Abstract
The rich structural dynamics of axonal arbors and neuronal circuitry can only be revealed through direct and repeated observations of the same neuron(s) over time, preferably in vivo. This protocol describes a long-term, high-resolution method for imaging neocortical neurons in vivo, using a combination of two-photon laser scanning microscopy (2PLSM) and a surgically implanted chronic cranial window. The window is used because the skull of most mammals is too opaque to allow high-resolution imaging of cortical neurons. Using this method, it is feasible to image the smallest neuronal structures in the superficial layers of the neocortex, such as dendritic spines and axonal boutons. Because the surface area of the craniotomy is relatively large, this technique is even suitable for use when labeled neurons are relatively uncommon. The surgery and imaging procedures are illustrated with examples from our studies of structural plasticity in the developing or adult mouse brain. The protocol is optimized for adult mice; we have used mice up to postnatal day 511 (P511). With minor modifications, it is possible to image neurons in rats and mice from P2. Most of our studies have used the Thy1 promoter to drive expression of fluorophores in subsets of cortical neurons.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPE: Please see the end of this article for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Agarose, 1.2% or 1.5% in cortex buffer (cooled to <37°C immediately before use)
Make the 1.2% agarose fresh for each experiment and keep it molten during the procedure (see Step 10).
Buprenorphine, 0.3 mg/mL (optional; see Step 15)
Carprofen, 50 mg/mL (optional; see Step 15)
Cortex buffer <R>
Cyanoacrylate (CA) glue (e.g., Vetbond or 3M)
Dental acrylic or cement (e.g., Jet or Lang Dental)
Dexamethasone sodium phosphate, 4 mg/mL (dexamethasone, final, 3 mg/mL)
Ethanol, 70%
Betadine can be used as an alternative for cleaning the scalp (see Step 4).
Eye ointment (sterile lubricant eye ointment [Stye, Del Pharmaceuticals] or optical gel [Nye Optical]) Ketamine (13 mg/mL) and Xylazine (1 mg/mL) used in combination for anesthesia
Isoflurane (2%) can be used as an alternative (see Step 1).
Saline
Transgenic mice, expressing fluorophore in cells of interest
Xylocaine (lidocaine), 1%
Equipment
Cotton swabs
Coverslips, glass, # 1, 5-mm-diameter (or custom-made cover glass)
Dental drill, high-speed, pneumatic, 1/4-in. bit (e.g., Dental Burs USA or Henry Schein)
Dissecting microscope
Gelfoam (e.g., Pharmacia & Upjohn/Baxter), cut into 7-mm2 squares and soaked in cortex buffer before use
Heating blanket
Imaging setup
In vivo images of fluorescently labeled neurons are best acquired using a custom-built 2PLSM (e.g., Lendvai et al. 2000; Helmchen and Denk 2005) with the animal positioned very close to the fluorescence detectors. We use a tunable Ti:sapphire laser (Tsunami; Spectra Physics/Newport); water-immersion objectives with a high numerical aperture (0.8–0.9 NA), 20×–60× magnification, optimized for infrared (IR) light; and photomultiplier tubes with high quantum efficiencies and low dark currents (Hamamatsu R3896 or H7422P). ScanImage software (Pologruto et al. 2003), custom written in MATLAB (Mathworks), is used for image acquisition and processing. Off-the-shelf 2PLSM setups are also available from major microscope manufacturers.
Razor (for shaving the scalp)
Stainless steel bars, 10 × 4 × 15-mm, custom, with screw holes
Stereotaxic frame (e.g., Stoelting)
Surgical instruments, including scalpel blade, dissection scissors, and fine forceps
Syringes and small-gauge injection needles
METHOD
Surgery
Humane treatment of animals must be observed at all times and should follow the local institutional guidelines for care and use of animals. All solutions should be sterile.
Anesthetize the mouse deeply with a mixture of ketamine and xylazine (intraperitoneal injection of 100 and 10 μg/g body weight, respectively) or with a continuous flow of gas anesthesia (e.g., 2% isoflurane).
-
Place the animal on a heating pad to maintain body temperature, and immobilize the head in a stereotaxic frame. Apply eye ointment to the eyes.
It is important to protect the eyes from dehydration and irritation by dental acrylic.
-
Administer dexamethasone (~2 μg/g body weight) by intramuscular injection to the quadriceps muscle.
This minimizes the cortical stress response during chronic experiments.
