Abstract
Cluster studies identified a subgroup of patients with psychosis whose premorbid adjustment deteriorates before the onset, which may reflect variation in genetic influence. However, other studies reported a complex relationship between distinctive patterns of cannabis use and cognitive and premorbid impairment that is worthy of consideration. We examined whether: (1) premorbid social functioning (PSF) and premorbid academic functioning (PAF) in childhood and adolescence and current intellectual quotient (IQ) define different clusters in 802 first-episode of psychosis (FEP) patients; resulting clusters vary in (2) polygenic risk scores (PRSs) for schizophrenia (SCZ_PRS), bipolar disorder (BD_PRS), major depression (MD_PRS), and IQ (IQ_PRS), and (3) patterns of cannabis use, compared to 1,263 population-based controls. Four transdiagnostic clusters emerged (BIC = 2268.5): (1) high-cognitive-functioning (n = 205), with the highest IQ (Mean = 106.1, 95% CI: 104.3, 107.9) and PAF, but low PSF. (2) Low-cognitive-functioning (n = 223), with the lowest IQ (Mean = 73.9, 95% CI: 72.2, 75.7) and PAF, but normal PSF. (3) Intermediate (n = 224) (Mean_IQ = 80.8, 95% CI: 79.1, 82.5) with low-improving PAF and PSF. 4) Deteriorating (n = 150) (Mean_IQ = 80.6, 95% CI: 78.5, 82.7), with normal-deteriorating PAF and PSF. The PRSs explained 7.9% of between-group membership. FEP had higher SCZ_PRS than controls [F(4,1319) = 20.4, P < .001]. Among the clusters, the deteriorating group had lower SCZ_PRS and was likelier to have used high-potency cannabis daily. Patients with FEP clustered according to their premorbid and cognitive abilities. Pronounced premorbid deterioration was not typical of most FEP, including those more strongly predisposed to schizophrenia, but appeared in a cluster with a history of high-potency cannabis use.
Keywords: premorbid, schizophrenia, cannabis, IQ, deterioration, bipolar
Introduction
Cognitive impairment is present in most patients with nonaffective psychosis, and a proportion of those with affective psychosis.1,2 The cognitive impairment in psychosis has been recently approached using clustering strategies.3 Research findings differ with some studies suggesting three cognitive clusters, among patients with nonaffective psychosis: those with high, intermediate, and low cognition,3,4 whereas others, including affective psychoses identified four.5 Cognitive clusters were also associated with symptoms and functional outcomes, indicating the utility of such classification for prognosis and treatment.6,7 Cluster analysis has also identified three cognitive-developmental trajectories from premorbid to current IQ: (a) stable impairment from childhood to current IQ, (b) normal premorbid function and high current IQ, and (c) IQ deteriorating from an average premorbid level.8–11
Studies exploring premorbid adjustment using the Premorbid Adjustment Scale (PAS) replicated similar clusters using it as a composite score, including social and academic premorbid adjustment.12,13 However, when the social and academic scales were divided into two scores, six clusters were obtained.14,15 These findings suggest different premorbid social and academic trajectories,16,17 and probably reflect differential development from childhood to adolescence. However, few studies examined patients with their first-episode of psychosis (FEP),6,18,19 and to the best of our knowledge, no studies clustered subjects according to their premorbid histories while also considering current IQ.
Other studies reported that patients with psychosis who were daily cannabis users constituted a less-impaired group who performed better in premorbid IQ20,21 and social, adjustment but had lower premorbid academic adjustment.22 Previous evidence revealed no significant differences in genetic load for schizophrenia between cannabis users and nonusers and between subjects smoking high-potency cannabis and other forms.23,24 Thus, we proposed that in such patients, deteriorating premorbid adjustment, when present, was due to substance abuse rather than a higher genetic susceptibility to schizophrenia.22
Recent research examined the potential of multiple PRSs for schizophrenia, IQ, educational attainment, and attention deficit hyperactivity disorder (ADHD) in differentiating cognitive antecedents and current IQ clusters in a group of patients with established schizophrenia. The results suggested that different trajectories of cognitive development in psychosis may reflect variation in genetic influence.11
The present study aimed to replicate these findings in a large, mostly European sample of FEP, compared with a group of population-based controls, cognitively characterized and profiled according to premorbid social and academic adjustment in childhood and early adolescence. In addition, we examined the discriminative ability of PRSs for schizophrenia (SCZ_PRS), bipolar disorders (BD_PRS), major depression (MD_PRS), and IQ (IQ_PRS) about cognitive profiles, as previous evidence in our sample suggests that different PRS differentiate between affective and nonaffective psychosis.25 The principal aims were: (1) to examine whether participants with FEP can cluster in different groups according to measures of premorbid social functioning (PSF) and premorbid academic functioning (PAF) in childhood and early adolescence and current IQ; (2) to describe and compare the different clusters by PRSs for SCZ, BD, MD, and IQ. As a secondary aim, we sought (3) to examine whether patterns of cannabis use differ between clusters.
Methods
Participants
The sample comprised patients with FEP and population-based controls who participated in the multi-centre EU-GEI study; all the participants provided written informed consent. In addition, ethics committees in each study site provided ethical approval for the study.26–31 Details are in Supplementary Material.
Measures
Instruments
To facilitate replicability, we used measures of development and IQ, which are well-established and widely used in research. Namely, an abbreviated version of the Wechsler Adult Intelligence Scale (WAIS)32 and the Premorbid Adjustment Scale (PAS).33,34 The Cannabis Experience Questionnaire (CEQ) collected cannabis and other substance use information.24 Researchers assessed patients as soon as they reached a stable mental state following treatment and ensured they were referring to the preonset period.27 The CEQ and the PAS interviews were completed by at least one corroborative source of information (eg, family, clinical notes, and other clinicians) to minimize the effect of the recall bias. Details are in Supplementary Material.
Cluster Analysis on FEP
We performed cluster analysis on childhood and early adolescence PSF, PAF, and current IQ to determine whether participants with FEP could be allocated into distinct membership classes. Controls were not included but used as a single group. We used a TwoStep Cluster Analysis procedure in SPSS, version 24 (details in Supplementary Material). We used the stepwise decrease in log- likelihood as the distance measure for identifying clusters and changes in the Bayesian Information Criterion (BIC) to determine the number of clusters to retain (best ratio change of cluster distance at least > 1.15).35 Fleiss’s kappa index established the extent of agreement in cluster assignment.36 We inserted the variable “self-ascribed ethnicity” in the “evaluation” field of the two-step cluster analysis to estimate its descriptive importance comparing clusters. To see if PSF and PAF changes in the two age ranges were significantly different within the formed clusters and controls, we ran repeated-measures ANOVAs. It was adjusted by age, sex, country, and self-ascribed ethnicity, having clusters of patients and controls as the between-group factor.
