Abstract
Objectives
Disengagement from treatment is common in first episode schizophrenia (FES) and is associated with poor outcomes. Our aim was to determine whether hippocampal subfield volumes predict disengagement during maintenance treatment of FES.
Methods
FES patients were recruited from sites in Boston, New York, Shanghai, and Changsha. After stabilization on antipsychotic medication, participants were randomized to add-on citalopram or placebo and followed for 12 months. Demographic, clinical and cognitive factors at baseline were compared between completers and disengagers in addition to volumes of hippocampal subfields.
Results
Baseline data were available for 95 randomized participants. Disengagers (n = 38, 40%) differed from completers (n = 57, 60%) by race (more likely Black; less likely Asian) and in more alcohol use, parkinsonism, negative symptoms and more impairment in visual learning and working memory. Bilateral dentate gyrus (DG), CA1, CA2/3 and whole hippocampal volumes were significantly smaller in disengagers compared to completers. When all the eight volumes were entered into the model simultaneously, only left DG volume significantly predicted disengagement status and remained significant after adjusting for age, sex, race, intracranial volume, antipsychotic dose, duration of untreated psychosis, citalopram status, alcohol status, and smoking status (P < .01). Left DG volume predicted disengagement with 57% sensitivity and 83% specificity.
Conclusions
Smaller left DG was significantly associated with disengagement status over 12 months of maintenance treatment in patients with FES participating in a randomized clinical trial. If replicated, these findings may provide a biomarker to identify patients at risk for disengagement and a potential target for interventions.
Keywords: dentate gyrus, retention, prediction, brain volume, biomarker
Introduction
Despite considerable progress in coordinated specialty care for individuals with early psychosis,1 disengagement from treatment remains a serious problem. Disengagement increases risk for relapse, which may in turn result in serious psychosocial disruption and a diminished response to subsequent antipsychotic treatment.2,3 Reported rates of disengagement during the first year of treatment have ranged from 12% to 53%.4 The most consistent predictors of disengagement have included lack of family support, living alone and substance use.4 Other patient characteristics, including age,5 sex,6 employment and educational status,7 symptom severity8 and level of insight9 have been found to be associated with disengagement, but not consistently.4 To the best of our knowledge, no biological marker has been associated with an increased risk of disengagement. Whereas clinical characteristics contributing to disengagement are highly variable and largely not predictive,4 identification of a biological marker might enhance the identification of patients at high risk for disengagement and guide therapeutic interventions.
Hippocampal volume loss is a promising marker in early psychosis that may predict clinical course.10,11 Hippocampal circuits are involved in several cognitive functions that are impaired in schizophrenia, including memory, novelty detection, attribution of salience and detection of prediction error. Within the hippocampus, the dentate gyrus (DG) and the CA1 and CA2/3 subfields have been implicated in schizophrenia because of their crucial roles in memory formation and recall, which, if disrupted, could result in delusions via formation of false memories, aberrant linking of memories, and inappropriate integration of memories into a framework of prior experiences.12,13 Hippocampal circuits are also necessary for inferential reasoning and decision making based on past experiences14; hence, impairment of hippocampal function could contribute to decisions to disengage from treatment that, to family and clinicians, may not seem rational. Low volume may be a marker for hippocampal dysfunction.15 Lower volume of the CA1 subfield compared to healthy controls has been associated with functional impairment in schizophrenia patients and attributed to glutamatergic excitotoxicity resulting from a deficit in inhibitory inputs,16 whereas lower volume in the DG has been attributed to stress-related elevation of glucocorticoids and to impaired neurogenesis.17 CA2 and CA3 subfield volumes have been less studied in schizophrenia, but are highly correlated with DG volume and these subfields are functionally linked to the DG in the performance of pattern recognition and pattern completion.12
We recently completed a series of studies examining potential molecular mechanisms underlying hippocampal volume loss in early psychosis and identifying clinical correlates.18–21 To explore hippocampal volume as a potential biomarker for risk of disengagement from treatment in early psychosis, we now examine data from the DECIFER Study, a 12-month placebo-controlled trial of add-on citalopram in first episode schizophrenia patients after initial stabilization on second generation antipsychotics.18 Because this study examined participants who disengaged from treatment under conditions of a randomized clinical trial rather than under usual clinical conditions, and since, to the best of our knowledge, there is no prior evidence linking hippocampal volume to treatment disengagement, the results of this study must be considered exploratory.
We hypothesized that disengagement would be associated with low baseline hippocampal volume and we focused our examination on DG, CA1, and CA2/3 volumes. To assess whether disengagement status was specifically associated with volumes of hippocampal structures versus generalized brain atrophy, we additionally examined intracranial volume (ICV), bilateral whole hippocampal volumes, whole brain volumes of each hemisphere, and cortical thickness of all brain regions.
