Abstract
Cutaneous melanoma remains the most lethal of the primary cutaneous neoplasms, and although the incidence of primary melanoma continues to rise, the mortality from metastatic disease remains unchanged, in part through advances in treatment. Major developments in immunomodulatory and targeted therapies have provided robust improvements in response and survival trends that have transformed the clinical management of patients with metastatic melanoma. Additional advances in immunologic and cancer cell biology have contributed to further optimization in (1) risk stratification, (2) prognostication, (3) treatment, (4) toxicity management, and (5) surveillance approaches for patients with an advanced melanoma diagnosis. In this review, we provide a comprehensive overview of the historical and future advances regarding the translational and clinical implications of advanced melanoma and share multidisciplinary recommendations to aid clinicians in the navigation of current treatment approaches for a variety of patient cohorts.
INTRODUCTION
Although the incidence of primary cutaneous melanoma has steadily increased for several decades and remains the most lethal of the primary cutaneous neoplasms, the 3-year overall survival (OS) rates have remained relatively constant from 26.4% to as low as 4.7% across the subcategories of stage IV metastatic disease from 2004 to 2009.1,2 As of 2018, the SEER database estimated that the 5-year survival rate is 29.8% in those with stage IV disease at the time of diagnosis in the United States.3 Noncutaneous forms of melanoma, including mucosal and ocular subtypes, classically portend an even worse prognosis.4 In 2021, it is estimated that there will be 106,110 new cases of invasive melanoma with 7,180 melanoma-related deaths in the United States, and according to GLOBOCAN for 2020, there were 324,635 cases of melanoma worldwide, representing 1.7% of all cancers and 57,043 melanoma deaths or 0.6% of cancer-related mortality.5
Once considered among the most refractory of cancers to traditional modes of therapy including chemotherapy, radiation therapy, and the early days of targeted therapy, the clinical and therapeutic approaches toward patients with melanoma have witnessed dramatic improvements in cancer cell biology and immunology over the past decade. Striking responses to novel targeted and immunotherapies, with resultant improvements in both quality of life and OS, have substantially altered our approach to patients with metastatic melanoma. In this review, we will provide oncologists with a navigational map to approach this powerful and growing armamentarium.
THE ROLE OF TARGET-SPECIFIC MOLECULAR TESTING
The v-raf murine sarcoma viral oncogene homolog B1 (BRAF) is a serine/threonine protein kinase that was described in 1983 as an oncogene playing a critical role in the RAS-RAF-MEK-ERK mitogen–activated protein kinase (MAPK) cell signaling pathway, with high frequencies of BRAF point mutations discovered in melanoma and other human cancers.6,7 The activating BRAF mutations observed in 37%-53% of patients with cutaneous malignant melanoma were identified as a therapeutic target early on with sorafenib, the first small-molecule inhibitor believed to be directed at BRAF, entering preliminary clinical trials in 2000. Three isoforms of RAF (A, B, and C) are now recognized, of which sorafenib principally targets CRAF. Therefore, randomized phase III studies with sorafenib failed to show benefit in patients with metastatic melanoma harboring BRAF mutations.8,9 Further investigation has appreciated the most common BRAF mutation to occur at the 600th position, the enzymatic pocket of the molecule, where it substitutes a glutamic acid (E) for a valine (V). The first second-generation BRAF inhibitor targeting BRAFV600E, PLX4032 (vemurafenib), remarkably provided rapid and deep responses in patients with metastatic melanoma and swiftly led to a foundational change in the therapeutic approach toward the inhibition of mutated, activated BRAF in metastatic melanoma.10 Although skin toxicity with induction of keratoacanthomas was noted, it subsequently became evident that this was related to unopposed CRAF activation, which abates upon the addition of downstream MEK inhibitors. Interestingly, the BRAFV600E mutation is not a UV-driven mutation and is also seen in the molecular profile of atypical benign nevi. Furthermore, although BRAFV600E remains the most common mutation, representing about 40% of melanomas, other rarer mutations in BRAF have also been identified. The current portfolio of BRAF inhibitors (vemurafenib, dabrafenib, and encorafenib) all targets the BRAFV600E pocket and is most active in tumors with this mutation. In tumors with rarer activating mutations such as the substitution of lysine (K) and aspartic acid (D) for valine, BRAF inhibitors exhibit reduced activity, although still maintaining therapeutic relevance.11
Immediately downstream of RAF in the MAPK pathway is MEK, for which constitutively activated mutations have been observed in approximately 8% of patients with cutaneous malignant melanoma.12 The first MEK inhibitor, trametinib, exhibited an OS benefit in patients with BRAF-mutant metastatic melanoma.13 The combination of BRAF and MEK inhibition with dabrafenib and trametinib was then observed to provide a progression-free survival (PFS) benefit compared with dabrafenib monotherapy for patients with advanced melanoma harboring BRAF V600E or V600K mutations, with additional observations to include similar tolerance and lower rates of cutaneous eruptions within the combination group likely by avoiding the aforementioned paradoxical MAPK activation and resultant dermatologic reactions with BRAF inhibitor monotherapy.14 There are currently three approved BRAF and MEK inhibitor combinations including dabrafenib plus trametinib, vemurafenib plus cobimetinib, and encorafenib plus binimetinib, all of which possess a comparable 18-month PFS range of 30%-40% with their own unique side effect profiles.15
Mutations of KIT, a proto-oncogene encoding a type III transmembrane receptor tyrosine kinase (c-KIT) with known expression during normal melanocyte development and resultant induction of both MAPK and phosphoinositide 3-kinase/AKT pathways upon constitutive activation via amplification or activating mutations, are commonly observed in melanomas arising from mucosal (39%), acral (36%), and chronically sun-damaged skin (28%).16 Interestingly, although only 5%-10% of KIT mutations are generally observed in patients with all melanoma subtypes, these mutations rarely occur in conjunction with BRAF or NRAS mutations and are typically not observed in cutaneous melanomas arising in the absence of chronic sun damage.17 KIT-targeted agents such as imatinib have been shown to provide benefit to patients whose tumor harbors KIT mutations, but not overexpression.18
Although the RAS oncogene, with its associated activating mutations within Ras GTPase proteins and downstream effects on MAPK and phosphoinositide 3-kinase/AKT pathways, has been well-documented within a variety of cancer types, the neuroblastoma ras viral oncogene homolog (NRAS) remains the predominantly mutated isoform in patients with melanoma with mutation rates of approximately 13%-25%.19 Clinically, NRAS mutations appear to be most commonly associated with elderly (age > 55 years) and chronically UV exposed patient cohorts and are related to a more aggressive prognosis including higher rates of visceral and CNS metastatic involvement.19,20 Treatment for patients with advanced melanoma harboring NRAS mutations is well outlined in a recent review19 and remains relatively controversial, as no clear targeted therapies are yet to exhibit clinical benefit; prospective utilization of downstream MEK inhibitors has failed to achieve OS benefits.19,21 To date, patients with a variety of melanoma subtypes harboring NRAS mutations appear to exhibit equivalent and potentially enhanced OS rates compared with NRAS wildtype cohorts after treatment with single-agent and combination immune checkpoint inhibitor (ICI) regimens, and further prospective efforts regarding biomarker and targeted NRAS therapeutic options are ongoing.19,22
Several additional genetic mutations in patients with melanoma, which currently lack direct targeted therapeutic agents, have been observed. Mutations in the NF1 gene with resultant loss of negative regulatory mechanisms in RAS-related MAPK pathways have exhibited high mutational rates of 12%-18% in patients with melanoma (45%-93% in desmoplastic clinicopathologic subtypes) and are now postulated to be the third most commonly observed driver mutation in these patients behind BRAF and NRAS, respectively.23 Alternative mutations in the PTEN tumor suppressor gene appear to be coexpressed in patients harboring BRAF mutations and may enhance tumor cell immune evasion via a variety of mechanisms independent of MAPK pathways including downregulation of antitumoral immune cells within the tumor microenvironment (TME) and tertiary lymphoid structures, thus contributing to suboptimal responses observed in subsets of patients receiving standard immune and targeted therapies.24 Another mutation of interest includes activating mutations of Pleckstrin homology domain interacting protein, which appears to promote metastatic transition in melanoma cells and has gained attention for potential targeted therapeutics given its unique molecular structure and predominance of mutations preserved to patients who are BRAF wildtype.25 It should be noted that this set of additional mutations are yet to provide clinically significant therapeutic targets and therefore are not routinely tested unless available in the setting of a clinical trial.
There are also several immunologic and genetic analytic modalities proven to provide clinical utility in a variety of solid tumor subtypes that have gained attention for patients with melanoma. For example, a high tumor mutational burden (TMB), although well established as a positive predictive biomarker for immunotherapeutic response in nonmelanoma solid tumor subtypes because of enhanced immunogenic neoantigen presentation and subsequent recognition by therapy-directed activated host T cells, is yet to clearly exhibit convincing clinical utility in guiding definitive treatment planning for patients with melanoma. However, combining additional modalities with TMB analytics may provide clinical benefits and is therefore under active investigation. For example, circulating tumor DNA (ctDNA) has been introduced as a predictive biomarker for patients with melanoma, postulating that persistent elevations of ctDNA during early phases of standard immunomodulating therapies exhibit a worse prognosis, with the highest rates of disease progression observed in patients harboring mutations in BRAF or NRAS.26 Encouraging prospective observations of combining TMB and ctDNA in patients with melanoma exhibited enhanced response and survival rates in those with high TMB tumors who also exhibited enhanced ctDNA clearance during early stages of treatment, for which further investigation and validation are anticipated.27 Additional immunologic modalities involving quantitative expression of programmed death ligand 1 (PD-L1), although beneficial in the prognosis and treatment for a variety of various solid tumor types, have only exhibited trends in OS benefits for patients with melanoma who possess high PD-L1 expression and therefore are yet to provide sufficient evidence of their clinical utility.28,29 Finally, tumor-infiltrating lymphocytes (TILs) provide a histologic reflection of host immune response within the TME, providing valuable prognostic and therapeutic insights into a variety of tumor types including melanoma.30 However, given the diverse pathologic subtypes of melanoma in addition to largely heterogenous TIL populations that include therapeutically desired cytotoxic T cells and combinations of undesired immune-dampening regulatory T cells (Tregs) and exhausted T-cell populations, a larger investment in single-cell analyses to better categorize the prognostic and therapeutic utility of TILs in patients with melanoma will likely provide substantial advances in treatment monitoring and future therapeutics.
