Abstract
Objective:
Surgical ablation of atrial fibrillation (AF) is indicated both in patients with AF undergoing concomitant cardiac surgery and in those who have not responded to medical and/or catheter-based ablation therapy. This study examined our long-term outcomes following the Cox-Maze IV procedure (CMP-IV).
Methods:
Between May 2003 and March 2018, 853 patients underwent either biatrial CMP-IV (n = 765) or a left-sided CMP-IV (n = 88) lesion set with complete isolation of the posterior left atrium. Freedom from atrial tachyarrhythmia (ATA) was assessed for up to 10 years. Rhythm outcomes were compared in multiple subgroups. Predictors of recurrence were determined using Fine–Gray regression, allowing for death as the competing risk.
Results:
The majority of patients (513/853, 60%) had nonparoxysmal AF. Twenty-four percent of patients (201/853) had not responded to at least 1 catheter-based ablation. Prolonged monitoring was used in 76% (647/853) of patients during their follow-up. Freedom from ATA was 92% (552/598), 84% (213/253), and 77% (67/87) at 1, 5, and 10 years, respectively. By competing risk analysis, incidence of first ATA recurrence was 11%, 23%, and 35% at 1, 5, and 10 years, respectively. On Fine–Gray regression, age, peripheral vascular disease, nonparoxysmal AF, left atrial size, early postoperative ATAs, and absence of sinus rhythm at discharge were the predictors of first ATA recurrence over 10 years of follow-up.
Conclusions:
The CMP-IV had an excellent long-term efficacy at maintaining sinus rhythm. At late follow-up, the results of the CMP-IV remained superior to those reported for catheter ablation and other forms of surgical ablation for AF. Age, left atrial size, and nonparoxysmal AF were the most relevant predictors of late recurrence.
Keywords: surgical ablation, Cox-Maze procedure, atrial fibrillation, long-term outcomes
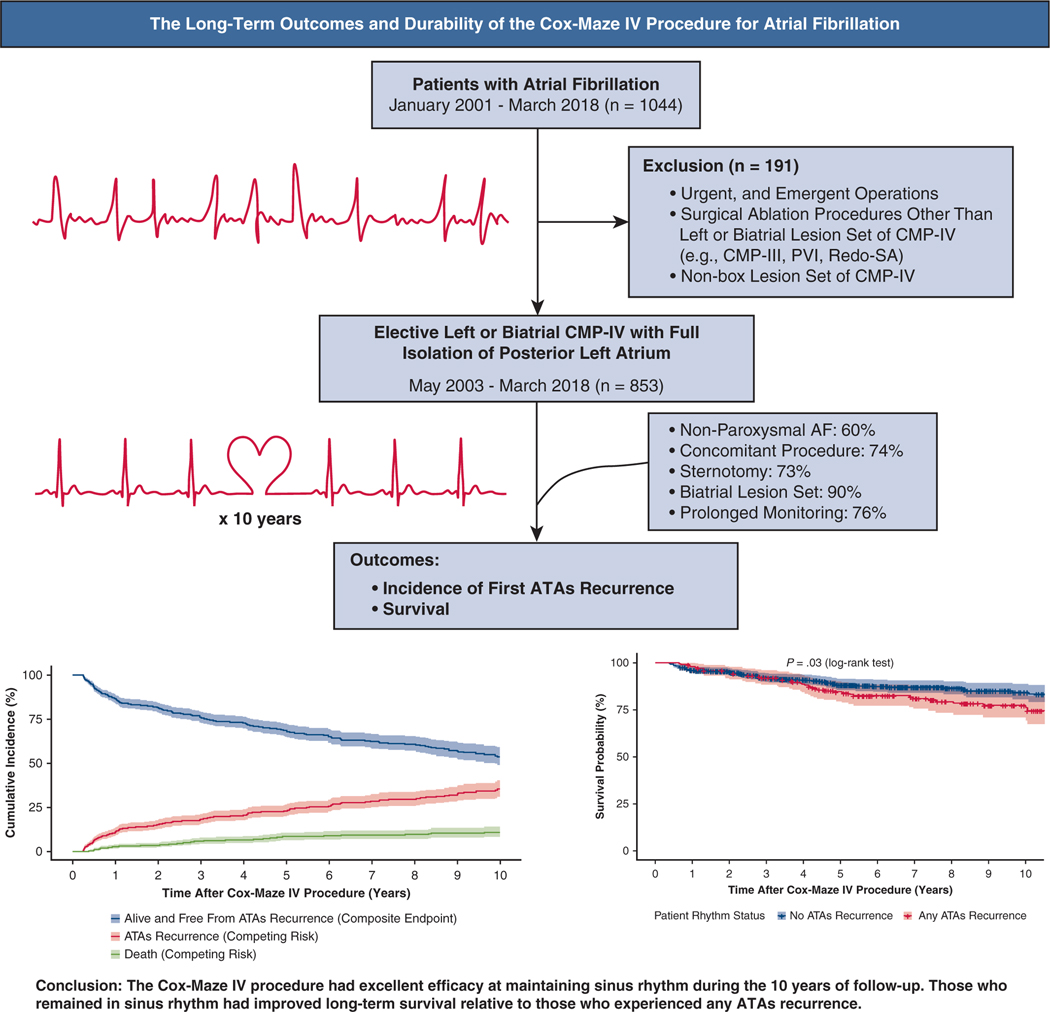
Graphical Abstract
ATA-free survival and prevalence of events (ATA recurrence and death) following CMP-IV.
Atrial fibrillation (AF) is the most common sustained arrhythmia in the world, with an increasing incidence corresponding to patient age. It occurs with a greater prevalence in men than women.1,2 Due to the poor efficacy of antiarrhythmic drugs and their adverse side effects, there has been considerable interest in the interventional treatment of AF, including both catheter ablation and surgical ablation (SA). The most effective surgical option for the management of AF has been the Cox-Maze procedure (CMP), introduced by James Cox and colleagues in 1987.3 The “cut-and-sew” version, known as the CMP-III, involves making surgical incisions in both the left and right atria to interrupt the macro-reentrant circuits thought to be responsible for AF propagation.3,4 This procedure has had excellent long-term success rates.4–8 Despite its proven efficacy, its widespread adoption was limited due to its technical complexity, a significant increase in cardiopulmonary bypass time, and its morbidity.9,10
On the basis of extensive clinical and ongoing animal investigations at our institution, revisions to the original “cut-and-sew” technique led to the fourth iteration of the procedure. Introduced clinically in 2002 by Damiano and colleagues, the Cox-Maze IV procedure (CMP-IV) uses bipolar radiofrequency and cryoablation devices to replace most of the surgical incisions of its predecessor.9,11,12 The current iteration includes superior and inferior connecting lesion between the isolated pulmonary veins, which forms a “box lesion,” and completely isolates the entire posterior left atrium (LA). The box lesion has been performed exclusively since 200411 (Appendix E1).
The modifications of the CMP-IV substantially simplified the CMP and made it technically easier to perform. This allowed for the development of minimally invasive approaches and more widespread adoption, allowing for many more patients to receive SA at the time of concomitant cardiac surgery. Before 2000, it was estimated that only several hundred SA procedures for AF were performed worldwide. In 2018, this number had increased to more than 30,000 by industry estimates. Using the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database, Badhwar and colleagues13 reported an overall increase of 50% in performing concomitant SA from July 2011 to June 2014.
The CMP-IV has proven to be as effective as its predecessor in restoring sinus rhythm while reducing operative morbidity and mortality.9,10,14–16 It has been shown to be equally as effective in patients undergoing SA for lone AF and concomitant cardiac surgery, including coronary artery bypass grafting, mitral valve, aortic valve, and septal myectomy procedures.16–20 In addition, the CMP-IV has been shown to have similar results for patients with both paroxysmal and nonparoxysmal (persistent and long-standing persistent) forms of AF.15,21
Our group and others have previously reported early- and mid-term outcomes following the CMP-IVand have demonstrated good results.21–24 Although a few studies with long-term follow-up are available (more than 7 years), data demonstrating long-term outcomes following SA for AF in a sizable patient cohort are extremely limited.10,25,26 In this report, we examined our 10-year outcomes in a large cohort of patients undergoing CMP-IV for AF at a single center.
METHODS
This study was approved by the Washington University School of Medicine institutional review board (#201105322, current approval date: December 18, 2018). Informed consent and permission for release of information were obtained from all patients. Our institutional STS database was used for preoperative demographic data, operative details, and perioperative results using STS definitions for complications. Rhythm and other follow-up data were prospectively entered into our institutional AF outcomes research database. Missing data and survival were ascertained through chart review, contact with patients, and from referring physicians when needed. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was calculated on the basis of age, sex, renal impairment, extracardiac arteriopathy, previous cardiac surgery, chronic lung disease, active endocarditis, diabetes, New York Heart Association class, left ventricular function, recent myocardial infarction, urgency of operation, and weight of intervention. If data were not available, the variable was omitted from the EuroSCORE II calculation. Preoperative diabetes was not distinguished between patients who were or were not insulin dependent.
Patient Population
Between January 2001 and March 2018, 1044 patients underwent SA for refractory AF. CMP-III, redo-SA, as well as any other SA techniques that did not completely isolate the posterior LA were excluded (n = 191). The remaining patients underwent either biatrial CMP-IV (n = 765) or a left-sided Cox-Maze (n = 88) lesion set, between May 2003 and March 2018. The operative details of the CMP-IV lesion set through a sternotomy or right minithoracotomy have been described previously by our group.10,27 Figures E1 and E2 depict the complete lesion set as performed via sternotomy or right minithoracotomy, respectively. Preoperative and perioperative variables were retrospectively evaluated and compared in multiple subgroups.
The majority of our patients were referred from cardiologists or cardiac surgeons familiar with our work and outcomes. In addition, this procedure can be performed via a right minithoracotomy, which is more attractive to both patients and referring physicians.
Follow-up and Postoperative Care
Follow-up visits occurred at 3, 6, and 12 months and annually thereafter. History and physical examinations as well as electrocardiograms were obtained at each visit. Since 2006, prolonged monitoring (ie, 24-hour Holter monitoring, pacemaker interrogation, or implantable loop recorders [ILRs]) has been used at 3, 6, and 12 months and annually thereafter. Throughout the entire series, the majority of patients (647/853, 76%) underwent prolonged monitoring during their follow-up. Recurrence was defined according to the most recent consensus statement as any episode of AF, atrial flutter, or atrial tachycardia that lasted longer than 30 seconds.28 For those with ILRs, due to the sensitivity of the device, arrhythmia of 2 minutes or longer was captured, reviewed, and analyzed to determine recurrence. Any patient requiring an interventional procedure for rhythm control after the 90-day blanking period was deemed a failure. Patients were considered to be a success if they were both free from AF and free from antiarrhythmic drugs (AADs; class I/III), according to HRS guidelines.29 In this study, we examined freedom from recurrence in 2 ways. In the first way and in our opinion the most valid, we looked at freedom from atrial tachyarrhythmia (ATA) recurrence at each time point (ie, 6 months, 1, 5, and 10 years). In this analysis, a patient could have a 30-second recurrence at 1 year but can still be free from ATA at 5 years. In the second analysis, we considered any recurrence a permanent failure, and just examined time to first recurrence, with death as the competing risk. This is somewhat problematic, in that a patient could have a 40-second single episode of AF at 6 months, and no further recurrence for the next 10 years, and still be considered a failure.
