Figure 3.
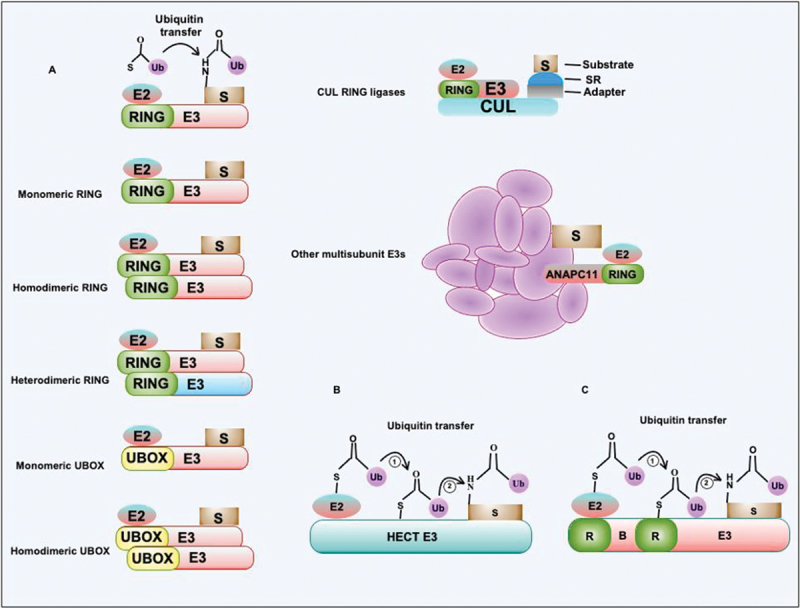
E3-Ub ligase uses a distinct mechanism for the transfer of Ub to substrates. (a) E3-Ub ligase containing the RING domain transfers Ub to target protein substrates from E2-Ub conjugating complex. RING or similar UBOX containing E3-Ub ligases exist in multiple oligomeric states such as monomers, homodimers, and heterodimers. CUL3-E3 RING ligase comprises multiple subunits that coordinate interaction with the substrate. The complex contains adapter protein and substrate-interacting protein together with various CUL isoforms. The anaphase-promoting complex/cyclosome contains multiple subunits that coordinate interaction between target substrates and RING domain-containing E3-Ub ligase together with E2-Ub conjugating enzyme. The role and functions of multi-subunit complex E3-Ub ligases are emerging now. These complexes perform more complex hetero-conjugation of Ub to the substrates. (b and c) The HECT and RBR type E3-Ub ligases transfer Ub from E2-Ub conjugating enzymes to HECT or RING domain conserved cysteines followed by the Ub transfer to target substrates. S, substrate; Ub, ubiquitin; SR, substrate receptor.
