Abstract
Background and Hypothesis
Throughout the life stages of women with schizophrenia-spectrum disorders (SSD), lower estrogen levels are associated with more severe disease course. At perimenopause in the mid-forties, estrogen levels decline to remain persistently low after menopause. This period is hypothesized to increase relapse risk and reduce antipsychotic effectiveness in preventing relapse.
Study Design
The cohort of persons with schizophrenia/schizoaffective disorder was identified from Finnish nationwide registers (N = 61 889) and stratified by sex and age <45 vs. ≥45 years. Hospitalizations for psychosis were defined per 5-year age group during the follow-up 1996–2017. Risk of psychosis hospitalization (Adjusted Hazard Ratio, aHR) was assessed using within-individual design, by comparing antipsychotic monotherapy use to nonuse periods in the same individuals for seven dose categories in defined daily doses (DDDs/day).
Results
Starting at age 45–50, women were consistently more often hospitalized for psychosis than their male peers. Women ≥45 had significantly higher aHRs than women <45 at antipsychotic monotherapy >0.6 DDDs/day, and than men at >1.1 DDDs/day. This female-specific age-dependent decrease in effectiveness was present for clozapine doses >0.6 DDDs/day, olanzapine doses >1.4 DDDs/day, and for specific doses of quetiapine (0.9–1.1 DDDs/day) and risperidone (0.6–0.9 DDDs/day).
Conclusions
While younger women have a lower risk of relapse and generally need a lower antipsychotic dose to prevent rehospitalization than men, antipsychotic effectiveness declines in women after the age of 45. Starting in mid-forties, older women with SSD should be regarded as a vulnerable group that deserve special attention.
Keywords: relapse, psychosis, menopause, sex, age
Introduction
Every woman with a schizophrenia spectrum disorder (schizophrenia or schizoaffective disorder, SSD) will eventually face a period of lasting impact on their endogenous estrogen production: menopause. The onset of menopause is defined as the cessation of natural menstrual cycles for 12 months and on average occurs at the age of 49 world-wide, with European mean age at menopause of 50.5.1 In the years preceding menopause (i.e. perimenopause), menstrual cycles become irregular with longer periods between menses and ovarian hormone production starts to decline.2
Throughout the life stages of women with SSD, an association between estrogen levels and disease severity is present. At the first psychotic episode, women tend to function better than men, a sex difference that has been attributed to estrogen’s protective effects.3 Ovarian hormones strongly impact brain and behavior of women, both in physiological and in pathological states. Estrogens support cognition and maintain high cognitive functioning under chronic stress.4 They act as antioxidants, increase neuroplasticity, and facilitate neurotransmission.5 As a result, women with natural menstrual cycles, on average have more severe psychotic symptoms and more admissions for psychosis during low estrogenic phases, which occurs preceding and during menses, compared to high estrogen periods surrounding ovulation.6 During pregnancy, high estrogen states are associated with relatively mild symptom severity, while the sharp drop in sex hormone levels after delivery forms the prelude of more severe symptoms.7 After menopause, estrogen levels remain low which is associated with a deterioration in the clinical course.8 Therefore, women with SSD have sex-specific psychiatric needs, that differ according to their life stage.9,10
Estrogens also affect pharmacokinetics of antipsychotic medication.11 They inhibit the liver enzyme cytochrome P-450 (CYP) 1A2, which metabolizes many antipsychotic drugs, especially olanzapine and clozapine. Lower activity of this enzyme leads to higher blood levels of these antipsychotics. A notable exception is quetiapine, which is mainly metabolized by an enzyme facilitated by estrogens, CYP3A4. Risperidone is metabolized by both CYP3A4 and CYP2D6, of which the latter is not dependent on estrogen levels. In addition, estrogens increase dopamine (D2) receptor sensitivity in the ventral tegmentum, which results in a higher D2 receptor occupancy of antipsychotics in women of fertile age, as compared to men, at equal plasma concentrations.12
After menopause, women with SSD lose these neuroprotective and pharmacokinetic and pharmacodynamic effects, as estrogen levels of postmenopausal women are no higher than those of men. While the process of aging causes a relative increase in plasma levels of antipsychotic medications in both sexes,13 the steep drop in natural estrogen production causes important additional changes in the CYP-metabolism of women. Low estrogen levels lead to lower activity of the CYP3A4 enzyme, while it leaves the CYP1A2 enzyme more active. The result may be that after menopause, plasma concentrations of specific drugs change. Due to the absence of estrogens, olanzapine and clozapine are metabolized more rapidly while that of quetiapine is slowed down.13 At the brain level, animal models of menopause show that dopamine receptor sensitivity declines in this life phase.14 This would suggest lower effectiveness of antipsychotic medication, especially of clozapine and olanzapine in postmenopausal women as compared to premenopausal women, but also as compared to men of similar age. Although the postmenopausal era is recognized as a period of increasing symptom severity, reduced efficacy of antipsychotic drugs and increased need for care,8,15 there is a remarkable paucity on quantitative data on this vulnerable period that all women with schizophrenia will experience.8 We here provide detailed, quantitative data on life-stage dependent clinical changes occurring in women with SSD, using an intra-individual design to prevent confounding. The main aims of this study are:
