Abstract
Listeria monocytogenes promotes the induction of the T-helper 1 (Th1) cell response, while ovalbumin (OVA) induces a Th2 cell response and allergic reactions, such as airway hyperreactivity and immunoglobulin E (IgE) production. When mice were immunized with OVA on day 7 after L. monocytogenes infection, eosinophilia in bronchoalveolar lavage and the production of total IgE, OVA-specific IgE, interleukin-4 (IL-4), and IL-5 in the circulation were markedly suppressed. Cytokine responses, including IL-4, IL-5, IL-10, IL-13, and gamma interferon, to OVA were decreased in the spleen cell cultures obtained from OVA-immunized mice that had been infected with L. monocytogenes. Conversely, when OVA-immunized mice were infected with L. monocytogenes, conversion from the nonlethal infection to the lethal infection occurred. Host resistance to L. monocytogenes infection in OVA-immunized mice was enhanced by the administration of anti–IL-10 monoclonal antibody. The present study indicates that striking interference is observed between Th1-inducing L. monocytogenes infection and Th2-driven OVA-induced airway hyperreactivity.
Antigen-specific CD4+ T-helper (Th)-cell responses can be divided into two types, based on cytokine production and effector function (1). Differentiation of Th1 cells, which can produce interleukin-2 (IL-2), gamma interferon (IFN-γ), and lymphotoxin, is driven by IL-12, while differentiation of Th2 cells, which can produce IL-4, IL-5, IL-10, and IL-13, is driven by IL-4. Listeria monocytogenes, a facultative intracellular bacterium, induces the Th1 cell response in the infected host (14); IFN-γ plays a critical role in antilisterial resistance (11), while IL-4 and IL-10 inhibit antilisterial resistance (3, 8).
Asthma is a disease caused by a type I allergic response to allergens and characterized by chronic airway inflammation, with recruitment of eosinophils. Th2 cells secreting IL-4, IL-5, and IL-13 have an important role in the development of asthma (12, 23). Previous reports indicated that IFN-γ-dominated immune responses to viral or mycobacterial infection in childhood are associated with a reduced incidence of asthma (25, 26). Recently, a mouse model of ovalbumin (OVA)-induced allergic airway hyperresponsiveness reportedly revealed a reduction in allergic responses, including eosinophilic airway inflammation and serum immunoglobulin E (IgE) production, as a result of infection with Mycobacterium bovis BCG (4) or injection of killed M. vaccae (29) or killed L. monocytogenes (10), which induces Th1 cells (24). Therefore, we were interested in investigating the interaction between Th2-polarized OVA-induced airway hyperreactivity and Th1-polarized L. monocytogenes infection in mice.
In this study, female C57BL/6 mice, 6 to 8 weeks old, were purchased from CLEA Japan, Inc., Tokyo, Japan. All animals were maintained under specific-pathogen-free conditions at the Institute for Animal Experiments, School of Medicine, Hirosaki University. Data were expressed as means and standard deviations (SD), and the Wilcoxon rank sum test was used to determine the significance of the differences in bacterial counts in the organs, cytokine titers, serum IgE levels, and leukocyte counts between the control and experimental groups. The generalized Wilcoxon test was used to determine the significance of differences in survival rates.
The effect of L. monocytogenes infection on the induction of OVA-induced allergic responses was investigated. L. monocytogenes 1b-1684 cells were prepared as described previously (18). Mice were infected intranasally with 108 CFU of L. monocytogenes; uninfected mice were given 0.01 M phosphate-buffered saline (pH 7.4) only. Both groups were immunized with OVA (Sigma Chemical Co., St. Louis, Mo.) on day 7 postinfection.
OVA-induced airway inflammation was induced according to a modification of the method of Kung et al. (16). Briefly, mice were injected intraperitoneally with 100 μg of OVA in 0.1 ml of alum adjuvant (Pierce, Rockford, Ill.) on day 7 postinfection (day 0 of immunization) and boosted intraperitoneally with 100 μg of OVA in alum on day 14 of immunization. The mice were given 20 μg of OVA in a 20-μl volume of 0.85% saline intranasally on days 25, 26, and 27 of immunization. As a nonallergic control, mice were infected with L. monocytogenes, injected with alum only on day 0, and exposed to saline only according to the schedule of OVA immunization.
