Abstract
Introduction
Older adults have the greatest burden of asthma and poorest outcomes. The pharmacogenetics of inhaled corticosteroid (ICS) treatment response is not well studied in older adults.
Methods
A genome-wide association study of ICS response was performed in asthmatics of European ancestry in Genetic Epidemiology Research on Adult Health and Aging (GERA) by fitting Cox proportional hazards regression models, followed by validation in the Mass General Brigham (MGB) Biobank and Rotterdam Study. ICS response was measured using two definitions in asthmatics on ICS treatment: (1) absence of oral corticosteroid (OCS) bursts using prescription records and (2) absence of asthma-related exacerbations using diagnosis codes. A fixed-effect meta-analysis was performed for each outcome. The validated single-nucleotide polymorphisms (SNPs) were functionally annotated to standard databases.
Results
In 5710 subjects in GERA, 676 subjects in MGB Biobank, and 465 subjects in the Rotterdam Study, four novel SNPs on chromosome six near PTCHD4 validated across all cohorts and met genome-wide significance on meta-analysis for the OCS burst outcome. In 4541 subjects in GERA and 505 subjects in MGB Biobank, 152 SNPs with p<5 × 10−5 were validated across these two cohorts for the asthma-related exacerbation outcome. The validated SNPs included methylation and expression quantitative trait loci for CPED1, CRADD and DST for the OCS burst outcome and GM2A, SNW1, CACNA1C, DPH1, and RPS10 for the asthma-related exacerbation outcome.
Conclusions
Multiple novel SNPs associated with ICS response were identified in older adult asthmatics. Several SNPs annotated to genes previously associated with asthma and other airway or allergic diseases, including PTCHD4.
INTRODUCTION
The prevalence of asthma has risen over the past six decades and is associated with significant morbidity, mortality and socioeconomic burden.1 2 Inhaled corticosteroids (ICS) are an important class of medications for the primary treatment and control of asthma. However, individual treatment responses vary, and up to one-third to one-half of patients have poor treatment response to ICS.3 4 Interindividual variability in ICS response is associated with genetics, but small sample sizes and heterogeneity of phenotypic outcomes have limited the large-scale discovery and validation of ICS pharmacogenetic loci.5
The burden of asthma is highest in adults ages 45 years and older.6 7 In particular, asthma exacerbation rates are highest in adults 55 years and older, and adults older than 65 years have the highest rate of hospitalisations for asthma and longest lengths of stay.7 Therefore, it is imperative to understand the basis of ICS treatment response in older individuals with asthma, yet the population of older adult asthmatics remains understudied. The objective of this study was to examine the genetics of ICS treatment response in older asthmatics by performing a large genome-wide association study (GWAS) in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort participants of European ancestry with independent validation in two large population-based cohorts.
METHODS
Study populations
GERA is a multiethnic cohort of over 110 000 subjects from the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC), Research Programme on Genes, Environment and Health who provided a saliva sample.8 In GERA, over 16 000 asthmatics have genotype data and longitudinal electronic medical records, including detailed diagnosis and medication records. All races and ethnicities were self-reported, and 80% of subjects were non-Hispanic white. Adult asthma cases were defined as patients at least 21 years of age with one or more of the following: physician-diagnosed asthma, self-reported asthma, or a report of an asthma exacerbation (ie, emergency department visit or hospitalisation due to asthma).9 Subjects with chronic obstructive pulmonary disease (COPD), pulmonary embolism, primary pulmonary hypertension, cystic fibrosis and bronchiectasis were excluded. Prescription fill data were available from outpatient and emergency department visits. GERA is enriched for older adults because overall, older adults seek care more frequently than younger patients. In fact, 74%–75% of the population were at least age 55 years, and only 8%–9% were younger than age 45 years. Due to small sample sizes in racial and ethnic minorities, only non-Hispanic white subjects were included in GERA and the subsequent validation cohorts.
Mass General Brigham (MGB) Biobank is a biorepository at MGB in Massachusetts with over 120 000 participants including 13 000 asthmatics and genotype data in over 40 000 subjects.10 The MGB Biobank is representative of the patient population at MGB, and at the time of analysis, the minimum age of asthmatics in MGB Biobank was 21 years and maximum age was 97 years. Patients with any lifetime history of asthma were selected using a validated phenotype algorithm that relies on coded diagnoses, medications, and vital signs (ie, body mass index (BMI)).11 The algorithm has a positive predictive value of 0.90 and negative predictive value of 0.99. Subjects with COPD, bronchiectasis and chronic bronchitis were excluded.
The Rotterdam Study is a prospective, population-based cohort study of inhabitants of the Ommoord district of Rotterdam, Netherlands aged 45 years and over that investigates chronic diseases in an ageing population.12 All participants were invited for periodic follow-up interviews and examinations at the research facility in addition to continuous follow-up through linkage of the database with data from the medical records of general practitioners, nursing homes, hospitals and pharmacies. Asthma cases were defined as subjects with a validated physician’s diagnosis of asthma, and 524 subjects had physician-diagnosed asthma on study entry.13 Subjects with validated COPD, defined as having a forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) ratio below 0.7 without having a diagnosis of asthma, were excluded.12 All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
ICS response
ICS response was defined by the absence of asthma exacerbations while on ICS treatment using two separate outcomes: (1) oral corticosteroid (OCS) bursts (figure 1) and (2) asthma-related exacerbations documented by International Classification of Diseases (ICD) diagnosis codes (figure 2). To filter out subjects who used ICS on a one-off basis, only subjects with least two ICS prescriptions ≥90 days apart were included. OCS bursts and asthma-related exacerbations were considered indicative of poor ICS response if they occurred within 30 days after filling an ICS prescription in GERA or 180 days after receiving an ICS prescription in MGB Biobank. A more stringent cut-off was used in the GERA to reduce misclassification bias in the discovery analysis. On sensitivity analysis, the extended cut-off in MGB Biobank resulted in a 6%–7% increase in classification of subjects with asthma exacerbations. Subjects whose first asthma exacerbation occurred prior to first ICS prescription in the longitudinal electronic medical record were removed, and subjects on chronic OCS were also removed for the OCS burst outcome. The Rotterdam Study only had data on OCS bursts and not asthma-related exacerbations. A time-to-event analysis was performed, and follow-up time for controls was calculated from their first ICS prescription to their last in the electronic medical record, whereas cases were censored at their first asthma exacerbation.
Figure 1.
