Abstract
Background
Prostate cancer (PrCa) is the second most prevalent malignancy in men worldwide. Observational studies have linked the use of low-density lipoprotein cholesterol (LDL-c) lowering therapies with reduced risk of PrCa, which may potentially be attributable to confounding factors. In this study, we performed a drug target Mendelian randomisation (MR) analysis to evaluate the association of genetically proxied inhibition of LDL-c-lowering drug targets on risk of PrCa.
Methods and findings
Single-nucleotide polymorphisms (SNPs) associated with LDL-c (P < 5 × 10−8) from the Global Lipids Genetics Consortium genome-wide association study (GWAS) (N = 1,320,016) and located in and around the HMGCR, NPC1L1, and PCSK9 genes were used to proxy the therapeutic inhibition of these targets. Summary-level data regarding the risk of total, advanced, and early-onset PrCa were obtained from the PRACTICAL consortium. Validation analyses were performed using genetic instruments from an LDL-c GWAS conducted on male UK Biobank participants of European ancestry (N = 201,678), as well as instruments selected based on liver-derived gene expression and circulation plasma levels of targets. We also investigated whether putative mediators may play a role in findings for traits previously implicated in PrCa risk (i.e., lipoprotein a (Lp(a)), body mass index (BMI), and testosterone).
Applying two-sample MR using the inverse-variance weighted approach provided strong evidence supporting an effect of genetically proxied inhibition of PCSK9 (equivalent to a standard deviation (SD) reduction in LDL-c) on lower risk of total PrCa (odds ratio (OR) = 0.85, 95% confidence interval (CI) = 0.76 to 0.96, P = 9.15 × 10−3) and early-onset PrCa (OR = 0.70, 95% CI = 0.52 to 0.95, P = 0.023). Genetically proxied HMGCR inhibition provided a similar central effect estimate on PrCa risk, although with a wider 95% CI (OR = 0.83, 95% CI = 0.62 to 1.13, P = 0.244), whereas genetically proxied NPC1L1 inhibition had an effect on higher PrCa risk with a 95% CI that likewise included the null (OR = 1.34, 95% CI = 0.87 to 2.04, P = 0.180). Analyses using male-stratified instruments provided consistent results.
Secondary MR analyses supported a genetically proxied effect of liver-specific PCSK9 expression (OR = 0.90 per SD reduction in PCSK9 expression, 95% CI = 0.86 to 0.95, P = 5.50 × 10−5) and circulating plasma levels of PCSK9 (OR = 0.93 per SD reduction in PCSK9 protein levels, 95% CI = 0.87 to 0.997, P = 0.04) on PrCa risk. Colocalization analyses identified strong evidence (posterior probability (PPA) = 81.3%) of a shared genetic variant (rs553741) between liver-derived PCSK9 expression and PrCa risk, whereas weak evidence was found for HMGCR (PPA = 0.33%) and NPC1L1 expression (PPA = 0.38%). Moreover, genetically proxied PCSK9 inhibition was strongly associated with Lp(a) levels (Beta = −0.08, 95% CI = −0.12 to −0.05, P = 1.00 × 10−5), but not BMI or testosterone, indicating a possible role for Lp(a) in the biological mechanism underlying the association between PCSK9 and PrCa. Notably, we emphasise that our estimates are based on a lifelong exposure that makes direct comparisons with trial results challenging.
Conclusions
Our study supports a strong association between genetically proxied inhibition of PCSK9 and a lower risk of total and early-onset PrCa, potentially through an alternative mechanism other than the on-target effect on LDL-c. Further evidence from clinical studies is needed to confirm this finding as well as the putative mediatory role of Lp(a).
Si Fang and colleagues use drug target Mendelian randomization to examine the association between lipid lowering drug targets and the risk of prostate cancer.
Author summary
Why was this study done?
Prostate cancer is the second most diagnosed malignancy in men globally.
Previous studies have provided conflicting evidence regarding a relationship between elevated low-density lipoprotein (LDL) cholesterol and prostate cancer risk.
The aim of this study was to examine the association between genetically proxied inhibition of lipid-lowering drug targets (i.e., PCSK9, NPC1L1, HMGCR) and prostate cancer using evidence from multiple datasets and analytical methods.
What did the researchers do and find?
Using genetic variants associated with LDL cholesterol, liver-derived gene expression, and plasma protein levels, the researchers applied drug target Mendelian randomisation (MR) and colocalization to examine the association between lipid-lowering drug targets and the risk of overall, early-onset, and advanced prostate cancer. Additional MR analyses were conducted to explore putative mediators of drug effects.
This study provided evidence of an association between genetically proxied PCSK9 inhibition and lower risk of overall and early-onset prostate cancer supported by both MR and colocalization approaches.
Follow-up analyses of genetically proxied PCSK9 inhibition highlighted a potential mediatory role for Lp(a) along the causal pathway to lower prostate cancer risk.
What do these findings mean?
PCSK9 inhibition may be involved in biological mechanisms that reduce the risk of overall and early-onset prostate cancer, potentially through the regulation of Lp(a).
However, functional validation is necessary to confirm these findings, as well as future research to further evaluate the relationship between lipid-lowering drug targets and advanced prostate cancer risk.
Introduction
Prostate cancer is the second most commonly diagnosed malignancy in men globally with over 1.4 million new cases in 2020 [1]. Findings from the literature have provided conflicting evidence of a relationship between elevated low-density lipoprotein (LDL) cholesterol and prostate cancer risk. For example, preclinical studies have suggested that high levels of extracellular LDL cholesterol (LDL-c) may promote the proliferation of prostate cancer cells [2,3]. Conversely, previous observational studies [4–8] have typically found limited evidence of an association between the levels of LDL-c and overall risk of prostate cancer, although some have reported that LDL-c lowering medications may reduce the risk of prostate cancer incidence [6,9–12]. Taken together, these findings suggest that although LDL-c may not directly contribute towards prostate tumorigenesis, biological pathways that regulate the biosynthesis and metabolism of LDL-c may influence prostate cancer risk through alternate mechanisms.
The use of human genetics to evaluate the efficacy and safety profiles of therapeutic targets is becoming increasingly popular in drug development, with recent evidence suggesting that targets with the support of genetics are approximately twice as likely to successfully make it to market [13]. Furthermore, the wealth of readily accessible data from genome-wide association studies (GWAS) means that these types of evaluations are typically inexpensive and quick to undertake. An approach to investigate genetic support for a target is Mendelian randomisation (MR) [14–16], a causal inference technique that harnesses randomly segregated genetic variants within a population as instrumental variables to proxy the perturbation of therapeutic targets [17,18].
The application of MR to examine the genetically proxied effects of drug targets (referred to as “drug target MR”) has demonstrated the validity of this approach to corroborate findings from clinical trials as long as the underlying assumptions are met [19]. For example, the efficacy of lipid-lowering drug targets in reducing risk of coronary artery disease has been shown by previous MR studies, for therapies such as statins (which target HMG-CoA reductase (HMGCR)), proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and Ezetimibe (which targets Niemann–Pick C1-Like 1 (NPC1L1)) [20,21]. Moreover, drug target MR analyses have provided evidence of adverse effects reported in trials, such as the effect of statins on increased risk of type 2 diabetes, as well as highlighting potential additional indications [22]. This approach has also been applied to suggest that statins may provide additional benefit towards the lowering of ovarian cancer risk [23], as well as a recent study by Sun and colleagues that investigated evidence of association between genetically proxied lipid-lowering drugs with breast and prostate cancer [12]. However, this study did not triangulate findings from multiple sources of data (such as molecular traits) or evaluate the robustness of MR estimates using different research methods, such as genetic colocalization.
