Abstract
Background and Hypothesis
Stress during adolescence is a major risk factor for schizophrenia. We have found previously in rats that adolescent stress caused, in adulthood, behavioral changes and enhanced ventral tegmental area (VTA) dopamine system activity, which were associated with dysregulation of the excitatory-inhibitory (E/I) balance in the ventral hippocampus (vHip). Levetiracetam, an anticonvulsant drug, regulates the release of neurotransmitters, including glutamate, via SV2A inhibition. It also modulates parvalbumin interneuron activity via Kv3.1 channels. Therefore, levetiracetam could ameliorate deficits in the E/I balance. We tested whether levetiracetam attenuate the adolescent stress-induced behavioral changes, vHip hyperactivity, and enhanced VTA dopamine system activity in adult rats.
Study Design
Male Sprague-Dawley rats were subjected to a combination of daily footshock (postnatal day [PD] 31–40), and three 1 h-restraint stress sessions (at PD31, 32, and 40). In adulthood (PD62), animals were tested for anxiety responses (elevated plus-maze and light-dark box), social interaction, and cognitive function (novel object recognition test). The activity of vHip pyramidal neurons and VTA dopamine neurons was also recorded.
Study Results
Adolescent stress produced anxiety-like responses and impaired sociability and cognitive function. Levetiracetam (10 mg/kg) reversed these changes. Levetiracetam also reversed the increased VTA dopamine neuron population activity and the enhanced firing rate of vHip pyramidal neurons induced by adolescent stress.
Conclusions
These findings suggest that levetiracetam attenuates the adverse outcomes associated with schizophrenia caused by stress during adolescence.
Keywords: levetiracetam, psychosis, adolescence, dopamine system, ventral hippocampus
Introduction
Adolescence is characterized by several dynamic changes in physiological and social processes.1 These changes can interfere with brain maturation, making adolescence a period of greater susceptibility to adverse socioenvironmental factors, such as stress.2 It is already well-established that stress is a major risk factor for developing psychiatric disorders, including depression, anxiety disorders, and schizophrenia.3–5
The time of stress exposure has been recognized as a critical factor in determining its consequences. For example, the exposure to adverse socioenvironmental factors, such as trauma and psychosocial adversities, during childhood, and adolescence is a well-recognized risk factor for stress for the development of psychotic disorders later in life.6,7 Individuals with psychosis report experiencing more frequent and severe abuse during childhood/adolescence than people without psychiatric conditions.8 In addition, childhood trauma is highly associated with schizophrenia even without a family history of psychosis.9
In rodent studies, stress during adolescence caused behavioral and electrophysiological changes in adulthood similar to those found in animal models relevant for the study of schizophrenia. Similar to the methylazoxymethanol acetate (MAM) developmental disruption model for schizophrenia,10 animals exposed to adolescent stress presented an increased activity of dopamine neurons in the ventral tegmental area (VTA) and the associated hyperactivity of the ventral hippocampus (vHip).11,12 The latter results from a loss of local parvalbumin (PV)-containing GABAergic interneurons.12 These findings are consistent with the proposal that the hyperdopaminergic state in schizophrenia results from an excitatory-inhibitory (E/I) imbalance in brain regions involved in regulating the VTA dopamine system, such as the vHip.13 In this context, drugs that act through the modulation of the E/I processes have emerged as candidates for treating disorders associated with deficits in this balance, including schizophrenia.14
Levetiracetam is a multi-target drug clinically used as an anticonvulsant. Levetiracetam is proposed to modulate processes related to the excitatory-inhibitory balance, such as GABA and glutamate release, through several mechanisms that include the modulation of the synaptic protein SV2A and Kv3.1 potassium channels, which are critical for the fast-spiking activity of parvalbumin interneurons.15,16 Drug repurposing studies using machine learning approaches revealed levetiracetam as a promising candidate for treating schizophrenia17 and it was recently proposed that levetiracetam could effectively attenuate schizophrenia symptoms by reducing the increased hippocampal activity found in the disease.18 Ongoing clinical trials are investigating this hypothesis (NCT02647437 and NCT03034356). In addition, a recent controlled, randomized clinical trial with 40 chronic schizophrenia patients indicated that the treatment with levetiracetam for 8 weeks produced significant effects on clinical symptoms, especially negative symptoms and cognitive functions.19
Here, we tested whether levetiracetam could attenuate long-term changes resembling schizophrenia caused by adolescent stress exposure, including behavioral deficits, increased VTA dopamine system activity, and vHip hyperactivity.
Methods
Animals
Adult male and female Sprague-Dawley rats obtained from Central Animal House of the University of São Paulo, Ribeirão Preto Campus, were housed together (1 male and 1 female) to copulate. After detecting spermatozoids in the vaginal smear, the male rat was removed, making pregnancy day 0. On a postnatal day (PD) 21, the offspring were weaned and randomly housed in groups of 3–4 per cage. Animals were housed in micro-isolated cages, with water and food available ad libitum, and under standard laboratory conditions. Offspring from several litters were used, and rats from the same litter were assigned to the different experimental groups. All of the animals in the same cage were devoted to the same experimental procedure. Only males were used in this study since we have previously found that female rats were resistant to present behavioral and electrophysiological changes after exposure to the same stress protocol.20 All procedures were performed following Brazilian and International regulations for the care and use of laboratory animals. The experimental protocols were approved by the Ribeirão Preto Medical School Ethical Committee (#155/2018).
