Abstract
Purpose:
Evidence suggests that visceral fat quantity may be associated with post-prostatectomy outcomes and risk of prostate cancer-related death. We aimed to evaluate if increased fat volume, normalized to prostate size, is associated with decreased risk of disease progression.
Materials and Methods:
Patients enrolled on a prospective active surveillance trial for at least 6 months who had an MRI within 2 years of enrollment were eligible. The surveillance protocol included a standardized follow-up regimen consisting of biennial PSA and exam and yearly biopsy. Clinicopathologic characteristics were collected at baseline. Three fat measurements were taken using prostate MRI images: subcutaneous, linear periprostatic (pubic symphysis to prostate) and volumetrically-defined periprostatic. Progression was defined as increase in Gleason grade group. Multivariable cox proportional hazards models were used to evaluate fat volumes normalized by prostate size (stratified into tertiles).
Results:
A total of 175 patients were included. Average age was 62.5 years (SD 7.4) and average PSA was 5.4 ng/dL (STD 3.9). Median follow-up was 42 months (IQR 18–60) and 50/175 patients (28.6%) progressed. Compared to the lowest tertile, the highest tertile of volumetric peri-prostatic fat measurement (HR 2.63, 95 CI 1.23–5.60, P=0.01) and linear peri-prostatic fat measurement (HR 2.30, 95 CI 1.01–5.22, P=0.05) were associated with worsened progression free survival, while subcutaneous fat measurement (P=0.97) was not. Importantly, the model did not substantively change when accounting for patient body mass index and other factors.
Conclusions:
Increased peri-prostatic fat volume, normalized to prostate size, may be associated with shortened progression-free survival in men with prostate cancer managed on active surveillance.
Keywords: Prostatic neoplasms, prostatic fat, progression
INTRODUCTION
Active surveillance (AS) is the standard management option for men with low risk prostate cancer (PCa) and select men with intermediate risk PCa.1 Accurate risk stratification and detection of disease progression on follow-up form the tenants of patient management for men enrolled on AS.2 While prostate biopsy remains the foundation of risk stratification in AS, these can be uncomfortable and place patients at risk for complications.3 The development of non-invasive biomarkers therefore represents an area of intense research focus, particularly among the use of genomic-based tests.4 However, no marker has been validated for use in this population.5
Evidence suggests that visceral fat volume may be associated with disease aggression and mortality following diagnosis of PCa on a population level.6 These data are consistent with observations that obesity may be associated with PCa aggression,7 an observation that has been specifically seen in AS cohorts.8 However, while these data are mixed,7 a number have studies have shown that peri-prostatic adipose tissue volume, as measured using axial imaging techniques, may be associated with PCa risk,9 disease aggression at diagnosis,10,11 and treatment.12 However, no investigations have examined the association between peri-prostatic fat volume and PCa progression on AS.
Given the use and rapid integration of magnetic resonance imaging (MRI) into routine PCa care13, measurement of peri-prostatic fat on MRI represents a potentially cost-effective and valuable factor in the determination of localized prostate cancer agression. We therefore hypothesized that increased peri-prostatic fat, measured on MRI and normalized to prostate size, would be independently associated with worsened progression-free survival in a large cohort of men enrolled on AS for localized PCa. We tested this hypothesis by examining the subset of men who underwent prostate MRI within 2 years of enrollment onto a prospective AS protocol at a single institution.
METHODS
Patient selection
The study was approved by the institutional review board and all patients signed informed consent. Patients were included who enrolled on a prospective AS protocol that began in 2006. The study was designed to observe outcomes of men enrolled on an AS and was conducted by a multidisciplinary team of urologic surgeons, radiation oncologists, and medical oncologists as previously described.14,15 The study was registered at clinicaltrials.gov (trial number NCT00490763). Prior to enrollment, men underwent a diagnostic biopsy at either an outside institution or UT MD Anderson Cancer Center. They then underwent an 11-core systematic confirmatory biopsy within 6 months of enrollment. Men with localized Gleason grade group (GG)1, GG2, or, in rare instances, GG3 prostate cancer were eligible. Patients enrolled on the AS protocol then underwent biannual evaluation with digital rectal examination and serum PSA measurement. Additionally, surveillance biopsies were repeated yearly, though biopsy was omitted if the previous year’s was negative. Patients with increasing tumor volume or GG were recommended to undergo treatment; however, patients could choose to stay on AS if approved by their physician.15 Prostate MRI was not standardized as part of the protocol. Rather, prostate MRI was ordered for men in the AS cohort at the discretion of the treating physician. Men with a prostate MRI within two years of study enrollment and over 6 months of follow-up were eligible for inclusion.
Clinical and pathologic data
Patient characteristics were collected at baseline, including patient age, PSA value, body mass index (BMI) and medication use. Tumor characteristics, including GG and core length were collected following confirmatory biopsy. Our outcome of interest was time to GG progression. Patients were followed until time of GG progression, treatment, loss to follow-up, study withdrawal, death, or December 31, 2016 (the censor date of the study), whichever came first.