Genotyping and PRS Calculation
Genotype procedure and genetic ancestry analysis are reported in Supplementary Material. PRSs were built with the clumping and thresholding method using the PRSice software37 and summary statistics from the largest available GWAS at the time of the analysis, excluding the current EU-GEI sample.38–41 A P-value threshold of 0.05 for SNP inclusion was chosen across phenotypes based on the variance explained in the original studies. The number of SNPs overlapping with the discovery summary was 11,022 for the SCZ_PRS, 9,564 for the BD_PRS, 11,014 for the MDD_PRS, and 13,691 for the IQ_PRS. The estimated heritability indexed by common alleles explains 7% of the phenotypic variance for SCZ_PRS,38 4% for BD_PRS,39 3% for MD_PRS,40 and 5.2% for IQ_PRS.41
PRSs Comparisons Between Groups
For PRS comparisons, we restricted our analysis to subjects with genetically European ancestry because the other groups were underpowered for such analyses. As a secondary analysis, we comprised all the multi-ancestry samples. We ran a multinomial logistic regression to estimate the effect of SCZ, BD, MD, and IQ PRSs in predicting group membership, including 10 PCs, age, sex, and the country as covariates and using controls as the reference category. The Nagelkerke R2 estimated the difference between models including PRS and models including covariates only. We then repeated this analysis by excluding controls and using the high-cognitive-functioning cluster as the reference category. Finally, a multivariate general linear model tested if SCZ, BD, MD, and IQ PRSs differed by the subgroups. PRSs for the graphs were adjusted by age, sex, country, and the 10 PCs. Of note, this analysis allows us to take into account all the different PRSs included. Finally, according to our secondary aim, a higher Cannabis Use Disorder PRS (CUD_PRS)42 could increase the probability of being a daily cannabis user.43 A posthoc exploratory analysis compared clusters and controls by CUD_PRS, adjusted for the same variables. Finally, we tested Height_PRS as a control analysis.
Results
Representativeness and Characteristics of the Sample
The sample comprised 802 patients with FEP (70.9% of the original sample, N = 1,130) and 1,263 population controls (84.4% of the original sample, N = 1,497), assessed with WAIS and PAS. The patients included in this analysis were younger compared to those not assessed patients [t(1,128) = 3.6, P < .001]. There were no meaningful differences between the controls and those not included (Supplementary Table 1). However, patients differed from controls, as detailed in Supplementary Table 2.
Cluster Results
Our optimal cluster analysis identified a four-cluster solution (obtained 36.5% of times; intermediate silhouette = 0.3; BIC = 2268.5; the ratio of distance measures = 1.591) ( Supplementary Figure 1). We termed the four clusters: high-cognitive-functioning, low-cognitive-functioning, intermediate, and deteriorating. Agreement in the assignment of subjects to the clusters was moderate (Fleiss’ kappa = 0.419, 95% CI: 0.418, 0.420, P < .005), ranging from good in the deteriorating cluster, moderate in the high- and low-functioning clusters, and poor in the intermediate cluster ( Supplementary Figure 2). Self-ascribed ethnicity had negligible descriptive power (predictor importance = 0.04).
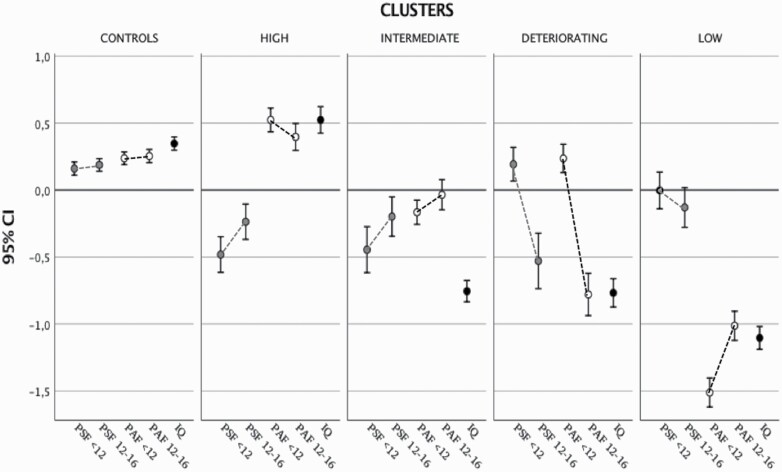
The high-cognitive-functioning cluster comprised 205 (25.5%) patients, who had the highest IQ (M = 106.1, 95% CI: 104.3, 107.9). They showed high PAF < 12, slightly deteriorating over time (Mdiff = −0.10, 95% CI: −0.20, −0.01), but low PSF < 12, somewhat improving in early adolescence (Mdiff = 0.24, 95% CI: 0.14, 0.33). Compared with controls, they had similar IQ (Mdiff = 2.7, 95% CI: −0.4, 6), and a higher PAF < 12 (Mdiff = 0.28, 95% CI: 0.16, 0.4) and PAF12-16 (Mdiff = 0.21, 95% CI: 0.02, 0.39).
Two hundred twenty-three patients (27.8%) clustered in the low-cognitive-functioning group, with the lowest IQ (M = 73.9, 95% CI: 72.2, 75.7), and PAF < 12 slightly improving in early adolescence (Mdiff = 0.51, 95% CI: 0.41, 0.6), but at the lowest level among groups. Their PSF < 12 was better than PAF < 12 and near normal but marginally deteriorating over time (Mdiff = −0.13, 95% CI: −0.28, 0.04).
Two hundred twenty-four patients (27.9%) showed an intermediate profile, compared with the previous two groups, in terms of IQ (M = 80.8, 95% CI: 79.1, 82.5) and PAF < 12. They had low PSF < 12; however, they improved in both PAS (Mdiff = 0.13, 95% CI: −0.03, 0.22) and PSF (Mdiff = 0.24, 95% CI: 0.15, 0.33) in early adolescence. They differed from the high-cognitive-functioning group only in having lower IQ and PAF at both ages.
