pDCs correlates with the appearance of spasmolytic polypeptide-expressing metaplasia (SPEM) during the chronic phase of an Helicobacter felis infection118. Moreover, TLR9-mediated induction of IFNα contributes to suppression of H. pylori by down-regulating TH1-mediated gastritis103. Although H. pylori DNA can gain entry into both gastric epithelial cells and pDCs, the overarching chronic effect of an H. pylori infection is to dampen the TH1/TH17 proinflammatory response by increasing the numbers of immunosuppressive cells such as Treg cells that secrete TGFβ and IL-10119 (Fig. 2). The ability of the H. pylori gastric infection to inhibit the TH1/TH17 immune response has systemic implications, as shown by H. pylori potently mitigating inflammation in the mouse colon (colitis) and lung (asthma)117,120-122. Thus, the phenotypic shift in the composition of immune infiltrates in response to H. pylori DNA motifs sets the initial stage for chronic gastritis. Moreover, genomic analysis of intestinal metaplasias from cancer-free patients demonstrated that CagA+ H. pylori DNA is present in these precursor lesions, suggesting that H. pylori makes some contribution to the progression of intestinal metaplasia to GAC, perhaps related to changes in telomere length123.
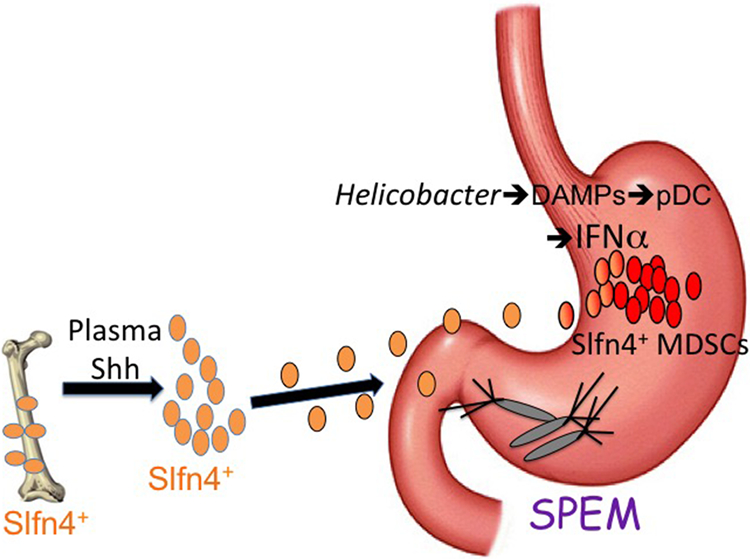
Figure 2 ∣. PMC-MDSC induction during spasmolytic polypeptide-expressing metaplasia (SPEM) development.
Acutely, Slfn4+ myeloid-derived suppressor cells (MDSCs) regulated by Shh signalling track to the stomach, but do not develop their T-cell suppressor function until levels of tissue IFNα produced by plasmacytoid dendritic cells (pDCs) increase. SPEM development coincides with maximal expression of Slfn4 and the Slfn4+ MDSCs acquire T-cell suppressor function 118,125. Slfn4+-MDSCs express NFκB and secrete regulatory microRNAs such as MIR130b203. DAMPs, damage-activated molecular patterns.
H. pylori DNA and heterogeneous byproducts of injury and inflammation produce various damage-activated molecular patterns (DAMPs), which are potent activators of TLR9-mediated IFNα production124. Host cell debris can induce an increase in TLR9 tissue levels of IFNα118,125. High mobility protein B1 (HMGB1), heat shock proteins 70 and 90 (HSP70 and HSP90), mitochondrial DNA, calreticulin, ATP, the IL-1 cytokine family and type I interferons (IFNs) are all examples of DAMPs released from host cells dying by various mechanisms including necroptosis, apoptosis, pyroptosis and ferroptosis126,127. In particular, in response to IL-17A and tamoxifen, apoptotic parietal cells likely release bioactive molecules that activate DAMP signalling128-130. In addition, tumour cells themselves generate DAMPs that perpetuate the immune suppressive microenvironment131.
The timeline in humans from H. pylori infection to disease complications — gastric atrophy, metaplasia and GAC — can span months to years132. During that time, the loss of acid-secreting parietal cells leaves the stomach relatively hypochlorhydric, which can shift the composition of the gastric microbiota133-135. Although a meta-analysis of studies investigating the gastric microbiome in gastric cancer inferred the presence of changes in the microbiota at different stages of the Correa cascade136 , none of the studies link the changes in the microbiome to specific inflammatory markers137-139. In humans with chronic gastritis, Prevotella, Streptococcus, Pseudomonas, Sphingomonas, Bacillus, and Acinetobacter were also identified in the normal mucosa adjacent to the tumour; however, H. pylori was still the organism consistently identified at different stages of progression136. This finding is consistent with mouse studies in which Acinetobacter species were shown to induce gastritis in mice and to cause a TH1 immune response140,141. Bacteroides and Haemophilus species have also shown elevated 16S rRNA genomic signatures in the blood of patients with GAC142. Therefore, although H. pylori seems necessary for the initial steps in the process of gastric carcinogenesis, once the mucosa becomes atrophic, it is unclear whether H. pylori, other bacteria or immune cells drive the mucosa towards metaplasia or cancer. As cell or bacterial debris is sufficient to sustain DAMP-mediated inflammation, further epithelial changes might not require live organisms and the microbial composition of the stomach at this stage might not influence what happens next. Establishing the microbiome pattern in gastric cancer has clinical consequences as it could indicate whether antibiotic-mediated eradication can prevent progression to GAC. We can only speculate what impact gastric microbiota other than H. pylori have on the immune microenvironment by correlating immunosuppressor subtypes with bacterial species in the atrophic stomach.
Impact of CagA on gastric epithelial cells
H. pylori CagA accumulates in GAC cell lines that express CD44, a cell surface marker associated with cancer stem cells, one isoform of which suppresses cell autophagy and enhances their resistance to reactive oxygen species (ROS)143. In cells not expressing this isoform of CD44, CagA is degraded by autophagy induced by the accumulation of ROS143. Thus, within the tumour environment, cancer cells become resistant to ROS-induced apoptosis through CD44144,145. The CD44 gene encodes several protein variants owing to alternative splicing and post-translational modifications146. CD44 variable isoforms have been implicated as key players in malignant transformation where their expression is highly restricted and specific, unlike the canonical CD44 full-length isoform147. Upon delivery into host cells by the T4SS, CagA translocates into the host cell cytoplasm where it can stimulate cell signalling through interaction with several host proteins92,148,149, including the tyrosine kinase c-MET receptor150-152. CagA exerts pro-proliferative effects within host cells, by inducing hyperproliferation and disrupting apical junctional complexes and cellular polarity153-155. Suzuki et al.156 demonstrated that H. pylori infection resulted in CagA CM motifs interacting with c-MET, leading to sustained PI3K–AKT signalling, which in turn resulted in β-catenin activation and cellular proliferation. We have shown that CD44 variant 6 (CD44v6) acts as a co-receptor for the function of c-MET in response to H. pylori infection and bacterial-induced epithelial proliferation157. CD44v9 has a central role in malignant transformation and chemoresistance in GAC158. Within GACs, CD44v9 has been reported to interact with the cystine–glutamate transporter xCT, thereby potentiating defence against ROS and promoting subsequent tumour growth158.
Using human organoid cultures, H. pylori-induced expression of the immune checkpoint molecule programmed cell death 1 ligand (PD-L1; also known as CD274) was shown to be mediated by the Shh signalling pathway159. In this study, CD44v9+ SPEM cells within the gastric epithelium of human tissue-derived or iPSC-derived organoids specifically expressed PD-L1159. Collectively, this study demonstrates that H. pylori-induced PD-L1 expression is an acute gastric epithelial response to infection in human cells, as previously observed in mice. By contrast, in a mouse model of PD-L1 expression, Wang and co-workers reported that the effectiveness of conventional platinum-fluoropyrimidine chemotherapy increases when PD-L1-expressing myeloid-derived suppressor cells (MDSCs) are depleted prior to treatment160. Their findings suggest that the site of PD-L1 expression, specifically when overexpressed on immune cells rather than epithelial cells, might improve the response to checkpoint inhibitors and we suggest that the level of PD-L1 expression on myeloid cells has potential as a predictive or prognostic biomarker. Therapeutic trials in GAC that include immune checkpoint inhibitors such PD-L1 in combination with standard chemotherapy are ongoing, but have not significantly improved the overall response rate161. These results highlight the need for GACs to be better stratified according to both histologic phenotype and genomic subtypes as well as the immune cell composition36,162,163.
Chronic gastritis
Chronic gastritis occurs when the inflammatory process fails to resolve 2. If the inflammation persists, parietal cells undergo apoptosis, atrophy ensues (chronic atrophic gastritis) and metaplastic cells emerge in their place 164,165.
Parietal cell death
Elevated levels of IL-1β are a consistent feature of both the acute and chronic immune microenvironment that can mediate transition of the mucosa from gastritis to atrophy26,166-168. As IL-1β suppresses Shh gene expression in parietal cells by inhibiting acid secretion 167, this finding suggests that parietal cells are key cellular targets that can trigger an imbalance in the immune response, tipping the mucosa towards metaplasia. However, Burclaff et al. showed that the targeted destruction of parietal cells via diphtheria toxin-mediated apoptosis in the absence of inflammation was not sufficient to induce gastric SPEM, consistent with the idea that specific cytokines have a central regulatory role in initiating the transition from atrophy to metaplasia169. This finding indicates that the induction of SPEM might require a mechanism of parietal cell destruction that induces inflammation. In contrast to the 6-month timeline for Helicobacter-induced gastritis to occur in mice170, chemical induction of SPEM with the protonophore DMP777 or its analogue L-635 is much more rapid, promoting the transdifferentiation of immature chief cells to become metaplastic once parietal cells have atrophied171. The L-635 analogue induces a more aggressive inflammatory response than DMP777 and results in full-blown SPEM within 3 days (versus 14 days with DMP777)172. DMP777 has neutrophil elastase inhibitory activity, a chemical property that L-635 lacks173, which explains why the L-635 analogue has a more intense inflammatory response than DMP777 that is similar to a Helicobacter infection174. Studies indicate that tamoxifen also acts as a parietal cell protonophore that causes a backwash of secreted acid into parietal cells, triggering cell death without prominent inflammation, similar to DMP777175,176. L-635, DMP777 and tamoxifen can all induce SPEM, but only L-635 induces notable gastritis and causes the most extensive SPEM changes of the three drugs 174. Bulk RNA-Seq profiling of macrophage markers in the corpuses of L-635-treated mice showed M2-like polarization with increased expression of Fizz1, Klf4 and IL-33177. The presence of inflammation has been shown to be required for intestinal metaplasia or SPEM to progress to dysplasia in both humans and in mouse models171. These studies demonstrate that inflammation is essential for the epithelium to progress to SPEM, and that the composition of the immune environment (whether caused by Helicobacter infection or by the chemical destruction of parietal cells) is one that favours infiltration of M2-like TAMs and the inhibition of effector T cells. As not all mechanisms of parietal cell death induce SPEM, it is important to understand how diphtheria toxin-mediated death differs from chemical or inflammatory-mediated mechanisms129.
Antral inflammation and gastrin
Compared with Helicobacter-infected mice where histologic changes start in the corpus of the stomach 174, H. pylori infection of Mongolian gerbils or chemical carcinogen treatment with the alkylating agent N-methyl-nitrosourea (MNU) more closely mimics the Correa cascade where inflammation in the gastric antrum predominates and leads to GAC178,179. The predominance of inflammation in the antrum is noteworthy since non-cardia cancer in humans typically develops in response to gastritis originating in the gastric antrum that then migrates proximally to the corpus30,132. Another possibility is that gastric atrophy originating in the antrum eliminates gastrin-expressing (G) cells and gastrin peptide, a growth factor for parietal cells, which in turn contributes to inflammation-driven atrophy in the corpus 180-182. Although most mouse models of SPEM have focused on events in the gastric corpus174, the transition of atrophy to metaplasia in the antrum follows a different pathway linked to the presence or absence of the G cell as studied in the gp130 and Tff1 mouse models 183-186. During non-atrophic gastritis, G-cell density and serum gastrin levels are initially elevated in response to a decrease in somatostatin-expressing antral (D) cells initially reported for human subjects 187,188. Owing to the acid-mediated feedback regulation of gastrin 189, corpus-dominant atrophy with preservation of the antrum correlates with hypergastrinaemia190,191. However, in humans, corpus atrophy from autoimmune gastritis (AIG) is more strongly associated with gastric carcinoids (G-NETs) than with GAC (Box 1) 189. By contrast, antral-predominant H. pylori-induced atrophic gastritis leads to decreased gastrin levels 192-194. Therefore, modelling the inflammatory microenvironment that inhibits gastrin is critical to our understanding of distal GAC development and might be useful for distinguishing between the induction of G-NETs and GAC.
BOX 1: Autoimmune gastritis: a tale of two phenotypes.
Autoimmune gastritis (AIG) occurs only in the gastric corpus and fundus and is caused by generation of anti-parietal cell or anti-intrinsic factor antibodies, and subsequent destruction of the oxyntic glands. Destruction of the parietal (oxyntic) cells results in a rise in the pH of the stomach, which stimulates production and secretion of the hormone gastrin from G cells located in the gastric antrum. Since parietal cells produce intrinsic factor and acid, which are essential for the absorption of vitamin B12 and iron, autoimmune gastritis is frequently associated with anaemia. Prior to understanding the aetiology of AIG, anaemia associated with parietal cell atrophy was termed ‘pernicious anaemia’338.