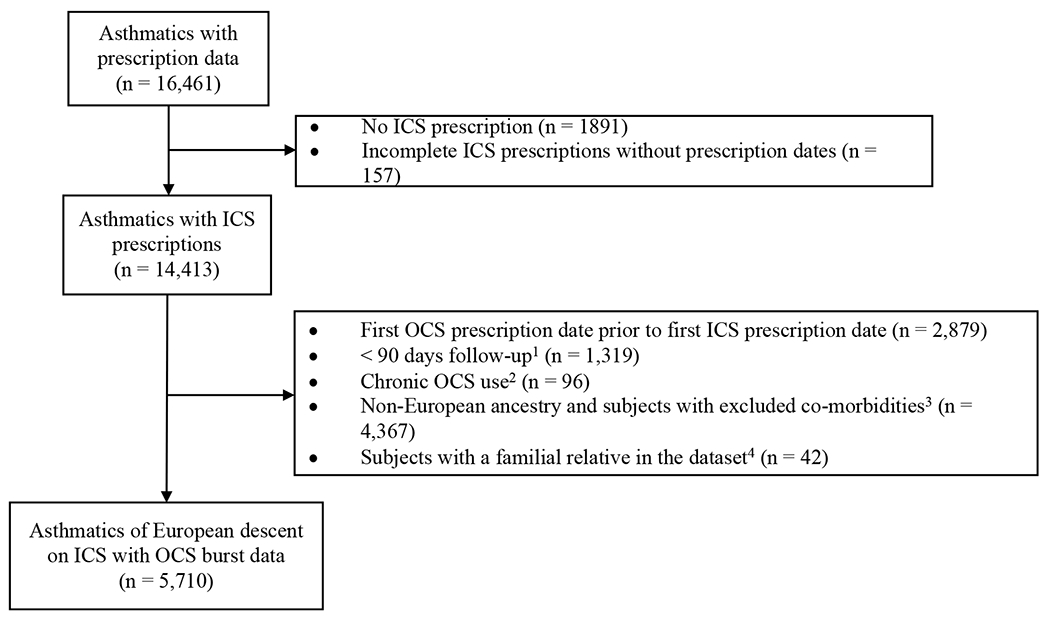
Derivation of the oral corticosteroid burst ICS response phenotype in GERA. 1Time period between the first and last ICS prescription dates; 2Subjects with days of OCS prescriptions ≥50% of ICS follow-up days were excluded, leaving subjects with OCS prescription coverage <50% of the ICS follow-up time; 3the following prespecified comorbidities were removed: cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease, pulmonary embolism, primary pulmonary hypertension; 4Subjects with identity-by-descent estimates >0.1875. GERA, Genetic Epidemiology Research on Adult and Aging; ICS, inhaled corticosteroid; OCS, oral corticosteroid.
Figure 2.
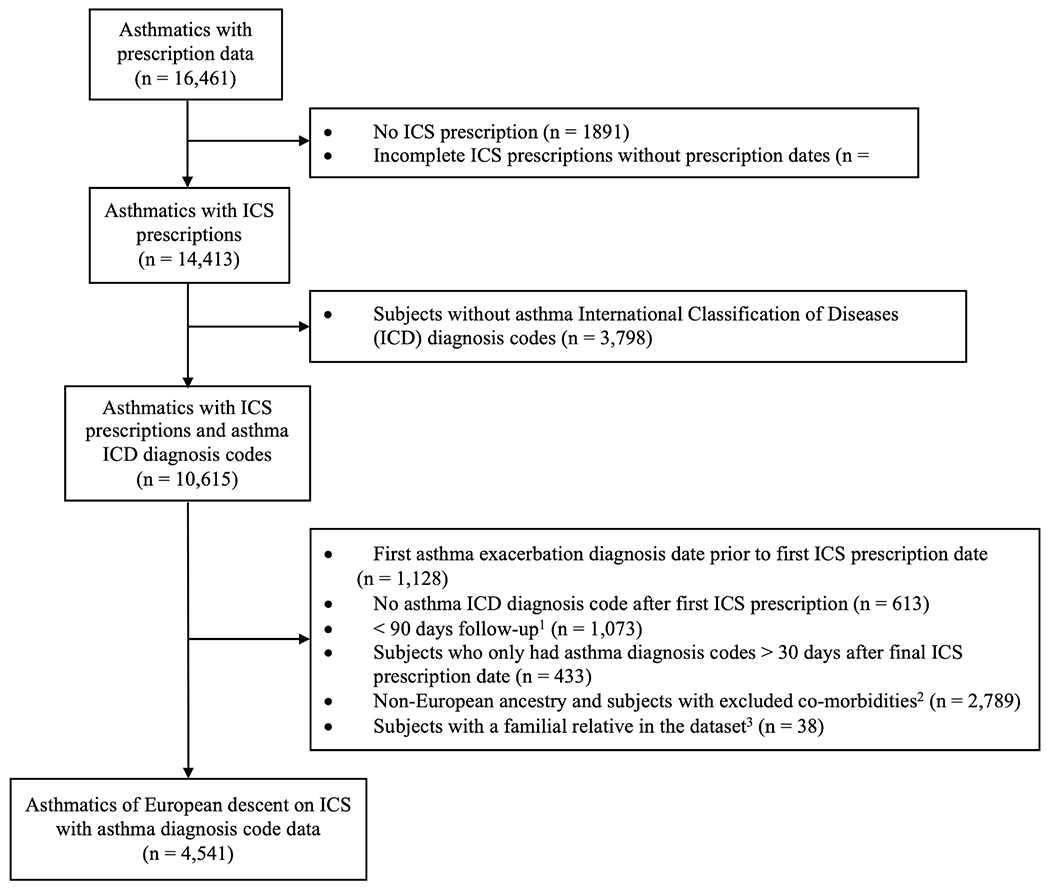
Derivation of the asthma-related exacerbation ICS response phenotype in GERA. 1Time period between the first and last asthma ICD diagnosis code dates; 2the following prespecified comorbidities were removed: cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease, pulmonary embolism, primary pulmonary hypertension; 3Subjects with identity-by-descent estimates >0.1875. GERA, Genetic Epidemiology Research on Adult and Aging; ICS, inhaled corticosteroid; OCS, oral corticosteroid.
Genotype data
Genotyping in GERA was performed on the Affymetric Axion Genotyping System and underwent standard quality control procedures as previously described.8 Subjects in the MGB Biobank were genotyped on the Illumina Multi-Ethnic Global Array (MEGA), Expanded MEGA (MEGA Ex), and MEGA with imputation to the 1000 Genomes phase 3 reference panel using the Michigan Imputation Server.14 Genotyping was performed using the Illumina HumanHap550-Duo and Human 610-Quad BeadChips in the Rotterdam Study. Single-nucleotide polymorphisms (SNPs) with minor allele frequencies<1% and Hardy-Weinberg equilibrium exact test p<10−5 were filtered using Plink V.1.9.15 Identity-by-descent estimates were calculated in each cohort, and one subject from each pair with an identity-by-descent estimate >0.1875 was removed. In GERA, the final dataset comprised 8 995 492 and 9 004 920 variants, respectively, for the OCS burst (online supplemental figure 1) and asthma-related exacerbation (online supplemental figure 2) phenotypes.
Statistical analysis
Univariate analyses between ICS response status and each of the following variables selected a priori were performed in R: smoking status, BMI, allergic rhinitis, gastro-oesophageal reflux disease, and concurrent asthma medication use. Concurrent asthma medication use was a composite binary variable consisting of any history of long-acting β-agonist, anti-leukotriene and anticholinergic prescriptions. Next, a multivariable Cox proportional hazards regression analysis examining the risk of asthma exacerbation on ICS was performed using the GWASTools R package controlling for age, sex, principal components as determined by scree plots, and additional variables statistically significant in the univariate analyses (online supplemental methods). Variables that violated the proportional hazards assumption were stratified. Additive models were applied with the minor allele as the effect allele. Systematic bias was assessed through quantile-quantile (QQ) plots and calculation of the genomic inflation factor (λgc). For each outcome, the analysis was performed first in GERA, and SNPs with p<5 × 10−5 were then evaluated for replication in MGB Biobank and the Rotterdam Study for the OCS burst outcome and only MGB Biobank for the asthma-related exacerbation outcome. A fixed-effect meta-analysis was performed in the replication cohorts using METAL, and a global fixed-effect meta-analysis across all cohorts was performed for the top identified SNPs from GERA.16 In the meta-analyses, weighting based on sample sizes and p values was performed over weighting based on effect sizes and SEs because the former effectively adjusts for both effect sizes and SEs in addition to sample sizes. The survival and survminer R packages were used to generate adjusted survival curves. Regional genomic plots were generated using LocusZoom.17 Sensitivity analyses stratifying by age <65 years and age ≥65 years were performed in GERA, in addition to separate interaction analyses for SNP interactions with obesity (BMI ≥30) and smoking status (current or former vs never smokers).