-
Shave the head using the razor, and wash the scalp with ethanol or Betadine. Using scissors, remove a 1-cm2 flap of skin to reveal the skull overlying the barrel cortex of both hemispheres.
All equipment should be sterile from this step on in the procedure.
Apply 1% xylocaine to the skull and exposed muscles using cotton-swab applicators.
-
Using a scalpel blade, scrape away the fascia covering the skull. Separate the right lateral muscle (temporalis) from the bone and push it ventrally.
It is important to avoid bleeding as much as possible, to prevent blood clots from obscuring the cranial window.
-
Mark an area of interest over the cortex. Surround this area with a thin layer of CA glue applied to the skull, muscle, and wound margins to stop bleeding and prevent seepage of serosanguineous fluid. After the glue has dried, apply a thin layer of dental acrylic on top of the glue.
The layer of CA glue improves the adherence of the dental acrylic.
-
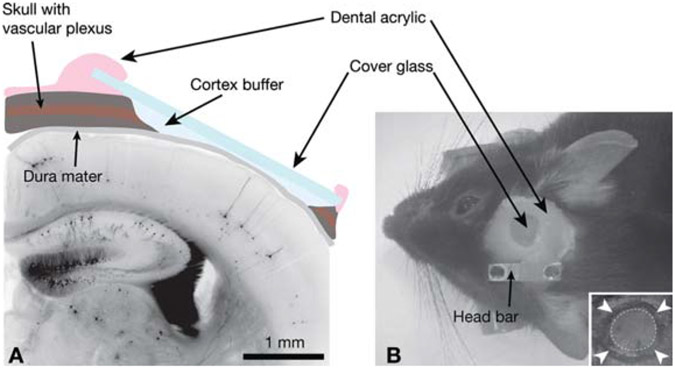
Use the dental drill to thin the circumference of an area of skull (2–3-mm-diameter) (Fig. 1B, inset). Work slowly to avoid heating the bone and damaging the dura mater. Keep the skull moist with cortex buffer.
A circular shape is the simplest, with an island of skull left intact in the center. The cortical surface vasculature should become visible where the skull is thinned, and there should be minimal bleeding from the skull and none from the brain. Take care not to apply pressure to the skull and brain or to puncture the skull while drilling.
-
Gently perforate the thinned skull with the sharp point of a pair of forceps, and lift up the island of bone within the drilled circle to expose the dura.
Do not exert any pressure on the brain. This removal is most successful if performed under a puddle of cortex buffer. Use Gelfoam to control any bleeding from the dura and skull; it can be applied directly to the dura when wet.
-
Cover the skull opening with the coverslip, which should lie flush with the skull. To reduce movement during imaging, a thin layer of molten 1.2% agarose can be applied to the brain surface before implanting the coverslip.
The coverslip should be ~100 μm from the brain surface and should be wider than the skull window. The agarose protects the brain from the air and the dental cement.
If agarose was used, carefully remove any excess from the edges of the window once it is set, and dry the remaining liquid on the skull.
Seal the optical window to the skull with dental acrylic, covering all exposed areas of skull, wound margins, and edges of the cover glass (Fig. 1B). Do not allow any glue to contact the dura, or it will lose its transparency.
-
Embed the stainless steel bar into the dental acrylic over the intact hemisphere (Fig. 1B).
The bar is used to stabilize the animal during imaging. Ensure that the screw threads are clear of acrylic, and allow the acrylic to set before manipulating the mouse or the bar (30 min is typically sufficient).
-
Perform imaging experiments for the duration of the anesthesia (typically up to 1 h after surgery using ketamine/xylazine anesthetic), if desired.
Go to Step 17.
- For chronic experiments, allow the mouse to recover on the heating pad before returning it to its cage. Keep the following points in mind:
- Buprenorphine (0.1 μg/g body weight, subcutaneous) and Carprofen (5 μg/g body weight, intraperitoneal) may be used to reduce postoperative pain and inflammation, respectively.
-
Allow 10–14 d for recovery before subsequent imaging sessions.The clarity of the cranial window often improves during the recovery period and the window can remain clear for months without the use of antibiotics. To reduce stress for the mouse, minimize the size of the stainless steel bar so that the mouse can freely move in its cage. A large cage with a high ceiling can help prevent the mice from knocking the dental acrylic and losing a bar or the entire window and headcap.