Relevant to the TIME, AIG is associated with GAC and type 1 gastric neuroendocrine tumours (G-NETs). G-NET incidence (4–12%) is slightly more frequent than the association of AIG with GAC (0.9–9%) 338. Moreover, the overall risk for developing GAC from AIG is lower than the association of H. pylori with GAC 339, possibly because of the lower prevalence of AIG when H. pylori organisms are present338,340. Thus, the epidemiology suggests that firstly, the inflammatory microenvironment generated by AIG is sufficient to generate both G-NETs and GAC and secondly, that the immune microenvironment of AIG differs significantly from H. pylori-induced GAC. An explanation for the differences in tumour type might be related to elevated serum gastrin, which in humans arguably targets the enterochromaffin-like (ECL) cell to a greater extent than non-endocrine epithelial cells 341,342. The autoreactive T-cell responses in AIG are TH1 and TH17-mediated, which produce IL-17A, IL-21, TNFα and IL-11 (an IL-6 family member) that contribute to the development of gastric atrophy 128,343. Thus, the cytokine profile does not seem to be a distinguishing feature of AIG-mediated GAC pathogenesis. However, aside from the role of elevated gastrin levels, TLR5 polymorphisms such as rs5744174 might explain the differential impact of whether AIG results in GAC development344. In summary, H. pylori does not seem to be a key risk factor for AIG, but instead exclusive corpus inflammation, sparing of the antrum and elevated gastrin levels favour the formation of G-NETs to a greater extent than GAC345,346.
Loss of Tff1 and constitutive activation of the IL-6–IL-11 co-receptor GP130 both inhibit gastrin expression and induce tumours in the gastric antrum via STAT3184-186,195. Tff1 becomes epigenetically silenced in MNU-dependent antral tumours, which in turn silences the expression of gastrin196. In addition, gastrin-null mice develop dysplastic antral tumours and show elevated levels of IL-1β, IFNγ, IL-11, IL-6 and STAT3181,182,197. Gastrin administration overrides the effect of MNU-induced antral cancers by inducing TFF1 expression 196,198. Loss of Apc gene function or MNU treatment also induces tumourigenesis in the antrum but not the corpus by increasing the activity of the Cxcl12–Cxcr4 signalling axis199. Β-catenin overexpression induces the chemokine CCL28, which contributes to immune suppression in the antrum by recruiting regulatory T cells (Treg cells)200-202. We conditionally overexpressed IFNγ and IL-1β in the gastric antrum of mice and found that IFNγ did not suppress gastrin but that IL-1β did suppress gastrin, suggesting that these two proinflammatory cytokines exert differential effects in the antrum168. Conditional overexpression of IL-1β in the antrum led to suppression of parietal cell acid secretion through to the suppression of gastrin gene expression, while IFNγ overexpression in the antrum induced gastric acidity by blocking local non-canonical HH signalling via GLI2 and increased G cell numbers and gastrin peptide secretion168,203. Therefore, as already observed in humans189,192,193, antral inflammation in mice also modulates gastrin peptide levels depending on the type of inflammatory cytokine 168.
Chronic gastritis to metaplasia
The transition of atrophy to intestinal metaplasia in the inflamed stomach is the most visible indication that chronic inflammation has induced a phenotypic change in the epithelium’s response to injury. Various reports indicate that the signalling pathways activated by H. pylori virulence factors induce CDX1 and CDX2, which are caudal-homeobox transcription factors essential to regulating intestinal identity and intestinal metaplasia96. Genomic profiling of intestinal metaplasia samples detected more cases of active H. pylori infection than did simple histologic examination123. The mere presence of H. pylori DNA, however, does not determine whether the bacteria or APC activation is ultimately responsible for the epithelial change. Whether H. pylori virulence factors or its byproducts alone are sufficient to induce intestinal metaplasia is unclear. However, the stochastic progression from chronic gastritis to intestinal metaplasia favours involvement of both mechanisms.
Genomic profiling of intestinal metaplasia
Genomic profiling of human gastric intestinal metaplasia revealed a low rate of clonal mutations compared with GAC; however, the tumour suppressor gene FBXW7 (which encodes an E3 ubiquitin ligase required for cMYC and cyclin E1 (encoded by CCNE1) protein degradation) showed missense point mutations in 4.7% of intestinal metaplasia samples (compared with 9.2–18.5% of GAC samples123) (Fig. 1). FBXW7 dominant negative mutations are increased in GAC compared to normal gastric epithelium, suggesting aberrant ubiquitin-mediated protein degradation123. Normally, wild-type FBXW7 promotes CDX2 ubiquitination and degradation when it docks at the WD domain of FBXW7204. Fbxw7−/+ mice treated with the alkylating agent N-methyl-nitrosourea (MNU) develop antral intestinal metaplasia and dysplasia within 8 weeks, which correlates with an increase in cMYC205. Thus, FBXW7 can suppress expression of CDX2 through protein degradation. Interestingly, cMYC and cyclin E1 are the same gene targets elevated in the immunologically cold chromosome instability GAC subtype35. Dominant-negative gastric intestinal metaplasia mutations in the WD40 domain of FBXW7 correlates with an increase in the immunohistochemical expression of cMYC. TP53 and ARID1A exhibited clonal mutations in GAC but not in intestinal metaplasia, whereas hypermethylation was more prevalent in intestinal metaplasia than in GAC123. Surprisingly, genomic analysis of GACs revealed that the CDX2 gene exhibits LOF mutations in GAC but that CDX2 amplification is present in oesophageal and colon adenocarcinomas206. Taken together, CDX2 protein expression can increase or decrease during the transition from intestinal metaplasia to GAC through increased protein turnover or altered epigenetic status (for example, DNA methylation or demethylation) of this locus207.
Gastric metaplasia in rodent models
Rodent models have provided detailed insight into GAC pathogenesis by mimicking the molecular steps occurring as the mucosa transitions from chronic gastritis through the intermediate lesions to cancer 170,208,209. Mapping the timeline of H. pylori infection from inoculation to SPEM revealed that infection in the mouse gastric corpus triggers the release of the sonic hedgehog (SHH) ligand from parietal cells into the circulation within 2 days, where it is sensed by circulating monocytes210. Parietal cell release of SHH is therefore one mechanism that contributes to the homing of myeloid cells to the infected stomach. During the acute TH1 response, IFNγ-driven inflammation increases211. After 2 months, the inflammatory milieu, specifically IL-1β, suppresses parietal cell expression of SHH and subsequently reduces gastric acidity167. H. pylori induction of SHH expression within parietal cells is mediated by NFκB signalling208,210. Furthermore, SHH directly stimulates the expression of H+,K+-ATPase through PKC-mediated signalling212,213. Thus, even before parietal cell atrophy occurs, inflammation-driven signalling suppresses parietal cell acid secretion167.
By 6 months, the gastric mucosa exhibits SPEM characterized by the expression of HE8, CD44v9, trefoil factor 2 (TFF2; also known as spasmolytic polypeptide (SP)). SPEM was first reported as a gastric mucous cell lineage expressing TFF2 in mice infected with Helicobacter felis214. In a 1999 review of archival samples from 22 resected GACs, Schmidt et al. confirmed that SPEM was present in most cases, typically within the corpus of the human stomach bordering the GAC215. More recent studies suggest that SPEM precedes intestinal metaplasia development in the human stomach216. Gastric glands in the antrum and mucous neck cells of the corpus express TFF2172,216,217 (Fig. 2). SPEM-expressing glands have subsequently been reported to express other markers including the GAC stem cell marker CD44v9159,218, a target of HH signalling219,220.
Central to the debate regarding GAC cell origin is the simple question of whether GAC arises from a metaplastic cell, a non-metaplastic stem cell, or both? Since Helicobacter infection alone in mice does not lead to progression to GAC, studying the linear progression from metaplasia to GAC is difficult to test experimentally in vivo. Perhaps the best example of non-linear progression from an inflamed gastric mucosa to cancer is non-hereditary diffuse GAC, which typically does not progress through the intestinal metaplasia histologic intermediate and could serve as an example of accumulating stem cell mutations221. This pattern of progression to GAC would support a stem cell that accumulates mutations under the inflammatory milieu initiated by a H. pylori infection. As discussed by Huang et al, the inflammatory microenvironment induces epigenetic changes, particularly alterations in DNA methylation that contribute to the accumulation of clonal mutations leading to GAC123.
Role of Hedgehog in immune cell signalling and metaplasia
SHH is a morphogen that has a key role in homeostasis of the stomach in addition to being involved in the transition of the gastric epithelium to GAC222. Originally identified for its role in cell patterning during embryonic development, SHH ligand is expressed in a number of adult organs, including the stomach223,224. In 2000, Ramalho-Santos et al. reported that Shh-null mice exhibit gastric metaplasia at birth225, indicating that Hedgehog (HH) signalling has an essential role in gastric homeostasis as well as in foregut malignancies222,226. Subsequent studies of H. pylori-infected individuals demonstrated a reciprocal relationship between SHH and CDX2 expression in which the SHH ligand was elevated in diffuse GAC227, while, loss of SHH correlates with the aberrant overexpression of CDX2228,229. One of the main aetiologies associated with chronic H. pylori infection and gastritis is atrophy of the acid-secreting parietal cells and the resulting loss of the major epithelial reservoir of SHH ligand230.
Suppression of Shh in the inflamed gastric corpus is an early change, which in the mouse occurs within 2 months of the infection and prior to parietal cell atrophy167,231. Although loss of acid secretion is not synonymous with parietal cell atrophy, it does reduce Shh expression167. CDX2 associated with intestinal metaplasia and intestinal-type cancers inhibits SHH expression232. Thus, loss of the SHH ligand correlates with an intestinal-type GAC phenotype (Fig. 1). By contrast, diffuse-type GACs, which typically do not progress through an intestinal metaplasia phenotype, express the SHH ligand, its receptor PTCH and its downstream target CD44v, suggesting autocrine regulation of cell growth by Hedgehog (HH) signalling in this cancer subtype233,234. YAP has been shown to regulate GAC growth through the HH effector protein GLI1, independently of SMO activation, consistent with non-canonical HH signalling235. In addition, non-canonical HH signalling activates RHOA236, the proto-oncogene mutated in some diffuse GACs. Although acute H. pylori gastritis is associated with elevated SHH secretion208,210,213,237-239, the reduction in SHH expression during chronic gastritis is associated with disruption of normal cell differentiation in the adult stomach and reduced parietal cell numbers167,231,240,241. By contrast, in a mouse model of Helicobacter infection, El-Zaatari et al. showed that NFκB mediates re-expression of SHH, which correlates with development of SPEM242. Taken together, HH signalling either through ligand-receptor dependent (canonical) or ligand-receptor independent mechanisms (non-canonical) has been implicated in both histologic subtypes of GAC pathogenesis243.
Canonical HH signalling begins with de-repression of the G-protein-coupled receptor (GPCR) Smoothened (SMO) once the HH ligand binds its receptor Patched (PTCH), another GPCR223. Of the three glioma (GLI)-associated zinc finger transcription factors activated by the pathway, GLI1 is widely accepted as an indicator of HH signalling244. In the uninfected stomach, GLI1 is expressed in myofibroblasts245; however, during H. felis infection, Gli1+-myeloid cells infiltrate the mucosa246. Moreover, H. felis-infected Gli1-deficient mice (Gli1+/LacZ (Gli+/−) and Gli1LacZ/LacZ (Gli1−/−) do not develop SPEM at 6 months despite the presence of inflammation, indicating that Gli1+-myeloid cells are required for chronic inflammation to progress to SPEM246. Gene profiling of wild-type versus Gli1LacZ/LacZ mice revealed elevated levels of several myeloid differentiation factors (for example, Slfn2 and Slfn4) at 6 months, but not at 2 months246. Between 2 months and 6 months, parietal cell atrophy occurs and SPEM develops, coincident with a shift in the immune microenvironment characterized by an increase in DAMP-induced type 1 cytokines, IFNβ and IFNα118 (Fig. 2). Lineage tracing revealed that the SLFN4+ cells are a subset of granulocytic or polymorphonuclear (PMN)-MDSCs that originate in the bone marrow and acquire their T-cell suppressive phenotype in the stomach at the same time as the emergence of SPEM at 6 months118. The level of Slfn4 expression reflects the presence of constitutive HH signalling, since Slfn4 was absent in myeloid cells from the infected Gli1 mutant mice246. Using chromatin immunoprecipitation, we showed that GLI1 binds to the Slfn4 promoter246. Both human (SLFN12L) and mouse (Slfn4) Schlafen loci are strongly induced by type 1 interferons, especially IFNα118,247, which explains the peak expression of Slfn4 in this subset of Cd11b+Gr1 MDSCs118,125. More recently, RNA-sequencing analysis of Slfn4+ MDSCs sorted by flow cytometry from H. felis-infected stomachs demonstrated elevated expression of Nos2, Arg1, Tnfα and Il-1α transcripts compared to Slfn4+ cells in the bone marrow or spleen203. Moreover, these Slfn4+ MDSCs secrete MiR130b, a microRNA that suppresses tumour suppressor genes such as Trp53INP1, Cyld, Runx3 and Pten203. Lineage tracing of this subset of HH-dependent myeloid cells enabled the identification and temporal tracking of their polarization within the H. felis-infected stomach and demonstrate how DAMP and HH signalling can shape the immune microenvironment during the progression from chronic gastritis to SPEM203.