1. To compare hospitalization rates for psychosis between women and men with SSD at different age groups.
2. To compare effectiveness of commonly used antipsychotic monotherapies in terms of dose-dependent psychosis hospitalization risk in men and women before and after the age of 45, as a proxy for menopausal status in women.16
Methods
We did a nationwide, register-based cohort study. The base cohort included all hospitalized patients with SSD during 1972–2014 in Finland (N = 61 889). SSD was defined using the ICD-10 codes F20 and F25, and with corresponding ICD-9 and ICD-8 codes. The cohort included 7676 persons with schizoaffective disorder at cohort entry (4512 women, 59%). A previous study showed similar profiles for effectiveness of antipsychotics in relapse prevention in the schizophrenia and the schizoaffective patients.17
Permissions for this study were granted by pertinent institutional authorities at the National Institute for Health and Welfare of Finland, The Social Insurance Institution of Finland, and Statistics Finland.
Study Design
We conducted a cohort study and the follow-up started at January 1, 1996 for persons diagnosed before that and at date of discharge from their first inpatient care due to SSD for those who were diagnosed after that date. The follow-up ended at death or on December 31, 2017 when data linkage ended. For analyses stratified by age of 45, this was defined time-dependently, and persons were kept in age group <45 years until one day before their 45th birthday and transferred to age group ≥45 on that birthday. This means that the same person contributed person-time to both age groups. The procedure was conducted for 5-year age groups.
Choice of Primary Measure
The primary outcome measure was relapse which was defined as inpatient hospital care for a F20–F29 diagnosis, recorded as the main discharge diagnosis. Hospitalization due to psychosis is a commonly used measure of relapse in register-based studies.18 Hospitalization captures severe relapses but not milder cases that could be successfully treated in outpatient care.
Hospital Admissions
The cohort was divided into 5-year age groups, starting at age 20 and ending at age 69 for men and women separately. Per age group, the proportion that needed hospital admission for psychosis (F20–F29) was defined. Women were compared to their male peers within each age group.
Antipsychotic Medication Use
Antipsychotic use (based on the Anatomical Therapeutic Chemical [ATC] classification code N05A excluding lithium) was derived from the prescription register, which includes all reimbursed medication dispensings from Finnish pharmacies to all residents since 1995. Prescription register data comprised date of dispensing, ATC code, product information, the number of packages dispensed, and dispensed amount in defined daily doses (DDDs) as defined by WHO. Antipsychotic use episodes were modelled with the PRE2DUP method,19 and temporal dose was estimated at each dispensing by using two previous dispensings.20 We investigated doses of antipsychotic monotherapies excluding time periods when two or more antipsychotics were used concomitantly. Antipsychotic nonuse periods were constructed between dose periods and further both nonuse and use periods were separated from hospital care periods. Separation of hospital care periods was done because patients are not at risk of having the study outcome (rehospitalization) during hospital care.
Antipsychotic monotherapies assigned by dose estimates were further categorized into the following defined daily doses per day categories: <0.4, 0.4–0.6, 0.6–0.9, 0.9–<1.1 (standard dose), 1.1–<1.4, 1.4–<1.6, and ≥1.6. The main exposure measures were dose categories, which vary in time for each patient and were re-evaluated at every dispensing. A patient might contribute person-time to multiple or even all dose categories during the follow-up. The main analyses were restricted to the four most frequently used oral antipsychotic monotherapies: clozapine, olanzapine, quetiapine, and risperidone. A combination measure of “any antipsychotic monotherapy” was constructed by pooling all specific antipsychotic monotherapies (also less frequently prescribed antipsychotics) of a certain dose category together. Additional analyses were performed for any long acting injectable (LAI) use and for the use of antipsychotic polytherapy. Polytherapy use periods also represent monotherapy transition periods and include the use of small and/or irregular doses.
Statistical Analysis
Sex ratios in hospital admissions for psychosis were calculated per age group and tested for significance using Pearson chi-square tests. Risk of relapse associated with each dose category was calculated in comparison with antipsychotic non-use as a reference and reported as adjusted Hazard Ratios (aHRs), stratified by age category and sex. Analyses were conducted using a within-individual design where all comparisons are conducted within the same individual and each person acts as his or her own control in a stratified Cox model. The design eliminates the impact of time-invariant factors (e.g. genetics, initial severity of illness) and only time-varying factors are adjusted for. These were time since cohort entry, temporal order of specific antipsychotics, and use of antidepressants, mood stabilizers, benzodiazepines and Z-drugs (nonbenzodiazepine sleeping medication). Non-overlapping confidence intervals signify significant differences between age and sex groups.
