Abstract
Matrix metalloproteinases (MMPs) may contribute to an impaired endothelial layer in several diseases. We examined the effect of heat-inactivated Streptococcus pneumoniae R6 on MMP-2 and MMP-9 release by cultured aortic and brain capillary endothelial cells. Treatment with heat-inactivated S. pneumoniae caused an increased release of MMP-2 by both cell types.
Under physiological conditions, matrix metalloproteinases (MMPs) contribute to the controlled degradation and remodeling of the extracellular matrix (17, 23). Under pathophysiological conditions, however, they can affect normal tissue function, e.g., by impairment of basal membranes. Overexpression of MMPs can be part of an inflammatory reaction and might contribute to the development of toxic shock-like syndrome, severe brain edema, and capillary leak syndrome (15). An increased expression of MMPs is demonstrated in diseases such as multiple sclerosis (18), intracerebral hemorrhage (20), and bacterial meningitis (1, 10, 11).
Cultured endothelial cells from different sources have a basal secretion of MMP-2 and MMP-9 which is regulated by various agents like tumor necrosis factor alpha and phorbol myristate acetate (PMA) (6, 9). So far, only little is known about the effect of bacteria on the expression and release of endothelial MMPs (2). Therefore, we investigated the effect of Streptococcus pneumoniae on MMP-2 and MMP-9 release by cultured endothelial cells (EC).
Endothelial cells were isolated and cultured form porcine aorta (AEC) and from porcine brain capillaries (BMVEC) and characterized as described previously (14), except that the cells were cultured in 24-well tissue culture dishes. The cells were grown to confluence and washed three times with 0.5 ml of medium 199 (BioWhittaker, Verviers, Belgium). Subsequently, 0.5 ml of medium 199 containing test substances, 0.1% fetal calf serum, 50 U of penicillin per ml, 0.05 mg of streptomycin per ml, 0.05 mg of gentamicin per ml, and 2.5 μg of amphotericin B per ml was added. Only primary cell cultures were used, because EC lose some of their typical antigens during passaging. S. pneumoniae R6 was grown in tryptic soy broth to a density of 108 CFU/ml; the bacterial density was determined by quantitative plating on blood agar. Bacteria were heat inactivated for 60 min at 70°C, concentrated by centrifugation at 10,000 × g, and washed twice in medium 199, before being adjusted to the appropriate density in culture medium. PMA (Sigma, St. Louis, Mo.) was prepared as a concentrated stock of 10 μmol/ml in dimethyl sulfoxide and stored at −20°C.
After 24 and 48 h of incubation, 50 μl of conditioned medium was removed from each well, centrifuged at 1,500 × g for 15 min to remove cell debris, and stored at −20°C until analyzed by zymography (7). Briefly, samples mixed with sample buffer (4.6 g of sodium dodecyl sulfate in 12.5 ml of 1 M Tris-HCl [pH 6.8], 20 ml of glycerol, 2 ml of 0.2% bromphenol blue, distilled water up to 100 ml) were applied to a 10% polyacrylamide gel containing 0.156% gelatin. The gels were run at 125 V for 2 h. Then they were rinsed in wash buffer (2.5% Triton X-100) for 30 min and in developing buffer (50 mM Tris, 0.2 M NaCl, 5 mM CaCl2, 0.02% [wt/vol] Brij 35 [Serva, Heidelberg, Germany], adjusted to pH 7.6 with HCl) for another 30 min. Subsequently, the gels were incubated with fresh developing buffer for 22 h at 37°C. After being stained in Coomassie brilliant blue for 15 min, the gels were destained twice for 30 min in a solution of 10% methanol and 15% acetic acid. MMP-2 and MMP-9 were identified as their pro-forms by their molecular masses and colocalization with human recombinant standards.
Scanning was done with a Saphir-Ultra scanner (Linotype-Hell AG, Kiel, Germany), and the intensities of the bands corresponding to the MMPs were quantitated with Quantity-One version 4.1.0 software (Bio-Rad, Richmond, Calif.).
The cells were washed twice with phosphate-buffered saline, lysed, and dissolved in phosphate-buffered saline, and the protein content was measured (22). No differences in protein content among controls and treated groups were observed except in groups treated with the highest concentration of heat-inactivated bacteria. These groups exhibited slightly higher protein concentrations than untreated controls did. Lactate dehydrogenase (LDH) was not detectable in heat-inactivated bacteria, and this did not differ between controls and these groups. Therefore, equal cell density of all analyzed groups can be assumed.
The data shown are representative of at least two further similar experiments. Statistical comparisons were made using the paired two-tailed t test adjusting for repeated testing by the Bonferroni method. P values below 0.05 were considered significant.
Confluent monolayers of AEC displayed a basal secretion of MMP-2 and MMP-9, whereas confluent monolayers of BMVEC showed a basal secretion only of MMP-2. In AEC, MMP-9 secretion was significantly up-regulated by up to 2-fold after 24 h and up to 2.3-fold after 48 h of challenge with 10 nM PMA, whereas the substance did not stimulate MMP-9 release in BMVEC (Fig. 1 shows the effect of PMA at 24 h). These results underline the differences in phenotype and gene expression between endothelial cells of brain capillaries and aorta.