Stress Protocol
Rats were subject to the stress protocol during adolescence (PD31–40) as previously described.11,12,21 Adolescent rats were exposed to a combination of daily inescapable footshocks for 10 days (PD31–40) and 3 restraint stress sessions (PD31, 32, and 40). In each footshock session, 25 scrambled footshocks (1.0 mA, 2 s) were delivered every 60 ± 20 s. Each animal was placed in a Plexiglas cylindrical size-adjusted restraint tube for 1 h for the restraint stress. Restraint stress was done immediately after the footshock session. After the stress protocol, animals were kept in their home cages until adulthood (PD65), when the behavioral tests and electrophysiological recordings were performed. Naïve animals were left undisturbed in their home cages.
Drugs
Levetiracetam (10 mg/kg i.p., Sigma, USA) was dissolved in saline (0.9% NaCl). All drugs were intraperitoneally (i.p.) injected in a 1 ml/kg final volume. The dose of levetiracetam was based on previous work showing that levetiracetam attenuated behavioral deficits in an animal model relevant for schizophrenia.22
Experimental Design
The work was carried out in 3 experimental cohorts. Adolescent male rats were subjected to a combination of footshocks and restraint stress. In the first experimental cohort, in adulthood, animals were tested for anxiety responses (elevated plus-maze, PD65) and cognitive function (novel object recognition test, PD66–67). In the second cohort, animals were tested again for anxiety responses (light-dark test, PD65) and social behavior (social interaction test, PD66). One week after the behavioral tests, animals were subjected to in vivo electrophysiological recordings of VTA dopamine neurons (PD73–105). In the third cohort, animals were subjected to in vivo electrophysiological recordings of vHip pyramidal neurons (PD65–85). Animals were treated with levetiracetam or saline 30 min before each behavioral test or electrophysiological recording, except for the novel object recognition test in which animals were treated immediately after the acquisition trial. The following experimental groups were evaluated: Naive rats treated with saline, naive rats treated with levetiracetam, stressed rats treated with saline, and stressed rats treated with levetiracetam.
Procedures
Elevated Plus-Maze.
Anxiety-like behaviors were evaluated in the elevated plus-maze and light-dark box tests. The elevated plus-maze consisted of 2 opposite wooden open arms (50 × 10 cm), crossed at a right angle by 2 arms of the same dimensions enclosed by 40-cm-high walls with no roof. The maze was located 50 cm above the floor, and a 1-cm high edge made of Plexiglas surrounded the open arms to prevent falls. Rat behaviors were filmed for 5 min and analyzed using the Any-Maze software (Stoelting, USA). The parameters analyzed were the percentage of entries and time in the open arms and the number of entries into the enclosed arms. The percentage of the time in open arms was calculated with time in open and closed arms, excluding time in the center of the EPM.
Light-Dark Box
The light-dark box consisted of an acrylic box containing 2 compartments (23 × 20 × 28 cm each), 1 dark and 1 light, interconnected by a small opening. The animal was placed in the light zone and allowed to move freely between the 2 compartments for 10 min. The number of entries and time spent in the light zone were recorded using the Any-Maze software.
Novel Object Recognition Test
The novel object recognition test is widely used to evaluate cognitive function. It was carried out in a circular arena (60 cm diameter and 65 cm height). Animals were habituated to the arena for 15 min. On the following day, animals were subjected to 2 trials separated by 1 h. During the first trial (acquisition trial), rats were placed in the arena containing 2 identical objects for 5 min. For the second trial (retention trial), one of the objects presented in the acquisition trial was replaced by an unknown (novel) object. Animals were then placed back in the arena for 5 min. The behavior was recorded on video for blind scoring of object exploration, which was defined as situations where the animal is directing its face to the object at a distance of approximately 2 cm while watching, licking, sniffing, or touching it with the forepaws. Recognition memory was assessed using the discrimination index, corresponding to the difference between the time exploring the novel and the familiar object, corrected for the total time exploring both objects (discrimination index = [novel − familiar/ novel + familiar]).
Social Interaction
The social interaction test is used to measure sociability. The test was performed in a circular arena (60 cm in diameter and 65 cm in height). Each animal was placed in the arena for 5 min for habituation. Immediately after habituation, an unfamiliar rat of the same strain, sex, and age was placed in the arena for 10 min. The time of active social behavior of the experimental rat as sniffing, following, grooming, and climbing on or under the other rat was recorded.
In Vivo Recordings of VTA Dopamine Neuron Activity
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma) and mounted on a stereotaxic frame. The coordinates for the VTA were 5.3–5.5 mm posterior from bregma, 0.6 mm lateral to the midline, and 6.5–9.0 mm ventral from the brain surface. Glass electrodes filled with 2% Chicago Sky Blue in 2 M NaCl were lowered through 6–9 vertical tracks in a predetermined pattern within the VTA of each rat to enable the comparison between dopamine neurons located throughout the medial, central, and lateral subregions of the VTA. Dopamine neurons were identified according to well-established electrophysiological features.23 Three parameters were measured: population activity (the number of spontaneously active dopamine neurons per electrode track), average firing rate, and the percentage of action potentials occurring in bursts (burst activity). Each identified dopamine neuron was recorded for 1–3 min. At the end of the recording, the recording sites were marked via iontophoretic ejection of Chicago Sky Blue dye from the electrode (20 μA constant negative current, 20 min) for posterior histological confirmation of the electrode sites.