Results
The cohort included 30 785 women and 31 104 men. The mean age of women was 49.8 years (SD 16.6) at the start of follow-up, which was somewhat higher than for men (43.6 years, SD 14.8). Mean time since their first SSD diagnosis was 9.0 (SD 8.9) for women and 8.6 years (SD 9.0) for men. Among both sexes, olanzapine was the most prescribed antipsychotic, used by 26.1% (N = 8039) of women and 26.0% (N = 8083) of men. Among women, risperidone was the second most common antipsychotic (23.6%, N = 7253) followed by quetiapine (20.1%, N = 6181) and clozapine (16.1%, N = 4941). Among men, the second most common antipsychotic was clozapine (22.1%, N = 6887), followed by risperidone (18.7%, N = 5828) and quetiapine (15.0%, N = 4652).
When stratified by sex and age of 45 years, 16 526 women contributed to the analyses of <45 years and 24 981 women contributed to the analyses of ≥45 years whereas the corresponding sample sizes for men were 21 378 and 21 451, respectively. Overall, women and men ≥45 years used higher proportion of their use time antipsychotic monotherapies with low dose (<0.4 DDDs/day) (30% and 23%, respectively) than younger women and men (17% and 11%), respectively, whereas standard dose (0.9–<1.1 DDDs/day) was equally distributed between all age and sex groups (11%–14%) and high dose (≥1.6 DDDs/day) was more common among younger women and men (supplementary figure 1). Of specific oral antipsychotic monotherapies, clozapine and olanzapine were more often used with higher doses whereas quetiapine and risperidone were more commonly used with lower doses (supplementary figures 2–5).
Hospital Admission for Psychosis Defined for 5-year Age Groups by Sex
When comparing men and women with SSD, age groups <45 show few consistent differences in proportions who needed hospital admissions for psychosis, with higher proportions of admissions in men aged 25–29 (P = .016) and in women aged 35–39 (P = .033) (table 1). Starting at age group of 45–49 and up until the oldest age group of 65–69, higher proportions of women are admitted to hospital for psychosis than their male peers (all P-values < .00001), with differences increasing at older age (table 1).
Table 1.
Percentage of Persons Who Had a Hospital Admission for Psychosis per Age Group and per Sex.
| Age group | All (%) | Men (%) | Women (%) | Women: men | X 2((2) | P value |
|---|---|---|---|---|---|---|
| 20–24 | 4408 (81.7) | 2773 (83.3) | 1635 (79.2) | 0.95 | 2.681 | .26 |
| 25–29 | 6575 (65.4) | 4220 (67.2) | 2355 (62.4) | 0.93 | 8.270 | .016 |
| 30–34 | 7752 (53.5) | 4716 (53.6) | 3036 (53.2) | 0.99 | 0.098 | .95 |
| 35–39 | 8534 (45.5) | 4880 (44.4) | 3654 (47.0) | 1.06 | 6.812 | .033 |
| 40–44 | 9268 (39.9) | 5103 (39.2) | 4165 (40.8) | 1.04 | 3.795 | .15 |
| 45–49 | 10016 (36.3) | 5097 (34.5) | 4919 (38.5) | 1.12 | 30.111 | <.00001 |
| 50–54 | 9976 (34.4) | 4870 (32.6) | 5106 (36.5) | 1.12 | 32.765 | <.00001 |
| 55–59 | 8516 (30.7) | 3995 (29.0) | 4521 (32.5) | 1.12 | 28.410 | <.00001 |
| 60–64 | 7042 (28.4) | 3085 (26.5) | 3957 (30.1) | 1.14 | 28.210 | <.00001 |
| 65-69 | 5426 (26.7) | 2059 (23.7) | 3367 (29.0) | 1.22 | 52.205 | <.00001 |
Effectiveness of Antipsychotic Monotherapy for Relapse Prevention
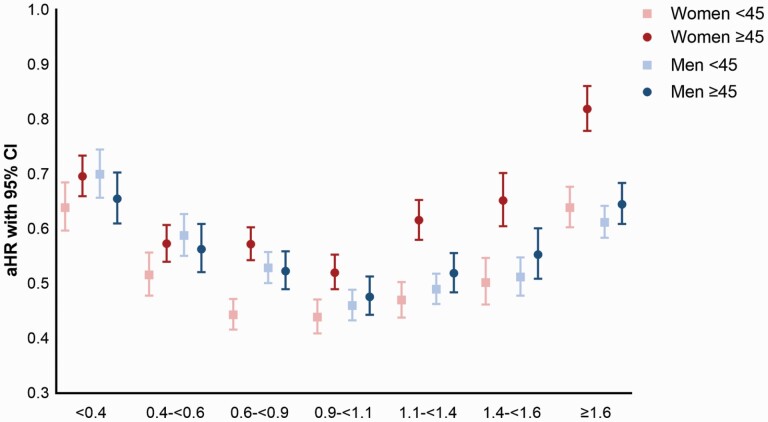
Risk of relapse (defined as aHR) associated with any antipsychotic monotherapy use in dose categories compared with nonuse, stratified by sex and age is shown in figure 1 and supplementary table 1. Dose–response relationship showed U-shaped curves for all groups, where standard dose (0.9–<1.1 DDDs per day, referring to about 1 DDD/day use) was associated with the lowest risk of relapse for most groups, but for women <45, doses of 0.6–0.9 DDD reached similar effectiveness. Women ≥45 had significantly higher risk of relapse associated with standard dose use (aHR 0.52, 95% CI 0.49–0.53) than any other group (women <45 aHR 0.44, 95% CI 0.41–0.47; men <45 aHR 0.46, 95% CI 0.43–0.49, and men ≥45 0.48, 0.44–0.51). Compared to women <45, women ≥45 showed significantly higher aHRs for all dose categories ≥0.6 DDDs/day. When comparing men and women below and above the age of 45, women <45 showed lower aHR at doses between 0.6 and 0.9 DDDs/day. For doses >1.1 DDDs/day, women ≥45 showed remarkably higher aHR when compared to women <45, but also when compared to men ≥45, and this difference increases with increasing dose.