Functional annotation
The validated SNPs from each global meta-analysis were annotated to genes from the Ensembl and HUGO Gene Nomenclature Committee databases using the biomaRt R package to the GRCh37 reference assembly. Annotation to Reactome pathways and regulatory elements from the National Institutes of Health Roadmap Epigenomics Project were performed using SNPnexus.18 SNPs were examined for methylation expression quantitative trait loci (meQTL) in two databases, the Avon Longitudinal Study of Parents and Children (ARIES) and Biobank-based Integrative Omics Study Consortium.19 20 The Gene-Tissue Expression Portal was used to evaluate the SNPs for eQTL in lung tissue. The NHGRI-EBI GWAS Catalogue was interrogated for overlap of the results with prior asthma, asthma corticosteroid response, COPD, lung function, allergic rhinitis, allergy, atopy and eosinophil count GWAS results.21
RESULTS
OCS burst outcome
In GERA, 5710 subjects were included in the OCS burst outcome discovery analysis, and validation analyses were performed in 676 subjects from MGB Biobank and 465 subjects from the Rotterdam Study (table 1). Subjects had a median follow-up time of 1131 (IQR1948) days in GERA, 1280 (IQR 2098) days in MGB Biobank and 1054 (IQR 1907) days in the Rotterdam Study. As expected, cases had a shorter follow-up time than controls; for example, in GERA, cases had a median follow-up time of 773 (IQR 1323) days, and controls had a median follow-up time of 1815 (IQR 2365) days. On genome-wide survival analysis in GERA, each copy of the minor allele in rs7247485 (ZNF536) conferred a 1.41 (95% CI 1.26 to 1.58) times increase in the HR of asthma exacerbations on ICS (p=2.93 × 10−9) (online supplemental figure 3a, online supplemental figure 4, online supplemental figure 5). The QQ plot and genomic inflation factor indicated adequate control of confounders (online supplemental figure 6), and 729 SNPs met a suggestive p<5 × 10−5 (online supplemental table 1). A sensitivity analysis stratifying by age <65 years or ≥65 years showed consistent results across age ranges (online supplemental table 2). No effect modification by obesity and smoking status met genome-wide significance (online supplemental tables 3 and 4). MGB Biobank had 611 (84%) of the 729 SNPs, and the Rotterdam Study had 606 (83%) of the 729 SNPs (online supplemental table 1). Five SNPs in linkage disequilibrium (rs138717703, rs77506063, rs116098116, rs116023293, rs145325916) on chromosome six near PTCHD4 comprised the top results from the meta-analysis of the independent replication cohorts MGB Biobank and the Rotterdam Study (online supplemental table 5). On global meta-analysis of all three cohorts, 116 (16%) of the top SNPs from GERA were validated with the same direction of effect in MGB Biobank and the Rotterdam Study (online supplemental table 6), and four SNPs (rs138717703, rs77506063, rs116023293, rs145325916) on chromosome six near PTCHD4 met genome-wide significance (table 2 and online supplemental figure 7). For the top SNP rs138717703 in the global meta-analysis, each copy of the minor allele was associated with a 1.73 (95% CI 1.39 to 2.16), 1.48 (95% CI 0.75 to 2.90) and 3.34 (95% CI 1.86 to 6.03) respective times increase in the HR of asthma exacerbations in GERA, MGB Biobank, and the Rotterdam Study (figure 3). Additional notable gene targets of the 116 validated SNPs (online supplemental table 6) include: (1) ANK3 (ankyrin 3) which is associated with lung function decline in COPD and is crucial to the biogenesis of bronchial epithelial cell lateral membranes22 23; (2) PRKCB (protein kinase C) involved in B cell activation, phagocytosis, and circadian regulation24 25; (3) DST (dystonin) involved in the assembly of hemidesmosomes in epithelial cells; (4) NCKAP1L (Nck-associated protein 1-like), an actin cytoskeleton regulator important for lymphocyte development, phagocytosis and neutrophil migration26 27 and (5) CPED1 (cadherin like and PC-esterase domain containing 1) whose expression is associated with FVC.28
Table 1.
Demographics of the subjects in GERA, MGB Biobank and Rotterdam Study analysed for the oral corticosteroid burst outcome
| GERA (n=5710) |
MGB biobank (n=676) |
Rotterdam study (n=465) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n=3097) | Controls (n=2613) | P value | Cases (n=229) | Controls (n=447) | P value | Cases (n=274) | Controls (n=191) | P value | |
| Age (years, mean±SD) | 63.8 (±14.0) | 63.4 (±15.2) | 0.24 | 57.7 (±15.4) | 59.9 (±15.9) | 0.09 | 63.7 (±8.7) | 62.5 (±9.0) | 0.16 |
|
| |||||||||
| Female sex | 2168 (70.0%) | 1765 (67.5%) | 0.046 | 160 (69.9%) | 274 (61.3%) | 0.03 | 191 (69.7%) | 132 (69.1%) | 0.97 |
|
| |||||||||
| Smoking status | <0.001 | 0.74 | 0.43 | ||||||
|
| |||||||||
| Never | 1622 (54.1%) | 1481 (59.1%) | 78 (59.1%) | 146 (60.8%) | 105 (38.3%) | 65 (34.0%) | |||
|
| |||||||||
| Current or Former | 1378 (45.9%) | 1027 (40.9%) | 54 (40.9%) | 94 (39.2%) | 168 (61.3%) | 124 (65.0%) | |||
|
| |||||||||
| BMI (kg/m2, mean±SD) | 29.2 (±6.2) | 28.6 (±6.3) | <0.05 | 30.6 (±7.7) | 29.0 (±7.6) | 0.06 | 28.1 (±4.4) | 28.2 (±4.9) | 0.9 |
|
| |||||||||
| Co-morbidities | |||||||||
|
| |||||||||
| Allergic rhinitis | 1404 (45.3%) | 1113 (42.6%) | <0.05 | 156 (68.1%) | 285 (63.8%) | 0.26 | – | – | – |
|
| |||||||||
| GERD* | 983 (31.7%) | 651 (24.9%) | <0.001 | 153 (66.8%) | 281 (62.9%) | 0.31 | – | – | – |
|
| |||||||||
| Concurrent asthma medications | |||||||||
|
| |||||||||
| Long-acting β-agonists | 333 (10.8%) | 117 (4.5%) | <0.001 | 158 (69.0%) | 181 (40.5%) | <0.001 | 239 (87.2%) | 125 (65.4%) | <0.001 |
|
| |||||||||
| Anti-leukotrienes | 149 (4.8%) | 71 (2.7%) | <0.001 | 87 (38.0%) | 77 (17.2%) | <0.001 | 56 (20.4%) | 15 (7.9%) | <0.001 |
|
| |||||||||
| Anti-cholinergics | 110 (3.6%) | 37 (1.4%) | <0.001 | 64 (27.9%) | 54 (12.1%) | <0.001 | 178 (65.0%) | 73 (38.2%) | <0.001 |
|
| |||||||||
| Biologics† | – | – | – | 6 (2.6%) | 0 (0.0%) | 0.001 | – | – | – |
Gastro-oesophageal reflux disease.
No subjects in GERA or Rotterdam Study on biologics. In MGB Biobank, omalizumab and mepolizumab only.
BMI, body mass index; GERA, Genetic Epidemiology Research on Adult and Aging; MGB, Mass General Brigham.