In Vivo Recordings of vHip Pyramidal Neurons
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma) and mounted on a stereotaxic frame. The coordinates for the vHip were 5.8 mm posterior from bregma, 4.8 mm lateral to the midline, and 6.0–8.5 mm ventral from the brain surface. Electrodes were lowered through 6 tracks inside the vHip. Pyramidal neurons were identified by typical electrophysiological characteristics such as firing rate (average up to 2 Hz) and action potential shape (half-width > 0.4 ms). The firing rate of these neurons was measured. Each identified pyramidal neuron was recorded for 1–3 min. At the end of the recording, the electrode sites were marked via iontophoretic ejection of Chicago Sky Blue dye from the electrode (20 μA constant negative current, 20 min) for posterior histological confirmation of the electrode sites.
Statistical Analysis
Data were presented as the mean ± the standard error of the mean (SEM). All the data were subjected to tests to verify the homogeneity of variances (Bartlett’s test) and if they followed a normal distribution (Shapiro–Wilk test). Those that met these parameters were subjected to parametric analysis (2 or 3-way ANOVA followed by the Tukey’s post-test or Student’s t-test). Otherwise, they were subjected to nonparametric analysis (Kruskal–Wallis followed by the Dunn’s test). P < .05 was considered significant.
Results
Levetiracetam Attenuates Adolescent Stress-induced Anxiety-like Responses in Rats as Adults
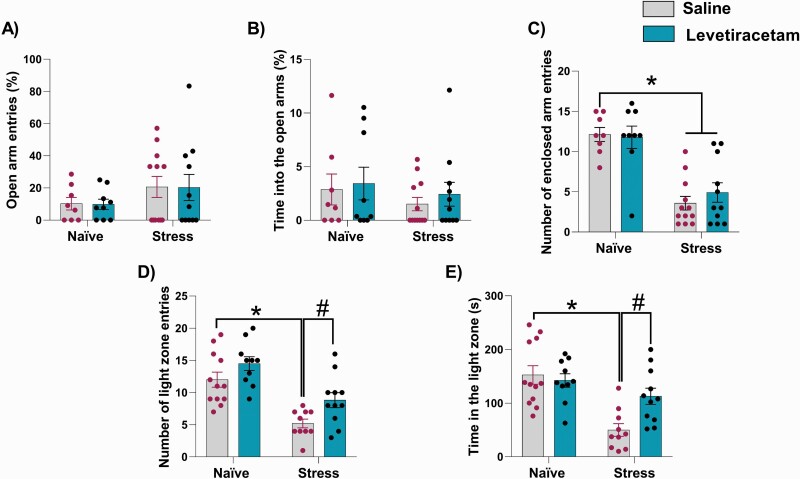
In the elevated plus-maze, no change in the percentage of entries and time spent in the open arms was found across all groups (% of open arm entries: H = 0.69, P = .87; % open arm time: H = 1.21, P = .74; Kruskal–Wallis test for both; figures 1A and B). Consistent with other studies,24 stress exposure decreased the number of entries into the enclosed arms. A 2-way ANOVA indicated an effect of stress (F1,36 = 47.54, P < .0001), but no treatment effect (F1,36 = 0.19, P = .66) or interaction between stress and treatment (F1,36 = 0.56, P = .46). Post hoc analysis indicated that stressed animals, regardless of treatment, showed a decrease in the number of entries into the closed arms (P < .05 vs naive-saline; Tukey’s post-test; figure 1C). Levetiracetam did not attenuate this change in stressed animals.
Fig. 1.
Effects of levetiracetam on adolescent stress-induced anxiety-like responses in rats as adults. In the elevated plus-maze (n = 8–12/group), stress during adolescence did not change (A) % of entries and (B) time spent in the open arms. However, it decreased (C) the number of entries into the enclosed arms, which was not attenuated by levetiracetam. In the light-dark box test (n = 10–11/group), adolescent stress decreased (D) the number of entries and (E) time in the light zone. Levetiracetam attenuated these changes. *P < .05 vs naive-salina and #P < .05 vs stress-saline, two-way ANOVA followed by Tukey’s post-test.
In the light-dark test, a 2-way ANOVA showed a significant effect of condition (stress) for the number of entries into the light zone (F1,39 = 34.13, P < .0001) and time in the light zone (F1,39 = 19.63, P < .0001). There was no treatment effect, but there was an interaction between the stress and treatment for the time spent in the light zone (F1,39 = 6.12, p = .02). Post hoc analysis indicated a decrease in the number of entries and time in the light zone in saline-treated stressed rats (P < .05 vs naive-saline; Tukey’s post-test). These changes were not found in levetiracetam-treated stressed rats (figures 1D and E), indicating that levetiracetam treatment in adulthood attenuated anxiety-like responses in the light-dark test induced by adolescent stress. In naïve rats, levetiracetam did not induce any change in the elevated plus-maze and light-dark test.