MRI protocol
Among men included in the analysis, multi-parametric MRI was performed on 1.5 GE MR scanner (GE Healthcare, Waukesha, WI) using an eight-channel abdominal array coil and endorectal coil (MR Innerva; Medrad, Pittsburgh, PA). A few patients (N=26) underwent scans without an endorectal coil. For suppression of bowel peristalsis, 1 mg of Glucagon was injected intramuscularly before the study. Acquisition specifications advanced over the study period, but typical sequences of the MR protocol included smaller field of view (FOV) axial, sagittal and coronal fast spin echo T2-weighted imaging, diffusion weighted imaging with b-value of 700 and 1000 s/mm2 apparent diffusion coefficient reconstruction, and dynamic contrast imaging, as well as whole pelvis T1-weighted imaging (Supplementary Table 1). DCE-MRI was performed after IV injection of gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals) at 0.1 mmol/kg of body weight at a rate of 3 mL/s via a power injector and consisted of 29 consecutive acquisitions over approximately 3.5 minutes. Images were reviewed by dedicated genitourinary radiologists.
Image Analysis
The large field of view pelvic axial T1 weighted images were reviewed on MIM software, version 6.6.6. Anterior subcutaneous fat was measured using a linear antero-posterior (AP) measurement from the superior aspect of pubis symphysis to skin in the axial plane (termed “subcutaneous fat measure”; Figure 1). For the volumetric analysis, the prostate and periprostatic fat were segmented on consecutives slices from the base of the gland to apex (termed “volumetric peri-prostatic measure”, Figure 2).12 A linear measure of periprostatic fat was measured as the shortest AP distance from the pubic symphysis (PS) to prostate on the small FOV sagittal T2 weighted image (termed “linear peri-prostatic fat measure”; Figure 3). Linear and volumetric periprostatic fat measures were normalized to prostate volume to account for variations in prostate size by dividing fat volume by prostate volume.12
Figure 1.
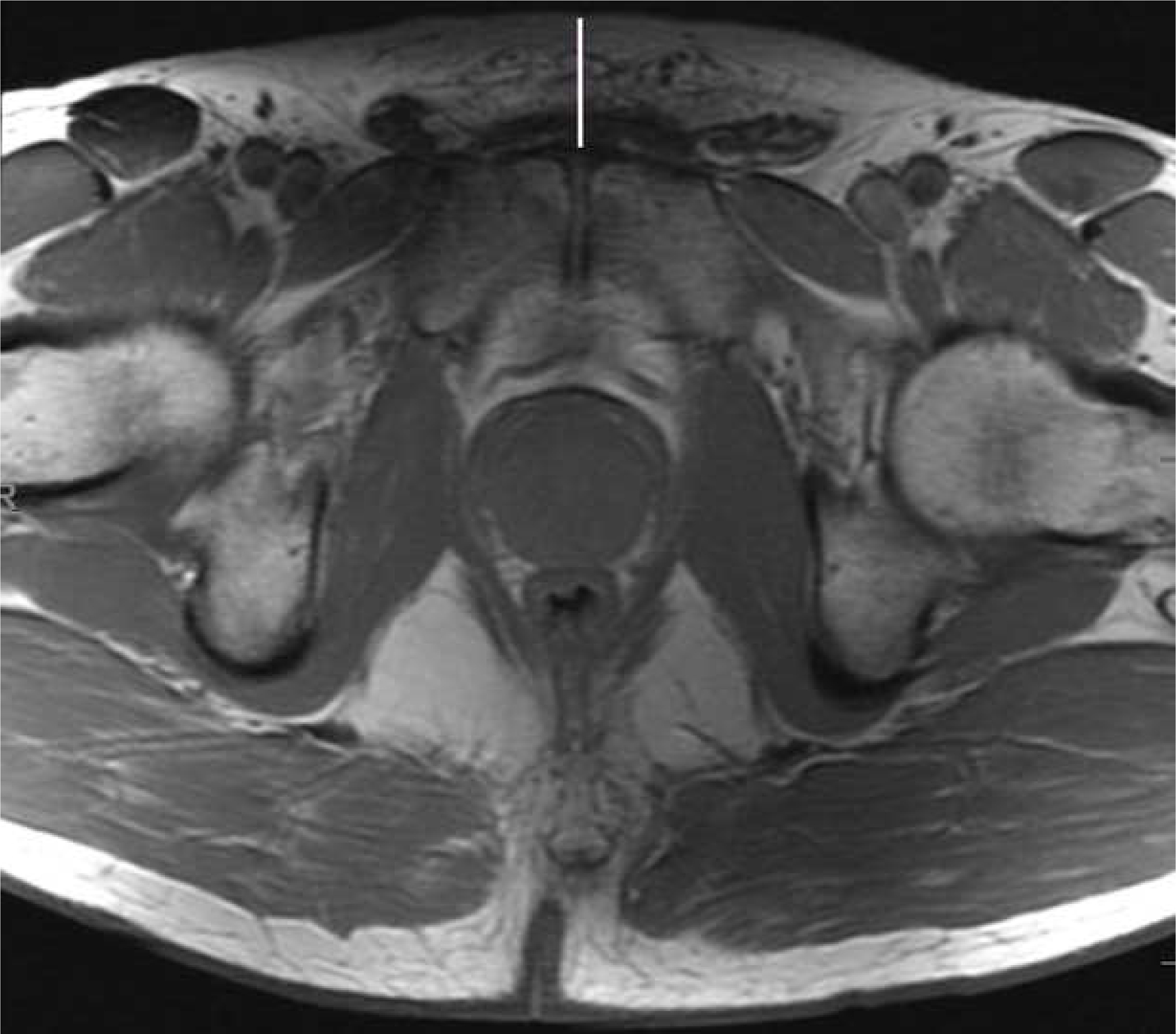
Anterior subcutaneous fat measurement Axial large FOV pelvic T1 weighted image demonstrating the linear antero-posterior measurement from the superior aspect of pubic symphysis to skin (white line).
