Abstract
The pathological features of ascending gonococcal infection suggest that proinflammatory mediators secreted by tissue-resident macrophages are important components of the host response. Challenge of fully differentiated, mature macrophages with variants of Neisseria gonorrhoeae strain P9 or purified bacterial surface components (pili, lipooligosaccharide, and outer membrane vesicles) induced the secretion of interleukin 6 (IL-6), tumor necrosis factor alpha, growth-related protein α, macrophage inflammatory protein 1α (MIP-1α), and RANTES cytokines but had no effect on IL-8 production. No secretion of IL-1β, epithelial-derived neutrophil attractant 78, granulocyte-macrophage colony-stimulating factor, IL-10, or IL-12 cytokines was observed. Notably, the P9-Opab protein, in comparison to P9-Opaa, increased the association of gonococci with macrophages and elevated the secretion of cytokines. Thus, variation in Opa protein expression by the gonococcus may be a determining factor in the severity of pelvic inflammatory disease.
In men, genital infection with Neisseria gonorrhoeae is typically characterized by acute urethritis, with the symptoms of urethral discharge and dysuria usually becoming manifest 2 to 5 days after infection (5). A similar clinical course has been observed in human male volunteers following urethral challenge with gonococci, and this was associated with the sequential secretion of several proinflammatory cytokines (interleukin-6 [IL-6], IL-8, tumor necrosis factor alpha [TNF-α], and IL-1β) that culminated in the influx of leukocytes (23). In women, localized infection with gonococci is frequently asymptomatic, although 25% of untreated individuals will develop pelvic inflammatory disease (PID) (1). Histopathologically, PID is also characterized by the infiltration of leukocytes, while macroscopically, fibrin deposition and intrapelvic adhesions appear as a result of persistent inflammation (31).
Although epithelial cells of the genitourinary mucosae are the primary targets for infection with gonococci (25), significant numbers of resident macrophages are found in the underlying tissue. In the fallopian tube, mononuclear phagocytes constitute more than 2% of the resident cell population (3), and it is likely that these cells also interact with gonococci in both the acute and persistent phases of PID. The interactions of gonococci with epithelial cells appear to be a multistage process. The first stage, attachment of the bacteria, is facilitated by pili (30); the second stage, intimate association of the bacteria with the host cell surface, is mediated by the opacity (Opa) outer membrane protein(s) (15); and the third stage, cellular invasion by the bacteria, is triggered by an Opa-dependent process (13). Additionally, the binding of gonococci via pili and Opa proteins has been reported to facilitate nonopsonic phagocytosis by monocytes (10).
The results of studies in vitro (21) have suggested that epithelial cells of the genitourinary tract are a significant source of the proinflammatory mediators associated with gonococcal infection. However, although cytokine production has been studied in fresh blood monocytes stimulated with purified gonococcal immunoglobulin A1 (IgA1) protease (18), the responses of mature, monocyte-derived macrophages to N. gonorrhoeae have not been investigated. In the present study, cultures of fully differentiated macrophages resembling the “tissue-resident” phenotype (9) were prepared, and the range and identity of the cytokine response from these cells following challenge with live gonococci or purified bacterial surface structures were determined.
Human buffy coats (80 ml total, per single donor) were obtained from the National Blood Service (Southampton Centre, Southampton, United Kingdom). Peripheral blood mononuclear cells were isolated by overlaying four 20-ml volumes of buffy coat onto equal volumes of HISTOPAQUE-1077 (Sigma) and centrifuging the mixtures at 700 × g for 30 min. The mononuclear fraction was removed and suspended in phosphate-buffered saline containing 10 mM glucose and 2.5 mM EDTA to a concentration of 1 × 107 to 4 × 107 cells per ml. To eliminate platelet contamination, the suspension was overlaid onto 3 volumes of fetal calf serum (Gibco-BRL) and centrifuged at 200 × g for 15 min, and the procedure was repeated. The resuspended cells were added at a density of 5 × 106 to 1 × 107 per cm2 to cell culture plates (24-well; Costar) that were precoated with Matrigel basement membrane matrix according to the manufacturer's instructions (Collaborative Biomedical Products). Following overnight incubation at 37°C in an atmosphere of 5% (vol/vol) CO2, nonadherent cells were removed, and differentiation into macrophages was allowed to proceed over 8 to 10 days in high-glucose Dulbecco's modified Eagle medium (Gibco-BRL) supplemented with 10% autologous human serum and 5% fetal calf serum. In order to determine culture purity, cells were fixed with citrate-acetone-methanol and stained using an α-naphthyl acetate esterase kit (Sigma), while macrophage maturity was assessed by incubating methanol-fixed cultures with anti-mannose receptor (22) antibody (10 μg ml−1; Pharmingen) followed by fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1/250 dilution; Sigma). The stained preparations were viewed on a Leica DM-RB microscope (Leitz).
