Abstract
Background
Several psychotropic drugs can induce weight gain and metabolic alterations. The authors compared metabolic evolutions of patients switching versus continuing psychotropic treatments with different risk profiles.
Methods
Patients either switched from a high- to a medium- (N = 36) or low-risk drug (N = 27), from a medium- to a low-risk drug (N = 71), or to a same-risk drug (N = 61). Controls were kept using either a high- (N = 35), medium- (N = 155), or low-risk drug (N = 47). The evolution over 2 years of weight and metabolic parameters was analyzed using linear mixed-effect models, also examining the influence of polygenic risk scores for body mass index (BMI) or BMI and psychiatric disorders.
Study Results
High-, medium-, or low-risk controls gained on average 1.32%, 0.42%, and 0.36% more weight per month than patients switching from or within these risk categories (P < .001, P < .001, and P = .003, respectively). High-to-high or high-to-medium switches resulted in a greater weight increase than switching to lower-risk categories (+0.77% and + 0.39% respectively, P < .001). No difference was found between switching medium-to-medium and medium-to-low (P ≈ 1). Switching high-to-low resulted in 10% weight loss after 2 years, with the greatest loss occurring the first 6 months after the switch. Compared with high-risk controls, lower total cholesterol (−0.27 mmol/l, P = .043) in the high-to-low group, and lower glucose (−0.44 mmol/l, P = .032) and systolic blood pressure (−5.50 mmHg, P = .034) in the low-to-low group were found. Polygenic scores were not associated with weight changes in controls or after switching.
Conclusion
Psychotropic switches to a lower- or same-risk drug can attenuate weight gain, with only switching high to low resulting in weight loss.
Keywords: psychotropic switch, metabolic risk, weight gain
Introduction
Several psychotropic drugs can induce cardiometabolic diseases such as type II diabetes, dyslipidemia, and/or obesity, contributing to the overall 10-year decrease in life expectancy among psychiatric patients.1 Within 6 to 12 months of therapy, weight increases can reach up to 12% from baseline,2 and lipid and/or glucose dysregulation may also occur,3–5 with some psychotropic drugs leading to more metabolic alterations than others.6 Among antipsychotics, clozapine, and olanzapine show the highest risk of inducing metabolic alterations.6 Quetiapine and risperidone follow as medium-risk drugs, while aripiprazole, amisulpride, and lurasidone are classified as low-risk.6,7 Mood stabilizers such as valproate and lithium can also induce weight gain, with valproate leading to more weight increase,8 blood lipid and/or glucose impairments than lithium.9,10 Among antidepressants, mirtazapine was identified as one of the most likely to induce metabolic side effects,11 with weight gain over 1 year comparable to quetiapine and/or risperidone.7 Along with psychotropic medication, risk factors for weight gain in the psychiatric population include female sex, young age, and low baseline weight.12–14
When nonpharmacological interventions (eg, diet, physical exercise) are insufficient for losing weight, switching psychotropic drugs has been used as a strategy to attenuate and/or reverse metabolic adverse effects.7 A 6-week randomized open-label study15 reported a weight loss of 2 kg and 0.7 kg among 173 and 112 patients switching from olanzapine or risperidone to aripiprazole, respectively, and similar results were reported in 2 open-label studies of 71 and 12 patients, respectively, reporting a weight loss of 1.3 kg after 20 weeks and 2.25 kg after 10 weeks following a switch from olanzapine to risperidone or quetiapine, respectively.16,17 Weight loss of 2.9 kg was also reported in a 6-month open-label study among 223 patients switching from risperidone to lurasidone.18 On the other hand, another 12-week open-label study did not find any significant changes in weight among 9 patients switching from risperidone to aripiprazole.19 No weight changes when switching to a same-risk molecule (eg, from quetiapine to risperidone20), and weight increases when switching to a higher-risk molecule have also been described.21 A decrease in triglycerides of 32.7 mg/dl after switching from olanzapine, quetiapine, or risperidone to aripiprazole was also reported for 109 patients in a 24-week randomized trial,22 along with an increase of 5.3 mg/dl of high-density lipoprotein (HDL) cholesterol levels and a decrease of 11.7 mg/dl in total cholesterol levels among 61 patients switching from mixed antipsychotics to aripiprazole in a 26-week open-label study.23 However, another 64-week open-label study did not find any differences in lipid levels among 79 patients switching from mixed antipsychotics to aripiprazole.24 Changes in glucose levels were either not reported or not detected in the previously mentioned studies.22–24 According to a meta-analysis, when compared with patients taking the same medication over the long term, only patients switching to aripiprazole lost weight and/or improved fasting glucose and/or triglyceride levels, while no glucose nor lipid level changes were detected after switching to amisulpride, paliperidone and/or risperidone, quetiapine, or lurasidone.21
Most of the abovementioned studies examined the metabolic consequences of switches over a period of up to 6 months only.15–20,22,23 In addition, control groups continuing to use the previous psychotropic medication was either absent15–17,19,20,23,24 or did not include switches to molecules presenting the same metabolic risk profile.18,22
Finally, because different classes of psychotropic drugs (antipsychotics, antidepressants, and mood stabilizers) can be prescribed to treat different psychiatric disorders (eg, antipsychotics for bipolar disorders25 and/or general anxiety26), it is important to consider metabolic changes after switching not only from one antipsychotic to another, but also from one class of psychotropic drugs to another, taking into account the class of risk for metabolic worsening.
In the present study, analyzing metabolic parameters before and after a switch and comparing them with a control group staying on the same medication, we evaluated whether switching psychotropic drugs for a lower- or same-risk molecule is a valid strategy for attenuating and/or reversing metabolic alterations in a large cohort of psychiatric patients in Switzerland.