THERAPEUTIC RECOMMENDATIONS FOR METASTATIC MELANOMA
Prognostic Considerations
In 2018, the eighth edition of the American Joint Committee on Cancer staging system for cutaneous melanoma outlined several revisions to aid in the clinical and prognostic management of this patient population. This included the incorporation of microsatellite, satellite, or in-transit metastases within the eight T subcategories and defining four separate distant metastatic (M) categories on the basis of the site of involvement.31 These changes have aided in further risk stratification and treatment planning, as metastatic involvement of viscera outside of the CNS, designated as M1c, and CNS-involved disease (M1d) are associated with a poorer prognosis than those with distant metastatic disease of the skin and soft tissue (M1a) and lung (M1b), respectively. Lactate dehydrogenase (LDH) was also included in each metastatic subgroup (designated as 1 or 0 if elevated or not, respectively), given the strong evidence of this biomarker to serve as a poor prognostic factor in patients with melanoma regardless of prior treatment or BRAF mutational status.32,33
Patient performance status (PS) measured through Eastern Cooperative Oncology Group scoring systems, although not directly affecting advanced melanoma American Joint Committee on Cancer staging, has also shown that a poor PS serves as an independent prognostic indicator given significantly worse survival rates observed in those with an Eastern Cooperative Oncology Group > 1 at time of diagnosis.34,35 This is not to be confused with advanced age, however, as dedicated studies have noted encouraging response rates and toxicity profiles of elderly patient cohorts receiving standard melanoma therapies.36,37
Oligometastatic Disease
Talimogene laherparepvec.
In 2015, talimogene laherparepvec (T-VEC) became the first oncolytic viral therapy to be approved by the US Food and Drug Administration (FDA) for the treatment of advanced (stage IIIB-IV) melanoma on the basis of the randomized phase III OPTiM trial.38 However, such a treatment modality remains reserved to those with locoregional (stage IIIB—IV [M1a]) disease, as the observed robust response rates remained preserved to this subgroup compared with those with pulmonary and visceral metastatic disease.39 Additional combination therapies with T-VEC have been implemented in more advanced disease and are discussed below.
Metastasectomy.
Intriguing phase III observations by Faries et al40 noted that although their primary intervention of intralesional bacillus Calmette-Guerin with or without allogenic whole-cell vaccines after complete resection of ≤ 5 distant metastases in patients with melanoma was terminated early because of low probability of efficacy, the 5-year OS rates of approximately 40% in both patient arms were appreciably higher than those in patients undergoing now historical standard-of-care systemic therapies without resection, thus supporting a long-term survival benefit in select patients with metastatic melanoma amenable to complete resection. Additional prospective and retrospective efforts have also observed long-term survival benefits after complete resection of distant metastases in a variety of gastrointestinal, hepatic, adrenal, and pulmonary sites of involvement for carefully selected patients, which include those with a favorable PS and extended disease-free interval.41 Finally, regardless of systemic therapy, resection of these isolated metastases may provide additional clinical benefit by alternating patient immune profiles given the immunosuppressive milieu established by melanoma tumor tissue.42 Further exploration regarding the potential clinical benefits of surgical resection in the era of now standard-of-care targeted agents and immunotherapies is anticipated.
Radiation modalities.
Outside of palliative intent, the utility of radiation therapy in the setting of advanced melanoma has remained a debated topic of study. Stereotactic radiosurgery (SRS) for patients with intracranial metastatic melanoma has exhibited expedited disease control and additional survival benefits when used in multimodality approaches that include surgery and systemic therapies.43,44 The use of whole brain radiation in the adjuvant setting, however, exhibited no clinical improvement in cognition or survival and therefore is not recommended.45 In the setting of extracranial oligometastatic disease, the use of SRS in patients unfit or otherwise ineligible for systemic options has provided durable rates of local disease control, particularly in pulmonary lesions, and thus is an appropriate therapeutic option in this subset of patients.46 Furthermore, the potential systemic reactivation of antitumor immunophenotypes after radiation therapy to targeted lesions, also referred to as the abscopal effect, has provided intriguing yet inconsistent data regarding the synergistic improvements of combining radiation therapy with immune-modulating therapies in patients with metastatic melanoma, for which additional prospective data remain desired.47
Advanced Metastatic Disease
Historic approaches.
High-dose (HD) interleukin (IL)-2 has been observed to enhance the expansion of an antitumoral immunophenotype including natural killer (NK) cells, NK T cells, antigen-activated CD-8+ T cells, mast cells, and dendritic cells.48 These findings coincided with favorable response rates and the potential for complete responses (CRs) in certain patients with metastatic melanoma, leading to its FDA approval within this patient population in 1998.49 This therapeutic modality has faced major shortcomings and associated challenges in achieving a prolonged therapeutic benefit given its short half-life with a relatively high toxicity profile, including a potentially fatal vascular leak syndrome, thus requiring inpatient management to administer. This regimen also exhibits eventual activation of counter-regulatory immune pathways, such as immune-dampening Tregs, with resultant therapeutic resistance.50 Although this modality may still provide clinical benefit in a subset of patients as a later-line option, HD IL2 has been largely replaced in the first-line setting given the aforementioned challenges in addition to the enhanced response rates of alternative immunologic treatments outlined below.
Before IL-2–based approaches, treatment of metastatic melanoma was mostly considered palliative with suboptimal partial response rates of 6%-15% observed through an approved regimen of the alkylating agent dacarbazine.51 The oral alkylating agent, temozolomide, gained additional attention for its oral route of administration, enhanced patient-reported quality of life, and potential ability to cross the blood-brain barrier, leading to enhanced responses for patients with CNS involvement, but ultimately provided no additional therapeutic benefits compared with those receiving dacarbazine.52 Subsequent attempts with combinational chemotherapy such as the Dartmouth regimen (dacarbazine, cisplatin, carmustine, and tamoxifen) exhibited enhanced toxicity without significant response or survival benefits when compared with dacarbazine.53 Additional efforts of combination chemotherapy included cisplatin, vinblastine, and dacarbazine, which was also unsuccessful in achieving clinically significant survival benefits compared with the standard single-agent regimen.54 Finally, the combination of carboplatin and paclitaxel, with or without vascular endothelial growth factor (VEGF) inhibition by bevacizumab, exhibited modest improvements in disease control but were also associated with high rates of toxicity and unclear survival benefits.55,56
These underwhelming observations led to the exploration of combining these chemotherapeutic agents and cytokines together, also referred to as biochemotherapy, which included cisplatin, vinblastine, and dacarbazine with IL-2 and interferon-alpha agents, among others. Although patients undergoing biochemotherapy regimens exhibited encouraging response rates compared with chemotherapy modalities, these regimens failed to translate into a clear long-term survival benefit.57 Given the suboptimal long-term responses and insignificant survival benefits of these agents, further exploration into alternative treatment options for this highly chemoresistant disease was strongly warranted.
Immunotherapy.
The initial observations of spontaneous regression of melanoma and lymphocyte infiltration led early investigators to posit that immunotherapy may serve a benefit in cancer therapeutics. The first studies of intralesional bacillus Calmette-Guerin or levamisole sparked interest, but it was not until the introduction of recombinant human cytokines, interferon-α2 and IL-2, that durable responses were noted, albeit in very small percentages of patients. The groundbreaking Nobel laureate winning observations of immune checkpoints (ICs) by Honjo (programmed cell death protein 1 [PD-1]) and Allison (cytotoxic T-cell lymphocyte [CTLA]4) paved the way for developing human-blocking antibodies. ICI entered clinical trials including the first phase I efforts with anti-CTLA4, ipilimumab, in 2007.58 Subsequent studies in other tumors led to expanded phase II and ultimately phase III trials, which uncovered a whole new profile of toxicities and immune-related adverse events (irAEs) that included occasionally lethal results. The durable responses of these therapeutic modalities were unprecedented, with their associated irAEs continuing to remain an often unavoidable burden to which extensive efforts in risk stratification, early identification, and mitigation are ongoing.59-61
The anti-CTLA4 agent, ipilimumab, was the first single-agent ICI approved by the FDA for the treatment of metastatic melanoma in March 2011 at a dose of 3 mg/kg once every 21 days for four doses,62 with eventual approval in the adjuvant setting at a dose of 10 mg/kg once every 3 weeks for four doses, then every 3 months for up to 3 years for advanced (stage III) disease in October 2015.63 However, anti-CTLA4 monotherapy is not considered a standard of care in the first-line setting given mature evidence of favorable PFS observed with both anti–PD-1 monotherapy or combined anti-CTLA4 and anti–PD-1 regimens.64,65 Further exploration of anti-CTLA4 agents is warranted, given their (1) durable dose responsiveness,66,67 (2) potential ability to shape the immune contexture including enhanced depletion of tumor-specific Tregs,68,69 (3) encouraging immune responses in preliminary neoadjuvant studies,70 and (4) reduced toxicity profiles in modified anti-CTLA4 antibodies.71
The phase III results of KEYNOTE-006 provided convincing evidence for the anti–PD-1 agent, pembrolizumab, at a dose of 10 mg/kg once every 2 weeks and once every 3 weeks, to exhibit superior PFS and OS in comparison with ipilimumab dosed at 3 mg/kg once every 3 weeks for four doses, for which durable benefits continue to be observed after a recent 5-year follow-up.72 The anti–PD-1 agent, nivolumab, gained FDA approval in patients with advanced BRAF wild-type melanoma shortly after at a dose of 3 mg/kg once every 2 weeks.73
The ICI combination of ipilimumab and nivolumab received FDA approval for patients with BRAFV600 wild-type unresectable or metastatic melanoma in October 2015, with expanded approval regardless of BRAF mutational status achieved less than one year later.74 This regimen of nivolumab 1 mg/kg and ipilimumab 3 mg/kg once every 3 weeks for four cycles followed by maintenance nivolumab at 3 mg/kg once every 2 weeks until disease progression, toxicity, or withdrawal was largely founded on the robust and durable responses observed in the CheckMate-067 trial, with most recent 6.5-year follow-up data continuing to exhibit improved outcomes in OS, PFS, and overall response rate (ORR) in patients treated with combination ICI compared with ipilimumab monotherapy.65 This regimen remains a standard of care in the frontline setting for patients with newly diagnosed metastatic melanoma who are fit and eligible for combination ICI, with the greatest suggested benefits observed in patients with (1) asymptomatic CNS involvement,75,76 (2) harboring of BRAF mutations, (3) advanced metastatic involvement, or (4) high serum LDH.65,77 These robust responses also carry a higher-risk irAE profile with a grade 3-4 irAE rate of 59%, and therefore clinical discretion, including consideration of ICI monotherapy or targeted treatment options, is strongly advised in select patient cohorts including those at high risk of ICI intolerance.65 It is also worth noting that all four combination ICI doses may not be necessary to achieve optimal response and survival rates, as clinical benefits have been observed in those with treatment intolerance78 and encouraging results from ongoing trials involving both shortened79 and inverted80 dosing regimens of this ICI combination appear to exhibit improved safety profiles without inferior responses for which long-term follow-up is eagerly awaited. Unfortunately, patients with metastatic uveal melanoma have only achieved modest 12-month OS improvements in the first-line setting after treatment with the aforementioned standardized ipilimumab and nivolumab dosing regimen on the basis of retrospective and phase II data, for which (1) alternative regimens, (2) long-term follow-up, (3) and phase III efforts in this high-risk cohort of patients are anticipated.81-83
The concept of combination ICI with targeted therapeutic agents is an enticing one given the potential benefits of adding ICI-related durability to the enhanced response rates achieved with targeted therapies. The first clinical trial investigating this treatment modality via a combination of anti-CTLA4 ICI with BRAF inhibition suffered major setbacks and early termination because of substantial liver toxicities, the etiology of which was suspected to be related to the well-established and previously mentioned paradoxical activation in MAPK pathways.84 Subsequent efforts have uncovered multiple synergistic immunophysiologic pathways in BRAF-mutant mouse models when treated with anti–PD-1 ICI and BRAF plus MEK inhibitor triple therapy combinations.84,85 There are currently several maturing phase II and III clinical trials using these combinations for patients with BRAF-mutant advanced melanoma including KEYNOTE-022 (pembrolizumab, dabrafenib, and trametinib), IMspire150 (atezolizumab, vemurafenib, and cobimetinib), and COMBI-i (spartalizumab, dabrafenib, and trametinib).86 Although only the IMspire150 trial met its primary end point of PFS for which the survival curves first separated after 6-8 months, a more matured analysis of the KEYNOTE-022 data exhibited numerically encouraging improvements in response duration, PFS, and OS.87 Furthermore, a subgroup analysis by the COMBI-i trial suggested that patients with higher serum LDH, increased metastatic sites, bulky disease, and higher mutational burden may represent a cohort with the greatest clinical benefit to triple therapy.88 Although the IMspire150 observations led to FDA approval of combination of anti–PD-1 (atezolizumab) with the BRAF plus MEK inhibitors vemurafenib and cobimetinib for patients with BRAFV600 mutation–positive unresectable or metastatic melanoma in July 2020, there remains little to no clinical scenarios where triple therapy is clinically suggested in the first-line setting given its higher toxicity profile, currently immature supportive evidence, and lack of comparative studies with standard-of-care sequential ICI followed by targeted therapy.89
Modified TILs have also proven to provide encouraging therapeutic benefits in addition to the prognostic insights outlined above. Single doses of the cryopreserved autologous TIL, lifileucel (LN-144), have provided strong phase II evidence of durable response rates in the high-risk cohort of patients with melanoma who have progressed on multiple lines of therapeutic agents.90 Such adoptive cell therapeutic approaches are very promising in re-establishing the desired endogenous antitumor immunophenotype for which additional phase III efforts are highly anticipated with additional consideration for earlier administration given potentially diminished responses in patients previously exposed to anti–PD-1 agents.90
Indoleamine-2,3 dioxygenase 1 is involved in enzymatic degradation of tryptophan, an amino acid that is crucial for T-cell activity. This observation ultimately led to a phase I to phase III effort with the small-molecule indoleamine-2,3 dioxygenase 1 inhibitor, epacadostat, in combination with pembrolizumab in patients with metastatic melanoma, which ultimately failed to exhibit a survival benefit.91 However, the lack of phase II efforts in these treatment modalities and advancements in the metabolomic pathways of these molecules warrants additional translational and clinical efforts.92
An exciting advancement in patients with metastatic uveal melanoma includes the melanoma-associated antigen, gp100. Phase III data have recently provided evidence that the first-in-class bispecific fusion protein, tebentafusp, targets melanoma-specific gp100 via specific T cell receptor portion with subsequent binding and activation of T cells via CD3 portion to elicit antitumor immune responses and achieve significant OS benefits when compared with standard-of-care ICI and chemotherapeutic regimens.93 Further investigation and clinical implementation of this regimen are eagerly anticipated given the suboptimal response to most standard-of-care options within this high-risk patient cohort, the pathophysiology and etiology of which are well described elsewhere.94,95
Targeted therapy.