In this study, we applied drug target MR to investigate the association between genetically proxied LDL-c lowering medications and risk of total, advanced, and early-onset prostate cancer using data from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium [24]. Genetic instruments were oriented to proxy the effect of statins, PCSK9 inhibitors, and Ezetimibe on lowering circulating LDL-c. The associations of genetically proxied LDL receptor (LDLR) mediated LDL-c and the genetically proxied levels of circulating LDL-c on prostate cancer outcomes were also examined to assess whether evidence of the findings for inhibiting drug targets may be generalisable to the lowering of LDL-c. Moreover, analyses using genetic variants to investigate the levels of circulating proteins and liver tissue-derived gene expression for targets were performed as sensitivity analyses and to triangulate evidence [25]. Effect estimates on advanced prostate cancer and early-onset prostate cancer were also investigated. Finally, we evaluated the genetically proxied effects of LDL-c-lowering drugs on several traits previously implicated to play a role in prostate cancer risk (body mass index (BMI) [26–28], lipoprotein A (Lp(a)) [8], and testosterone [29,30]) to discern whether they may reside along the pathways between therapeutic targets and risk of prostate cancer.
Methods
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline, specific for Mendelian randomisation [31] (S1 STROBE Checklist). An overview of the study can be found in Fig 1.
Fig 1. Study overview.
aThe LDL-c variants were oriented to be in the LDL-c lowering direction, i.e., effect estimates in Mendelian randomisation analysis are per 1 SD lowering of LDL-c. bThe original analysis was conducted by Ioannidou and colleagues [8]. LDL-c, LDL cholesterol; MR, Mendelian randomization; SD, standard deviation.
Ethics statement
Our work involved the previously collected genetic sequencing and phenotype data of human participants in the UK Biobank cohort study. The North West Multi-centre Research Ethics Committee (MREC) gave ethical approval for the UK Biobank.
Data sources
In our primary analysis, two-sample MR was applied to study the association of genetically proxied therapeutic inhibition for lipid-lowering drug targets PCSK9, NPC1L1, and HMGCR, as well as genetically proxied levels of LDLR and overall LDL-c, on the risk of prostate cancer.
Genetic instruments for drug targets were extracted from the latest summary statistics from the Global Lipids Genetics Consortium (GLGC) GWAS on LDL-c levels (n = 1,320,016) [32] from the online data repository (http://csg.sph.umich.edu/willer/public/glgc-lipids2021/). Final instruments for the 3 lipid-lowering drug targets (and genetically proxied LDLR) were genetic variants robustly associated with LDL-c (based on P < 5 × 10−8 and a pairwise linkage disequilibrium (LD) r2 < 0.1 using a reference panel consisting of individuals of European ancestry from the 1,000 Genomes Project Consortium [33]) and located within 100 kb around PCSK9 (Entrez Gene: 255738), NPC1L1 (Entrez Gene: 29881), HMGCR (Entrez Gene: 3156), and LDLR (Entrez Gene: 3949), respectively. Additionally, genome-wide variants associated with LDL-c were used as instrumental variables for overall levels of LDL-c (based on P < 5 × 10−8 and r2 < 0.001).
Given that genetic estimates on prostate cancer risk were derived using a male-only population, we repeated all analyses using male-stratified instruments identified by conducting a GWAS of LDL-c (ID: 30780) on 201,678 male participants of European ancestry in the UK Biobank study [34]. The levels of LDL-c were standardised to have a mean of 0 and SD of 1 prior to analysis. BOLT-LMM was implemented to conduct the GWAS analysis with adjustment for age and genotyping chip [35]. Further details of the GWAS analysis pipeline has been described previously [36,37]. After quality control and imputation, the GWAS results were clumped with 2 sets of different P-value and LD r2 thresholds as described above, i.e., (1) P < 5 × 10−8 and pairwise r2 < 0.1, or (2) P < 5 × 10−8 and r2 < 0.001.
For prostate cancer outcomes, summary statistics were obtained from GWAS meta-analyses on the risks of overall prostate cancer (n = 140,306 men including 79,194 cases and 61,112 controls), as well as 2 stratified meta-analysis, including: (1) the risk of early-onset prostate cancer (n = 51,244 including 6,988 prostate cancer cases diagnosed on or before age 55 years and 44,256 non-prostate cancer controls); (2) the risk of advanced prostate cancer (n = 73,475 including 15,167 advanced prostate cancer cases and 58,308 non-prostate cancer controls), all from the PRACTICAL consortium in which the majority of studies includes cases and controls without matching by clinical features [24]. Advanced prostate cancer cases include individuals with either metastatic prostate cancer, a Gleason score of 8 or higher, a prostate-specific antigen level greater than 100 ng/mL, or prostate cancer-related death [24]. All individuals involved in the prostate cancer GWAS analyses are of European ancestry.
We also used various datasets as part of our secondary analyses in this paper. Full details of these can be found in S1 Supplementary Note. A summary of all GWASs involved in this study is in S1 Table.
Statistical analysis
Two-sample Mendelian randomisation
In the primary analysis, two-sample MR was applied to investigate the associations of genetically proxied inhibition of lipid-lowering drug targets and overall LDL-c on the risk of prostate cancer. All analysis was performed using the TwoSampleMR R package (v0.5.6, https://github.com/mrcieu/TwoSampleMR). The application of MR must satisfy 3 key assumptions, including (1) the genetic instrumental variable are strongly associated with the exposure of interest; (2) they are associated with the outcome only through the exposure; and (3) the exposure and outcome does not have a shared cause [17,18]. The first assumption is the only testable assumption and could be assessed using instrument strength. The instrument strength (F statistics) for LDL-c-lowering drug targets and risk factors examined in this study were calculated using a formula previously described by Bowden and colleagues [38].
In the primary drug target MR analysis, effect estimates for genetically proxied inhibition of PCSK9, NPC1L1, and HMGCR were derived using the random-effects inverse-variance weighted (IVW) model [39]. Considering the weak LD between genetic variants (r2 < 0.1) used as instrumental variables, IVW analyses were adjusted for LD matrices between instruments based on the same reference panel as above to ensure they were independent of one and other [40]. Iterative leave-one-out analyses were conducted for PCSK9 to identify the presence of any single variants that may be driving identified effects on the outcome. In validation analysis using UKB male-stratified genetic instruments, the effects from inhibiting PCSK9 and HMGCR were analysed using IVW accounting for genetic correlations, whereas the effects from inhibition of NPC1L1 was estimated using Wald ratio based on rs2073547.
Next, we performed two-sample MR using the random-effects IVW method to investigate whether the association between genetically proxied LDL-c-lowering drug targets and total prostate cancer risk may be attributed to the inhibition of LDLR or due to overall changes in LDL-c. When analysing the effects of LDL-c levels, the weighted median model (which allows up to half of the included SNPs to be pleiotropic and is less influenced by outliers) [41], the weighted mode model (which assumes that the most common effect is consistent with the true causal effect) [42], and the MR-Egger model (which provides an estimate of association magnitude allowing all SNPs to be pleiotropic) [43] were used as sensitivity analysis.
As a secondary analysis, two-sample MR were performed using PCSK9 cis-eQTL and cis-pQTL to further examine results identified in the primary analyses. We firstly estimated the association between genetically proxied plasma levels of PCSK9 and LDL-c using the random-effects IVW methods accounting for LD between genetic variants. A Wald ratio was calculated to estimate the association between genetically proxied PCSK9 expression (instrumented using a single cis-eQTL) and prostate cancer outcomes. We applied the random-effects IVW method accounting for correlation structure between genetic variants to examine the associations between genetically proxied plasma PCSK9 (instrumented using cis-pQTLs) and prostate cancer outcomes. The pairwise LD correlation r2 between eQTL and pQTLs were calculated using LDmatrix from LDlink [44] based on the reference panel consisting of Utah Residents from North and West Europe (CEU) individuals. The PCSK9 cis-pQTL MR analysis was repeated using conditionally independent cis-pQTLs (based on the same Icelandic population they were derived from) using random-effect IVW method without the adjustment of correlation between SNPs.
In addition, LDL-c-associated genetic variants at the PCSK9 locus were functionally annotated using Ensembl Variant Effect Predictor (VEP) [45]. Regulatory pathway data for all targets analysed in this study were queried using the STRING (v11) database based on experimentally determined data [46].