Methods
Study Design
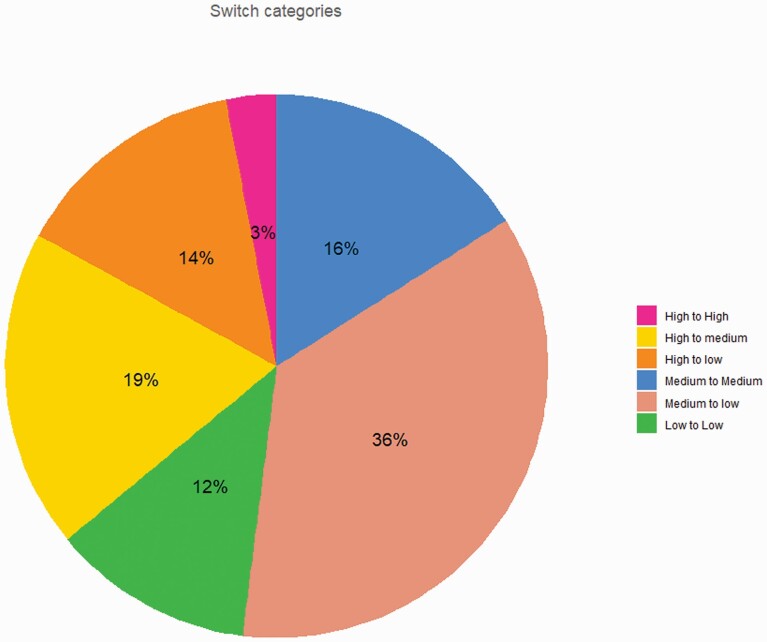
Patients were selected from the Psymetab cohort started in 2007 at the Department of Psychiatry of Lausanne University Hospital, in collaboration with a private mental health care center (Les Toises). As previously described,27 upon signature of an informed consent, PsyMetab collects clinical and genetic data from patients taking psychotropic treatments known to induce metabolic alterations. The Ethics Committee of the Canton of Vaud also granted access to clinical data of patients followed at the Department of Psychiatry of Lausanne University Hospital from 2007 to 2015 (PsyClin) because of the noninterventional post hoc analysis design. Patients switching psychotropic drugs were included and compared to patients maintaining the same medication. High-risk drugs included clozapine, olanzapine, and valproate; medium-risk drugs included levomepromazine, lithium, mirtazapine, quetiapine, risperidone/paliperidone, and zuclopenthixol; and low-risk drugs included amisulpride, aripiprazole, brexpiprazole, flupenthixol, haloperidol, and lurasidone. Patients either switched from a high-to-low, from a high-to-medium, from a medium-to-low risk drug, or for a molecule within the same risk category (see Figure 1 for proportions, and Supplementary Figure 1 for drug repartition). Switching was defined as starting a new medication within 30 days from the end of the previous one. If the previous psychotropic drug was quetiapine or aripiprazole, the gap between the 2 treatments had to be no longer than 14 and 60 days, respectively, due to the shorter and longer half-lives of the 2 drugs, respectively.28 Duration of treatments (before and after the switch) were available, and patients with treatment durations < 21 days for both first and second treatments were excluded, along with patients taking depot formulations during the first treatment. Controls were also classified as high-, medium-, or low-risk control groups.
Fig. 1.
Proportions of included patients in each switch category. Percentage of included patients in each switch category for the 195 patients switching psychotropic medication. High-risk drugs included clozapine, olanzapine, and valproate; medium-risk drugs included levomepromazine, lithium, mirtazapine, quetiapine, risperidone/paliperidone, and zuclopenthixol; and low-risk drugs included amisulpride, aripiprazole, brexpiprazole, flupenthixol, haloperidol, and lurasidone.
Measurements
Clinical data on age, sex, weight, height, diagnoses, lipids and/or glucose blood levels, and blood pressure were collected at the beginning of the treatment, after 1 and 3 months, and yearly. At 2 and 6 months, weight measurements were also scheduled. For hospitalized patients, supplementary observations of clinical data (eg, weight, lipid values) collected during the stay were also available. Weight change was calculated as the percentage of change from baseline value (ie, baseline weight at the beginning of the first treatment and/or at the beginning of the switch for the switch group).
Statistical Analysis
Descriptive statistics comparing patients switching versus controls were performed using the Wilcoxon rank-sum test for continuous variables and the Pearson χ2 or Fisher exact test for categorical variables as appropriate. The evolution of weight and other metabolic parameters over time was analyzed using linear mixed-effect models. Since the evolution of each metabolic parameter is strongly correlated with follow-up duration and age, the models included equal follow-up and age range for controls and switch groups (eg, for glucose observations available for 14-to-80-year-old controls and 15-to-80-year-old patients switching, only 15-to-80-year-old patients were included in the model). Linear mixed-effect models of weight change were adjusted by sex, age at baseline, baseline weight, medical environment (in- and outpatient), and by the interaction of both switch and control categories with time. Moreover, to compare switch groups versus their controls, and the different switches with one another, general linear hypothesis testing was used with contrast matrices, corrected for multiple testing by the “holm” method. Furthermore, partial r-square values indicated the share of variability explained by each covariate, and variable importance using t-statistics was reported. Since observations after 1 year may have included only patients experiencing mild metabolic disturbances (ie, patients with strong metabolic disturbances would have had their treatments changed), a quadratic model was applied including observations within 1 year to estimate the direction and the speed of weight changes over time. For glucose, total, HDL and low-density lipoprotein (LDL) cholesterol, triglycerides, and blood pressure, linear mixed-effect models over a 2-year follow-up were also performed, adjusting by the switch and control categories, time, sex, age at baseline and baseline body mass index (BMI), and excluding patients taking somatic-related drugs (eg, patients taking antidiabetic medication were excluded from models evaluating glucose). Models were also adjusted by fasting status for total, LDL, and HDL cholesterol, while for glucose and triglycerides only fasting observations were included. Additional linear mixed-effect models on weight change were performed for genotyped patients adjusting separately for 5 polygenic risk scores (941 and 97 BMI-associated single nucleotide polymorphisms (SNPs), 63 BMI- and schizophrenia-associated SNPs, 17 BMI- and bipolar disorder-associated SNPs, and 32 BMI-and major depression-associated SNPs; see Supplementary Methods). Smoking status, psychotropic co-medications, and the different diagnoses did not influence our outcomes (data not shown) and these covariates were therefore not included in the models. Stata 16.0 (StataCorp; College Station, TX) and R environment for statistical computing version 4.0.2 were used for the analysis, and P-values of ≤ .05 were considered statistically significant.