Transgenic expression of proinflammatory cytokines
Various mouse models have been used to determine the impact of specific proinflammatory cytokines on parietal cell atrophy. The evidence from human stomachs supports the observation that the pivotal transition of the epithelium towards transformation occurs with incomplete intestinal metaplasia (IIM) rather than with complete intestinal metaplasia (CIM) beginning at the incisura on the lesser curvature of the stomach with the metaplasia eventually extending bi-directionally into both the corpus and the antrum248,249. The dysplastic, hyperproliferative cells that overtake the mucosa presumably originate from the intestinal metaplasia in response to cells within the TIME that produce cytokines, growth factors and regulatory RNAs29,250. A major factor predisposing humans to neoplasia is their susceptibility to elevated levels of proinflammatory cytokines, especially IL-6, TNF and IL-1β25,26,251,252. In 2000, polymorphisms in the IL-1β promoter of patients with GAC were associated with increased circulating levels of this cytokine26. Subsequently, Tu et al. demonstrated that parietal cell overexpression of human IL-1β in mouse parietal cells using the H+, K+-Atpase-hIL-1β transgene induced corpus atrophy and dysplasia, confirming a pathogenic role for IL-1β. These researchers also reported that IL-1β is involved in the recruitment of Cd11b+Gr1+-MDSCs to the stomach 166. Macrophages recruited to the site of infection secrete the pro-inflammatory cytokine IL-1β253, which leads to inhibition of Shh expression167 and loss of normal epithelial cell differentiation237. IL-1β inhibits gastric acidity but IFNγ does not, suggesting that a TH1 response characterized by IFNγ maintains gastric acid production and might explain why the acute inflammatory response to H. pylori is linked to duodenal ulcer development167.
In H+,K+-Atpase-Ifnγ transgenic mice, TNF, IL-10, IL-6, IL-1β levels increased at 7 weeks remained elevated at 15 months, while IL-11 levels increased only at 15 months coincident with SPEM development254. The stomachs of H+,K+-Atpase-Ifnγ mice showed upregulation of phosphorylated STAT3 compared with the stomachs of control mice. Both IL-6 and IL-11 are important inducers of STAT3 phosphorylation and IL-11 has been showed to induce parietal cell apoptosis255. Transgenic expression of IFNγ from parietal cells or gastric organoids treated with IFNγ stimulates apoptosis, contributing to the progression of gastritis to atrophy and SPEM254,256. IFNγ−/− mice do not develop severe gastritis following H. pylori infection257, whereas infected IL-4−/− mice develop more severe gastritis compared to wild-type mice. Furthermore, a model of autoimmune gastritis in which gastritis is induced by CD4+ cells that are autoreactive against the H+,K+-ATPase antigen induces T-cell mediated destruction of parietal cells128,129,258 . In this autoimmune gastritis model, IL-17A is an important mediator of parietal cell death128. Taken together, either cytokine-induced apoptosis of parietal cells or T-cell-mediated autoimmune destruction of parietal cells is sufficient to induce inflammation and initiate gastric atrophy and SPEM 129.
Immune microenvironment and immune escape
A unique feature of H. pylori infection is that the bacteria itself orchestrate immune suppression through the early recruitment of Treg cells and myeloid cells to the stomach, in part as a result of the composition of its DNA, LPS and other bioactive molecules15,101. Treg cells, DCs and pro-inflammatory M1 macrophages, like neutrophils, are early inhabitants of the H. pylori-infected stomach17,211. Single-cell RNAsequencing of human GACs has been limited by the low number of samples, but has consistently shown active TNFα–NFκB and IL-6–JAK–STAT3 cytokine signalling259-261.
H. pylori and mechanisms of immune escape
The acute immune response to H. pylori consists of a mixture of neutrophils, pro-inflammatory M1 macrophages and CD4+ T lymphocytes that produce cytokines, which induce IFNγ, IL-17, IL-21 and IL-22211,262,263. IL-21 is required for the expansion and maintenance of the TH1 and TH17 responses 264. Ultimately, the balance between TH1, TH17 and immune suppressor cells such as Treg cells and tumour associated macrophages (TAMs) determine the switch from acute to chronic inflammation261, which is mediated in part by an increase in indoleamine 2,3-dioygenase-1 (IDO1) from DCs265. H. pylori eradication only moderately reduces GAC risk266-268 because inflammation persists in the gastric mucosa269. A 20-year follow-up study of pre-neoplastic lesions showed that IIM, when extended into the corpus, increases the risk of GAC compared with CIM, gastritis and antrum-restricted intestinal metaplasia132. However, the immune cell population was not analysed in that study. We performed a limited analysis of the immune microenvironment in tissue from a 13-year follow-up study of patients with GAC and found that increased SLFN5 expression in immune cells is associated with progression to GAC in patients with IIM270. Single-cell RNA sequencing of GAC as well as precursor lesions such as atrophic gastritis and metaplasias has revealed extensive heterogeneity within immune subpopulations259,261.
In individuals infected with H. pylori, the acute (neutrophilic) gastritis can progress to a chronic (monocytic) infiltrate271. H. pylori can initiate the acute immune response in a number of ways: through the activation of pathogen-activated molecular patterns (PAMPs) by bacterial antigens such as LPS and urease; through the injection of CagA into host cells with the subsequent induction of intracellular protein phosphorylation leading to changes in cell mobility; through the detection of soluble H. pylori antigens by resident APCs; and through the release of soluble factors from injured epithelial cells (for example, Shh from parietal cells)100,118,210,272-274. The transition from acute to chronic gastritis is orchestrated by a cascade of cytokines (including hepatocyte growth factor (HGF), IL-4 and macrophage colony-stimulating factor (M-CSF), which promotes polarization to resolve the inflammation and contribute to tissue remodelling99. The acute inflammatory response segues to a chronic infiltrate comprised of Treg cells (FOXP3+CD4+CD25hi), anti-inflammatory macrophages (M2 macrophages) and mononuclear cells99,275. Differences in the ratio of inflammatory cytokines initiated by H. pylori-specific molecules (for example, bacterial DNA and LPS) skew the cytokine profile to one that favours a muted immune response119. IDO1, a metabolite of tryptophan, is produced by activated DCs, and has a key role in creating an environment of immune tolerance and escape276,277. In addition to DCs, IFNγ induces IDO1 production by fibroblasts, macrophages, endothelial and stromal cells276. IDO1 induces differentiation of CD4+ T lymphocytes into Treg cells and promotes TAM polarization276. Thus accumulation of IFNγ also polarizes the cell population that will terminate the TH1 pro-inflammatory response.
GAC and mechanisms of immune escape
Once GAC is established, the tumours themselves sustain the immune suppressive environment, which enables them to escape immune surveillance123,206. Specifically, PD-L1, PD-1 and CTLA-4 are a subset of immune checkpoint receptors expressed on GAC and immune cells in the neoplastic microenvironment 278,279. These immune checkpoint receptors are targets for immunotherapy now approved for use in GAC280. Tumours can evade immune surveillance by expressing PD-L1 that interacts with the T-cell protein PD-1, subsequently inhibiting CD8+ cytotoxic T-lymphocyte proliferation, survival and effector function281-283. Inhibition of the PD-1–PD-L1 axis has produced effective response rates in various malignancies, including melanoma, renal and non-small cell lung cancer284-287. Despite the fact that PD-L1 is expressed in ~40% of GACs, the overall 5-year response rate to standard chemotherapy with or without checkpoint inhibitors is low at ~30%80,288-290. A plausible explanation for this low response rate is the overlap of immune suppressive mechanisms. Specifically, multiple immune suppressor populations, such as TAMs, tumour-associated neutrophils (TANs), MDSCs, regulatory B cells (Breg cells) and Treg cells, accumulate in GAC291 and facilitate tumour evasion by preventing cytotoxic T cell from attacking the tumour. Moreover, chemotherapy can also induce PD-L1 expression on GAC cells, reducing the effectiveness of immunotherapy when combined with standard chemotherapy regimens such as gemcitabine plus 5-fluorouracil160.
Helicobacter-mediated mouse models typically do not progress past the metaplastic stage (SPEM) 4–6 months after the infection is initiated depending on the genetic background 118, which has permitted extensive examination of how various suppressive immune populations interact with the epithelium during this process125. In humans, best estimates suggest a 6–10 year period for the initial immune response to H. pylori to polarize the inflammatory environment and alter epithelial cell fate132. Reports in humans indicate that the suppressive microenvironment exists before cancer develops80,123,260,261, a concept that has been confirmed in mouse models 118,125,170. Cytokines that are commonly detected prior to and after GAC develop include IL-1β, TNF, IL-6, TGFβ, type 1 interferons and WNTs. IL-6 and TNF secreted by TAMs induce PD-L1 expression on GACs through NFκB and STAT3 signalling292. TGFβ has a critical role in shaping the GAC immune microenvironment by inducing Breg cells, (CD19+CD24hiCD38hi), a subset of B lymphocytes that express IL-10, suppress TH1/TH17 proinflammatory mediators and promote the conversion of CD4+CD25− effector T cells to CD4+CD25+FoxP3+ Treg cells293,294. TGFβ1, a potent inducer of Foxp3 expression295, promotes Treg cell development296. Researchers have also shown that extracellular vesicles from tumours laden with HMGB1, a potent DAMP–TLR9 activator, induce neutrophils to suppress T-cell immunity by secreting CCL17297. Moreover, MDSCs and TAMs express regulatory RNAs (for example, microRNAs), which can be packaged and released from extracellular vesicles and modulate pre-cancerous gastric epithelial cells by targeting multiple signalling pathways203,298 (Fig. 3).
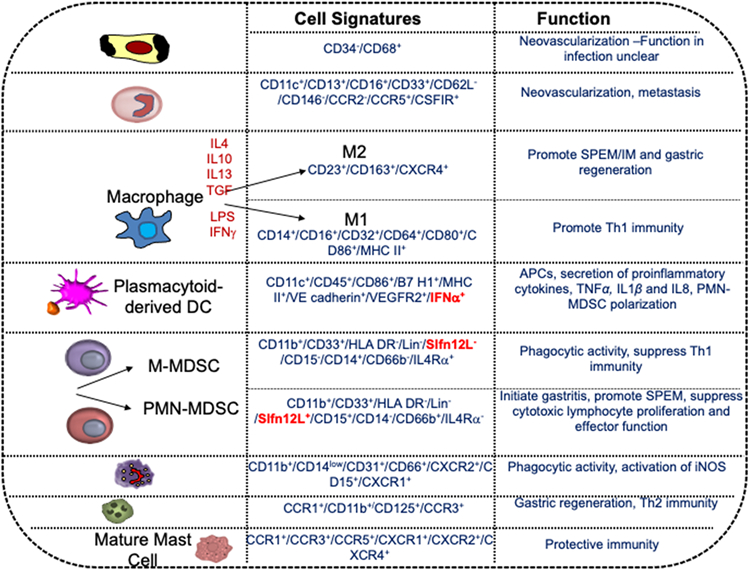
Figure 3 ∣. Differentiation and cell signatures of common immune cells recruited to the stomach in response to H. pylori infection.
Within the bone marrow, hematopoietic stem cells (HSCs) differentiate into common myeloid progenitor cells (CMPs) that then give rise to granulocyte-macrophage progenitors (GMPs). GMPs differentiate into myeloblasts, promyeloblasts, myelocytes and granulocytes in response to granulocyte colony-stimulating factor (G-CSF). By contrast, macrophage colony-stimulating factor (M-CSF) induce GMP differentiation towards monocytes, macrophages or dendritic cells via monocyte/macrophage and dendritic cell progenitors (MDPs)330. In response to specific environmental cues, hemangiocytes, TIE2-expressing monocytes (TEMs), monocytes, dendritic cells, MDSCs, neutrophils, eosinophils and immature mast cells all originate from the same HSC and circulate within the blood. Once recruited to the stomach in response to H. pylori infection or tissue injury, these immune cells express specific cell signatures that orchestrate neovascularization, create an immune suppressive environment and lead to development of metaplasia 177,203,210,217,323,331-337. APC, antigen- presenting cell; IM, intestinal metaplasia; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; M- MDSC, monocytic myeloid- derived suppressor cell; PMN, polymorphonuclear; SPEM, spasmolytic polypeptide- expressing metaplasia.
During the initial stages of an H. pylori infection, disruption of the epithelial apical-junction complex by the bacteria153 likely facilitates migration of DCs and their luminal and subepithelial interactions with CagA and other bacterial antigens299-301. H. pylori outer membrane proteins such as Omp18 and HpaA, intact bacteria and bacterial membrane preparations have all been shown to induce maturation of DCs in cell culture models302-304. Once activated, different subsets of DCs initiate either TH1, TH2 or Treg cell responses305. Mature DCs secrete IL-12 and IL-10 when stimulated by H. pylori306,307. Classic or conventional DCs (cDCs) are matured by TLR2-6 and TLR8 and induce TH1 effector cells, while DAMPs activate TLR7 and TLR9 which polarize pDCs115,308. In response to H. pylori infection, tolerogenic pDC populations that express CD11c, DC-SIGN and CDH1 expand, secrete type 1 interferons and prime Treg cells, which support immune evasion119,309. As with other immune cell types, single-cell profiling has revealed multiple Treg cell subsets, not all of which are immunosuppressive 310,311. In gastric tumours, Treg cell suppression requires the presence of the TCR-inducible co-stimulatory molecule (ICOS)312. Recruitment of immunosuppressive ICOS+ Treg cells requires activation of pDCs, which as previously stated is activated by either H. pylori DNA, unmethylated host DNA or specific molecules released from damaged epithelial cells101. Therefore, the subtype of DCs activated likely contributes to the persistence of suppressive Treg cells, which shifts the immune microenvironment from a pro-inflammatory environment to immune quiescence. In contrast to Treg cells, Breg cells are polarized by TGFβ, with the likely source of this cytokine from cancer-associated fibroblasts (CAFs), M2-like TAMs and the cancer itself313. In addition to monocytic-MDSCs and PMN-MDSCs314, a third category of MDSCs in GAC known as immature MDSCs (iMCs) has been reported315.The lectin-type oxidized LDL receptor 1 (LOX-1) is a unique marker for human polymorphonuclear (hPMN)-MDSCs316. hPMN-MDSCs upregulate genes associated with ER stress; for example, transcription factor genes encoded by spliced XBP1 and CHOP316. LOX+CD15+ hPMN-MDSCs are activated by endoplasmic reticulum stress and accumulate in the peripheral blood of patients with hepatocellular carcinoma317. In patients with gastric and pancreatic cancer, PMN-MDSCs accumulate in the spleen and can suppress T-cell proliferation, unlike PMN-MDSCs found in the peripheral blood, which do not display T-cell suppressor activity318. The conversion of mature neutrophils to PMN-MDSCs in the spleen can occur via two signalling pathways that induce immune suppressive activity in these cells. One of the pathways expands immature myeloid cells by inhibiting their terminal differentiation while the other pathway reprogrammes immature myeloid cells into suppressor cells 319,320.