Co-localization between eQTL and total prostate cancer risk
Given that single SNP MR analyses can be prone to high false discovery rates due to LD between the instrument and proximal variants, we conducted co-localization analyses to identify evidence of shared causal variants between liver-derived gene expression and risk of total prostate cancer. We constructed LocusZoom plots using gassocplot2 R package (https://github.com/jrs95/gassocplot2) to visualise the genetic variants associated with liver-derived PCSK9, HMGCR, and NPC1L1 gene expression (GTEx v8) at each of their corresponding loci and variants associated with risk of prostate cancer. The coloc (v5.1.0) [47] and eCAVIAR (v2.2) [48] approaches were applied to formally appraise evidence using the genetic correlation matrix generated using European individuals from the 1,000 genome reference panel [33]. Colocalization using the coloc method quantified the probability of a shared genetic variant between liver tissue-derived gene expression and prostate cancer (H4) for all 3 genes, based on genetic variants within 300 kb up- and downstream the lead cis-eQTLs. eCAVIAR was further applied to quantify the colocalization posterior probability (CLPP) between PCSK9 gene expression and prostate cancer, and the cut-off of CLPP to indicate evidence of a shared causal variant is >0.01 as proposed by the authors of this approach [48]. In addition, we estimated the genome-wide genetic correlation between LDL-c and prostate cancer using LD Score regression [49], as well as local genetic correlation between the 2 traits at the PCSK9 locus using LAVA [50].
Contrasting the genetically proxied associations between lipid-lowering drug targets and risk factors of prostate cancer
To examine whether the associations between genetically proxied inhibition of drug targets and prostate cancer risk could be attributed to a potential mediatory pathway involving changes in BMI, Lp(a), or testosterone, we performed two-sample MR to investigate effects from drug targets on those risk factors as a sensitivity analysis using the same methods as above. The associations between genetic variants and BMI were extracted (1) from the Pulit and colleagues GWAS meta-analysis on BMI (n = 806,834) [51] when using GLGC variants; and (2) from the Locke and colleagues GWAS on BMI (n = 339,224) [52] when analysed using UKB male-stratified genetic variants to avoid sample overlap. The associations between genetic instruments and Lp(a) were extracted from a GWAS on inverse rank normalised levels of Lp(a) in UKB participants (http://www.nealelab.is/uk-biobank/) from the IEU Open GWAS project. For BMI and Lp(a), replication analyses were conducted using summary statistics of male-stratified GWAS from the same cohorts mentioned above (n = 374,756 men in Pulit and colleagues GWAS on BMI, n = 152,893 men in Locke and colleagues GWAS on BMI, and n = 167,020 men in Neale lab UKB GWAS on inverse rank normalised levels of Lp(a)). Moreover, the associations between genetic variants and testosterone levels were from male-stratified GWAS on total and bioavailable testosterone levels (n = 199,569 and 184,205 men, respectively) in the UK Biobank accessed through the IEU Open GWAS project. Random-effects IVW models were used with adjustment for the LD between instruments as above. For drug targets instrumented using a single genetic variant, Wald ratio estimates were used to evaluate their genetically proxied associations with the outcome.
In addition, for potential risk factors of prostate cancer found to be associated with genetically proxied PCSK9 inhibition, we conducted MR to further examine their associations with prostate cancer risk. This includes univariable and multivariable MR to replicate the previously published findings on Lp(a) and prostate cancer. Univariable MR was performed (1) using IVW and weighted median model with 15 genome-wide significant genetic variants associated with Lp(a) as genetic instruments; and (2) using Wald ratio estimate with the cis-acting variant associated with Lp(a) on LPA gene as the genetic instrument. Multivariable MR was performed using the cis-acting variant for Lp(a) together with genome-wide significant variants associated with LDL-c from the latest GLGC GWAS [32].
Results
Genetic variant selection
In total, 28 genetic variants were used to proxy the inhibition of PCSK9, 4 for NPC1L1, 13 for HMGCR, 36 for the levels of LDLR-mediated LDL-c, and 424 for the levels of total LDL-c identified using the latest GLGC GWAS. Details of these genetic variants are in S2 Table. Male-stratified genetic instruments for each of these exposures are presented in S3 Table. Details of the cis-acting protein quantitative trait loci (cis-pQTL) for plasma levels of PCSK9 protein and liver-derived cis-acting expression quantitative trait loci (cis-eQTL) data for PCSK9 gene are listed in S4 Table. Functional annotations of PCSK9 variants are presented in S5 Table. Regulatory pathways between the 4 protein targets are presented in S1 Fig. The F statistics of genetic instruments for all drug targets and risk factors assessed in this study, including the eQTL and pQTLs, ranged from 26.9 to 629.7, suggesting that the results are unlikely to be biased due to weak instruments [53].
Mendelian randomisation analysis of lipid-lowering therapies and prostate cancer risk
We firstly applied drug target MR to investigate the association of genetically proxied lipid-lowering drug targets (HMGCR, PCSK9, and NPC1L1) with overall prostate cancer risk (Fig 2 and S6 Table). Genetically proxied inhibition of PCSK9 was strongly associated with a lower risk of developing prostate cancer (IVW MR odds ratio (OR) = 0.85, 95% confidence interval (95% CI) = 0.76 to 0.96, P = 0.009, per standard deviation (SD) reduction in LDL-c). Leave-one-out analyses provided consistent evidence of an association between genetically proxied PCSK9 inhibition and risk of prostate cancer, suggesting that the overall estimate was not driven by a single influential variant (S2 Fig and S7 Table). Genetically proxied inhibition of HMGCR provided evidence of a similar magnitude of effect on overall prostate cancer as PCSK9, although the 95% CI included the null (OR = 0.83, 95% CI = 0.62 to 1.13, P = 0.244). Genetically proxied inhibition of NPC1L1 was associated with higher overall prostate cancer risk which likewise had a 95% CI that included the null (OR = 1.34, 95% CI = 0.87 to 2.04, P = 0.180). MR analysis on the risk of early-onset and advanced prostate cancer identified strong evidence of an association between genetically proxied inhibition of PCSK9 and early-onset disease (OR = 0.70, 95% CI = 0.52 to 0.95, P = 0.023), but weaker evidence of association with advanced prostate cancer (OR = 0.91, 95% CI = 0.74 to 1.12, P = 0.381) (S6 Table). Similar findings were observed using male-stratified genetic instruments identified using the UKB data (S6 Table).
Fig 2. Results from MR analyses to estimate the effect of lipid-lowering therapies and risk factors on overall prostate cancer risk.
Effect estimates are odds ratios for prostate cancer per 1 SD reduction in LDL-c proxied using genetic instruments identified from the GLGC. In total, 28 genetic variants were used to proxy the inhibition of PCSK9, 4 for NPC1L1, 13 for HMGCR, 36 for the levels of LDLR-mediated LDL-c, and 424 for the levels of total LDL-c identified using the latest GLGC GWAS. F-statistics for the exposures ranges from 221.5 to 629.7. Detailed results can be found in S6 Table. GLGC, Global Lipids Genetics Consortium; LDL-c, LDL cholesterol; MR, Mendelian randomization; SD, standard deviation.
For comparative purposes, we investigated the effect of circulating LDL-c on overall prostate cancer risk by selecting genetic instruments at the LDLR locus as well as those associated with LDL-c across the genome. There was minimal evidence to suggest an effect of genetically proxied LDLR mediated LDL-c levels on overall prostate cancer risk (OR = 1.04, 95% CI = 0.96 to 1.12, P = 0.385, per SD reduction in LDL-c). Additionally, a set of 424 SNPs used as a genetic instrument for circulating LDL-c was weakly associated with prostate cancer risk (OR = 0.95, 95% CI = 0.88 to 1.01, P = 0.096) with similar effect estimates to those found for HMGCR inhibition (heterogeneity P = 0.432). The analysis for (overall) prostate cancer risk was repeated with similar conclusions using a male-specific 104 SNP LDL-c instrument derived in males only from the UKB (OR = 0.95, 95% CI = 0.89 to 1.01, P = 0.101). MR-Egger, weighted median, and weighted mode estimates were comparable to the IVW results (S7 Table).