Levetiracetam Reverses Impairments in Cognition and Sociability Induced By Adolescent Stress
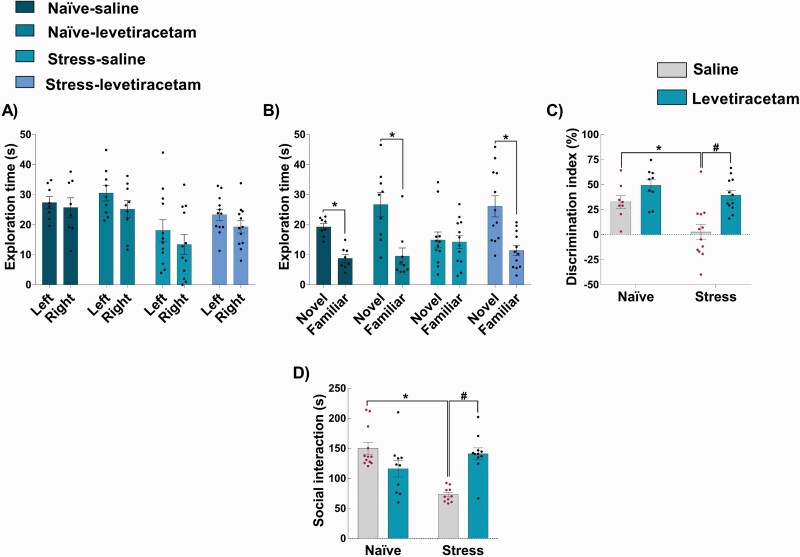
In the novel object recognition test, there was no difference between the exploration of the identical objects placed in the right or left side of the arena during acquisition session (naive-saline: t14 = 0.44, P = .66; naive-levetiracetam: t16 = 1.37, P = .19; stress-saline: t22 = 0.97, P = .34; stress-levetiracetam: t22 = 1.48, P = .15; figure 2A), indicating a lack of spatial preference. In the retention session, a greater exploration of the novel object was observed in naive-saline (t14 = 6.23, P < .0001), naive-levetiracetam (t16 = 3.57, P = .0026), and stress-levetiracetam (t22 =3.75, P = .0011), but not in stress-saline animals (t22 = 0.12, P = .84; figure 2B). These findings were reflected in the discrimination index (figure 2C). A 2-way ANOVA showed an effect of stress (F1,37 = 9.2, P = .0043) and treatment (F1,37 = 16.56, P = .0002). However, the interaction between stress and treatment factors did not reach the significance threshold (F1,37 = 2.41, P = .12). These findings indicate that stress exposure impaired object recognition memory and levetiracetam improved it (main effect of drug), irrespective of stress as the main result, a particularly pronounced improvement in stressed rats is indicated indirectly by paired testing (P < .05, stress-levetiracetam vs stress-saline, Tukey’s post-test; figure 2C).
Fig. 2.
Effects of levetiracetam on adolescent stress-induced changes in the novel object recognition test and social interaction in rats as adults. In the novel object recognition test (n = 8–12/group), (A) all groups explored equally the identical objects placed on the left and right sides of the arena in the acquisition trial. (B) In the retention trial, all groups explored more the novel object, except the stress-saline group. *P < .05, Student’s t-test between each group. This is reflected in (C) the discrimination index, with stress-saline animals showing a decrease in the discrimination index reversed by levetiracetam. Also, (D) adolescent stress decreased the social interaction time (n = 10–12/group) which was also reversed by levetiracetam. *P < .05 vs naive-saline and #P < .05 vs stress-saline, two-way ANOVA followed by Tukey’s post-test.
For the social interaction test, a 2-way ANOVA indicated an effect of stress (F1,38 = 5.60, P = .02), treatment (F1,38 = 4.70, P = .04), and an interaction between stress and treatment (F1,38 = 33.30, P < .0001). Post hoc analysis indicated a decrease in the social interaction time in saline-treated stressed rats compared to controls (P < .05 vs naive-saline, Tukey’s post-test). This impairment was reversed by levetiracetam (P < .05 vs stress-saline, Tukey’s post-test; figure 2D). In naïve rats, levetiracetam did not cause any effect in the novel object recognition test and social interaction.
Adolescent Stress Increased VTA Dopamine System Activity in Adults, Which was Reversed By Levetiracetam
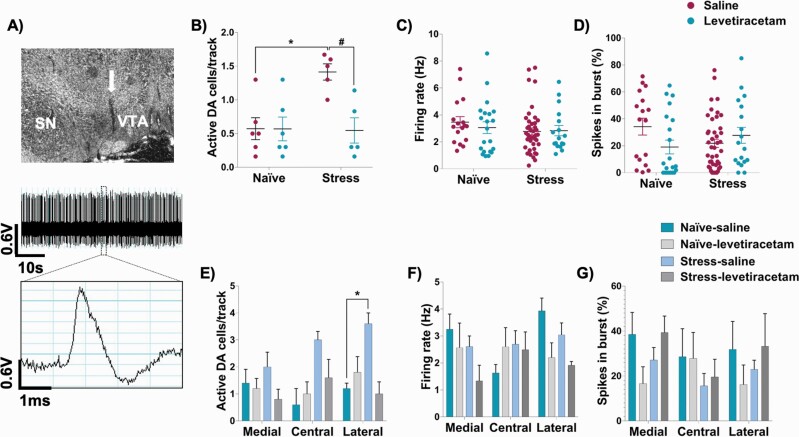
A representative trace of recordings from dopamine neurons in the VTA is shown in figure 3A. Stress exposure in adolescent rats increased the number of spontaneously active VTA dopamine neurons in adulthood. A 2-way ANOVA indicated a significant effect of stress (F1,18 = 6.07, P = .02), treatment (F1,18 = 6.85, P = .02), and an interaction between these factors (F1,18 = 6.75, P = .02). Tukey’s post-test indicated that the stress-saline group showed an increased number of spontaneously active VTA dopamine neurons (P < .05 vs naive-saline). This change was reversed by levetiracetam (P < .05 vs stress-saline; figure 3B). No significant effect was observed for the firing rate and % burst activity of the identified DA neurons (figures 3C and D).
Fig. 3.