As previously reported by our group, anticoagulation and prophylactic AADs were initiated in all patients early in the postoperative period unless contraindicated.17,21 Postoperative ATAs that were unresponsive to AADs were electrically cardioverted before discharge unless contraindicated. AADs were discontinued after 2 months in patients who were in sinus rhythm. Anticoagulation was discontinued in patients who were free from both ATAs and AADs on prolonged monitoring and who had no evidence of atrial stasis or thrombus on their postoperative transthoracic echocardiogram at the 3- to 6-month follow-up visit. Anticoagulation was discontinued 1 to 2 months after AADs were discontinued. This was done irrespective of patients’ CHA2DS2-VASc score.17,30
The average follow-up time was 4.1 ± 3.2 years (median 3.2 years, [interquartile range 1.1, 6.1]). At 1, 5, and 10 years, 83% (598/718), 73% (253/348), and 62% (87/141) of patients were available for follow-up, respectively. Only a few patients had ILRs. Less than 4% of patients at years 2 and 3 had data recovered from ILR. For years 5 and beyond, no data were collected from ILRs. This was not surprising, considering we do not routinely use ILR.
Freedom From ATAs and AADs
Early-, mid-, and long-term follow-up was defined as 1, 5, and 10 years, respectively, following CMP-IV. Freedom from ATAs on or off AADs at those time points was compared between paroxysmal (n = 340) and non-paroxysmal (n = 513) AF groups, and between stand-alone (n = 218) and concomitant (n = 635) CMP-IV. Of those who underwent concomitant operation, the majority (324/635, 51%), underwent a concomitant mitral valve procedure with or without tricuspid valve surgery. Concomitant procedures are outlined in Table E1.
Statistical Analysis
Continuous variables were reported as mean standard deviation, or median with interquartile range as appropriate. The Student t test was used to compare means of normally distributed continuous variables, whereas the Mann–Whitney U test was used for skewed distributions. Categorical variables were compared using either χ2 analysis or the Fisher exact test, as appropriate.
Composite end-point survival (freedom from first ATAs recurrence and death) was reported as a Kaplan–Meier estimate. The probability of being both alive and ATA-recurrence free is equivalent to the probability of experiencing neither of the competing risks, as described by Austin and colleagues.31 ATA recurrence was evaluated using competing risk methodology.32 Gray’s test was used to compare the incidence of first ATA recurrence between groups (paroxysmal vs nonparoxysmal AF and concomitant vs stand-alone CMP-IV). Death during the follow-up period served as the competing risk. Twenty-seven preoperative and perioperative characteristics that were deemed clinically relevant were evaluated using unadjusted univariable and adjusted multivariable Fine–Gray regression to identify predictors of first ATA recurrence. Patients who lacked rhythm follow-up beyond the 90-day blanking period were eliminated from the analysis. Lastly, binary logistic regression was used to identify predictors of postoperative pacemaker placement. Data analysis was performed using STATA, version 15.0 (Stata Statistical Software: Release 15; StataCorp LLC, College Station, Tex) and R 3.6.1 using the cmprsk package (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics
The average overall age at the time of surgery was 64.2 ± 11.5 years, and 516 of 853 (60.5%) patients were male. Of the overall cohort, 340 of 853 (40%) had paroxysmal AF and 513 of 853 (60%) had nonparoxysmal AF. The majority, 412 of 513 (80%), of patients in the nonparoxysmal group had long-standing persistent AF. Preoperative patient characteristics were compared between these groups and are shown in Table 1. Compared with the paroxysmal AF group, patients in the nonparoxysmal AF group tended to have a longer preoperative AF duration (median 4.8 [1.6, 9.0] years vs 2.0 [0.4, 5.8] years, P<.001), at least 1 failed catheter ablation (150/513 [29.2%] vs 51/340 [15.0%], P < .001), larger LA size (5.2 ± 1.0 cm vs 4.9 ± 1.0, P<.001), and lower left ventricular ejection fraction (54 ± 12 vs 57 ±11, P<.001). A greater proportion of patients in the nonparoxysmal group were male (328/513 [63.9%] vs 188/340 [55.3%], P=.012), and had a slightly lower median EuroSCORE II (median 2.6% [1.3, 5.2] vs 3.0% [1.8, 6.2], P =.008). However, the majority of patients with paroxysmal AF underwent concomitant operation, which increases EuroSCORE II (83.4% [285/340] vs 68.2% [350/513], P <.001).
TABLE 1.
Preoperative demographics
| Demographics | Overall (n = 853) | Paroxysmal AF (n = 340) | Nonparoxysmal AF (n = 513) | P value |
|---|---|---|---|---|
|
| ||||
| Age, y | 64.2 ± 11.5 | 64.0 ± 11.8 | 64.2 ± 11.3 | .793 |
| BMI, kg/m2 | 30.0 ± 6.9 | 29.7 ± 6.9 | 30.3 ± 6.9 | .247 |
| Male | 516 (60.5) | 188 (55.3) | 328 (63.9) | .012 |
| Long-standing persistent AF | 412 (48.3) | N/A | 412 (80.3) | N/A |
| Preoperative duration of AF, y, median [IQR] | 3.5 [0.8, 8.0] | 2.0 [0.4, 5.8] | 4.8 [1.6, 9.0] | <.001 |
| Left atrial size, cm | 5.1 ± 1.1 | 4.9 ± 1.0 | 5.2 ± 1.0 | <.001 |
| Failed catheter ablation | 201 (23.6) | 51 (15.0) | 150 (29.2) | <.001 |
| Preoperative pacemaker | 99 (11.6) | 39 (11.5) | 60 (11.7) | 1.000 |
| NYHA class III or IV symptoms | 490 (57.4) | 191 (56.2) | 299 (58.3) | .572 |
| LVEF, % | 56 ± 12 | 57 ± 11 | 54 ± 12 | <.001 |
| Hypertension | 608 (71.3) | 233 (68.5) | 375 (73.1) | .164 |
| Dyslipidemia | 501 (58.7) | 200 (58.8) | 301 (58.7) | 1.000 |
| Diabetes | 162 (19.0) | 70 (20.6) | 92 (17.9) | .373 |
| Chronic lung disease (moderate or greater) | 60 (7.0) | 20 (5.9) | 40 (7.8) | .339 |
| Peripheral vascular disease | 87 (10.2) | 35 (10.3) | 52 (10.1) | 1.000 |
| Renal failure | 37 (4.3) | 16 (5.0) | 21 (4.5) | .736 |
| Dialysis | 12 (1.4) | 5 (1.8) | 7 (1.6) | .774 |
| EuroSCORE II (%) (Median [IQR]) | 2.8 [1.4, 5.6] | 3.0 [1.8, 6.2] | 2.6 [1.3, 5.2] | .008 |
Values are n (%), or mean ± standard deviation, unless otherwise indicated. Statistically significant P values are in bold. AF, Atrial fibrillation; BMI, body mass index; N/A, not applicable; IQR, interquartile range; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; EuroSCORE, European System for Cardiac Operative Risk Evaluation.
Perioperative Results
The majority of patients underwent sternotomy (618/853 [72.5%]) and received CMP-IV with a concomitant operation (635/853 [74.4%]). Most patients received the biatrial lesion set (765/853 [89.7%]). Two hundred thirty-five (235/853 [27.5%]) patients underwent a right minithoracotomy approach. Table 2 summarizes the perioperative results and comparisons between the paroxysmal and nonparoxysmal groups. Stand-alone CMP-IV was used more commonly in patients with nonparoxysmal AF (163/513 [31.8%] vs 55/340 [16.2%], P <.001). The biatrial lesion set was used more often in patients with nonparoxysmal AF than in patients with paroxysmal AF (480/513 [93.6%] vs 285/340 [83.8%], P < .001). At our center, the left-sided only Cox-Maze lesion set was typically used on very selected patients with paroxysmal AF, no evidence of right atrial enlargement or tricuspid valve pathology, and a LA diameter of less than 5.0 cm.18,21 The only exceptions were in patients felt to be at prohibitive risk for a biatrial CMP-IV. Patients with nonparoxysmal AF were more likely to experience postoperative ATAs than patients with paroxysmal AF (308/513 [60.0%] vs 177/340 [52.1%], P = .024). More patients in the paroxysmal AF group were discharged home in sinus rhythm (310/340 [91.2%] vs 434/513 [84.6%], P = .002). The overall 30-day mortality was 3.3% (28/853). There were no differences between the 2 groups in 30-day mortality, postoperative pacemaker implantation, intensive care unit length of stay, hospital length of stay, or most major complications.
TABLE 2.
Perioperative data
| Demographics | Overall (n = 853) | Paroxysmal AF (n = 340) | Nonparoxysmal AF (n = 513) | P value |
|---|---|---|---|---|
|
| ||||
| Sternotomy* | 618 (72.5) | 242 (71.4) | 376 (73.4) | .530 |
| Concomitant procedure | 635 (74.4) | 285 (83.8) | 350 (68.2) | <.001 |
| Stand-alone CMP-IV | 218 (25.6) | 55 (16.2) | 163 (31.8) | <.001 |
| Biatrial CMP-IV lesion set | 765 (89.7) | 285 (83.8) | 480 (93.6) | <.001 |
| CBP time, min | 179 ± 50 | 182 ± 47 | 177 ± 52 | .216 |
| Crossclamp time, min | 87 ± 34 | 93 32 | 83 ± 35 | <.001 |
| Postoperative (early) atrial tachyarrhythmias | 485 (56.9) | 177 (52.1) | 308 (60.0) | .024 |
| ICU length of stay, d, median [IQR] | 2.9 [1.2, 5.0] | 2.8 [1.2, 5.1] | 3.0 [1.2, 5.0] | .951 |
| Hospital length of stay, d, median [IQR] | 9 [7, 13] | 9 [7, 14] | 9 [7, 13] | .545 |
| 30-d mortality | 28 (3.3) | 7 (2.1) | 21 (4.1) | .118 |
| Postoperative permanent pacemaker placement | 104 (12.2) | 46 (13.5) | 58 (11.3) | .338 |
| Sinus rhythm at discharge | 744 (87.2) | 310 (91.2) | 434 (84.6) | .002 |
| Major complication | ||||
| Renal failure requiring dialysis | 48 (5.6) | 17 (5.0) | 31 (6.0) | .356 |
| Myocardial infarction | 1 (0) | 0 (0) | 1 (0) | 1.000 |
| Cerebrovascular accident | 14 (1.6) | 6 (1.8) | 8 (1.6) | .791 |
| Mediastinitis | 4 (0) | 1 (0) | 3 (0) | 1.000 |
| Intra-aortic balloon pump | 34 (4.0) | 7 (2.1) | 27 (5.3) | .020 |
Values are n (%), or mean standard deviation, unless otherwise indicated. Statistically significant P values are in bold. AF, Atrial fibrillation; CMP-IV, Cox-Maze IV procedure; CBP, cardiopulmonary bypass; ICU, intensive care unit; IQR, interquartile range.