Finally, 150 patients (18.7%) were assigned to the deteriorating group, having an intermediate IQ (M = 80.6, 95% CI: 78.5, 82.7). However, in contrast to the intermediate group, started with good PAF < 12 (Mdiff = −0.001, 95% CI: 0.13, −0.13) and a PSF < 12 (Mdiff = −0.013, 95% CI: 0.13, −0.19) comparable to controls’; then, they deteriorated in early adolescence in both PSF (Mdiff = −0.74, 95% CI: −0.85, −0.62) and PAF (Mdiff = −0.96, 95% CI: −0.08, −0.85).
Repeated measures showed that all the patient groups had more significant change between childhood and early adolescence than in the general population controls; this change was more marked in the deteriorating group than in the other three clusters (table 1; figure 1; Supplementary Tables 3 and 4).
Table 1.
Comparisons in PSF, PAF, and IQ Scores
| Between-groups pairwise comparisons | Within-patient statisticsa | ||
|---|---|---|---|
| Dependent variable | Contrasts | Statistic F (3,789) | P-value |
| IQ | C = H > I = D > L | 221.7 | 2.5−10 |
| PAF < 12 | H > C = D > I > L | 316.2 | 8.81−12 |
| PAF12-16 | C > H > I > D > L | 121.1 | 1.5−64 |
| PSF < 12 | C = D > L > H = I | 18.7 | 8.91−13 |
| PSF12-16 | C > H = I = D = L | 3.2 | .021 |
C = CONTROLS; H = HIGH-COGNITIVE-FUNCTIONING; I = INTERMEDIATE; D = DETERIORATING; L = LOW-COGNITIVE-FUNCTIONING
Accounting for sex, age, country, and self-ascribed ethnicity.
aStatistics are relative to within-patient comparisons by assuming the high-cognitive-functioning group as the baseline category.
Fig. 1.
Trajectories of PSF and PAF, and IQ in childhood and early adolescence by clusters of FEP and controls. Legend: Y-axis represents z-scores for PSF, PAF, and IQ in each cluster of patients with FEP and controls. HIGH = high-cognitive-functioning; LOW = low-cognitive-functioning.
Subgroup Comparisons
The distribution by sex was similar among clusters [χ2(3) = 6.6, P = .084]. The low-cognitive-functioning and deteriorating groups were the youngest [F(3) = 6.5, P < .001], and the deteriorating group had the lowest percentage of European ancestry subjects [70%, (χ2(3) = 19.6, P > 0.001)], among those genotyped. Detailed between-group comparisons are in Supplementary Table 5. Patients in the low-cognitive-functioning (34.8%) and the deteriorating (36.9%) group had the highest percentage of daily cannabis smokers. Among patients, daily users of high-potency cannabis were more likely to belong to the deteriorating cluster (24.8%; log ODD = 0.422, 95% CI: 0.031, 0.834, P = .034) [χ2(3) = 11.8, P = .008]. The distribution of specific diagnoses [χ2(39) = 38, P = .515] was similar between different clusters ( Supplementary Table 6, Supplementary Figure 3). IQ subtests showed that processing speed was impaired in all clusters as compared to controls [F(4,2047) = 195.9, P < .001] (Supplementary Table 7). Finally, there were between-cluster differences in the distribution of the negative [F(3,778) = 4.1, P = .006], manic [F(3,778) = 2.8, P = .037] and depressive [F(3,778) = 6.1, P = .0003] symptom dimensions ( Supplementary Tables 8 and 9).
For the PRS analyses, 623 FEP and 1,000 controls, among those included in the study were genotyped. Twelve subjects were excluded from the original EU-GEI sample due to a sex discrepancy. The PRSs we measured (SCZ_PRS, BD_PRS, MDD_PR, and CUD_PRS) were all positively related to each other and SCZ_PRS and MDD_PRS were negatively related to IQ_PRS (Supplementary Table 10); additionally, SCZ_PRS, BD_PRS, MDD_PR, and CUD_PRS were all negatively related with PAF < 12 and PAF12-16, and IQ, whereas a positive correlation was present between these scores and IQ_PRS ( Supplementary Table 11).
After ancestry restriction to European subjects only, there were 139 high-cognitive-functioning, 142 intermediate, 77 deteriorating, and 133 low-cognitive-functioning patients compared with 836 controls. Compared to the covariates-only model, (Δχ2 = 271, df = 68, P = 4.8546−26, R2 = 0.205), the introduction of PRSs in the clusters-control analysis explained additional variance (R2 = 0.079) mostly due to SCZ_PRS (2-log Likelihood of reduced model χ2 (2-Logχ2) (4, 20) = 48.8, P = 6.18−10), BD_PRS [2-Logχ2(4,20) = 18.4, P = .001], and IQ PRS [2-Logχ2(4,20) = 10.9, P = .027]. MD_PRS did not explain additional variance in the model [2-Logχ2(4, 20) = 7.5, P = .108].
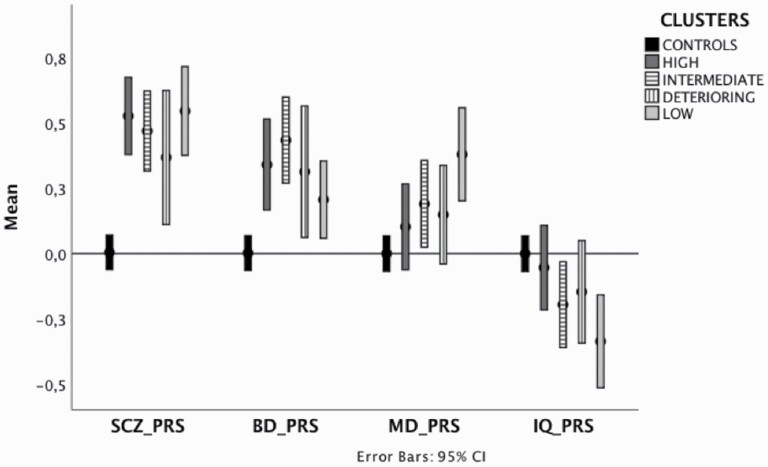
Pairwise comparisons revealed differences between controls and subgroups of patients in specific PRSs, with no substantial within-clusters differences (table 2). SCZ_PRS was higher in all clinical groups compared with controls, with the highest SCZ_PRS in the low- and high-cognitive-functioning patient groups and the lowest in the deteriorating group [F(4, 1,322) = 18.5, P > 0.001]. The intermediate- and high-cognitive-functioning groups had higher BD_PRS than controls, while the low-cognitive-functioning group had the lowest BD_PRS [F(4, 1,322) = 8.9, p > 0.001]. The high-cognitive-functioning and deteriorating groups had the most similar genetic predisposition to an average IQ, closer to controls. As expected, the low-cognitive-functioning group presented a worse IQ PRS [F(4, 1,322) = 4, P = .003] but unexpectedly higher MD_PRS than controls (table 2; figure 2; Supplementary Table 12).