Fig. 1.
Effectiveness of antipsychotic monotherapy by dose categories for relapse prevention in women and men, divided into below and above age 45.
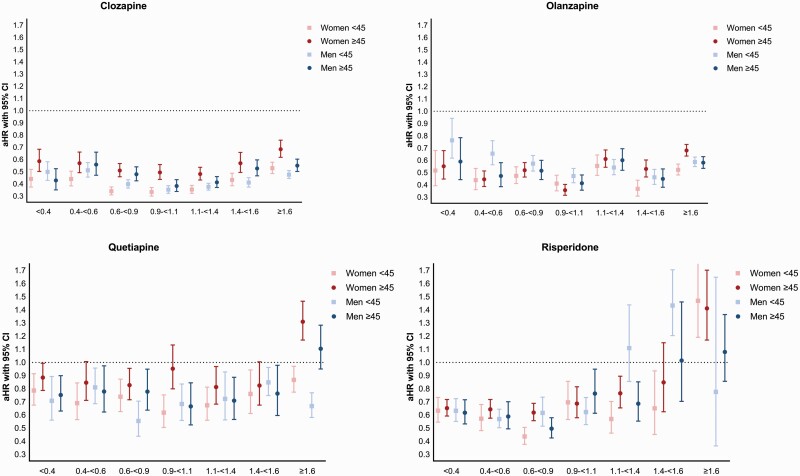
We also investigated aHRs for the four most used antipsychotic drugs: clozapine, olanzapine, quetiapine, and risperidone (figure 2, supplementary tables 2–5). When comparing the four panels of figure 2, clozapine and olanzapine showed higher effectiveness in preventing relapse than quetiapine and risperidone, which was present for all age and sex categories. Lower numbers of risperidone users in the higher dose groups makes these data less reliable, as reflected in wide confidence intervals.
Fig. 2.
Effectiveness of oral antipsychotic medication (clozapine, olanzapine, quetiapine and risperidone) for relapse prevention in women and men, divided into below and above age 45.
The higher aHR for women above vs. below 45 was most pronounced for clozapine, where it was significant in all dose categories >0.6 DDDs/day (e.g. aHR for 0.9–<1.1 DDDs/day 0.33, 95% CI 0.30–0.37 for women <45; vs. 0.49, 95% CI 0.44-0.56 for women ≥45). Men ≥45 also had lower benefit of clozapine than men <45, but this effect was only present for daily doses 1.4–1.6 DDDs. For olanzapine, aHRs for the dose categories >1.4 DDDs/day were lower for women <45 than for women ≥45. For quetiapine monotherapies, lower effectiveness in relapse prevention for women ≥45 than in women <45 was seen in the dose categories 0.9–<1.1 and >1.6 DDDs/day. For risperidone, significance was only reached at 0.6–0.9 DDDs/day (aHR 0.44, 95% CI 0.38–0.51 for women <45 and 0.62, 95% CI 0.56–0.69 for women ≥45). Of note, high dose (≥1.6 DDDs/day) risperidone was not associated with reduced risk of relapse in any age/sex category and was associated with an increased risk among women ≥45 (aHR 1.41, 95% CI 1.17–1.70) compared to lower doses.
The aHRs associated with any LAI use in comparison with nonuse and the aHRs associated with antipsychotic polytherapy in comparison with nonuse showed similar trends as the aHRs for any antipsychotic monotherapy (supplementary table 6-7, supplementary figure 6-7).