Results
Table 1 displays the clinical and demographic characteristics of the switch and control groups regardless of the risk categories. Patients who switched were younger (P < .001), had shorter follow-ups (P < .001), were mostly inpatients (P < .001), and were diagnosed with psychotic disorders (P < .001). Switch group BMI at baseline and between follow-ups did not differ from controls baseline BMI (P = .43 and P = .10, respectively).
Table 1.
Descriptive Statistics of Switch and Control Groups
| Switch (N = 195) |
Control (N = 237) |
P-value | Totala (N = 432) |
|
|---|---|---|---|---|
| Age at baseline (years) | 33 (23–50) | 44 (30–58) | <.001 | 39 (27–54) |
| Sex | .61 | |||
| Men | 102 (52.3%) | 117 (49.4%) | 219 (50.7%) | |
| Women | 93 (47.7%) | 120 (50.6%) | 213 (49.3%) | |
| Diagnosesb | <.001 | |||
| Psychotic disorders | 101 (51.8%) | 64 (27.0%) | 165 (38.2%) | |
| Depression | 29 (14.9%) | 42 (17.7%) | 71 (16.4%) | |
| Bipolar disorder | 20 (10.3%) | 45 (19.0%) | 65 (15.0%) | |
| Schizoaffective disorders | 26 (13.3%) | 13 (5.5%) | 39 (9.0%) | |
| Others | 10 (5.1%) | 23 (9.7%) | 33 (7.6%) | |
| Missing | 9 (4.6%) | 50 (21.1%) | 59 (13.7%) | |
| Duration of 1st follow-upc (days) | 92 (45–170) | 380 (360–430) | <.001 | 350 (100–390) |
| Duration of 2nd follow-upc (days) | 140 (61–340) | 380 (360–430) | <.001 | 360 (170–390) |
| Total follow-up durationd (days) | 290 (160–520) | 380 (360–430) | <.001 | 370 (290–460) |
| BMI at baselinee (Kg/m2) | 23 (21–26) | 24 (21–27) | .43 | 23 (21–26) |
| Missing | 3 (1.5%) | 21 (8.9%) | 24 (5.6%) | |
| BMI between follow-upse (kg/m2) | 24 (22–28) | 24 (21–27) | .10 | 24 (21–27) |
| Missing | 3 (1.5%) | 21 (8.9%) | 24 (5.6%) | |
| Smoking | .81 | |||
| Yes | 95 (48.7%) | 95 (40.1%) | 190 (44.0%) | |
| No | 89 (45.6%) | 83 (35.0%) | 172 (39.8%) | |
| Missing | 11 (5.6%) | 59 (24.9%) | 70 (16.2%) | |
| Psychotropic co-medicationf | .060 | |||
| Yes | 50 (25.6%) | 42 (17.7%) | 92 (21.3%) | |
| No | 145 (74.4%) | 195 (82.3%) | 340 (78.7%) | |
| Medical environment | <.001 | |||
| Inpatients | 134 (68.7%) | 30 (12.7%) | 164 (38.0%) | |
| Outpatients | 61 (31.3%) | 207 (87.3%) | 268 (62.0%) |
Note: BMI, body mass index.
Note: Information follows.
aMedian with quartiles 1 - 3 and proportions are reported for continuous and categorical variables, respectively.
bInternational Classification of Diseases-10th Revision classification: organic disorders, anxiety, personality disorder, intellectual disability, dementia, and substance use disorder were classified together as “other.”
cFirst (before switch) and second (after switch) follow-up duration is the same for controls.
dFor the switch group, it refers to the sum of the 2 follow-ups durations (ie, first and second).
eControls have the same BMI at baseline and between follow-ups. For the switch group, BMI at baseline refers to the BMI at the beginning of the 1st follow-up, and BMI between follow-ups refers to the BMI at the moment of the switch.
fPsychotropic comedication with potential for increasing weight: haloperidol, pipamperone, flupentixol, asenapine, amisulpride, aripiprazole, lurasidone, zuclopenthixol, levomepromazine, risperidone/paliperidone, quetiapine, lithium, mirtazapine, valproate, olanzapine, and clozapine.
Note: Significant P-values are in bold.