FIGURE 1.
Experimental preparation for in vivo imaging. (A) Schematic view of the cranial window in cross section superposed on a histologic section of neocortex from a GFP-M transgenic mouse. (B) External view of a cranial window implant; (inset) the circle of thinned bone (arrowheads) around an island of skull (dotted circle) that will be removed during the craniotomy.
Image Acquisition
-
16.
Anesthetize animals with ketamine and xylazine (65 and 6.5 μg/g, respectively). Place the animals under the microscope on a heating pad, and stabilize the head using screws placed through the bars.
-
17.
Trail 1.5% agarose around the edge of the imaging window, making a small enclosure to hold water for the objective during imaging.
-
18.
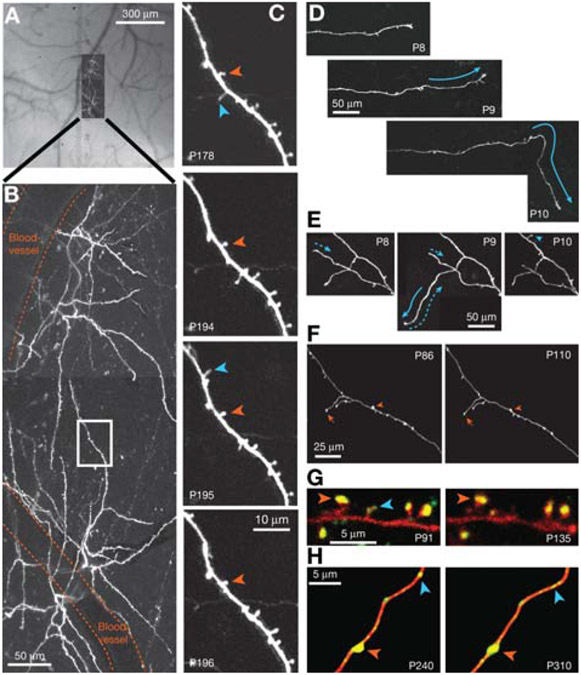
In the first imaging session, acquire a bright-field image of the vasculature in the dura mater (Fig. 2A).
This provides important anatomical landmarks that will be used to relocate previously imaged neurons in subsequent imaging sessions.
See Troubleshooting.
-
19.
Acquire images of apical dendritic tufts of fluorescently labeled pyramidal neurons using 2PLSM. To relocate previously imaged cells, use the unique vascular pattern near the cell to match fluorescent images to the bright-field image (Fig. 2A,B).
For high-magnification spine imaging of second and higher order branches, we typically select seven to 15 fields, each 50 × 50 μm, for each cell (Fig. 2C). Our image stacks typically consist of sections (512 × 512 pixels; μ0.09 μm/pixel, 2 msec/line) collected in 1-μm steps (three to five samples per resolution element).
See Troubleshooting.
-
20.
Collect low-magnification fluorescent images (512 × 512 pixels; ~0.3 μm/pixel; 3-μm steps) for overview (Fig. 2B). Take care to achieve close to identical fluorescence levels across imaged regions and imaging sessions by adjusting the excitation power.
FIGURE 2.
Long-term imaging of GFP-expressing pyramidal neurons in transgenic mice. (A) Composite bright-field image of the exposed dura mater and the high-resolution 2PLSM image in B, demonstrating how the vascular tree is used as a reference to locate specific neurons. (B) Top view of dendritic tufts on two pyramidal neurons in layer 5 (projection of a stack of optical sections). Blood vessels and a region of interest (white box) are indicated. (C) Long-term images revealing stable (orange) and transient (blue) dendritic spines from the region of interest (boxed in A). (D,E) Long-term images of postnatal thalamocortical neurons in the developing mouse neocortex showing axonal growth (solid arrows), retraction (dotted arrows), and a new terminaux bouton (blue arrowhead). (Modified, with permission, from Portera-Cailliau et al. 2005.) (F) Examples of stable branches (arrows) and boutons (arrowheads) on thalamocortical axons in the adult mouse neocortex. (Modified, with permission of Elsevier, from De Paola et al. 2006.) (G) Presumptive synaptic contacts indicated by the presence of PSD95-GFP (green) on DsRed-labeled dendritic spines (red); stable (orange arrowhead) and transient (blue arrowhead) synapses are indicated. (H) Synaptophysin-GFP (green) identifies synaptic contacts on axonal boutons expressing DsRed (red). Some synapses are stable (orange arrowhead), but other varicosities appear and disappear (blue arrowheads), possibly indicating synaptic changes. (G and H, Modified, with permission of Macmillan Publishers Ltd., from Holtmaat and Svoboda 2009.) In all images, (P) postnatal day. Panels A–C and F are GFP-M transgenics; D and F are mGFP-L21 transgenics; neurons in F and G were transfected in utero. All images are best projections, as described (Holtmaat et al. 2005; Portera-Cailliau et al. 2005; De Paola et al. 2006).