Table 2.
Model Summary of Group Comparisons in Terms of PRSs
| Controls | High | Intermediate | Deteriorating | Low | η 2 | Contrasts | Within-patient statisticsa | ||
|---|---|---|---|---|---|---|---|---|---|
| PRS | (N = 836) | (N = 139) | (N = 142) | (N = 77) | (N = 133) | F | P | ||
| SZ | −0.185 −0.25, −0.11 |
0.343 0.18, 0.50 |
0.285 0.12, 0.44 |
0.184 −0.02, 0.39 |
0.362 0.20, 0.52 |
0.059 | L = H = I = D > C | 0.65 | .583 |
| BD | −0.113 −0.18, −0.04 |
0.218 0.06, 0.37 |
0.313 0.15, 0.46 |
0.192 −0.02, 0.40 |
0.084 −0.07, 0.24 |
0.028 | I = H > C = D = L | 1.22 | .301 |
| MD | −0.089 −0.15, −0.02 |
0.025 −0.13, 0.18 |
0.113 −0.04, 0.27 |
0.071 −0.14, 0.28 |
0.303 0.14, 0.46 |
0.017 | L > C = I = D = H | 2 | .112 |
| IQ | 0.072 0.03, 0.14 |
0.015 −0.14, 0.17 |
−0.127 −0.28, 0.03 |
−0.07 −0.29, 0.14 |
−0.268 −0.43, −0.10 |
0.013 | L < C = H = D = I | 1.9 | .123 |
Estimated Marginal Means and 95% CIs. The model is corrected by age, sex, country, and the ten ancestry PC as covariates and weighted by case/control.
High = high-cognitive-functioning; Low = low-cognitive-functioning
aStatistics are relative to within-patient only comparisons by assuming the high-cognitive-functioning group as the baseline category. Between-patient contrasts are represented in the “contrasts” column.
Fig. 2.
Polygenic risk scores across different clusters of FEP and controls. Legend: The Y-axis indicates z-scores for PRSs. Higher standardized scores indicate higher genetic risk for the SCZ_PRS, BD_PRS, and MD_PRS. For IQ_PRS, lower standardized scores indicate a predisposition to worse IQ. All the PRSs were adjusted to account for age, sex, country, and ten PCs and standardized around controls’ means and standard deviations. Each controls’ PRS represents a mean of 0 and a standard deviation of 1. Error bars represent 95% CIs. HIGH = high-cognitive-functioning; LOW = low-cognitive-functioning.
PRS scores did not differ in between-cluster comparisons in FEP only, and the four PRSs explained only 2.7% of the between-clusters variance. These results were primarily consistent with the total multi-ancestry sample, but the deteriorating sample had a higher BD_PRS than the restricted sample ( Supplementary Table 14, Supplementary Figure 4). Moreover, a sensitivity analysis verified the tenure of results by running the same PRSs at the threshold of 1.0, and the results stayed consistent ( Supplementary Figure 5).
Posthoc analysis on CUD_PRS [F(4, 1,303) = 3.25, P = .011, effect size = 0.010) revealed higher scores in the low-cognitive-functioning group with respect to controls (Mdiff = −0.000113, 95% CI: −0.000215, −1.20E-05, P = .017) but no within-cluster differences (Supplementary Table 13, Supplementary Figure 6). Finally, the Height_PRS that we used as a control PRS was not related to group membership [F(4, 1,274) = 2.122, P = .079].
Discussion
Main Findings
Using cluster analysis of patients based on the premorbid adjustment and current IQ, we identified four independent clusters, in line with previous studies with mixed-diagnosis samples.5 However, unlike previous studies, we did not find segregation of those with bipolar diagnoses into the high-cognitive-functioning group and schizophrenia in the intermediate and low-cognitive-functioning groups, nor more symptoms in patients with lower IQ.5,44 Recruiting patients at FEP may have considerably overcome the problems of illness-related social impairment and symptom duration influencing cognitive abilities. Moreover, research on those with long-established diagnoses11 could not have studied the entire patient sample included in FEP studies because a proportion of patients with psychosis recovered.45
PRSs explained 7.9% of the total variance in comparing the four clusters to controls and 2.7% of the variance of the within-patient cluster. Interestingly, the cluster of patients showing functional deterioration did not have a higher genetic liability to schizophrenia than other groups of patients.
However, this cluster was more likely composed of heavy cannabis users. Altogether, these findings might reflect that deterioration is a complex phenomenon involving other genetic culprits and environmental risk factors, able to reduce the subject’s social and cognitive capacities.24
Finally, we did not find more cognitive impairment among patients with higher SCZ_PRS.46–49 Instead, cognitive function was mainly related to variations in IQ_PRS.50
The High Cognitive-Functioning Cluster
In line with other studies,4 25% of patients fell into the high-cognitive-functioning cluster. They were well-educated, cognitively preserved, apart from processing speed,1,10,51–53 and outperformed other patients and controls in PAF, total IQ, and cognitive subdomains. Patients in this group had the highest between-patients IQ_PRS and very high SCZ_PRS. In contrast to their preserved cognitive function, this group of patients showed childhood impairment in PSF, slightly improving in adolescence, possibly impairing social problem-solving capacities.54
The Low-Cognitive-Functioning Cluster
The low-cognitive-functioning group included 28% of patients, similarly to studies describing patients with constant poor-cognitive-functioning before the onset,4 rather than deterioration, as was reflected in their PAF.16 The inclusion of all those with reasonably reliable IQ scores (≥45) in our study55 described a genuinely low-cognitive-functioning group, probably enclosing neurodevelopmentally impaired patients.56,57 However, they were not necessarily the most globally compromised over their lifespan. They showed better PSF in childhood than high-cognitive-functioning and intermediate patients, although slightly deteriorating in early adolescence.