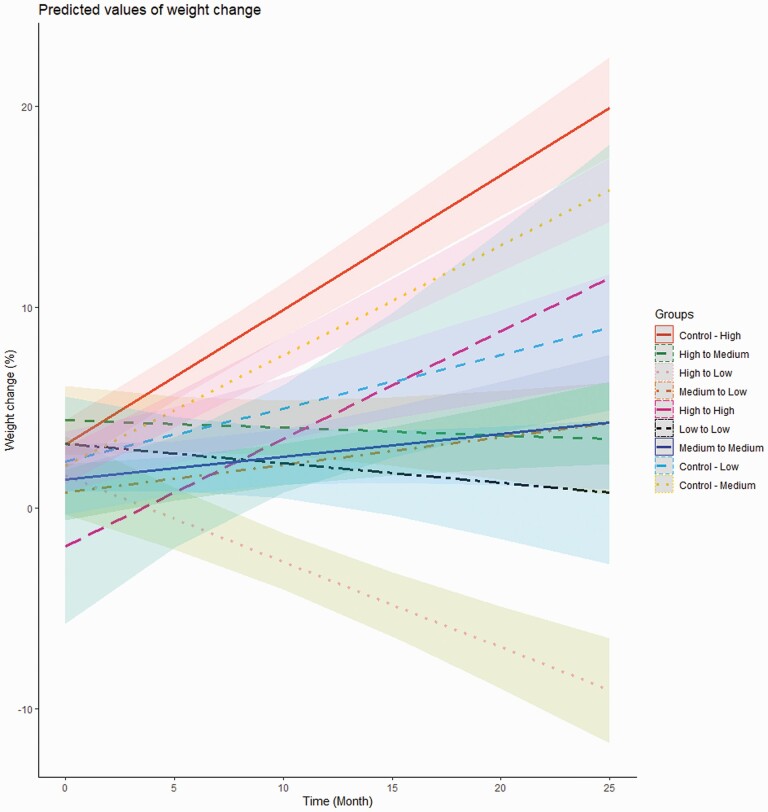
For high-risk controls, the linear mixed-effect model (Table 2) showed a positive correlation between time and weight change with +0.67% of weight per month of treatment (P < .001). Weight change was also negatively correlated with baseline weight (−0.10% for each additional kilogram at baseline, P < 0.001), and with age (−0.05% for each additional year, P < .001). Inpatients gained less weight (mean −1.54%) than outpatients (P < .001), and patients switching high-to-high gained less weight (mean −5.07%) than high-risk controls (P = .007, data not shown). For patients switching high-to-medium and high-to-low (Table 2), weight changes of −0.04% and −0.43% for each additional month were found, respectively (P < .001). Patients switching medium-to-low and medium-to-medium showed −0.41% and a −0.44% per month as compared to controls taking medium-risk drugs, respectively (P < .001), whereas patients switching low-to-low drugs showed −0.36% compared to controls taking low-risk drugs (P < .001). Predicted values of weight change over time (Figure 2) showed weight loss only for patients switching high-to-low, with around 10% weight loss predicted after 2 years, which was the same amount of weight gain (+10%) predicted for high-risk controls over one year. Moreover, for patients switching high-to-low and their controls, the quadratic model (Supplementary Figure 2) predicted that the greatest weight decrease or increase, respectively, occurred during the first 6 months after the switch, or treatment start, followed by a flattening of weight evolution over time. Interestingly, switching high-to-medium or low-to-low led to a weight gain attenuation only after a moderate weight increase occurring during the first 6 months after the switch. On the other hand, patients switching medium-to-medium and medium-to-low experienced a moderate but constant weight increase over time. Since early weight gain (≥5% in one month) is a risk factor for further weight increase in the long term,27 an additional analysis including this variable in the model and excluding baseline weight was performed, reporting similar results as in Table 2 (data not shown). A sensitivity analysis also was performed excluding patients taking metformin (N = 17),29 this drug being also prescribed to attenuate psychotropic-induced weight gain, reporting similar results as in Table 2 (data not shown).
Table 2.
Linear Mixed-Effect Models of Weight Changes Over A 2-Year Follow-up
| Weight Change Over 2-Year Follow-upa | |||
|---|---|---|---|
| Predictors | Estimatesb | CI | P |
| Time [Control High]c | 0.67 | 0.57 to 0.77 | <.001 |
| Control Medium * Timed | −0.12 | −0.24 to −0.00 | .045 |
| Control Low * Timee | −0.41 | −0.56 to −0.25 | <.001 |
| Switch High-to-Medium * Time | −0.71 | −0.87 to −0.55 | <.001 |
| Switch High-to-Low * Time | −1.10 | −1.26 to −0.94 | <.001 |
| Switch Medium-to-Low * Time | −0.53 | −0.68 to −0.39 | <.001 |
| Switch High-to-High * Time | −0.14 | −0.51 to 0.24 | .47 |
| Switch Medium-to-Medium * Time | −0.56 | −0.76 to −0.36 | <.001 |
| Switch Low-to-Low * Time | −0.77 | −0.98 to −0.55 | <.001 |
| N Patients | 432 | ||
| N Observations | 5348 | ||
Note: CI: confidence interval; P, P-value (significant values in bold); N: number.
Note: Information follows.
aLinear mixed-effect model adjusted by sex, age, medical environment, and baseline weight.
bEstimates indicate the mean weight change size per month.
cReference group. Time is expressed in months. Weight change for patients switching from high-to-low risk is −0.43% (ie, 0.67%–1.10%) for each additional month, whereas for patients switching from high-to-medium risk weight change is −0.04% (ie, 0.67%–0.71%) for each additional month. No significant difference was found between controls taking high-risk drugs and patients switching within the high-risk category. Medium- and low-risk controls gained 0.55% (ie, 0.67–0.12) and 0.26% (ie, 0.67–0.41) in weight for each additional month.
dSwitching medium-to-low and medium-to-medium showed -0.41% [(−0.53%) − (−0.12%)] and−0.44% [(−0.56%) − (−0.12%)] weight change compared to controls taking medium-risk drugs.
eSwitching low-to-low drugs showed −0.36% [(−0.77%) − (−0.41%)] weight change compared to controls taking low-risk drugs.
Fig. 2.
Predicted values of weight change in control patients and in the switch group. Gradual weight loss is observed only in patients switching high-to-low, with a prediction of around 10% weight loss after 2 years, which is the same amount of weight gain in high-risk controls over one year. High-risk drugs included clozapine, olanzapine, and valproate; medium-risk drugs included levomepromazine, lithium, mirtazapine, quetiapine, risperidone/paliperidone, and zuclopenthixol; and low-risk drugs included amisulpride, aripiprazole, brexpiprazole, flupenthixol, haloperidol, and lurasidone.