N. gonorrhoeae strain P9 and variants P9–1 (Opa− Pil−), P9–2 (Opa− α-Pil+), P9–13 (Opaa+ Pil−), and P9–16 (Opab+ Pil−) have been described previously (15). Culture wells containing 2.5 × 104 macrophages were challenged with 107 gonococci in 0.5 ml of low-glucose Dulbecco's modified Eagle medium (Gibco-BRL) supplemented with 2% (vol/vol) fetal calf serum or with medium alone (control) and incubated at 37°C in an atmosphere of 5% (vol/vol) CO2. Total cell association of each gonococcal variant was quantified by colony counting following lysis of the monolayer with saponin (28). Confocal microscopy was subsequently used to confirm gonococcal association with cells: macrophages were challenged with an isogenic transformant of strain P9 expressing green fluorescent protein (2) and counterstained with mannose receptor antibody and tetramethyl rhodamine isothiocyanate-conjugated goat anti-mouse IgG (1/250 dilution; Sigma). In addition, the viability of macrophages following challenge with gonococci was assessed with the LIVE/DEAD reduced biohazard viability-cytotoxicity assay from Molecular Probes, according to the manufacturer's instructions. All macrophage monolayers were examined on a Leica model TCS 4D confocal microscope (Leitz).
In order to measure cytokine secretion by macrophages, culture medium from challenged and control wells was also removed during the experiments, diluted in assay buffer (2), and stored at −70°C. Cytokines were quantified by a sandwich immunosorbent assay, using capture antibodies, biotinylated detector antibodies, and recombinant cytokine standards supplied by R&D Systems (IL-1β, IL-6, IL-8, IL-12, macrophage inflammatory protein [MIP-1α], RANTES, and TNF-α) or Biosource International (granulocyte-macrophage colony-stimulating factor and IL-10). Bound, labeled antibodies were detected by the Delfia time-resolved fluorometry system (Wallac) as described previously (2). In addition, growth-related protein α (GRO-α) and epithelial-derived neutrophil attractant 78 (ENA-78) cytokines were detected using Quantikine enzyme-linked immunosorbent assay kits (R&D Systems).
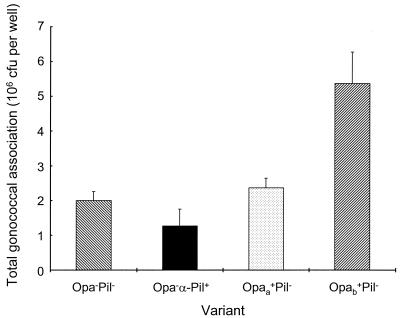
In the present study, the degree of association between gonococci and macrophages was clearly affected by the dominant surface phenotype of the bacterial inoculum (Fig. 1). At 6 h, the Opa− Pil− variant exhibited considerable cell-specific association, and this was not enhanced by the presence of α-pili or Opaa protein. In contrast, expression of Opab protein conferred a significant (P < 0.05) increase in the association of Pil− gonococci, when compared to the Opa− Pil− and Opaa+ Pil− variants. The overall association of gonococci with macrophages was confirmed by confocal microscopy. However, gonococcal association was clearly not homogeneous, in that some macrophages did not have adherent bacteria, whereas others were profusely covered (data not shown). The viability of macrophages following challenge with gonococci was also investigated, using a cell viability assay and visualization by confocal microscopy. After 6 h of bacterial challenge, there was considerable rounding and shrinkage of cells with adherent gonococci, although the great majority of macrophages were still attached to the Matrigel substrate and viability was greater than 90%.
FIG. 1.