Triangulation of evidence using data on circulating protein levels and liver-derived gene expression
To further examine the association between PCSK9 and prostate cancer, two-sample MR analyses were performed using cis-acting pQTLs to instrument inhibition of circulating PCSK9 protein levels (S8 Table). These cis-pQTLs are strongly associated with LDL-c levels (Beta = −0.54, 95% CI = −0.59 to −0.49, P = 3.81 × 10−90, SD change in LDL-c per SD reduction in PCSK9 levels). MR results provided evidence of an effect of lower levels of circulating PCSK9 protein on overall prostate cancer (OR = 0.93, 95% CI = 0.87 to 0.997, P = 0.040, per SD reduction in plasma PCSK9 levels), and early-onset prostate cancer (OR = 0.86, 95% CI = 0.74 to 0.98, P = 0.030) but not advanced prostate cancer (OR = 0.98, 95% CI = 0.89 to 1.07, P = 0.600) using the IVW method accounting for genetic correlation structure, consistent with findings from our initial analyses. Repeating the MR analysis using conditionally independent cis-pQTLs on overall PrCa risk provided a very similar magnitude of effect (OR = 0.92, 95% CI = 0.85 to 0.999, P = 0.048).
LDL-c removal occurs primarily in the liver, which is also the organ where PCSK9 is strongly expressed based on the latest release (v8) of the Genotype-Tissue Expression (GTEx) project [54]. Based on our variant selection criteria (i.e., in and around the PCSK9 gene and pairwise r2 < 0.1) and a false-discovery rate threshold of 0.05 defined by GTEx, there was only 1 eQTL in liver tissue using this dataset (rs553741), which is in strong LD with one of the SNPs used as a genetic instrument to proxy the effects of PCSK9 inhibition in the primary drug target MR (rs472495, r2 = 0.909) (for full LD matrix see S9 Table). MR estimates were supportive of an association between lower levels of PCSK9 gene expression (instrumented using the eQTL) and a lower risk of overall prostate cancer (OR = 0.90, 95% CI = 0.86 to 0.95, P = 5.50 × 10−5, per SD reduction in PCSK9 transcript levels). Analysis on disease subtypes provided similar magnitude of association with genetically proxied PCSK9, although confidence intervals overlapped the null (early-onset prostate cancer: OR = 0.91, 95% CI = 0.91 to 1.03, P = 0.139; advanced prostate cancer: OR = 0.93, 95% CI = 0.86 to 1.01, P = 0.102).
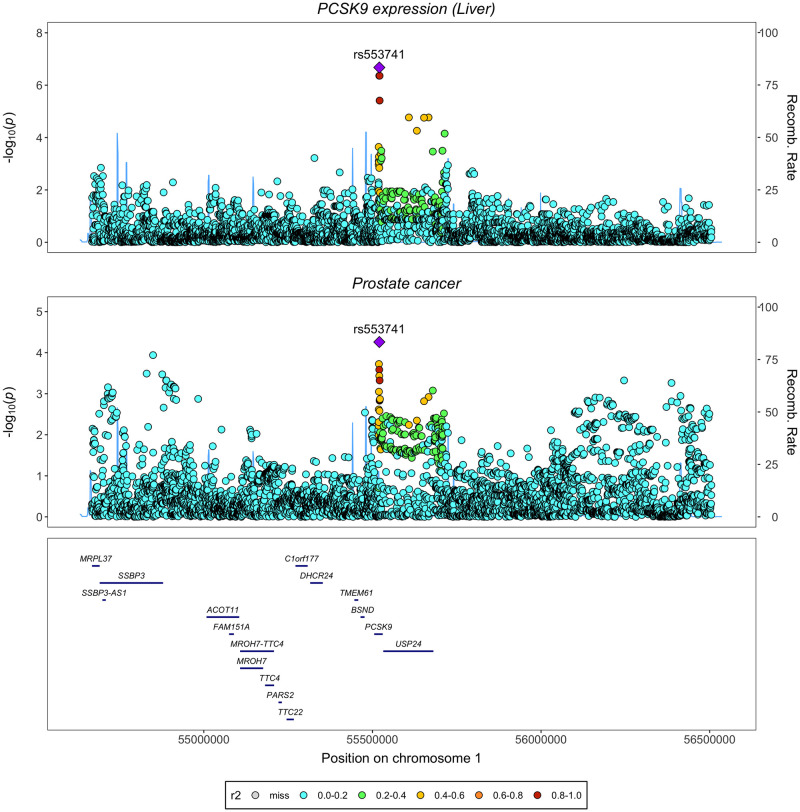
As drug target MR analyses, particularly when using only a single cis-acting variant as an instrument, are susceptible to false-positive findings due to LD structure with nearby genes [55,56], we conducted genetic colocalization at this locus to evaluate evidence of a shared causal variant between PCSK9 expression in liver tissue and prostate cancer. A LocusZoom plot comparing the cis-acting eQTLs associated with liver tissue-derived PCSK9 expression and SNPs associated with risk of prostate cancer (Fig 3) identified a shared top SNP (rs553741) in the PCSK9 gene region. Analysis using coloc method found a posterior probability of colocalization (H4) of 81.3%, providing strong evidence for colocalization between the 2 traits. Full results from coloc are presented in S10 Table. Using the eCAVIAR method [48], we found a CLPP of 0.103 for the variant rs553741, which suggests there is strong evidence of a shared causal variant at this locus based on a threshold of CLPP > 0.01 as proposed by the authors of this approach [48]. Detailed CLPP for every candidate SNP are presented in S11 Table.
Fig 3. LocusZoom plots illustrating evidence of genetic colocalization between PCSK9 gene expression in the liver and prostate cancer risk at the PCSK9 gene locus.
In addition, LocusZoom plots for liver tissue-derived HMGCR and NPC1L1 gene expression and prostate cancer did not find evidence supporting shared top hits in their respective gene regions (S3 and S4 Figs). Formal evaluations using coloc also found little evidence for colocalization between the expression of HMGCR or NPC1L1 in liver and prostate cancer at the respective genes (H4 = 0.33% for HMGCR, H4 = 0.38% for NPC1L1). Additional LocusZoom plots visualising genetic variants associated with LDL-c and prostate cancer at PCSK9, HMGCR, and NPC1L1 genes provided similar results (S5–S7 Figs). Genetic correlation results identified evidence for correlation between LDL-c and prostate cancer risk at the PCSK9 loci (correlation coefficient rho = 1) (S12 Table).
Contrasting the genetically proxied associations between lipid-lowering drug targets and risk factors of prostate cancer
We hypothesised that the association between genetically proxied lipid-lowering drug target inhibition and prostate cancer may be mediated through prostate cancer risk factors, such as BMI, Lp(a), or testosterone. Therefore, we examined the association between genetically proxied inhibition of drug targets and risk factors for prostate cancer using drug target MR (Fig 4). To maximise power, all effect estimates for BMI and Lp(a) reported in the main text are from analysis using males and females combined GWAS as the outcome. For validation analysis results using males only GWAS on BMI and Lp(a), see supporting files S13 and S14 Tables.
Fig 4. Results from drug target MR analyses to investigate the effect of lipid-lowering therapies on BMI, lipoprotein A, and testosterone.
Estimates of effects from genetically proxied inhibition of PCSK9, NPC1L1, and HMGCR on BMI, levels of lipoprotein A, total testosterone, and bioavailable testosterone. Effect estimates were in SD change in the outcome per drug target inhibition effect equivalent to an SD reduction in LDL-c. Results were from analysis using instruments identified from the GLGC data. BMI, body mass index; GLGC, Global Lipids Genetics Consortium; LDL-c, LDL cholesterol; MR, Mendelian randomization; SD, standard deviation.
Repeating our primary MR analyses to investigate the genetically proxied association of each lipid-lowering target on BMI (S13 Table) provided little evidence for genetically proxied inhibition of PCSK9 (Beta = 0.02, 95% CI = −0.01 to 0.05, P = 0.212, SD change in BMI per SD reduction in LDL-c) as well as NPC1L1 (Beta = −0.02, 95% CI = −0.23 to 0.18, P = 0.819) on prostate cancer risk. However, genetically proxied inhibition of HMGCR provided strong evidence for an association with elevated BMI (Beta = 0.28, 95% CI = 0.18 to 0.37, P = 1.61 × 10−8). Replication using males-only GWAS on BMI provided similar evidence (S13 Table).