Using general linear hypothesis testing (Table 3), high-, medium-, or low-risk controls gained on average +1.32%, +0.42%, and +0.36% more weight per month than patients switching from these categories (P < .001, P < .001, and P = .003, respectively). Furthermore, switching high-to-high was associated with greater weight increase than switching from high-to lower-risk categories on average (+0.77% per month, P < .001), and patients switching high-to-medium gained +0.39% more weight per month than patients switching high-to-low (P < .001). No difference was found between switching medium-to-medium and switching medium-to-low (P ≈ 1).
Table 3.
Test of Linear Hypothesesa
| Tested Hypothesesb,c,d | Estimatese | P |
|---|---|---|
| Control High vs. average of Switch High-to-Low, -Medium, and -Highb | 1.32 | <.001 |
| Control Medium vs average of Switch Medium-to-Low and -Mediumb | 0.42 | <.001 |
| Control Low vs Switch Low-to-Lowb | 0.36 | .003 |
| Switch High-to-High vs. average Switch High-to-Medium and -Lowc | 0.77 | <.001 |
| Switch Medium-to-Medium vs Switch Medium-to-Lowc | −0.02 | ≈1 |
| Switch High-to-Medium vs Switch High-to-Lowd | 0.39 | <.001 |
Note: P, P-value (significant values in bold).
Note: Information follows.
aInteractions of time with both switch and control categories shown in Table 2 are tested using the matrix of contrasts.
bHypothesis: after switch, mean weight change over time of controls equals weight change over time of patients switching from a molecule within the same risk category of controls.
cHypothesis: after switch, mean weight change over time of patients switching within the same category of risk equals weight change over time of patients switching to a lower-risk molecule.
dHypothesis: after switch, mean weight change over time of patients switching high-to-low equals weight change over time of patients switching high-to-medium.
eControls taking high-risk drugs gained +1.32% more weight for each additional month than patients switching from a high-risk drug. Moreover, patients switching high-to-high gained +0.77% more weight for each additional month than the other switch groups starting with a high-risk molecule, and patients switching high-to-medium gained +0.39% more weight for each additional month than patients switching high-to-low.
Considering partial r-square values (Supplementary Figure 3), 5.8% of the variance was explained by baseline weight, followed by the interaction of time with both high-risk controls (3.4%) and patients switching high-to-low (3.3%). These last 2 covariates also showed the highest levels of importance according to the t-statistics (Supplementary Figure 4).
Due to a significant interaction of age and sex with switch and/or control groups and time (data not shown), stratified models (data not shown) were created, and linear hypotheses were tested (Supplementary Tables 1 and 2). Among young adults (≤25 years), only medium-risk controls gained significantly more weight per month when compared with patients switching from a medium-risk molecule (+0.89% per month, P < .001). In addition, patients switching high-to-medium gained more weight per month than patients switching high-to-low (+1.31% per month, P < .001). Concerning adults (>25 and <65 years), controls taking a medium-or low-risk molecule gained more weight than patients switching, +0.24% and+0.54% per month, respectively (P = .029 and P < .001, respectively), with no difference in weight change between the switch groups. On the other hand, old-age (≥65 years) controls taking low-risk drugs gained less weight per month than patients switching within this category (P < .001), and switching within the medium-risk category resulted in greater weight increase than switching medium-to-low (+1.32% per month, P = .003). Each control group among women gained more weight than the switch groups (P < .001) whereas, among men, only medium-risk controls gained more weight per month than men switching from a medium-risk molecule (P < .001). Moreover, switching within the high category was associated with greater weight increase per month than switching high-to-lower among women (+0.76%, P = .001), as well as switching high-to-medium versus high-to-low (+0.46%, P = .049), which was also found among men (+0.40%, P = .006).
Polygenic risk scores for BMI or BMI and psychiatric disorders were not associated with weight changes in controls (N = 241) or after a switch (N = 93, Supplementary Table 3).
Metabolic Parameters
No significant interaction between time and switch or control groups was found in linear mixed-effect models over a 2-year follow-up on glucose, total, HDL, LDL cholesterol, triglycerides, and systolic blood pressure, whereas a significant interaction for patients switching medium-to-medium was found on diastolic blood pressure, probably due to chance finding (−0.44 mmHg per month, P = .029, data not shown). When compared with high-risk controls (Supplementary Table 4), a mean of −0.27 mmol/l (P = .043) in total cholesterol for patients switching high-to-low (P = .043) was found, as well as a mean −0.44 mmol/l (P = .032) in glucose and −5.50 mmHg (P = .034) in systolic blood pressure for patients switching low-to-low. No difference was found among the 3 control groups within each model.
Discussion
With a 2-year naturalistic longitudinal study design, different weight patterns were found between controls continuing on the same psychotropic medication and patients switching for either a lower-risk or a same-risk molecule. Controls gained more weight per month than patients switching from their same risk category. This result is in line with a previous 24-week randomized trial including 215 patients, reporting −2.9kg for patients switching to aripiprazole when compared with patients continuing to use either olanzapine, risperidone, or quetiapine.22 However, those patients were randomized into switching or staying on the same medication with the outcome being the weight difference 24 weeks after switching, while in the present study switching was due to clinical needs (eg, poor treatment response and excessive weight gain), and weight evolution before and after switching was modeled over 2 years. Moreover, since weight evolution is baseline-weight-dependent, percentages of weight change rather than absolute weight are more informative. In addition, to the best of our knowledge, the present study is the first in which patients switching low-to-low were directly compared to low-risk controls, the latter group showing greater weight increase. In other words, switching to a drug in the same risk category could result in weight gain attenuation. This result is in agreement with a 12-week open-label observational study, with 19 patients switching from low-risk aripiprazole to low-risk ziprasidone, resulting in a mean loss of 3 kg.30 Given that higher weight gain is observed among antipsychotic-naïve patients,31 a progressive adaptation after each psychotropic therapy would partially explain why a same-risk switch could attenuate the weight increase of the previous psychotropic therapy, regardless of antipsychotic-naïve status.