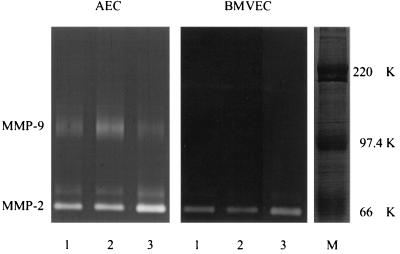
FIG. 1.
Zymograms of cell culture medium derived from AEC and BMVEC after 24 h of challenge with either 10 nM PMA (lane 2) or 1010 CFU of heat-inactivated S. pneumoniae R6 per ml (lane 3) or without treatment (lane 1). There is an increase in the level of MMP-2 as a result of the S. pneumoniae R6 challenge in supernatants of AEC and BMVEC and of MMP-9 by PMA in AEC culture medium. M, molecular mass markers (Rainbow coloured protein weight marker [Amersham, Little Chalfont, United Kingdom]) in kilodaltons.
The effect of heat-inactivated S. pneumoniae R6 on MMP-2 release by AEC and BMVEC is summarized in Table 1. At high concentrations, heat-inactivated S. pneumoniae R6 significantly stimulated MMP-2 release in both types of ECs. Since far higher concentrations of stimulants are often necessary in vitro than in vivo, the observed phenomenon is likely to be of physiological significance. In contrast, S. pneumoniae R6 effected MMP-9 expression in neither AEC nor in BMVEC (Fig. 1).
TABLE 1.
MMP-2 release by BMVEC and AEC after treatment with different concentrations of heat-inactivated S. pneumoniaea
| Treatment | % MMP-2 release (mean ± SD) byb:
|
|||
|---|---|---|---|---|
| AEC
|
BMVEC
|
|||
| 24 h | 48 h | 24 h | 48 h | |
| None (control) | 100 ± 15 | 100 ± 21 | 100 ± 29 | 100 ± 31 |
| 1010 CFU of R6 | 140 ± 24 (∗) | 160 ± 20 (∗∗) | 160 ± 41 (∗) | 140 ± 27 (∗) |
| 109 CFU of R6 | 97 ± 39 | 130 ± 37 | 98 ± 35 | 120 ± 24 |
| 108 CFU of R6 | 112 ± 15 | 120 ± 15 | 77 ± 16 | 110 ± 25 |
The supernatants of 10 independent samples of conditioned medium harvested from AEC or BMVEC were analyzed for MMP-2 expression by zymography.
Data listed within one column have been normalized to the results for the corresponding control group (100%) of the respective experiment. Significant differences between treatment groups and corresponding control groups are indicated by asterisks: (∗), P = 0.01; (∗∗), P ≤ 0.001 (only results within one column can be compared). The results are representative of at least three similar experiments.
During bacterial meningitis, increased levels of MMP-9 can be detected in cerebrospinal fluid (CSF) (1, 10, 11), but its cellular origin is unknown. Possible sources might be monocytes, macrophages (16), granulocytes (8), astrocytes, microglia (4), and EC (3). Our data, however, exclude BMVEC as possible sources of increased MMP-9 levels in CSF, and recent experiments identified migrating immune cells from the blood as the major source of MMP-9 in CSF (24).
In contrast to pro-MMP-9, the release of pro-MMP-2 in AEC and BMVEC is stimulated by heat-inactivated S. pneumoniae R6. Pro-MMPs, including pro-MMP-2, can be activated by several mechanisms such as membrane-linked proteases of intact bacteria (2, 13, 21). They can also be activated by EC in three-dimensional type I collagen gels involving membrane type 1 MMP (MT-1-MMP), resembling the in vivo situation (5, 23). The observed increase in MMP-2 release by endothelial cells upon stimulation by heat-inactivated pneumococci points to a role of MMP-2 in the pathopysiology of bacterial infections. In contrast, the MMP-2 level was not increased in sera of meningitic rabbits during antibiotic treatment (1). In these animals, however, viable bacteria were not present in the circulation. In accordance to the present in vitro data, an increased MMP-2 activity has been observed in lung tissue and lavage fluid of rats after endotoxin challenge and perfusion with N-formyl-methionyl-leucyl-phenylalanine (12). It is conceivable that in vivo the elevated release of pro-MMP-2 by heat-inactivated S. pneumoniae in endothelial cells results in an increased activity of MMP-2, facilitating the disruption of basal membranes of the blood-brain barrier (19) or capillaries of other organs.
Acknowledgments
We thank E. Wieland, Department of Clinical Chemistry, University of Göttingen, for kindly measuring LDH activities in cell culture extracts.
The study was supported in part by the Deutsche Forschungsgemeinschaft (grant Na 165/4-1) and by the Verein zur Förderung von Forschung, Wissenschaft und Lehre der Neurologischen Abteilung der Universitätsklinik, Göttingen, Germany.