Evaluating effects on Lp(a) for each target (S14 Table) suggested that there was strong evidence of an effect of PCSK9 inhibition on lower levels of this lipoprotein particle (IVW accounting for LD matrix: Beta = −0.08, 95% CI = −0.12 to −0.05, P = 1.00 × 10−5, SD change in Lp(a) levels per SD reduction in LDL-c). The association was supported in an analysis using PCSK9 pQTLs as the genetic instruments (Beta = −0.03 SD change in the levels of Lp(a) per SD reduction in plasma PCSK9 levels, 95%CI = −0.05 to −0.02, P = 1.47 × 10−4). Conversely, investigating the effects of genetically proxied inhibition of HMGCR (Beta = −0.05, 95% CI = −0.13 to 0.02, P = 0.150) and NPC1L1 (Beta = 0.08, 95% CI = −0.44 to 0.02, P = 0.080) on Lp(a) levels found that their CIs included the null despite similar central magnitudes of effect compared with PCSK9. Replication using males-only GWAS on Lp(a) provided similar evidence (S14 Table).
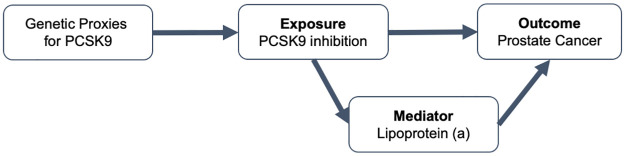
In addition, we examined the association between genetically proxied Lp(a) levels on prostate cancer risk from using male-stratified GWAS on Lp(a) by replicating MR analysis conducted by Ioannidou and colleagues [8]. Using 15 Lp(a)-associated variants from across the genome (r2 < 0.001, P < 5 × 10−8), we found consistent effect estimates on the association between genetically proxied Lp(a) and prostate cancer in the univariable setting using IVW (OR = 1.06, 95% CI = 0.95 to 1.20, P = 0.305, per SD increase in Lp(a) levels) and weighted median methods (OR = 1.07, 95% CI = 1.004 to 1.13, P = 0.036). Analysis using the genetic variant associated with Lp(a) located within the LPA gene (rs73596816) provided evidence with a consistent magnitude of association (OR = 1.07, 95% CI = 1.00 to 1.14, P = 0.056) based on the Wald ratio method. Multivariable MR using the cis-variant for Lp(a) adjusting for LDL-c levels provided strong evidence for genetically proxied Lp(a) on lower prostate cancer risk (OR = 1.05, 95% CI = 1.01 to 1.08, P = 0.013). The putative causal relationship between PCSK9 inhibition, Lp(a), and prostate cancer risk is illustrated in a directed acyclic graph (Fig 5).
Fig 5. A DAG showing the putative association between PCSK9 inhibition, lipoprotein A, and prostate cancer.
DAG, directed acyclic graph.
Examining the effects from drug targets on testosterone levels (S15 Table) suggest genetically proxied inhibition of both PCSK9 and NPC1L1 contributed very little to alterations in total testosterone (PCSK9: Beta = 0.04, 95% CI = −0.01 to 0.09, P = 0.146, SD change in testosterone per SD reduction in LDL-c; NPC1L1: Beta = 0.02, 95% CI = −0.19 to 0.22, P = 0.876) or bioavailable testosterone (PCSK9: Beta = −0.02, 95% CI = −0.07 to 0.03, P = 0.341; NPC1L1: Beta = 0.08, 95% CI = −0.08 to 0.25, P = 0.327) in men. On the contrary, genetic variants proxying the inhibition of HMGCR showed strong correlation with both measurements of testosterone (total: Beta = −0.21, 95% CI = −0.29 to −0.12, P = 2.36 × 10−6; bioavailable: Beta = −0.14, 95% CI = −0.25 to −0.03, P = 0.014) in men. However, associations between genetically proxied HMGCR inhibition with testosterone and BMI require further evaluations, such as genetic colocalization analyses to investigate potential pleiotropic effects via neighbouring genes.
Discussion
In this work, we have identified strong evidence using large-scale genetic data to suggest that therapeutic inhibition of lipid-lowering drug target PCSK9 may reduce prostate cancer risk. Estimates based on circulating PCSK9 protein and liver tissue-derived PCSK9 expression data further support this finding. Taken together, these findings suggest that the genetically proxied association between PCSK9 inhibition and a lower risk of prostate cancer is unlikely to be due to a mechanism involving the lowering of LDL-c levels. We postulate that one potential explanation for this finding is due to the lowering of Lp(a), which genetically proxied PCSK9 inhibition provided stronger evidence of achieving in comparison to statin and Ezetimibe therapies in this study.
PCSK9 is known as a regulator of the metabolism of LDL-c, although thus far its role in cancer susceptibility has yet to be comprehensively evaluated and characterised. There are various preclinical studies based on tumour tissue or mouse models that report the direct effects of PCSK9 or PCSK9 inhibitors on multiple types of cancer [57], including hepatocellular carcinoma [58,59], lung carcinoma [60,61], colorectal cancer [62], and breast cancer [63]. Additionally, Liu and colleagues inoculated Pcsk9 knockout mouse cancer cells into syngeneic mouse hosts and observed delayed tumour growth, as well as a synergistic effect between PCSK9 inhibition and anti-PD1 antibody treatment to promote the efficacy of tumour growth suppression [64]. In contrast, few preclinical studies have linked PCSK9 to prostate cancer [65,66], although Gan and colleagues previously demonstrated that PCSK9 siRNA protects human prostate cancer cells from ionising radiation-induced cell damage [67].
With excellent efficacy and safety profiles, 2 monoclonal antibodies against PCSK9 have been developed to lower elevated LDL-c levels and subsequently help prevent coronary heart disease (CHD) [68,69]. Our findings add to growing evidence suggesting that PCSK9 inhibitors may provide the most benefit towards reducing disease risk in comparison to statins and Ezetimibe that inhibit HMGCR and NPC1L1, respectively. Furthermore, recent evaluations involving CRISPR base editing in primates suggests that complete knockdown of PCSK9 in the liver results in approximately a 60% reduction of LDL cholesterol [70]. Further research is required to investigate the consequences of this approach towards prostate cancer risk, although our findings using genetic proxies of PCSK9 inhibition predict that this would have a beneficial effect, especially on early-onset prostate cancer. This supports the recently identified favourable overall effects from genetically proxied PCSK9 inhibition on the lifespan [71].
MR analyses provided a similar central effect estimate for genetically proxied HMGCR estimates and prostate cancer as those found for PCSK9 inhibition, although with wider corresponding confidence intervals resulting in weaker evidence of an effect. Furthermore, as was the case for NPC1L1, there was also weak evidence of a shared causal variant based on colocalization analyses. This could be due to the lack of power provided by the prostate cancer GWAS used in this study, meaning that investigations should be repeated in the future once findings from larger prostate cancer case-control GWAS are available.
The evidence for associations between genetically proxied inhibition of lipid-lowering drug targets and prostate cancer is consistent with a recently published drug target MR study [12], in which the authors applied a smaller set of genetic instruments for drug targets (11 for PCSK9, 3 for NPC1L1, and 5 for HMGCR) identified from the GWAS on LDL-c (n = 173,082) published in 2013 [72] to study the effects of lipid-lowering drugs on prostate and breast cancer risk. In addition to the use of a larger set of genetic instruments identified from a much recent and larger GWAS meta-analysis, our study has provided a more comprehensive and robust evaluation. Firstly, we examined the association between on-target effects of lipid-lowering drugs and cancer risk by including genetically proxied LDLR and LDL-c as the exposures. Secondly, we conducted various sensitivity analyses to further investigate the finding between genetically proxied PCSK9 inhibition and prostate cancer risk. For example, leave-one-out analyses found consistent effect estimates for genetically proxied PCSK9 on prostate cancer risk, suggesting the association is unlikely to be driven by any single variant in our instrument. This mitigates the likelihood that individual pleiotropic variants at the PCSK9 locus are influencing prostate cancer risk via alternate biological pathways. Thirdly, we have triangulated evidence from liver tissue-derived gene expression and plasma protein data to further support findings from our primary MR approach. Moreover, we included further evidence from genetic colocalization analyses using liver tissue-derived gene expression data, which suggests that our findings are unlikely to be explained by a pathway involving a neighbouring gene as opposed to PCSK9. Furthermore, we included validation analyses using male-stratified datasets where possible.