To the best of our knowledge, the present study is the first to compare weight change after switching from a high to a high-risk drug to both switchings from a high-to-medium and low-risk drug, indicating that the first alternative leads to a greater weight change per month. Weight gain attenuation and weight loss were also reported for high-to-medium and high-to-low patients, respectively, when compared to high-risk controls. Similarly, a meta-analysis reported a mean weight increase of 2.8kg when switching to a high-risk drug (eg, olanzapine or clozapine), and no significant weight changes when switching to medium-risk drugs (eg, to quetiapine and/or risperidone), and 2 kg weight loss when switching to a low-risk drug (ie, aripiprazole).21 On the other hand, our results did not show differences in the evolution of weight between patients switching medium-to-medium versus medium-to-low, probably because of the moderate difference in the metabolic risk between the 2 drug categories. Ultimately, our results show that only switching high-to-low resulted in weight loss, the amount of weight loss predicted after 2 years being the same amount gained by controls in half the time (ie, 1 year). In addition, for patients switching high-to-low and their controls, the greatest weight decrease or increase, respectively, occurred during the first 6 months of treatment (switch or start). These results are in line with previous studies reporting a weight gain plateau after 9 months32 of olanzapine treatment and after 1 year33 of a psychotropic treatment. Our results are also in line with another study reporting that the greatest weight loss is reached within 6 months among obese patients undergoing diet and anti-obesity pharmacological treatments.34 To our knowledge, the present study is the first to predict that the greatest amount of weight loss is reached within the first 6 months after switching high-to-low.
Partial r-squared values highlighted the importance of accounting for baseline weight when considering weight evolutions, as this covariate was the most explicative of our model variance. This is in agreement with other studies showing that a low baseline weight is a major risk factor for important weight gain induced by psychotropic drugs.14,35 The second and third most explicative covariates were weight evolutions over time of both high-controls and patients switching high-to-low, probably because the most important weight gain and loss, respectively, were found in these two groups.
Among both young adults and adults, no difference between high-risk controls and patients switching from a high-risk molecule was found, most probably because of the very low number of patients switching high-to-high (ie, 2 young adults and 3 adults, data not shown). However, a difference in weight gain was found for patients switching high-to-medium versus high-to-low only among young adults. Since young age is a risk factor for psychotropic-induced weight gain,14 younger patients could be the age category most benefitting from switching high-to-low. Interestingly, elderly patients switching low-to-low gained more weight than elderly controls staying on the same low-risk drugs, and elderly patients switching medium-to-medium also gained more weight per month than elderly patients switching medium-to-low, these results probably be explained by the lower sample size in the elderly group (ie, 68 patients included) versus the others (ie, 102 and 260 patients included in the young adult and adult groups, respectively). Of note, partial r-squared values indicated age as the 4th most explicative covariate of our main model variance, underlying the need of further studies evaluating weight evolution among controls and patients switching in larger age-categorized sample sizes.
Concerning sex-stratified analysis, weight changes in women were similar to those found in the whole cohort. On the other hand, among men, only medium-risk controls showed greater weight gain per month than patients switching from a medium-risk drug. A trend was, however, found of higher weight change among high-risk male controls versus patients switching high-to-lower. Moreover, male patients switching high-to-medium gained more weight per month than patients switching high-to-low. Since female sex is a risk factor for psychotropic-induced weight gain, women could benefit more from switching drugs.36 Moreover, similar results were found for young adults and men, probably because men were statistically younger than women (39 vs 46 years, P < .001, data not shown), which would contribute to the different results between the sexes.
No differences in the evolution of blood glucose and/or lipid levels were found within control and switch groups. However, switching high-to-low resulted in lower concentrations of total cholesterol, in accordance with a previous 26-week open-label study reporting total cholesterol decrease after switching to aripiprazole.23 On the other hand, our results are in contrast with a previous 24-week randomized trial reporting a decrease in triglycerides after switching to aripiprazole,22 and with a 26-week meta-analysis, detecting fasting glucose and/or triglyceride improvements when switching to aripiprazole.21 This discrepancy could be due to the shorter duration of treatment after a switch (ie, median of 20 weeks in the present study versus 24 and 26 weeks), to the risk defined before and after switching, and/or to the lower statistical power within each control and switch group (eg, our 19 patients switching high-to-low with triglyceride levels vs 89 in the previously mentioned trial22).
Of note, polygenic risk scores for BMI or BMI and psychiatric disorders were not associated with weight changes either in controls or after a switch, probably due to the limited sample size and/or the limited effect sizes of genetic factors included in the scores and/or an overall limited influence of genetic factors. Further studies with greater sample sizes focusing on BMI and psychiatric-related polygenic risk scores are needed.