Methods
The DECIFER study has been previously described18 and was registered at clinicaltrials.gov NCT01041274. The study was a 12 month, four-site, randomized, parallel-group, placebo-controlled trial of add-on citalopram in the maintenance treatment of first episode schizophrenia patients who were first stabilized with clinician-determined second generation antipsychotic medication. The study was conducted at the Massachusetts General Hospital in Boston, the NYU Langone Medical Center in New York, the Shanghai Mental Health Center in Shanghai, and the Second Xiangya Hospital of Central South University, Changsha. Entry criteria included: age 15–40 years, onset of psychosis before age 35, cumulative antipsychotic exposure of at least 4 weeks and fewer than 24 weeks, a diagnosis of schizophrenia or schizophreniform disorder confirmed by the Structured Clinical Interview for DSMIV-TR (SCID), and absence of major depression, suicidal ideation, unstable medical illness or substance abuse (other than cannabis) over the preceding 3 months.
After obtaining written informed consent, participants were assessed with the Brief Psychiatric Rating Scale (BPRS), the modified Scale for Assessment of Negative Symptoms (SANS), the Calgary Depression Scale for Schizophrenia (CDSS), the Heinrichs Carpenter Quality of Life Scale (HCQLS), the Simpson Angus Scale (SAS) for assessment of parkinsonism, the Barnes Akathisia Scale (BAS), and the MATRICS Consensus Cognitive Battery (MCCB). At the Boston and New York sites only, the following rating scales were also administered at baseline: Behavior and Symptom Identification Scale (Basis-24), Macarthur Perceived Coercion Scale (PCS), Birchwood Insight Scale (BIS), Subjective Well Being Under Neuroleptic Treatment (SWN-S), and World Health Organization Quality of Life Scale: Short Version (WHOQOL-BREF). Raters at all sites were initially trained on-site in the administration of rating scales and the MCCB; rater training was maintained by conference calls every 3 months. In addition, at all four sites participants were scanned twice at baseline on 3T Siemens scanners using similar pulse-sequences, which were harmonized before study initiation. Detailed MRI sequences can be found in supplementary table S1. Following completion of baseline assessments, participants were randomly assigned to citalopram or placebo in identical 20 mg capsules that were increased after 1 week to 40 mg/d if tolerated. All participants also received psychoeducation and relapse prevention planning provided by a doctoral level clinician weekly for 16 sessions followed by 8 monthly sessions.
The FreeSurfer v6.0 automated hippocampal subfield extraction tool was used to segment the hippocampal subfields CA1, CA2/3, and DG and the mean value of the two scans was used for analyses. Hippocampal subfield segmentation has been found to be highly reliable with less than a 3% mean difference in DG, CA1 and whole hippocampus estimated volumes between repeated scans and less than a 5% mean difference in CA3 estimated volumes; reliability was higher in left compared to right structures.22 Cortical thickness was measured using the cortical surface stream of FreeSurfer v6.0 based on the T1-weighed structural MRI described in detail previously.23 We used the cortical parcellation based on the Desikan–Killiany atlas to determine the average cortical thickness in 70 cortical regions (34 regions per hemisphere plus the mean thickness of each hemisphere). All segmentations were visually inspected for accuracy by an experienced rater blind to the treatment and to the disengagement status of participants. No segmentations required manual adjustment.
Statistical Analyses
Completers and disengagers were compared with respect to baseline demographic and clinical characteristics and baseline cortical thickness using t-tests for continuous variables and chi-square tests for categorical variables. Significance was judged at a two-sided 0.05 level, without adjustment for multiple testing as we wanted to identify all potential differences between completers and disengagers.
The relationship of hippocampal subfield volumes and whole hippocampal brain volumes to disengagement status was assessed by modeling disengagement status as a function of brain volume, using logistic regression. To evaluate the presence and role of potential confounders in this relationship, a series of additional models that included progressively expanding sets of baseline patient covariates were employed and the effects of brain volume on disengagement status were compared across models. For clinical purposes, we sought to qualify the relationship between brain volume and disengagement status in terms of the ability of brain volume to predict completion of patients’ participation in the 12-month randomized controlled trial. This was accomplished by obtaining the receiver operating characteristics (ROC) of the logistic regression model for disengagement status as a function of brain volume only. The area under the curve (AUC) together with sensitivity and specificity are reported.
We performed two sensitivity analyses. The first was an equivalent analysis with the outcome being time to disengagement (using Cox proportional hazards regression) rather than disengagement status (yes/no) using logistic regression. The second sensitivity analysis repeated logistic regression analyses of the relationship between left DG brain volume and disengagement status after excluding six patients: three who were terminated by research staff due to nonadherence; one who disengaged due to incarceration and two patients who disengaged due to hospitalization. This analysis was intended to provide a sample more representative of patients electively disengaging under typical clinical conditions.