Discussion
We showed that the proportion of patients needing hospitalizations for psychosis is approximately evenly distributed among men and women until the age of 45. After that age, more women than men with SSD need hospitalizations, with strongest sex differences for the age group between 65 and 69. We also showed that antipsychotic monotherapy is most effective in preventing relapse in women below 45, as compared to women above that age, and also as compared to men of all ages. For these younger women, dose of 0.6–0.9 DDDs/day was as effective as standard dose 0.9–1.1 DDDs/day, and for risperidone even more effective at this lower dose. This suggests that younger women may be over-medicated when adhering to the WHO-recommended DDD for antipsychotic medication. In an earlier paper,9 we argued that high estrogen levels in women of fertile age make them more sensitive to most antipsychotic drugs in terms of efficacy and side-effects, and that lower dosing is probably appropriate for most antipsychotics, except for quetiapine and lurasidone.
After the age of 45, the tide appears to turn for women, as medicated women above this age showed higher risk of relapse compared to women below this age, but also in comparison to men in the same age group. The lower effectiveness for older women was most pronounced for clozapine, where these women had significantly more rehospitalizations at all doses compared to younger women. For olanzapine, older women did particularly poorly at the higher dose groups. Quetiapine and risperidone appear to be less effective than olanzapine and clozapine in preventing hospitalization in all age and sex groups. Differences between the sex and age groups were smaller for quetiapine and risperidone, with older women doing significantly worse only at daily doses between 0.9 and 1.1, and above 1.6 for quetiapine, and daily doses between 0.6 and 0.9 for risperidone.
The timing when women start to show higher hospitalization rates than men coincides with perimenopause, usually starting in the mid-forties, when menses becomes less frequent and natural estrogen production declines. Exact menopausal dates were not known from the female participants. In Europe, menopause on average occurs at 49 years of age, but smoking and less physical activity tend to lower the mean age of menopause,1 while obesity, which is also common among women with SSD, tends to increase the age at menopause.1 We therefore do not expect significant differences in age at menopause for women with SSD. During the years of menopausal transition (i.e. perimenopause), the lower estrogen production not only affects the reproductive system but also the central nervous system,21 which worsens the course of neuropsychiatric disorders including psychosis. The sex difference in hospitalizations after age 45 may partly be caused by reduced efficacy of antipsychotic medication to prevent relapse in women with low estrogen levels. Menopause also affects the metabolism of selected antipsychotics, which could explain the antipsychotic-specific effects reported in this study. With much lower average estrogen levels after menopause, the induction of CYP1A2 decreases, leading to more rapid conversion of antipsychotics that are mainly metabolized by this enzyme (i.e. clozapine and olanzapine). This pharmacokinetic effect could explain the pronounced effect of age in women using clozapine. Our findings oppose an earlier study reporting that female sex was associated with more frequent admissions and lower responsiveness to clozapine.22 We showed that clozapine is similarly or even more effective in women before the age of 45 as compared to men of all ages. The earlier study did not account for age categories as a proxy for menopausal status, while this could have had a major impact on their conclusions. We found significant differences in effectiveness between younger and older women using olanzapine only for the higher dose groups. For olanzapine, the risk of overdosing women is very high before menopause,11 as blood levels are not monitored routinely as they are for clozapine, which could have affected the results.
Quetiapine is metabolized by CYP3A4, which becomes somewhat less active after menopause, potentially resulting in higher blood levels after menopause and changes in effectiveness after menopause may therefore be limited. Risperidone is dependent on both CYP2D6 and CYP3A4, but its metabolite paliperidone is also active, so that changes in metabolism do not affect efficacy for relapse prevention. However, we did find lower effectiveness of quetiapine and risperidone after presumed menopause specifically for the optimal doses of these antipsychotics for younger women (0.9–1.1 DDD and 0.6–0.9 DDD, respectively), which may indicate that pharmacodynamic effects of estrogen decline (i.e. decreasing dopamine receptor sensitivity) may be more important than pharmacokinetic effects.
The effect of age as a proxy for menopause on women with SSD has been described before, but not in such a large cohort and in such great detail. This relative paucity in studies on menopausal women with SSD was recently mentioned by González-Rodríguez et al.8 who found only 49 studies on this topic. This group did important research on menopause themselves and showed a negative effect of time since menopause on antipsychotic response in a sample of 64 postmenopausal women with SSD,23 which parallels our finding of increasing women: men ratio for relapse risk across the older age groups.
Shlomi-Polachek et al.24 compared the frequency hospitalizations in a sample of 5411 women and men. Women younger than 45 years had lower proportions of voluntary hospitalizations than men in the same age group (33.5% vs. 66.5%), while women older than 55 years had higher proportions of hospitalizations than men (57.2% vs. 42.8%). The age group 45–55 was excluded in this study. This study also assessed prescribed drug doses for intramuscular risperidone (n = 280) and clozapine (n = 192). Women younger than 45 years were prescribed similar doses of intramuscular risperidone and lower doses of clozapine (345.8 vs. 380.2 mg/day) and women older than 55 were prescribed higher doses of intramuscular risperidone (44.8 vs. 34.4 mg/2 weeks) and clozapine (164.3 vs. 154.5 mg/day) than men in the same age group. Seeman et al.25 carried out a survey of schizophrenia patients who were treated by the same psychiatrist over a 3-year period. The sample consisted of 43 women and 58 men. When stratified by age, women under 40 were prescribed lower doses than age-matched men, while women over age 40 were given higher doses than men.