This group was the most different from controls in terms of polygenic liability. In addition to higher SCZ_PRS, this cluster presented lower IQ and higher MD_PRSs than controls. The first finding confirms the role of IQ as an independent risk factor for developing psychosis,50,58 and at an earlier age of onset.59,60 According to emerging evidence,61 some people with a high MD_PRS and a neurodevelopmental genetic vulnerability (ie, higher SCZ and lower IQ PRS) may develop psychosis, whereas others develop major depression at similar ages.62 This differentiation raises an interesting question about the role of additional risk factors.63 Notably, almost 35% of patients in this group smoked cannabis daily, which at least triple the odds of developing psychosis24 and contributes to lower age of onset.64,65 Accordingly, CUD_PRS in the low-cognitive-functioning group was the highest among clusters and differentiated them from controls.
The Intermediate Cluster
The intermediate group included 28% of our patients. Accordingly, their IQ PRS was effectively intermediate between the high and deteriorating groups from one side and the low group from the other. Despite their lower intellectual functioning (Supplementary Table 13), intermediate patients reported the same employment rates as the high-cognitive-functioning patients. Thus, they probably had adequate functioning before onset.66 However, this group presented SCZ_PRS and BD_PRS higher than controls, the latter being the highest among the patient groups. Unlike previous studies,4,15 we found a precise premorbid characterization of this group, different from the other clusters: like the high-cognitive-functioning group, these patients presented very low PSF but, like the low-cognitive-functioning cluster, they presented low PAF; both these scores improved over time, as also occurred in the control group. The lowest educational attainment, a higher percentage of non-white subjects, and differential national representativeness of this group (mainly from Spain and Brazil), compared with the high cognitive-functioning cluster (the UK and the Netherlands), could indicate that their intermediate IQ reflects the combined effect of genetic, cultural, and other environmental differences.21,67,68
The Deteriorating Cluster
These patients had normal childhood function (PSF and PAF) similar to controls but showed deterioration during early adolescence. The SCZ_PRS in this cluster was higher than controls, but the lowest among patients and PRSs for IQ, BD, and MD were not different from controls. In line with a recent study with non-schizophrenic older adults,69 this finding contradicts the hypothesis of an intrinsic cognitive deterioration due to a higher schizophrenia genetic predisposition. Dickinson et al.11 revealed higher SCZ_PRS and lower IQ_PRS in patients with “adolescent declining” from premorbid to current IQ. However, they excluded patients with a history of substance abuse. We observed that deteriorating patients were likelier to have smoked high-potency cannabis daily, both known risk factors for psychosis.24 This pattern could be responsible for the lower age of onset of this group,64,65 as compared with the high-cognitive-functioning and the intermediate group, and could have contributed to deteriorating PAF and, hypothetically, current IQ as described in our previous study.22 However, their CUD_PRS was not different from controls and was lower than the low- the high-cognitive-functioning patients. This finding is in line with previous analyses suggesting that the probability of smoking high-potency cannabis, which increases tolerance and the frequency of cannabis use, is more dependent on the market availability than on any polygenic predispositions.23,24 Also, nearly half of patients in this cluster ascribed to a minority ethnic group, which may have represented an additional risk factor reflecting sociocultural disadvantage.70–72
Nevertheless, the current study cannot provide definitive causal evidence of the relationship between heavy cannabis use and a deteriorating pattern in premorbid adjustment. Cannabis was the only environmental risk factor explored because of its relationship to cognition and premorbid adjustment.22 Additionally, environmental risk factors often correlate with other environmental and polygenic risk factors,73 like cannabis abuse and minority ethnic groups, urbanization, and social isolation.74,75 Thus, one of the most recent approaches using environmental risk score algorithm76 would help explore the hypothesis of higher environmental exposure in this cluster of FEP.
Nonetheless, we have to acknowledge some limitations of the study. First, there were marginal variations in the PRS profile between-patient groups. Although the GWASs used different power and predictive values,77 we cannot directly compare the discriminative ability between different PRSs. Higher power would be necessary to find differences between groups with high-superimposable genetic characteristics.78 PRSs should be further considered as dynamic sources of weighting effects of risk allele effects, as they vary each time more powered GWASs are released. In the interim, we may consider the lack of additional explanatory power of the MD_PRS40 is due to its lower predictive value, which is less than half that SZ_PRS.38
Additionally, differences could emerge in more sophisticated analyses based on subsets of genes or addressing the role of other components of the human genomic variation (eg, copy number variations), which could be present more in a cluster than in another.79 The restriction of ancestry to solely white Europeans excluded a group of subjects, particularly at risk for psychosis, and limited the generalizability of our findings.70 Furthermore, it is a cross-sectional study, and the reconstruction of the premorbid development is subject to self-report. However, this is not especially critical when using the PAS.80 Equally, it would be interesting to look at the cognitive trajectories of our clusters at follow-up. The clusters resulting from a multicultural sample could only be partially replicated in different subsamples. However, the variability resulting from multi-site samples yields light on differences that studies using different methodologies in more homogeneous samples should not be able to catch. Finally, we only looked at cannabis use as an environmental risk factor and did not have the opportunity to control for socioeconomic status. Nonetheless, in future directions of this study, we could examine the distribution of other environmental risk factors between clusters.
This is, to our knowledge, the first study clustering patients with FEP by premorbid and cognitive-functioning, which suggests four discrete subgroups across the psychosis spectrum. First, cognitive impairment and premorbid deterioration were not reflected in a higher polygenic risk for schizophrenia. Indeed, the group of low-cognitive-functioning at FEP seemed to reflect neurodevelopmental impairment but no childhood social impairment. In contrast, the high-cognitive-functioning group showed childhood social impairment. Second, an intermediate cognitive group showed poor but improving childhood social and academic functioning. In contrast, a deteriorating group showed a marked deterioration in social and academic function in early adolescence, from normal functioning in childhood, probably related to an environmental impact, such as daily use of high-potency cannabis.
This study argues against the view that a pronounced and generalized deterioration in premorbid adjustment is typical of most patients who develop psychosis, especially in those more strongly predisposed to schizophrenia. Our findings could also inform care and prevention strategies81 focusing on patients’ resources and on preventable risk factors, such as cannabis misuse.
Supplementary Material
Acknowledgements
Special acknowledgment to all the patients and the EU-GEI team. A special thanks to Prof. Jonathan Rabinowitz who gave his useful insights in a very early stage of this work. This study represents independent research. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Contributor Information
Laura Ferraro, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy.