On day 28 of immunization, mice in the three groups were sacrificed, and the numbers of leukocytes in bronchoalveolar lavage fluids (BALF) and serum IgE levels were estimated. To obtain BALF, mice were killed, the trachea was cannulated, and bronchoalveolar lavage was performed by flushing lungs and airways three times each with 0.5 ml of phosphate-buffered saline. BALF were pooled and centrifuged at 200 × g for 10 min at 4°C. The cell pellet was resuspended in 0.1 ml of saline containing 10% bovine serum albumin (fraction V; Sigma). A total cell count was performed manually with a Fuchs-Rosenthal chamber and staining with Türk solution. An eosinophil count was performed manually with a Fuchs-Rosenthal chamber and staining with Hinkelman's solution. Total IgE was determined by a double-sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (PharMingen, San Diego, Calif.). OVA-specific IgE was also measured with an ELISA. Pooled sera from OVA-immunized C57BL/6 mice, arbitrarily assigned an OVA-specific IgE titer of 10 U/ml, were included in each assay as a standard.
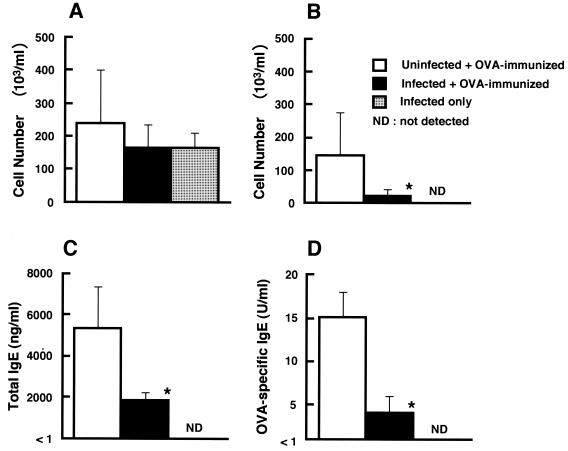
Total numbers of leukocytes in BALF were comparable in OVA-immunized mice in the L. monocytogenes-infected group and the uninfected group (Fig. 1A). Infiltration of leukocytes into BALF was also observed in mice with L. monocytogenes infection only. However, eosinophilia in BALF was reduced in OVA-immunized mice infected with L. monocytogenes compared with uninfected OVA-immunized mice (P < 0.01) (Fig. 1B). Eosinophilia was also reduced in lung tissues of OVA-immunized mice by L. monocytogenes infection (data not shown). High titers of total IgE were detected in the sera of uninfected OVA-immunized mice, and the titers were reduced in OVA-immunized mice infected with L. monocytogenes (Fig. 1C). Similarly, OVA-specific IgE was induced in the sera of uninfected OVA-immunized mice, but the level was decreased in OVA-immunized mice infected with L. monocytogenes (Fig. 1D). Total IgE and OVA-specific IgE were not detected in the sera of L. monocytogenes-infected mice without OVA immunization.
FIG. 1.
Effect of L. monocytogenes infection on eosinophilia in BALF and serum IgE levels in OVA-immunized mice. Mice were infected with 108 CFU of viable L. monocytogenes cells intranasally and immunized with OVA 7 days later as described in the text. BALF and sera were collected 24 h after the last intranasal exposure with OVA or saline on day 28 of immunization. Total numbers of leukocytes (A) and total numbers of eosinophils (B) in BALF and total IgE (C) and OVA-specific IgE (D) levels in sera were determined. Uninfected and immunized mice as well as infected and unimmunized mice were also used. Each result represents the mean and SD for a group of four mice. An asterisk indicates a significant difference from the result for the uninfected OVA-immunized group at a P value of <0.01. The results were reproduced in three repeat experiments.
Next, the titers of IL-4 and IL-5 in the sera of these mice were determined. IL-4 titers were determined by an ELISA as described previously (18). The IL-5 assay was carried out using an OptEIATM set (PharMingen). IL-4 was detected in uninfected OVA-immunized mice (66 ± 9 pg/ml) but was not detected in OVA-immunized mice infected with L. monocytogenes (less than 10 pg/ml). Similarly, IL-5 was induced in the circulation of OVA-immunized mice (166 ± 36 pg/ml), and IL-5 production was decreased by L. monocytogenes infection (117 ± 15 pg/ml) (P < 0.05).