Table 2.
Genome-wide significant SNPs on meta-analysis of the oral corticosteroid burst ICS response outcome in asthmatics in GERA, MGB Biobank and Rotterdam Study
| rsID | Chr | Nearest gene | Nearest coding gene | Minor allele | Major allele | Minor allele frequency | Discovery |
Replication |
Global meta-analysis p value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GERA HR (n=5710) | GERA p value | MGB biobank HR (n=676) | Rotterdam study HR (n=465) | Replication meta-analysis p value | ||||||||
| rs138717703 | 6 | RBMXP1 | PTCHD4 | G | C | 0.01 | 1.73 (1.39–2.16) | 7.91×10−7 | 1.48 (0.75–2.90) | 3.34 (1.86–6.03) | 5.78×10−4 | 3.09×10−9 |
|
| ||||||||||||
| rs77506063 | 6 | RBMXP1 | PTCHD4 | C | T | 0.01 | 1.73 (1.39–2.16) | 7.91×10−7 | 1.48 (0.75–2.90) | 3.34 (1.86–6.03) | 5.78×10−4 | 3.09×10−9 |
|
| ||||||||||||
| rs116023293 | 6 | HNRNPA3P4 | PTCHD4 | G | A | 0.01 | 1.74 (1.40–2.16) | 5.28×10−7 | 1.27 (0.66–2.42) | 3.19 (1.73–5.90) | 3.56×10−3 | 7.65×10−9 |
|
| ||||||||||||
| rs145325916 | 6 | RBMXP1 | PTCHD4 | C | T | 0.01 | 1.74 (1.40–2.16) | 6.18×10−7 | 1.27 (0.66–2.42) | 3.19 (1.73–5.90) | 3.56×10−3 | 9.00×10−9 |
GERA, Genetic Epidemiology Research on Adult and Aging; ICS, inhaled corticosteroid; MGB, Mass General Brigham; SNPs, single-nucleotide polymorphisms.
Figure 3.
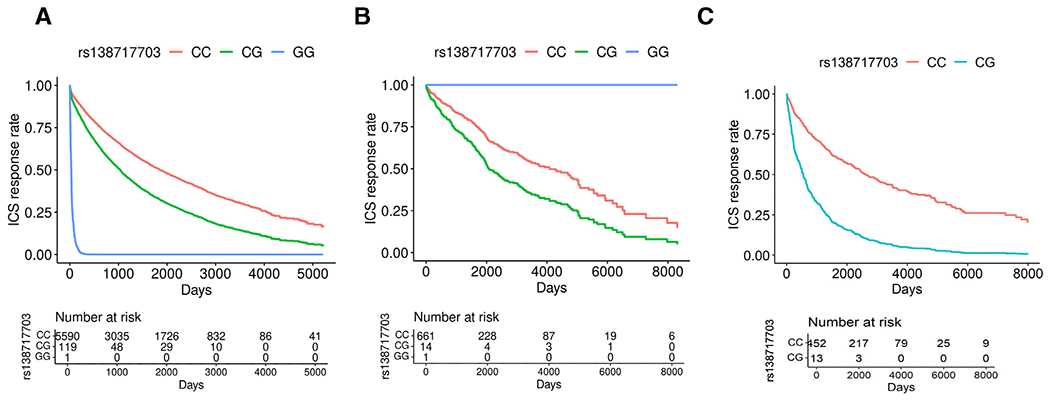
Expected survival curves of rs138717703, representative of the four genome-wide significant SNPs in linkage disequilibrium (rs138717703, rs77506063, rs116023293 and rs145325916) associated with poor ICS response as measured by OCS bursts in a meta-analysis across GERA, MGB Biobank, and the Rotterdam Study.1 (A) GERA (HR 1.73 (95% CI 1.39 to 2.16), p=7.91×10−7, MAF 0.01), (B) MGB Biobank (HR 1.48 (95% CI 0.75 to 2.90), p=2.55×10−1, MAF 0.01), (C) Rotterdam Study (HR 3.34 (95% CI 1.86 to 6.03), p=5.86×10−5, MAF 0.01). 1One subject each in GERA and MGB Biobank had the minor allele homozygous genotype (GG) for all four SNPs. The direction of effect for the homozygous minor allele genotype differed across these two cohorts due to low minor allele frequency. No subjects in the Rotterdam Study had the homozygous minor allele genotype. C, cytosine; G, guanineGERA, Genetic Epidemiology Research on Adult and Aging; ICS, inhaled corticosteroid; MGB, Mass General Brigham; OCS, oral corticosteroid; SNPs, single-nucleotide polymorphisms.
Pathway analysis of the 116 validated SNPs demonstrated an enrichment of pathways related to cell signalling, neuronal trafficking and plasticity, activation of B cells, and extracellular matrix organisation (online supplemental table 7), and 95 SNPs annotated to epigenomic regulatory elements in relevant tissues and cell types, including the lung, adrenal glands, thymus and immune cells (online supplemental table 8). In addition, 12 SNPs were meQTL for 7 genes, including CRADD (CASP2 and RIPK1 domain containing adaptor with death domain) which regulates endothelial barrier function, suppresses proinflammatory cytokines and chemokines, and is correlated with disease activity and eosinophilia in eosinophilic granulomatosis with polyangiitis and the aforementioned CPED1 (online supplemental table 9).29 30 Twenty-one SNPs were human lung eQTL for DST (online supplemental table 10). All of the validated SNPs in the meta-analysis were novel for asthma, asthma corticosteroid response, and additional traits examined in the NHGRI-EBI GWAS Catalogue.
Asthma-related exacerbation outcome
In GERA, 4541 subjects were included in the asthma-related exacerbation outcome discovery analysis with validation in 505 subjects from the MGB Biobank (table 3). Subjects had a median follow-up time of 1547 (IQR 2228) days in GERA and 1092 (IQR 1730) days in MGB Biobank. On genome-wide Survival analysis in GERA, two SNPs in ADAMTS17 met genome-wide significance. Each copy of the minor allele in rs140187287 and rs141689337 conferred a respective 2.43 (95% CI 1.80 to 3.27) (p=5.37 × 10−9) and 2.59 (95% CI 1.86 to 3.62) times increase (p=2.09 × 10−8) in the HR of asthma exacerbations on ICS (online supplemental figure 3b, online supplemental figure 8, online supplemental figure 9). The QQ plot and genomic inflation factor indicated adequate control of confounders (online supplemental figure 10), and 489 SNPs had a suggestive p<5 × 10−5 (online supplemental table 11). On sensitivity analyses, including tobacco smoking as a confounder yielded similar results (online supplemental table 12) and stratifying by age identified 45 variants in the age ≥65 years strata that met genome-wide significance (online supplemental table 13). Thirty-two out of the 45 SNPs annotated to TPD52L1 on chromosome 6. No effect modification by obesity and smoking status met genome-wide significance (online supplemental tables 14 and 15). MGB Biobank had 376 (77%) of the 489 SNPs for validation analysis (online supplemental table 11), and the Rotterdam Study did not have data on asthma-related exacerbations. On global meta-analysis, 152 (31%) of the top SNPs from GERA were validated with the same direction of effect across MGB Biobank, but none met genome-wide significance (online supplemental table 16). Notable gene targets of the 152 validated SNPs include: (1) KCNQ3 (potassium voltage-gated channel subfamily Q member 3) which encodes a potassium channel that regulates bronchiole relaxation, enhances bronchodilator therapy, and is inhibited by histamine31 32; (2) DNAH11 (dynein axonemal heavy chain 11) and (3) ARMC4 (armadillo repeat containing 4) that control respiratory cilia movement33 34; (4) CACNA1C (calcium voltage-gated channel subunit alpha1 C) which is associated with histamine hypersensitivity and upregulated in chronic hypoxia35 36; (5) KDM4C (lysine demethylase 4 c) which encodes a histone demethylase that regulates B cell activation and proliferation37; (6) MMP16 (matrix metallopeptidase 16) involved in alveolarisation, airway remodelling and alveolar disruption in COPD, susceptibility to bronchopulmonary dysplasia in premature infants, and chronic respiratory morbidity and lung function in infants with respiratory syncytial virus infection38–40; (7) CD36 (cluster of differentiation 36) which mediates phagocytosis of apoptotic eosinophils by bronchial epithelial cells, promotes allergic responses to house dust mite, and is elevated in the bronchoalveolar lavage fluid exosomes of asthmatics41–43; (8) TEC (Tec protein tyrosine kinase) required for T cell receptor activation, IL-2 and IL-17 induction, myeloid cell growth and differentiation, activation of mast cells on high-affinity IgE receptor stimulation, and regulation of neutrophil activation and degranulation44–49 and (9) ADAMTS12 (a disintegrin and metalloproteinase with thrombospondin type 1 motif 12) an asthma susceptibility gene that is protective against airway eosinophilic inflammation and hyperresponsiveness and is induced by glucocorticoids in lung fibroblasts.50–52
Table 3.