Image Analysis
-
21.Use predetermined criteria to score the images, as follows.
- Align related images from the same time point using stable anatomical reference points, such as dendritic branch points.
- Assign a single-blind code to the images to mask the experimental parameters (e.g., cortical region, developmental age, and experimental manipulation) before scoring by a naïve analyst.
- Use length measurements to quantify the appearance and disappearance of dendritic spines and terminaux axonal boutons.
-
Use threshold intensity ratio measurements to determine changes in the volume of spines or en passant boutons that are continuous with the axonal shaft (Holtmaat et al. 2005; De Paola et al. 2006).Threshold intensity ratio measurements are relatively easy to acquire from 2PLSM images, because the point-spread function in the z-axis typically exceeds the size of small structures such as dendritic spines and axonal boutons. Therefore, the integrated fluorescence intensity of these microstructures in a single optical section is a good indicator of their volumes (Holtmaat et al. 2005).
TROUBLESHOOTING
Problem (Step 18): The imaging window is obscured or the image quality is poor.
Solution: Consider the following:
Poor surgical technique is the most common source of problems with high-resolution chronic imaging. Surgical operations must be clean and performed consistently and quickly. The imaging window may become obscured by bleeding from the dura, edema of the neocortex, and infection. These adverse events can be avoided by keeping the operation sterile and by being as gentle as possible with dura and brain to minimize tissue damage and reaction.
Regrowth of the skull is inevitable and may occur after a few weeks or months.
Aberrant structural distortions and glial cell reactivity may also contribute to poor image quality and should be assessed at several time points after surgery using routine histological methods and immunostaining. These processes should be mild and transient (<2 wk for astrocytes, <1 wk for microglia) (Xu et al. 2007; Lee et al. 2008; Holtmaat et al. 2009).
Problem (Step 19): Boutons or dendritic spines are difficult to resolve.
Solution: Fluorophore expression may not be sufficiently high. Switch to a transgenic line with a stronger promoter to drive higher-level expression in the cell of interest.
DISCUSSION
To thoroughly understand adaptive changes in the brain, it is essential to monitor the structure and function of individual, identified neurons in the intact brain over long timescales. Using 2PLSM of fluorescently labeled cortical neurons, synaptic structures can be monitored in vivo over periods of minutes or months (Denk et al. 1990; Svoboda and Yasuda 2006). Typically, cortical neurons can be visualized to a depth of ~500 μm, deep enough to include layers 1–4 of the mouse neocortex.
For imaging neurons in vivo with 2PLSM, the fluorophores of choice are the green fluorescent protein (GFP) and its derivatives (XFPs). The spectral characteristics of these proteins are comparable to some of the better synthetic fluorophores, they have large extinction ratios and quantum efficiencies over a broad spectral range (e.g., 830–1020 nm for enhanced GFP [EGFP]), and they are quite resistant to photobleaching (Giepmans et al. 2006). One potential drawback is that high levels of fluorophore expression are necessary to visualize small cellular compartments, such as dendritic spines. It has been estimated that cytoplasmic GFP must be >1 μm to reliably detect dendritic spines (Niswender et al. 1995), which exceeds the concentration of the vast majority of endogenous proteins expressed by neurons.
Germline transgenesis has been the most common method for labeling neurons in vivo. Spatial and temporal control of fluorophore expression can be achieved using either a minimal promoter (Holtmaat et al. 1998) or engineered bacterial artificial chromosomes for transgenesis (Heintz 2004). The most commonly used transgenes (in our studies and others) for imaging neuronal structure in vivo use the Thy1 promoter to drive high-level expression of the fluorophore (e.g., GFP-M, yellow fluorescent protein [YFP]-H, mGFP-L21) in subpopulations of neurons (e.g., Caroni 1997; Feng et al. 2000; De Paola et al. 2003; Portera-Cailliau et al. 2005).