Additionally, we performed subsequent analyses to explore the potential mediatory role of BMI, Lp(a), and testosterone in the associations between drug targets and prostate cancer risk. Elevated BMI has been found to associate with increased prostate cancer risk in multiple observational studies [26–28], whereas MR study identified evidence for an inversed association between them [73]. In addition, levels of testosterone were found to be strongly correlated with the risk of prostate cancer in both observational [29] and MR [30] studies. Our results found little evidence supporting effects of genetically proxied inhibition of PCSK9 on these 2 risk factors. Moreover, a higher levels of Lp(a) has previously been reported to associate with poor prognosis of prostate cancer [74] as well as a higher risk of overall and early-onset prostate cancer [8]. The evidence for associations between Lp(a) and overall prostate cancer risk was replicated in our study using male-stratified genetic instruments. Our results provided strong evidence that PCSK9 inhibition may lower levels of Lp(a), whereas effect estimates from the inhibition of NPC1L1 or HMGCR again had wider CIs compared to PCSK9 estimates. This finding has also been supported by randomised control trials [68,75] which found that PCSK9 inhibitors significantly reduced levels of Lp(a) (e.g., Alirocumab lead to 25.6% reduction in Lp(a) compared with placebo group at week 24 [68]), whereas statins and Ezetimibe had little to mild effects on Lp(a) [76]. Furthermore, genetically proxied PCSK9 exhibited the strongest magnitude of association with the risk of early-onset prostate cancer than that of overall prostate cancer, and there is weak evidence for association with advanced disease. This is consistent with the association between genetically predicted Lp(a) and the risk of prostate cancer outcomes identified in the recent multivariable MR [8] and replicated in our study. Although our findings suggest that Lp(a) may play a mediatory role along the pathway between PCSK9 inhibition and prostate cancer risk, further functional work is required to robustly demonstrate this.
This study has noteworthy limitations. Firstly, although the estimates derived in this MR study are based on a standard deviation change in LDL-c that is clinically achievable (e.g., in randomised controlled trials, PCSK9 inhibitors on average reduced LDL-c by 60 to 70 mg/dL compared with placebo group at week 24 in addition to the use of statins [68,69]), these are based on genetic proxies of therapeutic targets that may not be equivalent to those reported by randomised controlled trials. This is because drugs are often taken for a defined period, whereas estimates from MR analyses are conventionally interpreted as lifelong exposures to risk factors given that genetic variants are typically fixed at conception. Furthermore, there is increasing evidence to suggest that associations between genetic instruments and exposures may vary throughout the life course [77]. Additionally, conventional MR methods assume a linear relationship between genetically proxied exposures and outcomes; however, drugs may not trigger any biological response until a drug dose exceeds a certain level. Secondly, we leveraged 13 cis-pQTLs to instrument plasma levels of PCSK9 in this work due to the availability of a large-scale dataset for whole blood measures (n = 35,559) [78]. However, analyses using cis-pQTL derived from liver tissue would be valuable to further examine the effect of PCSK9 inhibition on prostate cancer risk once these data are available in sufficient samples. Thirdly, using genetic instruments at the PCSK9 locus extracted from a GWAS of LDL-c, liver-derived PCSK9 expression, and circulating PCSK9 protein, this work focuses on the indirect association between PCSK9 inhibition and prostate cancer risk. Future studies with prostate derived PCSK9 cis-pQTL are essential for evaluating the direct role of PCSK9 in prostate cancer cells, especially the effect on advanced prostate cancer.
Additionally, replication of MR estimates using males-stratified PCSK9 cis-eQTL and cis-pQTL would be worthwhile to support this finding in the future, even though we did not find large differences using LDL-c stratified instruments to overall conclusions. We also note that multivariable MR cannot be applied to explore the mediatory role of Lp(a) due to the lack of genome-wide significant genetic variants in PCSK9 gene region from the UK Biobank GWAS on Lp(a). It is worth further investigation when a larger GWAS on Lp(a) is available in the future. Moreover, the lack of evidence for an association between PCSK9 inhibition and the risk of advanced prostate cancer, as well as comparatively wider confidence intervals for associations between HMGCR, NPC1L1, and prostate cancer (compared to genetically proxied PCSK9 inhibition) could potentially be due to lack of power. These should be further explored when larger datasets are available. Furthermore, we did not explore the genetically proxied association between drug target inhibition and other stratified prostate cancer phenotypes, such as Gleason score, cancer aggressiveness, or recurrence. Although the PRACTICAL consortium has published GWASs on some of these phenotypes, these GWASs are conducted among prostate cancer cases without the inclusion of non-cancer controls. MR analysis using such datasets may induce index event bias [79], leading to false-negative results or even spurious inverse associations. These could be investigated by following up participants of randomised controlled trials for PCSK9 inhibitors. Similarly, leveraging large-scale summary-level data from PRACTICAL meant that we were unable to evaluate the time-varying effects of PCSK9 inhibition on risk of prostate cancer at separate stages in the life course or in population subgroups undergoing different treatment regimens. These are therefore important areas of future research to investigate either in a clinical trial setting or when large-scale genetic association datasets become accessible.
In summary, our study demonstrates that genetically proxied inhibition of PCSK9 is strongly associated with a lower risk of overall and early-onset prostate cancer, potentially through a mechanism involving the lowering of Lp(a) levels. Further evidence from clinical studies on prostate cancer incidence and progression among patients taking PCSK9 inhibitors is needed to confirm this finding.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Estimates are effects equivalent to 1 SD reduction in LDL-c levels.
(XLSX)
These analyses were performed using genetic instruments identified from the Global Lipids Genetics Consortium.
(XLSX)
Estimates are per 1 SD reduction in plasma protein levels of PCSK9 for pQTLs or per 1 SD reduction in the levels of PCSK9 transcripts in the liver tissue.
(XLSX)
The pairwise r2 were calculated using the reference panel consisting of Utah Residents from North and West Europe (CEU) individuals from the 1,000 Genomes project.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Estimates are effects equivalent to 1 SD reduction in LDL-c levels or 1 SD reduction in plasma protein levels of PCSK9.
(XLSX)
Estimates are effects equivalent to 1 SD reduction in LDL-c levels or 1 SD reduction in plasma protein levels of PCSK9.
(XLSX)
Estimates are effects equivalent to 1 SD reduction in LDL-c levels or 1 SD reduction in plasma protein levels of PCSK9.
(XLSX)
(TIFF)
SD, standard deviation.
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(DOCX)
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CLPP
colocalization posterior probability
- GLGC
Global Lipids Genetics Consortium
- GTEx
Genotype-Tissue Expression
- GWAS
genome-wide association study
- IVW
inverse-variance weighted
- LD
linkage disequilibrium
- LDL
low-density lipoprotein
- LDL-c
LDL cholesterol
- MR
Mendelian randomization
- OR
odds ratio
- PRACTICAL
Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome
- SD
standard deviation
- SNP
single-nucleotide polymorphism
Data Availability
Several summary statistics of genome wide association studies (GWAS) used in this study are publicly available on the MRC Integrative Epidemiology Unit (IEU) Open GWAS project (https://gwas.mrcieu.ac.uk/), including LDL cholesterol GWAS from the Global Lipids Genetics (GLGC) Consortium, overall prostate cancer risk GWAS from the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium, Locke et al. GWAS on body mass index, GWAS on lipoprotein A, male-stratified total and bioavailable testosterone from the Neale lab, as well as the newly performed male-stratified GWAS on LDL cholesterol on UK Biobank participants. Pulit et al. GWAS summary statistics on body mass index is available from https://zenodo.org/record/1251813#.YlhVzZPMJhE. Liver-derived gene expression eQTL data is available from the GTEx project via https://www.gtexportal.org/home/. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 12/12/2021. PCSK9 plasma pQTL data is available from https://www.decode.com/summarydata/. Summary statistics of GWAS on advanced and early onset prostate cancer risks are under restricted access. They are available from the PRACTICAL consortium upon application (contact: PRACTICAL@icr.ac.uk).