Both studies indicate that the deteriorating clinical course of women of postmenopausal age may have prompted their physicians to increase the dose. This dose increase is an understandable reaction to a declining clinical status, but it is unsure if this strategy is successful, as we showed that at higher levels, the effectiveness of antipsychotics in preventing relapse in postmenopausal women is very low, especially for quetiapine and risperidone. While relapse may not be prevented with higher doses, the risk of side-effects will increase, which is particularly worrisome for postmenopausal women as they are most vulnerable for cardiovascular and metabolic side-effects.26 A better strategy could be to provide hormonal replacement therapy (HRT) or augmentation with a selective estrogen receptor modulator (SERM), which mimics the effect of estrogens in the brain.27 The SERM raloxifene has proven to ameliorate positive, negative and cognitive symptoms and may even allow a lower dose of prescribed antipsychotics by improving treatment efficacy.9,28 While both raloxifene and HRT slightly increase the risk for thrombo-embolic complications, raloxifene reduces the risk for breast cancer, while HRT increases this risk.29 Age for starting HRT or raloxifene is critical, as beneficial effects of estrogenic therapy are probably absent when started several years after menopause.30 As estrogen levels and clinical course already start to decline several years before actual menopause, mid-forties may be the optimal age for such preventive actions.
Strengths and Limitations
A clear strength of this study is its large sample size and the naturalistic design, which enables translation to real-world settings in similar populations. Importantly, analyses were conducted in a within-individual design where each person (within each age group) acts as their own control, minimizing confounding by selection. However, within subject analysis also has its limitations, as we could only include patients with a variation in exposure and experiencing relapse in pharmacological treatment. In addition, we used hospitalizations with psychotic disorders as the main diagnosis at discharge, thereby excluding hospitalizations for other reasons (e.g. somatic disorders), but the exact reasons for hospitalization were not available. The permanent decline of estrogens is a common denominator of women of menopausal age and the need for rehospitalization may have been triggered by combinations of potential consequences of this phenomenon (e.g. lower effectiveness of antipsychotics, psychotic exacerbations or menopause-associated stresses). Other limitations concern the use of age as an estimation of menopausal status and the absence of information on clinical outcome measures. Individual characteristics that influence drug efficacy, such as body-mass index, smoking status and protein binding of the antipsychotics were unavailable. In addition, aging may come with other sex-specific changes that are difficult to disentangle from menopause effects and may be intertwined with its hormone-related changes (for a review, see Hägg and Jylhävä31). In future studies, assessment of follicle-stimulation hormone levels would be helpful for estimating menopausal status, as estrogen levels have strong circadian variation. Finnish mental health care may resemble those of other European countries, Canada and Oceania, but may be different from that in US and non-western countries,32 which limits the generalizability of our findings.
Conclusion
We showed that women with SSD aged 45 and older consistently need more hospitalizations for psychosis than their male peers. The effectiveness of antipsychotic drugs in preventing rehospitalizations for psychosis was much lower in women older than 45, as compared to women below this age, but also as compared to male peers. The most pronounced sex-specific effect of age was observed for clozapine, for higher doses of olanzapine, and for optimal doses of quetiapine and risperidone.
It is important to recognize that perimenopause is a critical period during which symptoms increase, while the effectiveness of antipsychotics declines as a consequence of estrogen deficiency. These processes are likely to reinforce each other, together leading to a major increase in relapse risk. Dose increase is a logical clinical response to symptom deterioration but may not be the best strategy, as effectiveness of antipsychotics in women over 45 was very low for higher dose ranges. Earlier studies showed that estrogenic treatment (either HRT or raloxifene) reduces symptoms and increases the effectiveness of antipsychotic medication, but also provide protection against cognitive decline, cardiovascular comorbidity, and osteoporosis, in postmenopausal women with SSD.27,33–35 As estrogen decline and deterioration of the clinical course already start in the mid-forties, this is the optimal age to consider such preventive actions.
Supplementary Material
Contributor Information
Iris E Sommer, Department of Psychiatry, Rijksuniversiteit Groningen (RUG), University Medical Center Groningen (UMCG), Groningen, Netherlands.
Bodyl A Brand, Department of Psychiatry, Rijksuniversiteit Groningen (RUG), University Medical Center Groningen (UMCG), Groningen, Netherlands.
Shiral Gangadin, Department of Psychiatry, Rijksuniversiteit Groningen (RUG), University Medical Center Groningen (UMCG), Groningen, Netherlands.