As outlined above, the therapeutic combination of BRAF and MEK inhibitors has provided rapid and deep responses that are now widely accepted and used in the clinical setting as an acceptable standard of care in patients with metastatic melanoma harboring BRAF mutations, for which encouraging long-term durability studies continue to mature. Clinical use of BRAF monotherapy is no longer standard practice given the PFS and OS benefit of BRAF and MEK inhibitor combinations with additional secondary benefits of reducing the aforementioned dermatologic toxicities observed with BRAF inhibitor monotherapy.14 Trojaniello et al15 recently shared a comprehensive review including a summary of the most updated follow-up of these combinations, which observed comparable 5-year OS rates of 26%-32% and grade 3-4 toxicity rates of 54%-68% across the various approved regimens (dabrafenib plus trametinib, vemurafenib plus cobimetinib, and encorafenib plus binimetinib). Although numerically longer OS trends have been noted with the combination of encorafenib and binimetinib in side-by-side analyses, no combination of these approved combinations has proven clinical superiority, and treatment decisions should be made on a case-by-case basis that accounts for comorbidities, ease of use, and side effect profiles while awaiting further supportive evidence including the utilization of biomarkers.96
An additional targeted therapy clinically investigated in patients with melanoma includes KIT mutations. Early observations by Carvajal et al97 in 2011 showed that treatment with imatinib mesylate led to significant response rates in patients with metastatic melanoma harboring KIT alterations, including two CRs in a pair of patients that harbored a combination of the most common point mutation of exon 11 (L576P) and a KIT amplification mutation. It is further postulated that activating KIT mutations, specifically within exons 11 and 13, have the highest response rates to imatinib compared with KIT amplification mutations.98 Additional small-molecule inhibitors of c-KIT exhibiting a range of mostly limited response rates in patients with KIT mutation harboring melanomas include nilotinib, dasatinib, and sunitinib. Therapeutic approaches therefore require further prospective studies including more carefully defined patient cohorts, with current considerations of imatinib versus clinical trials for patients with acral or mucosal melanomas harboring activating KIT mutations who progress on or are ineligible for ICI therapy.98
Recommended clinical approaches.
On the basis of the evidence provided above, our institution implements a frontline clinical approach to patients with advanced melanoma, which is summarized in Figure 1. We continue to encourage patients and physicians to first consider participation in clinical trials as a means to allow advancement of therapeutic options. When considering treatments for patients with high-risk advanced locoregional and metastatic disease, testing for molecular targets should be performed when clinical decision forks are reached. Currently, standard molecular testing includes BRAF mutational analyses at the time of diagnosis. In the infrequent clinical scenario of rapidly progressing metastatic disease where initiation of targeted therapy may provide lifesaving control and occasional cost considerations of molecular testing, a prompt and reliable result of BRAFV600E mutation status can be achieved via immunohistochemical techniques. However, the clinician should be alerted that current immunohistochemical testing only identifies the V600E variant, and a more nuanced understanding of other BRAF mutational variants is worthy of additional molecular testing given their less active but clinically relevant activity to BRAF-targeted therapies.99-101 Additional molecular testing of CKIT mutations may also be considered in rare cases within the small subset of patients who are BRAF wildtype and progress on or are ineligible for ICI therapy.97
FIG 1.
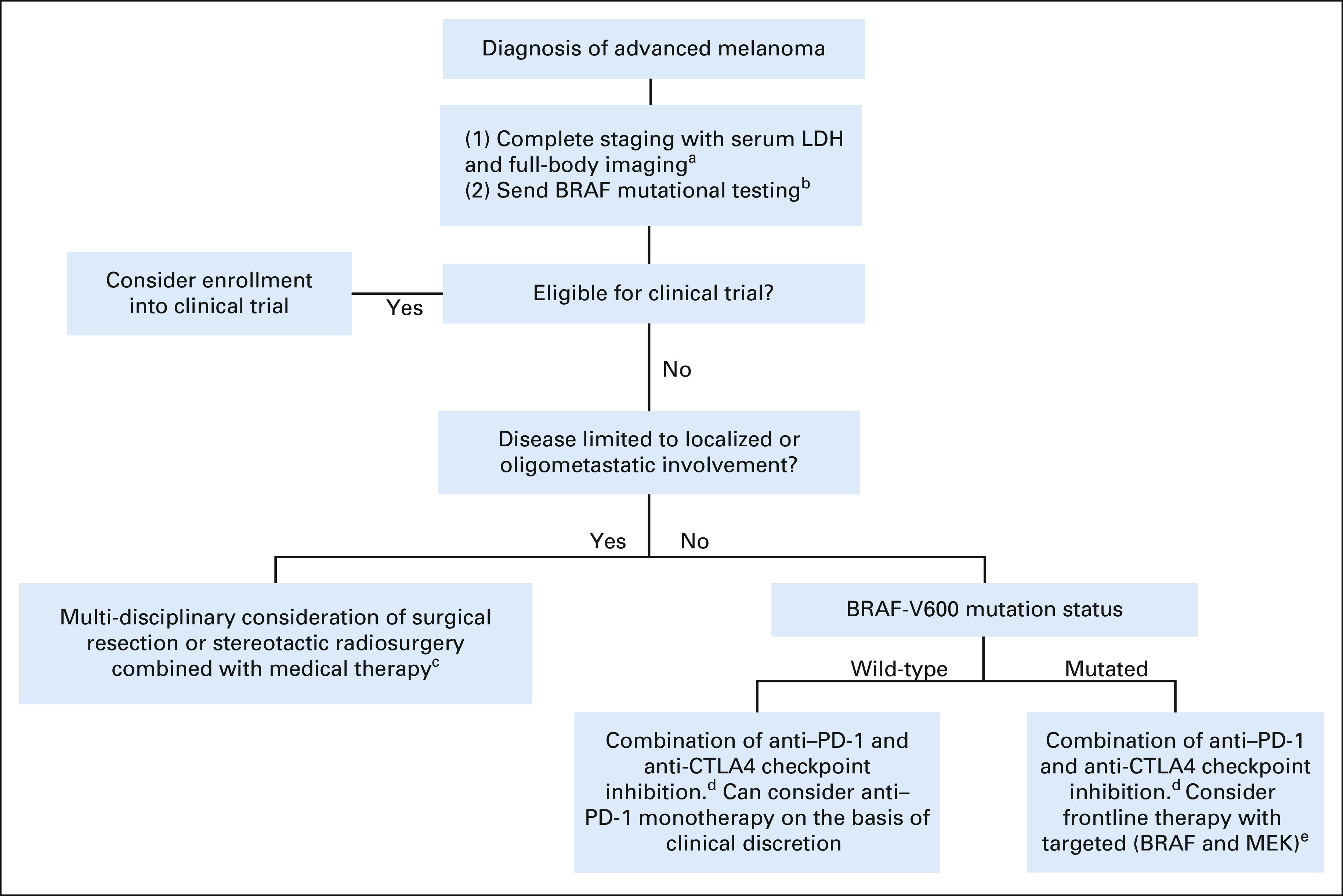
Recommended current clinical approach to patients with treatment-naive advanced metastatic melanoma. aInclude magnetic resonance imaging of brain if clinically indicated. bAdditional molecular analyses including mutational status of RAS, CKIT, and NF1 as well as expression of programmed death ligand 1 and quantification of tumor-infiltrating lymphocyte infiltration, tumor mutational burden, and circulating tumor DNA are not currently used as the standard of care but likely to provide clinical utility in the future and should be considered in appropriate experimental settings. cMedical therapies include talimogene laherparepvec for patients with locoregional (stage IIIB-IV-M1a) disease and systemic therapy (most commonly combined anti–PD-1 and anti-CTLA4 checkpoint inhibition) if visceral involvement. These treatments should not be delayed while undergoing consideration of additional interventions. dCombination of anti–PD-1 and anti-CTLA4 ICI is encouraged as standard first-line systemic therapy regardless of BRAF mutational status, with most enhanced benefits observed in patients with (1) CNS involvement, (2) elevated LDH, and/or (3) visceral metastatic disease, which warrants an enhanced response rate. Can consider anti–PD-1 monotherapy for clinical and/or toxicity concerns from combination ICI. eAppropriate to consider as frontline therapy with targeted (BRAF and MEK) inhibition in the subset of patients with BRAFV600 mutation who require brisk response because of rapid and/or symptomatic disease progression. CTLA, cytotoxic T-cell lymphocyte; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; PD-1, programmed cell death protein 1.