All patients who did not undergo sternotomy underwent right minithoracotomy.
Freedom From ATA On or Off AADs
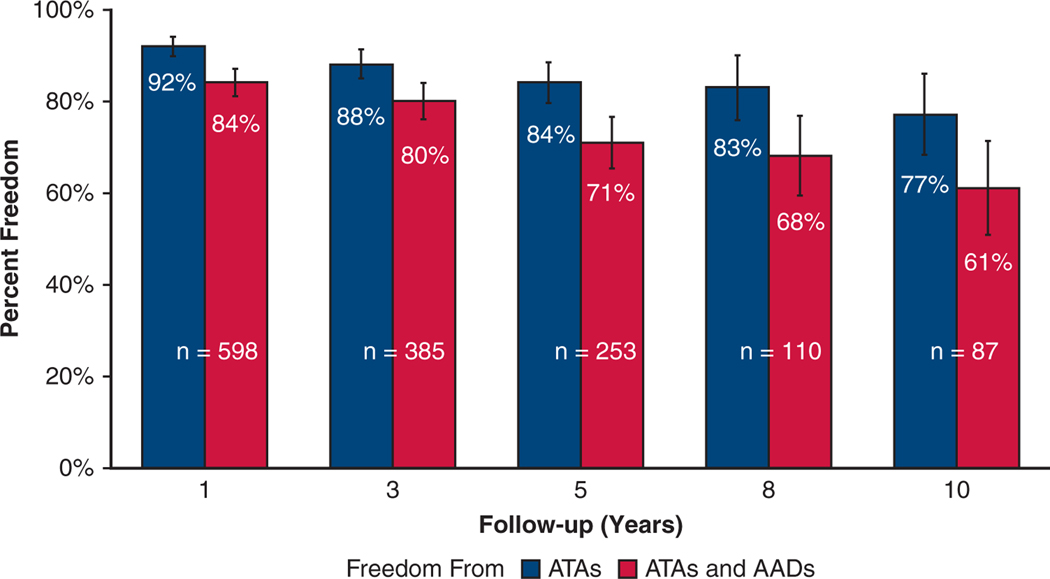
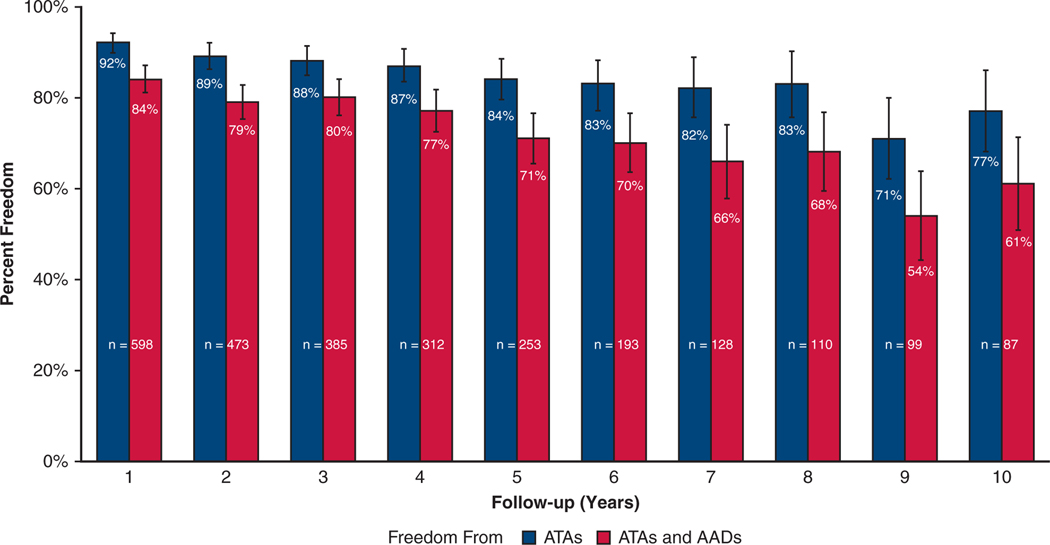
The overall freedom from recurrent ATAs at 1, 3, 5, 8, and 10 years was 92% (552/598), 88% (340/385), 84% (213/253), 83% (91/110), and 77% (67/87), respectively. The overall freedom from ATAs off AADs at 1, 3, 5, 8, and 10 years was 84% (505/598), 80% (309/385), 71% (180/253), 68% (75/110), and 61% (53/87), respectively (Figure 1). Figure E3 depicts freedom from ATAs and freedom from ATAs off AADs at each year following CMP-IV. Subgroup analysis of patients who had complete 10-year follow-up (n = 42) revealed similar freedom from ATAs: 88% (37/42), 86% (36/42), and 69% (29/42) at 1, 5, and 10 years, respectively. Symptomatic recurrence occurred in only 5 of 253 patients (2%) at 5 years, and 4 of 87 patients (5%) at 10 years following the CMP-IV.
FIGURE 1.
Early-, mid-, and long-term freedom from ATAs on or off AADs with 95% confidence intervals following the CMP-IV. ATA, Atrial tachyarrhythmia; AADs, antiarrhythmic drugs.
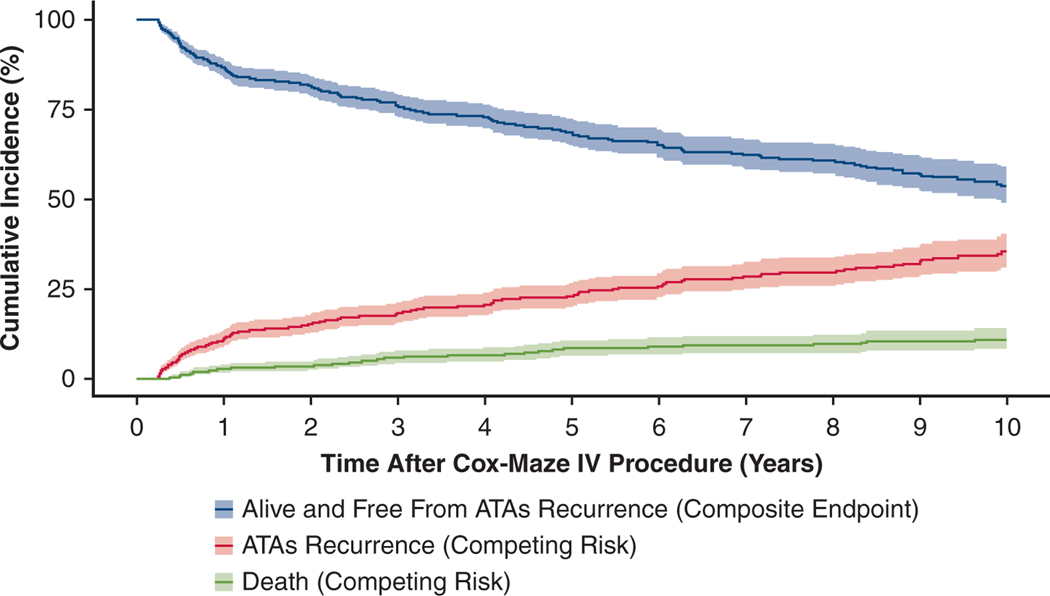
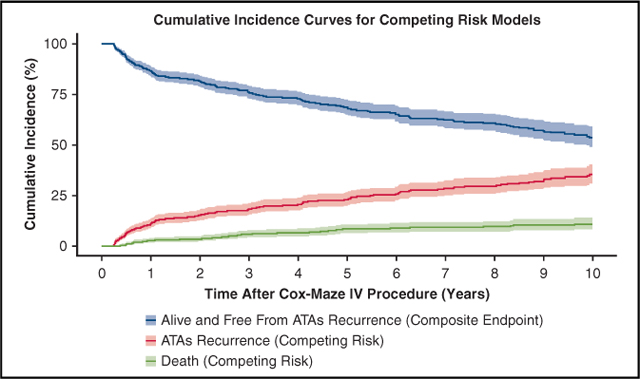
By competing risk analysis, the estimated incidence of first ATAs recurrence was 11%, 18%, 23%, 30% and 35% at 1, 3, 5, 8, and 10 years, respectively (Figure 2, Table E2).
FIGURE 2.
ATAs recurrence-free survival and CIF curves for competing events (ATA recurrence and death) following CMP-IV. Patients were assumed to be in 1 of 3 distinct states: alive and having experienced first ATAs recurrence (CIF) in red, dead before ATA recurrence (CIF) in green, or alive and free from ATA recurrence (composite end point) in blue. Percentage of patients who were in each category at each year after CMP-IV can be found in Table E2. ATA, Atrial tachyarrhythmia.
Paroxysmal Versus Nonparoxysmal AF
There was no significant difference in the overall freedom from ATAs between the paroxysmal and nonparoxysmal AF groups at 5 and 10 years, which were, respectively, 86.7% (91/105) versus 82.4% (122/148), P = .388 and 79.5% (35/44) versus 74.4% (32/43), P = .620. Early-, mid-, and long-term freedom from ATAs off AADs was not significantly different between patients with paroxysmal and nonparoxysmal AF (1 year: 87.3% [213/244] vs 82.5% [292/354], P = .135; 5 years: 74.3% [78/105] vs 68.9% [102/148], P = .399; 10 years: 61.4% [27/44] vs 60.5% [26/43], P = 1.000).