Diego Quattrone, Department of Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; National Institute for Health Research, Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College, London, UK; South London and Maudsley Mental Health NHS Trust, London, UK.
Daniele La Barbera, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy.
Caterina La Cascia, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy.
Craig Morgan, Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK.
James B Kirkbride, Division of Psychiatry, University College London, Psylife Group, London, UK.
Alastair G Cardno, Division of Psychological and Social Medicine, University of Leeds, Leeds, UK.
Pak Sham, Li KaShing Faculty of Medicine, The University of Hong Kong, Centre for Genomic Sciences, Hong Kong, China.
Giada Tripoli, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy; Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Lucia Sideli, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy; LUMSA University, Department of Human Science, Rome.
Fabio Seminerio, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy.
Crocettarachele Sartorio, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy.
Andrei Szoke, University of Paris Est Creteil, INSERM, IMRB, AP-HP, Hôpitaux Universitaires, H. Mondor, DMU IMPACT, F-94010 Creteil, France.
Ilaria Tarricone, Department of Medical and Surgical Science, Psychiatry Unit, Alma Mater Studiorum, University of Bologna, Bologna, Italy.
Miquel Bernardo, Department of Medicine, IDIBAPS, CIBERSAM, Barcelona Clinic Schizophrenia Unit, Neuroscience Institute, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Victoria Rodriguez, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Simona A Stilo, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; Department of Mental Health and Addiction Services, ASP Crotone, Crotone, Italy.
Charlotte Gayer-Anderson, Department of Health Service and Population Research, Institute of Psychiatry, King’s College London, London, UK.
Lieuwe de Haan, Department of Psychiatry, Early Psychosis Section, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands.
Eva Velthorst, Department of Psychiatry and Seaver Center for Research and Treatment, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Center for Transcultural Psychiatry Veldzicht, Balkbrug, Overijssel, The Netherlands.
Hannah Jongsma, Division of Psychiatry, University College London, Psylife Group, London, UK; Center for Transcultural Psychiatry Veldzicht, Balkbrug, Overijssel, The Netherlands; University Centre for Psychiatry, University Medical Centre Groningen, Groningen, The Netherlands.
Rutten B P Bart, Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands.
Alexander Richards, Division of Psychological Medicine and Clinical Neurosciences, MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, Cardiff, UK.
Celso Arango, Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, CIBERSAM, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain.
Paulo Rossi Menezez, Department of Preventive Medicine, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil.
Antonio Lasalvia, Section of Psychiatry, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Sarah Tosato, Section of Psychiatry, Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy.
Andrea Tortelli, Institut Mondor de recherché biomedicale, Creteil, France; Etablissement Public de Sante Maison Blanche, Paris, France.
Cristina Marta Del Ben, Neuroscience and Behaviour Department, Ribeirão Preto Medical School, University of São Paulo, Brazil.
Jean-Paul Selten, University Centre for Psychiatry, University Medical Centre Groningen, Groningen, The Netherlands; Rivierduinen Institute for Mental Health Care, Leiden, The Netherlands.
Peter B Jones, Department of Psychiatry, University of Cambridgeshire and Peterborough NHS Foundation Trust, CAMEO Early Intervention Service, Cambridge, UK.
Jim van Os, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, The Netherlands; UMC Utrecht Brain Centre Rudolf Magnus, Utrecht University, Utrecht, The Netherlands.
Marta Di Forti, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy; Department of Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; National Institute for Health Research, Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College, London, UK.
Evangelos Vassos, Department of Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; National Institute for Health Research, Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College, London, UK.
Robin M Murray, Department of Biomedicine, Neuroscience and Advanced Diagnostics (BiND), Psychiatry Section, University of Palermo, Palermo, Italy; National Institute for Health Research, Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College, London, UK; Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Funding
The European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-GEI) Project is funded by grant agreement HEALTH-F2-2010–241909 (Project EU-GEI) from the Seventh Framework Programme. The Brazilian part of the study was funded by the Fundacion de Amparo . Pesquisa do Estado de Sao Paulo (grant n. 2012/0417-0); this study was further part funded by the National Institute for Health and Care Research (NIHR) Maudsley Biomedical Research Centre (BRC) at South London and the South London and Maudsley (SLaM) National Health Service (NHS) Foundation Trust, and the King's College, London, UK.
References
- 1. Kravariti E, Morgan K, Fearon P, et al. Neuropsychological functioning in first-episode schizophrenia. Br J Psychiatry. 2009;195(4):336–345. [DOI] [PubMed] [Google Scholar]
- 2. Russo M, Van Rheenen TE, Shanahan M, et al. Neurocognitive subtypes in patients with bipolar disorder and their unaffected siblings. Psychol Med. 2017;47(16):2892–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter CS. Clusters, dimensions, and hierarchies: finding a path forward for the neuroscience of mental disorders? Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(1):2–3. [DOI] [PubMed] [Google Scholar]
- 4. Carruthers SP, Van Rheenen TE, Gurvich C, Sumner PJ, Rossell SL.. Characterising the structure of cognitive heterogeneity in schizophrenia spectrum disorders. A systematic review and narrative synthesis. Neurosci Biobehav Rev. 2019;107:252–278. [DOI] [PubMed] [Google Scholar]
- 5. Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quidé Y.. A systematic review of studies reporting data-driven cognitive subtypes across the psychosis spectrum. Neuropsychol Rev. 2020;30(4):446–460. [DOI] [PubMed] [Google Scholar]
- 6. Uren J, Cotton SM, Killackey E, Saling MM, Allott K.. Cognitive clusters in first-episode psychosis: overlap with healthy controls and relationship to concurrent and prospective symptoms and functioning. Neuropsychology. 2017;31(7):787–797. [DOI] [PubMed] [Google Scholar]