In patients with advanced melanoma harboring a BRAF mutation without contraindications to ICI, we advise the prioritization of combination immunotherapy over approved targeted agents in the first-line setting. This approach has been previously founded on currently available long-term durable response and survival rates of ICI, even when prematurely discontinued, in addition to retrospectively observed improvements in patient outcomes and OS when initially treated with ICI before exposure of targeted agents.102-106 However, updates of two prospective studies (SECOMBIT and DREAMseq) presented in late 2021 have now further supported the use of combination ICI over targeted therapies in the frontline setting for patients with untreated, metastatic BRAFV600-mutated melanoma. The SECOMBIT trial has observed that patients treated with ICI combination (ipilimumab plus nivolumab) before BRAF and MEK inhibition (encorafenib plus binimetinib) exhibited 3-year PFS (53% v 41%) and OS (62% v 54%) benefits when compared with the reverse sequence of those regimens, along with additional PFS (54%) and OS (60%) benefits through a sandwich approach of BRAF and MEK inhibition both before and after combination ICI.107 It is worth noting that the SECOMBIT trial's phase II noncomparative design was not powered to compare the arms of (1) combination ICI until progression followed by dual BRAF and MEK inhibition, (2) dual BRAF and MEK inhibition until progression followed by combination ICI, and (3) eight weeks of BRAF and MEK inhibition followed by combination ICI and returning to BRAF and MEK inhibition at disease progression, for which long-term follow-up is ongoing. The DREAMseq study was designed to specifically compare the sequence of initial combination ICI versus targeted therapies in this cohort, which recently reported a 20% OS benefit at the 2-year mark in treatment-naive patients with BRAFV600-mutant metastatic melanoma if initially treated with combination ICI ipilimumab plus nivolumab (72% OS; 95% CI, 62 to 81) compared with initial treatment with BRAF and MEK inhibitors dabrafenib plus trametinib (52% OS; 95% CI, 42 to 62).108 These studies strongly support the use of dual ICI therapy as first-line therapy in patients who possess tumors harboring BRAF mutations and who are eligible for combination ICI regimens.
Consideration of ICI monotherapy is reserved for anti–PD-1 agents, pembrolizumab and nivolumab, which are approved and reasonable options in certain scenarios such as patients with advanced or metastatic melanoma who are ineligible for clinical trials and deemed unfit for combination ICI therapies. Although ICI monotherapies do not appear to provide as durable of a response compared with combination ICI regimens, prospective phase III data failed to exhibit a significant OS benefit for combination ICI compared with nivolumab monotherapy and also noted PFS and OS benefits for both combination ICI and nivolumab monotherapy when compared with ipilimumab.109 Similar response rates and toxicity profiles have been observed with pembrolizumab as ICI monotherapy, and therefore, both pembrolizumab and nivolumab are appropriate options for anti–PD-1 monotherapy.110,111 Conversely, combination ICI appears to be of particular utility over anti–PD-1 monotherapy in those with (1) CNS involvement, (2) elevated LDH, and/or (3) visceral involvement that warrants enhanced response rates.75,112,113 A reasonable exception to consider BRAF and MEK-targeted therapy in the frontline setting involves patients harboring a BRAF mutation who may benefit from a brisk response because of rapid and/or symptomatic disease progression, with the timing of ICI in this cohort currently under investigation in the aforementioned SECOMBIT trial.102,103,107 The recommended sequence of these agents and the utility of ICI monotherapy remain major topics of interest currently under prospective investigation (ClinicalTrials.gov identifier: NCT02224781 and NCT02631447) with further exploration of predictive biomarkers and patterns of therapeutic resistance as additional fields of clinical necessity in guiding the optimal management of this cohort.102,103
In regard to the duration of systemic therapy, encouraging observations of continuous durable responses have been observed after early discontinuation of single-agent and combination ICI therapy and relatively low rates of disease progression in those discontinuing targeted therapy.114,115 However, the most durable responses after treatment discontinuation appear to be limited to patients who have achieved a CR during their initial treatment, and therefore, a much more extensive understanding of the clinical characteristics and biomarkers associated with continuous responses after the discontinuation of systemic therapies is required before safely endorsing the discontinuation of these life-prolonging agents.106,116,117 A recent example of these advances includes the observation that levels of ctDNA appear to remain undetectable in patients with prolonged responses after discontinuation of treatment in those who initially achieved a CR.118 We therefore currently advise the continuation of ICI therapy in patients who are responding but yet to achieve a CR and continuation of targeted therapies for even responding patients who may achieve a CR until disease progression or dose-limiting toxicity, whereas more reliable methods in predictive risk stratification and biomarker monitoring may eventually permit discontinuation in select cohorts of patients. In ICI-treated patients who achieve a CR, we recommend that discontinuing therapy after 6-12 months beyond the date of CR may be considered on the basis of a shared decision-making approach with the patient.117
Special considerations in CNS disease.
Melanoma with metastatic involvement of the CNS classically portends a grim prognosis and remains a common exclusion criterion in clinical trials including a majority of those listed above. In the nonsurgical setting, targeted therapies with combination BRAF and MEK inhibition exhibited PFS and OS benefits in patients with asymptomatic CNS involvement, but these lesions also exhibited suboptimal durations of response compared with their extracranial disease sites.119 Immunotherapies have more robust data supporting OS benefits of ICI in CNS disease, with initial prospective data providing durable OS benefits with ipilimumab in 2012 followed by enhanced benefits observed through combination of ipilimumab and nivolumab, which were particularly notable within asymptomatic and/or steroid-independent patient populations.75,120,121 Combination ICI has exhibited an intracranial ORR of 56%, with 19% of these patients achieving a CR.121 Targeted therapy combinations have exhibited similar intracranial ORR rates ranging from 42% to 50%.122 Furthermore, as opposed to targeted therapies mentioned above, ICI-treated patients exhibited equivalent CNS response rates when compared with their extracranial sites, which imparts additional preference for an ICI-based approach in long-term therapeutic decision making. Furthermore, multimodality techniques appear to provide the greatest survival benefits, as retrospective evidence supports the combination of ICI in conjunction with SRS therapy for which clinical trials involving radiation combined with ICI (ClinicalTrials.gov identifier: NCT03340129) and targeted therapies (ClinicalTrials.gov identifier: NCT02974803) are ongoing.123,124
NOVEL APPROACHES AND FUTURE CLINICAL CONSIDERATIONS
Toll-Like Receptors
Toll-like receptor (TLR) therapies appear to promote intratumoral antigen cross-presentation with resultant activation and tumoral infiltration by adaptive immune cells that ultimately enhances the antitumor immune response and sensitivity to ICI therapies.125 Intratumoral injections with TLR9 agonists have provided encouraging preclinical evidence of both local and systemic responses within melanoma mouse models, with intriguing dose-responsive patterns when exposed to more potent TLR9 agonists in combination with modified anti-CTLA4 therapies that are capable of enhanced depletion of tumor-specific Tregs.69 Although phase I/II efforts involving the intratumoral TLR9 agonist, tilsotolimod, combined with systemic ipilimumab in patients with anti–PD-1-resistant advanced melanoma exhibited sufficient tolerability to this regimen with an encouraging response to treated and distant lesions, their phase III ILLUMINATE-301 trial recently shared that their primary end point of ORR was not met.126 More recently, phase I data from dose-escalated intratumoral virus-like TLR9 agonist, vidutolimod (formerly CMP-001), in combination with systemic anti–PD-1 therapy (pembrolizumab) in patients with advanced melanoma on previous ICI therapy have observed encouraging toxicity profiles with durable responses in 25% of patients and enhanced expression of antitumoral immune profiles within distant metastatic lesions.127 Given the therapeutic challenges of patients with progressive melanoma despite standard ICI therapy, these adjunctive agents may provide crucial type I interferon immune responses associated with overcoming immunologic resistance to PD-1 blockade within the TME.127
Novel ICIs
In addition to the detrimental psychosocial implications of stress in patients with cancer, the multifactorial etiologies and dynamic biochemical pathways associated with the physiologic state of stress have also been shown to promote tumor growth and metastasis within multiple solid tumor cell lines through induction of tumorigenic matrix metalloproteinases, VEGF, IL-8, and IL-6 via increased β-adrenergic receptor signaling pathways modulated through the sympathetic autonomic nervous system.128-130 In melanoma cell lines, Yang et al131 showed that the VEGF, IL-6, and IL-8 pathways associated with the neuroendocrine stress response were abrogated by the use of propranolol, a nonselective inhibitor of β-adrenergic receptor signaling pathways. Synergistic antitumor activity of standard-of-care anti–PD-1 treatment combined with propranolol was then observed within melanoma mouse models, ultimately leading to a phase I clinical trial, which exhibited a very encouraging overall response rate of 78% in the propranolol plus pembrolizumab arm with an improved safety profile compared with those observed with combination checkpoint inhibitors in the first-line setting.64,72,132,133 Multicenter phase II efforts to further validate these findings are ongoing (ClinicalTrials.gov identifier: NCT03384836).
Lymphocyte-activation gene 3 (LAG-3) has recently gained support as a targetable IC on the basis of its expression on TILs with a distinct contribution to tumor-mediated T-cell exhaustion. The phase III RELATIVITY-047 study (ClinicalTrials.gov identifier: NCT03470922) investigating a novel immunotherapeutic combination with standard-of-care anti–PD-1 (nivolumab) in addition to the anti–LAG-3 agent, relatlimab, in treatment-naive patients with advanced melanoma exhibited the improved PFS of 10.1 months compared with 4.6 months for those on nivolumab monotherapy (P = .0055) with a resultant HR of 0.75 (95% CI, 0.6 to 0.9).134 The grade 3-4 toxicity profile of anti–PD-1 plus anti–LAG-3 combination therapy was 18.9% as compared with 9.7% receiving nivolumab monotherapy. Although these data currently lack the maturity of providing an interpretation in OS benefits and are yet to be prospectively compared with standard-of-care anti–PD-1 and anti-CTLA4 combination immunotherapy, these encouraging observations support the potential utility of this novel regimen especially in patients ineligible for combination ICI.