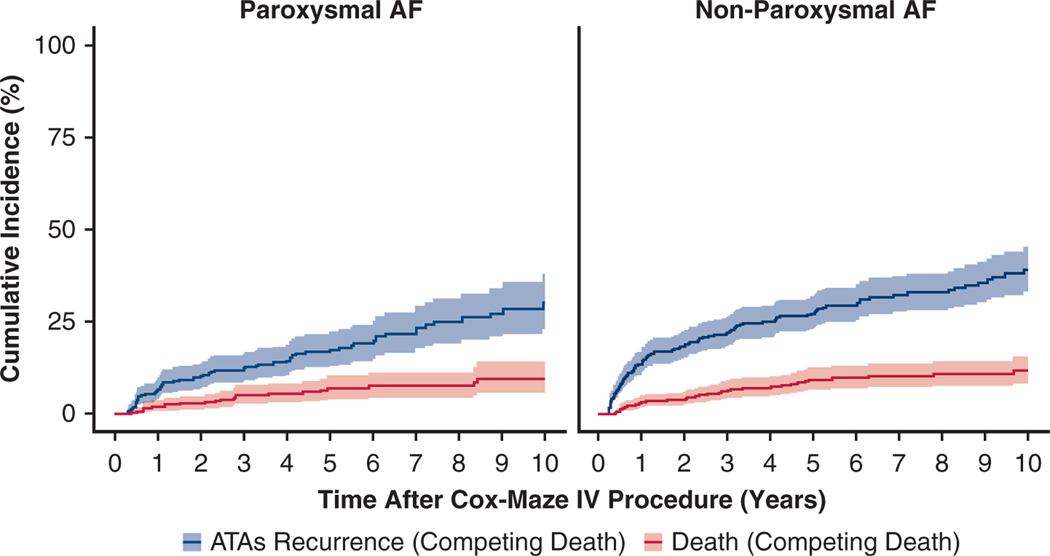
However, by competing risk analysis, the estimated incidence of first ATAs recurrence (Figure 3, Table E3) at 1, 5, and 10 years was 7%, 17%, and 30% in patients with paroxysmal AF, respectively. This incidence was significantly greater in patients with nonparoxysmal AF at 14%, 27%, and 39% at 1, 5, and 10 years, respectively (Gray’s test, P = .003). There was no significant difference in the all-cause mortality rate between the 2 groups (Gray’s test, P = .329). Moreover, patients with nonparoxysmal AF experienced a significantly shorter time to the first recurrence of ATAs compared with the paroxysmal AF group (median 1.1 [0.5, 3.3] years vs 2.1 [0.7, 5.0] years, P = .036).
FIGURE 3.
Cumulative incidence function curves showing the competing risks of ATA recurrence in blue and death in red for patients with paroxysmal AF and nonparoxysmal AF in the first 10 years following the CMP-IV. Estimated incidence of first ATAs recurrence was greater in the nonparoxysmal AF group (Gray’s test, P = .003). There was no significant difference in the all-cause mortality rate between the 2 groups (Gray’s test, P = .329). Percentage of patients who were in each category at each year after CMP-IV can be found in Table E3. AF, Atrial fibrillation; ATA, atrial tachyarrhythmia.
Stand-Alone Versus Concomitant CMP-IV
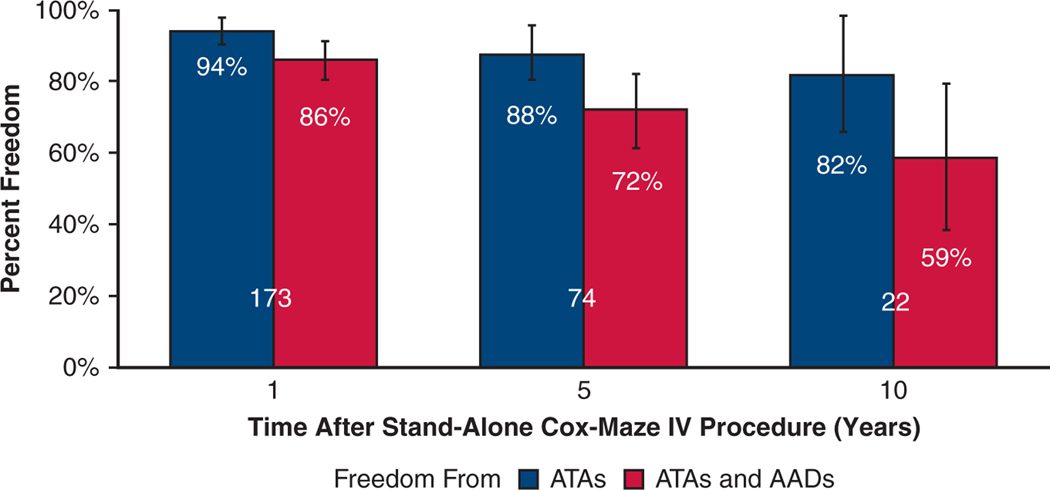
No significant differences were found in freedom from ATAs, and freedom from ATAs off AADs, at early-, mid-, and long-term follow-up at each time point between those who underwent stand-alone CMP-IV versus concomitant procedures. Freedom from ATAs at 1 year was 93.6% (162/173) versus 91.8% (390/425), P=.501; at 5 years, 87.8% (65/74) versus 82.7% (148/179), P=.349; and at 10 years, 81.8% (18/22) versus 75.4% (49/65), P=.770. Freedom from ATAs off AADs at 1 year was 86.1% (149/173) versus 83.8% (356/425), P =.470; at 5 years, 71.6% (53/74) versus 70.9% (127/179), P = 1.000; and at 10 years, 59.1% (13/22) versus 61.5% (40/65), P = 1.000. Figure E4 depicts freedom from ATAs on or off AADs for patients who underwent stand-alone CMP-IV.
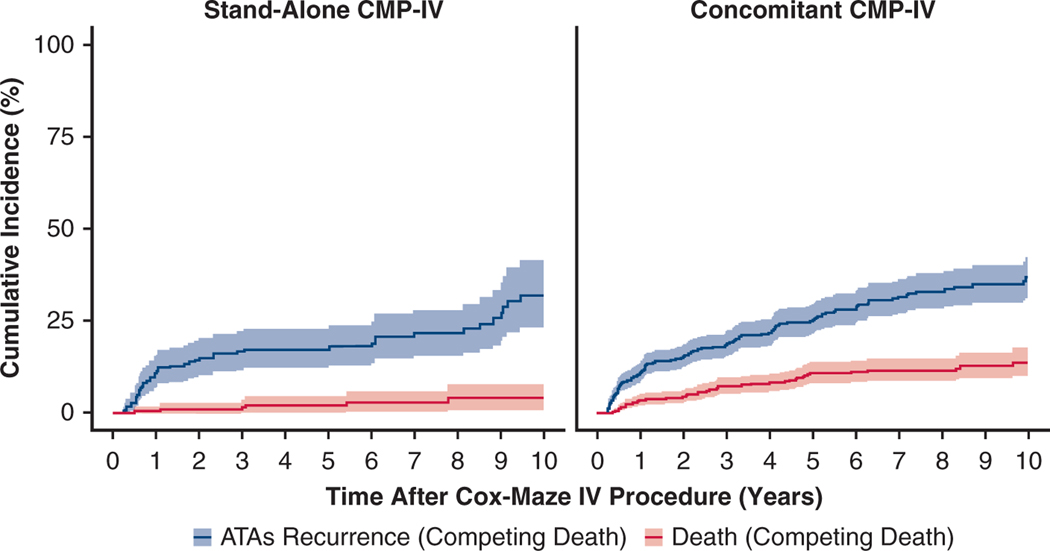
By competing risk analysis, the estimated incidence of first ATA recurrence (Figure 4, Table E4) at 1, 5, and 10 years were 11%, 17%, and 32%, respectively, in patients who underwent stand-alone CMP-IV. This was not significantly different relative to patients who underwent concomitant CMP-IV; 11%, 25%, and 37% at 1, 5, and 10 years, respectively (Gray’s test, P =.096). There was, however, a significant difference in mortality rate between the 2 groups (Gray’s test, P < .001). At 10 years, the mortality rate was 4% for the stand-alone group and 14% for the concomitant group.
FIGURE 4.
Cumulative incidence function curves showing the competing risks of ATA recurrence in blue and death in red for patients who underwent stand-alone and concomitant CMP-IV in the first 10 years following the procedure. There was no significant difference in incidence of first ATAs recurrence between the 2 groups (Gray’s test, P = .096). Concomitant CMP-IV patients had greater mortality rate, relative to stand-alone CMP-IV (Gray’s test, P <.001). Percentage of patients who were in each category at each year after CMP-IV can be found in Table E4. CMP-IV, Cox-Maze IV procedure; ATA, atrial tachyarrhythmia.
Predictors of Recurrence
Twenty-seven preoperative and perioperative characteristics that were deemed clinically relevant were evaluated using univariable and multivariable Fine–Gray regression to identify potential predictors of first ATAs recurrence within 10-year follow up to CMP-IV (Table 3; risk-adjusted Table E5). The following were univariable predictors of first recurrence by Fine–Gray regression: age (subdistribution hazard ratio [SHR], 1.03; 95% confidence interval [CI], 1.01–1.04; P <.001), peripheral vascular disease (SHR, 2.32; 95% CI, 1.61–3.35; P <.001), New York Heart Association class III/IV symptoms (SHR, 1.45; 95% CI, 1.08–1.94; P =.012), nonparoxysmal AF (SHR, 1.58; 95% CI, 1.17–2.13; P =.003), left atrial size (SHR, 1.25; 95% CI, 1.11–1.41; P <.001), longer preoperative time in AF (SHR, 1.00; 95% CI, 1.00–1.00; P =.047), postoperative early ATA (SHR, 1.95; 95% CI, 1.44–2.62; P <.001), and absence of sinus rhythm at the time of hospital discharge (SHR, 2.50; 95% CI, 1.78–3.52; P <.001).
TABLE 3.
Univariable and multivariable predictors of ATA recurrence over 10 years after CMP-IV (Fine-Gray regression)
| Univariable regression |
Multivariable regression |
|||
|---|---|---|---|---|
| Variable | SHR (95% CI) | P value | SHR (95% CI) | P value |
|
| ||||
| Age, y | 1.03 (1.01–1.04) | <.001 | 1.02 (1.00–1.03) | .047 |
| Sex (female) | 1.24 (0.94–1.65) | .130 | ||
| BMI, kg/m2 | 0.99 (0.98–1.02) | .910 | ||
| Diabetes | 1.16 (0.82–1.63) | .400 | ||
| Dyslipidemia | 0.96 (0.72–1.27) | .780 | ||
| Hypertension | 1.22 (0.89–1.67) | .210 | ||
| Renal failure | 0.89 (0.42–1.90) | .770 | ||
| Chronic lung disease (moderate or greater) | 0.45 (0.20–0.99) | .048 | 0.35 (0.15–0.79) | .011 |
| History/current smoker | 1.21 (0.91–1.61) | .200 | ||
| Peripheral vascular disease | 2.32 (1.61–3.35) | <.001 | 1.92 (1.31–2.82) | .001 |
| Cerebrovascular disease | 1.11 (0.77–1.60) | .570 | ||
| Myocardial infarction | 1.32 (0.87–1.99) | .190 | ||
| LVEF, % | 0.99 (0.98–1.00) | .047 | 0.99 (0.98–1.00) | .140 |
| NYHA class III or IV symptoms | 1.45 (1.08–1.94) | .012 | 1.19 (0.87–1.65) | .280 |
| Nonparoxysmal AF | 1.58 (1.17–2.13) | .003 | 1.41 (1.03–1.94) | .034 |
| Left atrial size, cm | 1.25 (1.11–1.41) | <.001 | 1.17 (1.02–1.34) | .025 |
| Preoperative duration of AF, y | 1.00 (1.00–1.00) | .047 | 1.00 (1.00–1.00) | .110 |
| Failed catheter ablation | 0.98 (0.71–1.37) | .930 | ||
| Sternotomy | 1.35 (0.96–1.90) | .088 | 1.03 (0.72–1.47) | .870 |
| Concomitant procedure | 1.32 (0.95–1.83) | .096 | 1.02 (0.70–1.49) | .910 |
| Biatrial CMP-IV lesion set | 1.01 (0.61–1.66) | .980 | ||
| Postoperative mediastinitis | 0.73 (0.09–6.00) | .770 | ||
| Prolonged ventilation | 1.21 (0.84–1.74) | .310 | ||
| Postoperative pneumonia | 1.33 (0.74–2.41) | .350 | ||
| Postoperative (early*) atrial tachyarrhythmias | 1.95 (1.44–2.62) | <.001 | 1.51 (1.07–2.12) | .018 |
| Absence of sinus rhythm at discharge | 2.50 (1.78–3.52) | <.001 | 1.62 (1.10–2.41) | .016 |
| Postoperative permanent pacemaker placement | 1.04 (0.69–1.57) | .850 | ||
Statistically significant P values are in bold. SHR, Subdistribution hazard ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AF, atrial fibrillation; CMP-IV, Cox-Maze IV procedure.