As immunosuppressive cells, TAMs persist within the gastric immune microenvironment following H. pylori-mediated chronic inflammation because DCs and other immune cells express IDO1321,322. Single-cell RNA sequencing of the gastric TIME revealed that the macrophage population is quite heterogeneous and does not necessarily conform to categorizing them simply as M1 or M2261. Mouse models have demonstrated that M2-polarized macrophages emerge with the appearance of SPEM323 and are associated with IL-33-mediated induction of IL-13 signalling177,324, demonstrating that M2-like polarization in mice occurs during the transition of the mucosa to SPEM. Taken together, M2-polarized macrophages likely create a suppressive immune environment before GAC develops, in part because of arginase1 (ARG1) catalyses the hydrolysis of L-arginine, an essential substrate for T-cell proliferation325-327.
Conclusions
Bacterial and viral infections collectively have a key role in the initiation of GACs and early detection, eradication and prevention of these infections is therefore important. Most people carrying these infectious agents will not develop symptoms, and only a small but clinically significant percentage of those infected will develop complications. By delving deeper into the suppressive nature of the immune response and understanding host risk factors, we should eventually be able to predict which individuals are at risk of these complications and develop biomarkers to detect the epithelial changes early and/or prevent progression to GAC. EBV-positive and MSI-H GACs represent a small subset of GACs that generate a robust inflammatory response from neo-antigens. The generation of neo-antigens and the type of immune response that results makes EBV-positive and MSI-H GACs more susceptible to chemotherapies in combination with immune checkpoint inhibitors58,76,85. In comparison, most GACs are immunologically ‘cold’ and include cancers initiated by LOF mutations in CDH1 and other tight junction protein components (diffuse-type GACs) and intestinal-type GACs initiated by H. pylori infection57,58. The fact that H. pylori-induced GACs are so unresponsive is somewhat surprising, but possibly results from the organism initiating immune evasive strategies119,328. A central question is by what mechanism does the organism dampen the immune response, which benefits its persistence yet allows GAC to emerge. In short, both bone-marrow-derived and tissue resident cells become activated and over time differentiate into immune suppressive phenotypes under the instruction of the microenvironment before GAC develops. The type and extent of the metaplasia is a crucial inflection point. Although H. pylori DNA persists in intestinal metaplasia, what role it has, if any, is unclear123. One possible explanation is that H. pylori DNA contains immunoregulatory sequences that contribute to immune suppression by inducing Treg cells119. Another possibility is that the debris created by injured and dying cells (e.g., parietal cells) accumulates to a threshold level that eventually activates pDC DAMP signalling125,217. Owing to the multiple immune cell types involved, their phenotypic plasticity and metabolic signatures will need to be temporally studied using single-cell RNA-sequencing and other unbiased analyses of the multiple immune cell populations recruited to the stomach prior to GAC. Understanding the transitions between atrophy, SPEM, CIM and IIM in the human stomach will provide insight into the conditions under which the heterogeneous immune populations develop and become dominant, since once GAC has emerged, the dampened immune environment has already existed for several years261.
ACKNOWLEDGEMENTS
This work was supported by Public Service Grants R01 DK118563 (PI: Merchant), 5U19AI11649105 (PIs: Weiss and Wells, Project Leader 1: Zavros), R01DK083402-10 (PI: Zavros) and NCI P30CA023074, U54CA143924. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to sincerely thank Olivia Q. Merchant at the University of Michigan for her assistance with the figures.
Footnotes
Competing interests
- Helicobacter pylori (H. pylori) is a major risk factor for gastric adenocarcinoma (GAC).
- Early detection and eradication of H. pylori are critical for stopping progression from chronic inflammation to GAC.
- Immune cell populations driving the epithelial transition from normal to metaplasia to dysplasia are largely undefined.
- Since the host’s dampened immune environment can exist for several years prior to GAC, identifying critical inflection points and associated biomarkers might improve prevention of GAC.
References
- 1.De Palma M, Biziato D & Petrova TV Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17, 457–474, doi: 10.1038/nrc.2017.51 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52, 6735–6740 (1992). [PubMed] [Google Scholar]
- 3.Marques-Lespier JM, Gonzalez-Pons M & Cruz-Correa M Current Perspectives on Gastric Cancer. Gastroenterol Clin North Am 45, 413–428, doi: 10.1016/j.gtc.2016.04.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helicobacter & Cancer Collaborative, G. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49, 347–353, doi: 10.1136/gut.49.3.347 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uemura N et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345, 784–789, doi: 10.1056/NEJMoa001999 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Bray F et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424, doi: 10.3322/caac.21492 (2018). [DOI] [PubMed] [Google Scholar]
- 7. de Martel C, Georges D, Bray F, Ferlay J & Clifford GM Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 8, e180–e190, doi: 10.1016/S2214-109X(19)30488-7 (2020). Most recent data linking GAC to H. pylori infection.
- 8.Mayne ST & Navarro SA Diet, obesity and reflux in the etiology of adenocarcinomas of the esophagus and gastric cardia in humans. J Nutr 132, 3467S–3470S, doi: 10.1093/jn/132.11.3467S (2002). [DOI] [PubMed] [Google Scholar]
- 9.Olefson S & Moss SF Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 18, 23–32, doi: 10.1007/s10120-014-0425-4 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Laszkowska M et al. Racial and ethnic disparities in mortality from gastric and esophageal adenocarcinoma. Cancer Med 9, 5678–5686, doi: 10.1002/cam4.3063 (2020). Racial disparities in GAC linked to distal GAC
- 11.Gupta S et al. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 156, 59–62 e54, doi: 10.1053/j.gastro.2018.09.045 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Metz DC, Ellenberg S, Kaplan DE & Goldberg DS Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 158, 527–536 e527, doi: 10.1053/j.gastro.2019.10.019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XZ, Chen H, Castro FA, Hu JK & Brenner H Epstein-Barr virus infection and gastric cancer: a systematic review. Medicine (Baltimore) 94, e792, doi: 10.1097/MD.0000000000000792 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy G, Pfeiffer R, Camargo MC & Rabkin CS Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 137, 824–833, doi: 10.1053/j.gastro.2009.05.001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson K & Atherton JC The Spectrum of Helicobacter-Mediated Diseases. Annu Rev Pathol, doi: 10.1146/annurev-pathol-032520-024949 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Roszczenko-Jasinska P, Wojtys MI & Jagusztyn-Krynicka EK Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl Microbiol Biotechnol 104, 9891–9905, doi: 10.1007/s00253-020-10945-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amieva M & Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 150, 64–78, doi: 10.1053/j.gastro.2015.09.004 (2016). Comprehensive review of H. pylori mechanisms to suppress host immunity and favor its sustained colonization.
- 18.Jencks DS et al. Overview of Current Concepts in Gastric Intestinal Metaplasia and Gastric Cancer. Gastroenterol Hepatol (N Y) 14, 92–101 (2018). [PMC free article] [PubMed] [Google Scholar]
- 19.Noto JM et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 123, 479–492, doi: 10.1172/JCI64373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuipers EJ, Thijs JC & Festen HP The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther 9 Suppl 2, 59–69 (1995). [PubMed] [Google Scholar]
- 21.Yao X, Ajani JA & Song S Molecular biology and immunology of gastric cancer peritoneal metastasis. Transl Gastroenterol Hepatol 5, 57, doi: 10.21037/tgh.2020.02.08 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Oliveira IA & Corvelo TCO ABH and Lewis blood group systems and their relation to diagnosis and risk of Helicobacter pylori infection. Microb Pathog 152, 104653, doi: 10.1016/j.micpath.2020.104653 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Chakrani Z, Robinson K & Taye B Association Between ABO Blood Groups and Helicobacter pylori Infection: A Meta-Analysis. Sci Rep 8, 17604, doi: 10.1038/s41598-018-36006-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negovan A, Iancu M, Fulop E & Banescu C Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol 25, 4105–4124, doi: 10.3748/wjg.v25.i30.4105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahara T et al. Effect of IL-1beta and TNF-alpha polymorphisms on the prognosis and survival of gastric cancer patients. Clin Exp Med 11, 211–217, doi: 10.1007/s10238-010-0129-y (2011). [DOI] [PubMed] [Google Scholar]
- 26. El-Omar EM et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402, doi: 10.1038/35006081 (2000). Linking a pro-inflammatory cytokine to increased GAC risk.
- 27.Walter E & Scott M The life and work of Rudolf Virchow 1821-1902: "Cell theory, thrombosis and the sausage duel". J Intensive Care Soc 18, 234–235, doi: 10.1177/1751143716663967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkwill F & Mantovani A Inflammation and cancer: back to Virchow? Lancet 357, 539–545, doi: 10.1016/S0140-6736(00)04046-0 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Lochhead P & El-Omar EM Molecular Predictors of Gastric Neoplastic Progression. Cancer Cell 33, 9–11, doi: 10.1016/j.ccell.2017.12.006 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Correa P, Haenszel W, Cuello C, Tannenbaum S & Archer M A model for gastric cancer epidemiology. Lancet 2, 58–60, doi: 10.1016/s0140-6736(75)90498-5 (1975). [DOI] [PubMed] [Google Scholar]
- 31.Marshall BJ & Warren JR Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315, doi: 10.1016/s0140-6736(84)91816-6 (1984). [DOI] [PubMed] [Google Scholar]
- 32.Graham DY History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol 20, 5191–5204, doi: 10.3748/wjg.v20.i18.5191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaser MJ & Parsonnet J Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest 94, 4–8, doi: 10.1172/JCI117336 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209, doi: 10.1038/nature13480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Derks S et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol 31, 1011–1020, doi: 10.1016/j.annonc.2020.04.011 (2020). Characterization of tumor immune microenvironment in GACs.
- 36. Kim TS, da Silva E, Coit DG & Tang LH Intratumoral Immune Response to Gastric Cancer Varies by Molecular and Histologic Subtype. Am J Surg Pathol 43, 851–860, doi: 10.1097/PAS.0000000000001253 (2019). Immune microenvironment in TCGA GACs.
- 37.Dixon MF, Genta RM, Yardley JH & Correa P Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20, 1161–1181, doi: 10.1097/00000478-199610000-00001 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Parsonnet J et al. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst 83, 640–643, doi: 10.1093/jnci/83.9.640 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Araujo-Filho I et al. Prevalence of Helicobacter pylori infection in advanced gastric carcinoma. Arq Gastroenterol 43, 288–292, doi: 10.1590/s0004-28032006000400009 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Baek SM et al. Role of Serum Pepsinogen II and Helicobacter pylori Status in the Detection of Diffuse-Type Early Gastric Cancer in Young Individuals in South Korea. Gut Liver 14, 439–449, doi: 10.5009/gnl19091 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handa Y et al. Association of Helicobacter pylori and diffuse type gastric cancer. J Gastroenterol 31 Suppl 9, 29–32 (1996). [PubMed] [Google Scholar]
- 42.Li JH, Shi XZ, Lv S, Liu M & Xu GW Effect of Helicobacter pylori infection on p53 expression of gastric mucosa and adenocarcinoma with microsatellite instability. World J Gastroenterol 11, 4363–4366, doi: 10.3748/wjg.v11.i28.4363 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashiwagi K et al. Clinical usefulness of microsatellite instability for the prediction of gastric adenoma or adenocarcinoma in patients with chronic gastritis. Br J Cancer 82, 1814–1818, doi: 10.1054/bjoc.1999.1154 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dos Santos Pereira E et al. Helicobacter pylori cagE, cagG, and cagM can be a prognostic marker for intestinal and diffuse gastric cancer. Infect Genet Evol 84, 104477, doi: 10.1016/j.meegid.2020.104477 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Ferrasi AC et al. Helicobacter pylori and EBV in gastric carcinomas: methylation status and microsatellite instability. World J Gastroenterol 16, 312–319, doi: 10.3748/wjg.v16.i3.312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Post RS et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 52, 361–374, doi: 10.1136/jmedgenet-2015-103094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho SY et al. Sporadic Early-Onset Diffuse Gastric Cancers Have High Frequency of Somatic CDH1 Alterations, but Low Frequency of Somatic RHOA Mutations Compared With Late-Onset Cancers. Gastroenterology 153, 536–549 e526, doi: 10.1053/j.gastro.2017.05.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H et al. Gain-of-Function RHOA Mutations Promote Focal Adhesion Kinase Activation and Dependency in Diffuse Gastric Cancer. Cancer Discov 10, 288–305, doi: 10.1158/2159-8290.CD-19-0811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kakiuchi M et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 46, 583–587, doi: 10.1038/ng.2984 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Hayakawa Y, Wang TC & Bass AJ RhoA mutations identified in diffuse gastric cancer. Cancer Cell 26, 9–11, doi: 10.1016/j.ccr.2014.06.022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki A et al. Defined lifestyle and germline factors predispose Asian populations to gastric cancer. Sci Adv 6, eaav9778, doi: 10.1126/sciadv.aav9778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blair VR et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21, e386–e397, doi: 10.1016/S1470-2045(20)30219-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corso G et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet 55, 431–441, doi: 10.1136/jmedgenet-2018-105337 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Shin Y et al. A functional polymorphism (−347 G-->GA) in the E-cadherin gene is associated with colorectal cancer. Carcinogenesis 25, 2173–2176, doi: 10.1093/carcin/bgh223 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Carneiro F et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 203, 681–687, doi: 10.1002/path.1564 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Rocha JP et al. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology 73, 878–886, doi: 10.1111/his.13715 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Iyer P, Moslim M, Farma JM & Denlinger CS Diffuse gastric cancer: histologic, molecular, and genetic basis of disease. Transl Gastroenterol Hepatol 5, 52, doi: 10.21037/tgh.2020.01.02 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ajani JA et al. Gastric adenocarcinoma. Nat Rev Dis Primers 3, 17036, doi: 10.1038/nrdp.2017.36 (2017). Clinical review of GACs and treatment options.