Effects of levetiracetam on the adolescent stress-induced changes in the VTA dopamine neuron activity. (A) The final location of the last track was marked by electrophoretic ejection of dye (indicated by the arrow) for histological verification. A 1-min segment of spontaneous activity and a representative waveform of VTA dopamine neuron neurons are shown. (B) Adolescent stress increased VTA dopamine neuron population activity, which was reversed by levetiracetam. No changes in (C) firing rate and (D) burst activity was found (naive-saline: 6 rats, 17 dopamine neurons; naive-levetiracetam: 6 rats, 21 dopamine neurons; stress-saline: 5 rats, 43 dopamine neurons; stress-levetiracetam: 6 rats, 18 dopamine neurons). *P < .05 vs naive-saline and #P < .05 vs stress-saline; two-way ANOVA, followed by Tukey’s post-test. Subregion analysis indicated that adolescent stress-induced changes (E) in population activity were confined to the lateral VTA, with no change in (F) firing rate, and (G) burst activity across VTA subregions. *P < .05 vs naive-saline; three-way ANOVA followed by Tukey’s post-test.
We further evaluate possible differences across VTA subregions (medial, central, and lateral). For population activity, 3-way ANOVA indicated an effect of stress (F1,16 = 4.64, P = .046) and interactions between VTA subregion and stress (F2,32 = 3.74, P = .035), and stress and treatment (F1,16 = 7.26, P = 0.016), reflecting an increase in the number of spontaneously active dopamine neurons only in the lateral VTA of the saline-treated stressed rats (P < .05 vs naive-saline; Tukey post-test; figure 3E). No significant effect was observed for the firing rate and % burst activity across the VTA subregions (figures 3F and G).
Levetiracetam Reversed Ventral Hippocampus Hyperactivity Induced By Adolescent Stress
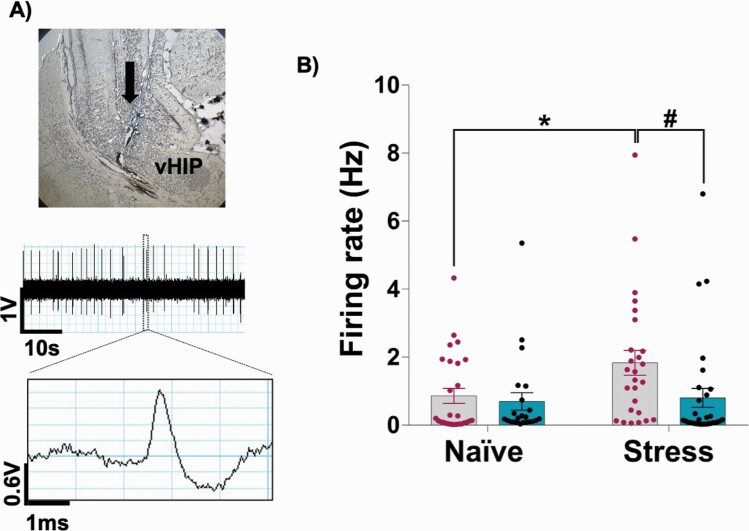
A representative trace of recordings from pyramidal neurons in the vHip is shown in figure 4A. Adolescent stress increased the firing rate of vHip pyramidal neurons in rats as adults, which was reversed by acute levetiracetam. The Kruskal–Wallis test indicated a significant effect (H = 10.19, P = .01). The Dunn’s post-test revealed that saline-treated stressed animals showed an increased firing rate of vHip pyramidal neurons (P < .05 vs naive-saline). This hyperactivity was reversed by levetiracetam (P < .05 vs stress-saline; figure 4B).
Fig. 4.
Effects of levetiracetam on the firing rate of vHip pyramidal neurons in adult animals exposed to adolescent stress. (A) The final location of the last track was marked by electrophoretic ejection of dye (indicated by the arrow) for histological verification. A 1-min segment of spontaneous activity and a representative waveform of vHip pyramidal neuron are shown. (B) Saline-treated stressed animals showed an increased firing rate of vHip pyramidal neurons, which was reversed by levetiracetam (naive-saline: 7 rats, 27 pyramidal neurons; naive-levetiracetam: 5 rats, 23 pyramidal neurons; stress-saline: 6 rats, 26 pyramidal neurons; stress-levetiracetam: 6 rats, 31 pyramidal neurons). *P < .05 vs naive-saline and #P < .05 vs stress-saline, Kruskal–Wallis test followed by the Dunn’s post-test.
Discussion
This study sought to evaluate if acute levetiracetam administration in adult rats would attenuate the behavioral changes and the increase of the VTA dopamine system and vHip activity induced by stress exposure during adolescence. As has already been described,11,12 adolescent stress increased anxiety-like behaviors, decreased sociability, and causes cognitive deficits in adulthood. These changes were associated with an increase in VTA dopamine neuron population activity and the firing rate of vHip pyramidal neurons. These changes are similar to those found in animal models relevant for schizophrenia.25,26 Acute treatment with levetiracetam in adult rats attenuated most of the changes induced by stress in adolescence. It also reduced the increased VTA dopamine neuron population activity and vHip hyperactivity observed in these animals.