Figure 2.
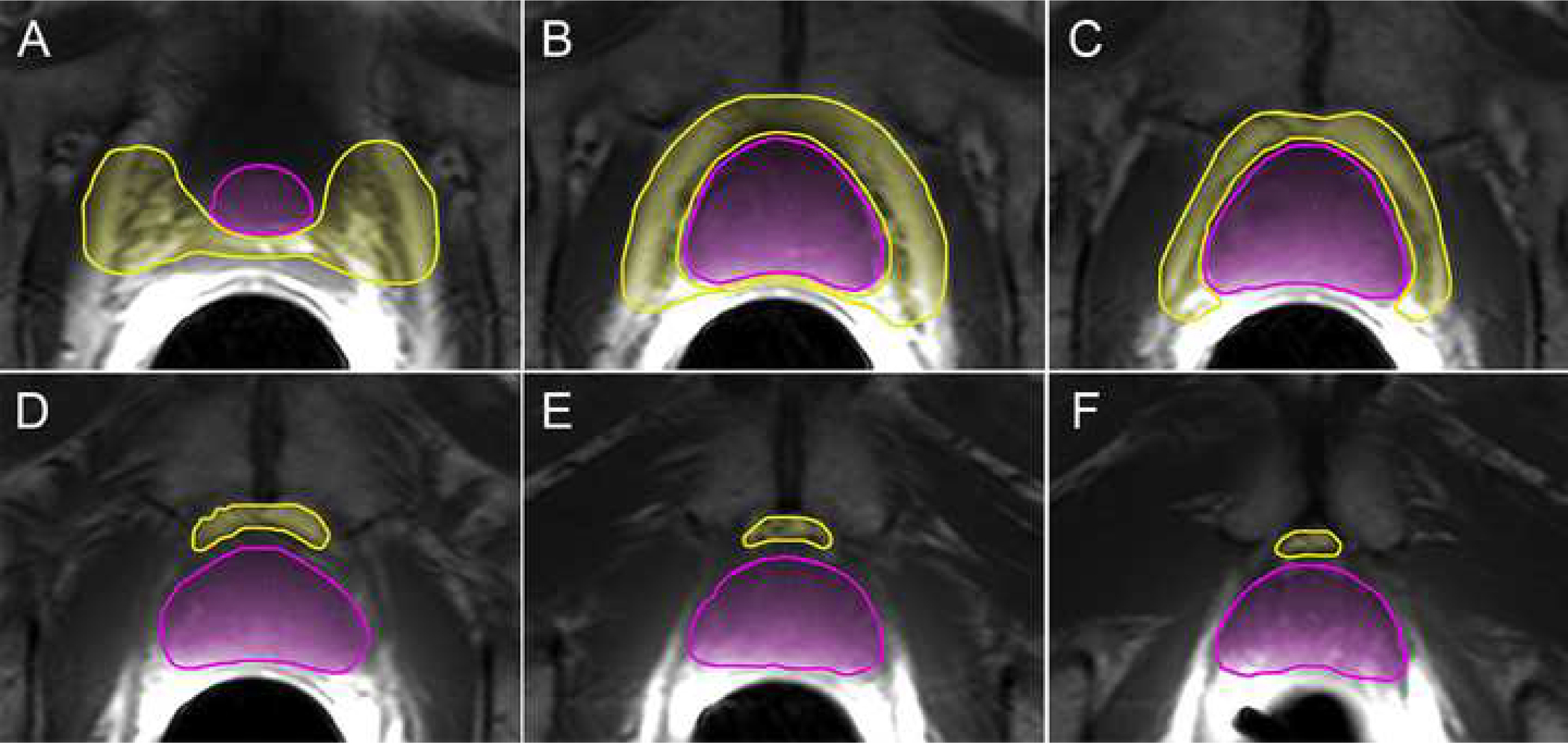
Volumetric segmentation of prostate and peri-prostatic fat volume Axial consecutive T1 weighted images from the base of the gland to apex (A-F) demonstrating the prostate (pink) and periprostatic (yellow) volumes.
Figure 3.