Association of variants of N. gonorrhoeae strain P9 with mature human macrophages. Macrophages were cultured (2.5 × 104 cells, ≈1.25 × 104 per cm2) in 24-well tissue culture plates coated with Matrigel and challenged with approximately 107 CFU of the Opa− Pil−, Opa− α-Pil+, Opaa+ Pil−, or Opab+ Pil− variant of N. gonorrhoeae strain P9 per well. To correct for attachment of bacteria to the Matrigel substrate, each gonococcal suspension was also added in duplicate to culture wells lacking macrophages. Data are shown for corrected association after 6 h and are represented as the mean plus standard error for triplicate wells. Statistical comparisons between means were conducted using independent t tests, with P < 0.05 considered significant.
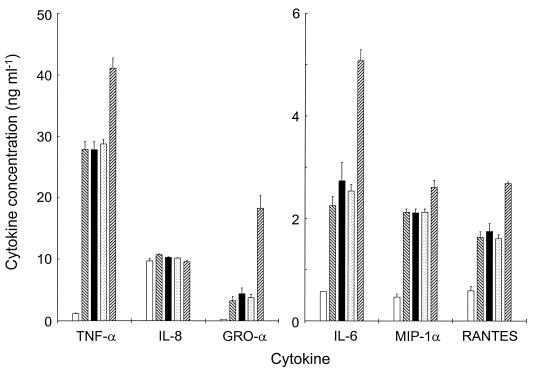
Time course experiments, in which macrophages were challenged with the gonococcal variants for up to 27 h, showed that a 6-h challenge period allowed cytokine concentrations to reach appreciable levels without excessive bacterial replication in the medium. At 6 h, all gonococcal variants induced significant (P < 0.01) secretion of IL-6, TNF-α, GRO-α, MIP-1α, and RANTES from macrophages, relative to that of the uninfected control (Fig. 2). In particular, Pil− gonococci that expressed Opab protein elicited significantly (P < 0.01) higher levels of IL-6, TNF-α, GRO-α, and RANTES cytokines in comparison to that elicited by the Opa− Pil− and Opaa+ Pil− variants (Fig. 2). However, the presence of α-pili or Opaa protein did not increase the macrophage cytokine response above levels observed following challenge with Opa− Pil− gonococci. In addition, none of the gonococcal variants stimulated macrophage secretion of IL-1β, IL-10, IL-12, granulocyte-macrophage colony-stimulating factor, and ENA-78 cytokines.
FIG. 2.
Induction of proinflammatory cytokine secretion in mature human macrophages challenged with gonococci. Supernatants were removed from the macrophage cultures challenged with the gonococcal variants, and cytokine release was quantified by specific immunoassays. Protein secretion was detected for TNF-α, IL-8, GRO-α, IL-6, MIP-1α, and RANTES following challenge with Opa− Pil− (▧), Opa− α-Pil+ (▪ ), Opaa+ Pil− (░⃞), and Opab+ Pil− (▨) gonococci. Cytokine production at 6 h was compared with levels in unchallenged cultures (□). Individual bars represent the geometric mean cytokine concentration plus standard error from six independent wells. One-way analysis of variance was used to compare mean values, with P < 0.05 considered significant.
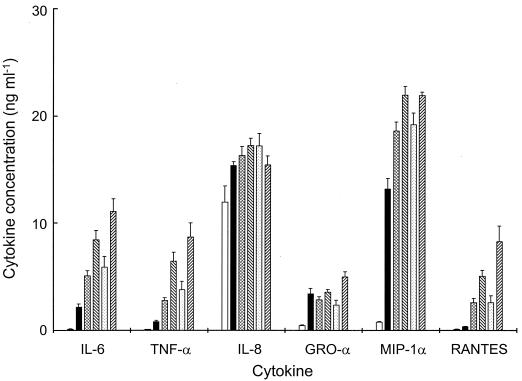
In order to investigate whether cytokine induction was independent of gonococcal metabolic activity, surface components were purified from gonococci and used as macrophage stimuli. α-Pili were sheared from the surface of intact gonococci in a cell homogenizer and purified by cycles of repeated disaggregation and precipitation with ammonium sulfate (16), soluble lipooligosaccharide (LOS) was obtained by phenol-water extraction (14), and outer membrane vesicles (OMV) were prepared from Opa− Pil−, Opaa+ Pil−, and Opab+ Pil− variants by extraction of intact gonococcal cells with lithium acetate (8). Macrophages were challenged for different periods of time with the gonococcal components (each at 10 μg ml−1) with OMV preparations also containing LOS at a concentration equivalent to that of protein, as previously reported for strain P9 (8). As early as 3 h after challenge, OMV containing Opab protein stimulated greater levels of production of IL-6, TNF-α, and MIP-1α cytokines compared to levels of secretion induced by all other gonococcal components (data not shown). Peak cytokine concentrations were reached at 18 h, at which time the purified pili, LOS, and OMV (with or without Opaa or Opab protein) elicited significantly higher (P < 0.01) levels of IL-6, TNF-α, GRO-α, MIP-1α, and RANTES from macrophages, compared to those from unchallenged cells (Fig. 3). In general, purified LOS induced lower levels of cytokine secretion from macrophages than did Opa− OMV. However, since these stimuli contained similar doses of LOS, the observed effects were probably due to differences in the physical structure of LOS and/or the presence of other stimulatory component(s) of the outer membrane. Notably, incubation with Opab+ OMV was associated with increased (approximately two- to threefold) generation of IL-6, TNF-α, RANTES, and GRO-α when compared to Opaa+ OMV (Fig. 3).