- 7. Habtewold TD, Rodijk LH, Liemburg EJ, et al. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl Psychiatry. 2020;10(1): 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badcock JC, Dragović M, Waters FAV, Jablensky A.. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and compromised intellect. J Psychiatr Res. 2005;39(1):11–19. [DOI] [PubMed] [Google Scholar]
- 9. Leeson VC, Harrison I, Ron M, Barnes T, Joyce E.. The effect of cannabis use and cognitive reserve on age at onset and psychosis outcomes in first-episode schizophrenia. Schizophr Bull. 2011;38(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR.. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913. [DOI] [PubMed] [Google Scholar]
- 11. Dickinson D, Zaidman SR, Giangrande EJ, Eisenberg DP, Gregory MD, Berman KF.. Distinct polygenic score profiles in schizophrenia subgroups with different trajectories of cognitive development. Am J Psychiatry. 2020;177(4):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan CC, Shanahan M, Ospina LH, Larsen EM, Burdick KE.. Premorbid adjustment trajectories in schizophrenia and bipolar disorder: a transdiagnostic cluster analysis. Psychiatry Res. 2019;272:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole VT, Apud JA, Weinberger DR, Dickinson D.. Using latent class growth analysis to form trajectories of premorbid adjustment in schizophrenia. J Abnorm Psychol. 2012;121(2):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsen TK, Friis S, Haahr U, et al. Premorbid adjustment in first-episode non-affective psychosis: distinct patterns of pre-onset course. Br J Psychiatry. 2004;185:108–115. [DOI] [PubMed] [Google Scholar]
- 15. Quee PJ, Meijer JH, Islam MA, et al. Premorbid adjustment profiles in psychosis and the role of familial factors. J Abnorm Psychol. 2014;123(3):578–587. [DOI] [PubMed] [Google Scholar]
- 16. Allen DN, Kelley ME, Miyatake RK, Gurklis J, van Kammen DP.. Confirmation of a two-factor model of premorbid adjustment in males with schizophrenia. Schizophr Bull. 2001;27(1):39–46. [DOI] [PubMed] [Google Scholar]
- 17. Allen DN, Frantom LV, Strauss GP, van Kammen DP.. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophr Res. 2005;75(2):389–397. [DOI] [PubMed] [Google Scholar]
- 18. Roser MP, Allott K, Killackey E, Farhall J, Cotton SM.. Exploring cognitive heterogeneity in first-episode psychosis: what cluster analysis can reveal. Psychiatry Res. 2015;229(3):819–827. [DOI] [PubMed] [Google Scholar]
- 19. Leeson VC, Sharma P, Harrison M, Ron M, Barnes TRE, Joyce EM.. IQ trajectory, cognitive reserve, and clinical outcome following a first episode of psychosis: a 3-year longitudinal study. Schizophr Bull. 2011;37(4):768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferraro L, Russo M, O’Connor J, et al. Cannabis users have higher premorbid IQ than other patients with first onset psychosis. Schizophr Res. 2013;150(1):129–135. [DOI] [PubMed] [Google Scholar]
- 21. Ferraro L, Murray RM, Di Forti M, et al. IQ differences between patients with first episode psychosis in London and Palermo reflect differences in patterns of cannabis use. Schizophr Res. 2019;210:81–88. [DOI] [PubMed] [Google Scholar]
- 22. Ferraro L, La Cascia C, Quattrone D, et al. Premorbid adjustment and IQ in patients with first-episode psychosis: a multisite case-control study of their relationship with cannabis use. Schizophr Bull. 2020;46(3):517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Forti M, Vassos E, Lynskey M, Craig M, Murray RM.. Cannabis and psychosis—authors’ reply. Lancet Psychiatry. 2015;2(5):382. [DOI] [PubMed] [Google Scholar]
- 24. Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez V, Alameda L, Quattrone D, et al. Use of multiple polygenic risk scores for distinguishing schizophrenia-spectrum disorder and affective psychosis categories in a first-episode sample; the EU-GEI study. Psychol Med. 2022:1–10. doi: 10.1017/S0033291721005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jongsma HE, Gayer-Anderson C, Lasalvia A, et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry. 2018;75(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gayer-Anderson C, Jongsma HE, Di Forti M, et al. The EUropean Network of National Schizophrenia Networks Studying Gene–Environment Interactions (EU-GEI): incidence and first-episode case–control programme. Soc Psychiatry Psychiatr Epidemiol. 2020;55(5):645–657. [DOI] [PubMed] [Google Scholar]
- 28. CORDIS. Final Report Summary—EU-GEI (European Network of National Schizophrenia Networks Studying Gene-Environment Interactions) _Report Summary _EU-GEI FP7 CORDIS European Commission.pdf. 2019. https://cordis.europa.eu/project/id/241909/reporting/it. Accessed February 8, 2021.
- 29. van Os J, Rutten BP, Poulton R.. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34(6):1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Os J, Rutten BP, Myin-Germeys I, et al. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40(4):729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Published 1992.
- 32. Velthorst E, Levine SZ, Henquet C, et al. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in Schizophrenia—a control-case family study. Cogn Neuropsychiatry. 2013;18(6):574–593. [DOI] [PubMed] [Google Scholar]
- 33. Cannon-Spoor HE, Potkin SG, Wyatt RJ.. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8(3):470–484. [DOI] [PubMed] [Google Scholar]
- 34. Rabinowitz J, Levine SZ, Brill N, Bromet EJ.. The Premorbid Adjustment Scale Structured Interview (PAS-SI): preliminary findings. Schizophr Res. 2007;90(1):255–257. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Li QL, Dong LY, Wen BC.. Combination clustering analysis method and its application. J Appl Sci. 2013;13(8):1251–1255. [Google Scholar]
- 36. Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 37. Choi SW, O’Reilly PF.. PRSice-2: polygenic Risk Score software for biobank-scale data. GigaScience. 2019;8(7):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stahl EA, Breen G, Forstner AJ, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Demontis D, Rajagopal VM, Thorgeirsson TE, et al. Genome-wide association study implicates CHRNA2 in cannabis use disorder. Nat Neurosci. 2019;22(7):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson EC, Demontis D, Thorgeirsson TE.. A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry. 2020;7(12):1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Legge SE, Cardno AG, Allardyce J, et al. Associations between schizophrenia polygenic liability, symptom dimensions, and cognitive ability in schizophrenia. JAMA Psychiatry. 2021:e211961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgan C, Lappin J, Heslin M, et al. Reappraising the long-term course and outcome of psychotic disorders: the AESOP-10 study. Psychol Med. 2014;44(13):2713–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Habtewold TD, Liemburg EJ, Islam MA, et al. Association of schizophrenia polygenic risk score with data-driven cognitive subtypes: a six-year longitudinal study in patients, siblings and controls. Schizophr Res. 2020;223:135–147. [DOI] [PubMed] [Google Scholar]