Funding Statement
This research was conducted in the Medical Research Council (MRC) Integrative Epidemiology Unit at the University of Bristol (MC_UU_00011/1 to GDS, MC_UU_00011/4 to TRG) (https://www.ukri.org/councils/mrc/). SF is supported by a Wellcome Trust PhD studentship in Molecular, Genetic and Lifecourse Epidemiology (108902/Z/15/Z) (https://wellcome.org/). JY is supported by a Cancer Research UK Population Research Postdoctoral Fellowship (C68933/A28534) (https://www.cancerresearchuk.org/). CJB is supported by the World Cancer Research Fund (WCRF UK) (https://www.wcrf.org/), as part of the World Cancer Research Fund International grant program (IIG_2019_2009). TRG and GDS conduct research at the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. Epub 2021/02/05. doi: 10.3322/caac.21660 . [DOI] [PubMed] [Google Scholar]
- 2.Teemu JM, Heimo S, Pasi P, Merja B, Tiina S, Timo Y, et al. The importance of LDL and cholesterol metabolism for prostate epithelial cell growth. PLoS ONE. 2012. doi: 10.1371/journal.pone.0039445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung YY, Ko JH, Um JY, Chinnathambi A, Alharbi SA, Sethi G, et al. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J Cell Physiol. 2021;236(7):5253–64. Epub 2020/12/29. doi: 10.1002/jcp.30229 . [DOI] [PubMed] [Google Scholar]
- 4.Liu Yp, Zhang Y, Li P, Cheng C, Zhao Y, Li D, et al. Cholesterol Levels in Blood and the Risk of Prostate Cancer: A Meta-analysis of 14 Prospective Studies. Cancer Epidemiol Biomarkers Prev. 2015. doi: 10.1158/1055-9965.epi-14-1329 [DOI] [PubMed] [Google Scholar]
- 5.Jamnagerwalla J, Howard LE, Allott EH, Vidal AC, Moreira DM, Castro-Santamaria R, et al. Serum cholesterol and risk of high-grade prostate cancer: results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018. doi: 10.1038/s41391-017-0030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull CJ, Bonilla C, Holly JM, Perks CM, Davies N, Haycock P, et al. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med. 2016;5(6):1125–36. Epub 2016/03/20. doi: 10.1002/cam4.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orho-Melander M, Hindy G, Borgquist S, Schulz CA, Manjer J, Melander O, et al. Blood lipid genetic scores, the HMGCR gene and cancer risk: a Mendelian randomization study. Int J Epidemiol. 2018;47(2):495–505. Epub 2017/11/23. doi: 10.1093/ije/dyx237 . [DOI] [PubMed] [Google Scholar]
- 8.Ioannidou A, Watts EL, Perez-Cornago A, Platz EA, Mills IG, Key TJ, et al. The relationship between lipoprotein A and other lipids with prostate cancer risk: A multivariable Mendelian randomisation study. PLoS Med. 2022;19(1):e1003859. Epub 2022/01/28. doi: 10.1371/journal.pmed.1003859 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eric JJ, Carmen R, Elizabeth BB, Yiting W, Michael JT, Eugenia EC. Cholesterol-Lowering Drugs and Advanced Prostate Cancer Incidence in a Large U.S. Cohort. Cancer Epidemiol Biomarkers Prev. 2007. doi: 10.1158/1055-9965.epi-07-0448 [DOI] [PubMed] [Google Scholar]
- 10.Farwell WR, LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103(11):885–92. Epub 2011/04/19. doi: 10.1093/jnci/djr108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ping T, Shiyou W, Zhuang T, Liang G, Chen Z, Pan N, et al. LDL-lowering therapy and the risk of prostate cancer: a meta-analysis of 6 randomized controlled trials and 36 observational studies. Sci Rep. 2016. doi: 10.1038/srep24521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, Ding H, Jia Y, Shi M, Guo D, Yang P, et al. Associations of genetically proxied inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with breast cancer and prostate cancer. Breast Cancer Res. 2022;24(1):12. Epub 2022/02/14. doi: 10.1186/s13058-022-01508-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King EA, Davis JW, Degner JF. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019;15(12):e1008489. Epub 2019/12/13. doi: 10.1371/journal.pgen.1008489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey Smith G, Ebrahim S. ’Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. Epub 2003/04/12. doi: 10.1093/ije/dyg070 . [DOI] [PubMed] [Google Scholar]
- 15.Richmond RC, Davey Smith G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb Perspect Med. 2022;12(1). Epub 2021/08/25. doi: 10.1101/cshperspect.a040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2(1):6. doi: 10.1038/s43586-021-00092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill D, Georgakis MK, Walker VM, Schmidt AF, Gkatzionis A, Freitag DF, et al. Mendelian randomization for studying the effects of perturbing drug targets. Wellcome Open Res. 2021;6:16. Epub 2021/03/03. doi: 10.12688/wellcomeopenres.16544.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng J, Haberland V, Baird D, Walker V, Haycock PC, Hurle MR, et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet. 2020;52(10):1122–31. Epub 2020/09/09. doi: 10.1038/s41588-020-0682-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes MV, Richardson TG, Ference BA, Davies NM, Davey Smith G. Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development. Nat Rev Cardiol. 2021;18(6):435–453. doi: 10.1038/s41569-020-00493-1 [DOI] [PubMed] [Google Scholar]
- 20.Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N Engl J Med. 2016;375(22):2144–53. Epub 2016/12/14. doi: 10.1056/NEJMoa1604304 . [DOI] [PubMed] [Google Scholar]
- 21.Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–61. Epub 2015/03/17. doi: 10.1016/j.jacc.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daghlas I, Karhunen V, Ray D, Zuber V, Burgess S, Tsao PS, et al. Genetic Evidence for Repurposing of GLP1R (Glucagon-Like Peptide-1 Receptor) Agonists to Prevent Heart Failure. J Am Heart Assoc. 2021;10(13):e020331. Epub 2021/06/30. doi: 10.1161/JAHA.120.020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Davey Smith G, et al. Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer. JAMA. 2020;323(7):646–55. Epub 2020/02/19. doi: 10.1001/jama.2020.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–36. Epub 2018/06/13. doi: 10.1038/s41588-018-0142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munafo MR, Davey Smith G. Robust research needs many lines of evidence. Nature. 2018;553(7689):399–401. Epub 2018/01/26. doi: 10.1038/d41586-018-01023-3 . [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Chen X, Gerke TA, Bird VY, Ghayee HK, Prosperi M. BMI trajectories and risk of overall and grade-specific prostate cancer: An observational cohort study among men seen for prostatic conditions. Cancer Med. 2018;7(10):5272–80. Epub 2018/09/13. doi: 10.1002/cam4.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavalette C, Cordina Duverger E, Artaud F, Rebillard X, Lamy PJ, Tretarre B, et al. Body mass index trajectories and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2020;9(17):6421–9. Epub 2020/07/09. doi: 10.1002/cam4.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal AC, Oyekunle T, Howard LE, De Hoedt AM, Kane CJ, Terris MK, et al. Obesity, race, and long-term prostate cancer outcomes. Cancer. 2020;126(16):3733–41. Epub 2020/06/05. doi: 10.1002/cncr.32906 . [DOI] [PubMed] [Google Scholar]
- 29.Watts EL, Appleby PN, Perez-Cornago A, Bueno-de-Mesquita HB, Chan JM, Chen C, et al. Low Free Testosterone and Prostate Cancer Risk: A Collaborative Analysis of 20 Prospective Studies. Eur Urol. 2018;74(5):585–94. Epub 2018/08/06. doi: 10.1016/j.eururo.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammadi-Shemirani P, Chong M, Pigeyre M, Morton RW, Gerstein HC, Pare G. Effects of lifelong testosterone exposure on health and disease using Mendelian randomization. Elife. 2020;9. Epub 2020/10/17. doi: 10.7554/eLife.58914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. Epub 2021/10/28. doi: 10.1136/bmj.n2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, et al. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600(7890):675–9. Epub 2021/12/11. doi: 10.1038/s41586-021-04064-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. Epub 2015/10/04. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9. Epub 2018/10/12. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loh PR, Tucker G, Bulik-Sullivan BK, Vilhjalmsson BJ, Finucane HK, Salem RM, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 2015;47(3):284–90. Epub 2015/02/03. doi: 10.1038/ng.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell R, Hemani G, Dudding T, Paternoster L. UK Biobank Genetic Data: MRC-IEU Quality Control, Version 2. data.bris; 2018.