Early defined as in-hospital (before discharge) ATAs.
In multivariable Fine–Gray regression, the following were identified as predictors of first ATA recurrence: age (SHR, 1.02; 95% CI, 1.00–1.03; P = .047), peripheral vascular disease (SHR, 1.92; 95% CI, 1.31–2.82; P = .001), nonparoxysmal AF (SHR, 1.41; 95% CI, 1.03–1.94; P= .034), left atrial size (SHR, 1.17; 95% CI, 1.02–1.34; P =.025), postoperative early ATA (SHR, 1.51; 95% CI, 1.07–2.12; P =.018), and absence of sinus rhythm at the time of hospital discharge (SHR, 1.62; 95% CI, 1.10–2.41; P =.016). Interestingly, presence of chronic lung disease (moderate or greater) was found to be a “protective” predictor for first ATA recurrence (SHR, 0.35; 95% CI, 0.15–0.79; P = .011). This predictor was excluded from subsequent additional consideration, as the predictive finding of chronic lung disease was considered statistically random. The same variables were identified as predictors of first ATA recurrence, when adjusted for all covariates (Table E5).
Predictors of Postoperative Pacemaker Placement
Twenty-three preoperative and perioperative characteristics that were deemed clinically relevant were evaluated using univariable and multivariable binary logistic regression to identify predictors of postoperative pacemaker placement (Table E6). In multivariable logistic regression, the following were found as predictors of postoperative pacemaker placement: age (odds ratio [OR], 1.03; 95% CI, 1.01–1.06; P = .003), female sex (OR, 2.48; 95% CI, 1.61–3.82; P < .001), sternotomy (OR, 2.20; 95% CI, 1.24–3.90; P = .007), biatrial CMP-IV lesion set (OR, 3.61; 95% CI, 1.27–10.30; P = .016), and postoperative early ATAs (OR, 1.84; 95% CI, 1.14–2.98; P = .013).
Other Findings
The results of the composite end-point survival analysis can be seen in Figure 2 and Table E2. ATA recurrence-free survival was estimated to be 87%, 69%, and 54% at 1, 5, and 10 years, respectively (ie, alive and free from any ATA recurrence).
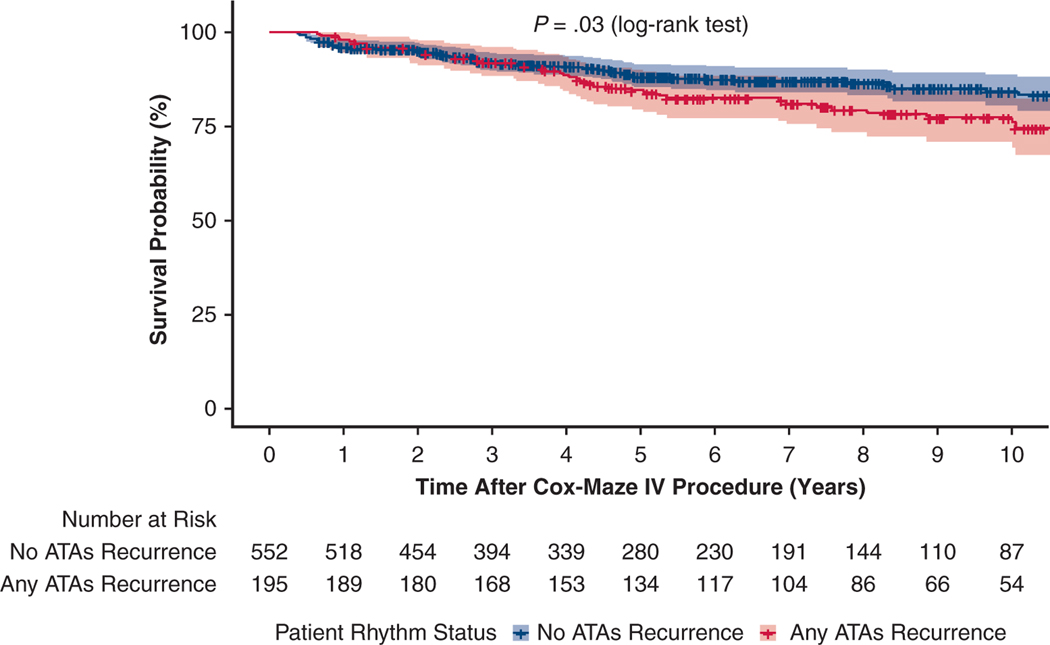
Moreover, although the majority of patients remained alive and free from ATA recurrence throughout the study time, significant difference was noted in mortality rate in those who experienced at least 1 ATA episode. At 10 years, the survival in those who remained in sinus rhythm was 84% (95% CI, 80%−89%) versus 77% (95% CI, 71%−84%) in those who experienced at least 1 ATA recurrence (Log-rank test, P = .03) (Figure E5).
Lastly, performing the CMP-IV through a right minithoracotomy was found to be as effective as sternotomy (SHR, 1.35; 95% CI, 0.96–1.90; P = .088).
DISCUSSION
Since its introduction, the CMP has been demonstrated to be the most effective treatment for AF.5–8,10,14,15,21 The most recent iteration, the CMP-IV, introduced in 2002, has made the operation simpler, technically easier to perform, and has led to widespread adoption. This has been achieved while maintaining the excellent late success rates of the CMP, and significantly reducing its morbidity and mortality.10,12,14–16 It is the only surgical operation to receive a Food and Drug Administration indication for the treatment of AF. However, there have been very few reports looking at late outcomes.
In this study, we examined the 10-year outcomes for a large cohort of patients undergoing the CMP-IV at a single center (Figure 5). The major finding of our study was that the CMP-IV provided excellent freedom from ATAs at early-, mid-, and long-term follow-up. Furthermore, the CMP-IV was equally effective in patients undergoing both stand-alone and concomitant AF ablation. The indication for performing stand-alone CMP-IV comes from the STS, The Heart Rhythm Society, European Heart Rhythm Association, and the European Cardiac Arrhythmia Society that have recommended SA as a primary stand-alone procedure to restore sinus rhythm for patients who are refractory to class I/III AADs and/or catheter-based therapy (Class IIA, Level B, nonrandomized).13,28,29 In contradistinction to catheter ablation and more limited surgical lesion sets, the CMP-IV was found to be equally effective in patients with both paroxysmal and nonparoxysmal AF at mid- and long-term follow-up. However, patients with nonparoxysmal AF tended to experience the first AF episode earlier than those with paroxysmal AF. By competing risk analysis, the estimated incidence of first ATA recurrence was also greater in patients with nonparoxysmal AF, relative to those with paroxysmal AF.
FIGURE 5.
Overview of study design including total number of study patients undergoing elective CMP-IV with full box lesion set for symptomatic AF (n = 853). Primary outcome was incidence of first ATA recurrence. By competing risk analysis, the estimated incidence of first ATAs recurrence was 11%, 18%, 23%, 30% and 35% at 1, 3, 5, 8, and 10 years, respectively. At 10 years, the survival in those who remained in sinus rhythm was 84% versus 77% in those who experienced at least 1 ATA recurrence (log-rank test, P = .03). Overall, the CMP-IV had excellent efficacy at maintaining sinus rhythm during the 10 years of follow-up. CMP-IV, Cox-Maze IV procedure; PVI, pulmonary vein isolation; SA, surgical ablation; AF, atrial fibrillation; ATA, atrial tachyarrhythmia.
Our outcomes are consistent with the excellent early- and mid-term results of the CMP-IV (complete lesion set), previously reported by our group and others.16,19–23 Our results are particularly favorable when compared with catheter ablation studies.33,34 In a study of 255 patients who underwent catheter ablation, of whom 43% had paroxysmal AF, the AF-free survival after a single ablation procedure was 32% for the overall cohort at 10 years (39% for paroxysmal vs 24% for nonparoxysmal AF, P =.001). Allowing for multiple catheter ablations in those with failure improved the AF-free survival to 52% at 10 years (61% paroxysmal vs 44% nonparoxysmal, P =.002).34 Several other studies have reported early- and mid-term success rates after catheter ablations with equally poor results.33,35 In our study, the freedom from ATAs at 1, 5, and 10 years was 92% (552/598), 84% (213/253), and 77% (67/87), respectively, following a single ablation procedure, the CMP-IV. Taking into account death during the follow-up period by competing risk analysis, the incidence of first ATA recurrence was 11%, 23%, and 35% at 1, 5, and 10 years, respectively. Furthermore, subgroup analysis of patients who had complete 10-year follow-up (n 42) revealed similar freedom from ATAs: 88%, 86%, and 69% at 1, 5, and 10 years, respectively. This was despite the relatively large average LA size (5.1 ± 1.1 cm) in our patients and the fact that 24% (201/853) had not responded to 1 or more catheter ablations. Even more remarkable was the low incidence of symptomatic recurrent ATAs. Symptomatic recurrence occurred in only 5 of 253 patients (2%) at 5 years, and 4 of 87 patients (5%) at 10 years following CMP-IV.
The success of the CMP-IV in the concomitant setting is of great importance when considering that most SAs in the world are currently performed as part of concomitant cardiac procedures.13 We found no significant difference in freedom from ATAs, and freedom from ATAs off AADs, at early-, mid-, and long-term follow-up between those who underwent stand-alone CMP-IV and concomitant procedures. Both groups had high rates of restoration of sinus rhythm, which is consistent with results previously reported by our group and others.16,21–23 These findings support our recommendation that the CMP-IV should be considered in all patients undergoing concomitant cardiac surgery, as long as the procedure can be performed without adding morbidity or mortality to the procedure.
Using multivariable Fine–Gray regression, we identified several predictors of first ATA recurrence. Age, peripheral vascular disease, nonparoxysmal AF, left atrial size, postoperative early ATAs, and absence of sinus rhythm at the time of hospital discharge were found to increase the risk of first ATA recurrence over 10 years of follow-up. Several of these factors have been previously shown to predict recurrence after both catheter ablation and SA.14,16,21–24,31,32 It is important to note that these are the predictors to the first recurrence of ATAs using Fine–Gray regression, with death as the competing risk, but not at a specific time point as previously reported by our group and others. A patient who had the first ATA event after the 90-day blanking period (eg, at 1 year) was considered a failure, irrespective of what the rhythm was at a later time point (eg, at 10 years). Transient short runs of ATAs in long-term follow-up did not always predict persistent failure, particularly in patients requiring no intervention to restore sinus rhythm.