V-domain immunoglobulin suppressor of T-cell activation (VISTA), a negative regulator of T-cell function, is expressed by T cells, myeloid cells, and tumor cells with common associations of poorer prognostic factors in patients with melanoma such as increased Breslow thickness and advanced stage.135 Furthermore, preclinical studies have observed that combined blockade of both PD-1 and VISTA appears to exhibit synergistic effects in the restoration of T-cell activity and antitumor pathways in a variety of cancer cell types for which a first-in-class oral dual anti–PD-1 and anti-VISTA small-molecule ICI (CA-170) has been developed with a dose-escalation phase I clinical trial currently ongoing (ClinicalTrials.gov identifier: NCT02812875).136
T-cell immunoglobulin and mucin domain 3 (Tim-3) is a cell surface molecule expressed on CD4+ T cells, cytotoxic CD8+ T cells, NK cells, dendritic cells, and a variety of cancer-specific cells including melanoma with several ligands that appear to provide inhibitory responses in IC pathways including diminished T-cell activation and impaired antitumor immunity.137 Several studies using monoclonal antibodies against Tim-3 for a variety of cancer types are ongoing with early observations of a phase II trial for patients with advanced melanoma previously treated with anti–PD-1 ICI exhibiting suboptimal response rates after a combination of anti–Tim-3 antibody (MDB453) and anti–PD-1 (spartalizumab) therapies, for which preliminary results exhibited a 84.8% discontinuation rate because of disease progression (60.6%) or cancer-related death (12.1%).137 More extensive studies with longer-term follow-up are therefore required to adequately investigate the utility of this therapeutic modality.
The metabolomic changes associated with immune activation and inhibition, or immunometabolism, may provide a variety of biomarkers toward the assessment of treatment response and a potential target for a variety of cancer therapeutics. In patients with melanoma, extracellular adenosine signaling pathways involving an interplay of CD39 and CD73 have been observed to protect melanoma cells through multiple dynamic changes within the TME including enhancement of tumor cell escape through direct antiproliferative effects on CD4+ and CD8+ T cells.138 Therapeutic modalities targeting these pathways, which include small-molecule inhibition and humanized monoclonal antibodies directed toward the inhibition of CD73, often in combination with ICI, are currently ongoing within early phase and preclinical trials.139 In addition, combinations of CD73 pathway inhibitors with BRAF and MEK inhibitors appear to potentiate antitumor effects within melanoma mouse models harboring BRAF mutations, warranting further clinical exploration within this clinical cohort.140
Cytokines
A variety of cytokines provide paracrine and autocrine impacts on the complex regulatory pathways associated with the innate and adaptive immune system as it is related to tumorigenesis and anticancer therapeutics, which has therefore cultivated a vested interest in the prognostic and therapeutic benefits of these molecules. One such cytokine is IL-12, which although highly toxic when administered systemically, has provided phase II evidence of local and distant responses after intratumoral injection with plasmid IL-12, tavokinogene telseplasmid, when administered in combination with anti–PD-1 ICI for patients with ICI-refractory advanced melanoma as per KEYNOTE-695.141 Given the encouraging response and toxicity profiles of this combination, tavokinogene telseplasmid has now received Orphan Drug and Fast-Track designation by the FDA, and phase III efforts are underway (ClinicalTrials.gov identifier: NCT02453594).
Another cytokine of interest is IL-7, which possesses pleiotropic effects on both adaptive and innate immune pathways with known regulatory effects on the proliferation and survival of CD4+ and CD8+ T cells and preclinical evidence with various IL-7 formulations that exhibit enhanced TME T-cell invasion, increased activation of dendritic cells within tumor-draining lymph nodes, and enhanced efficacy of approved anticancer immunotherapeutic agents.50,142 However, there remains no clear clinical evidence to support the utilization of IL-7 therapies at this time, as the only currently available study that involved a group of 11 patients with metastatic melanoma exhibited no objective response to recombinant IL-7.143
The cytokine IL-2 possesses a wealth of historical evidence supporting its elaborate antitumor activity as outlined above, and current clinical efforts invested in protein engineering as a means to enhance the bioavailability, safety, and efficacy of these pathways are ongoing.144 For example, Bempegaldesleukin is a CD122-preferential agonist of IL-2 found to enhance CD8+ T-cell and NK cell infiltration within the TME while simultaneously enhancing PD-1 expression of intratumoral lymphocytes and PD-L1 of tumor cells. The PIVOT-02 phase II trial's recent 29-month follow-up results of combination of Bempegaldesleukin with nivolumab in the first-line setting for patients with advanced melanoma exhibited an ORR of 52.6%, with CR in 34.2%, and a median PFS of 30.9 months while also providing encouraging toxicity profiles (grade 3 to 4 irAEs occurring in 17.1% and 4.9% of patients, respectively) for which additional efficacy data are to be explored upon completion of their currently enrolling phase III efforts.145 A cytokine functionally similar to IL-2 is IL-15, which shares CD8+ T-cell and NK cell activation and promotion pathways, but lacks the stimulatory signals to immune-dampening Tregs. Low in vivo expression of IL-15's activating proteins contributed to an enhanced toxicity profile, given the HDs required to reach a biologic response, and ultimately led to the development of a novel superagonist fusion protein complex (ALT-803), which is now under clinical investigation as monotherapy (ClinicalTrials.gov identifier: NCT01946789) and as a combination with anti–PD-1 ICI (ClinicalTrials.gov identifier: NCT03228667).146,147
Transforming growth factor-β (TGF-β) signaling pathways have been observed to promote cancer cell invasion and metastatic spread via epithelial-mesenchymal transition and thus are considered a major driver of cancer progression and malignant transformation.148 Despite encouraging preclinical data involving the blockade of these pathways, four separate categories of anti–TGF-β therapies have been unable to provide clear evidence of survival benefits in the clinical setting, with additional concerns of dose-limiting cardiovascular toxicity.149 Additional preclinical efforts have suggested that TGF-β1 isoform–specific blockade may enhance antitumor immune profiles, allowing more effective responses to ICI while avoiding the previously observed cardiovascular toxicities seen in nonselective TGF-β blockade.150
Viral Oncolytics
Genetic modification of certain nonpathogenic viral species transformed to express antitumorigenic factors, or oncolytic viruses, is a therapeutic modality to target and kill specific tumor cell types. One of the most successful modalities of viral oncolytics to date for patients with melanoma includes the aforementioned T-VEC therapy, which expresses granulocyte-macrophage colony–stimulating factor (GM-CSF) to induce complex immunomodulatory effects including dendritic cell activation with subsequent enhancement of antitumor T-cell activation. Those treated with T-VEC have exhibited repeated evidence of systemic responses to uninjected lesions and provocative evidence of enhanced systemic responses when combined with systemic ICI.151,152 The clinical benefits of this approach require further investigation, as the phase Ib/III KEYNOTE-034 trial of combined T-VEC and pembrolizumab was recently terminated because of futility.153 Through a related mechanism of action to T-VEC, ONCOS-102, an adenovirus-based tumor-specific GM-CSF intratumoral injection therapy, has recently received fast-track FDA designation given its encouraging phase I evidence of immune activation and potential systemic resensitization to ICI for patients with anti–PD-1 refractory melanoma with an ORR of 35% after receiving treatment with this agent in combination with pembrolizumab.154 Additional encouraging phase I data in patients with anti-PD-1 refractory melanoma include treatment with PVSRIPO, a live-attenuated, type I poliovirus (Sabin) vaccine with a modified internal ribosomal entry site of human rhinovirus type 2, which appears to produce sustained type I/III interferon inflammatory changes within the injected TME and subsequent enhancement of response to anti–PD-1 ICI, for which phase II efforts in the PD-1 refractory setting are ongoing (ClinicalTrials.gov identifier: NCT04577807).155 Other oncolytic viral therapies in various stages of clinical development for patients with melanoma, mostly in combination with systemic ICI, include Coxsackievirus A21, modified herpes simplex virus (RP1 and RP2), GM-CSF–expressing poxvirus JX-594 (Pexa-Vec), and a chimeric poxvirus (CF33-hNIS).156-159 Additional viral oncolytic vectors that target and manipulate a variety of pathways in the melanoma-specific TME are currently under investigation in the preclinical setting.160,161 The clinical utility of viral oncolytic therapies, both as single agent and combined with systemic ICI, is yet to be adequately defined, and therefore, the results of the studies outlined above are highly anticipated.
Additional GM-CSF Modalities
The yeast-derived recombinant human GM-CSF, sargramostim, has achieved orphan drug designation by the FDA in February 2019 for patients with advanced melanoma given convincing evidence of its additive and synergistic antitumor immune responses in combination with ICI.162 These findings have been most notable in the phase II E1608 study, where patients randomly assigned to receive sargramostim (250 μg subcutaneously once daily for 14 days every 3 weeks) and high-dose ipilimumab (10 mg/kg once every 3 weeks) for four cycles followed by maintenance ipilimumab and sargramostim exhibited a significant improvement in 1-year OS rates (68.9%) compared with those receiving ipilimumab monotherapy (52.9%, P = .01).163 Interestingly, patients on combination of sargramostim and ipilimumab also exhibited significant reductions in grade 3-5 irAEs compared with those on ipilimumab monotherapy (44.9 v 58.3%; P = .04), suggesting that additional mechanisms in toxicity reduction and improved treatment tolerance may be achieved with the addition of GM-CSF to ICI therapies.164 Additional efforts toward formal approval of this regimen are ongoing, including a phase II/III trial with GM-CSF combined with ipilimumab and nivolumab ICI as frontline therapy for patients with advanced melanoma (ClinicalTrials.gov identifier: NCT02339571).
Adoptive Cellular Therapies
Given the common therapeutic targets of activating T cells through a variety of modalities outlined above, adoptive cellular therapy (ACT) of TILs has introduced an attractive modality of enhancing the endogenous antitumoral immunophenotype with resultant clinical responses. These approaches have now achieved promising clinical results with the aforementioned Lifileucel in heavily pretreated patients with metastatic melanoma.165 Additional ACT with T-cell receptor gene transfer to human lymphocytes with resultant binding to the cognate epitope of major histocompatibility complex molecules on target cells provided reasonable response rates but with added risks of severe toxicity profiles because of shared antigen expression and therefore will benefit from investigation and development of germline-specific antigens for future melanoma-specific efforts through this modality.166,167 The use of chimeric antigen receptors (CARs) in engineering T-cell specificity has historically found success in B-cell malignancies, likely given solid tumor's immunosuppressive milieu expressed within the TME, which has been observed clinically after a near complete lack of response noted in a phase I effort with CAR-T–targeted antigens to VEGF2 in patients with solid tumors including melanoma.168 Further advances in alternative CAR-T targets as well as lymphodepletion before ACT and combinations of anti–PD-1 therapies with either TIL or CAR therapies are anticipated to provide further advances in ACT as a successful treatment modality in patients with solid tumors.167,168
Microbiome
The gastrointestinal tract microbiome has provided a fascinating and evolving field of study in patients with cancer, as growing evidence supports the hypothesis that specific species and overall diversity of the gut microbiome are capable of influencing antitumor immune responses through innate and adaptive immune pathways and may be modified through diet and fecal microbiota transplants in select patients as a means of improving their therapeutic response.169 Furthermore, Routy et al170 observed that patients with advanced cancer exhibited significantly reduced immunologic responses to immunotherapeutic PD-1 blockade and reduced OS after receiving oral antibiotics, suggesting that even transient dysbiosis may affect the therapeutic efficacy of ICIs. Similar observations have noted that patients with melanoma using over-the-counter probiotics exhibit reduced gut biodiversity, which appears to correlate with a reduced response to ICI.171 A favorable gut microbiome in patients with melanoma, identified as highly diverse with abundant Ruminococcaceae and Faecalibacterium species, was found to provide enhanced systemic and antitumor immune responses mediated by increased antigen presentation and improved effector T-cell function within peripheral and TMEs and was reproducible in mouse models receiving fecal microbiota transplants stool collected from mice who exhibited a favorable response to their anti–PD-L1 regimen.172 Conversely, recent observations have noted a predominance of Bacteroides intestinalis species within the microbiota of patients with melanoma who developed intestinal toxicities on combination of anti-CTLA4 and anti–PD-1 ICI and further postulated that the resultant GI upregulation of IL-1b inflammatory pathways may serve as a target for future modalities in irAE surveillance and treatment.173
Targeted Therapies
Additional targeted therapies directed toward various MAPK pathways are a field of active exploration for patients with advanced melanoma. Various novel tyrosine kinase inhibitors are yet to gain the durable responses achieved with the aforementioned BRAF and MEK inhibitors. The targeted KIT inhibitor, imatinib, maintains minimal evidence of durable responses in patients with melanoma harboring KIT amplification mutations and is not considered the standard of care given high rates of progression and potential resistance.97 Furthermore, agents targeting VEGF have also failed to provide clinically significant responses as monotherapy in patients with melanoma, but the phase II LEAP-004 trial using the VEGF tyrosine kinase inhibitor, lenvatinib, in combination with anti–PD-1, pembrolizumab, for patients with advanced melanoma refractory to frontline ICI therapy has recently shared updated follow-up results exhibiting clinically meaningful and durable responses, for which ongoing exploration is necessary.174 The second-generation VEGF inhibitor, axitinib, has exhibited encouraging phase II data in the second-line setting for patients with advanced melanoma, for which multiple phase III studies including combinations with ICI are ongoing (ClinicalTrials.gov identifier: NCT04493203).175 Additional details of novel targeted agents are highlighted in great detail elsewhere.15,113
Antigen Modulation
Intriguing advances in cancer epigenetics in patients with melanoma have presented the possibility to identify and manipulate specific MAPK signaling pathways as a means to prolong melanoma cell sensitivity to targeted therapies.176 Such an approach appears to be in its infancy, and therefore, additional therapeutic alternations investigated through single-cell analyses and prospective clinical trials are warranted.