Advantages and Disadvantages of the Cranial Window
High-resolution chronic imaging in vivo can be achieved using either a glass-covered cranial window (Levasseur et al. 1975; Brown et al. 2001; Trachtenberg et al. 2002) or a thin-skull preparation (Christie et al. 2001; Grutzendler et al. 2002). Both techniques yield comparable results (Majewska et al. 2006; Holtmaat et al. 2009; Livneh et al. 2009) but have their own advantages and disadvantages. The cranial window provides excellent optical access and exposes a relatively large area (~0.8–12 mm2), and imaging can be repeated with unlimited frequency so long as the window does not become obscured, as discussed above. The cranial window is the method of choice for analysis of animals with a low proportion of fluorescently labeled cells (e.g., GFP-M), cell reconstructions, and post hoc anatomy (Knott et al. 2006). See Mostany and Portera-Cailliau (2008) and Holtmaat et al. (2009) for detailed descriptions and discussions of this method. In contrast, thinning of the skull (to ~20 μm) is usually performed over a very small area (~0.1–0.3 mm2) to avoid contusion of the underlying cortex and is therefore more suitable for mice with a high proportion of labeled cells (e.g., YFP-H). Although the thinning procedure must be repeated before every imaging session and can only be performed fewer than four times, it can be performed at any time during the life of the animal.
Quantification of Morphological Changes
The cranial window provides excellent optical access to fluorescently labeled neurons. In control experiments, including direct comparisons with electron microscopy, we have confirmed that even the smallest dendritic spines can be reliably detected in vivo using this method, with certain limitations (Holtmaat et al. 2005). First, analysis should be limited to protrusions with fluorescence intensity that is greater than severalfold above background, based on the standard deviation of the background fluorescence close to the dendrite. Second, a major drawback of long-working-distance, water-immersion objectives is their poor resolving power in the (optical) z-axis, owing to their low NA. Therefore, analysis should be restricted to protrusions that emanate laterally from the dendritic shaft, irrespective of apparent shape, whereas those that project above or below the dendrite in the z-axis should not be scored. We use custom software to analyze dendritic spines and axonal boutons in three dimensions rather than in projections, as discussed in detail in Holtmaat et al. (2005) and De Paola et al. (2006).
The reliability of measurements acquired in vivo should be assessed as follows: Estimates of spine and bouton densities acquired from in vivo images should be comparable with estimates from the same region in the fixed, dissected brain. Structural dynamics and spine or bouton densities should be stable over long time frames in naive adult mice (Trachtenberg et al. 2002; Holtmaat et al. 2005,2006, 2009; De Paola et al. 2006; Lee et al. 2006, 2008; Keck et al. 2008; Hofer et al. 2009).
Chronic in vivo imaging of the neocortex has revealed how dendritic and axonal structures can be either remarkably dynamic or stable during development and experience-dependent learning in adults (Fig. 2C-F; Grutzendler et al. 2002; Trachtenberg et al. 2002; Mizrahi and Katz 2003; Holtmaat et al. 2005; Portera-Cailliau et al. 2005; Zuo et al. 2005a; De Paola et al. 2006; Lee et al. 2006; Majewska et al. 2006; Stettler et al. 2006; Nishiyama et al. 2007). In vivo 2PLSM can also be used to study the distributions of synaptic proteins (Fig. 2G,H; Gray et al. 2006). Structural dynamics in developing and young-adult tissue often result in net changes of neuronal complexity and synaptic density (Fig. 2D,E; Holtmaat et al. 2005; Portera-Cailliau et al. 2005; Zuo et al. 2005a), whereas in naive adults there is a dynamic balance between neurite growth and retraction, and synapse formation and loss (Fig. 2C,F; Grutzendler et al. 2002; Trachtenberg et al. 2002; Holtmaat et al. 2005; De Paola et al. 2006; Lee et al. 2006; Majewska et al. 2006). Because growth and retraction of spines and boutons are linked to synapse formation and elimination (De Paola et al. 2006; Knott et al. 2006), the structural dynamics associated with new experiences or changes in sensory input may alter connectivity within neuronal networks, at least temporarily (Zuo et al. 2005b; Holtmaat et al. 2006; Keck et al. 2008; Hofer et al. 2009).