- 59.Wang R et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut 69, 18–31, doi: 10.1136/gutjnl-2018-318070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos SN et al. O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget 7, 83570–83587, doi: 10.18632/oncotarget.13192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamura F et al. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric Cancer 19, 85–97, doi: 10.1007/s10120-014-0454-z (2016). [DOI] [PubMed] [Google Scholar]
- 62.Woo HJ, Joo HG, Song SW, Sohn YS & Chae C Immunohistochemical detection of galectin-3 in canine gastric carcinomas. J Comp Pathol 124, 216–218, doi: 10.1053/jcpa.2000.0442 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Kim SJ et al. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology 138, 1035–1045 e1031-1032, doi: 10.1053/j.gastro.2009.09.061 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Wang Z et al. Upregulation of T-cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Monocytes/Macrophages Associates with Gastric Cancer Progression. Immunol Invest 46, 134–148, doi: 10.1080/08820139.2016.1229790 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Chou FC, Chen HY, Kuo CC & Sytwu HK Role of Galectins in Tumors and in Clinical Immunotherapy. Int J Mol Sci 19, doi: 10.3390/ijms19020430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ajani JA et al. Galectin-3 expression is prognostic in diffuse type gastric adenocarcinoma, confers aggressive phenotype, and can be targeted by YAP1/BET inhibitors. Br J Cancer 118, 52–61, doi: 10.1038/bjc.2017.388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang GH, Lee S, Kim JS & Jung HY Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest 83, 635–641, doi: 10.1097/01.lab.0000067481.08984.3f (2003). [DOI] [PubMed] [Google Scholar]
- 68.Chan AO et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 52, 502–506, doi: 10.1136/gut.52.4.502 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura G et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 92, 569–573, doi: 10.1093/jnci/92.7.569 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Fornasarig M et al. Molecular and Pathological Features of Gastric Cancer in Lynch Syndrome and Familial Adenomatous Polyposis. Int J Mol Sci 19, doi: 10.3390/ijms19061682 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silva-Fernandes IJL et al. The intricate interplay between MSI and polymorphisms of DNA repair enzymes in gastric cancer H.pylori associated. Mutagenesis 32, 471–478, doi: 10.1093/mutage/gex013 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Fang DC et al. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol 8, 787–791, doi: 10.3748/wjg.v8.i5.787 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ebert MP et al. Increased beta-catenin mRNA levels and mutational alterations of the APC and beta-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis 23, 87–91, doi: 10.1093/carcin/23.1.87 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Park WS et al. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res 59, 4257–4260 (1999). [PubMed] [Google Scholar]
- 75.Budczies J et al. Integrated analysis of the immunological and genetic status in and across cancer types: impact of mutational signatures beyond tumor mutational burden. Oncoimmunology 7, e1526613, doi: 10.1080/2162402X.2018.1526613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shin SJ et al. Mismatch Repair Status of Gastric Cancer and Its Association with the Local and Systemic Immune Response. Oncologist 24, e835–e844, doi: 10.1634/theoncologist.2018-0273 (2019). MSI-H status, immune microenvironment and GAC response to treatment.
- 77.Cristescu R et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21, 449–456, doi: 10.1038/nm.3850 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Seo HM et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol 99, 143–147, doi: 10.1002/jso.21220 (2009). [DOI] [PubMed] [Google Scholar]
- 79.De Souza A Finding the hot spot: identifying immune sensitive gastrointestinal tumors. Transl Gastroenterol Hepatol 5, 48, doi: 10.21037/tgh.2019.12.11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwak Y, Seo AN, Lee HE & Lee HS Tumor immune response and immunotherapy in gastric cancer. J Pathol Transl Med 54, 20–33, doi: 10.4132/jptm.2019.10.08 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zepeda-Najar C, Palacios-Astudillo RX, Chavez-Hernandez JD, Lino-Silva LS & Salcedo-Hernandez RA Prognostic impact of microsatellite instability in gastric cancer. Contemp Oncol (Pozn) 25, 68–71, doi: 10.5114/wo.2021.104939 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanland LJ & Luftig MA The Role of EBV-Induced Hypermethylation in Gastric Cancer Tumorigenesis. Viruses 12, doi: 10.3390/v12111222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho J, Kang MS & Kim KM Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response. J Gastric Cancer 16, 1–7, doi: 10.5230/jgc.2016.16.1.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Enomoto S et al. Lack of association between CpG island methylator phenotype in human gastric cancers and methylation in their background non-cancerous gastric mucosae. Cancer Sci 98, 1853–1861, doi: 10.1111/j.1349-7006.2007.00625.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panda A et al. Immune Activation and Benefit From Avelumab in EBV-Positive Gastric Cancer. J Natl Cancer Inst 110, 316–320, doi: 10.1093/jnci/djx213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grabsch HI & Tan P Gastric cancer pathology and underlying molecular mechanisms. Dig Surg 30, 150–158, doi: 10.1159/000350876 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Hino R et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res 69, 2766–2774, doi: 10.1158/0008-5472.CAN-08-3070 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA & Sood AK Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15, 563–572, doi: 10.1038/nrc3978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luongo F et al. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers (Basel) 11, doi: 10.3390/cancers11081076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pyo JS, Kim NY & Kang DW Clinicopathological Significance of EBV-Infected Gastric Carcinomas: A Meta-Analysis. Medicina (Kaunas) 56, doi: 10.3390/medicina56070345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Souza CRT et al. Association between Helicobacter pylori, Epstein-Barr virus, human papillomavirus and gastric adenocarcinomas. World J Gastroenterol 24, 4928–4938, doi: 10.3748/wjg.v24.i43.4928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Odenbreit S et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287, 1497–1500, doi: 10.1126/science.287.5457.1497 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Fujii Y et al. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci U S A 109, 20584–20589, doi: 10.1073/pnas.1208651109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fischer AS & Sigal M The Role of Wnt and R-spondin in the Stomach During Health and Disease. Biomedicines 7, doi: 10.3390/biomedicines7020044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiurillo MA Role of the Wnt/beta-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med 5, 84–102, doi: 10.5493/wjem.v5.i2.84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen HY, Hu Y, Lu NH & Zhu Y Caudal type homeoboxes as a driving force in Helicobacter pylori infection-induced gastric intestinal metaplasia. Gut Microbes 12, 1–12, doi: 10.1080/19490976.2020.1809331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rau TT et al. Methylation-dependent activation of CDX1 through NF-kappaB: a link from inflammation to intestinal metaplasia in the human stomach. Am J Pathol 181, 487–498, doi: 10.1016/j.ajpath.2012.04.028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tegtmeyer N, Neddermann M, Asche CI & Backert S Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA. Mol Microbiol 105, 358–372, doi: 10.1111/mmi.13707 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Ito N, Tsujimoto H, Ueno H, Xie Q & Shinomiya N Helicobacter pylori-Mediated Immunity and Signaling Transduction in Gastric Cancer. J Clin Med 9, doi: 10.3390/jcm9113699 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varga MG et al. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene 35, 6262–6269, doi: 10.1038/onc.2016.158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varga MG & Peek RM DNA Transfer and Toll-like Receptor Modulation by Helicobacter pylori. Curr Top Microbiol Immunol 400, 169–193, doi: 10.1007/978-3-319-50520-6_8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melit LE, Marginean CO, Marginean CD & Marginean MO The Relationship between Toll-like Receptors and Helicobacter pylori-Related Gastropathies: Still a Controversial Topic. J Immunol Res 2019, 8197048, doi: 10.1155/2019/8197048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Otani K et al. Toll-like receptor 9 signaling has anti-inflammatory effects on the early phase of Helicobacter pylori-induced gastritis. Biochem Biophys Res Commun 426, 342–349, doi: 10.1016/j.bbrc.2012.08.080 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Lin AS et al. Bacterial Energetic Requirements for Helicobacter pylori Cag Type IV Secretion System-Dependent Alterations in Gastric Epithelial Cells. Infect Immun 88, doi: 10.1128/IAI.00790-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cui L, Wang X & Zhang D TLRs as a Promise Target Along With Immune Checkpoint Against Gastric Cancer. Front Cell Dev Biol 8, 611444, doi: 10.3389/fcell.2020.611444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.West AC & Jenkins BJ Investigating the Role of Toll-Like Receptors in Mouse Models of Gastric Cancer. Methods Mol Biol 1390, 427–449, doi: 10.1007/978-1-4939-3335-8_25 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Kasurinen A et al. Evaluation of toll-like receptors as prognostic biomarkers in gastric cancer: high tissue TLR5 predicts a better outcome. Sci Rep 9, 12553, doi: 10.1038/s41598-019-49111-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crump KE & Sahingur SE Microbial Nucleic Acid Sensing in Oral and Systemic Diseases. J Dent Res 95, 17–25, doi: 10.1177/0022034515609062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Castano-Rodriguez N, Kaakoush NO & Mitchell HM Pattern-recognition receptors and gastric cancer. Front Immunol 5, 336, doi: 10.3389/fimmu.2014.00336 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Akira S, Uematsu S & Takeuchi O Pathogen recognition and innate immunity. Cell 124, 783–801, doi: 10.1016/j.cell.2006.02.015 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Rad R et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology 136, 2247–2257, doi: 10.1053/j.gastro.2009.02.066 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Cui S et al. The C-terminal regulatory domain is the RNA 5'-triphosphate sensor of RIG-I. Mol Cell 29, 169–179, doi: 10.1016/j.molcel.2007.10.032 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Schlee M, Barchet W, Hornung V & Hartmann G Beyond double-stranded RNA-type I IFN induction by 3pRNA and other viral nucleic acids. Curr Top Microbiol Immunol 316, 207–230, doi: 10.1007/978-3-540-71329-6_11 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmausser B et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol 136, 521–526, doi: 10.1111/j.1365-2249.2004.02464.x (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koucky V, Boucek J & Fialova A Immunology of Plasmacytoid Dendritic Cells in Solid Tumors: A Brief Review. Cancers (Basel) 11, doi: 10.3390/cancers11040470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoshino K et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature 440, 949–953, doi: 10.1038/nature04641 (2006). [DOI] [PubMed] [Google Scholar]
- 117. Luther J et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 60, 1479–1486, doi: 10.1136/gut.2010.220087 (2011). Immunosuppressive property of H. pylori DNA.
- 118. Ding L et al. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest 126, 2867–2880, doi: 10.1172/JCI82529 (2016). Lineage tracing of myeloid cell from bone marrow to stomach where it polarizes to MDSC by DAMP signaling.
- 119.Kao JY et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138, 1046–1054, doi: 10.1053/j.gastro.2009.11.043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Higgins PD et al. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis 17, 1398–1408, doi: 10.1002/ibd.21489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnold IC et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 121, 3088–3093, doi: 10.1172/JCI45041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kyburz A & Muller A Helicobacter pylori and Extragastric Diseases. Curr Top Microbiol Immunol 400, 325–347, doi: 10.1007/978-3-319-50520-6_14 (2017). [DOI] [PubMed] [Google Scholar]
- 123. Huang KK et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 33, 137–150 e135, doi: 10.1016/j.ccell.2017.11.018 (2018). sc-RNA-Seq of intestinal metaplasia in the human stomach.