The effect of L. monocytogenes infection on cytokine responses in cultures of spleen cells obtained from OVA-immunized mice was investigated. Spleen cell culturing was carried out as reported previously (20). Spleen cells in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal calf serum, 200 U of penicillin G per ml, and 200 μg of streptomycin per ml were placed in a 24-well tissue culture plate (Greiner, Frickenhausen, Germany) at a cell density of 107 cells/well in a final volume of 1 ml. The cells were stimulated with OVA at a final concentration of 1 mg/ml or with heat-killed L. monocytogenes (20) at a final concentration of 107 organisms/ml for 72 h. IFN-γ and IL-10 assays were carried out with ELISAs as described previously (18, 19). An IL-13 ELISA was performed with rat anti-mouse IL-13 monoclonal antibody (MAb) (38213.11; R&D Systems, Minneapolis, Minn.) and biotinylated rabbit anti-mouse IL-13 antibody (R&D Systems).
The levels of IL-4, IL-5, IL-10, IL-13, and IFN-γ produced in spleen cell cultures were compared for infected OVA-immunized mice, uninfected OVA-immunized mice, and infected and unimmunized mice (Table 1). IL-4, IL-5, and IL-13 titers in OVA-stimulated cultures of spleen cell obtained from OVA-immunized mice infected with L. monocytogenes were markedly lower than those in samples from uninfected OVA-immunized mice (P < 0.01). IL-10 production was completely suppressed in these cells. These cytokines were not induced by OVA in mice only infected with L. monocytogenes. Spleen cells obtained from uninfected OVA-immunized mice produced IFN-γ in response to OVA, whereas IFN-γ production was suppressed in OVA-immunized mice infected with L. monocytogenes. In contrast, the production of IFN-γ in response to heat-killed L. monocytogenes was comparable between OVA-immunized mice and unimmunized mice infected with L. monocytogenes.
TABLE 1.
Effect of L. monocytogenes infection on cytokine production induced by OVA in cultures of spleen cells obtained from OVA-immunized mice
| Infection with L. monocytogenesa | Immunization with OVAa | Titer (pg/ml)b of the indicated cytokine after stimulation with:
|
|||||
|---|---|---|---|---|---|---|---|
| OVA
|
Heat-killed L. monocytogenes (IFN-γ) | ||||||
| IL-4 | IL-5 | IL-10 | IL-13 | IFN-γ | |||
| No | Yes | 38 ± 11 | 508 ± 380 | 388 ± 143 | 737 ± 175 | 1,464 + 551 | <10 |
| Yes | Yes | 13 ± 4c | 231 ± 42c | <10 | 151 ± 121c | <10 | 1,904 ± 1,501 |
| Yes | No | <10 | <10 | <10 | 36 ± 28 | <10 | 2,426 ± 1,928 |
Mice were infected intranasally with 108 CFU of L. monocytogenes and immunized with OVA 7 days later.
Spleen cells were collected 24 h after the last intranasal exposure to OVA or saline. Spleen cells were stimulated with OVA or heat-killed L. monocytogenes for 72 h, and cytokine titers in the supernatant fluids were determined. The results were reproduced in three repeat experiments.
Significantly different from the result for the uninfected OVA-immunized group at a P value of <0.01.