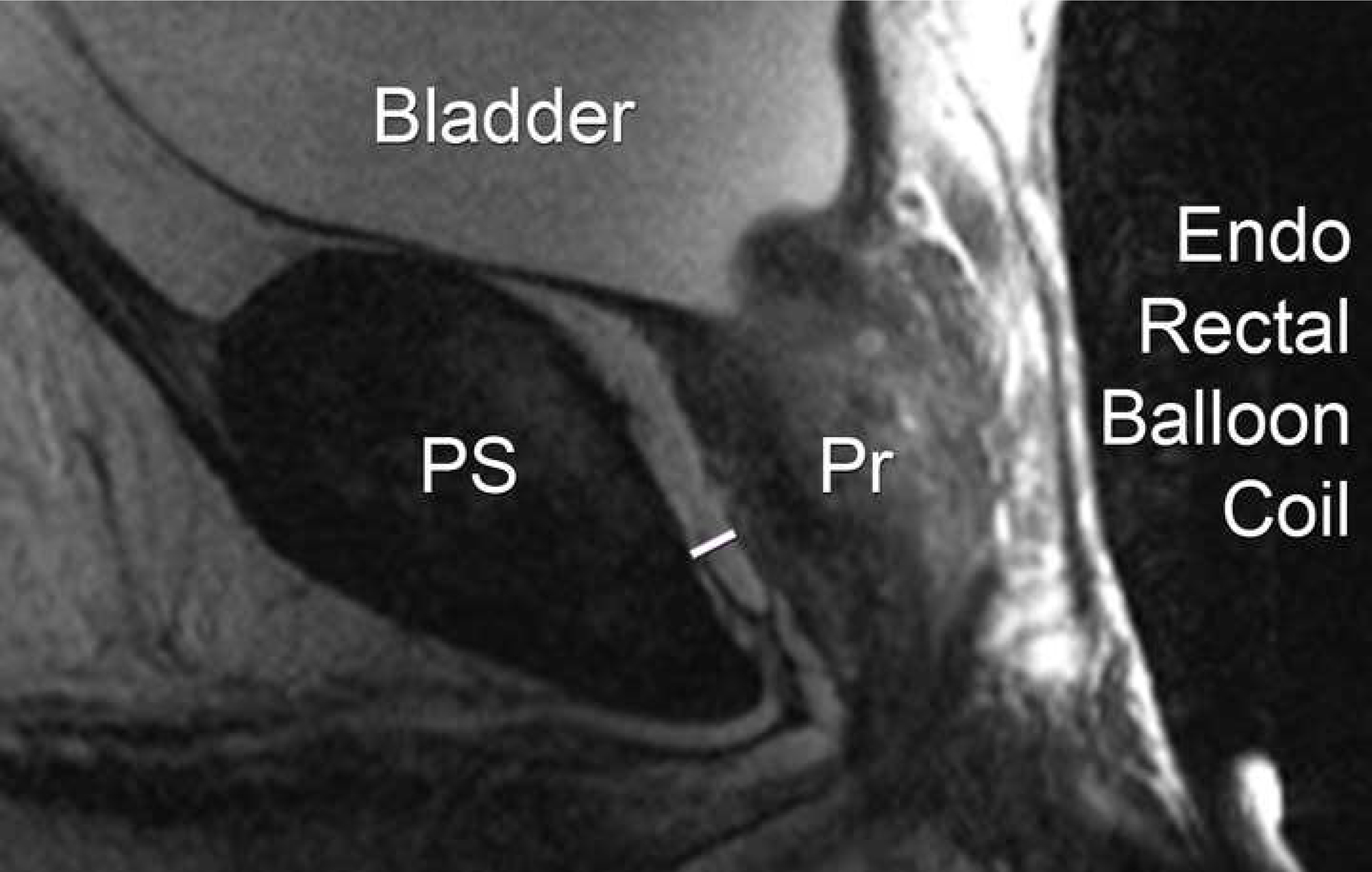
Linear peri-prostatic fat measurement Sagittal small FOV T2 weighted image demonstrating the linear measurement (white arrow) from the posterior surface of pubic symphysis (PS) to anterior surface of prostate (Pr).
Statistical analysis
Descriptive statistics were used to describe the patient cohort. Fat measures, including subcutaneous fat and both measures of peri-prostatic fat, were categorized into tertiles (high, medium, low) based on the population distribution. Differences across tertiles were tested using the X2 and analysis of variance tests for categorical and continuous variables, respectively. We evaluated the association between fat measures and progression free survival using Cox proportional hazards models with person-years as the underlying time metric. Hazard ratios (HR), 95% confidence intervals (95 CI), and P values for linear trend (using median tertile values) are reported, with the lowest tertile as the referent group. We confirmed that the proportional hazards assumption was met through assessment of interaction terms for exposures with follow-up time.
We examined two models for each analysis: 1) a base model adjusted for age, only, and, 2) a clinical model that included age, BMI, PSA and summation tumor length. Summation tumor length, a measure strongly associated with GG progression in this cohort,15 was defined as the sum total tumor length as measured on all baseline and confirmatory biopsy cores. We additionally evaluated other lifestyle and demographic factors, including smoking status, race, hypertension, DM and statin use for association with GG progression. However, none of these factors modified the crude hazard ratios or final models and were therefore not included. Statistical significance was considered if p≤0.05. Statistical analysis was performed using Stata/SE version 15.1 statistical software (Stata Corp. LP, College Station, TX).
RESULTS
A total of 175 patients were identified who had over 6 months of follow-up and MRI performed within 2 years of enrollment. Average age was 62.5 years (standard deviation [SD] 7.4 years) and 83.6% were white. A total of 138/175 (78.9%) patients had GG 1 disease based on diagnostic and confirmatory biopsies. The average PSA was 5.4 (SD 3.9)
Baseline characteristics by volumetric peri-prostatic fat measurements are listed in Table 1. The average volumetric peri-prostatic fat measure, was 1.01 (SD 0.27). All fat measurements by tertile are shown in Table 2. Volumetric peri-prostatic fat measurements correlated with linear peri-prostatic fat measure measurements (Spearman’s rho = 0.33, P<0.01) and subcutaneous fat measurements (Spearman’s rho = 0.40, P<0.01), while pubic-symphysis based and subcutaneous measures were strongly correlated (Spearman’s rho = 0.53, P<0.01).