Antti Tanskanen, Department of Forensic Psychiatry, University of Eastern Finland, Niuvanniemi Hospital, Kuopio, Finland; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Center for Psychiatry Research, Stockholm City Council, Stockholm, Sweden.
Jari Tiihonen, Department of Forensic Psychiatry, University of Eastern Finland, Niuvanniemi Hospital, Kuopio, Finland; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Center for Psychiatry Research, Stockholm City Council, Stockholm, Sweden.
Heidi Taipale, Department of Forensic Psychiatry, University of Eastern Finland, Niuvanniemi Hospital, Kuopio, Finland; Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden; Center for Psychiatry Research, Stockholm City Council, Stockholm, Sweden.
Funding
This work was supported by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital and the Academy of Finland (grant numbers 315969, 320107, 345326). ZonMW (Dutch Medical Research Association) for I. Sommer, project nr. 836041008.
Conflict of Interest
JT, HT, and AT have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution. JT has been a consultant, advisor, or has received honoraria from Eli Lilly, Evidera, Janssen-Cilag, Lundbeck, Orion, Otsuka, Mediuutiset, Sidera, and Sunovion. HT reports personal fees from Janssen-Cilag and Otsuka. CUC has been a consultant, advisor, or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Cardio Diagnostics, Damitsa, Gedeon Richter, Hikma, IntraCellular Therapies, Janssen/Johnson & Johnson, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Relmada, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris; provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Lundbeck, Relmada, Rovi, and Teva; received grant support from Janssen and Takeda; received royalties from UpToDate; and is a stock option holder of LB Pharma. IS, BB and SG declare no conflicts of interest.
References
- 1. Schoenaker DAJM, Jackson CA, Rowlands JV, Mishra GD.. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol. 2014;43(5):1542–1562. doi: 10.1093/ije/dyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burger H. The menopausal transition--endocrinology. J Sex Med. 2008;5(10):2266–2273. doi: 10.1111/j.1743-6109.2008.00921.x. [DOI] [PubMed] [Google Scholar]
- 3. Riecher-Rössler A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. The Lancet Psychiatry 2017;4(1):63–72. doi: 10.1016/S2215-0366(16)30379-0. [DOI] [PubMed] [Google Scholar]
- 4. Wei J, Yuen EY, Liu W, et al. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- 5. Barth C, Villringer A, Sacher J.. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reilly TJ, Sagnay de la Bastida VC, Joyce DW, Cullen AE, McGuire P.. Exacerbation of psychosis during the perimenstrual phase of the menstrual cycle: systematic review and meta-analysis. Schizophr Bull. 2020;46(1):78–90. doi: 10.1093/schbul/sbz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen A, Fedechko T, Schwartz E, et al. Psychiatric risk assessment from the clinician’s perspective: lessons for the future. Community Ment Health J. 2019;55:1165–1172. doi: 10.1007/s10597-019-00411-x. [DOI] [PubMed] [Google Scholar]
- 8. González-Rodríguez A, Guàrdia A, Monreal JA.. Peri- and post-menopausal women with schizophrenia and related disorders are a population with specific needs: a narrative review of current theories. J Pers Med 2021;11(9):849. doi: 10.3390/jpm11090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brand BA, de Boer JN, Sommer IEC.. Estrogens in schizophrenia: progress, current challenges and opportunities. Curr Opin Psychiatry. 2021;34(3):228–237. doi: 10.1097/YCO.0000000000000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lange B, Mueller JK, Leweke FM, Bumb JM.. How gender affects the pharmacotherapeutic approach to treating psychosis – a systematic review. Expert Opin Pharmacother. 2017;18(4):351–362. doi: 10.1080/14656566.2017.1288722. [DOI] [PubMed] [Google Scholar]
- 11. Brand BA, Haveman YRA, de Beer F, de Boer JN, Dazzan P, Sommer IEC.. Antipsychotic medication for women with schizophrenia spectrum disorders. Psychol Med. Published online November 12, 2021;52:1–15. doi: 10.1017/S0033291721004591 [DOI] [Google Scholar]
- 12. Eugene AR, Masiak J.. A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord J Psychiatry. 2017;71(6):417–424. doi: 10.1080/08039488.2017.1314011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castberg I, Westin AA, Skogvoll E, Spigset O.. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr Scand. 2017;136(5):455–464. doi: 10.1111/acps.12794. [DOI] [PubMed] [Google Scholar]