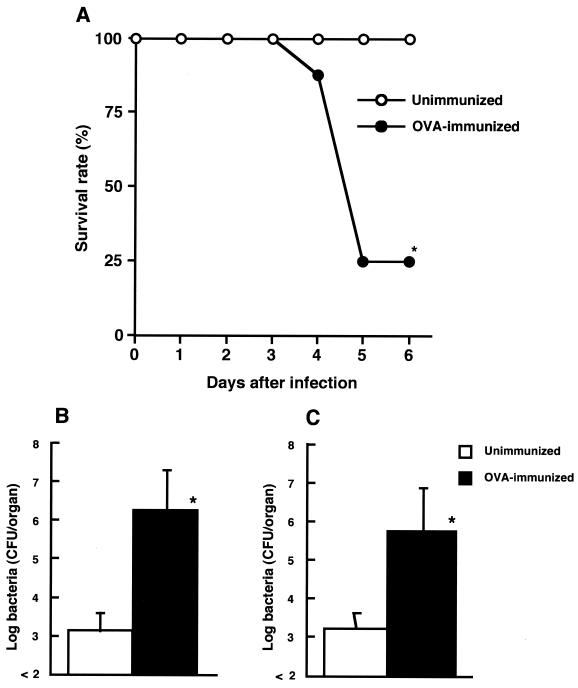
Next, we investigated the effect of OVA immunization on host resistance to L. monocytogenes infection. On day 28 of immunization, OVA-immunized and unimmunized mice were infected with 5 × 104 CFU of viable L. monocytogenes, and survival was observed (Fig. 2A). Most of the OVA-immunized mice succumbed to L. monocytogenes infection, but all of the unimmunized mice survived because the 50% lethal dose of L. monocytogenes used here for the unimmunized mice was 5 × 105 CFU. No mice were dead after 6 to 14 days of infection (data not shown). To address whether the elimination of bacteria from the organs of OVA-immunized mice was inhibited, OVA-immunized mice and unimmunized mice were infected intravenously with 104 CFU of L. monocytogenes on day 28 of immunization, and bacterial numbers in the spleens and livers were determined 5 days later by culturing on tryptic soy agar (Difco Laboratories, Detroit, Mich.). The numbers of L. monocytogenes were significantly increased in both organs of OVA-immunized mice compared with those of unimmunized mice (P < 0.01) (Fig. 2B and C).
FIG. 2.
Effect of OVA-induced allergy on susceptibility to an intravenous infection with L. monocytogenes. (A) OVA-immunized mice and unimmunized mice were infected with 5 × 104 CFU of viable L. monocytogenes, and survival was observed. Eight mice were used in each group. (B and C) Similarly, OVA-immunized mice and unimmunized mice were infected intravenously with 104 CFU of viable L. monocytogenes, and bacterial numbers in the spleens (B) and livers (C) were determined on day 5 of infection. Each result represents the mean and SD for a group of four mice. An asterisk indicates a significant difference from the result for the unimmunized group at a P value of <0.01. The results were reproduced in three repeat experiments.
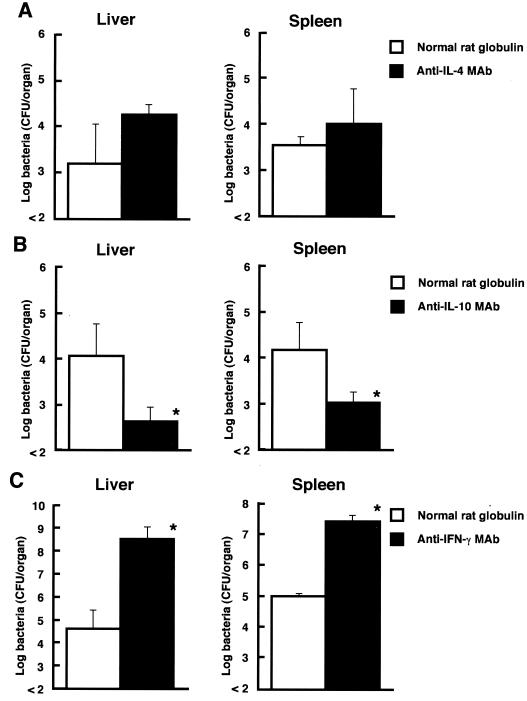
To determine whether IL-4 and IL-10 would be involved in the suppression of antilisterial resistance in OVA-immunized mice, the effects of the in vivo administration of anti–IL-4 MAb and anti–IL-10 MAb on bacterial growth in the spleens and livers of OVA-immunized mice were investigated. Ascitic fluid containing MAb against mouse IL-4 (11B11; rat IgG1) (21), mouse IL-10 (JES5-2A5; rat IgG1) (17), or mouse IFN-γ (R4-6A2; rat IgG1) (27) was partially purified by (NH4)2SO4 precipitation (18). The mice were given a single intravenous injection of 1 mg of anti–IL-4 MAb or anti–IL-10 MAb 1 h before infection. Normal rat globulin was injected as a control. All MAbs and normal rat globulin contained endotoxin at less than 0.1 ng per injected dose, as determined by use of the Limulus amoebocyte lysate assay. On day 28 of immunization with OVA, mice were injected with anti–IL-4 MAb, anti–IL-10 MAb, or normal rat globulin 1 h before infection with 2 × 103 CFU of viable L. monocytogenes, and the numbers of bacteria in the spleens and livers of these animals were determined on day 5 of infection. In vivo administration of anti–IL-4 MAb had no significant effect on the elimination of L. monocytogenes cells from the organs (Fig. 3A). In contrast, the numbers of bacteria in the organs of OVA-immunized mice significantly decreased when anti–IL-10 MAb was injected (Fig. 3B). The effect of the in vivo administration of anti–IFN-γ MAb on antilisterial resistance was also examined. The numbers of bacteria in the organs of OVA-immunized mice treated with anti–IFN-γ MAb were significantly increased compared with those in normal rat globulin-treated mice (Fig. 3C).