Demographics of the subjects in GERA and MGB Biobank analysed for the asthma-related exacerbation outcome
| GERA (n=4541) |
MGB biobank (n=505) |
|||||
|---|---|---|---|---|---|---|
| Cases (n=646) | Controls (n=3895) | P value | Cases (n=202) | Controls (n=303) | P value | |
| Age (years, mean±SD) | 62.6 (±14.2) | 62.8 (±14.5) | 0.5 | 59.4 (±16.0) | 56.9 (±16.6) | 0.1 |
|
| ||||||
| Female sex | 502 (77.7%) | 2686 (69.0%) | <0.001 | 130 (64.4%) | 194 (64.0%) | 0.94 |
|
| ||||||
| Smoking status | 0.58 | 0.23 | ||||
|
| ||||||
| Never | 387 (61.6%) | 2282 (60.5%) | 61 (60.4%) | 92 (52.9%) | ||
|
| ||||||
| Current or former | 241 (38.4%) | 1492 (39.5%) | 40 (39.6%) | 82 (47.1%) | ||
|
| ||||||
| BMI (kg/m2, mean±SD) | 29.6 (±6.2) | 28.7 (±6.0) | <0.001 | 31.2 (±8.9) | 28.4 (±6.9) | 0.01 |
|
| ||||||
| Co-morbidities | ||||||
|
| ||||||
| Allergic rhinitis | 300 (46.4%) | 1659 (42.6%) | 0.07 | 137 (67.8%) | 164 (54.1%) | 0.002 |
|
| ||||||
| GERD | 216 (33.4%) | 1053 (27.0%) | <0.001 | 133 (65.8%) | 170 (56.1%) | 0.03 |
|
| ||||||
| Concurrent asthma medications* | ||||||
|
| ||||||
| Long-acting β-agonists | 55 (8.5%) | 187 (4.8%) | <0.001 | 116 (57.4%) | 137 (45.2%) | 0.01 |
|
| ||||||
| Antileukotrienes | 31 (4.8%) | 101 (2.6%) | <0.05 | 66 (32.7%) | 84 (27.7%) | 0.23 |
|
| ||||||
| Anticholinergics | 14 (2.2%) | 61 (1.6%) | 0.27 | 55 (27.2%) | 41 (13.5%) | <0.001 |
|
| ||||||
| Biologics* | – | – | – | 8 (4.0%) | 3 (1.0%) | 0.03 |
In MGB Biobank, omalizumab and mepolizumab only.
BMI, body mass index; GERA, Genetic Epidemiology Research on Adult and Aging; MGB, Mass General Brigham.
Pathway analysis of the validated SNPs demonstrated an enrichment of pathways related to protein and fatty acid metabolism, toll-like receptor signalling, antigen cross-presentation, cell cycle control and vesicle-mediated transport (online supplemental table 17), and 130 SNPs annotated to epigenomic regulatory elements in relevant tissues and cell types (online supplemental table 18). Furthermore, 28 SNPs were meQTL for 11 genes (online supplemental table 19), including: (1) PTPRS (protein tyrosine phosphatase receptor type S) which encodes an inhibitory receptor on human plasmacytoid dendritic cells and is involved in the developmental regulation of the late fetal and neonatal lung53 54; (2) GM2A (GM2 ganglioside activator) a biomarker for COPD55; (3) SNW1 (SNW domain containing 1) a vitamin D receptor and nuclear receptor coactivator that enhances glucocorticoid-mediated gene transcription56; and the aforementioned (4) CACNA1C. Fourteen SNPs were human lung eQTF for DPH1, RPS10, and CTBP1-AS2 (online supplemental table 20). Both rs1051322 and rs55815331 on chromosome 17 were cis-meQTL and eQTL for DPH1, suggesting a role of these variants in regulating both methylation and gene expression. All of the validated SNPs were novel for asthma, asthma corticosteroid response, and additional traits examined in the NHGRI-EBI GWAS Catalogue.
DISCUSSION
Older adults are at risk for higher morbidity and mortality due to asthma, and resistance ICS treatment further compounds these poor outcomes.6 7 In this large GWAS of older adult asthmatics of European ancestry, several novel genetic variants associated with ICS treatment response were identified in three population-based cohorts. Poor ICS response was measured as asthma exacerbations on ICS using two methods, OCS bursts from prescription data and asthma-related exacerbations from ICD diagnosis codes. Four novel SNPs on chromosome 6 (rs138717703, rs77506063, rs116023293 and rs145325916) near PTCHD4 were significantly associated at the genome-wide level with OCS bursts despite ICS treatment, and although the minor allele frequencies were low, each copy of the minor allele conferred a large effect size, increasing the risk of asthma exacerbations by 1.48 to 3.34 times. PTCHD4 encodes the protein patched domain containing four that represses hedgehog signaling.57 PTCHD4 is upregulated in atopic asthma, chronic rhinosinusitis with nasal polyps, and chronic hypersensitivity pneumonitis and enriched in differentially methylated CpG sites associated with COPD, and our results demonstrate a novel association of PTCHD4 with poor ICS response in older adult asthmatics.58–61 These four genome-wide significant SNPs also annotated to histone modifications in the adrenal gland, immune cells (monocytes and T helper 17 cells), and mesenchymal and endodermal cells, suggesting a role in regulating chromatin configurations in cell types relevant to corticosteroid response in asthma.
In the discovery cohort GERA, rs7247485 near ZNF536 met genome-wide significance for the OCS burst outcome, but the direction of effect replicated in only MGB Biobank and not the Rotterdam Study (online supplemental table 1). ZNF536 (zinc finger protein 536) expression has been shown to be downregulated in non-severe asthmatics after treatment with dexamethasone.62 Furthermore, rs140187287 and rs141689337 near ADAMTS17 met genome-wide significance for the asthma-related exacerbation outcome in GERA, but these two SNPs were not present in the MGB Biobank for replication (online supplemental table 11). ADAMTS17 (a disintegrin and metalloproteinase with thrombospondin type 1 motif 17) is involved in maintenance of the extracellular matrix, and its expression is upregulated in CD4+ T cells and associated with respiratory symptoms in subjects with dust mite rhinitis.63 Further replication of these loci are needed to draw conclusions about their role in regulating ICS response in older adult asthmatics.