- 124.Krysko DV et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12, 860–875, doi: 10.1038/nrc3380 (2012). [DOI] [PubMed] [Google Scholar]
- 125.Merchant JL & Ding L Hedgehog Signaling Links Chronic Inflammation to Gastric Cancer Precursor Lesions. Cell Mol Gastroenterol Hepatol 3, 201–210, doi: 10.1016/j.jcmgh.2017.01.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahmed A & Tait SWG Targeting immunogenic cell death in cancer. Mol Oncol 14, 2994–3006, doi: 10.1002/1878-0261.12851 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sarhan M, Land WG, Tonnus W, Hugo CP & Linkermann A Origin and Consequences of Necroinflammation. Physiol Rev 98, 727–780, doi: 10.1152/physrev.00041.2016 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Bockerstett KA et al. Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis. Cell Mol Gastroenterol Hepatol 5, 678–690 e671, doi: 10.1016/j.jcmgh.2017.12.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Merchant JL Parietal Cell Death by Cytokines. Cell Mol Gastroenterol Hepatol 5, 636–637, doi: 10.1016/j.jcmgh.2018.01.019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huh WJ et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142, 21–24 e27, doi: 10.1053/j.gastro.2011.09.050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jang GY et al. Interactions between tumor-derived proteins and Toll-like receptors. Exp Mol Med, doi: 10.1038/s12276-020-00540-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Piazuelo MB et al. The Colombian chemoprevention trial. Twenty-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology, doi: 10.1053/j.gastro.2020.11.017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lahner E, Conti L, Annibale B & Corleto VD Current Perspectives in Atrophic Gastritis. Curr Gastroenterol Rep 22, 38, doi: 10.1007/s11894-020-00775-1 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Takahashi-Kanemitsu A, Knight CT & Hatakeyama M Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol Immunol 17, 50–63, doi: 10.1038/s41423-019-0339-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li J & Perez Perez GI Is There a Role for the Non-Helicobacter pylori Bacteria in the Risk of Developing Gastric Cancer? Int J Mol Sci 19, doi: 10.3390/ijms19051353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barra WF et al. Gastric Cancer Microbiome. Pathobiology, 1–14, doi: 10.1159/000512833 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Thorell K et al. In Vivo Analysis of the Viable Microbiota and Helicobacter pylori Transcriptome in Gastric Infection and Early Stages of Carcinogenesis. Infect Immun 85, doi: 10.1128/IAI.00031-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caguazango JC & Pazos AJ Microbiota according to gastric topography in patients with low or high risk of gastric cancer in Narino, Colombia. Biomedica 39, 157–171, doi: 10.7705/biomedica.v39i4.4520 (2019). [DOI] [PubMed] [Google Scholar]
- 139. Schulz C, Schutte K, Mayerle J & Malfertheiner P The role of the gastric bacterial microbiome in gastric cancer: Helicobacter pylori and beyond. Therap Adv Gastroenterol 12, 1756284819894062, doi: 10.1177/1756284819894062 (2019). Role of the gastric microbiome.
- 140.Ofori-Darko E et al. An OmpA-like protein from Acinetobacter spp. stimulates gastrin and interleukin-8 promoters. Infect Immun 68, 3657–3666, doi: 10.1128/iai.68.6.3657-3666.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zavros Y, Rieder G, Ferguson A & Merchant JL Gastritis and hypergastrinemia due to Acinetobacter lwoffii in mice. Infect Immun 70, 2630–2639, doi: 10.1128/iai.70.5.2630-2639.2002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dong Z et al. Detection of Microbial 16S rRNA Gene in the Serum of Patients With Gastric Cancer. Front Oncol 9, 608, doi: 10.3389/fonc.2019.00608 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsugawa H et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12, 764–777, doi: 10.1016/j.chom.2012.10.014 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Ishimoto T et al. Macrophage-derived reactive oxygen species suppress miR-328 targeting CD44 in cancer cells and promote redox adaptation. Carcinogenesis 35, 1003–1011, doi: 10.1093/carcin/bgt402 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Fu L et al. Gastric Cancer Stem Cells: Current Insights into the Immune Microenvironment and Therapeutic Targets. Biomedicines 8, doi: 10.3390/biomedicines8010007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomari MM et al. CD44 polymorphisms and its variants, as an inconsistent marker in cancer investigations. Mutat Res Rev Mutat Res 787, 108374, doi: 10.1016/j.mrrev.2021.108374 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Zavros Y Initiation and Maintenance of Gastric Cancer: A Focus on CD44 Variant Isoforms and Cancer Stem Cells. Cell Mol Gastroenterol Hepatol 4, 55–63, doi: 10.1016/j.jcmgh.2017.03.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Higashi H et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295, 683–686, doi: 10.1126/science.1067147 (2002). [DOI] [PubMed] [Google Scholar]
- 149.Selbach M, Moese S, Backert S, Jungblut PR & Meyer TF The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics 4, 2961–2968, doi: 10.1002/pmic.200400915 (2004). [DOI] [PubMed] [Google Scholar]
- 150.Oliveira MJ et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J Biol Chem 281, 34888–34896, doi: 10.1074/jbc.M607067200 (2006). [DOI] [PubMed] [Google Scholar]
- 151.Oliveira MJ et al. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J Infect Dis 200, 745–755, doi: 10.1086/604727 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Churin Y et al. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol 161, 249–255, doi: 10.1083/jcb.200208039 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amieva MR et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300, 1430–1434, doi: 10.1126/science.1081919 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Franco AT et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A 102, 10646–10651, doi: 10.1073/pnas.0504927102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saadat I et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333, doi: 10.1038/nature05765 (2007). [DOI] [PubMed] [Google Scholar]
- 156.Suzuki M et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe 5, 23–34, doi: 10.1016/j.chom.2008.11.010 (2009). [DOI] [PubMed] [Google Scholar]
- 157. Bertaux-Skeirik N et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog 11, e1004663, doi: 10.1371/journal.ppat.1004663 (2015). Role of H. pylori in modulating CD44+ cells (a putative gastric stem cell marker).
- 158.Ishimoto T et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19, 387–400, doi: 10.1016/j.ccr.2011.01.038 (2011). [DOI] [PubMed] [Google Scholar]
- 159.Holokai L et al. Increased Programmed Death-Ligand 1 is an Early Epithelial Cell Response to Helicobacter pylori Infection. PLoS Pathog 15, e1007468, doi: 10.1371/journal.ppat.1007468 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim W et al. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology, doi: 10.1053/j.gastro.2020.10.036 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harada K et al. Recent advances in the management of gastric adenocarcinoma patients. F1000Res 7, doi: 10.12688/f1000research.15133.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cho CJ et al. Poor prognosis in Epstein-Barr virus-negative gastric cancer with lymphoid stroma is associated with immune phenotype. Gastric Cancer 21, 925–935, doi: 10.1007/s10120-018-0820-3 (2018). [DOI] [PubMed] [Google Scholar]
- 163.Gullo I et al. New insights into the inflamed tumor immune microenvironment of gastric cancer with lymphoid stroma: from morphology and digital analysis to gene expression. Gastric Cancer 22, 77–90, doi: 10.1007/s10120-018-0836-8 (2019). [DOI] [PubMed] [Google Scholar]
- 164.Goldenring JR & Mills JC Cellular Plasticity, Reprogramming, and Regeneration: Metaplasia in the Stomach and Beyond. Gastroenterology, doi: 10.1053/j.gastro.2021.10.036 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spechler SJ et al. A Summary of the 2016 James W. Freston Conference of the American Gastroenterological Association: Intestinal Metaplasia in the Esophagus and Stomach: Origins, Differences, Similarities and Significance. Gastroenterology 153, e6–e13, doi: 10.1053/j.gastro.2017.05.050 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tu S et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14, 408–419, doi: 10.1016/j.ccr.2008.10.011 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Waghray M et al. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138, 562–572, 572 e561-562, doi: 10.1053/j.gastro.2009.10.043 (2010). IL-1β inhibits Shh and parietal cell acid secretion.
- 168.Ding L, Sontz EA, Saqui-Salces M, Merchant JL Interleukin-1b Suppresses Gastrin via Primary Cilia and Induces Antral Hyperplasia. Cell Mol Gastroenterol Hepatol 11, 1251–1266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burclaff J, Osaki LH, Liu D, Goldenring JR & Mills JC Targeted Apoptosis of Parietal Cells Is Insufficient to Induce Metaplasia in Stomach. Gastroenterology 152, 762–766 e767, doi: 10.1053/j.gastro.2016.12.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ding L, El Zaatari M & Merchant JL Recapitulating Human Gastric Cancer Pathogenesis: Experimental Models of Gastric Cancer. Adv Exp Med Biol 908, 441–478, doi: 10.1007/978-3-319-41388-4_22 (2016). Overview of rodent models of GAC.
- 171.Weis VG et al. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 312, G67–G76, doi: 10.1152/ajpgi.00326.2016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weis VG et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 62, 1270–1279, doi: 10.1136/gutjnl-2012-302401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nam KT et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139, 2028–2037 e2029, doi: 10.1053/j.gastro.2010.09.005 (2010). SPEM in the human stomach.
- 174.Petersen CP, Mills JC & Goldenring JR Murine Models of Gastric Corpus Preneoplasia. Cell Mol Gastroenterol Hepatol 3, 11–26, doi: 10.1016/j.jcmgh.2016.11.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Manning EH, Lapierre LA, Mills JC & Goldenring JR Tamoxifen Acts as a Parietal Cell Protonophore. Cell Mol Gastroenterol Hepatol 10, 655–657 e651, doi: 10.1016/j.jcmgh.2020.04.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saenz JB, Burclaff J & Mills JC Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss. Methods Mol Biol 1422, 329–339, doi: 10.1007/978-1-4939-3603-8_28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Petersen CP et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut 67, 805–817, doi: 10.1136/gutjnl-2016-312779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Noto JM, Romero-Gallo J, Piazuelo MB & Peek RM The Mongolian Gerbil: A Robust Model of Helicobacter pylori-Induced Gastric Inflammation and Cancer. Methods Mol Biol 1422, 263–280, doi: 10.1007/978-1-4939-3603-8_24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsukamoto T, Mizoshita T & Tatematsu M Animal models of stomach carcinogenesis. Toxicol Pathol 35, 636–648, doi: 10.1080/01926230701420632 (2007). [DOI] [PubMed] [Google Scholar]
- 180.Kang W, Rathinavelu S, Samuelson LC & Merchant JL Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest 85, 702–715, doi: 10.1038/labinvest.3700260 (2005). [DOI] [PubMed] [Google Scholar]
- 181.Zavros Y et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24, 2354–2366, doi: 10.1038/sj.onc.1208407 (2005). [DOI] [PubMed] [Google Scholar]
- 182.Friis-Hansen L, Rieneck K, Nilsson HO, Wadstrom T & Rehfeld JF Gastric inflammation, metaplasia, and tumor development in gastrin-deficient mice. Gastroenterology 131, 246–258, doi: 10.1053/j.gastro.2006.04.031 (2006). [DOI] [PubMed] [Google Scholar]
- 183.Judd LM et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126, 196–207, doi: 10.1053/j.gastro.2003.10.066 (2004). [DOI] [PubMed] [Google Scholar]
- 184.Tebbutt NC et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med 8, 1089–1097, doi: 10.1038/nm763 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Znalesniak EB, Salm F & Hoffmann W Molecular Alterations in the Stomach of Tff1-Deficient Mice: Early Steps in Antral Carcinogenesis. Int J Mol Sci 21, doi: 10.3390/ijms21020644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Soutto M et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat Commun 10, 3039, doi: 10.1038/s41467-019-11011-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kamada T et al. The association between antral G and D cells and mucosal inflammation, atrophy, and Helicobacter pylori infection in subjects with normal mucosa, chronic gastritis, and duodenal ulcer. Am J Gastroenterol 93, 748–752, doi: 10.1111/j.1572-0241.1998.218_a.x (1998). [DOI] [PubMed] [Google Scholar]
- 188.Liu Y, Vosmaer GD, Tytgat GN, Xiao SD & Ten Kate FJ Gastrin (G) cells and somatostatin (D) cells in patients with dyspeptic symptoms: Helicobacter pylori associated and non-associated gastritis. J Clin Pathol 58, 927–931, doi: 10.1136/jcp.2003.010710 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Smith JP, Nadella S & Osborne N Gastrin and Gastric Cancer. Cell Mol Gastroenterol Hepatol 4, 75–83, doi: 10.1016/j.jcmgh.2017.03.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chuang CH et al. Hypergastrinemia after Helicobacter pylori infection is associated with bacterial load and related inflammation of the oxyntic corpus mucosa. J Gastroenterol Hepatol 19, 988–993, doi: 10.1111/j.1440-1746.2004.03416.x (2004). [DOI] [PubMed] [Google Scholar]
- 191.Haruma K et al. Old and New Gut Hormone, Gastrin and Acid Suppressive Therapy. Digestion 97, 340–344, doi: 10.1159/000485734 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Agreus L et al. Rationale in diagnosis and screening of atrophic gastritis with stomach-specific plasma biomarkers. Scand J Gastroenterol 47, 136–147, doi: 10.3109/00365521.2011.645501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kotelevets SM & Chekh SA Screening, Monitoring, and Treatment of Precancerous Atrophic Gastritis in the Prospective Study for Seven Years. Asian Pac J Cancer Prev 21, 331–336, doi: 10.31557/APJCP.2020.21.2.331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.de Vries AC & Kuipers EJ Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter 12 Suppl 2, 22–31, doi: 10.1111/j.1523-5378.2007.00562.x (2007). [DOI] [PubMed] [Google Scholar]
- 195.Howlett M et al. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology 129, 1005–1018, doi: 10.1053/j.gastro.2005.06.068 (2005). [DOI] [PubMed] [Google Scholar]
- 196.Tomita H et al. Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology 140, 879–891, doi: 10.1053/j.gastro.2010.11.037 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saqui-Salces M et al. Inflammation and Gli2 suppress gastrin gene expression in a murine model of antral hyperplasia. PLoS One 7, e48039, doi: 10.1371/journal.pone.0048039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Khan ZE, Wang TC, Cui G, Chi AL & Dimaline R Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology 125, 510–521, doi: 10.1016/s0016-5085(03)00908-9 (2003). [DOI] [PubMed] [Google Scholar]
- 199.Sakitani K et al. CXCR4-expressing Mist1(+) progenitors in the gastric antrum contribute to gastric cancer development. Oncotarget 8, 111012–111025, doi: 10.18632/oncotarget.22451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ji L et al. Blockade of beta-Catenin-Induced CCL28 Suppresses Gastric Cancer Progression via Inhibition of Treg Cell Infiltration. Cancer Res 80, 2004–2016, doi: 10.1158/0008-5472.CAN-19-3074 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Li X et al. WNT/beta-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front Immunol 10, 2293, doi: 10.3389/fimmu.2019.02293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tomita H et al. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res 67, 4079–4087, doi: 10.1158/0008-5472.CAN-06-4025 (2007). [DOI] [PubMed] [Google Scholar]
- 203.Ding L et al. MiR130b from Schlafen4(+) MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut 69, 1750–1761, doi: 10.1136/gutjnl-2019-318817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kumar Y et al. Ubiquitin Ligase, Fbw7, Targets CDX2 for Degradation via Two Phosphodegron Motifs in a GSK3beta-Dependent Manner. Mol Cancer Res 14, 1097–1109, doi: 10.1158/1541-7786.MCR-16-0138 (2016). [DOI] [PubMed] [Google Scholar]
- 205.Jiang Y et al. Fbxw7 haploinsufficiency loses its protection against DNA damage and accelerates MNU-induced gastric carcinogenesis. Oncotarget 8, 33444–33456, doi: 10.18632/oncotarget.16800 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu Y et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell 33, 721–735 e728, doi: 10.1016/j.ccell.2018.03.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim HJ et al. Methylation of the CDX2 promoter in Helicobacter pylori-infected gastric mucosa increases with age and its rapid demethylation in gastric tumors is associated with upregulated gene expression. Carcinogenesis 41, 1341–1352, doi: 10.1093/carcin/bgaa083 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Schumacher MA et al. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol 593, 1809–1827, doi: 10.1113/jphysiol.2014.283028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Seidlitz T et al. Mouse Models of Human Gastric Cancer Subtypes With Stomach-Specific CreERT2-Mediated Pathway Alterations. Gastroenterology 157, 1599–1614 e1592, doi: 10.1053/j.gastro.2019.09.026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Schumacher MA et al. Gastric Sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology 142, 1150–1159 e1156, doi: 10.1053/j.gastro.2012.01.029 (2012). SHH as a chemoattractant for myeloid cells induced by H. pylori infection.