Table 1:
Selected baseline characteristics of men with localized prostate cancer on active surveillance by volumetric peri-prostatic fat measures
| Characteristic | Lowest Tertile (N=59) | Middle Tertile (N=58) | Highest Tertile (N=58) | P Value |
|---|---|---|---|---|
| Age (mean, STD) | 61.1 (7.4) | 62.2 (7.4) | 64.2 (7.0) | 0.07 |
| Race | 0.67 | |||
| White | 49 (83.1)) | 48 (85.7) | 46 (82.1) | |
| Black | 4 (6.8) | 5 (8.9) | 7 (12.5) | |
| Other/unknown | 6 (10.2) | 3 (5.4) | 3 (5.4) | |
| PSA (mean, STD) | 5.1 (3.2) | 5.5 (3.3) | 5.6 (5.0) | 0.81 |
| Summation tumor length (mm) | 4.0 (7.8) | 3.3 (4.0) | 5.2 (7.0) | 0.29 |
| Baseline Gleason Score (N,%) | 0.18 | |||
| Gleason 6 | 49 (83.1) | 48 (82.8) | 41 (70.7) | |
| Gleason 7 | 10 (17.0) | 10 (17.2) | 17 (29.3) | |
| BMI (mean,STD) | 29.4 (3.9) | 29.0 (4.0) | 29.0 (3.7) | 0.81 |
| Statin use (N,%) | 0.21 | |||
| Yes | 23 (39.0) | 23 (39.7) | 31 (53.5) | |
| No | 36 (61.0) | 35 (60.3) | 27 (46.5) | |
| Smoking/tobacco status | 0.37 | |||
| Ever | 35 (59.3) | 32 (55.2) | 27 (46.6) | |
| Never | 24 (40.7) | 26 (44.8) | 31 (53.5) | |
| Hypertension | 0.15 | |||
| Yes | 21 (35.6) | 26 (44.8) | 31 (53.5) | |
| No | 38 (64.4) | 32 (55.2) | 27 (46.6) | |
| Diabetes Mellitus | 0.77 | |||
| Yes | 5 (8.5) | 7 (12.1) | 7 (12.1) | |
| No | 54 (91.5) | 51 (87.9) | 51 (87.9) | |
| Hyperlipidemia | 0.15 | |||
| Yes | 14 (23.7) | 12 (20.7) | 6 (10.3) | |
| No | 45 (76.3) | 46 (79.3) | 52 (89.7) |
Table 2:
Fat measurements normalized to prostate size
| Fat measurement | Total | Lowest Tertile | Middle Tertile | Highest Tertile |
|---|---|---|---|---|
| Total calculated peri-prostatic fat volume* (mean, STD) | 1.01 (0.27) | 0.75 (0.10) | 0.98 (0.06) | 1.31 (0.21) |
| Distance from pubic symphysis to prostate* (mean, STD) | 0.045 (0.061) | 0.15 (0.005) | 0.032 (0.007) | 0.089 (0.090) |
| Subcutaneous fat measurement* (mean, STD) | 1.01 (0.58) | 0.51 (0.14) | 0.90 (0.10) | 1.61 (0.61) |
normalized to prostate size
Over a median follow-up of 42 months (interquartile range 18–60 months), 50/175 (28.6%) patients had GG progression. Supplementary Table 2 lists fat measurements stratified by progression status. Volumetric peri-prostatic fat (P=0.01), but not linear peri-prostatic fat measure (P=0.88) or subcutaneous fat (P=0.78), was associated with progression status following normalization to prostate size.
Figure 4 demonstrates five-year GG progression free survival rates by tertile of volumetric peri-prostatic fat volume. Supplementary Figures 1 and 2 demonstrate five-year progression free survival rates by tertile of linear peri-prostatic fat and subcutaneous fat measures, respectively. Univariable logrank test showed that volumetric peri-prostatic fat measurement by tertile (P=0.01) and linear peri-prostatic fat measurement (P=0.03) were associated with worsened progression free survival, while subcutaneous fat measurement (P=0.44) was not.
Figure 4:
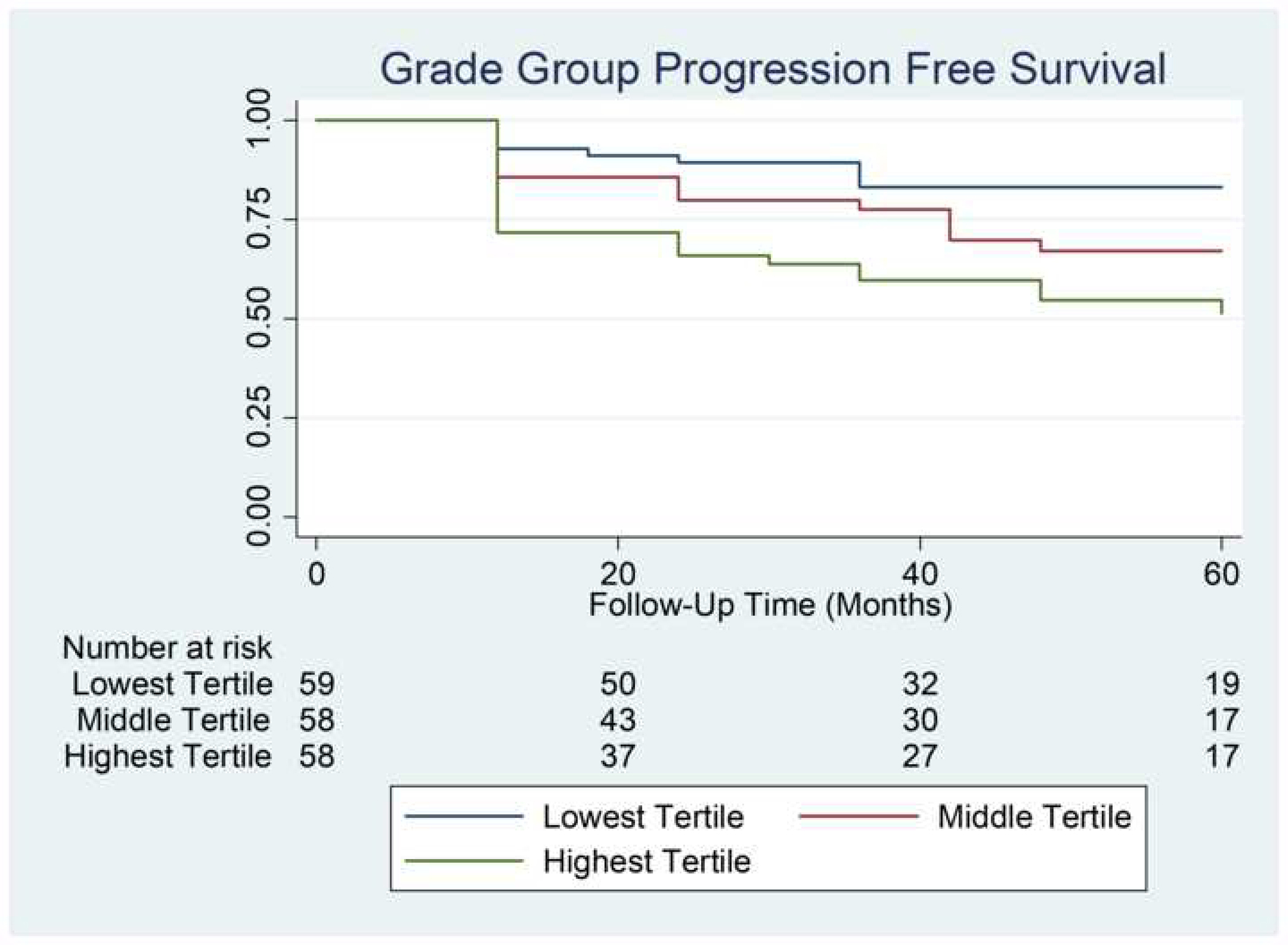