On global meta-analysis, 116 SNPs with suggestive statistical significance (p<5 × 10−5) in GERA validated across MGB Biobank and the Rotterdam Study for the OCS burst outcome, including the four genome-wide significant variants in PTCHD4. Several of the validated SNPs annotated to genes previously associated with lung function (ANK3, CPED1), COPD (ANK3), immune regulation (PRKCB, NCKAP1L, CRADD), bronchial epithelial cell membrane biogenesis and adhesion (ANK3, DST), and eosinophilia (CRADD).22–28 In addition, most of the SNPs annotated to epigenomic regulatory elements, meQTL and/or eQTL suggesting potential functional roles. Twenty-one SNPs on chromosome 6 were lung eQTL for DST which encodes dystonin, a cytoskeletal and cell junction cross-linking protein that is overexpressed in combined pulmonary fibrosis and emphysema and may represent a marker of pulmonary disease severity.64 65
For the asthma-related exacerbation outcome, 152 SNPs with suggestive statistical significance in GERA were validated in MGB Biobank, but none met genome-wide significance on meta-analysis. Several of the SNPs annotated to genes previously associated with asthma (CD36, ADAMTS12), airway eosinophilic inflammation and hyperresponsiveness (ADAMTS12), bronchiole relaxation and enhancement of bronchodilator therapy (KCNQ3), COPD (MMP16, GM2A), chronic hypoxia (CACNA1C), control of respiratory cilia movement (DNAH11, ARMC4), excitability of vagal airway C-fibres and sodium flux across lung epithelial cells (KCNQ3), dust mite allergy (CD36), atopic dermatitis (TEC), histamine hypersensitivity (CACNA1C), mast cell activation (TEC), B cell activation and proliferation (KDM4C), T cell and neutrophil activation (TEC), bronchial epithelial cell and macrophage phagocytosis (CD36), and glucocorticoid signalling (ADAMTS12, SNW1).31–38 41–45 48–52 55 56 66–69 Fourteen SNPs were lung eQTL for three genes: DPH1 (diphthamide biosynthesis protein 1), RPS10 (ribosomal protein S10), and CTBP1-AS2 (C-terminal-binding protein 1 divergent transcript). DPH1 has been associated with diisocyante-induced asthma.70 RPS10 is a gene regulatory network node in children with asthma, and its expression is positively regulated by IL-6 and soluble IL-6 receptor in bronchial epithelial cells and negatively regulated by IL-4 in serum macrophages of asthmatics.71–73 Stratification at an age cut-off of 65 years identified 32 out of 45 SNPs that met genome-wide significance in subjects≥age 65 years that annotated to TPD52L1 (tumour protein D52 like 1). TPD52L1 is downregulated in more severe stages of COPD, upregulated by IL-6 in human bronchial epithelial cells, and upregulated in postinjury repair of human airway epithelial cells.72 74 75 Additional studies are needed in the future to confirm the effects of these SNPs on ICS response in adults≥age 65 years.
Our results detected large effect sizes associated with ICS response in older asthmatics, in addition to novel associations of ICS response with several genes previously associated with susceptibility to asthma and other airway and allergic diseases. Because a prior asthma exacerbation is the strongest predictor of a future exacerbation despite asthma controller medication use, subjects who had an asthma exacerbation prior to their first ICS prescription were excluded, and all analyses adjusted for the prescribed use of other asthma medications to further reduce confounding by asthma severity and indication.76 GERA is based on claims data which includes medication fills, whereas MGB Biobank and the Rotterdam Study only had prescription data. In the latter, prescriptions not filed through the electronic medical record (eg, called in by a clinician) would be missed. Additionally, we were unable to address different doses of ICS as this information was not in the datasets. Most prior GWASes on ICS response measured improvement in lung function and airway responsiveness, but these outcomes were not available across the cohorts in this study.77 Genetic polymorphisms associated with ICS response vary by the outcome measured, and the differences in how the two outcomes in this study were derived (ie, using prescription data with or without ICD diagnosis codes) could explain for the absence of overlap in the top SNPs.78
This study was limited in only including individuals of European-ancestry due to sample size constraints, and further research and replication across other races and ethnicities are needed. The definition of asthma in GERA included both physician-diagnosed and self-reported asthma, which may have led to the inclusion of more subjects with mild vs moderate or severe persistent asthma compared with MGB Biobank and the Rotterdam Study. Furthermore, prescription dispensing from GERA was only available for outpatient and emergency department visits, which may not have captured more severe inpatient asthma exacerbations. Electronic medical record data are susceptible to misclassification bias, which we attempted to minimise by stringent inclusion and exclusion criteria for phenotype classification. Furthermore, prescription data from electronic medical records may not fully reflect actual drug adherence and utilisation, and the time-to-event analytical approach used does not account for the time varying effects of ICS and other asthma controller treatments. These limitations may have led to unmeasured confounding of our results. Due to relatively small sample sizes in MGB Biobank and the Rotterdam Study (each were only 10% the size of GERA), only the top SNPs from GERA underwent validation in these cohorts, and it is possible that if independent GWASes were performed in each cohort, additional suggestive or genome-wide significant SNPs would have been identified on meta-analysis. Finally, functional annotation using standard databases was performed, and the inferred findings need further validation in older adult asthmatics.
The burden of asthma is highest in older adults who also have the poorest outcomes, and few studies have focused on the pharmacogenetics of ICS treatment response in the ageing adult population.6 7 77 This study identified multiple novel SNPs associated with exacerbations in older adults with asthma on ICS treatment and discovered new associations with ICS response for several genes previously linked to asthma and other airway and allergic diseases. These identified SNPs may potentially serve as biomarkers to guide the future individualised treatment of asthma in older adults. Our results also inferred complex genetic, epigenetic and transcriptomic interactions regulating ICS treatment response in older individuals with asthma, and future investigations of these complex genomic interactions may help reveal the biological mechanisms of ICS response and lead to the development of more effective treatments for asthma in older adults.
Supplementary Material
Key messages.
What is already known on this topic
Older adults with asthma carry the highest burden of disease with the poorest outcomes but remain understudied. Inhaled corticosteroids remain one of the primary treatments for the control of asthma. However, the pharmacogenetics of inhaled corticosteroid treatment response in older adult asthmatics is not well understood.
What this study adds
Multiple novel genetic variants associated with poor treatment response to inhaled corticosteroids were identified across three population-based cohorts of older adults with asthma. Four single-nucleotide polymorphisms near PTCHD4 met genome-wide significance as predictors of oral corticosteroid bursts in older asthmatics on inhaled corticosteroids. Functional annotation of the identified single-nucleotide polymorphisms inferred complex genetic, epigenetic and transcriptomic interactions regulating inhaled corticosteroid treatment response.
How this study might affect research, practice and/or policy
This study identified genetic predictors of inhaled corticosteroid treatment response that may help guide the individualised treatment of asthma in older adults in the future. Further research into these genetic variants may increase our understanding of the biological mechanisms regulating inhaled corticosteroid treatment response in older individuals with asthma.
Acknowledgements
The Mass General Brigham Biobank is supported by Mass General Brigham Personalised Medicine. Within the Rotterdam study, E. de Roos and B. Stricker are acknowledged for the validation of asthmatics and medication data respectively, and the study participants, staff from the Rotterdam Study, and participating general practitioners and pharmacists are thanked for making scientific research possible. The GTEx Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Funding
NIH R01 HL127332, R01 HL129935, R01 HL139634, R01 NR013391, R01 HL152244, U01 HL65899, P01 HL132825, K01 HL125858, K23 HL151819, Thrasher Research Fund Award #15115, European Respiratory Society (LTRF 201801-00302). The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organisation for Scientific Research (NWO); the Netherlands Organisation for Health Research and Development (ZonMW); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative; the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
Footnotes
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/thoraxjnl-2021-217674).