Radiologic Adjuncts to Therapy
Enhanced radiologic approaches in patients with advanced melanoma may also provide clinical benefits in interpreting treatment responses. For example, a prospective study using dynamic 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging during early phases of systemic ICI in patients with advanced melanoma provided clinical impacts on PFS on the basis of several semiquantitative and quantitative parameters.177 Additional efforts in examining such an approach may provide further insights into these PFS trends with simultaneous improvements in monitoring treatment responses and guiding therapeutic decisions within this patient population.
In conclusion, this review provides a broad summary regarding the plethora of active and future therapeutic modalities involved in the management and treatment of patients with metastatic melanoma. Although the landscape of this disease has dramatically improved over the past several decades, limited targeted therapeutic options and increasing rates of disease progression on currently accepted standard-of-care regimens warrant continuous diligence in this field. Prospective efforts are therefore necessary to (1) further characterize the long-term response rates and optimal sequencing of our current therapeutic standards of care, (2) identify clinically significant novel targeted therapeutic agents, (3) explore the immunophenotypes associated with multiple biomarkers as a means to predict and monitor the efficacy and tolerance of various therapeutic modalities, and (4) recognize and ameliorate the pathways involved in therapeutic toxicity and resistance.
Igor Puzanov
Stock and Other Ownership Interests: Celldex (I)
Consulting or Advisory Role: Amgen, Iovance Biotherapeutics, Merck, Roche, Nouscom, Seneca Therapeutics
Lamya Hamad
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Marc S. Ernstoff
Stock and Other Ownership Interests: GE Healthcare, Bristol Myers Squibb
Research Funding: Alkermes (Inst)
No other potential conflicts of interest were reported.
Footnotes
See accompanying commentary on page 353
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin Switzer, Marc S. Ernstoff
Administrative support: Igor Puzanov
Collection and assembly of data: Igor Puzanov, Marc S. Ernstoff
Data analysis and interpretation: Igor Puzanov, Joseph J. Skitzki, Lamya Hamad, Marc S. Ernstoff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Managing Metastatic Melanoma in 2022: A Clinical Review
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Igor Puzanov
Stock and Other Ownership Interests: Celldex (I)
Consulting or Advisory Role: Amgen, Iovance Biotherapeutics, Merck, Roche, Nouscom, Seneca Therapeutics
Lamya Hamad
Consulting or Advisory Role: Bristol Myers Squibb Foundation
Marc S. Ernstoff
Stock and Other Ownership Interests: GE Healthcare, Bristol Myers Squibb
Research Funding: Alkermes (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Song X, Zhao Z, Barber B, et al. : Overall survival in patients with metastatic melanoma. Curr Med Res Opin 31:987-991, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Matthews NH, Li WQ, Qureshi AA, et al. : Epidemiology of melanoma, in Ward WH, Farma JM (eds): Cutaneous Melanoma: Etiology and Therapy. Brisbane, AU, Codon Publications, 2017 [PubMed] [Google Scholar]
- 3.SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. https://seer.cancer.gov/explorer [Google Scholar]
- 4.Wong VK, Lubner MG, Menias CO, et al. : Clinical and imaging features of noncutaneous melanoma. AJR Am J Roentgenol 208:942-959, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Viale PH: The American Cancer Society's facts & figures: 2020 edition. J Adv Pract Oncol 11:135-136, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapp UR, Goldsborough MD, Mark GE, et al. : Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci USA 80:4218-4222, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H, Bignell GR, Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Flaherty KT, Schiller J, Schuchter LM, et al. : A phase I trial of the oral, multikinase inhibitor sorafenib in combination with carboplatin and paclitaxel. Clin Cancer Res 14:4836-4842, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KT, Lee SJ, Zhao F, et al. : Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31:373-379, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty KT, Puzanov I, Kim KB, et al. : Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809-819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies AM, Wilmott JS, Drummond M, et al. : Clinicopathologic features associated with efficacy and long-term survival in metastatic melanoma patients treated with BRAF or combined BRAF and MEK inhibitors. Cancer 121:3826-3835, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Kunz M, Dannemann M, Kelso J: High-throughput sequencing of the melanoma genome. Exp Dermatol 22:10-17, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Flaherty KT, Robert C, Hersey P, et al. : Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367:107-114, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Long GV, Stroyakovskiy D, Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877-1888, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Trojaniello C, Luke JJ, Ascierto PA: Therapeutic advancements across clinical stages in melanoma, with a focus on targeted immunotherapy. Front Oncol 11:670726, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez-Castaneda LD, Nova JA, Tovar-Parra JD: Frequency of mutations in BRAF, NRAS, and KIT in different populations and histological subtypes of melanoma: A systemic review. Melanoma Res 30:62-70, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham DDM, Guhan S, Tsao H: KIT and melanoma: Biological insights and clinical implications. Yonsei Med J 61:562-571, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, Corless CL, Giobbie-Hurder A, et al. : Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 31:3182-3190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Alvarez A, Ortiz C, Munoz-Couselo E: Current perspectives and novel strategies of NRAS-mutant melanoma. Onco Targets Ther 14:3709-3719, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakob JA, Bassett RL Jr, Ng CS, et al. : NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 118:4014-4023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dummer R, Schadendorf D, Ascierto PA, et al. : Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:435-445, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Rose AAN, Armstrong SM, Hogg D, et al. : Biologic subtypes of melanoma predict survival benefit of combination anti-PD1+anti-CTLA4 immune checkpoint inhibitors versus anti-PD1 monotherapy. J Immunother Cancer 9:e001642, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiuru M, Busam KJ: The NF1 gene in tumor syndromes and melanoma. Lab Invest 97:146-157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrita R, Mitra S, Sanna A, et al. : The role of PTEN loss in immune escape, melanoma prognosis and therapy response. Cancers (Basel) 12:742, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Semir D, Bezrookove V, Nosrati M, et al. : PHIP as a therapeutic target for driver-negative subtypes of melanoma, breast, and lung cancer. Proc Natl Acad Sci USA 115:E5766-E5775, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe SP, Luber B, Makell M, et al. : From validity to clinical utility: The influence of circulating tumor DNA on melanoma patient management in a real-world setting. Mol Oncol 12:1661-1672, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forschner A, Battke F, Hadaschik D, et al. : Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma—Results of a prospective biomarker study. J Immunother Cancer 7:180, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Dong M, Shui Y, et al. : A pooled analysis of the prognostic value of PD-L1 in melanoma: Evidence from 1062 patients. Cancer Cell Int 20:96, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Wang F, Yan Y, et al. : Prognostic and clinicopathological value of PD-L1 in melanoma: A meta-analysis. Am J Med Sci 359:339-346, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Fu Q, Chen N, Ge C, et al. : Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 8:1593806, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keung EZ, Gershenwald JE: The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert Rev Anticancer Ther 18:775-784, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershenwald JE, Scolyer RA, Hess KR, et al. : Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472-492, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascierto PA, Ribas A, Larkin J, et al. : Impact of initial treatment and prognostic factors on postprogression survival in BRAF-mutated metastatic melanoma treated with dacarbazine or vemurafenib +/- cobimetinib: A pooled analysis of four clinical trials. J Transl Med 18:294, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong A, Williams M, Milne D, et al. : Clinical and palliative care outcomes for patients of poor performance status treated with antiprogrammed death-1 monoclonal antibodies for advanced melanoma. Asia Pac J Clin Oncol 13:385-390, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Kitano S, Takahashi A, et al. : Nivolumab for advanced melanoma: Pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 7:77404-77415, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridolfi L, De Rosa F, Petracci E, et al. : Anti-PD1 antibodies in patients aged >/= 75 years with metastatic melanoma: A retrospective multicentre study. J Geriatr Oncol 11:515-522, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Johnpulle RA, Conry RM, Sosman JA, et al. : Responses to immune checkpoint inhibitors in nonagenarians. Oncoimmunology 5:e1234572, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andtbacka RH, Kaufman HL, Collichio F, et al. : Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33:2780-2788, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Harrington KJ, Andtbacka RH, Collichio F, et al. : Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: Subanalysis of the phase III OPTiM trial. Onco Targets Ther 9:7081-7093, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faries MB, Mozzillo N, Kashani-Sabet M, et al. : Long-term survival after complete surgical resection and adjuvant immunotherapy for distant melanoma metastases. Ann Surg Oncol 24:3991-4000, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Gamboa AC, Lowe M, Yushak ML, et al. : Surgical considerations and systemic therapy of melanoma. Surg Clin North Am 100:141-159, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nevala WK, Vachon CM, Leontovich AA, et al. : Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 15:1931-1939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janavicius M, Lachej N, Anglickiene G, et al. : Outcomes of treatment for melanoma brain metastases. J Skin Cancer 2020:7520924, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto M, Serizawa T, Shuto T, et al. : Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol 15:387-395, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Hong AM, Fogarty GB, Dolven-Jacobsen K, et al. : Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: A multicenter, randomized phase III trial. J Clin Oncol 37:3132-3141, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Palma DA, Olson R, Harrow S, et al. : Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 38:2830-2838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodet O, Nemejcova K, Strnadova K, et al. : The abscopal effect in the era of checkpoint inhibitors. Int J Mol Sci 22:7204, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conlon KC, Miljkovic MD, Waldmann TA: Cytokines in the treatment of cancer. J Interferon Cytokine Res 39:6-21, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkins MB, Lotze MT, Dutcher JP, et al. : High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105-2116, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Kim JH, Lee KJ, Lee SW: Cancer immunotherapy with T-cell targeting cytokines: IL-2 and IL-7. BMB Rep 54:21-30, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim C, Lee CW, Kovacic L, et al. : Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist 15:765-771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwala SS, Kirkwood JM: Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 5:144-151, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Chapman PB, Einhorn LH, Meyers ML, et al. : Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 17:2745-2751, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Bedikian AY, Johnson MM, Warneke CL, et al. : Systemic therapy for unresectable metastatic melanoma: Impact of biochemotherapy on long-term survival. J Immunotoxicol 5:201-207, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Pflugfelder A, Eigentler TK, Keim U, et al. : Effectiveness of carboplatin and paclitaxel as first- and second-line treatment in 61 patients with metastatic melanoma. PLoS One 6:e16882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez DG, Suman VJ, Fitch TR, et al. : Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: A North Central Cancer Treatment Group study, N047A. Cancer 115:119-127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ives NJ, Stowe RL, Lorigan P, et al. : Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: A meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol 25:5426-5434, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Small EJ, Tchekmedyian NS, Rini BI, et al. : A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 13:1810-1815, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Brahmer JR, Lacchetti C, Schneider BJ, et al. : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical practice guideline. J Clin Oncol 36:1714-1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puzanov I, Diab A, Abdallah K, et al. : Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5:95, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander W, Attwood K, Catalfamo K, et al. : Using the tumor microenvironment to identify predictors of immunotoxicity to checkpoint inhibitors. J Clin Oncol 39, 2021. (abstr 2545) [Google Scholar]
- 62.Hodi FS, O'Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol 16:522-530, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 65.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol 39, 2021. (abstr 9506) [Google Scholar]
- 66.Ascierto PA, Del Vecchio M, Robert C, et al. : Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 18:611-622, 2017 [DOI] [PubMed] [Google Scholar]
- 67.Ascierto PA, Del Vecchio M, Mackiewicz A, et al. : Overall survival at 5 years of follow-up in a phase III trial comparing ipilimumab 10 mg/kg with 3 mg/kg in patients with advanced melanoma. J Immunother Cancer 8:e000391, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romano E, Kusio-Kobialka M, Foukas PG, et al. : Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA 112:6140-6145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reilley MJ, Morrow B, Ager CR, et al. : TLR9 activation cooperates with T cell checkpoint blockade to regress poorly immunogenic melanoma. J Immunother Cancer 7:323, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tarhini A, Lin Y, Lin H, et al. : Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-alpha2b in locally/regionally advanced melanoma: Safety, efficacy and impact on T-cell repertoire. J Immunother Cancer 6:112, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stone EL, Carter KP, Wagner EK, et al. : Lack of blocking activity in anti-CTLA-4 antibodies reduces toxicity, but not anti-tumor efficacy. bioRxiv 10.1101/2021.07.12.452090 [DOI] [Google Scholar]
- 72.Robert C, Ribas A, Schachter J, et al. : Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239-1251, 2019 [DOI] [PubMed] [Google Scholar]
- 73.Robert C, Long GV, Brady B, et al. : Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320-330, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Hodi FS, Chesney J, Pavlick AC, et al. : Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558-1568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long GV, Atkinson V, Lo S, et al. : Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol 19:672-681, 2018 [DOI] [PubMed] [Google Scholar]
- 76.Tawbi HA, Forsyth PA, Algazi A, et al. : Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379:722-730, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schvartsman G, Taranto P, Glitza IC, et al. : Management of metastatic cutaneous melanoma: Updates in clinical practice. Ther Adv Med Oncol 11:1758835919851663, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. : Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol 4:98-101, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Postow MA, Goldman DA, Shoushtari AN, et al. : A phase II study to evaluate the need for > two doses of nivolumab + ipilimumab combination (combo) immunotherapy. J Clin Oncol 38, 2020. (abstr 10003) [Google Scholar]
- 80.Lebbe C, Meyer N, Mortier L, et al. : Two dosing regimens of nivolumab (NIVO) plus ipilimumab (IPI) for advanced (adv) melanoma: Three-year results of CheckMate 511. J Clin Oncol 39, 2021. (abstr 9516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piulats JM, Espinosa E, de la Cruz Merino L, et al. : Nivolumab plus ipilimumab for treatment-naive metastatic uveal melanoma: An open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol 39:586-598, 2021 [DOI] [PubMed] [Google Scholar]
- 82.Najjar YG, Navrazhina K, Ding F, et al. : Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: A multicenter, retrospective study. J Immunother Cancer 8:e000331, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelster MS, Gruschkus SK, Bassett R, et al. : Nivolumab and ipilimumab in metastatic uveal melanoma: Results from a single-arm phase II study. J Clin Oncol 39:599-607, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ribas A, Hodi FS, Callahan M, et al. : Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 368:1365-1366, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Hu-Lieskovan S, Mok S, Homet Moreno B, et al. : Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med 7:279ra41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lelliott EJ, McArthur GA, Oliaro J, et al. : Immunomodulatory effects of BRAF, MEK, and CDK4/6 inhibitors: Implications for combining targeted therapy and immune checkpoint blockade for the treatment of melanoma. Front Immunol 12:661737, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. : KEYNOTE-022 part 3: A randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 8:e001806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Long GV, Lebbe C, Atkinson V, et al. : The anti–PD-1 antibody spartalizumab (S) in combination with dabrafenib (D) and trametinib (T) in previously untreated patients (pts) with advanced BRAF V600–mutant melanoma: Updated efficacy and safety from parts 1 and 2 of COMBI-i. J Clin Oncol 37, 2019. (abstr 9531) [Google Scholar]
- 89.Stachyra-Strawa P, Ciesielka M, Janiszewski M, et al. : The role of immunotherapy and molecular-targeted therapy in the treatment of melanoma (Review). Oncol Rep 46:158, 2021 [DOI] [PubMed] [Google Scholar]
- 90.Larkin J, Sarnaik A, Chesney JA, et al. : Lifileucel (LN-144), a cryopreserved autologous tumor infiltrating lymphocyte (TIL) therapy in patients with advanced melanoma: Evaluation of impact of prior anti-PD-1 therapy. J Clin Oncol 39, 2021. (abstr 9505) [Google Scholar]
- 91.Long GV, Dummer R, Hamid O, et al. : Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol 20:1083-1097, 2019 [DOI] [PubMed] [Google Scholar]
- 92.Van den Eynde BJ, van Baren N, Baurain J-F: Is there a clinical future for IDO1 inhibitors after the failure of epacadostat in melanoma? Annu Rev Cancer Biol 4:241-256, 2020 [Google Scholar]
- 93.Nathan P, Hassel JC, Rutkowski P, et al. : Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 385:1196-1206, 2021 [DOI] [PubMed] [Google Scholar]
- 94.Middleton MR, McAlpine C, Woodcock VK, et al. : Tebentafusp, A TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res 26:5869-5878, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fallico M, Raciti G, Longo A, et al. : Current molecular and clinical insights into uveal melanoma (Review). Int J Oncol 58:10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hamid O, Cowey CL, Offner M, et al. : Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel) 11:1642, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carvajal RD, Antonescu CR, Wolchok JD, et al. : KIT as a therapeutic target in metastatic melanoma. JAMA 305:2327-2334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nassar KW, Tan AC: The mutational landscape of mucosal melanoma. Semin Cancer Biol 61:139-148, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tetzlaff MT, Pattanaprichakul P, Wargo J, et al. : Utility of BRAF V600E immunohistochemistry expression pattern as a surrogate of BRAF mutation status in 154 patients with advanced melanoma. Hum Pathol 46:1101-1110, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanni I, Tanda ET, Spagnolo F, et al. : The current state of molecular testing in the BRAF-mutated melanoma landscape. Front Mol Biosci 7:113, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng H, Liu F, Zhou H, et al. : Individualized treatment strategy for cutaneous melanoma: Where are we now and where are we going? Front Oncol 11:775100, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavlick AC, Fecher L, Ascierto PA, et al. : Frontline therapy for BRAF-mutated metastatic melanoma: How do you choose, and is there one correct answer? Am Soc Clin Oncol Ed Book 39:564-571, 2019 [DOI] [PubMed] [Google Scholar]
- 103.Pavlick AC, Zhao R, Lee CH, et al. : First-line immunotherapy versus targeted therapy in patients with BRAF-mutant advanced melanoma: A real-world analysis. Future Oncol 17:689-699, 2021 [DOI] [PubMed] [Google Scholar]
- 104.Ackerman A, Klein O, McDermott DF, et al. : Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer 120:1695-1701, 2014 [DOI] [PubMed] [Google Scholar]
- 105.Ascierto PA, Simeone E, Sileni VC, et al. : Sequential treatment with ipilimumab and BRAF inhibitors in patients with metastatic melanoma: Data from the Italian cohort of the ipilimumab expanded access program. Cancer Invest 32:144-149, 2014 [DOI] [PubMed] [Google Scholar]
- 106.Corti F, Randon G, Bini M, et al. : Risk of disease progression (PD) following discontinuation of BRAF±MEK targeted therapies for reasons other than PD in patients (pts) with metastatic or unresectable melanoma. J Clin Oncol 38, 2020. (abstr 10053) [Google Scholar]
- 107.Ascierto PA, Mandala M, Ferrucci PF, et al. : SECOMBIT: The best sequential approach with combo immunotherapy (ipilimumab/nivolumab) and combo target therapy (encorafenib/binimetinib) in patients with BRAF mutated metastatic melanoma: A phase II randomized study. Presented at ESMO Congress 2021, Paris, France, September 20, 2021 (abstr LBA40)
- 108.Atkins MB, Lee SJ, Chmielowski B, et al. : DREAMseq (doublet, randomized evaluation in advanced melanoma sequencing): A phase III trial—ECOG-ACRIN EA6134. Presented at American Society of Clinical Oncology (ASCO) Plenary Series 2021, November 16, 2021 (abstr 356154)
- 109.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eggermont AMM, Blank CU, Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 [DOI] [PubMed] [Google Scholar]
- 111.Sullivan RJ, Atkins MB, Kirkwood JM, et al. : An update on the Society for Immunotherapy of Cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: Version 2.0. J Immunother Cancer 6:44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knispel S, Gassenmaier M, Menzies AM, et al. : Outcome of melanoma patients with elevated LDH treated with first-line targeted therapy or PD-1-based immune checkpoint inhibition. Eur J Cancer 148:61-75, 2021 [DOI] [PubMed] [Google Scholar]