Using multivariable binary logistic regression, we found age, female sex, sternotomy, biatrial CMP-IV lesion set, and postoperative early ATAs to be predictors of postoperative pacemaker placement. Several of these risk factors have previously been found by our group and others to predict postoperative pacemaker placement, likely associated with the presence of preoperative sick sinus syndrome, or procedural sinus node dysfunction secondary to the right sided lesion set or associated valvular procedures.
Another interesting finding of this study was the survival benefit seen in patients who remained in sinus rhythm throughout the follow-up period. We and others have previously shown a survival benefit to performing concomitant AF ablation as opposed to not treating the AF,36 but this also suggests a further benefit to successful restoration of sinus rhythm, over and above that seen with just managing the LAA.
Limitations
The excellent outcomes of the CMP-IVat 10 years should be viewed in light of several limitations of the study. This study was retrospective and nonrandomized by nature, and thus it is subject to bias inherent to this design, such as selection bias and interval censoring. The operations were performed at a single institution with the majority of cases performed by a single highly experienced surgeon. Thus, the results may not be generalizable to all centers. In addition, incomplete follow-up and lack of prolonged monitoring on every patient could have led to an overestimation of our success rates. At 10 years, more than 30% of patients had been lost to follow-up, and prolonged monitoring was used in only 31% (27/87) of patients. The use of prolonged monitoring at each time point was as follows: 1 year: 72% (432/598); 2 years: 67% (319/473); 3 years: 66% (255/385); 4 years: 63% (198/312); 5 years: 60% (153/253); 6 years: 51% (99/193); 7 years: 52% (66/128); 8 years: 39% (43/110); 9 years: 39% (39/99); and 10 years: 31% (27/87).
In addition, while the early postoperative cerebrovascular accidents (including transient ischemic attacks) were low (1.6% [14/853]), the long-term freedom from cerebrovascular accident was not available to report. However, we have previously reported a very low rate of incident of stroke in patients undergoing CMP.30 Lastly, survival data were missing for 9% of patients (not available in social security database, medical records, primary care physician office, or obituary search). For these reasons, our study may have suffered from attrition and reporting biases.
Despite these limitations, this is one of the largest studies in the literature and one of the very few to have adequate late follow-up and comprehensive statistical analysis. Our results clearly demonstrate the efficacy and durability of the CMP-IV in both stand-alone and concomitant AF ablation and for all types of AF. Subgroup analysis of patients who had complete 10-year follow-up revealed similar freedom from ATAs.
CONCLUSIONS
The CMP-IV has been demonstrated to be the most successful surgical treatment for AF, with excellent 10-year efficacy. Patients with nonparoxysmal AF experienced a first ATA episode of recurrence sooner than those with paroxysmal AF. There was no difference in rhythm outcomes between patients who underwent stand-alone or concomitant CMP-IV. Using multivariable Fine-–Gray regression, we found that age, peripheral vascular disease, nonparoxysmal AF, left atrial size, postoperative early ATAs, and absence of sinus rhythm at the time of hospital discharge to be the relevant predictors of first ATAs recurrence over 10 years of follow-up. Those who remained in sinus rhythm had improved long-term survival relative to those who experienced any ATA recurrence. Given the survival benefit and exceptional freedom from symptomatic recurrence, the CMP-IV should be considered in all patients with medically refractory, symptomatic AF who have not responded to or who are poor candidates for catheter ablation, and in all patients undergoing concomitant cardiac surgery, as long as the procedure can be performed without adding morbidity to the operation.
CENTRAL MESSAGE.
The Cox-Maze IV procedure had excellent efficacy in restoring sinus rhythm during 10 years of follow-up. This was accomplished in patients with both paroxysmal and nonparoxysmal AF.
PERSPECTIVE.
There is a paucity of data describing long-term outcomes and durability of surgical ablation for AF. The Cox-Maze procedure continues to be the gold standard treatment for AF with excellent 10-year efficacy. Over 10 years of follow-up, age, peripheral vascular disease, nonparoxysmal AF, left atrial size, postoperative ATAs, and the absence of sinus rhythm at the time of discharge predicted recurrence.
Acknowledgments
This work was supported by the National Institutes of Health R01-HL032257 to R. J. Damiano and R. B. Schuessler; T32-HL007776 to R. J. Damiano, A. J. Khiabani, R. M. MacGregor, and J. L. Manghelli; and the Barnes-Jewish Hospital Foundation.
Abbreviations and Acronyms
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- ATA
atrial tachyarrhythmia
- CI
confidence interval
- CMP
Cox-Maze procedure
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- ILR
implantable loop recorder
- LA
left atrium
- OR
odds ratio
- SA
surgical ablation
- SHR
subdistribution hazard ratio
- STS
Society of Thoracic Surgeons
Discussion Presenter: Dr Ali J. Khiabani

Dr Vinay Badhwar (Morgantown, WVa). I thank the Association for the privilege of discussing this paper, and the authors for providing the manuscript in advance. I would like to thank you, young Dr Khiabani, for an elegant presentation without developing atrial flutter yourself, given the pressure cooker you just entered. With that comment, this represents an outstanding experience from the Washington University group and the leaders in the field to help us learn about this main problem. I’d like to summarize what you shared. This is a study of 765 patients with biatrial Cox-Maze IV, and 88 with left-sided Cox-Maze IV lesions. Importantly, 513, or 60%, were for persistent atrial fibrillation (AF), but continuous electrocardiogram (EKG) monitoring was only incorporated in 2006—the series started in 2001—resulting in only 647 patients, or 76% of your series that had acceptable guideline-directed rhythm reporting.
Also, this is a 10-year look. When rhythm was documented at the time of follow-up, it looked very good at 77%. But when Kaplan–Meier analysis was performed, it was 47%. I think the way you’ve reported it is very appropriate. This is an all-inclusive, multivariable regression analysis, and as we just discussed in the last session, there are some issues with that sometimes. The attempt to identify predictors of recurrence was unfortunately not very successful. Little was gleaned, other than left ventricular dysfunction and the hint of AF at discharge.
With that, I have 3 focused questions for you.
First, AF at discharge was strongly predictive of long-term failure, and in your manuscript, you had an odds ratio of 7 at 5 years and an odds ratio of 1.85 at the 10-year analysis. Have you applied this knowledge to implement a more-aggressive approach to electrical cardioversion before discharge, as many other centers in the world have adopted?

Dr Ali J. Khiabani (St Louis, Mo). Thank you for your questions. Overall, the majority of our patients, 87%, were discharged home in sinus rhythm. 85% in the nonparoxysmal AF group, and 91% in the paroxysmal AF group. Unfortunately, I don’t have the exact number of how many patients required cardioversion before the time of hospital discharge. However, we do cardiovert patients routinely postoperatively if they develop AF. With these findings, we are now more aggressively cardiovert patients while they’re in the hospital if they develop AF. I agree with you, it is important to advise surgeons to try to send these patients home in sinus rhythm.
Dr Badhwar. My second question is: A large proportion of these patients had EKG monitoring. If you analyze the 647 that had continuous EKG monitoring at the 24-hour time point or other modalities, this is still a huge dataset. If you were to do so, how do think that would inform the literature?
Dr Khiabani. I think the short answer is: we are probably overestimating our success rate. Unfortunately, we didn’t perform that kind of analysis to determine the freedom from atrial tachyarrhythmias in those with only prolonged monitoring. Having said that, prolonged monitoring in our study, as you alluded to, was used in 76% of our patients at any time point after the 90-day blanking period. Prolonged monitoring was used in 60% of patients at 5 years and of course lower at 10 years. But I do agree with you, the lack of prolonged monitoring in every patient could have led to our overestimation.
Dr Badhwar. Lastly, we talked about the Cox model. In this particular analysis, I would have hoped to see something more informative for the literature, such as nonparoxysmal versus paroxysmal, and biatrial versus left atrial information, since you’ve had such a vast experience. Do you plan this analysis, or if you have done some subset information, can you share that with us?
Dr Khiabani. I agree with you that we should do a more in-depth analysis, given that we have probably the largest series in the literature with patients following the Cox-maze IV procedure. One analysis that I didn’t get a chance to go over during this presentation was that when we looked at the patients with nonparoxysmal AF and compared them with the paroxysmal group, we found that patients with nonparoxysmal AF experienced an atrial tachyarrhythmia episode earlier, with a median time of 1 year, compared with the patients with paroxysmal AF, who experienced their first episode of ATA at 2 years. But we will do more analysis, and encourage all our audience to read our accepted manuscript.
Dr Badhwar. Congratulations.
Dr Khiabani. Thank you.
APPENDIX E1.
The most effective surgical treatment for the management of AF has been the Cox-Maze procedure (CMP), introduced by James Cox and colleagues in 1987. The original maze procedure (maze I) was introduced clinically in September 1987.E1 However, it caused chronotropic inadequacy of the sinoatrial node and prolonged intra-atrial conduction delay. Because of these problems, the maze II procedure was developed. This technique, however, was extremely difficult to perform because of the need to transect the superior vena cava. As a result, the operation was modified to the maze III procedure.E1 This procedure had excellent short- and long-term outcomes. Despite its proven efficacy, its widespread adoption was limited due to a significant increase in cardiopulmonary bypass time, increase in morbidity, and its technical complexity. In 1996, James Cox and colleagues performed their first minimally invasive maze III.E2 The technique was first published in 2000. In this technique, the procedure was performed using right-sided thoracotomy and the Heartport system (Heartport, Inc, Redwood City, Calif). Cryoablation was used as an alternative energy source. Unfortunately, this procedure did not gain widespread acceptance, and there are no reports comparing the outcomes with the traditional sternotomy approach. At Washington University, on the basis of extensive clinical and ongoing animal investigations, revisions to the original “cut-and-sew” technique led to the fourth iteration of the procedure. Introduced clinically in 2002 by Damiano and colleagues,E3 the maze IV procedure uses bipolar radiofrequency and cryoablation devices to replace most of the surgical incisions of its predecessor. The current iteration, introduced in 2004, includes superior and inferior connecting lesion between the isolated pulmonary veins, which forms a “box lesion,” and completely isolates the entire posterior left atrium.E4 The maze IV has proven to be as effective as its predecessor in restoring sinus rhythm, while also reducing operative morbidity and mortality.E5,E6 It is important to note that while the electrophysiological consequences of both the CMP-III and CMP-IVare similar, the introduction of the CMP-IV has led to a greater acceptance and performance of surgical ablation for atrial fibrillation over the last 2 decades.
FIGURE E1.