Competing interests None declared.
Patient consent for publication Not applicable.
Ethics approval GERA was approved by the institutional review boards at KPNC and Brigham and Women’s Hospital (2002P000331). Use of the MGB Biobank was approved by the institutional review board at Brigham and Women’s Hospital (2014P001109). The Rotterdam Study was approved by the Medical Ethics Committee of Erasmus Medical Center (MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, licence number 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC171200112.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data from the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort may be obtained by contacting the the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC), Research Program on Genes, Environment and Health (rpgeh-collab@kp.org). Data from the Mass General Brigham (MGB) Biobank can be obtained by contacting biobank@partners.org. Data from the Rotterdam Study can be obtained by contacting the Department of Epidemiology at Erasmus University Medical Center via telephone 0031 (0)10 704 34 88 or through the contact form on their website.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report 2011;32:1–14. [PubMed] [Google Scholar]
- 2.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11:404–6. [DOI] [PubMed] [Google Scholar]
- 3.Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol 2002;109:410–8. [DOI] [PubMed] [Google Scholar]
- 4.Tantisira KG, Lake S, Silverman ES, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet 2004;13:1353–9. [DOI] [PubMed] [Google Scholar]
- 5.Davis JS, Weiss ST, Tantisira KG. Asthma pharmacogenomics: 2015 update. Curr Allergy Asthma Rep 2015;15:42. [DOI] [PubMed] [Google Scholar]
- 6.Global Asthma Network. The global asthma report 2018. Auckland, New Zealand: Global Asthma Network, 2018. [Google Scholar]
- 7.Busse PJ, McDonald VM, Wisnivesky JP, et al. Asthma across the ages: adults. J Allergy Clin Immunol Pract 2020;8:1828–38. [DOI] [PubMed] [Google Scholar]
- 8.Kvale MN, Hesselson S, Hoffmann TJ, et al. Genotyping informatics and quality control for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics 2015;200:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlin A, Sordillo JE, Ziniti J, et al. Large-Scale, multiethnic genome-wide association study identifies novel loci contributing to asthma susceptibility in adults. J Allergy Clin Immunol 2019;143:1633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlson EW, Boutin NT, Hoffnagle AG, et al. Building the partners healthcare Biobank at partners personalized medicine: informed consent, return of research results, recruitment lessons and operational considerations. J Pers Med 2016;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu S, Liao KP, Shaw SY, et al. Toward high-throughput phenotyping: unbiased automated feature extraction and selection from knowledge sources. J Am Med Inform Assoc 2015;22:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Roos EW, Lahousse L, Verhamme KMC, et al. Asthma and its comorbidities in middle-aged and older adults; the Rotterdam study. Respir Med 2018;139:6–12. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Forer L, Schönherr S, et al. Next-Generation genotype imputation seivice and methods. Nat Genet 2016;48:1284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CC, Chow CC, Tellier LC, et al. Second-Generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willer CJ, Li Y, Abecasis GR. Metal: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26:2190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010;26:2336–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oscanoa J, Sivapalan L, Gadaleta E, et al. SNPnexus: a web server for functional annotation of human genome sequence variation (2020 update). Nucleic Acids Res 2020;48:W185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaunt TR, Shihab HA, Hemani G, et al. Systematic identification of genetic influences on methylation across the human life course. Genome Biol 2016;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonder MJ, Luijk R, Zhernakova DV, et al. Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet 2017;49:131–8. [DOI] [PubMed] [Google Scholar]
- 21.Buniello A, MacArthur JAL, Cerezo M, et al. The NHGRI-EBI GWAS catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res 2019;47:D1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansel NN, Ruczinski I, Rafaels N, et al. Genome-Wide study identifies two loci associated with lung function decline in mild to moderate COPD. Hum Genet 2013;132:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizhatil K, Yoon W, Mohler PJ, et al. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem 2007;282:2029–37. [DOI] [PubMed] [Google Scholar]
- 24.Ondee T, Jaroonwitchawan T, Pisitkun T, et al. Decreased protein kinase C-β type II associated with the prominent endotoxin exhaustion in the macrophage of FcGRIIb−/− lupus prone mice is revealed by phosphoproteomic analysis. Int J Mol Sci 2019;20:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venneri MA, Hasenmajer V, Fiore D, et al. Circadian rhythm of glucocorticoid administration Entrains clock genes in immune cells: a dream trial ancillary study. J Clin Endocrinol Metab 2018;103:2998–3009. [DOI] [PubMed] [Google Scholar]
- 26.Castro CN, Rosenzwajg M, Carapito R, et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. J Exp Med 2020;217:e20192275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook SA, Comrie WA, Poli MC, et al. HEM1 deficiency disrupts mTORC2 and F-actin control in inherited immunodysregulatory disease. Science 2020;369:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minelli C, Dean CH, Hind M, et al.Association of forced vital capacity with the developmental gene NCOR2. PLoS One 2016;11:e0147388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao H, Liu Y, Veach RA, et al. The adaptor CRADD/RAIDD controls activation of endothelial cells by prainflammatory stimuli. J Biol Chem 2014;289:21973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakiela B, Szczeklik W, Sokolowska B, et al. Intrinsic pathway of apoptosis in peripheral blood eosinophils of Churg-Strauss syndrome. Rheumatology 2009;48:1202–7. [DOI] [PubMed] [Google Scholar]
- 31.Brueggemann LI, Haick JM, Neuburg S, et al. Kcnq (Kv7) potassium channel activators as bronchodilators: combination with a β2-adrenergic agonist enhances relaxation of rat airways. Am J Physiol Lung Cell Mol Physiol 2014;306:L476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B, Liang H, Liu L, et al. Phosphatidylinositol 4,5-bisphosphate hydrolysis mediates histamine-induced KCNQ/M current inhibition. Am J Physiol Cell Physiol 2008;295:C81–91. [DOI] [PubMed] [Google Scholar]
- 33.Schultz R, Elenius V, Lukkarinen H, et al. Two novel mutations in the DNAH11 gene in primary ciliary dyskinesia (CILD7) with considerable variety in the clinical and beating cilia phenotype. BMC Med Genet 2020;21:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onoufriadis A, Shoemark A, Munye MM, et al. Combined exome and whole-genome sequencing identifies mutations in ARMC4 as a cause of primary ciliary dyskinesia with defects in the outer dynein arm. J Med Genet 2014;51:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyler AL, Raza A, Krementsov DN, et al. Network-Based functional prediction augments genetic association to predict candidate genes for histamine hypersensitivity in mice. G3 2019;9:4223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan J, Yamamura A, Zimnicka AM, et al. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol 2013;305:L154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung K-H, Woo YH, Lin I-Y, et al. The KDM4A/KDM4C/NF-κB and WDR5 epigenetic cascade regulates the activation of B cells. Nucleic Acids Res 2018;46:5547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Wang J, Zheng Z, et al. A protective polymorphism in MMP16, improved blood gas levels, and chronic obstructive pulmonary diseases: family and two population-based studies. Hum Mutat 2020;41:1280–97. [DOI] [PubMed] [Google Scholar]