- 211.Bamford KB et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114, 482–492, doi: 10.1016/s0016-5085(98)70531-1 (1998). [DOI] [PubMed] [Google Scholar]
- 212.Stepan V et al. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem 280, 15700–15708, doi: 10.1074/jbc.M413037200 (2005). [DOI] [PubMed] [Google Scholar]
- 213.El-Zaatari M et al. Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion. Gastroenterology 139, 2061–2071 e2062, doi: 10.1053/j.gastro.2010.08.047 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wang TC et al. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114, 675–689, doi: 10.1016/s0016-5085(98)70581-5 (1998). Initial report of SPEM in mice.
- 215.Schmidt PH et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79, 639–646 (1999). [PMC free article] [PubMed] [Google Scholar]
- 216.Can N et al. Mucins, trefoil factors and pancreatic duodenal homeobox 1 expression in spasmolytic polypeptide expressing metaplasia and intestinal metaplasia adjacent to gastric carcinomas. Arch Med Sci 16, 1402–1410, doi: 10.5114/aoms.2013.36923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meyer AR & Goldenring JR Injury, repair, inflammation and metaplasia in the stomach. J Physiol 596, 3861–3867, doi: 10.1113/JP275512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Engevik AC et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell Mol Gastroenterol Hepatol 2, 605–624, doi: 10.1016/j.jcmgh.2016.05.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yoon C et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin Cancer Res 20, 3974–3988, doi: 10.1158/1078-0432.CCR-14-0011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 220.Song Z et al. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One 6, e17687, doi: 10.1371/journal.pone.0017687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Solcia E et al. Intestinal and diffuse gastric cancers arise in a different background of Helicobacter pylori gastritis through different gene involvement. Am J Surg Pathol 20 Suppl 1, S8–22, doi: 10.1097/00000478-199600001-00003 (1996). [DOI] [PubMed] [Google Scholar]
- 222. Berman DM et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425, 846–851, doi: 10.1038/nature01972 (2003). HH signaling important in GAC and other foregut tumors.
- 223.Johnson RL, Riddle RD, Laufer E & Tabin C Sonic hedgehog: a key mediator of anterior-posterior patterning of the limb and dorso-ventral patterning of axial embryonic structures. Biochem Soc Trans 22, 569–574, doi: 10.1042/bst0220569 (1994). [DOI] [PubMed] [Google Scholar]
- 224.Nusslein-Volhard C & Wieschaus E Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801, doi: 10.1038/287795a0 (1980). [DOI] [PubMed] [Google Scholar]
- 225.Ramalho-Santos M, Melton DA & McMahon AP Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127, 2763–2772 (2000). [DOI] [PubMed] [Google Scholar]
- 226.Watkins DN & Peacock CD Hedgehog signalling in foregut malignancy. Biochem Pharmacol 68, 1055–1060, doi: 10.1016/j.bcp.2004.04.025 (2004). [DOI] [PubMed] [Google Scholar]
- 227.Shiotani A et al. Sonic hedgehog and CDX2 expression in the stomach. J Gastroenterol Hepatol 23 Suppl 2, S161–166, doi: 10.1111/j.1440-1746.2008.05406.x (2008). [DOI] [PubMed] [Google Scholar]
- 228.Shiotani A et al. Helicobacter pylori-induced atrophic gastritis progressing to gastric cancer exhibits sonic hedgehog loss and aberrant CDX2 expression. Aliment Pharmacol Ther 24 Suppl 4, 71–80, doi: 10.1111/j.1365-2036.2006.00028.x (2006). [DOI] [PubMed] [Google Scholar]
- 229.Shiotani A et al. Re-expression of sonic hedgehog and reduction of CDX2 after Helicobacter pylori eradication prior to incomplete intestinal metaplasia. Int J Cancer 121, 1182–1189, doi: 10.1002/ijc.22835 (2007). [DOI] [PubMed] [Google Scholar]
- 230.Venerito M et al. Oxyntic gastric atrophy in Helicobacter pylori gastritis is distinct from autoimmune gastritis. J Clin Pathol 69, 677–685, doi: 10.1136/jclinpath-2015-203405 (2016). [DOI] [PubMed] [Google Scholar]
- 231.Shiotani A et al. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol 100, 581–587, doi: 10.1111/j.1572-0241.2005.41001.x (2005). [DOI] [PubMed] [Google Scholar]
- 232. Mutoh H, Hayakawa H, Sashikawa M, Sakamoto H & Sugano K Direct repression of Sonic Hedgehog expression in the stomach by Cdx2 leads to intestinal transformation. Biochem J 427, 423–434, doi: 10.1042/BJ20091177 (2010). SHH repression and CDX2 expression in intestinal type GAC.
- 233.Konstantinou D, Bertaux-Skeirik N & Zavros Y Hedgehog signaling in the stomach. Curr Opin Pharmacol 31, 76–82, doi: 10.1016/j.coph.2016.09.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Fukaya M et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology 131, 14–29, doi: 10.1053/j.gastro.2006.05.008 (2006). SHH increased in diffuse GAC.
- 235.Han T et al. Yes-Associated Protein Contributes to Cell Proliferation and Migration of Gastric Cancer via Activation of Gli1. Onco Targets Ther 13, 10867–10876, doi: 10.2147/OTT.S266449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Polizio AH et al. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286, 19589–19596, doi: 10.1074/jbc.M110.197111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xiao C et al. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138, 550–561, 561 e551-558, doi: 10.1053/j.gastro.2009.11.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zavros Y, Orr MA, Xiao C & Malinowska DH Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol 295, G99–G111, doi: 10.1152/ajpgi.00389.2007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zavros Y et al. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282, 33265–33274, doi: 10.1074/jbc.M707090200 (2007). [DOI] [PubMed] [Google Scholar]
- 240.van den Brink GR et al. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 51, 628–633, doi: 10.1136/gut.51.5.628 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Suzuki H et al. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol 206, 186–197, doi: 10.1002/path.1763 (2005). [DOI] [PubMed] [Google Scholar]
- 242.El-Zaatari M et al. De-regulation of the sonic hedgehog pathway in the InsGas mouse model of gastric carcinogenesis. Br J Cancer 96, 1855–1861, doi: 10.1038/sj.bjc.6603782 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu Y, Song S, Wang Z & Ajani JA The role of hedgehog signaling in gastric cancer: molecular mechanisms, clinical potential, and perspective. Cell Commun Signal 17, 157, doi: 10.1186/s12964-019-0479-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Didiasova M, Schaefer L & Wygrecka M Targeting GLI Transcription Factors in Cancer. Molecules 23, doi: 10.3390/molecules23051003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kolterud A et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology 137, 618–628, doi: 10.1053/j.gastro.2009.05.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.El-Zaatari M et al. Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets. PLoS One 8, e58935, doi: 10.1371/journal.pone.0058935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mavrommatis E, Fish EN & Platanias LC The schlafen family of proteins and their regulation by interferons. J Interferon Cytokine Res 33, 206–210, doi: 10.1089/jir.2012.0133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pimentel-Nunes P et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 51, 365–388, doi: 10.1055/a-0859-1883 (2019). [DOI] [PubMed] [Google Scholar]
- 249.Pittayanon R et al. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment Pharmacol Ther 46, 40–45, doi: 10.1111/apt.14082 (2017). [DOI] [PubMed] [Google Scholar]
- 250.Giroux V & Rustgi AK Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer 17, 594–604, doi: 10.1038/nrc.2017.68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A & Svennerholm AM Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun 66, 5964–5971, doi: 10.1128/IAI.66.12.5964-5971.1998 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Morse HR, Olomolaiye OO, Wood NA, Keen LJ & Bidwell JL Induced heteroduplex genotyping of TNF-alpha, IL-1beta, IL-6 and IL-10 polymorphisms associated with transcriptional regulation. Cytokine 11, 789–795, doi: 10.1006/cyto.1999.0491 (1999). [DOI] [PubMed] [Google Scholar]
- 253.Wilson KT & Crabtree JE Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 133, 288–308, doi: 10.1053/j.gastro.2007.05.008 (2007). [DOI] [PubMed] [Google Scholar]
- 254.Syu LJ et al. Transgenic expression of interferon-gamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol 181, 2114–2125, doi: 10.1016/j.ajpath.2012.08.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Putoczki TL et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271, doi: 10.1016/j.ccr.2013.06.017 (2013). [DOI] [PubMed] [Google Scholar]
- 256.Osaki LH et al. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol 247, 513–523, doi: 10.1002/path.5214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Smythies LE et al. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol 165, 1022–1029, doi: 10.4049/jimmunol.165.2.1022 (2000). [DOI] [PubMed] [Google Scholar]
- 258.McHugh RS, Shevach EM, Margulies DH & Natarajan K A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol 31, 2094–2103, doi: (2001). [DOI] [PubMed] [Google Scholar]
- 259.Zhang M et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut, doi: 10.1136/gutjnl-2019-320368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang P et al. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell Rep 27, 1934–1947 e1935, doi: 10.1016/j.celrep.2019.04.052 (2019). [DOI] [PubMed] [Google Scholar]
- 261.Sathe A et al. Single-Cell Genomic Characterization Reveals the Cellular Reprogramming of the Gastric Tumor Microenvironment. Clin Cancer Res 26, 2640–2653, doi: 10.1158/1078-0432.CCR-19-3231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bagheri N, Salimzadeh L & Shirzad H The role of T helper 1-cell response in Helicobacter pylori-infection. Microb Pathog 123, 1–8, doi: 10.1016/j.micpath.2018.06.033 (2018). [DOI] [PubMed] [Google Scholar]
- 263.Dixon B, Hossain R, Patel RV & Algood HMS Th17 Cells in Helicobacter pylori Infection: a Dichotomy of Help and Harm. Infect Immun 87, doi: 10.1128/IAI.00363-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yasmin S, Dixon B, Olivares-Villagomez D & Algood HMS Interleukin-21 (IL-21) Downregulates Dendritic Cell Cytokine Responses to Helicobacter pylori and Modulates T Lymphocyte IL-17A Expression in Peyer's Patches during Infection. Infect Immun 87, doi: 10.1128/IAI.00237-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Larussa T et al. Enhanced expression of indoleamine 2,3-dioxygenase in Helicobacter pylori-infected human gastric mucosa modulates Th1/Th2 pathway and interleukin 17 production. Helicobacter 20, 41–48, doi: 10.1111/hel.12174 (2015). [DOI] [PubMed] [Google Scholar]
- 266.Ford AC, Yuan Y, Forman D, Hunt R & Moayyedi P Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev 7, CD005583, doi: 10.1002/14651858.CD005583.pub3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Venerito M, Ford AC, Rokkas T & Malfertheiner P Review: Prevention and management of gastric cancer. Helicobacter 25 Suppl 1, e12740, doi: 10.1111/hel.12740 (2020). [DOI] [PubMed] [Google Scholar]
- 268.Sugano K Effect of Helicobacter pylori eradication on the incidence of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 22, 435–445, doi: 10.1007/s10120-018-0876-0 (2019). [DOI] [PubMed] [Google Scholar]
- 269.Obayashi Y et al. Risk Factors for Gastric Cancer after the Eradication of Helicobacter pylori Evaluated based on the Background Gastric Mucosa : A Propensity Score-matched Case-Control Study. Intern Med, doi: 10.2169/internalmedicine.5486-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Companioni Napoles O et al. SCHLAFEN 5 expression correlates with intestinal metaplasia that progresses to gastric cancer. J Gastroenterol 52, 39–49, doi: 10.1007/s00535-016-1202-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Park YH & Kim N Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev 20, 25–40, doi: 10.15430/JCP.2015.20.1.25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mueller D et al. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest 122, 1553–1566, doi: 10.1172/JCI61143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Blosse A, Lehours P, Wilson KT & Gobert AP Helicobacter: Inflammation, immunology, and vaccines. Helicobacter 23 Suppl 1, e12517, doi: 10.1111/hel.12517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kim DJ, Park JH, Franchi L, Backert S & Nunez G The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur J Immunol 43, 2650–2658, doi: 10.1002/eji.201243281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Amedei A, Della Bella C, Silvestri E, Prisco D & D'Elios MM T cells in gastric cancer: friends or foes. Clin Dev Immunol 2012, 690571, doi: 10.1155/2012/690571 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Prendergast GC et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63, 721–735, doi: 10.1007/s00262-014-1549-4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Azadegan-Dehkordi F et al. Increased Indoleamine 2, 3-Dioxygenase expression modulates Th1/Th17/Th22 and Treg pathway in humans with Helicobacter Pylori-Infected gastric mucosa. Hum Immunol, doi: 10.1016/j.humimm.2020.10.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Li P et al. GC-Derived EVs Enriched with MicroRNA-675-3p Contribute to the MAPK/PD-L1-Mediated Tumor Immune Escape by Targeting CXXC4. Mol Ther Nucleic Acids 22, 615–626, doi: 10.1016/j.omtn.2020.08.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Reyes VE & Peniche AG Helicobacter pylori Deregulates T and B Cell Signaling to Trigger Immune Evasion. Curr Top Microbiol Immunol 421, 229–265, doi: 10.1007/978-3-030-15138-6_10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ilson DH Immunotherapy in esophagogastric cancer. Clin Adv Hematol Oncol 19, 639–647 (2021). [PMC free article] [PubMed] [Google Scholar]