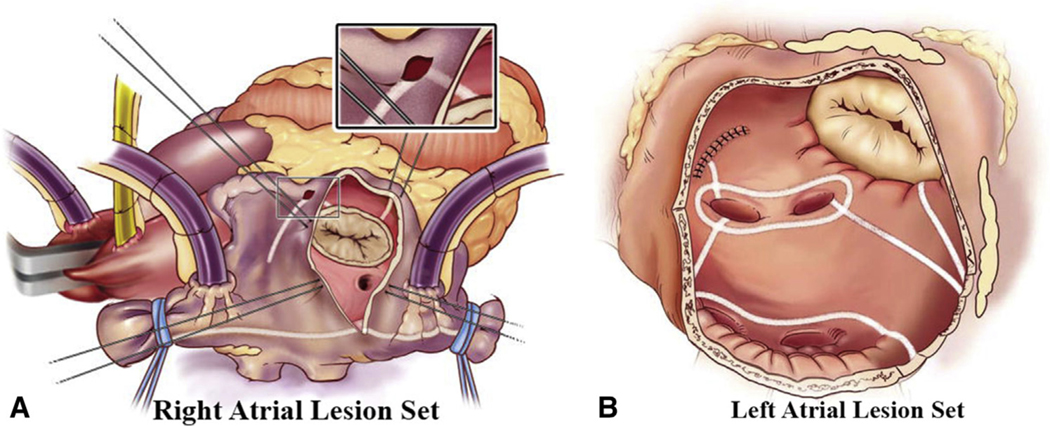
Cox-Maze IV lesion set via median sternotomy approach. Right (A) and left (B) atrial lesion sets used for the Cox-Maze IV. In the right atrium (A), radiofrequency ablation lines (white lines) extend from the superior vena cava to the inferior vena cava and along the right atrial free wall down to the tricuspid valve annulus. In the left atrium (B), all ablation lines are performed with a bipolar radiofrequency clamp except for an endocardial cryoablation at the mitral annulus and an epicardial cryoablation over the coronary sinus. Reproduced with permission from Weimar and colleagues.10
FIGURE E2.
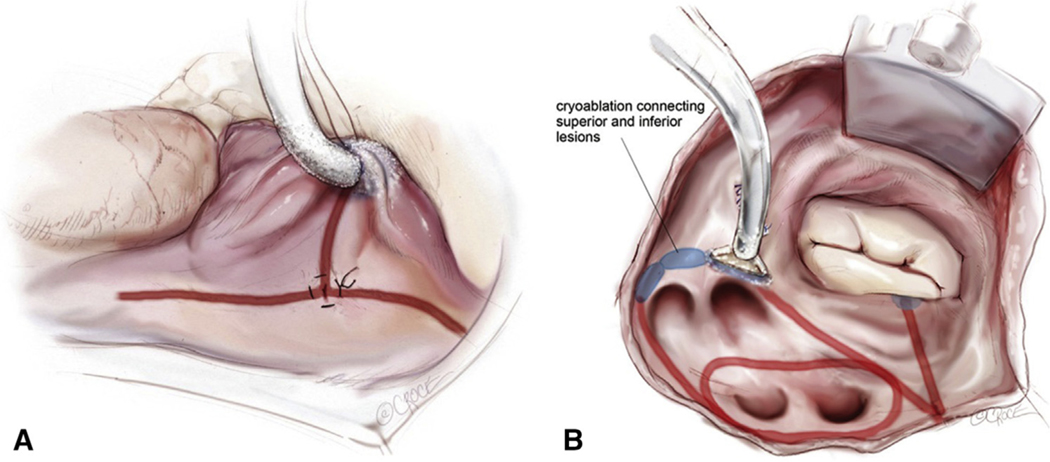
Cox-Maze IV lesion set via minimally invasive right minithoracotomy approach. Right atrial (A) and left atrial (B) lesion sets of the minimally invasive CM IV. Ablation lines are made using a combination of bipolar radiofrequency (red lines) and cryoablation (blue). Reproduced with permission from Beth Croce, Bioperspective.com.
FIGURE E3.
Freedom from ATAs on or off AADs with 95% confidence intervals after the CMP-IV. ATA, Atrial tachyarrhythmia; AAD, antiarrhythmic drugs.
FIGURE E4.
Freedom from ATAs on or off AADs with 95% confidence intervals after the stand-alone CMP-IV. ATA, Atrial tachyarrhythmia; AAD, antiarrhythmic drugs.
FIGURE E5.
Kaplan–Meier curve estimating the survival of patients who experienced any ATA recurrence in orange and those who remained in sinus rhythm in blue (log-rank test, P = .03). ATA, Atrial tachyarrhythmia.
TABLE E1.
Concomitant procedures
| Procedures in addition to CMP-IV | Number of patients, n (%; of 635) |
|---|---|
|
| |
| Mitral valve ± tricuspid valve | 324 (51) |
| CABG ± mitral valve | 126 (20) |
| Aortic valve ± CABG | 104 (16) |
| Aortic valve ± mitral valve | 27 (4) |
| Other | 54 (9) |
CMP-IV, Cox-Maze IV procedure; CABG, coronary artery bypass grafting.
TABLE E2.
Percentage of patients estimated to be alive and have experienced first ATA recurrence (CIF), dead before ATA recurrence (CIF), or alive and free from ATA recurrence (composite end point) at each year following the CMP-IV
| Year | First ATA recurrence (CIF) | Death (CIF) | Alive and free from any ATA (composite end point) | Number at risk |
|---|---|---|---|---|
|
| ||||
| 1 | 11% | 3% | 87% | 633 |
| 2 | 15% | 3% | 82% | 539 |
| 3 | 18% | 6% | 76% | 459 |
| 4 | 21% | 6% | 73% | 390 |
| 5 | 23% | 8% | 69% | 318 |
| 6 | 26% | 9% | 65% | 256 |
| 7 | 29% | 9% | 62% | 207 |
| 8 | 30% | 9% | 61% | 157 |
| 9 | 32% | 10% | 58% | 117 |
| 10 | 35% | 11% | 58% | 88 |
Also see Figure 2. ATA, Atrial tachyarrhythmia; CIF, cumulative incidence function.
TABLE E3.
Percentage of patients estimated to be alive and have experienced first ATA recurrence (CIF), or dead before ATA recurrence (CIF) for the 2 groups (paroxysmal AF vs nonparoxysmal AF) at each year following the CMP-IV
| Year | Paroxysmal AF first ATA recurrence (CIF) | Paroxysmal death (CIF) | Paroxysmal number at risk | Nonparoxysmal first ATA recurrence (CIF) | Nonparoxysmal death (CIF) | Nonparoxysmal number at risk |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 7% | 2% | 271 | 14% | 3% | 362 |
| 2 | 10% | 3% | 241 | 18% | 4% | 298 |
| 3 | 12% | 5% | 210 | 22% | 6% | 249 |
| 4 | 14% | 5% | 175 | 25% | 7% | 215 |
| 5 | 17% | 7% | 141 | 27% | 9% | 177 |
| 6 | 20% | 8% | 111 | 30% | 10% | 145 |
| 7 | 23% | 8% | 87 | 32% | 10% | 120 |
| 8 | 25% | 8% | 68 | 33% | 11% | 89 |
| 9 | 27% | 10% | 48 | 36% | 11% | 69 |
| 10 | 30% | 10% | 36 | 39% | 12% | 52 |
Also see Figure 3. AF, Atrial fibrillation; ATA, atrial tachyarrhythmia; CIF, cumulative incidence function.
TABLE E4.
Percentage of patients estimated to be alive and have experienced first ATA recurrence (CIF), or dead before ATA recurrence (CIF) for the 2 groups (stand-alone vs concomitant CMP-IV procedure) at each year following the CMP-IV
| Year | Stand-alone first ATA recurrence (CIF) | Stand-alone death (CIF) | Stand-alone number at risk | Concomitant first ATA recurrence (CIF) | Concomitant death (CIF) | Concomitant number at risk |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 11% | 0% | 177 | 11% | 4% | 456 |
| 2 | 14% | 1% | 152 | 15% | 4% | 387 |
| 3 | 17% | 2% | 137 | 19% | 7% | 322 |
| 4 | 17% | 2% | 124 | 22% | 8% | 266 |
| 5 | 17% | 2% | 111 | 25% | 11% | 207 |
| 6 | 19% | 3% | 91 | 29% | 11% | 165 |
| 7 | 22% | 3% | 78 | 31% | 11% | 129 |
| 8 | 22% | 4% | 61 | 33% | 11% | 96 |
| 9 | 26% | 4% | 45 | 35% | 13% | 72 |
| 10 | 32% | 4% | 37 | 37% | 14% | 51 |
Also see Figure 4. ATA, Atrial tachyarrhythmia; CIF, cumulative incidence function.
TABLE E5.
Risk adjusted predictors of ATA recurrence over 10 years after CMP-IV (Fine–Gray regression)
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| Variable | SHR (95% CI) | P value | SHR (95% CI) | P value |
|
| ||||
| Age, y | 1.03 (1.01–1.04) | <.001 | 1.02 (1.00–1.04) | .027 |
| Sex (female) | 1.24 (0.94–1.65) | .130 | 1.26 (0.89–1.79) | .190 |
| BMI, kg/m2 | 0.99 (0.98–1.02) | .910 | 1.00 (0.98–1.03) | .850 |
| Diabetes | 1.16 (0.82–1.63) | .400 | 0.98 (0.67–1.44) | .910 |
| Dyslipidemia | 0.96 (0.72–1.27) | .780 | 0.79 (0.57–1.10) | .160 |
| Hypertension | 1.22 (0.89–1.67) | .210 | 1.02 (0.72–1.43) | .930 |
| Renal failure | 0.89 (0.42–1.90) | .770 | 0.77 (0.33–1.81) | .550 |
| Chronic lung disease (moderate or greater) | 0.45 (0.20–0.99) | .048 | 0.33 (0.15–0.75) | .008 |
| History/current smoker | 1.21 (0.91–1.61) | .200 | 1.13 (0.82–1.56) | .460 |
| Peripheral vascular disease | 2.32 (1.61–3.35) | <.001 | 1.89 (1.26–2.83) | .002 |
| Cerebrovascular disease | 1.11 (0.77–1.60) | .570 | 1.04 (0.70–1.53) | .860 |
| Myocardial infarction | 1.32 (0.87–1.99) | .190 | 1.26 (0.79–2.03) | .330 |
| LVEF, % | 0.99 (0.98–1.00) | .047 | 1.01 (0.98–1.01) | .097 |
| NYHA class III or IV symptoms | 1.45 (1.08–1.94) | .012 | 1.45 (0.81–1.62) | .430 |
| Nonparoxysmal AF | 1.58 (1.17–2.13) | .003 | 1.45 (1.04–2.01) | .029 |
| Left atrial size, cm | 1.25 (1.11–1.41) | <.001 | 1.18 (1.02–1.36) | .022 |
| Preoperative duration of AF, y | 1.00 (1.00–1.00) | .047 | 1.00 (1.00–1.00) | .120 |
| Failed catheter ablation | 0.98 (0.71–1.37) | .930 | 1.33 (0.87–2.02) | .190 |
| Sternotomy | 1.35 (0.96–1.90) | .088 | 1.05 (0.71–1.55) | .810 |
| Concomitant procedure | 1.32 (0.95–1.83) | .100 | 1.12 (0.73–1.73) | .600 |
| Biatrial CMP-IV lesion set | 1.01 (0.61–1.66) | .980 | 0.80 (0.47–1.39) | .430 |
| Postoperative mediastinitis | 0.73 (0.09–6.00) | .770 | 0.97 (0.19–4.93) | .970 |
| Prolonged ventilation | 1.21 (0.84–1.74) | .310 | 0.88 (0.54–1.42) | .590 |
| Postoperative pneumonia | 1.33 (0.74–2.41) | .350 | 1.47 (0.70–3.06) | .310 |
| Postoperative (early*) atrial tachyarrhythmias | 1.95 (1.44–2.62) | <.001 | 1.48 (1.04–2.11) | .030 |
| Absence of sinus rhythm at discharge | 2.50 (1.78–3.52) | <.001 | 1.76 (1.18–2.65) | .006 |
| Postoperative permanent pacemaker placement | 1.04 (0.69–1.57) | .850 | 0.92 (0.58–1.47) | .740 |
Statistically significant P values are in bold. SHR, Subdistribution hazard ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AF, atrial fibrillation; CMP-IV, Cox-Maze IV procedure. *Early defined as in-hospital (prior to discharge) atrial tachyarrhythmias.