FIG. 3.
Induction of proinflammatory cytokine secretion in mature human macrophages incubated with gonococcal components. Supernatants were removed from the macrophage cultures incubated with various gonococcal components (10 μg ml−1), and cytokine release was quantified by specific immunoassays. Protein secretion was detected for IL-6, TNF-α, IL-8, GRO-α, MIP-1α, and RANTES following incubation with purified pili (▪), purified LOS (▩), Opa− OMV (▧), Opaa+ OMV (░⃞), and Opab+ OMV (▨). Peak cytokine production at 18 h was compared with levels in unchallenged cultures (□). Individual bars represent the geometric mean cytokine concentration plus standard error from six independent wells. One-way analysis of variance was used to compare mean values, with P < 0.05 considered significant.
In this study, release of a significant level of IL-8 from unchallenged macrophages was observed and neither challenge with viable bacteria nor incubation with surface components of the gonococcus elevated IL-8 secretion above control levels (Fig. 2 and 3). Adherence of macrophages to the culture substrate(s) in vitro has been shown to induce considerable secretion of IL-8 (24), and in the present study it is likely that long-term adherence during maturation resulted in maximal production of this cytokine, which could not be increased by additional stimulation.
The secretion of proinflammatory mediators by tissue-resident macrophages is likely to play a pivotal role in the host response to ascending gonococcal infection. Chemoattractant cytokines (chemokines) belong to several functionally distinct families, including the CXC family (whose members predominantly act upon neutrophils) and the CC family (classically associated with mononuclear cell infiltration) (19). In the present study, challenge of macrophages with viable gonococci markedly increased generation of the CXC chemokine GRO-α and also the CC chemokines MIP-1α and RANTES. These CC chemokines do not appear to be significant components of the epithelial cell cytokine response induced by gonococci (2, 21); therefore, macrophages resident in the reproductive tract probably represent the primary source for their production. In response to gonococcal stimulation, secretion of GRO-α is likely to contribute significantly to the infiltration of polymorphonuclear leukocytes (PMNL) observed during uncomplicated gonorrhea and the acute phase of PID (6, 31); whereas MIP-1α may trigger the influx of mononuclear cells, and possibly neutrophils (17), into the female upper reproductive tract. In addition, RANTES production by resident macrophages may lead to the recruitment of primed monocytes from the circulation (29), resulting in further release of inflammatory mediators at the site of infection (32).
In this study, macrophages challenged with gonococci secreted high levels of the proinflammatory, pleiotropic cytokines TNF-α and IL-6. Production of TNF-α has been reported for epithelial cells challenged with whole gonococci (2, 21). However, the protein concentrations determined in the present study suggest that macrophages challenged with gonococci generate several thousand times more TNF-α per cell than do epithelial cells (2). TNF-α appears to be of central importance in the pathogenesis of PID, as it causes sloughing of ciliated epithelial cells in the fallopian tube culture model (20), and high concentrations of TNF-α in peritoneal fluid are associated with fallopian tube occlusion (4). The established role of IL-6 in other diseases involving fibrosis (7) suggests that in chronic PID it acts in concert with TNF-α, precipitating the pelvic adhesions that are known to contribute to infertility (31). Furthermore, a common feature of ascending gonococcal infection is plasma cell endometritis (12), and since IL-6 stimulates B-cell differentiation (11), it is likely to play a significant role in the development of this condition.