RECIPE
Cortex Buffer
125 mm NaCl
5 mm KCl
10 mm glucose
10 mm HEPES
2 mm MgSO4
2 mm CaCl2
Adjust pH to 7.4.
ACKNOWLEDGMENTS
This work was supported by the Howard Hughes Medical Institute (HHMI), National Institutes of Health (NIH), the Swiss National Science Foundation, the IRP Foundation, the Larry L. Hillblom Foundation, the March of Dimes Foundation, FRAXA, and the Dana Foundation.
REFERENCES
- Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. 2001. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 7: 864–868. [DOI] [PubMed] [Google Scholar]
- Caroni P 1997. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 71: 3–9. [DOI] [PubMed] [Google Scholar]
- Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, Hyman BT. 2001. Growth arrest of individual senile plaques in a model of Alzheimer’s disease observed by in vivo multiphoton microscopy. J Neurosci 21: 858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. 1990. Two-photon laser scanning microscopy. Science 248: 73–76. [DOI] [PubMed] [Google Scholar]
- De Paola V, Arber S, Caroni P. 2003. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci 6: 491–500. [DOI] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. 2006. Cell type–specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49: 861–875. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. 2006. The fluorescent toolbox for assessing protein location and function. Science 312: 217–224. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. 2006. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol 4: e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan WB. 2002. Long-term dendritic spine stability in the adult cortex. Nature 420: 812–816. [DOI] [PubMed] [Google Scholar]
- Heintz N 2004. Gene Expression Nervous System Atlas (GENSAT). Nat Neurosci 7: 483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W. 2005. Deep tissue two-photon microscopy. Nat Methods 2: 932–940. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. 2009. Experience leaves a lasting structural trace in cortical circuits. Nature 457: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658 (Erratum 10: 759). [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Oestreicher AB, Gispen WH, Verhaagen J. 1998. Manipulation of gene expression in the mammalian nervous system: Application in the study of neurite outgrowth and neuroregeneration-related proteins. Brain Res Brain Res Rev 26: 43–71. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45: 279–291. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. 2006. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature 441: 979–983. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee W-CA. 2009. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4: 1128–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hubener M. 2008. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat Neurosci 11: 1162–1167. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. 2006. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci 9: 1117–1124. [DOI] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. 2006. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol 4: e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Chen JL, Huang H, Leslie JH, Amitai Y, So PT, Nedivi E. 2008. A dynamic zone defines interneuron remodeling in the adult neocortex. ProcNatlAcadSci 105: 19968–19973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern E, Chen B, Svoboda K. 2000. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404: 876–881. [DOI] [PubMed] [Google Scholar]
- Levasseur JE, Wei EP, Raper AJ, Kontos AA, Patterson JL. 1975. Detailed description of a cranial window technique for acute and chronic experiments. Stroke 6: 308–317. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Feinstein N, Klein M, Mizrahi A. 2009. Sensory input enhances synaptogenesis of adult-born neurons. J Neurosci 29: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. 2006. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci 26: 3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. 2003. Dendritic stability in the adult olfactory bulb. Nat Neurosci 6: 1201–1207. [DOI] [PubMed] [Google Scholar]
- Mostany R, Portera-Cailliau C. 2008. A craniotomy surgery procedure for chronic brain imaging. J Vis Exp 12: 680. doi: 10.3791/680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama H, Fukaya M, Watanabe M, Linden DJ. 2007. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron 56: 472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Blackman SM, Rohde L, Magnuson MA, Piston DW. 1995. Quantitative imaging of green fluorescent protein in cultured cells: Comparison of microscopic techniques, use in fusion proteins and detection limits. J Microsc 180: 109–116. [DOI] [PubMed] [Google Scholar]
- Pologruto TA, Sabatini BL, Svoboda K. 2003. ScanImage: Flexible software for operating laser-scanning microscopes. Biomed Eng Online 2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C, Weimer RM, Paola VD, Caroni P, Svoboda K. 2005. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol 3: e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. 2006. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron 49: 877–887. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. 2006. Principles oftwo-photon excitation microscopy and its applications to neuroscience. Neuron 50: 823–839. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. 2002. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420: 788–794. [DOI] [PubMed] [Google Scholar]
- Xu HT, Pan F, Yang G, Gan WB. 2007. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci 10: 549–551. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. 2005a. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46: 181–189. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. 2005b. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436: 261–265. [DOI] [PubMed] [Google Scholar]