Kaplan-Meier analysis of Gleason grade group survival by normalized volumetric peri-prostatic fat measurement
Table 3 demonstrates Cox proportional hazards models evaluating association of fat measurements with time to GG progression. In the multivariable model including clinical factors, compared to the lowest tertile, the highest tertile of volumetric peri-prostatic fat measurement (HR 2.63, 95 CI 1.23–5.60, P=0.01) and linear peri-prostatic fat measurement (HR 2.30, 95 CI 1.01–5.22, P=0.05) were associated with worsened progression free survival, while subcutaneous fat measurement (HR 1.01, 95 CI 0.51–2.02, P=0.97) was not. P trend for linearity was 0.01 for the volumetric peri-prostatic fat measurement, 0.37 for the linear peri-prostatic fat measurement, and 0.19 for the subcutaneous fat measurement, respectively.
Table 3 –
Cox proportional hazards models evaluating association of fat measures with time to Gleason grade group progression.
| Frequency | Base modela | Clinical Modelb | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Number of events | N | HR | 95%CI | P-value | HR | 95%CI | P-value | |
|
| ||||||||
| Volumetric Per-Peri-Prostatic Fat Measure (Tertile) | ||||||||
| Lowest | 10 | 59 | Reference | Reference | ||||
| Middle | 16 | 58 | 1.73 | 0.78–3.82 | 0.17 | 1.81 | 0.82 | 4.00 |
| Highest | 24 | 58 | 2.72 | 1.29–5.73 | 0.01 | 2.63 | 1.23–5.60 | 0.01 |
| p-value for trend | 0.01 | 0.01 | ||||||
| Linear Peri-Prostatic Fat Measure (Tertile) | ||||||||
| Lowest | 9 | 57 | Reference | Reference | ||||
| Middle | 22 | 57 | 2.78 | 1.27–6.07 | 0.01 | 2.71 | 1.23–5.95 | 0.01 |
| Highest | 18 | 57 | 2.51 | 1.12–5.62 | 0.03 | 2.30 | 1.01–5.22 | 0.05 |
| p-value for trend | 0.47 | 0.37 | ||||||
| Subcutaneous Fat Measure (Tertile) | ||||||||
| Lowest | 16 | 59 | Reference | Reference | ||||
| Middle | 15 | 58 | 0.66 | 0.33–1.33 | 0.25 | 0.82 | 0.39–1.71 | 0.59 |
| Highest | 19 | 58 | 0.83 | 0.43–1.59 | 0.57 | 1.01 | 0.51–2.02 | 0.97 |
| p-value for trend | 0.24 | 0.19 | ||||||
– base model includes adjustment for age, only.
– clinical model includes adjustment for PSA, summation tumor length (please see text for description and age
DISCUSSION
Our study demonstrates peri-prostatic fat volume, as measured on routine MRI imaging, is independently associated with time to GG PCa progression in men enrolled on AS. These findings are notable in that measurements using both labor-intensive volumetric software (“volumetric peri-prostatic fat volume”) and those determined using a simple measurement in the sagittal plane (“linear peri-prostatic fat volume”) were well correlated and similarly associated with outcomes following enrollment on AS. While, to our knowledge, this study represents the first to demonstrate the association between peri-prostatic fat volume and GG progression on AS, multiple prior studies have suggested that varying measures of peri-prostatic fat and visceral fat are associated with PCa aggression and outcomes.