Adolescent stress-induced anxiety responses were evaluated in 2 widely used tests, the elevated plus-maze and the light-dark box test.27,28 In the elevated plus-maze, we did not observe changes in the open arms exploration, which is used as an index of anxiety. However, stressed animals showed fewer entries into the closed arms. Validation studies using factorial analysis29,30 have associated the number of enclosed arm entries with general exploratory activity. Based on these studies, it was proposed that anxiety-specific effects should be reflected by changes in open arms exploratory activity without changes in enclosed arm entries. Despite this suggestion, however, some authors have proposed that stress exposure could also change (decrease) this latter variable in the EPM.24,31,32 Even if this was the case, levetiracetam failed to change this parameter in the present study. However, in another animal test to evaluate anxiety, the light-dark box, levetiracetam did reverse the anxiety-like responses, indicated by a decrease in the exploration (entries and time) of the light compartment caused by adolescent stress exposure. These results corroborate findings of other studies suggesting that this compound may relieve anxiety.33,34
Similar to previous studies from our group,11 the combination of footshock and restraint stress applied to adolescent rats impaired novel object recognition in adulthood, indicating that this adolescent stress caused deficits in cognitive function. Levetiracetam had a general procognitive effect in this test, reversing these changes in stressed animals. These findings agree with results showing that levetiracetam reversed cognitive impairments in an animal model for schizophrenia based on NMDA receptor hypofunction.22 In addition to anxiety responses and cognition deficits, adolescent stress resulted in decreased social interaction in adulthood, which was also reversed by levetiracetam.
Adolescent stress also causes long-term changes in the dopamine system.35,36 With the same stress protocol used in the present study, we have previously found that the exposure of adolescent rats to stress increases the number of spontaneously active dopamine neurons in the VTA and enhances the locomotor response to amphetamine that persists until adulthood.11,12 Similar findings have been consistently found in the MAM model for schizophrenia.25,26 Here, we found that adolescent stress increased VTA dopamine neuron population activity, which was reversed by levetiracetam.
In rodents, the VTA can be anatomically divided in a mediolateral manner based on an overall topography in the efferent projections to the forebrain.37 Dopamine neurons in the medial and central VTA subregions project to the prefrontal cortex, amygdala, and reward-related nucleus accumbens shell, whereas dopamine neurons in the lateral VTA project to the nucleus accumbens core and associative striatum.37,38 Similar to our previous study,11 VTA subregion analyses indicated that an increase in the number of spontaneously active VTA dopamine neurons in stressed animals is significant only in the lateral VTA. The lateral VTA projects to the associative striatum,37 the same region in which the hyperdopaminergic state in schizophrenia patients seems to be more pronounced.37,39
Increases in the VTA dopamine system activity are driven by vHip hyperactivity through the dysregulation of a vHip-nucleus accumbens-ventral pallidum-VTA pathway.13,40 For instance, in the MAM model for schizophrenia, MAM rats show increases in VTA dopamine neuron population activity and the firing rate of vHip pyramidal neurons. In these animals, vHip inhibition normalizes VTA dopamine neuron activity.10 In schizophrenia patients, hippocampal hyperactivity has also been associated with striatal hyperdopaminergic states and psychotic symptoms.41 Similar to the MAM model, we have previously found that stress during adolescence increases VTA dopamine neuron activity and produces vHip hyperactivity in adult rats. Here, we replicated these findings. We also showed that the acute levetiracetam treatment before the in vivo recordings reversed adolescent stress-induced changes in the activity of dopamine neurons in the VTA and pyramidal neurons in the vHip. Similarly, levetiracetam attenuated age-related hippocampal hyperactivity and cognitive impairment in animal models42 and elderly individuals with mild cognitive impairment.43
In schizophrenia, an abnormally high hippocampal activity appears to result from a functional loss of parvalbumin-containing GABAergic interneurons.44–46 A decreased number of parvalbumin interneurons in the hippocampus is a robust finding in the postmortem brain of schizophrenia patients47 and several rodent models for schizophrenia.48 In addition, exposure to stress during adolescence negatively impacts parvalbumin interneuron function.35 We have found previously that adolescent stress decreases the number of parvalbumin interneurons in the vHip.12 This change underlies long-term circuit-level disruptions (ie, hippocampal hyperactivity and the associated VTA dopamine system dysregulation) and behavioral deficits. In addition to the dopamine dysregulation, a hyperactive vHip can also interfere with other circuits. For instance, the vHip projects to the prefrontal cortex and amygdala. Disruption of these regions could lead to cognitive deficits and interfere with emotional responses.49,50 Thus, the anxiety responses, decreased sociability, and cognitive deficits caused by adolescent stress may be associated with vHip activity. By reversing aberrant vHip activity, levetiracetam could attenuate the behavioral changes caused by adolescent stress.
Although we have not explored how levetiracetam produces its effects, several studies suggest that this drug attenuates changes related to E/I imbalance by decreasing glutamate-dependent excitatory transmission and/or facilitating GABAergic neurotransmission.18 These effects are modulated by levetiracetam’s actions on different pharmacological targets, including voltage-gated calcium channels,51 the SV2A glycoprotein,52,53 facilitation of GABAergic neurotransmission,54 and modulation of the potassium channels Kv3.1.15 In vitro studies showed that levetiracetam inhibits voltage-gated calcium channels, which are essential for action potential maintenance in the hippocampal pyramidal neurons, resulting in decreased excitatory neurotransmission.51 This mechanism could contribute to normalizing the increased firing rate of vHip pyramidal neurons caused by adolescence stress exposure.
Levetiracetam effects may involve interference in the GABAergic neurotransmission. Levetiracetam modulates Kv3.1 potassium channels,15 of which are abundant in parvalbumin interneurons and play a critical role in regulating their fast-spiking activity.55 Preclinical and human studies have indicated the potential of Kv3.1 modulators to treat schizophrenia symptoms.56,57 Therefore, levetiracetam could also attenuate adolescent stress-induced changes by normalizing the functional loss of parvalbumin interneurons.