To understand the potential mechanisms by which brain volume is related to disengagement status, we assessed the magnitude and significance of the effect of brain volume on disengagement after adjusting for baseline measures of cognition and symptoms severity. To explore possible clinical correlates of DG volume that might contribute to the association with disengagement status, we tested for interactions between engagement status and clinical variables that differed between disengagers and completers, explaining DG volume. We included the MCCB composite score rather than multiple cognitive domains. This was done by logistic regressions, modeling disengagement status as a function of brain volume, clinical measures, and their interactions. The significance of the interaction terms was judged at a level of α = 0.05 without adjustment for multiple testing; this was done to avoid omitting potentially important effects in the process of generating hypotheses pertaining to mechanisms underlying the association between brain volume and disengagement. All statistical analyses were conducted using R.24
Results
Of the 95 first episode schizophrenia patients who were randomized, 57 (60%) completed the 12-month trial and 38 (40%) disengaged (table 1). Reasons for disengagement are provided in supplemental table S2. Imaging data were available for 88 participants (93%), demographic information and clinical ratings (BPRS, SANS, CDRS, SAS, and BAS) were available for 90 (95%), and MCCB assessments were available for 89 (94%). The battery of scales targeting additional factors hypothesized to contribute to disengagement (BIS, PCS, SWN-S, WHOQOL-BREF) were available for the 44 (46%) participants at the US sites. At baseline, disengagers did not differ from completers by age or sex, but did differ by race; disengagers were more likely to be Black and less likely to be Asian (table 1). Disengagers were also more likely to use alcohol, had more severe negative symptoms (SANS total score) and Parkinsonism (SAS total score) and exhibited greater impairments in working memory and visual learning (table 1). Disengagers did not differ significantly from completers on measures of psychosis, insight, subjective or objective quality of life, perceived coercion, medication adherence, treatment assignment (citalopram vs placebo) or akathisia (table 1 & supplemental table S3). At a trend level of significance, disengagers were prescribed a lower dose of antipsychotic medication, had higher BPRS total scores, performed more poorly on MCCB tests of attention and vigilance and were more likely to smoke cigarettes (table 1).
Table 1.
Baseline Characteristics of Disengagers vs Completers
| Patient Characteristics | Whole Sample | Disengagers | Completers | Engagers vs. Completers Comparison | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD)/n (%) | N | Mean (SD)/n (%) | N | Mean (SD)/n (%) | P-value | Effect Size | |
| Age | 95 | 23.4 (4.83) | 38 | 23.7 (5.19) | 57 | 23.3 (4.62) | 0.699 | 0.08 |
| Female | 95 | 36 (37.9%) | 38 | 12(31.6%) | 57 | 24 (42.1%) | 0.412 | |
| Race | ||||||||
| White | 95 | 29 (30.5%) | 38 | 12 (31.6%) | 57 | 17 (29.8%) | 0.006 | |
| Black | 12 (12.6%) | 10 (26.3%) | 2 (3.5%) | |||||
| Asian | 50 (52.6%) | 14 (36.8%) | 36 (63.2%) | |||||
| Other | 4 (4.2%) | 2 (5.3%) | 2 (3.5%) | |||||
| Hispanic ethnicity | 95 | 28 (29.5%) | 38 | 12 (31.6%) | 57 | 16 (28.1%) | 0.890 | |
| Alcohol use currently | 95 | 27 (28.4%) | 38 | 16 (42.1%) | 57 | 11 (19.3%) | 0.047 | |
| Tobacco use currently | 95 | 23 (24.2%) | 38 | 14 (36.8%) | 57 | 9 (15.8%) | 0.062 | |
| DUP (weeks) | 88 | 43.6 (72.3) | 35 | 47.3 (76.0) | 53 | 41.1 (70.3) | 0.693 | 0.09 |
| Length of treatment (weeks) | 81 | 87.9 (158) | 32 | 106 (248) | 49 | 76.3 (41.5) | 0.419 | 0.19 |
| Drug use currently | 95 | 13 (13.7%) | 38 | 8 (21.1%) | 57 | 5 (8.8%) | 0.170 | |