The present study has several limitations. Weight-impacting variables such as physical activity, diet, alcohol consumption, and/or psychotropic-naïve status were unavailable. Adherence to treatment could not be ascertained, although for inpatients the record of daily-administered drugs was taken into account. Moreover, we could not account for the psychotropic dose, which could influence the weight change,37–39 nor for confounding factors such as age of onset for psychiatric illness and/or duration of total psychotropic treatment prior to the study entry and/or the initial weight before any psychotropic treatment. Concomitant prescription of all weight-impacting drugs could not be taken into account, but a sensitivity analysis excluding patients with metformin, a drug which could be prescribed to attenuate psychotropic-induced weight gain,29 was performed. An inclusion bias could be that controls may have stayed on their medication due to milder metabolic adverse effects than in patients who switched, the present results could therefore underestimate the effects of switching. On the other hand, patients switching due to excessive weight gain could have been advised to increase physical activity and/or be under diet supervision (ie, first-line clinical approaches to reverse psychotropic-induced weight gain7), possibly leading to an overestimation of our results. For the metabolic parameters, the duration of follow-up after the switch was probably insufficient to detect differences among groups. Moreover, a limited sample size was available for certain switch categories (eg, high-to-high), and for investigating the influence of polygenic risk scores. On the other hand, our study could benefit from real-world data, as it models for the first time weight changes of controls and patients switching from the same-risk drugs as the controls, and compares same-risk switches versus lower-risk ones.
Conclusion
Our results suggest that psychotropic switching to a lower or to a same-risk drug can attenuate psychotropic-induced weight gain, while only switching high-to-low resulted in weight loss occurring mainly during the first 6 months after the switch. Because of the slow effect of a switch on weight evolution, the cost-benefit ratio of a psychotropic switch should be rapidly evaluated, in particular among patients experiencing early weight gain (ie, ≥5% from baseline after one month27).
Supplementary Material
Acknowledgment
The authors thank L. Maw for editorial assistance and the medical staff involved in the data collection.
Contributor Information
Marianna Piras, Unit of Pharmacogenetics and Clinical Psychopharmacology, Centre for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Setareh Ranjbar, Center for Psychiatric Epidemiology and Psychopathology, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Nermine Laaboub, Unit of Pharmacogenetics and Clinical Psychopharmacology, Centre for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Claire Grosu, Unit of Pharmacogenetics and Clinical Psychopharmacology, Centre for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Franziska Gamma, Les Toises Psychiatry and Psychotherapy Center, Lausanne, Switzerland.
Kerstin Jessica Plessen, Service of Child and Adolescent Psychiatry, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Armin von Gunten, Service of Old Age Psychiatry, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Philippe Conus, Service of General Psychiatry, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland.
Chin Bin Eap, Unit of Pharmacogenetics and Clinical Psychopharmacology, Centre for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital, University of Lausanne, Prilly, Switzerland; School of Pharmaceutical Sciences, University of Geneva, University of Lausanne, Geneva, Switzerland; Center for Research and Innovation in Clinical Pharmaceutical Sciences, University of Lausanne, Lausanne, Switzerland; Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, University of Lausanne, Geneva, Switzerland.
Funding
This work was funded by the Swiss National Research Foundation (CBE and PC: 320030-120686, 324730-144064, and 320030-173211; CBE, PC, and KJP: 320030-200602). The funding source had no role in the writing of the manuscript or in the decision to submit it for publication.
Previous Presentation
An E-Poster of the present work was presented at the European Congress of Psychiatry (04-07/06/2022).
Disclosure
CBE received honoraria for conferences from Forum pour la formation médicale, Janssen-Cilag, Lundbeck, Otsuka, Sandoz, Servier, Sunovion, Sysmex Suisse AG, Takeda, Vifor-Pharma, and Zeller in the past 3 years.
Conflict of Interest
All authors declare that they have no conflict of interest in relation to the content of this work.
Author Contributions
CBE had full access to the data in the study and takes responsibility for its integrity and accuracy. Study concept and design were provided by CBE. Acquisition of data was provided by MP, NL, CG, and by FG, KJP, AvG, and PC. MP and SR provided statistical analyses and interpretation. Drafting of the manuscript was provided by MP. Each author provided critical revision of the manuscript. CBE, PC, and KJP obtained funding for the study. FG, KJP, AvG, PC, and CE provided administrative, technical, or material support.
Ethical Statement
This study was carried out in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics committee of Vaud (CER-VD).
References
- 1. Laursen TM, Munk-Olsen T, Vestergaard M.. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83–88. [DOI] [PubMed] [Google Scholar]
- 2. Foley DL, Morley KI.. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68(6):609–616. [DOI] [PubMed] [Google Scholar]
- 3. Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K.. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olfson M, Marcus SC, Corey-Lisle P, Tuomari AV, Hines P, L’Italien GJ.. Hyperlipidemia following treatment with antipsychotic medications Am J Psychiatry. 2006;163(10):1821–1825. [DOI] [PubMed] [Google Scholar]
- 5. Grajales D, Ferreira V, Valverde ÁM.. Second-generation antipsychotics and dysregulation of glucose metabolism: beyond weight gain. Cells. 2019;8(11):1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillinger T, McCutcheon RA, Vano L, et al. . Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi: 10.1016/s2215-0366(19)30416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasnain M, Vieweg WV.. Weight considerations in psychotropic drug prescribing and switching. Postgrad Med. 2013;125(5):117–129. [DOI] [PubMed] [Google Scholar]