- 14. Arad M, Weiner I.. Contrasting effects of increased and decreased dopamine transmission on latent inhibition in ovariectomized rats and their modulation by 17beta-estradiol: an animal model of menopausal psychosis? Neuropsychopharmacology. 2010;35(7):1570–1582. doi: 10.1038/npp.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. González-Rodríguez A, Seeman MV.. Pharmacotherapy for schizophrenia in postmenopausal women. Expert Opin Pharmacother. 2018;19(8):809–821. doi: 10.1080/14656566.2018.1465563. [DOI] [PubMed] [Google Scholar]
- 16. Barentsen R, Van De Weijer PHM, Van Gend S, Foekema H.. Climacteric symptoms in a representative Dutch Population sample as measured with the greene climacteric scale. Maturitas. Published online 2001;38:123–128. doi: 10.1016/S0378-5122(00)00212-7 [DOI] [PubMed] [Google Scholar]
- 17. Lintunen J, Taipale H, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J, Lähteenvuo M.. Long-term real-world effectiveness of pharmacotherapies for schizoaffective disorder. Schizophr Bull. 2021;47(4):1099–1107. doi: 10.1093/schbul/sbab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gleeson JFM, Alvarez-Jimenez M, Cotton SM, Parker AG, Hetrick S.. A systematic review of relapse measurement in randomized controlled trials of relapse prevention in first-episode psychosis. Schizophr Res. 2010;119(1-3):79–88. doi: 10.1016/j.schres.2010.02.1073. [DOI] [PubMed] [Google Scholar]
- 19. Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods – a second generation method (PRE2DUP). BMC Med Inform Decis Mak. 2015;15:21. doi: 10.1186/s12911-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taipale H, Tanskanen A, Luykx JJ, et al. Optimal doses of specific antipsychotics for relapse prevention in a nationwide cohort of patients with schizophrenia. Schizophr Bull. 2022;48:774–784. doi: 10.1093/schbul/sbac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E.. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielsen J, Nielsen RE, Correll CU.. Predictors of clozapine response in patients with treatment-refractory schizophrenia. J Clin Psychopharmacol. 2012;32(5):678–683. doi: 10.1097/JCP.0b013e318267b3cd. [DOI] [PubMed] [Google Scholar]
- 23. González-Rodríguez A, Catalán R, Penadés R, et al. antipsychotic response worsens with postmenopausal duration in women with schizophrenia. J Clin Psychopharmacol. 2016;36(6):580–587. doi: 10.1097/JCP.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 24. Shlomi Polachek I, Manor A, Baumfeld Y, et al. Sex differences in psychiatric hospitalizations of individuals with psychotic disorders. J Nerv Ment Dis. 2017;205(4):313–317. doi: 10.1097/NMD.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 25. Seeman MV. Interaction of sex, age, and neuroleptic dose. Compr Psychiatry. 1983;24(2):125–128. doi: 10.1016/0010-440X(83)90100-1. [DOI] [PubMed] [Google Scholar]
- 26. Newson L. Menopause and cardiovascular disease. Post Reprod Heal. 2018;24(1):44–49. doi: 10.1177/2053369117749675. [DOI] [PubMed] [Google Scholar]
- 27. Kulkarni J, Butler S, Riecher-Rössler A.. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53(July 2018):100743. doi: 10.1016/j.yfrne.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 28. de Boer J, Prikken M, Lei WU, Begemann M, Sommer I.. The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis. NPJ Schizophr. 2018;4(1):1. doi: 10.1038/s41537-017-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vinogradova Y, Coupland C, Hippisley-Cox J.. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 2020;371:m3873. doi: 10.1136/bmj.m3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langer RD, Hodis HN, Lobo RA, Allison MA.. Hormone replacement therapy - where are we now? Climacteric 2021;24(1):3–10. doi: 10.1080/13697137.2020.1851183. [DOI] [PubMed] [Google Scholar]
- 31. Hägg S, Jylhävä J.. Sex differences in biological aging with a focus on human studies. Elife 2021;10:e63425. doi: 10.7554/eLife.63425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM.. Outcomes of Nordic mental health systems: life expectancy of patients with mental disorders. Br J Psychiatry. 2011;199(6):453–458. doi: 10.1192/bjp.bp.110.085100. [DOI] [PubMed] [Google Scholar]
- 33. Lindamer LA, Buse DC, Lohr JB, Jeste DV.. Hormone replacement therapy in postmenopausal women with schizophrenia: positive effect on negative symptoms? Biol Psychiatry. 2001;49(1):47–51. doi: 10.1016/s0006-3223(00)00995-1. [DOI] [PubMed] [Google Scholar]
- 34. Wang Q, Dong X, Wang Y, Li X.. Raloxifene as an adjunctive treatment for postmenopausal women with schizophrenia: a meta-analysis of randomized controlled trials. Arch Womens Ment Health. 2018;21(1):31–41. doi: 10.1007/s00737-017-0773-2. [DOI] [PubMed] [Google Scholar]
- 35. Gurvich C, Gavrilidis E, Worsley R, Hudaib A, Thomas N, Kulkarni J.. Menstrual cycle irregularity and menopause status influence cognition in women with schizophrenia. Psychoneuroendocrinology. 2018;96(April):173–178. doi: 10.1016/j.psyneuen.2018.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.