| Antipsychotic dose in CPZ mg/d equivalents | 89 | 382 (179) | 34 | 339 (187) | 55 | 409 (170) | 0.071 | -0.39 |
| BPRS total | 90 | 38.4(10.4) | 34 | 40.9 (12.2) | 56 | 36.9 (8.96) | 0.072 | 0.38 |
| BPRS positive subscale | 90 | 11.2 (4.80) | 34 | 11.9 (5.50) | 56 | 10.8 (4.33) | 0.307 | 0.23 |
| SANS total | 90 | 20.0 (13.2) | 34 | 23.6 (15.1) | 56 | 17.9 (11.6) | 0.045 | 0.43 |
| CDRS total | 90 | 2.17 (2.47) | 34 | 2.38 (2.52) | 56 | 2.04 (2.45) | 0.521 | 0.14 |
| HQOL | 95 | 73.5 (24.9) | 38 | 66.4 (25.9) | 57 | 78.2 (23.2) | 0.023 | -0.47 |
| MARS | 90 | 7.44 (1.91) | 34 | 7.18 (2.15) | 56 | 7.61 (1.75) | 0.303 | -0.23 |
| BAS | 86 | 0.419 (0.759) | 35 | 0.429 (0.850) | 51 | 0.412 (0.698) | 0.920 | 0.02 |
| SAS | 83 | 0.916 (1.74) | 34 | 1.38 (2.32) | 49 | 0.592 (1.10) | 0.041 | 0.45 |
| MCCB composite | 89 | 34.4 (13.0) | 35 | 31.8 (12.3) | 54 | 36.1 (13.3) | 0.125 | -0.33 |
| Speed of processing | 92 | 33.2 (13.3) | 36 | 32.1 (13.2) | 56 | 33.9 (13.4) | 0.532 | -0.14 |
| Attention & vigilance | 90 | 37.7 (12.0) | 35 | 34.7 (12.9) | 55 | 39.7 (11.1) | 0.059 | -0.42 |
| Working memory | 93 | 38.1 (12.0) | 37 | 34.9 (11.7) | 56 | 40.1 (11.9) | 0.040 | -0.43 |
| Verbal learning | 93 | 36.3 (10.0) | 37 | 36.1 (8.18) | 56 | 36.4 (11.2) | 0.898 | -0.03 |
| Visual learning | 91 | 43.8 (11.7) | 36 | 40.7 (11.1) | 55 | 45.8 (11.8) | 0.039 | -0.44 |
| Reasoning and problem solving | 92 | 40.2(10.7) | 36 | 38.1 (10.6) | 56 | 41.6 (10.7) | 0.118 | -0.33 |
| Social cognition | 92 | 42.8 (18.5) | 36 | 46.1 (19.5) | 56 | 40.7 (17.7) | 0.177 | 0.29 |
| ICV | 91 | 1560 (168) | 35 | 1530 (194) | 56 | 1570 (150) | 0.303 | -0.24 |
| Left DG | 88 | 315 (37.2) | 35 | 295 (34.5) | 53 | 328 (33.1) | <0.001 | -0.89 |
| Right DG | 88 | 330 (41.2) | 35 | 313 (43.2) | 53 | 342 (35.8) | 0.001 | -0.70 |
| Left CA1 | 88 | 624 (70.4) | 35 | 604 (73.5) | 53 | 637 (65.7) | 0.028 | -0.47 |
| Right CA1 | 88 | 662 (70.8) | 35 | 643 (77.2) | 53 | 675 (63.8) | 0.037 | -0.45 |
| Left CA2/3 | 88 | 210 (30.8) | 35 | 197 (31.2) | 53 | 219 (27.5) | 0.001 | -0.71 |
| Right CA2/3 | 88 | 231 (33.6) | 35 | 221 (35.9) | 53 | 238 (30.4) | 0.016 | -0.51 |
| Left total hippocampus | 88 | 3500 (333) | 35 | 3390 (342) | 53 | 3580 (308) | 0.009 | -0.57 |
| Right total hippocampus | 88 | 3680 (332) | 35 | 3550 (352) | 53 | 3760 (295) | 0.005 | -0.63 |
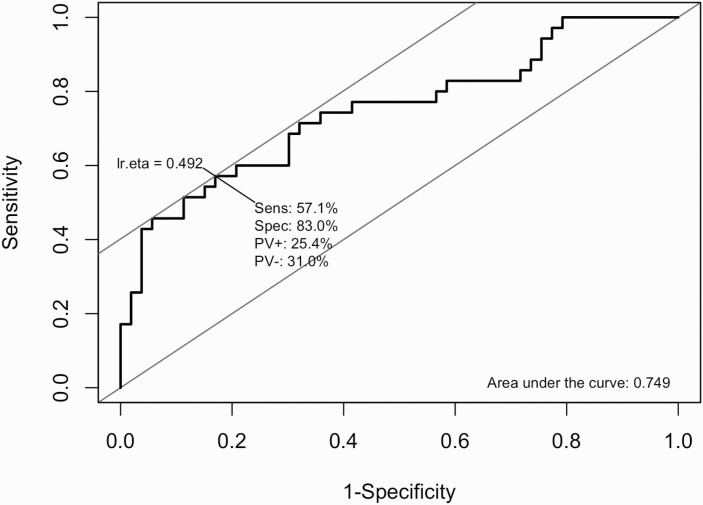
Compared to completers, disengagers exhibited significantly smaller volumes in bilateral DG, CA1, and CA2/3 subfields and bilateral whole hippocampi (table 1). A logistic regression predicting disengagement status with all eight hippocampal volumes as predictors found that only the left DG was significantly associated with disengagement status in the presence of the other measures (supplemental table S4; P = .01). The differences between completers and disengagers in left DG volume remained significant after adjusting for age, sex, ICV, race, antipsychotic dose, duration of untreated psychosis (DUP), alcohol use and cigarette smoking (table 2; P < .01), which were considered potential confounders. The sensitivity analysis after exclusion of patients who were terminated from study due to nonadherence, incarceration or hospitalization produced analogous results (supplemental table S5). The Cox proportional hazards regression demonstrated that time to disengagement was also significantly associated with left DG volume after adjusting for all potentially confounding variables (supplemental table S6). ROC analysis of left DG volume and disengagement status resulted in an AUC of 0.749 and identified a left DG volume cut-off of 301 mm3 as the optimal predictor of disengagement status during the 12-month trial, with a sensitivity of 57.1% and specificity of 83.0% (figure 1). All six patients (15.4%) with left DG volume less than 260 mm3 were dropouts and all 11 patients (19.3%) with left DG volume greater than 356 mm3 were completers (supplemental figure S1).