- 8. Bowden CL, Mosolov S, Hranov L, et al. . Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12-week trial. Int Clin Psychopharmacol. 2010;25(2):60–67. [DOI] [PubMed] [Google Scholar]
- 9. Abosi O, Lopes S, Schmitz S, Fiedorowicz JG.. Cardiometabolic effects of psychotropic medications. Horm MolBiol Clin Investig. 2018;36(1). doi: 10.1515/hmbci-2017-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verrotti A, la Torre R, Trotta D, Mohn A, Chiarelli F.. Valproate-induced insulin resistance and obesity in children. Horm Res. 2009;71(3):125–131. [DOI] [PubMed] [Google Scholar]
- 11. Gartlehner G, Hansen RA, Morgan LC, et al. . Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. Comparative Effectiveness Review No. 48. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 12. Singh R, Bansal Y, Medhi B, Kuhad A.. Antipsychotics-induced metabolic alterations: recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur J Pharmacol. 2019;844:231–240. [DOI] [PubMed] [Google Scholar]
- 13. Ward A, Quon P, Abouzaid S, Haber N, Ahmed S, Kim E.. Cardiometabolic consequences of therapy for chronic schizophrenia using second-generation antipsychotic agents in a medicaid population: clinical and economic evaluation. P T. 2013;38(2):109–115. [PMC free article] [PubMed] [Google Scholar]
- 14. Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. . Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. J Psychiatr Res. 2009;43(6):620–626. [DOI] [PubMed] [Google Scholar]
- 15. Casey DE, Carson WH, Saha AR, et al. . Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl). 2003;166(4):391–399. [DOI] [PubMed] [Google Scholar]
- 16. Meyer JM, Pandina G, Bossie CA, Turkoz I, Greenspan A.. Effects of switching from olanzapine to risperidone on the prevalence of the metabolic syndrome in overweight or obese patients with schizophrenia or schizoaffective disorder: analysis of a multicenter, rater-blinded, open-label study. Clin Ther. 2005;27(12):1930–1941. [DOI] [PubMed] [Google Scholar]
- 17. Gupta S, Masand PS, Virk S, et al. . Weight decline in patients switching from olanzapine to quetiapine. Schizophr Res. 2004;70(1):57–62. [DOI] [PubMed] [Google Scholar]
- 18. Mattingly GW, Haddad PM, Tocco M, et al. . Switching to Lurasidone following 12 months of treatment with Risperidone: results of a 6-month, open-label study. BMC Psychiatry. 2020;20(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishitobi M, Kosaka H, Takahashi T, et al. . Effectiveness and tolerability of switching to aripiprazole from risperidone in subjects with autism spectrum disorders: a prospective open-label study. Clin Neuropharmacol. 2013;36(5):151–156. [DOI] [PubMed] [Google Scholar]
- 20. Lindenmayer JP, Eerdekens E, Berry SA, Eerdekens M.. Safety and efficacy of long-acting risperidone in schizophrenia: a 12-week, multicenter, open-label study in stable patients switched from typical and atypical oral antipsychotics. J Clin Psychiatry. 2004;65(8):1084–1089. [PubMed] [Google Scholar]
- 21. Siskind D, Gallagher E, Winckel K, et al. . Does switching antipsychotics ameliorate weight gain in patients with severe mental illness? A systematic review and meta-analysis. Schizophr Bull. 2021;47(4):948–958. doi: 10.1093/schbul/sbaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stroup TS, McEvoy JP, Ring KD, et al. . A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SW, Shin IS, Kim JM, et al. . Effectiveness of switching to aripiprazole from atypical antipsychotics in patients with schizophrenia. Clin Neuropharmacol. 2009;32(5):243–249. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh MH, Lin WW, Chen ST, et al. . A 64-week, multicenter, open-label study of aripiprazole effectiveness in the management of patients with schizophrenia or schizoaffective disorder in a general psychiatric outpatient setting. Ann Gen Psychiatry. 2010;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G.. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord. 2020;269:154–184. [DOI] [PubMed] [Google Scholar]
- 26. Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N.. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–777. [DOI] [PubMed] [Google Scholar]
- 27. Vandenberghe F, Gholam-Rezaee M, Saigí-Morgui N, et al. . Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417–e1423. [DOI] [PubMed] [Google Scholar]
- 28. Mauri MC, Paletta S, Di Pace C, et al. . Clinical pharmacokinetics of atypical antipsychotics: an update. Clin Pharmacokinet. 2018;57(12):1493–1528. [DOI] [PubMed] [Google Scholar]
- 29. de Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R.. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SW, Shin IS, Kim JM, Bae KY, Yang SJ, Yoon JS.. Effectiveness of switching from aripiprazole to ziprasidone in patients with schizophrenia. Clin Neuropharmacol. 2010;33(3):121–125. [DOI] [PubMed] [Google Scholar]
- 31. Bak M, Drukker M, Cortenraad S, Vandenberk E, Guloksuz S.. Antipsychotics result in more weight gain in antipsychotic naive patients than in patients after antipsychotic switch and weight gain is irrespective of psychiatric diagnosis: a meta-analysis. PLoS One. 2021;16(2):e0244944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinon BJ, Kaiser CJ, Ahmed S, Rotelli MD, Kollack-Walker S.. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol. 2005;25(3):255–258. [DOI] [PubMed] [Google Scholar]
- 33. Hasnain M, Vieweg WV, Hollett B.. Weight gain and glucose dysregulation with second-generation antipsychotics and antidepressants: a review for primary care physicians. Postgrad Med. 124(4):154–167. [DOI] [PubMed] [Google Scholar]
- 34. Garcia Ulen C, Huizinga MM, Beech B, Elasy TA.. Weight regain prevention. Clin Diabetes. 2008;26(3):100–113. [Google Scholar]
- 35. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU.. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. [DOI] [PubMed] [Google Scholar]
- 36. Lee S-Y, Park M-H, Patkar AA, Pae C-U.. A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):490–496. [DOI] [PubMed] [Google Scholar]
- 37. Schoretsanitis G, Dubath C, Grosu C, et al. . Olanzapine-associated dose-dependent alterations for weight and metabolic parameters in a prospective cohort. Basic Clin Pharmacol Toxicol. 2022;130(4):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dubath C, Piras M, Gholam M, et al. . Effect of quetiapine, from low to high dose, on weight and metabolic traits: results from a prospective cohort study. Pharmacopsychiatry. 2021;54(6):279–286. [DOI] [PubMed] [Google Scholar]
- 39. Piras M, Dubath C, Gholam M, et al. . Daily dose effects of risperidone on weight and other metabolic parameters: a prospective cohort study. J Clin Psychiatry. 2022;83(4):21m14110. doi: 10.4088/JCP.21m14110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.