Another mechanism of action potentially involved in the effects of levetiracetam in the present study is the modulation of the SV2A glycoprotein. The anticonvulsant effects of levetiracetam are thought to involve the modulation of this glycoprotein, attenuating an increased excitatory neurotransmission found in epilepsy.58 Although its function is not entirely known, SV2A could participate in calcium-dependent exocytosis-related processes, maintenance of neurotransmitters in synaptic vesicles, and transport of vesicle constituents.53 Thus, through these mechanisms, levetiracetam could produce its effects in adolescence-stressed animals by modulating the dysregulation of the vHip-nucleus accumbens-ventral pallidum-VTA pathway. Corroborating this proposal, it was found that levetiracetam reduces excitatory neurotransmission in the nucleus accumbens.59 However, further studies are needed to advance our understanding of the mechanisms underlying adolescent stress-induced abnormalities and by which levetiracetam attenuated these changes.
In conclusion, we found that stress exposure during adolescence leads to long-term behavioral changes and circuit-level disruptions similar to those observed in schizophrenia patients. Acute treatment with levetiracetam attenuates these changes. Therefore, our findings support the continuation of preclinical and clinical research that investigated levetiracetam as a potential drug for the treatment of stress-related developmental psychiatric disorders such as schizophrenia.
Acknowledgments
The authors thank Marco Antonio de Carvalho, Eleni Tamburus Gomes, and Eliane Aparecida Antunes Maciel for technical assistance. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Andreza M Cavichioli, Department of Pharmacology, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Thamyris Santos-Silva, Department of Pharmacology, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Anthony A Grace, Departments of Neuroscience, Psychiatry, and Psychology, University of Pittsburgh, Pittsburgh, PA, USA.
Francisco S Guimarães, Department of Pharmacology, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Felipe V Gomes, Department of Pharmacology, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, São Paulo, Brazil.
Funding
This study was funded by the São Paulo Research Foundation (FAPESP – 2018/ 17597-3 to F.V.G.), International Brain Research Organization (IBRO Return Home Fellowship to F.V.G.), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. AMC received a Master fellowship from the São Paulo Research Foundation (FAPESP – 2020/ 04241-6). F.V.G. receives a Research Productivity Fellowship – Level 2 granted by the National Council for Scientific and Technological Development (CNPq – 303137/2021-5).
Disclosures
A.A.G. has received consulting fees from Alkermes, Lundbeck, Takeda, Roche, Lyra, Concert, and SynAgile, and research funding from Lundbeck.
References
- 1. Sisk CL, Foster DL.. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. [DOI] [PubMed] [Google Scholar]
- 2. Paus T, Keshavan M, Giedd JN.. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [DOI] [PubMed] [Google Scholar]
- 4. Ray A, Gulati K, Rai N.. Stress, anxiety, and immunomodulation: a pharmacological analysis. Vitam Horm. 2017;103:1–25. [DOI] [PubMed] [Google Scholar]
- 5. Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 6. van Os J, Kenis G, Rutten BP.. The environment and schizophrenia. Nature. 2010;468(7321):203–212. [DOI] [PubMed] [Google Scholar]
- 7. Rosenfield PJ, Jiang D, Pauselli L.. Childhood adversity and psychotic disorders: epidemiological evidence, theoretical models and clinical considerations. Schizophr Res. 2021. doi: 10.1016/j.schres.2021.06.005. In Press. [DOI] [PubMed] [Google Scholar]
- 8. Mauritz MW, Goossens PJ, Draijer N, van Achterberg T.. Prevalence of interpersonal trauma exposure and trauma-related disorders in severe mental illness. Eur J Psychotraumatol. 2013;4:19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popovic D, Schmitt A, Kaurani L, et al. Childhood trauma in schizophrenia: current findings and research perspectives. Front Neurosci. 2019;13:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lodge DJ, Grace AA.. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomes FV, Grace AA.. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr Bull. 2017;43(3):592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes FV, Zhu X, Grace AA.. The pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry. 2020;25(12):3278–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grace AA, Gomes FV.. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr Bull. 2019;45(1):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomes FV, Grace AA.. Beyond dopamine receptor antagonism: new targets for schizophrenia treatment and prevention. Int J Mol Sci . 2021;22(9):4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang CW, Tsai JJ, Huang CC, Wu SN.. Experimental and simulation studies on the mechanisms of levetiracetam-mediated inhibition of delayed-rectifier potassium current (KV3.1): contribution to the firing of action potentials. J Physiol Pharmacol. 2009;60(4):37–47. [PubMed] [Google Scholar]
- 16. Nowack A, Malarkey EB, Yao J, Bleckert A, Hill J, Bajjalieh SM.. Levetiracetam reverses synaptic deficits produced by overexpression of SV2A. PLoS One. 2011;6(12):e29560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lüscher Dias T, Schuch V, Beltrão-Braga PCB, et al. Drug repositioning for psychiatric and neurological disorders through a network medicine approach. Transl Psychiatry. 2020;10(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kätzel D, Wolff AR, Bygrave AM, Bannerman DM.. Hippocampal hyperactivity as a druggable circuit-level origin of aberrant salience in schizophrenia. Front Pharmacol. 2020;11:486811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Behdani F, Hassanzadeh B, Eslamzadeh M, et al. Can levetiracetam improve clinical symptoms in schizophrenic patients? A randomized placebo-controlled clinical trial. Int Clin Psychopharmacol. 2022;37(4):159–165. [DOI] [PubMed] [Google Scholar]