Table 2.
Logistic Regression Models for Prediction of Disengagement Status
| Model 1 (N = 88) | Model 2 (N = 75) | Model 3 (N = 75) | ||||
|---|---|---|---|---|---|---|
| Coef (St.Err) | P-value | Coef (St.Err) | P-value | Coef (St.Err) | P-value | |
| Left dentate gyrus volume | −0.0312 (0.0084) | 0.0002 | −0.0421 (0.0137) | 0.0021 | −0.0394 (0.0148) | 0.0079 |
| ICV | 6.194e−07 (2.817e−06) | 0.8260 | −5.046e−07 (2.977e−06) | 0.8654 | ||
| Antipsychotic dose | −0.0046 (0.0022) | 0.0315 | −0.0058 (0.0024) | 0.0159 | ||
| DUP (in weeks) | −0.0039 (0.0041) | 0.3458 | −0.0038 (0.0042) | 0.3622 | ||
| Treatment | 0.4224 (0.6417) | 0.5104 | 0.5030 (0.7004) | 0.4727 | ||
| Age | 0.0172 (0.0671) | 0.7975 | 0.0246 (0.0698) | 0.7243 | ||
| Sex (Female) | −1.1770 (0.8683) | 0.1752 | −1.6720 (0.9621) | 0.0823 | ||
| Race (reference: white) | ||||||
| Black | 2.7900 (1.3140) | 0.0337 | 3.4030 (1.4700) | 0.0206 | ||
| Asian | −0.0368 (0.8196) | 0.9642 | −0.0230 (1.6070) | 0.8489 | ||
| Other | −0.9126 (1.3890) | 0.5112 | −1.1040 (1.5400) | 0.4733 | ||
| Alcohol use (ref: none) | ||||||
| Past but not current | −0.2461 (1.6070) | 0.8783 | ||||
| Current | −0.5386 (1.3360) | 0.6869 | ||||
| Tobacco use (ref: none) | ||||||
| Past but not current | −1.4569 (1.1540) | 0.2072 | ||||
| Current | −0.0639 (1.1570) | 0.9560 |
Fig. 1.
Area under the curve analysis: Baseline left DG volume prediction of engagement status.
To examine whether the effect of left DG volume on disengagement was mediated by cognition or symptom severity, we augmented the three sequential models in table 2 with baseline measures of cognition (MCCB) and symptom severity (SANS and BPRS). The left DG volume remained a disengagement predictor of similar or larger magnitude and significance after adjusting for those measures (supplement table S7).
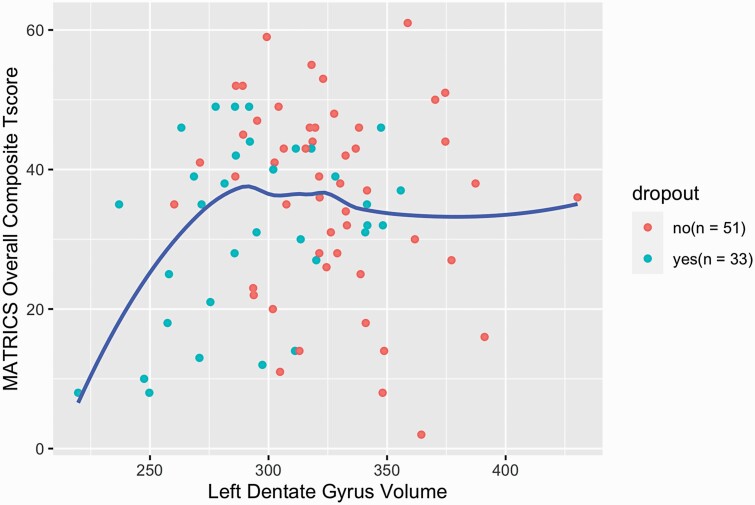
Of all the clinical variables that differed between disengagers and completers, only the MCCB composite score had significant interaction with left DG volume in explaining disengagement status (table 3, P = .02) such that left DG volume was significantly positively associated with the MATRICS composite score in disengagers (P = .04), whereas there was no association in completers. A plot of MCCB composite score by left DG volume for the whole sample is provided in figure 1. Similar curves are provided for all MCCB cognitive domains in supplemental figure S2.