- 281.Ahmadzadeh M et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544, doi: 10.1182/blood-2008-12-195792 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chen X et al. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol 44, 2603–2616, doi: 10.1002/eji.201344423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Reissfelder C et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest 125, 739–751, doi: 10.1172/JCI74894 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hamid O & Carvajal RD Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 13, 847–861, doi: 10.1517/14712598.2013.770836 (2013). [DOI] [PubMed] [Google Scholar]
- 285.Hamid O et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369, 134–144, doi: 10.1056/NEJMoa1305133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iwai Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99, 12293–12297, doi: 10.1073/pnas.192461099 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Topalian SL, Drake CG & Pardoll DM Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24, 207–212, doi: 10.1016/j.coi.2011.12.009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Muro K et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30, 19–33, doi: 10.1093/annonc/mdy502 (2019). [DOI] [PubMed] [Google Scholar]
- 289.Subhash VV, Yeo MS, Tan WL & Yong WP Strategies and Advancements in Harnessing the Immune System for Gastric Cancer Immunotherapy. J Immunol Res 2015, 308574, doi: 10.1155/2015/308574 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Muro K et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 17, 717–726, doi: 10.1016/S1470-2045(16)00175-3 (2016). [DOI] [PubMed] [Google Scholar]
- 291.Wang L et al. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 190, 794–804, doi: 10.4049/jimmunol.1202088 (2013). [DOI] [PubMed] [Google Scholar]
- 292.Ju X, Zhang H, Zhou Z, Chen M & Wang Q Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-a signaling. Exp Cell Res 396, 112315, doi: 10.1016/j.yexcr.2020.112315 (2020). [DOI] [PubMed] [Google Scholar]
- 293.Wang WW et al. CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6, 33486–33499, doi: 10.18632/oncotarget.5588 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Rincon-Arevalo H, Sanchez-Parra CC, Castano D, Yassin L & Vasquez G Regulatory B Cells and Mechanisms. Int Rev Immunol 35, 156–176, doi: 10.3109/08830185.2015.1015719 (2016). [DOI] [PubMed] [Google Scholar]
- 295.Pyzik M & Piccirillo CA TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leukoc Biol 82, 335–346, doi: 10.1189/jlb.1006644 (2007). [DOI] [PubMed] [Google Scholar]
- 296.Yuan XL et al. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-beta1. World J Gastroenterol 17, 2019–2027, doi: 10.3748/wjg.v17.i15.2019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shi Y et al. Extracellular Vesicles From Gastric Cancer Cells Induce PD-L1 Expression on Neutrophils to Suppress T-Cell Immunity. Front Oncol 10, 629, doi: 10.3389/fonc.2020.00629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Whiteside TL Exosomes and tumor-mediated immune suppression. J Clin Invest 126, 1216–1223, doi: 10.1172/JCI81136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Buret AG, Fedwick JP & Flynn AN Host epithelial interactions with Helicobacter pylori: a role for disrupted gastric barrier function in the clinical outcome of infection? Can J Gastroenterol 19, 543–552, doi: 10.1155/2005/940213 (2005). [DOI] [PubMed] [Google Scholar]
- 300.Fedwick JP, Lapointe TK, Meddings JB, Sherman PM & Buret AG Helicobacter pylori activates myosin light-chain kinase to disrupt claudin-4 and claudin-5 and increase epithelial permeability. Infect Immun 73, 7844–7852, doi: 10.1128/IAI.73.12.7844-7852.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wroblewski LE, Peek RM Jr. & Wilson KT Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23, 713–739, doi: 10.1128/CMR.00011-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Voland P et al. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. Am J Physiol Gastrointest Liver Physiol 284, G96–G106, doi: 10.1152/ajpgi.00160.2002 (2003). [DOI] [PubMed] [Google Scholar]
- 303.Rathinavelu S, Kao JY, Zavros Y & Merchant JL Helicobacter pylori outer membrane protein 18 (Hp1125) induces dendritic cell maturation and function. Helicobacter 10, 424–432, doi: 10.1111/j.1523-5378.2005.00350.x (2005). [DOI] [PubMed] [Google Scholar]
- 304.Hafsi N et al. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol 173, 1249–1257, doi: 10.4049/jimmunol.173.2.1249 (2004). [DOI] [PubMed] [Google Scholar]
- 305.Banchereau J et al. Immunobiology of dendritic cells. Annu Rev Immunol 18, 767–811, doi: 10.1146/annurev.immunol.18.1.767 (2000). [DOI] [PubMed] [Google Scholar]
- 306.Guiney DG, Hasegawa P & Cole SP Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect Immun 71, 4163–4166, doi: 10.1128/iai.71.7.4163-4166.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kranzer K et al. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect Immun 72, 4416–4423, doi: 10.1128/IAI.72.8.4416-4423.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Colonna M, Trinchieri G & Liu YJ Plasmacytoid dendritic cells in immunity. Nat Immunol 5, 1219–1226, doi: 10.1038/ni1141 (2004). [DOI] [PubMed] [Google Scholar]
- 309.Blaser N, Backert S & Pachathundikandi SK Immune Cell Signaling by Helicobacter pylori: Impact on Gastric Pathology. Adv Exp Med Biol 1149, 77–106, doi: 10.1007/5584_2019_360 (2019). [DOI] [PubMed] [Google Scholar]
- 310.Miyara M et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911, doi: 10.1016/j.immuni.2009.03.019 (2009). [DOI] [PubMed] [Google Scholar]
- 311.Sakaguchi S, Miyara M, Costantino CM & Hafler DA FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10, 490–500, doi: 10.1038/nri2785 (2010). [DOI] [PubMed] [Google Scholar]
- 312.Nagase H et al. ICOS(+) Foxp3(+) TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer 140, 686–695, doi: 10.1002/ijc.30475 (2017). [DOI] [PubMed] [Google Scholar]
- 313.Oya Y, Hayakawa Y & Koike K Tumor microenvironment in gastric cancers. Cancer Sci 111, 2696–2707, doi: 10.1111/cas.14521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gabrilovich DI, Ostrand-Rosenberg S & Bronte V Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 12, 253–268, doi: 10.1038/nri3175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ren W et al. Circulating and tumor-infiltrating arginase 1-expressing cells in gastric adenocarcinoma patients were mainly immature and monocytic Myeloid-derived suppressor cells. Sci Rep 10, 8056, doi: 10.1038/s41598-020-64841-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Condamine T et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 1, doi: 10.1126/sciimmunol.aaf8943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nan J et al. Endoplasmic reticulum stress induced LOX-1(+ ) CD15(+) polymorphonuclear myeloid-derived suppressor cells in hepatocellular carcinoma. Immunology 154, 144–155, doi: 10.1111/imm.12876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tavukcuoglu E et al. Human splenic polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) are strategically located immune regulatory cells in cancer. Eur J Immunol 50, 2067–2074, doi: 10.1002/eji.202048666 (2020). [DOI] [PubMed] [Google Scholar]
- 319.Condamine T, Mastio J & Gabrilovich DI Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol 98, 913–922, doi: 10.1189/jlb.4RI0515-204R (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Karin N The Development and Homing of Myeloid-Derived Suppressor Cells: From a Two-Stage Model to a Multistep Narrative. Front Immunol 11, 557586, doi: 10.3389/fimmu.2020.557586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Huang G et al. Indoleamine 2,3-dioxygenase (IDO) downregulates the cell surface expression of the CD4 molecule. Int J Mol Sci 13, 10863–10879, doi: 10.3390/ijms130910863 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhao Q et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol 188, 1117–1124, doi: 10.4049/jimmunol.1100164 (2012). [DOI] [PubMed] [Google Scholar]
- 323.Petersen CP et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 146, 1727–1738 e1728, doi: 10.1053/j.gastro.2014.02.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.De Salvo C et al. Interleukin 33 Triggers Early Eosinophil-Dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology, doi: 10.1053/j.gastro.2020.09.040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kielbassa K, Vegna S, Ramirez C & Akkari L Understanding the Origin and Diversity of Macrophages to Tailor Their Targeting in Solid Cancers. Front Immunol 10, 2215, doi: 10.3389/fimmu.2019.02215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Marvel D & Gabrilovich DI Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 125, 3356–3364, doi: 10.1172/JCI80005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ugel S, De Sanctis F, Mandruzzato S & Bronte V Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest 125, 3365–3376, doi: 10.1172/JCI80006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Owyang SY, Luther J, Owyang CC, Zhang M & Kao JY Helicobacter pylori DNA's anti-inflammatory effect on experimental colitis. Gut Microbes 3, 168–171, doi: 10.4161/gmic.19181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Veglia F, Perego M & Gabrilovich D Myeloid-derived suppressor cells coming of age. Nat Immunol 19, 108–119, doi: 10.1038/s41590-017-0022-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Murdoch C, Muthana M, Coffelt SB & Lewis CE The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8, 618–631, doi: 10.1038/nrc2444 (2008). [DOI] [PubMed] [Google Scholar]
- 331.Shaked Y & Voest EE Bone marrow derived cells in tumor angiogenesis and growth: are they the good, the bad or the evil? Biochim Biophys Acta 1796, 1–4, doi: 10.1016/j.bbcan.2009.07.002 (2009). [DOI] [PubMed] [Google Scholar]
- 332.Venneri MA et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109, 5276–5285, doi: 10.1182/blood-2006-10-053504 (2007). [DOI] [PubMed] [Google Scholar]
- 333.Lewis CE, De Palma M & Naldini L Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res 67, 8429–8432, doi: 10.1158/0008-5472.CAN-07-1684 (2007). [DOI] [PubMed] [Google Scholar]
- 334.Teal E, Dua-Awereh M, Hirshorn ST & Zavros Y Role of metaplasia during gastric regeneration. Am J Physiol Cell Physiol 319, C947–C954, doi: 10.1152/ajpcell.00415.2019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.De Salvo C et al. Interleukin 33 Triggers Early Eosinophil-Dependent Events Leading to Metaplasia in a Chronic Model of Gastritis-Prone Mice. Gastroenterology 160, 302–316 e307, doi: 10.1053/j.gastro.2020.09.040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Holokai L et al. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 12, doi: 10.3390/cancers12123816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rojas A, Araya P, Gonzalez I & Morales E Gastric Tumor Microenvironment. Adv Exp Med Biol 1226, 23–35, doi: 10.1007/978-3-030-36214-0_2 (2020). [DOI] [PubMed] [Google Scholar]
- 338.Lenti MV et al. Autoimmune gastritis. Nat Rev Dis Primers 6, 56, doi: 10.1038/s41572-020-0187-8 (2020). [DOI] [PubMed] [Google Scholar]
- 339.Mahmud N et al. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: a renewed call for surveillance. Ann Gastroenterol 32, 67–72, doi: 10.20524/aog.2018.0325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Terao S et al. Multicenter study of autoimmune gastritis in Japan: Clinical and endoscopic characteristics. Dig Endosc 32, 364–372, doi: 10.1111/den.13500 (2020). [DOI] [PubMed] [Google Scholar]
- 341.Waldum H & Mjones PG Correct Identification of Cell of Origin May Explain Many Aspects of Cancer: The Role of Neuroendocrine Cells as Exemplified from the Stomach. Int J Mol Sci 21, doi: 10.3390/ijms21165751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Dockray GJ, Moore A, Varro A & Pritchard DM Gastrin receptor pharmacology. Curr Gastroenterol Rep 14, 453–459, doi: 10.1007/s11894-012-0293-1 (2012). [DOI] [PubMed] [Google Scholar]
- 343.D'Elios MM et al. H(+),K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology 120, 377–386, doi: 10.1053/gast.2001.21187 (2001). [DOI] [PubMed] [Google Scholar]
- 344.De Re V et al. Polymorphism in Toll-Like Receptors and Helicobacter Pylori Motility in Autoimmune Atrophic Gastritis and Gastric Cancer. Cancers (Basel) 11, doi: 10.3390/cancers11050648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Role of TLRs in polarizing the immune microenvironment prior GAC. [Google Scholar]
- 345.Tsuboi M et al. Distinct Features of Autoimmune Gastritis in Patients with Open-Type Chronic Gastritis in Japan. Biomedicines 8, doi: 10.3390/biomedicines8100419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Notsu T et al. Prevalence of Autoimmune Gastritis in Individuals Undergoing Medical Checkups in Japan. Intern Med 58, 1817–1823, doi: 10.2169/internalmedicine.2292-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]