Cytokine production by macrophages was not dependent solely on the interactions with viable bacteria. Challenge with intact gonococci and purified surface antigens suggested that essential components of the bacterial outer membrane were the principal stimuli, with Opa proteins, but not pili, modifying the magnitude of the observed cytokine responses. Accordingly, compared with Pil− Opa− P9 gonococci, pilus expression did not influence bacterial association with macrophages, which is in agreement with previous observations of PMNL challenged with this strain (27). In contrast, P9-Opab protein, compared to P9-Opaa, increased interactions between macrophages and gonococci and enhanced subsequent cytokine secretion. Expression of P9-Opab, compared to that of P9-Opaa, has also been found to increase adherence to Chang epithelial cells (26), whereas Opaa+ P9 gonococci displayed a greater association with PMNL (27) and endometrial epithelium cells (2). The Opa50 protein of gonococcal strain MS11 has been shown to exhibit a similar predilection for Chang epithelial cells and monocytes, while associating poorly with PMNL (10, 13), suggesting that a functionally conserved Opa variant plays an important role in virulence. However, comparisons between the amino acid sequences of P9-Opaa, P9-Opab, and individual Opa proteins from other strains, including MS11, failed to demonstrate a straightforward relationship between sequence and function with respect to cell tropism, which agrees with the observations of Kupsch et al. (13).
In summary, macrophages residing in the female genitourinary tract contribute significantly to the immunopathology of gonococcal PID. The initial interactions between gonococci and epithelial cells trigger an inflammatory response that is amplified by both direct involvement of these macrophages and subsequent recruitment of leukocytes from the circulation. Indeed, resident macrophages may respond to the changing expression of the gonococcal Opa repertoire by releasing higher levels of chemokines and pleiotropic cytokines, exacerbating tissue damage in the genitourinary tract, and sustaining the chronic sequelae associated with PID.
Acknowledgments
This work was supported by the Wellcome Trust and the Medical Research Council. B. L. Makepeace was the recipient of a Wellcome Trust studentship.
REFERENCES
- 1.Anonymous. Fact sheet. Geneva, Switzerland: Office of HIV/AIDS and Sexually Transmitted Diseases, World Health Organization; 1996. [Google Scholar]
- 2.Christodoulides M, Everson J S, Liu B, Lambden P R, Watt P J, Thomas E J, Heckels J E. Interaction of primary human endometrial cells with Neisseria gonorrhoeaeexpressing green fluorescent protein. Mol Microbiol. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- 3.Givan A L, White H D, Stern J E, Colby E, Gosselin E J, Guyre P M, Wira C R. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of Fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Guerra-Infante F M, Flores-Medina S, Lopez-Hurtado M, Zamora-Ruiz A, Gonzalez I S, Reyes M N, Villagrana-Zessati R. Tumor necrosis factor in peritoneal fluid from asymptomatic infertile women. Arch Med Res. 1999;30:138–143. doi: 10.1016/s0188-0128(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 5.Handsfield H H, Sparling P F. Neisseria gonorrhoeae. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Vol. 2. New York, N.Y: Churchill Livingstone; 1995. pp. 1909–1926. [Google Scholar]
- 6.Harkness A H. The pathology of gonorrhoea. Br J Ven Dis. 1948;24:137–147. [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa M, Sato S, Ihn H, Takehara K. Enhanced production of interleukin-6 (IL-6), oncostatin M and soluble IL-6 receptor by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Rheumatology. 1999;38:612–617. doi: 10.1093/rheumatology/38.7.612. [DOI] [PubMed] [Google Scholar]
- 8.Heckels J E. The surface properties of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977;99:333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan G, Gaudernack G. In vitrodifferentiation of human monocytes—differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982;156:1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knepper B, Heuer I, Meyer T F, van Putten J P M. Differential response of human monocytes to Neisseria gonorrhoeaevariants expressing pili and opacity proteins. Infect Immun. 1997;65:4122–4129. doi: 10.1128/iai.65.10.4122-4129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kono Y, Beagley K W, Fujihashi K, McGhee J R, Taga T, Hirano T, Kishimoto T, Kiyono H. Cytokine regulation of localized inflammation—induction of activated B-cells and IL-6-mediated polyclonal IgG and IgA synthesis in inflamed human gingiva. J Immunol. 1991;146:1812–1821. [PubMed] [Google Scholar]