REFERENCES
- 1.Azeh I, Mäder M, Smirnov A, Beuche W, Nau R, Weber F. Experimental pneumococcal meningitis in rabbits: the increase of matrix metalloproteinase-9 in cerebrospinal fluid correlates with leucocyte invasion. Neurosci Lett. 1998;256:127–130. doi: 10.1016/s0304-3940(98)00776-9. [DOI] [PubMed] [Google Scholar]
- 2.Burns E H, Jr, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A J, O'Connell J, Crabbe T, Angal S, Murphy G. The matrix metalloproteinases and their natural inhibitors: prospects for treating degenerative tissue diseases. Trends Biotechnol. 1992;10:200–207. doi: 10.1016/0167-7799(92)90214-g. [DOI] [PubMed] [Google Scholar]
- 4.Gottschall P E, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res. 1995;42:335–342. doi: 10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- 5.Haas T L, Davis S J, Madri J A. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- 6.Harkness K A, Adamson P, Sussman J D, Davies-Jones G A, Greenwood J, Woodroofe M N. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123:698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 7.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 8.Hibbs M S, Hasty K A, Kang A H, Mainardi C L. Secretion of collagenolytic enzymes by human polymorphonuclear leukocytes. Coll Relat Res. 1984;4:467–477. doi: 10.1016/s0174-173x(84)80013-8. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C J, Nguyen M. Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. Int J Biochem Cell Biol. 1997;29:1167–1177. doi: 10.1016/s1357-2725(97)00061-7. [DOI] [PubMed] [Google Scholar]
- 10.Kieseier B C, Paul R, Koedel U, Seifert T, Clements J M, Gearing A J, Pfister H W, Hartung H P. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain. 1999;122:1579–1587. doi: 10.1093/brain/122.8.1579. [DOI] [PubMed] [Google Scholar]
- 11.Leib S L, Leppert D, Clements J, Tauber M G. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun. 2000;68:615–620. doi: 10.1128/iai.68.2.615-620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lois M, Brown L A, Moss I M, Roman J, Guidot D M. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med. 1999;160:1354–1360. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Shams N B, Hanninen L A, Kenyon K R. Proteolytic activation of corneal matrix metalloproteinase by Pseudomonas aeruginosa elastase. Curr Eye Res. 1992;11:1105–1109. doi: 10.3109/02713689209015082. [DOI] [PubMed] [Google Scholar]
- 14.Michel U, Schneider O, Kirchhof C, Meisel S, Smirnov A, Wiltfang J, Rieckmann P. Production of follistatin in porcine endothelial cells: differential regulation by bacterial compounds and the synthetic glucocorticoid RU 28362. Endocrinology. 1996;137:4925–4934. doi: 10.1210/endo.137.11.8895365. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Ebihara I, Shimada N, Shoji H, Koide H. Modulation of plasma metalloproteinase-9 concentrations and peripheral blood monocyte mRNA levels in patients with septic shock: effect of fiber-immobilized polymyxin B treatment. Am J Med Sci. 1998;316:355–360. doi: 10.1097/00000441-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen B S, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer. 1996;65:57–62. doi: 10.1002/(SICI)1097-0215(19960103)65:1<57::AID-IJC10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Partridge C A, Jeffrey J J, Malik A B. A 96-kDa gelatinase induced by TNF-alpha contributes to increased microvascular endothelial permeability. Am J Physiol. 1993;265:L438–L447. doi: 10.1152/ajplung.1993.265.5.L438. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg G A, Dencoff J E, Correa N, Jr, Reiners M, Ford C C. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996;46:1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg G A, Kornfeld M, Estrada E, Kelley R O, Liotta L A, Stetler-Stevenson W G. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 1992;576:203–207. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg G A, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 1997;48:921–926. doi: 10.1212/wnl.48.4.921. [DOI] [PubMed] [Google Scholar]
- 21.Santala A, Saarinen J, Kovanen P, Kuusela P. Activation of interstitial collagenase, MMP-1, by Staphylococcus aureus cells having surface-bound plasmin: a novel role of plasminogen receptors of bacteria. FEBS Lett. 1999;461:153–156. doi: 10.1016/s0014-5793(99)01440-4. . (Erratum, 469:213, 2000.) [DOI] [PubMed] [Google Scholar]
- 22.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. . (Erratum, 163:279, 1987.) [DOI] [PubMed] [Google Scholar]
- 23.Stetler-Stevenson W G. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Investig. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yushchenko M, Weber F, Mäder M, Schöll U, Maliszewska M, Tumani H, Felgenhauer K, Beuche W. Matrix metalloproteinase-9 (MMP-9) in human cerebrospinal fluid (CSF): elevated levels are primarily related to CSF cell count. J Neuroimmunol. 2000;110:244–251. doi: 10.1016/s0165-5728(00)00339-8. [DOI] [PubMed] [Google Scholar]