TABLE E6.
Univariable and multivariable predictors of postoperative pacemaker placement (binary logistic regression)
| Univariable regression |
Multivariable regression |
|||
|---|---|---|---|---|
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
|
| ||||
| Age, y | 1.04 (1.02–1.06) | <.001 | 1.03 (1.01–1.06) | .003 |
| Sex (female) | 2.43 (1.06–3.69) | <.001 | 2.48 (1.61–3.82) | <.001 |
| BMI, kg/m2 | 0.97 (0.94–1.01) | .056 | ||
| Diabetes | 1.43 (0.82–1.63) | .520 | ||
| Dyslipidemia | 0.76 (0.51–1.15) | .197 | ||
| Hypertension | 0.99 (0.63–1.56) | .976 | ||
| Renal failure | 1.12 (0.80–1.90) | .540 | ||
| Chronic lung disease (moderate or greater) | 1.23 (0.81–1.34) | .870 | ||
| History/current smoker | 0.86 (0.56–1.32) | .487 | ||
| Peripheral vascular disease | 0.82 (0.40–1.68) | .579 | ||
| Cerebrovascular disease | 1.30 (0.78–2.18) | .320 | ||
| Myocardial infarction | 1.09 (0.91–1.18) | .238 | ||
| LVEF, % | 1.00 (0.99–1.02) | .690 | ||
| NYHA class III or IV symptoms | 1.70 (1.09–2.62) | .018 | 1.11 (0.69–1.78) | .503 |
| Nonparoxysmal AF | 0.82 (0.54–1.23) | .332 | ||
| Left atrial size, cm | 0.97 (0.80–1.18) | .773 | ||
| Preoperative duration of AF, y | 1.00 (0.99–1.00) | .062 | ||
| Failed catheter ablation | 0.43 (0.23–0.78) | .006 | 0.63 (0.32–1.24) | .070 |
| Sternotomy | 2.25 (1.29–3.93) | .004 | 2.20 (1.24–3.90) | .007 |
| Concomitant procedure | 2.64 (1.44–4.82) | .002 | 1.34 (0.67–2.69) | .143 |
| Biatrial CMP-IV lesion set | 3.16 (1.13–8.80) | .028 | 3.61 (1.27–10.3) | .016 |
| Postoperative (early*) atrial tachyarrhythmias | 2.38 (1.50–3.78) | <.001 | 1.84 (1.14–2.98) | .013 |
| Absence of sinus rhythm at discharge | 1.03 (0.55–1.91) | .938 | ||
Statistically significant P values are in bold. OR, Odds ratio; CI, confidence interval; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; AF, atrial fibrillation; CMP-IV, Cox-Maze IV procedure.
Early defined as in-hospital (before discharge) atrial tachyarrhythmias.
Footnotes
Conflict of Interest Statement
Dr Damiano reported AtriCure, Inc: speaker and receives research funding; LivaNova, Inc: speaker; Medtronic: consultant; and Edwards Lifesciences: speaker. M.R.M. reported Medtronic: consultant. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/19%20AM/Saturday_May4/Plenary/P1_4.mp4.

References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–7. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RV, et al. Life-time risk for development of atrial fibrillation: the Framingham heart study. Circulation. 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL, Schuessler RB, D’Agostino HJ Jr, Stone CM, Chang BC, Cain ME, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–83. [PubMed] [Google Scholar]
- 4.Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:2–14. [DOI] [PubMed] [Google Scholar]
- 5.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM III, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. [DOI] [PubMed] [Google Scholar]
- 6.Damiano RJ, Gaynor SL, Bailey M, Prasad S, Cox JL, Boineau JP, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg. 2003;126:2016–21. [DOI] [PubMed] [Google Scholar]
- 7.Schaff HV, Dearani JA, Daly RC, Orszulak TA, Danielson GK. Cox-Maze procedure for atrial fibrillation: Mayo clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:30–7. [DOI] [PubMed] [Google Scholar]
- 8.Mccarthy PM, Gillinov AM, Castle L, Chung M, Cosgrove D. The Cox-maze procedure: the Cleveland clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25–9. [DOI] [PubMed] [Google Scholar]
- 9.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–96. [DOI] [PubMed] [Google Scholar]
- 10.Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, et al. The Cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voeller RK, Bailey MS, Zierer A, Lall SC, Sakamoto S, Aubuchon K, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–7. [DOI] [PubMed] [Google Scholar]
- 12.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. [DOI] [PubMed] [Google Scholar]
- 13.Badhwar V, Rankin JS, Ad N, Grau-Sepulveda M, Damiano RJ, Gillinov AM, et al. Surgical ablation of atrial fibrillation in the United States: trends and propensity matched outcomes. Ann Thorac Surg. 2017;104:493–500. [DOI] [PubMed] [Google Scholar]
- 14.Schill MR, Sinn LA, Greenberg JW, Henn MC, Lancaster TS, Schuessler RB, et al. A minimally invasive stand-alone Cox-maze procedure is as effective as median sternotomy approach. Innovations (Phila). 2017;12:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ. The Cox-maze IV procedure in its second decade: still the gold standard? Eur J Cardiothorac Surg. 2018;53(suppl_1):i19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrance CP, Henn MC, Miller JR, Sinn LA, Schuessler RB, Maniar HS, et al. A minimally invasive Cox maze IV procedure is as effective as sternotomy while decreasing major morbidity and hospital stay. J Thorac Cardiovasc Surg. 2014;148:955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adademir T, Khiabani AJ, Schill MR, Sinn LA, Schuessler RB, Moon MR, et al. Surgical ablation of atrial fibrillation in patients with tachycardia-induced cardiomyopathy. Ann Thorac Surg. 2019;108:443–50. [DOI] [PubMed] [Google Scholar]
- 18.Khiabani AJ, Adademir T, Schuessler RB, Melby SJ, Moon MR, Damiano RJ. Management of atrial fibrillation in patients undergoing coronary artery bypass grafting: review of the literature. Innovations (Phila). 2018;13:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schill MR, Musharbash FN, Hansalia V, Greenberg JW, Melby SJ, Maniar HS, et al. Late results of the Cox-maze IV procedure in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2017;153:1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henn MC, Lawrance CP, Sinn LA, Miller JR, Schuessler RB, Moon MR, et al. Effectiveness of surgical ablation in patients with atrial fibrillation and aortic valve disease. Ann Thorac Surg. 2015;100:1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henn MC, Lancaster TS, Miller JR, Sinn LA, Schuessler RB, Moon MR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2015;150:1168–76. 1178.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ad N, Holmes SD, Massimiano PS, Rongione AJ, Fornaresio LM. Long-term outcome following concomitant mitral valve surgery and Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2018;155:983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ad N, Holmes SD, Friehling T. Minimally invasive stand-alone Cox maze procedure for persistent and long-standing persistent atrial fibrillation: perioperative safety and 5-year outcomes. Circ Arrhythm Electrophysiol. 2017;10(11). [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Chang JP, Chen MC, Cheng CI, Chung WJ. Long-term results of radio-frequency maze procedure for persistent atrial fibrillation with concomitant mitral surgery. J Thorac Dis. 2017;9:5176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaita F, Ebrille E, Scaglione M, Caponi D, Garberoglio L, Vivalda L, et al. Very long-term results of surgical and transcatheter ablation of long-standing persistent atrial fibrillation. Ann Thorac Surg. 2013;96:1273–8. [DOI] [PubMed] [Google Scholar]
- 26.Boulad N, Shammas NW, Early G, Roberts S, Shammas GA, Hu YL, et al. Ten-year outcome of intraoperative treatment of atrial fibrillation using radiofrequency ablation. Ther Clin Risk Manag. 2017;13:1233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson JO, Saint LL, Leidenfrost JE, Damiano RJ. Illustrated techniques for performing the Cox-Maze IV procedure through a right mini-thoracotomy. Ann Cardiothorac Surg. 2014;3:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
- 30.Pet M, Robertson JO, Bailey M, Guthrie TJ, Moon MR, Lawton JS, et al. The impact of CHADS2 score on late stroke after the Cox maze procedure. J Thorac Cardiovasc Surg. 2013;146:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huebner M, Wolkewitz M, Enriquez-Sarano M, Schumacher M. Competing risks need to be considered in survival analysis models for cardiovascular outcomes. J Thorac Cardiovasc Surg. 2017;153:1427–31. [DOI] [PubMed] [Google Scholar]
- 33.Gökoğlan Y, Mohanty S, Güneş MF, Trivedi C, Santangeli P, Gianni C, et al. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow-up. Circ Arrhythm Electrophysiol. 2016;9:e003660. [DOI] [PubMed] [Google Scholar]
- 34.Gaita F, Scaglione M, Battaglia A, Matta M, Gallo C, Galatà M, et al. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up? Europace. 2018;20:443–50. [DOI] [PubMed] [Google Scholar]
- 35.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
E-References
- E1.Cox JL.The first maze procedure. J Thorac Cardiovasc Surg. 2011;141:1093–7. [DOI] [PubMed] [Google Scholar]
- E2.Cox JL.The minimally invasive maze-III procedure. Op Tech Thorac Cardiovasc Surg. 2000;5:79–92. [Google Scholar]
- E3.a SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MA, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–42. [DOI] [PubMed] [Google Scholar]
- E4.Voeller RK, Bailey MS, Zierer A, Lall SC, Sakamoto S, Aubuchon K, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg. 2008;135:870–7. [DOI] [PubMed] [Google Scholar]
- E5.Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–96. [DOI] [PubMed] [Google Scholar]
- E6.Weimar, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL, et al. The Cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]