- 12.Korn A P, Hessol N A, Padian N S, Bolan G A, Donegan E, Landers D V, Schachter J. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178:987–990. doi: 10.1016/s0002-9378(98)70536-8. [DOI] [PubMed] [Google Scholar]
- 13.Kupsch E M, Knepper B, Kuroki T, Heuer I, Meyer T F. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeaefor human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambden P R, Heckels J E. Synthesis of immunogenic oligosaccharide-protein conjugates from the lipopolysaccharide of Neisseria gonorrhoeaeP9. J Immunol Methods. 1982;48:233–240. doi: 10.1016/0022-1759(82)90197-1. [DOI] [PubMed] [Google Scholar]
- 15.Lambden P R, Heckels J E, James L T, Watt P J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979;114:305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- 16.Lambden P R, Robertson J N, Watt P J. The preparation and properties of alpha-pili and beta-pili from variants of Neisseria gonorrhoeaeP9. J Gen Microbiol. 1981;124:109–117. doi: 10.1099/00221287-124-1-109. [DOI] [PubMed] [Google Scholar]
- 17.Lee S C, Brummet M E, Shahabuddin S, Woodworth T G, Georas S N, Leiferman K M, Gilman S C, Stellato C, Gladue R P, Schleimer R P, Beck L A. Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes. J Immunol. 2000;164:3392–3401. doi: 10.4049/jimmunol.164.6.3392. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzen D R, Dux F, Wolk U, Tsirpouchtsidis A, Haas G, Meyer T F. Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines. J Exp Med. 1999;190:1049–1058. doi: 10.1084/jem.190.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 20.McGee Z A, Clemens C M, Jensen R L, Klein J J, Barley L R, Gorby G L. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb Pathog. 1992;12:333–341. doi: 10.1016/0882-4010(92)90096-7. [DOI] [PubMed] [Google Scholar]
- 21.Naumann M, Wessler S, Bartsch C, Wieland B, Meyer T F. Neisseria gonorrhoeaeepithelial cell interaction leads to the activation of the transcription factors nuclear factor kappa b and activator protein 1 and the induction of inflammatory cytokines. J Exp Med. 1997;186:247–258. doi: 10.1084/jem.186.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorman F, Braat E M, Barrett-Bergshoeff M, Barbe E, van Leeuwen A, Lindeman J, Rijken D C. Monoclonal antibodies against the human mannose receptor as a specific marker in flow cytometry and immunohistochemistry for macrophages. J Leukoc Biol. 1997;61:63–72. doi: 10.1002/jlb.61.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey K H, Schneider H, Cross A S, Boslego J W, Hoover D L, Staley T L, Kuschner R A, Deal C D. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 24.Standiford T J, Kunkel S L, Kasahara K, Milia M J, Rolfe M W, Strieter R M. Interleukin 8 gene expression from human alveolar macrophages—the role of adherence. Am J Respir Cell Mol Biol. 1991;5:579–585. doi: 10.1165/ajrcmb/5.6.579. [DOI] [PubMed] [Google Scholar]
- 25.van Putten J P M, Duensing T D. Infection of mucosal epithelial cells by Neisseria gonorrhoeae. Rev Med Microbiol. 1997;8:51–59. [Google Scholar]
- 26.Virji M, Everson J S. Comparative virulence of opacity variants of Neisseria gonorrhoeaestrain P9. Infect Immun. 1981;31:965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virji M, Heckels J E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeaewith human polymorphonuclear leukocytes. J Gen Microbiol. 1986;132:503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- 28.Virji M, Kayhty H, Ferguson D J P, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseriawith cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 29.Volin M V, Shah M R, Tokuhira M, Haines G K, Woods J M, Koch A E. RANTES expression and contribution to monocyte chemotaxis in arthritis. Clin Immunol Immunopathol. 1998;89:44–53. doi: 10.1006/clin.1998.4590. [DOI] [PubMed] [Google Scholar]
- 30.Ward M E, Watt P J, Robertson J N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974;129:650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- 31.Westrom L, Mardh P A. Acute pelvic inflammatory disease (PID) In: Holmes K K, Mardh P A, Sparling P F, Weisner P J, Cates W, Lemon S M, Stamm W E, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill; 1990. pp. 593–613. [Google Scholar]
- 32.Witkin S S, Toth M, Jeremias J, Ledger W J. Increased inducibility of inflammatory mediators from peripheral blood mononuclear cells of women with salpingitis. Am J Obstet Gynecol. 1991;165:719–723. doi: 10.1016/0002-9378(91)90316-j. [DOI] [PubMed] [Google Scholar]