- 113.Moreira A, Heinzerling L, Bhardwaj N, et al. : Current melanoma treatments: Where do we stand? Cancers (Basel) 13:221, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stege H, Haist M, Schultheis M, et al. : Discontinuation of BRAF/MEK-directed targeted therapy after complete remission of metastatic melanoma—A retrospective multicenter ADOReg study. Cancers (Basel) 13:2312, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marron TU, Ryan AE, Reddy SM, et al. : Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer 9:e001901, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mulder E, de Joode K, Litiere S, et al. : Early discontinuation of PD-1 blockade upon achieving a complete or partial response in patients with advanced melanoma: The multicentre prospective Safe Stop trial. BMC Cancer 21:323, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davies MA: Is it safe to stop anti–PD-1 immunotherapy in patients with metastatic melanoma who achieve a complete response? J Clin Oncol 38:1645-1647, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Warburton L, Meniawy TM, Calapre L, et al. : Stopping targeted therapy for complete responders in advanced BRAF mutant melanoma. Sci Rep 10:18878, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davies MA, Saiag P, Robert C, et al. : Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 18:863-873, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Margolin K, Ernstoff MS, Hamid O, et al. : Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol 13:459-465, 2012 [DOI] [PubMed] [Google Scholar]
- 121.Tawbi HA, Forsyth PA, Hodi FS, et al. : Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro Oncol 23:1961-1973, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Becco P, Gallo S, Poletto S, et al. : Melanoma brain metastases in the era of target therapies: An overview. Cancers (Basel) 12:1640, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liermann J, Winkler JK, Syed M, et al. : Stereotactic radiosurgery with concurrent immunotherapy in melanoma brain metastases is feasible and effective. Front Oncol 10:592796, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makawita S, Tawbi HA: Nonsurgical management of melanoma brain metastasis: Current therapeutics, challenges, and strategies for progress. Am Soc Clin Oncol Ed Book 41:79-90, 2021 [DOI] [PubMed] [Google Scholar]
- 125.Kapp K, Volz B, Oswald D, et al. : Beneficial modulation of the tumor microenvironment and generation of anti-tumor responses by TLR9 agonist lefitolimod alone and in combination with checkpoint inhibitors. Oncoimmunology 8:e1659096, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Idera Pharmaceuticals Announces Results From ILLUMINATE-301 Trial of Tilsotolimod + Ipilimumab in Anti-PD-1 Refractory Advanced Melanoma. Press Release. Idera Pharmaceuticals, 2021. http://bit.ly/3r5gxDg [Google Scholar]
- 127.Ribas A, Medina T, Kirkwood JM, et al. : Overcoming PD-1 blockade resistance with CpG-A toll-like receptor 9 agonist Vidutolimod in patients with metastatic melanoma. Cancer Discov 11:2998-3007, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreno-Smith M, Lutgendorf SK, Sood AK: Impact of stress on cancer metastasis. Future Oncol 6:1863-1881, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohammadpour H, MacDonald CR, Qiao G, et al. : Beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest 129:5537-5552, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thaker PH, Lutgendorf SK, Sood AK: The neuroendocrine impact of chronic stress on cancer. Cell Cycle 6:430-433, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Yang EV, Kim SJ, Donovan EL, et al. : Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav Immun 23:267-275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gandhi S, Pandey MR, Attwood K, et al. : Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: Safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res 27:87-95, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schachter J, Ribas A, Long GV, et al. : Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 390:1853-1862, 2017 [DOI] [PubMed] [Google Scholar]
- 134.Lipson EJ, Tawbi HA-H, Schadendorf D, et al. : Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: Primary phase III results from RELATIVITY-047 (CA224-047). J Clin Oncol 39, 2021. (abstr 9503) [Google Scholar]
- 135.Choi JW, Kim YJ, Yun KA, et al. : The prognostic significance of VISTA and CD33-positive myeloid cells in cutaneous melanoma and their relationship with PD-1 expression. Sci Rep 10:14372, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Powderly J, Patel MR, Lee JJ, et al. : CA-170, a first in class oral small molecule dual inhibitor of immune checkpoints PD-L1 and VISTA, demonstrates tumor growth inhibition in pre-clinical models and promotes T cell activation in phase 1 study. Ann Oncol 28:v405-v406, 2017 [Google Scholar]
- 137.Acharya N, Sabatos-Peyton C, Anderson AC: Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer 8:e000911, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Umansky V, Shevchenko I, Bazhin AV, et al. : Extracellular adenosine metabolism in immune cells in melanoma. Cancer Immunol Immunother 63:1073-1080, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Passarelli A, Tucci M, Mannavola F, et al. : The metabolic milieu in melanoma: Role of immune suppression by CD73/adenosine. Tumour Biol 42:1010428319837138, 2019 [DOI] [PubMed] [Google Scholar]
- 140.Young A, Ngiow SF, Madore J, et al. : Targeting adenosine in BRAF-mutant melanoma reduces tumor growth and metastasis. Cancer Res 77:4684-4696, 2017 [DOI] [PubMed] [Google Scholar]
- 141.Algazi AP, Twitty CG, Tsai KK, et al. : Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res 26:2827-2837, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim JH, Kim YM, Choi D, et al. : Hybrid Fc-fused interleukin-7 induces an inflamed tumor microenvironment and improves the efficacy of cancer immunotherapy. Clin Transl Immunol 9:e1168, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. : IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother 29:313-319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mullard A: Restoring IL-2 to its cancer immunotherapy glory. Nat Rev Drug Discov 20:163-165, 2021 [DOI] [PubMed] [Google Scholar]
- 145.Diab A, Tykodi SS, Daniels GA, et al. : Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J Clin Oncol 39:2914-2925, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bentebibel S-E, Diab A: Cytokines in the treatment of melanoma. Curr Oncol Rep 23:83, 2021 [DOI] [PubMed] [Google Scholar]
- 147.Wrangle JM, Awad MM, Badin FB, et al. : Preliminary data from QUILT 3.055: A phase 2 multi-cohort study of N803 (IL-15 superagonist) in combination with checkpoint inhibitors (CPI). J Clin Oncol 39, 2021. (abstr 2596) [Google Scholar]
- 148.Hao Y, Baker D, Ten Dijke P: TGF-beta-mediated epithelial-mesenchymal transition and cancer metastasis. Int J Mol Sci 20:2767, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Teixeira AF, Ten Dijke P, Zhu HJ: On-target anti-TGF-beta therapies are not succeeding in clinical cancer treatments: What are remaining challenges? Front Cell Dev Biol 8:605, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cane S, Van Snick J, Uyttenhove C, et al. : TGFbeta1 neutralization displays therapeutic efficacy through both an immunomodulatory and a non-immune tumor-intrinsic mechanism. J Immunother Cancer 9:e001798, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gellrich FF, Schmitz M, Beissert S, et al. : Anti-PD-1 and novel combinations in the treatment of melanoma—An update. J Clin Med 9:223, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Puzanov I, Milhem MM, Minor D, et al. : Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol 34:2619-2626, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gogas HJ, Ribas A, Chesney J, et al. : MASTERKEY-265: A phase III, randomized, placebo (Pbo)-controlled study of talimogene laherparepvec (T) plus pembrolizumab (P) for unresectable stage IIIB–IVM1c melanoma (MEL) [abstract]. Presented at European Society for Medical Oncology Congress, Paris, France, September 15-21, 2021 (abstr 1037O)
- 154.Kuryk L, Moller AW, Jaderberg M: Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 8:e1532763, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beasley GM, Nair SK, Farrow NE, et al. : Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J Immunother Cancer 9:e002203, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Andtbacka RHI, Curti B, Daniels GA, et al. : Clinical responses of oncolytic coxsackievirus A21 (V937) in patients with unresectable melanoma. J Clin Oncol 39:3829-3838, 2021 [DOI] [PubMed] [Google Scholar]
- 157.Aroldi F, Sacco J, Harrington K, et al. : 421 Initialresults of a phase 1 trial of RP2, a first in class, enhanced potency, anti-CTLA-4 antibody expressing, oncolytic HSV as single agent and combined with nivolumab in patients with solid tumors. J Immunother Cancer 8:A256-A257, 2020 [Google Scholar]
- 158.Breitbach CJ, Bell JC, Hwang TH, et al. : The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother 4:25-31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fein S, Guttman L, Marino MT, et al. : Abstract CT243: A first-in-human phase 1 ascending, multiple dose, safety and tolerance study of Vaxinia (CF33-hNIS), a novel chimeric oncolytic poxvirus, administered intratumorally or intravenously in adult patients with mixed advanced solid tumors (MAST). Cancer Res 80, 2020. (abstr CT243) [Google Scholar]
- 160.Ring SS, Cupovic J, Onder L, et al. : Viral vector-mediated reprogramming of the fibroblastic tumor stroma sustains curative melanoma treatment. Nat Commun 12:4734, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Middleton MR, Hoeller C, Michielin O, et al. : Intratumoural immunotherapies for unresectable and metastatic melanoma: Current status and future perspectives. Br J Cancer 123:885-897, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tarhini AA, Joshi I, Garner F: Sargramostim and immune checkpoint inhibitors: Combinatorial therapeutic studies in metastatic melanoma. Immunotherapy 13:1011-1029, 2021 [DOI] [PubMed] [Google Scholar]
- 163.Hodi FS, Lee S, McDermott DF, et al. : Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 312:1744-1753, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.An Q, Liu Z: Comparative efficacy and safety of combination therapies for advanced melanoma: A network meta-analysis. BMC Cancer 19:43, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chesney JA, Larkin JM, Kirkwood JM, et al. : Abstract CT008: Lifileucel (LN-144), a cryopreserved autologous tumor infiltrating lymphocyte (TIL) therapy in patients with advanced (unresectable or metastatic) melanoma: Durable duration of response at 28 month follow up. Cancer Res 81, 2021. (abstr CT008) [Google Scholar]
- 166.Johnson LA, Morgan RA, Dudley ME, et al. : Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114:535-546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merhavi-Shoham E, Itzhaki O, Markel G, et al. : Adoptive cell therapy for metastatic melanoma. Cancer J 23:48-53, 2017 [DOI] [PubMed] [Google Scholar]
- 168.Li J, Li W, Huang K, et al. : Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J Hematol Oncol 11:22, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iida N, Dzutsev A, Stewart CA, et al. : Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342:967-970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Routy B, Le Chatelier E, Derosa L, et al. : Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359:91-97, 2018 [DOI] [PubMed] [Google Scholar]
- 171.Spencer CN, Gopalakrishnan V, McQuade JL, et al. : Abstract 2838: The gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors. Tumor Biol 79, 2019. (abstr 2838) [Google Scholar]
- 172.Gopalakrishnan V, Spencer CN, Nezi L, et al. : Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359:97-103, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andrews MC, Duong CPM, Gopalakrishnan V, et al. : Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med 27:1432-1441, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Arance AM, de la Cruz-Merino L, Petrella TM, et al. : Lenvatinib (len) plus pembrolizumab (pembro) for patients (pts) with advanced melanoma and confirmed progression on a PD-1 or PD-L1 inhibitor: Updated findings of LEAP-004. J Clin Oncol 39, 2021. (abstr 9504) [Google Scholar]
- 175.Fruehauf J, Lutzky J, McDermott D, et al. : Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res 17:7462-7469, 2011 [DOI] [PubMed] [Google Scholar]
- 176.Khaliq M, Manikkam M, Martinez ED, et al. : Epigenetic modulation reveals differentiation state specificity of oncogene addiction. Nat Commun 12:1536, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sachpekidis C, Hassel JC, Kopp-Schneider A, et al. : Quantitative dynamic (18)F-FDG PET/CT in survival prediction of metastatic melanoma under PD-1 inhibitors. Cancers (Basel) 13:1019, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]