Table 3.
Interaction Analysis of Left DG Volume as a Function of Disengagement Status
| Interaction term with dropout status | P-value |
|---|---|
| Race | 0.51 |
| Alcohol use | 0.89 |
| SANS total | 0.32 |
| HQOL | 0.97 |
| SAS | 0.87 |
| MCCB composite | 0.02 |
| Speed of processing | 0.25 |
| Attention & vigilance | 0.02 |
| Working memory | 0.48 |
| Verbal learning | 0.04 |
| Visual learning | 0.01 |
| Reasoning and problem solving | 0.74 |
| Social cognition | 0.65 |
To assess the specificity of the association between DG volume and disengagement status, disengagers and completers were compared on ICV, total brain volumes and on cortical thickness of 70 brain regions parcellated by FreeSurfer 6.0. Using a significance threshold of P < .01, none differed between groups, whereas when a significance threshold of P < .05 was used, nine brain regions differed between groups (supplemental table S8).
Discussion
We found that 40% of first episode schizophrenia patients participating in a randomized controlled trial disengaged during 12 months of maintenance treatment, despite the provision of individualized psychoeducation and relapse planning in addition to usual care. This rate of disengagement falls within the range of disengagement rates reported in previous longitudinal studies.4 Consistent with the clinical literature, alcohol use predicted disengagement status. Race also predicted disengagement, although race may have been confounded by possible country effects (China vs. USA). The degree to which the higher rate of disengagement in Black participants may be attributed to socioeconomic status or the effects of structural racism cannot be determined by available data. Our finding that cognitive performance, negative symptoms and Parkinsonism also predicted disengagement is consistent with some but not all prior studies.4 Because the number of participants who completed additional measures of insight, perceived coercion, and self-rated quality of life was small (n = 44), we can only conclude that if any of these factors contributed to disengagement risk, it was not a large effect.
The association of baseline volumes of DG with disengagement status was highly significant after adjusting for relevant variables and was a stronger predictor of disengagement than any clinical predictors. This association was unlikely to be solely the result of generalized cortical volume loss since cortical thickness was not strongly associated with disengagement in the additional brain regions tested. While this finding requires replication, the predictive specificity of DG for disengagement status was remarkable, given the large number of demographic and clinical variables that may contribute to a patient’s decision to disengage, including drug side effects, cognitive deficits, lack of insight, paranoia, apathy and socioeconomic factors. DG volume may represent a vulnerability factor that interacts with many demographic, clinical and environmental factors that contribute to disengagement.
The mechanism by which lower hippocampal volume is associated with disengagement status is unclear. Of the variables that we measured, only cognitive performance significantly interacted with left DG volume in predicting disengagement status. Analysis of the MCCB composite score suggested that low DG volume was only associated with impaired cognition below a threshold of approximately 290 mm3; the slope of the association was quite steep below this threshold and plateaued above it (figure 2). It is possible that cognitive impairment associated with low DG volume below a critical threshold may contribute to the decision to discontinue treatment. However, DG volume remained a significant predictor of disengagement after adjusting for cognitive impairment and other measures of symptom severity, suggesting that, while cognition may moderate the effect of DG volume on disengagement, DG volume exerts an effect on disengagement independent of cognition. The nature of this putative cognitive impairment and its relationships to hippocampal volume and functioning require further study. Given the very high rate of disengagement that we observed in patients with DG volumes below this threshold, it is quite possible that this subgroup of early psychosis patients may not be well represented in longitudinal studies and may require special efforts to retain in treatment and in research protocols.
Fig. 2.
Baseline MCCB composite score and left dentate gyrus volume by engagement status.