Increased peri-prostatic fat measures are associated with worsened biopsy and post-prostatectomy outcomes. A 2004 case-control study demonstrated that generalized visceral fat (measured on CT scan) was associated with risk of PCa diagnosis. Studies evaluating pelvic visceral fat, as measured using a single axial CT slice, showed that increased fat volume was associated with diagnosis of high risk16 PCa17 and increased GG on biopsy.10 Studies have also demonstrated that peri-prostatic fat measures using a single sagittal measurement from PS to prostate (as done in our analysis) is associated with risk of PCa9 and GG2 or higher disease.9,11 Furthermore, in the study by Woo et al., this fat measure was associated with GG on final pathology after accounting for multiple clinical factors.11 Finally, a 2017 study by Dahran et al. used manual of segmentation of peri-prostatic fat to investigate its association with post-operative GG, demonstrating that fat volume was positively correlated with GG.12
Evidence also suggests that visceral adiposity plays a role in advanced prostate cancer. In a population-based analysis, Dickerman et al. demonstrated that increased visceral fat adiposity (as measured by a single axial CT slice) was associated with advanced and lethal prostate cancer.6 Further evidence suggests that increases in peri-prostatic fat as measured from PS to prostate may be associated with worsened progression free survival and progression to castration resistant disease in men started on androgen deprivation therapy for metastatic disease.18
The association between peri-prostatic fat thickness and progression on AS has potential clinical relevance to the large proportion of men who are enrolled on AS for low risk prostate cancer. Clinical factors such as age and pathologic biopsy results currently guide AS entry criteria and follow-up; however, these measures are limited in sensitivity for aggressive disease and may also lead to overtreatment.19 Commercial tissue-based genomic tests have been developed to improve PCa risk stratification,20,21 but these tests are less than ideal in that they require patient tissue, lack sensitivity due to PCa heterogeneity,22 and have not been validated in AS cohorts.23 Recent work additionally suggests that a 17-gene prostate score test result may improve clinically-based predictive models of subsequent aggressive disease in AS.5 If validated, our findings may offer a straightforward, non-invasive measure to help inform disease aggression in men considering AS.5
There are a number of potential mechanisms through which peri-prostatic fat levels directly influence prostate cancer aggression. Early data suggest that circulating leptin levels may be associated with PSA levels and GG score on prostate biopsy.24 Analyses of peri-prostatic fat collected at the time of radical prostatectomy demonstrate that fat cells directly secrete high levels of IL-6, and may activate downstream factors that can affect aggressiveness, such as STAT3.25 This suggests that paracrine signaling may play an important role, especially given that detected IL-6 levels were over 300-fold higher in the peri-prostatic fat than circulating plasma. These data are substantiated by findings that periprostatic fat can enhance proliferation and migration when added to the media of human PCa cell lines.26 A gene expression microarray study suggests that peri-prostatic fat in men with PCa may have increased expression of anti-apoptotic, cell-cycle activating, and proliferation-related genes compared to men with benign prostatic disease.26 Furthermore, in likely the strongest evidence published to date, Laurent et al. demonstrated that peri-prostatic fat secretes the chemokine CCL7, which stimulates the migration of CCL3 positive prostate tumors.27 CCL3 was found to be over-expressed in aggressive PCa27, a finding that was replicated in biopsies of patients with metastatic PCa who failed androgen deprivation therapy.28 Models investigating the interaction of peri-prostatic fat with tumor that has extended beyond the prostate capsule suggest that bidirectional crosstalk stimulates cancer aggression, including peri-prostatic fat lipolysis and fatty acid uptake by prostate tumor cells, a process that is enhanced by obesity.29 Further work investigating peri-prostatic fat content and signaling is needed to define mechanisms through which peri-prostatic fat-mediated PCa aggression may be modified.
This study is limited due to its retrospective design and performance at a single large referral center with significant experience among radiologists, pathologists and urologists. While patient follow-up and biopsy regimens were standardized, improving study homogeneity, selection of men on the AS protocol was at the discretion of the treating physician and therefore introduced potential selection bias. Furthermore, this study was completed before MRI fusion biopsy techniques were widely used at our institution, indicating some tumors may have been understaged in this population.30 This limitation, however, is expected to apply to the entire cohort included in this study, potentially mitigating this bias. The study additionally did not evaluate changes in peri-prostatic fat measures overtime. While a subgroup analysis did not demonstrate longitudinal changes in peri-prostatic fat measures, including in men with weight changes (data not shown), it is not known if behavioral changes can influence peri-prostatic fat in men with prostate cancer. Prior to potential application to clinical practice, further studies are needed to validate these results, particularly in the current MRI-era with advanced biopsy techniques.
CONCLUSIONS
We describe the association between peri-prostatic fat volume, as measured using volumetric software and a straightforward, pubic symphysis to prostate measurement, and Gleason GG progression in men with localized PCa managed on AS. Peri-prostatic fat volume normalized to prostate size was associated with shorter progression free survival, an observation that was consistent across a number of patient factors, including BMI. Peri-prostatic fat volume may serve as a valuable independent factor associated with disease aggression in AS, and future studies are needed to determine both its clinical and mechanistic roles in determining PCa progression.
Supplementary Material
Funding:
JR Gregg is funded (in part) through Department of Defense Prostate Cancer Research Program Early Career Award, grant number W81XWH-18-1-0173 (PI: J. Gregg)
CR Daniel is funded (in part) through the National Cancer Institute Cancer Center Support Grant (CCSG 5P30 CA016672-37) to MD Anderson (PI: P. Pisters).