FIG. 3.
Effect of in vivo administration of anticytokine MAbs on host resistance against L. monocytogenes infection in OVA-immunized mice. Mice were injected with anti–IL-4 MAb (A), anti–IL-10 MAb (B), or anti–IFN-γ MAb (C) on day 28 of immunization. Control mice were injected with normal rat globulin instead of anticytokine MAbs. Mice were infected with 2 × 103 CFU of viable L. monocytogenes cells 1 h later, and the numbers of bacteria in the spleens and livers of these animals were determined on day 5 of infection. Each result represents the mean and SD for a group of four mice. An asterisk indicates a significant difference from the result for the normal rat globulin-treated group at a P value of <0.01. The results were reproduced in three repeat experiments.
Recent studies indicated that the induction of OVA-induced allergic airway hyperresponsiveness and the serum IgE response are suppressed by prior or simultaneous infection with M. bovis BCG (4) or injection of killed M. vaccae (29) or killed L. monocytogenes (10). All of these bacteria can induce Th1 polarization or can act as Th1-inducing adjuvants (24). Moreover, the administration of IFN-γ or IL-12, which are critical cytokines in the induction of Th1 polarization (28), has been reported to inhibit the induction of eosinophilia in airways and serum IgE production in mice (6, 13). In the present study, prior infection with L. monocytogenes inhibited eosinophilia in BALF (Fig. 1) and lungs (data not shown) and serum IgE production (Fig. 1). IL-4, IL-5, and IL-13 are reportedly critical in the induction of airway hyperresponsiveness and IgE production by allergens (2, 5, 7, 30). In addition, IL-5 is known to be a critical eosinophilia-inducing agent (2, 15). Moreover, IFN-γ is reportedly involved in the induction of airway inflammation with lymphocytes and monocytes (9, 22). Here, the serum IL-4 and IL-5 levels were reduced in OVA-immunized mice infected with L. monocytogenes. Moreover, IL-4, IL-5, IL-10, IL-13, and IFN-γ production in response to OVA was markedly reduced in the spleen cells obtained from these mice (Table 1). These results indicate that the suppression of OVA-induced Th1 and Th2 cell responses might be involved in the inhibition of OVA-induced allergic responses by prior infection with L. monocytogenes.
Erb et al. (4) reported that M. bovis BCG-induced inhibition of airway eosinophilia was strongly reduced in IFN-γ receptor-deficient mice, suggesting that IFN-γ plays an important role in M. bovis BCG-induced suppression. Hansen et al. (10) reported that heat-killed L. monocytogenes as an adjuvant inhibited eosinophilia and antigen-specific IgE and IL-4 production and dramatically increased antigen-specific IFN-γ production. In the present study, OVA-specific IFN-γ production was not observed in spleens cells obtained from OVA-immunized mice infected with L. monocytogenes, although L. monocytogenes-specific IFN-γ production was comparable to that in mice exposed only to L. monocytogenes infection (Table 1). These results suggest that OVA-specific IFN-γ production might not trigger the suppression of allergic responses in L. monocytogenes-infected mice.