- 37.Mitchell R, Elsworth B, Mitchell R, Raistrick C, Paternoster L, Hemani G, et al. MRC IEU UK Biobank GWAS pipeline version 2. databris; 2019.
- 38.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–74. Epub 2016/09/13. doi: 10.1093/ije/dyw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65. Epub 2013/10/12. doi: 10.1002/gepi.21758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess S, Zuber V, Valdes-Marquez E, Sun BB, Hopewell JC. Mendelian randomization with fine-mapped genetic data: Choosing from large numbers of correlated instrumental variables. Genet Epidemiol. 2017;41(8):714–25. Epub 2017/09/26. doi: 10.1002/gepi.22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–14. Epub 2016/04/12. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98. Epub 2017/10/19. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25. Epub 2015/06/08. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–7. Epub 2015/07/04. doi: 10.1093/bioinformatics/btv402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26(16):2069–70. Epub 2010/06/22. doi: 10.1093/bioinformatics/btq330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D13. Epub 2018/11/27. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383. Epub 2014/05/17. doi: 10.1371/journal.pgen.1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hormozdiari F, van de Bunt M, Segre AV, Li X, Joo JWJ, Bilow M, et al. Colocalization of GWAS and eQTL Signals Detects Target Genes. Am J Hum Genet. 2016;99(6):1245–60. Epub 2016/11/22. doi: 10.1016/j.ajhg.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics C, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–5. Epub 2015/02/03. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werme J, van der Sluis S, Posthuma D, de Leeuw CA. An integrated framework for local genetic correlation analysis. Nat Genet. 2022;54(3):274–82. Epub 2022/03/16. doi: 10.1038/s41588-022-01017-y . [DOI] [PubMed] [Google Scholar]
- 51.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28(1):166–74. Epub 2018/09/22. doi: 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. Epub 2015/02/13. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65(3):557–586. doi: 10.2307/2171753 [DOI] [Google Scholar]
- 54.The GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369(6509):1318–30. Epub 2020/09/12. doi: 10.1126/science.aaz1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richardson TG, Zheng J, Davey Smith G, Timpson NJ, Gaunt TR, Relton CL, et al. Mendelian Randomization Analysis Identifies CpG Sites as Putative Mediators for Genetic Influences on Cardiovascular Disease Risk. Am J Hum Genet. 2017;101(4):590–602. Epub 2017/10/07. doi: 10.1016/j.ajhg.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuber V, Grinberg NF, Gill D, Manipur I, Slob EAW, Patel A, et al. Combining evidence from Mendelian randomization and colocalization: Review and comparison of approaches. Am J Hum Genet. 2022;109(5):767–82. Epub 2022/04/23. doi: 10.1016/j.ajhg.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhattacharya A, Chowdhury A, Chaudhury K, Shukla PC. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188581. Epub 2021/06/19. doi: 10.1016/j.bbcan.2021.188581 . [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14(12):1122–31. Epub 2013/01/12. doi: 10.1593/neo.121252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang SZ, Zhu XD, Feng LH, Li XL, Liu XF, Sun HC, et al. PCSK9 promotes tumor growth by inhibiting tumor cell apoptosis in hepatocellular carcinoma. Exp Hematol Oncol. 2021;10(1):25. Epub 2021/04/02. doi: 10.1186/s40164-021-00218-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Cui Y, Cao L, Zhang Y, Yin Y, Hu X. PCSK9 regulates apoptosis in human lung adenocarcinoma A549 cells via endoplasmic reticulum stress and mitochondrial signaling pathways. Exp Ther Med. 2017;13(5):1993–9. Epub 2017/06/02. doi: 10.3892/etm.2017.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suh JM, Son Y, Yoo JY, Goh Y, Seidah NG, Lee S, et al. Proprotein convertase subtilisin/kexin Type 9 is required for Ahnak-mediated metastasis of melanoma into lung epithelial cells. Neoplasia. 2021;23(9):993–1001. Epub 2021/08/06. doi: 10.1016/j.neo.2021.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang K, Zhu J, Luo HH, Yu SW, Wang L. Pro-protein convertase subtilisin/kexin type 9 promotes intestinal tumor development by activating Janus kinase 2/signal transducer and activator of transcription 3/SOCS3 signaling in Apc(Min/+) mice. Int J Immunopathol Pharmacol. 2021;35:20587384211038345. Epub 2021/09/30. doi: 10.1177/20587384211038345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowak C, Arnlov J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018;9(1):3957. Epub 2018/09/29. doi: 10.1038/s41467-018-06467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588(7839):693–8. Epub 2020/11/13. doi: 10.1038/s41586-020-2911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdelwahed KS, Siddique AB, Mohyeldin MM, Qusa MH, Goda AA, Singh SS, et al. Pseurotin A as a novel suppressor of hormone dependent breast cancer progression and recurrence by inhibiting PCSK9 secretion and interaction with LDL receptor. Pharmacol Res. 2020;158:104847. Epub 2020/05/22. doi: 10.1016/j.phrs.2020.104847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdelwahed KS, Siddique AB, Qusa MH, King JA, Souid S, Abd Elmageed ZY, et al. PCSK9 Axis-Targeting Pseurotin A as a Novel Prostate Cancer Recurrence Suppressor Lead. ACS Pharmacol Transl Sci. 2021. doi: 10.1021/acsptsci.1c00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gan SS, Ye JQ, Wang L, Qu FJ, Chu CM, Tian YJ, et al. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. Onco Targets Ther. 2017;10:2139–46. Epub 2017/04/27. doi: 10.2147/OTT.S129413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. Epub 2015/03/17. doi: 10.1056/NEJMoa1501031 . [DOI] [PubMed] [Google Scholar]
- 69.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713–22. Epub 2017/03/18. doi: 10.1056/NEJMoa1615664 . [DOI] [PubMed] [Google Scholar]
- 70.Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–34. Epub 2021/05/21. doi: 10.1038/s41586-021-03534-y . [DOI] [PubMed] [Google Scholar]
- 71.Daghlas I, Gill D. Low-density lipoprotein cholesterol and lifespan: A Mendelian randomization study. Br J Clin Pharmacol. 2021;87(10):3916–24. Epub 2021/03/12. doi: 10.1111/bcp.14811 . [DOI] [PubMed] [Google Scholar]
- 72.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83. Epub 2013/10/08. doi: 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vithayathil M, Carter P, Kar S, Mason AM, Burgess S, Larsson SC. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: A mendelian randomisation study. PLoS Med. 2021;18(7):e1003706. Epub 2021/07/30. doi: 10.1371/journal.pmed.1003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang FM, Zhang Y. High Lipoprotein(a) Level Is Independently Associated with Adverse Clinicopathological Features in Patients with Prostate Cancer. Dis Markers. 2019;2019:9483935. Epub 2019/12/31. doi: 10.1155/2019/9483935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–92. Epub 2018/12/28. doi: 10.1161/CIRCULATIONAHA.118.037184 . [DOI] [PubMed] [Google Scholar]
- 76.Sabatine MS. PCSK9 inhibitors: clinical evidence and implementation. Nat Rev Cardiol. 2019;16(3):155–65. Epub 2018/11/14. doi: 10.1038/s41569-018-0107-8 . [DOI] [PubMed] [Google Scholar]
- 77.Richardson TG, Sanderson E, Elsworth B, Tilling K, Davey Smith G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: mendelian randomisation study. BMJ. 2020;369:m1203. Epub 2020/05/08. doi: 10.1136/bmj.m1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferkingstad E, Sulem P, Atlason BA, Sveinbjornsson G, Magnusson MI, Styrmisdottir EL, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet. 2021;53(12):1712–21. Epub 2021/12/04. doi: 10.1038/s41588-021-00978-w . [DOI] [PubMed] [Google Scholar]
- 79.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305(8):822–3. Epub 2011/02/24. doi: 10.1001/jama.2011.163 [DOI] [PMC free article] [PubMed] [Google Scholar]