- 20. Klinger K, Gomes FV, Rincón-Cortés M, Grace AA.. Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. Eur Neuropsychopharmacol. 2019;29(10):1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uliana DL, Gomes FV, Grace AA.. Stress impacts corticoamygdalar connectivity in an age-dependent manner. Neuropsychopharmacology. 2021;46(4):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koh MT, Shao Y, Rosenzweig-Lipson S, Gallagher M.. Treatment with levetiracetam improves cognition in a ketamine rat model of schizophrenia. Schizophr Res. 2018;193:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ungless MA, Grace AA.. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padovan CM, Guimarães FS.. Restraint-induced hypoactivity in an elevated plus-maze. Braz J Med Biol Res. 2000;33(1):79–83. [DOI] [PubMed] [Google Scholar]
- 25. Modinos G, Allen P, Grace AA, McGuire P.. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38(3):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomes FV, Rincón-Cortés M, Grace AA.. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci Biobehav Rev. 2016;70:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bourin M, Hascoët M.. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1–3):55–65. [DOI] [PubMed] [Google Scholar]
- 28. Carobrez AP, Bertoglio LJ.. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29(8):1193–1205. [DOI] [PubMed] [Google Scholar]
- 29. Pellow S, Chopin P, File SE, Briley M.. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. [DOI] [PubMed] [Google Scholar]
- 30. Hogg SA. review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54(1):21–30. [DOI] [PubMed] [Google Scholar]
- 31. Rodgers RJ, Dalvi A.. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21(6):801–810. [DOI] [PubMed] [Google Scholar]
- 32. Augustsson H, Meyerson BJ.. Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol Behav. 2004;81(4):685–698. [DOI] [PubMed] [Google Scholar]
- 33. Kinrys G, Worthington JJ, Wygant L, Nery F, Reese H, Pollack MH.. Levetiracetam as adjunctive therapy for refractory anxiety disorders. J Clin Psychiatry. 2007;68(7):1010–1013. [DOI] [PubMed] [Google Scholar]
- 34. Gower AJ, Falter U, Lamberty Y.. Anxiolytic effects of the novel anti-epileptic drug levetiracetam in the elevated plus-maze test in the rat. Eur J Pharmacol. 2003;481(1):67–74. [DOI] [PubMed] [Google Scholar]
- 35. Gomes FV, Zhu X, Grace AA.. Stress during critical periods of development and risk for schizophrenia. Schizophr Res. 2019;213:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gomes FV, Grace AA.. Adolescent stress as a driving factor for schizophrenia development-a basic science perspective. Schizophr Bull. 2017;43(3):486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J.. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760–773. [DOI] [PubMed] [Google Scholar]
- 39. McCutcheon R, Beck K, Jauhar S, Howes OD.. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr Bull. 2018;44(6):1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sonnenschein SF, Gomes FV, Grace AA.. Dysregulation of midbrain dopamine system and the pathophysiology of schizophrenia. Front Psychiatry. 2020;11:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lieberman JA, Girgis RR, Brucato G, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23(8):1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haberman RP, Koh MT, Gallagher M.. Heightened cortical excitability in aged rodents with memory impairment. Neurobiol Aging. 2017;54:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lodge DJ, Behrens MM, Grace AA.. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heckers S, Konradi C.. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167(1–3):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Konradi C, Yang CK, Zimmerman EI, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang ZJ, Reynolds GP.. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 48. Steullet P, Cabungcal JH, Coyle J, et al. Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry. 2017;22(7):936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Herman JP, Mueller NK.. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174(2):215–224. [DOI] [PubMed] [Google Scholar]
- 50. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lukyanetz EA, Shkryl VM, Kostyuk PG.. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia. 2002;43(1):9–18. [DOI] [PubMed] [Google Scholar]
- 52. Yang XF, Rothman SM.. Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure. 2009;18(9):615–619. [DOI] [PubMed] [Google Scholar]
- 53. Löscher W, Gillard M, Sands ZA, Kaminski RM, Klitgaard H.. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs. 2016;30(11):1055–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wakita M, Kotani N, Kogure K, Akaike N.. Inhibition of excitatory synaptic transmission in hippocampal neurons by levetiracetam involves Zn2+-dependent GABA type A receptor-mediated presynaptic modulation. J Pharmacol Exp Ther. 2014;348(2):246–259. [DOI] [PubMed] [Google Scholar]
- 55. Boddum K, Hougaard C, Xiao-Ying Lin J, et al. Kv3.1/Kv3.2 channel positive modulators enable faster activating kinetics and increase firing frequency in fast-spiking GABAergic interneurons. Neuropharmacology. 2017;118:102–112. [DOI] [PubMed] [Google Scholar]
- 56. Leger M, Alvaro G, Large CH, Harte MK, Neil JC.. Efficacy of AUT6, a novel and selective Kv3 channel modulator, to alleviate cognitive and neurobiological dysfunction in the sub-chronic PCP rat model of schizophrenia symptomatology. J Psychopharmacol. 2015;29:A66. [Google Scholar]
- 57. Deakin B, Perini F, Nazimek J, et al. AUT00206, a novel kv3 channel modulator, reduces ketamine-induced bold signalling in healthy male volunteers: a randomised placebo-controlled crossover trial. Schizophr Bull. 2019;45(suppl S2):S245–S246. [Google Scholar]
- 58. Sourbron J, Chan H, Wammes-van der Heijden EA, et al. Review on the relevance of therapeutic drug monitoring of levetiracetam. Seizure. 2018;62:131–135. [DOI] [PubMed] [Google Scholar]
- 59. Robinson JE, Chen M, Stamatakis AM, et al. Levetiracetam has opposite effects on alcohol- and cocaine-related behaviors in C57BL/6J mice. Neuropsychopharmacology. 2013;38(7):1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]