When mice in which airway hyperreactivity had been induced by OVA were infected intravenously with L. monocytogenes, conversion from the nonlethal infection to the lethal infection occurred (Fig. 2A). Bacterial growth was significantly enhanced in the spleens and livers of OVA-immunized mice (Fig. 2B and C). IL-4 and IL-10 are known to play a detrimental role in host resistance against L. monocytogenes infection (3, 8). In the present study, administration of anti–IL-4 MAb resulted in no significant effect on antilisterial resistance, while neutralization of endogenous IL-10 by the corresponding MAb augmented the elimination of bacteria from the spleens and livers of OVA-immunized mice (Fig. 3). These results suggest that IL-10 might play a critical role in the suppression of antilisterial resistance in OVA-immunized mice.
In conclusion, interference between host resistance to L. monocytogenes infection and OVA-induced allergic responses was observed. We are now attempting to study whether listerial cell components and products can inhibit OVA-induced airway hyperreactivity and whether host resistance to other Th1-inducing bacterial infections is attenuated in OVA-immunized mice.
Acknowledgments
This work was supported in part by a grant-in-aid for general scientific research (10670247) provided by the Japanese Ministry of Education, Science, Sports, and Culture.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;87:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai W J, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 4.Erb K J, Holloway J W, Sobeck A, Moll H, Gros G L. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. Interleukin 5 deficiency abolishes eosinophilia, airway hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavett S H, O'Hearn D, Li X, Huang S-K, Finkelman F D, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grünig G, Warnock M, Wakil A E, Venkayya R, Brombacher F, Rennick D M, Sheppard D, Mohrs M, Donaldson D D, Locksley R M, Corry D B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haak-Frendsho M, Brown J F, Iizawa Y, Wagner R D, Czuprynski C J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992;148:3978–3985. [PubMed] [Google Scholar]
- 9.Hansen G, Berry G, DeKruyff R H, Umetsu D T. Allergen specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Investig. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen G, Yeung V P, Berry G, Umetsu D T, DeKruyff R H. Vaccination with heat-killed Listeria as an adjuvant reverses established allergen induced airway hyperreactivity and inflammation: role of CD8+ T cells and IL-18. J Immunol. 2000;164:223–230. doi: 10.4049/jimmunol.164.1.223. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vicek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 12.Huang S K, Xiao H Q, Kleine-Tebbe J, Paciotti G, Marsh D G, Lichtenstein L M, Liu M C. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 13.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the filtration of CD4+ T cells. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 15.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G, Matthaei K I. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 16.Kung T T, Jones H, Adams G K, Umland S P, Kreutner W, Egan R W, Chapman R W, Watnick A S. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T R, Schumacher H J H, Fiorentio D F, Leverah J, Moore K W, Bond M W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- 18.Nakane A, Nishikawa S, Sasaki S, Miura T, Asano M, Kohanawa M, Ishiwata K, Minagawa T. Endogenous interleukin-4, but not interleukin-10, is involved in suppression of host resistance against Listeria monocytogenes infection in gamma interferon-depleted mice. Infect Immun. 1996;64:1252–1258. doi: 10.1128/iai.64.4.1252-1258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakane A, Yamada K, Hasegawa S, Mizuki D, Mizuki M, Sasaki S, Miura T. Endogenous cytokines during a lethal infection with Listeria monocytogenes in mice. FEMS Microbiol Lett. 1999;175:133–142. doi: 10.1111/j.1574-6968.1999.tb13612.x. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa S, Nakane A. Host resistance against Listeria monocytogenes is reciprocal during the course of infection in alymphoplastic aly mutant mice. Cell Immunol. 1998;187:88–94. doi: 10.1006/cimm.1998.1329. [DOI] [PubMed] [Google Scholar]
- 21.Ohara J, Paul W E. Production of a monoclonal antibody and molecular characterization of B-cell stimulatory factor. Nature. 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 22.Randolph D A, Stephens R, Carruthers C J L, Chaplin D D. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Investig. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D S, Hamid Q, Ying S, Tsicopoulus A, Barkans J, Bentley A M, Corrigan C, Durham S R, Kay A B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 24.Schaible U E, Collins H L, Kaufmann S H E. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 25.Shaheen S O, Araby P, Hall A J, Barker D J, Hayes C B, Shiell A W, Goudiaby A. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 26.Shirakawa T, Enomoto T, Shimazu S, Hopkin J M. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 27.Spintalny G L, Havell E A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activity. J Exp Med. 1984;159:1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C-C, Rook G A W. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–313. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbasi J, Xu X, Schofield B, Neben T Y, Karp C L, Donaldson D D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]