- 47. Sniekers S, Stringer S, Watanabe K, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 2017;49(7):1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kendler KS, Ohlsson H, Sundquist J, Sundquist K.. IQ and schizophrenia in a Swedish National Sample: their causal relationship and the interaction of IQ with genetic risk. 2015. Doi: 10.1176/appi.ajp.2014.14040516 [DOI] [PMC free article] [PubMed]
- 49. Engen MJ, Lyngstad SH, Ueland T, et al. Polygenic scores for schizophrenia and general cognitive ability: associations with six cognitive domains, premorbid intelligence, and cognitive composite score in individuals with a psychotic disorder and in healthy controls. Transl Psychiatry. 2020;10(1):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Richards AL, Pardiñas AF, Frizzati A, et al. The relationship between polygenic risk scores and cognition in Schizophrenia. Schizophr Bull. 2020;46(2):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cobia DJ, Csernansky JG, Wang L.. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr Res. 2011;133:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruiz JC, Soler MJ, Fuentes I, Tomás P.. Intellectual functioning and memory deficits in schizophrenia. Compr Psychiatry. 2007;48(3):276–282. [DOI] [PubMed] [Google Scholar]
- 53. Lim K, Smucny J, Barch DM, Lam M, Keefe RSE, Lee J.. Cognitive subtyping in schizophrenia: a latent profile analysis. Schizophr Bull. 2021;47(3):712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vaskinn A, Sundet K, Hultman CM, Friis S, Andreassen OA.. Social problem-solving in high-functioning schizophrenia: specific deficits in sending skills. Psychiatry Res. 2009;165(3):215–223. [DOI] [PubMed] [Google Scholar]
- 55. Wechsler D. WAIS-R: Manual: Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 56. Howes OD, Murray RM.. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murray RM, O’Callaghan E, Castle DJ, Lewis SW.. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18(2):319–332. [DOI] [PubMed] [Google Scholar]
- 58. Toulopoulou T, Zhang X, Cherny S, et al. Polygenic risk score increases schizophrenia liability through cognition-relevant pathways. Brain. 2019;142(2):471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joyce E. Origins of cognitive dysfunction in schizophrenia: clues from age at onset. Br J Psychiatry. 2005;186:93–95. [DOI] [PubMed] [Google Scholar]
- 60. Baeza I, de la Serna E, Amoretti S, et al. Premorbid characteristics as predictors of early onset versus adult onset in patients with a first episode of psychosis. J Clin Psychiatry. 2021;82(6). doi: 10.4088/JCP.21M13907. [DOI] [PubMed] [Google Scholar]
- 61. Rice F, Riglin L, Thapar AK, et al. Characterizing developmental trajectories and the role of neuropsychiatric genetic risk variants in early-onset depression. JAMA Psychiatry. 2019;76(3):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Power RA, Tansey KE, Buttenschøn HN, et al. Genome-wide association for major depression through age at onset stratification: major depressive disorder working group of the psychiatric genomics consortium. Biol Psychiatry. 2017;81(4):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Agerbo E, Trabjerg BB, Børglum AD, et al. Risk of early-onset depression associated with polygenic liability, parental psychiatric history, and socioeconomic status. JAMA Psychiatry. 2021;78(4):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Large M. Cannabis use and earlier onset of psychosis: a systematic meta-analysis cannabis use and earlier onset of psychosis. Arch Gen Psychiatry. 2011;68(6):555–561. [DOI] [PubMed] [Google Scholar]
- 65. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wieland J, Zitman FG.. It is time to bring borderline intellectual functioning back into the main fold of classification systems. BJPsych Bull. 2016;40(4):204–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickens WT, Flynn JR, Aaron H, et al. Heritability estimates versus large environmental effects: the IQ paradox resolved. Psychol Rev. 2001;108(2):346–369. [DOI] [PubMed] [Google Scholar]
- 68. Ferraro L, Capuccio V, La Cascia C, et al. Better IQ but worse Premorbid Academic Adjustment in cannabis-users psychotic patients: another brick in the intuition. In: Early Intervention in Psychiatry. Vol 10; 2016:7–61, Wiley-Blackwell Publishing Ltd,United Kingdom. [Google Scholar]
- 69. Kępińska AP, MacCabe JH, Cadar D, Steptoe A, Murray RM, Ajnakina O.. Schizophrenia polygenic risk predicts general cognitive deficit but not cognitive decline in healthy older adults. Transl Psychiatry. 2020;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tarricone I, D’Andrea G, Jongsma HE, et al. Migration history and risk of psychosis: results from the multinational EU-GEI study. Psychol Med. 2021. doi: 10.1017/S003329172000495X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jongsma HE, Gayer-Anderson C, Tarricone I, et al. Social disadvantage, linguistic distance, ethnic minority status and first-episode psychosis: results from the EU-GEI case-control study. Psychol Med. 2020. doi: 10.1017/S003329172000029X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kirkbride JB, Barker D, Cowden F, et al. Psychoses, ethnicity and socio-economic status. Br J Psychiatry. 2008;193(1):18–24. [DOI] [PubMed] [Google Scholar]
- 73. Murray RM, Vassos E.. Nature, nurture, and the polygenic risk score for schizophrenia. Schizophr Bull. 2020;46(6):1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murray RM, Mondelli V, Stilo SA, et al. The influence of risk factors on the onset and outcome of psychosis: what we learned from the GAP study. Schizophr Res. 2020;225:63–68. [DOI] [PubMed] [Google Scholar]
- 75. Guloksuz S, Rutten B, Pries L, et al. The complexities of evaluating the exposome in psychiatry: a data-driven illustration of challenges and some propositions for amendments. Schizophr Bull. 2018;44:1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vassos E, Sham P, Kempton M, et al. The Maudsley environmental risk score for psychosis. Psychol Med. 2020;50(13):2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ripke S, Walters JTR, O’Donovan MC.. Mapping genomic loci prioritises genes and implicates synaptic biology in schizophrenia. medRxiv. 2020. doi: 10.1101/2020.09.12.20192922. [DOI] [Google Scholar]
- 78. Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grama S, Willcocks I, Hubert JJ, et al. Polygenic risk for schizophrenia and subcortical brain anatomy in the UK Biobank cohort. Transl Psychiatry. 2020;10(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brill N, Reichenberg A, Weiser M, Rabinowitz J.. Validity of the premorbid adjustment scale. Schizophr Bull. 2008;34(5):981–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shah JL, Scott J, McGorry PD, et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. 2020;19(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.