If our finding of a highly specific association between small DG volume and subsequent disengagement is replicated, this finding could help clinicians identify individuals at high risk for disengagement. While we cannot conclude from this study that the association between DG volume and disengagement is causal, it is possible that impairment of hippocampal function might represent a target to improve retention in treatment. For example, if the associated cognitive deficits underlie this association, they might be targeted with a cognitive behavioral approach. Interventions targeting hippocampal volume loss might also improve retention. Exercise is a well-established intervention to increase DG volume,25,26 an effect believed to reflect enhanced neurogenesis.27,28 Exercise has similarly been associated with increased hippocampal volume and improved working memory in individuals with schizophrenia.29 In depression, antidepressant efficacy has been linked to neurogenesis30 and increased hippocampal volume.31 However, in the DECIFER study, in which citalopram improved negative symptoms, depressive symptoms were not improved by citalopram and neither change in hippocampal volume nor disengagement status differed between citalopram and placebo groups.18,20 This possible differential effect of antidepressants on hippocampal volume in individuals with depression versus individuals without depression was also found in a study of nonhuman primates.32 In this study, a selective serotonin reuptake inhibitor increased anterior hippocampal volume in animals exhibiting depressive behaviors following stress but was associated with hippocampal volume loss in animals that were not previously stressed.32 These data suggest that the mechanism underlying low hippocampal volume in schizophrenia may differ from depression and may not be similarly responsive to antidepressant treatment. In animal models, several second generation antipsychotics, including clozapine, olanzapine, quetiapine and aripiprazole, have been reported to increase neurogenesis33,34; these drugs also appear to be associated with lower rates of all cause discontinuation compared to first generation agents in randomized controlled trials35 and in naturalistic studies.36 Whether these agents increase hippocampal volume in association with improved retention remains to be established.
Limitations
Because this study occurred within a randomized controlled trial, it is unclear the degree to which results can be generalized to routine clinical care of early psychosis patients. Notably, disengagement rates did not differ between placebo and citalopram groups,18 and our findings remained significant after excluding patients who were dropped from study due to nonadherence or due to incarceration or hospitalization.18 Because the association of hippocampal volume with disengagement status in early psychosis has not been reported previously, these results require replication. Only a randomized study of an intervention that increases hippocampal volume will allow us to draw inferences as to whether the relationship between reduced hippocampal volume and disengagement is causal.
Conclusion
In a 12-month study of maintenance treatment in patients with first episode schizophrenia, baseline volumes of the left DG hippocampal subfield significantly predicted disengagement status after adjusting for relevant demographic and clinical factors. If confirmed by future studies, the volume of hippocampal structures may provide a biomarker to identify patients at risk for disengagement and may provide a target for interventions to reduce risk of disengagement.
Supplementary Material
Acknowledgments
Authors indicated the following disclosures. O.F. has received research grants from Alkermes, Avanir, Janssen, Otsuka, and Saladax. He has served on the advisory board or received consultant honoraria from Alkermes, Janssen, Neurocrine, Novartis, Roche, Elsevier, Global Medical Education, UpToDate, American Psychiatric Association, and Medscape. He receives royalties from Wolters-Kluwer and UpToDate. In the past 3 years, D.C.G. has received research funding from the National Institutes of Health, Stanley Medical Research Institute, and Avanir Pharmaceuticals. He has participated on advisory boards for Avanir Pharmaceuticals and Takeda Pharmaceuticals but has accepted no honoraria from commercial entities. No other disclosures were reported.
Contributor Information
Wei Qi, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA.
Julia Marx, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA.
Michael Zingman, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA.
Yi Li, Department of Population Health, Division of Biostatistics, NYU School of Medicine, 180 Madison Avenue, New York, NY, USA.
Eva Petkova, Department of Population Health, Division of Biostatistics, NYU School of Medicine, 180 Madison Avenue, New York, NY, USA; Nathan Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY, USA.
Esther Blessing, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA.
Babak Ardekani, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA; Nathan Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY, USA.
Ayse Sakalli Kani, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA; 4New York State Psychiatric Institute, Columbia University Medical Center, 601 West 168th St., New York, NY, USA.
Corinne Cather, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA, USA.
Oliver Freudenreich, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA, USA.
Daphne Holt, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston, MA, USA.
Jingping Zhao, National Clinical Research Center for Mental Disorders, Mental Health Institute, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China.
Jijun Wang, Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Donald C Goff, Department of Psychiatry, NYU Langone Health, 1 Park Avenue, New York, NY, USA; Nathan Kline Institute for Psychiatric Research, 140 Old Orangeburg Road, Orangeburg, NY, USA.
Funding
This work was supported by the National Institute of Mental Health (R01 MH084900 to D.C.G.). The funding source had no role in the design of this study or in the analysis of data and submission for publication.