- 39.Hadchouel A, Decobert F, Franco-Montoya M-L, et al. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS One 2008;3:e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drysdale SB, Prendergast M, Alcazar M, et al. Genetic predisposition of RSV infection-related respiratory morbidity in preterm infants. Eur J Pediatr 2014;173:905–12. [DOI] [PubMed] [Google Scholar]
- 41.Sexton DW, Al-Rabia M, Blaylock MG, et al. Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin Exp Allergy 2004;34:1514–24. [DOI] [PubMed] [Google Scholar]
- 42.Patel PS, Kearney JF. Cd36 and platelet-activating factor receptor promote house dust mite allergy development. J Immunol 2017;199:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torregrosa Paredes P, Esser J, Admyre C, et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 2012;67:911–9. [DOI] [PubMed] [Google Scholar]
- 44.Yang WC, Ching KA, Tsoukas CD, et al. Tec kinase signaling in T cells is regulated by phosphatidylinositol 3-kinase and the Tec pleckstrin homology domain. J Immunol 2001;166:387–95. [DOI] [PubMed] [Google Scholar]
- 45.Altman A, Kaminski S, Busuttil V, et al. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol 2004;34:2001–11. [DOI] [PubMed] [Google Scholar]
- 46.Boucheron N, Sharif O, Schebesta A, et al. The protein tyrosine kinase Tec regulates a CD44highCD62L-Th17 subset. J Immunol 2010;185:5111–9. [DOI] [PubMed] [Google Scholar]
- 47.Miyazato A, Yamashita Y, Hatake K, et al. Tec protein tyrosine kinase is involved in the signaling mechanism of granulocyte colony-stimulating factor receptor. Cell Growth Differ 1996;7:1135–9. [PubMed] [Google Scholar]
- 48.Ellmeier W, Abramova A, Schebesta A. Tec family kinases: regulation of FcεRI-mediated mast-cell activation. Febs J 2011;278:1990–2000. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes MJG, Lachance G, Paré G, et al. Signaling through CD16b in human neutrophils involves the Tec family of tyrosine kinases. J Leukoc Biol 2005;78:524–32. [DOI] [PubMed] [Google Scholar]
- 50.Kurz T, Hoffjan S, Hayes MG, et al. Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J Allergy Clin Immunol 2006;118:396–402. [DOI] [PubMed] [Google Scholar]
- 51.Paulissen G, El Hour M, Rocks N, et al. Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12. J Immunol 2012;189:4135–43. [DOI] [PubMed] [Google Scholar]
- 52.Short KL, Bird AD, Seow BKL, et al. Glucocorticoid signalling drives reduced versican levels in the fetal mouse lung. J Mol Endocrinol 2020;64:155–64. [DOI] [PubMed] [Google Scholar]
- 53.Bunin A, Sisirak V, Ghosh HS, et al. Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine plasmacytoid dendritic cells. Immunity 2015;43:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim H, Yeger H, Han R, et al. Expression of LAR-PTP2 in rat lung is confined to proliferating epithelia lining the airways and air sacs. Am J Physiol 1996;270:L566–76. [DOI] [PubMed] [Google Scholar]
- 55.Maghsoudloo M, Azimzadeh Jamalkandi S, Najafi A, et al. Identification of biomarkers in common chronic lung diseases by co-expression networks and drug-target interactions analysis. Mol Med 2020;26:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baudino TA, Kraichely DM, Jefcoat SC, et al. Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem 1998;273:16434–41. [DOI] [PubMed] [Google Scholar]
- 57.Chung JH, Larsen AR, Chen E, et al. A PTCH1 homolog transcriptionally activated by p53 suppresses hedgehog signaling. J Biol Chem 2014;289:33020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forno E, Zhang R, Jiang Y, et al. Transcriptome-Wide and differential expression network analyses of childhood asthma in nasal epithelium. J Allergy Clin Immunol 2020;146:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Y, Zi X-X, Tian T-F, et al. Whole-transcriptome sequencing reveals heightened inflammation and defective host defence responses in chronic rhinosinusitis with nasal polyps. Eur Respir J 2019;54:1900732. [DOI] [PubMed] [Google Scholar]
- 60.Horimasu Y, Ishikawa N, Iwamoto H, et al. Clinical and molecular features of rapidly progressive chronic hypersensitivity pneumonitis. Sarcoidosis Vasc Diffuse Lung Dis 2017;34:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrow JD, Cho MH, Hersh CP, et al. Dna methylation profiling in human lung tissue identifies genes associated with COPD. Epigenetics 2016;11:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Austin PJ, Tsitsiou E, Boardman C, et al. Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J Allergy Clin Immunol 2017;139:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones AC, Anderson D, Troy NM, et al. Rewiring of gene networks underlying mite allergen-induced CD4 + Th-cell responses during immunotherapy. Allergy 2020;75:2330–41. [DOI] [PubMed] [Google Scholar]
- 64.Künzli K, Favre B, Chofflon M, et al. One gene but different proteins and diseases: the complexity of dystonin and bullous pemphigoid antigen 1. Exp Dermatol 2016;25:10–16. [DOI] [PubMed] [Google Scholar]
- 65.Hanaoka M, Ito M, Droma Y, et al. Comparison of gene expression profiling between lung fibrotic and emphysematous tissues sampled from patients with combined pulmonary fibrosis and emphysema. Fibrogenesis Tissue Repair 2012;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawakami Y, Yumoto K, Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int 2007;56:403–9. [DOI] [PubMed] [Google Scholar]
- 67.Moodley Y, Rigby P, Bundell C, et al. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol 2003;162:771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun H, Lin A-H, Ru F, et al. KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity. JCI Insight 2019;4:e124467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenwood IA, Yeung SYM, Hettiarachi S, et al. KCNQ-encoded channels regulate Na+ transport across H441 lung epithelial cells. Pflugers Arch 2009;457:785–94. [DOI] [PubMed] [Google Scholar]
- 70.Yucesoy B, Kaufman KM, Lummus ZL, et al. Genome-Wide association study identifies novel loci associated with diisocyanate-induced occupational asthma. Toxicol Sci 2015;146:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C, Li H, Cao L, et al. Identification of differentially expressed genes associated with asthma in children based on the bioanalysis of the regulatory network. Mol Med Rep 2018;18:2153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jevnikar Z, Östling J, Ax E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol 2019;143:577–90. [DOI] [PubMed] [Google Scholar]
- 73.Martinez FO, Helming L, Milde R, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013;121:e57–69. [DOI] [PubMed] [Google Scholar]
- 74.Singh D, Fox SM, Tal-Singer R, et al. Induced sputum genes associated with Spirometric and radiological disease severity in COPD ex-smokers. Thorax 2011;66:489–95. [DOI] [PubMed] [Google Scholar]
- 75.Heguy A, Harvey B-G, Leopold PL, et al. Responses of the human airway epithelium transcriptome to in vivo injury. Physiol Genomics 2007;29:139–48. [DOI] [PubMed] [Google Scholar]
- 76.Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008;122:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keskin O, Farzan N, Birben E, et al. Genetic associations of the response to inhaled corticosteroids in asthma: a systematic review. Clin Transl Allergy 2019;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers AJ, Tantisira KG, Fuhlbrigge AL, et al. Predictors of poor response during asthma therapy differ with definition of outcome. Pharmacogenomics 2009;10:1231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data from the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort may be obtained by contacting the the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC), Research Program on Genes, Environment and Health (rpgeh-collab@kp.org). Data from the Mass General Brigham (MGB) Biobank can be obtained by contacting biobank@partners.org. Data from the Rotterdam Study can be obtained by contacting the Department of Epidemiology at Erasmus University Medical Center via telephone 0031 (0)10 704 34 88 or through the contact form on their website.