Key of Definitions for Abbreviations
- AS
Active surveillance
- FOV
Field of view
- GG
Gleason grade group
- PCa
Prostate cancer
- PS
Pubic symphysis
Footnotes
There are no conflicts of interest
Protection of Human and Animal Subjects:
This study is registered on clinical.trials.gov (trial number NCT00490763), and use of de-identified data in this study for analysis was approved as exempt by the University of Texas MD Anderson Cancer Center Institutional Review Board. The study was performed in accordance with the Declaration of Helsinki.
REFERENCES
- 1.Mohler JL, Antonarakis ES, Armstrong AJ, et al. : Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019; 17: 479–505. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Bruinsma SM, Nicholson J, et al. : Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur. Urol. 2015; 67: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loeb S, Vellekoop A, Ahmed HU, et al. : Systematic review of complications of prostate biopsy. Eur. Urol. 2013; 64: 876–892. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S and Tosoian JJ: Biomarkers in active surveillance. Transl. Androl. Urol. 2018; 7: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin DW, Zheng Y, McKenney JK, et al. : 17-Gene Genomic Prostate Score Test Results in the Canary Prostate Active Surveillance Study (PASS) Cohort. J. Clin. Oncol. 2020: JCO.19.02267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerman BA, Torfadottir JE, Valdimarsdottir UA, et al. : Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer 2019: cncr.32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allott EH, Masko EM and Freedland SJ: Obesity and Prostate Cancer: Weighing the Evidence. Eur. Urol. 2013; 63: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal AC and Freedland SJ: Obesity and Prostate Cancer: A Focused Update on Active Surveillance, Race, and Molecular Subtyping. Eur. Urol 2017; 72: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Cao M, Chen Y, et al. : The combination of prostate imaging reporting and data system version 2 (PI-RADS v2) and periprostatic fat thickness on multi-parametric MRI to predict the presence of prostate cancer. Oncotarget 2017; 8. Available at: http://www.oncotarget.com/fulltext/17182, accessed April 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Sun L, Qi J, et al. : Periprostatic adiposity measured on magnetic resonance imaging correlates with prostate cancer aggressiveness. Urol. J. 2014; 11: 1793–1799. [PubMed] [Google Scholar]
- 11.Woo S, Cho JY, Kim SY, et al. : Periprostatic Fat Thickness on MRI: Correlation With Gleason Score in Prostate Cancer. Am. J. Roentgenol. 2015; 204: W43–W47. [DOI] [PubMed] [Google Scholar]
- 12.Dahran N, Szewczyk-Bieda M, Wei C, et al. : Normalized periprostatic fat MRI measurements can predict prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localised disease. Sci. Rep. 2017; 7: 4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberlin DT, Casalino DD, Miller FH, et al. : Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom. Radiol. N. Y. 2017; 42: 1255–1258. [DOI] [PubMed] [Google Scholar]
- 14.Davis JW, Ward JF, Pettaway CA, et al. : Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int. 2016; 118: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg JR, Davis JW, Reichard C, et al. : Determining Clinically Based Factors Associated With Reclassification in the Pre-MRI Era using a Large Prospective Active Surveillance Cohort. Urology 2020; 138: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amico AV, Whittington R, Malkowicz SB, et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280: 969–974. [DOI] [PubMed] [Google Scholar]
- 17.van Roermund JGH, Hinnen KA, Tolman CJ, et al. : Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int. 2011; 107: 1775–1779. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Chen S, Li W, et al. : Periprostatic Fat Thickness on MRI is an Independent Predictor of Time to Castration-resistant Prostate Cancer in Chinese Patients With Newly Diagnosed Prostate Cancer Treated With Androgen Deprivation Therapy. Clin. Genitourin. Cancer 2019; 17: e1036–e1047. [DOI] [PubMed] [Google Scholar]
- 19.Simpkin AJ, Tilling K, Martin RM, et al. : Systematic Review and Meta-analysis of Factors Determining Change to Radical Treatment in Active Surveillance for Localized Prostate Cancer. Eur. Urol. 2015; 67: 993–1005. [DOI] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Simko JP, Cowan JE, et al. : Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 2013; 31: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 21.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. : A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol 2014; 66: 550–560. [DOI] [PubMed] [Google Scholar]
- 22.Wei L, Wang J, Lampert E, et al. : Intratumoral and Intertumoral Genomic Heterogeneity of Multifocal Localized Prostate Cancer Impacts Molecular Classifications and Genomic Prognosticators. Eur. Urol. 2017; 71: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen HG, Welty CJ and Cooperberg MR: Diagnostic associations of gene expression signatures in prostate cancer tissue: Curr. Opin. Urol 2015; 25: 65–70. [DOI] [PubMed] [Google Scholar]
- 24.Saglam K, Aydur E, Yilmaz M, et al. : Leptin influences cellular differentiation and progression in prostate cancer. J. Urol. 2003; 169: 1308–1311. [DOI] [PubMed] [Google Scholar]
- 25.Finley DS, Calvert VS, Inokuchi J, et al. : Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J. Urol. 2009; 182: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro R, Monteiro C, Catalán V, et al. : Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. BMC Med 2012; 10. Available at: 10.1186/1741-7015-10-108, accessed January 30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent V, Guérard A, Mazerolles C, et al. : Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016; 7: 10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salji M, Hendry J, Patel A, et al. : Peri-prostatic Fat Volume Measurement as a Predictive Tool for Castration Resistance in Advanced Prostate Cancer. Eur. Urol. Focus 2018; 4: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurent V, Toulet A, Attané C, et al. : Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. MCR 2019; 17: 821–835. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