References
- 1. Dixon LB, Goldman HH, Srihari VH, Kane JM. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emsley R, Oosthuizen P, Koen L, Niehaus D, Martinez L. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80–83. [DOI] [PubMed] [Google Scholar]
- 3. Emsley R. Non-adherence and its consequences: understanding the nature of relapse. World Psychiatry 2013;12(3):234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mascayano F, van der Ven E, Martinez-Ales G, et al. . Disengagement from early intervention services for psychosis: a systematic review. Psychiatr Serv. 2021;72(1):49–60. [DOI] [PubMed] [Google Scholar]
- 5. Maraj A, Veru F, Morrison L, et al. . Disengagement in immigrant groups receiving services for a first episode of psychosis. Schizophr Res. 2018;193:399–405. [DOI] [PubMed] [Google Scholar]
- 6. Hamilton JE, Srivastava D, Womack D, et al. . Treatment retention among patients participating in coordinated specialty care for first-episode psychosis: a mixed-methods analysis. J Behav Health Serv Res. 2019;46(3):415–433. [DOI] [PubMed] [Google Scholar]
- 7. Kim DJ, Brown E, Reynolds S, et al. . The rates and determinants of disengagement and subsequent re-engagement in young people with first-episode psychosis. Soc Psychiatry Psychiatr Epidemiol. 2019;54(8):945–953. [DOI] [PubMed] [Google Scholar]
- 8. Macbeth A, Gumley A, Schwannauer M, Fisher R. Service engagement in first episode psychosis: clinical and premorbid correlates. J Nerv Ment Dis. 2013;201(5):359–364. [DOI] [PubMed] [Google Scholar]
- 9. Sint K, Rosenheck R, Robinson DG, et al. . Accounting for group differences in study retention in a randomized trial of specialized treatment for first episode psychosis. Schizophr Res. 2018;195:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Erp TG, Hibar DP, Rasmussen JM, et al. . Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHugo M, Armstrong K, Roeske MJ, Woodward ND, Blackford JU, Heckers S. Hippocampal volume in early psychosis: a 2-year longitudinal study. Transl Psychiatry. 2020;10(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. [DOI] [PubMed] [Google Scholar]
- 13. Hainmueller T, Bartos M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat Rev Neurosci. 2020;21(3):153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: building memories to navigate future decisions. Front Hum Neurosci. 2012;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vargas T, Dean DJ, Osborne KJ, et al. . Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44(5):1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schobel SA, Chaudhury NH, Khan UA, et al. . Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013;78(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry. 2017;82(12):914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goff DC, Freudenreich O, Cather C, et al. . Citalopram in first episode schizophrenia: the DECIFER trial. Schizophr Res. 2019;208:331–337. [DOI] [PubMed] [Google Scholar]
- 19. Goff DC, Zeng B, Ardekani BA, et al. . Association of hippocampal atrophy with duration of untreated psychosis and molecular biomarkers during initial antipsychotic treatment of first-episode psychosis. JAMA Psychiatry 2018;75(4):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qi W, Blessing E, Li C, et al. . Effect of citalopram on hippocampal volume in first-episode schizophrenia: structural MRI results from the DECIFER trial. Psychiatry Res Neuroimaging 2021;312:111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang J, Hart KL, Qi W, et al. . Association of aripiprazole with reduced hippocampal atrophy during maintenance treatment of first-episode schizophrenia. J Clin Psychopharmacol. 2021;41(3):244–249. [DOI] [PubMed] [Google Scholar]
- 22. Brown EM, Pierce ME, Clark DC, et al. . Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 2020;210:116563. [DOI] [PubMed] [Google Scholar]
- 23. Dale AM, Fischl B, Sereno M. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 24. Team RC. R: A language and Environment for statistical computing. In: Computing RFfS, ed. Vienna, Austria; 2020. https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 [Google Scholar]
- 25. Erickson KI, Voss MW, Prakash RS, et al. . Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nauer RK, Dunne MF, Stern CE, Storer TW, Schon K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus 2020;30(5):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pereira AC, Huddleston DE, Brickman AM, et al. . An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suwabe K, Byun K, Hyodo K, et al. . Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Natl Acad Sci USA. 2018;115(41):10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pajonk FG, Wobrock T, Gruber O, et al. . Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–143. [DOI] [PubMed] [Google Scholar]
- 30. Santarelli L, Saxe M, Gross C, et al. . Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003;301(5634):805–809. [DOI] [PubMed] [Google Scholar]
- 31. Boldrini M, Santiago AN, Hen R, et al. . Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willard SL, Uberseder B, Clark A, et al. . Long term sertraline effects on neural structures in depressed and nondepressed adult female nonhuman primates. Neuropharmacology 2015;99:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chikama K, Yamada H, Tsukamoto T, Kajitani K, Nakabeppu Y, Uchimura N. Chronic atypical antipsychotics, but not haloperidol, increase neurogenesis in the hippocampus of adult mouse. Brain Res. 2017;1676:77–82. [DOI] [PubMed] [Google Scholar]
- 34. Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29(6):1063–1069. [DOI] [PubMed] [Google Scholar]
- 35. Soares-Weiser K, Bechard-Evans L, Lawson AH, Davis J, Ascher-Svanum H. Time to all-cause treatment discontinuation of olanzapine compared to other antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2013;23(2):118–125. [DOI] [PubMed] [Google Scholar]
- 36. Haro JM, Novick D, Suarez D, Roca M. Antipsychotic treatment discontinuation in previously untreated patients with schizophrenia: 36-month results from the SOHO study. J Psychiatr Res. 2009;43(3):265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.