Abstract
Purpose
We evaluated the evolution of thyroid function and autoimmunity among COVID-19 survivors over 6 months in relation to interferon beta-1b treatment and long COVID.
Methods
We included COVID-19 survivors managed in a major COVID-19 centre between July 2020 and May 2021 who were reassessed three and/or six months after acute COVID-19. Thyroid function tests (TFTs) and anti-thyroid antibody titres were measured at acute COVID-19, 3-month and 6-month.
Results
250 COVID-19 survivors were included (mean age 52.7 years, 50.4% men). Persistent thyroid function abnormalities were more likely in those with abnormal TFTs in acute COVID-19 (P < 0.001). Among 51 patients with abnormal TFTs in acute COVID-19, 82.4% resolved upon follow-up. Of 199 patients with normal TFTs in acute COVID-19, only 4.5% had incident abnormal TFTs, more likely in interferon-treated patients (P = 0.044) and none clinically overt. Among 129 patients with complete 6-month follow-up for anti-thyroid antibody titres, there was no significant change overall, except for modest increase in anti-thyroid antibody titres among the 84 interferon-treated patients (P < 0.05 at both 3 months and 6 months). Long COVID occurred in 19.5% and 10.4% at 3 and 6 months respectively, where TFTs and anti-thyroid antibody titres were not predictive of its occurrence.
Conclusion
Over 6 months, most abnormal TFTs in acute COVID-19 resolved, with no significant incident thyroid dysfunction. SARS-CoV-2 infection did not lead to change in thyroid autoimmunity, while interferon treatment was associated with modest increase in anti-thyroid antibody titres. Thyroid function and anti-thyroid antibodies did not play a significant role in long COVID.
Keywords: post-acute COVID-19 syndrome, COVID-19, thyroid function tests, autoimmunity, interferons
Introduction
The spectrum of thyroid dysfunction in acute COVID-19 has been well reported, including mainly thyroiditis and non-thyroidal illness syndrome (NTIS) [1]. The causal link between COVID-19 and thyroid dysfunction has been strengthened by observations of SARS-CoV-2 in the thyroid tissues of patients who died from COVID-19 [2], and studies demonstrating ACE2 mRNA expression in thyroid cells [3, 4]. Data on the longer-term effect of COVID-19 on the thyroid are limited. Clarke et al. evaluated the thyroid function among 68 adult COVID-19 survivors without known thyroid disease at a median of 7 months after acute COVID (largely with at least moderate severity), and found normal thyroid function in all [5]. Another longitudinal cohort study of 240 pregnant women followed up for glycaemic status postpartum showed that those with SARS‐CoV‐2 seropositivity had a comparable risk of thyroid dysfunction or new‐onset thyroid autoimmunity within 1 year to those without [6]. Nonetheless, follow-up data from a non-selective group of survivors from predominantly non-severe COVID-19 is lacking. These data are important since most COVID-19 survivors experience mild symptoms, and evidence-based recommendations for endocrine surveillance are currently lacking in this population.
Interferons are one of the immunotherapies in COVID-19, showing benefits in reducing clinical severity and hastening viral clearance in randomised controlled trials (RCT) [7], particularly when initiated during the early stage of viraemia [8]. Our group previously showed that interferon beta-1b therapy in acute COVID-19 was associated with a modest increase in anti-thyroid antibody titres upon a 3-month follow-up [9]. Further follow-up is warranted to delineate whether this change is transient and to identify any subsequent thyroid dysfunction as the occurrence of anti-TPO can precede thyroid dysfunction [10].
Even after recovery from acute COVID-19, many COVID-19 survivors continue to experience various symptoms [11], described as ‘long COVID’, ‘post-acute sequelae of SARS-CoV-2’ or ‘post-acute COVID-19 syndrome’ [12]. As the population of COVID-19 survivors is growing, long COVID may evolve into a ‘pandemic of the pandemic’ [13]. Since fatigue is a typical presentation [11], it is relevant to examine whether thyroid function plays a role in long COVID given that thyroid dysfunctions are readily treatable.
Hence, we carried out a prospective follow-up study of COVID-19 survivors with the primary objective of delineating the evolution of thyroid function and autoimmunity. Secondary objectives were to further evaluate the effect of interferon beta-1b treatment on the thyroid, and to assess the role of thyroid function and autoimmunity in long COVID.
Materials and methods
Study cohort
This study included all COVID-19 survivors of our study cohort, recruited with the protocol described below, who have been reassessed at least once at three or six months after acute COVID-19.
Consecutive adults (aged ≥18 years) admitted to Queen Mary Hospital, a major COVID-19 centre, for COVID-19 between 21 July 2020 and 20 May 2021 were prospectively recruited [14]. During the recruitment period, public health ordinance in Hong Kong required all patients tested positive for COVID-19 to be hospitalised [15], including those detected on contact tracing and the Universal Community Testing Programme [16], regardless of symptoms. Our cohort has been shown to be representative of COVID-19 patients in the entire territory [17]. Presence of SARS-CoV-2 was confirmed in all patients by RT-PCR from the nasopharyngeal swab (NPS) and/or deep throat saliva (DTS), using the LightMix SarbecoV E-gene assay (TIB Molbiol, Berlin, Germany) [18]. Patients were excluded if they (i) had a history of thyroid/pituitary/hypothalamic disorders; (ii) were on anti-thyroid drugs/thyroid hormone replacement; or (iii) were on medications with potential impact on thyroid function, including systemic steroids. Each patient had blood tests within 24 h after admission, before the initiation of COVID-19 treatments.
Serum TSH, fT4 and fT3 were measured with immunoassays ADVIA Centaur® TSH3-Ultra, FT4 and FT3 assays, respectively (Siemens Healthcare Diagnostics Inc., Erlangen, Germany). The reference ranges for TSH, fT4 and fT3 were 0.35–4.8 mIU/L, 12–23 pmol/L and 3.2–6.5 pmol/L, respectively. Anti-thyroglobulin (anti-Tg) and anti-thyroid peroxidase (anti-TPO) antibody titres were measured with QUANTA Lite® Thyroid T and TPO enzyme-linked immunosorbent assay, respectively (Inova Diagnostics, San Diego, CA, USA). Positive anti-Tg and anti-TPO were defined by >100 units [19].
Demographics and major comorbidities were recorded. COVID-19-related symptoms were evaluated with a standard checklist. Respiratory rate, baseline oxygen saturation by pulse oximetry and oxygen requirement on admission were captured. Chest x-ray was performed on admission. Cycle threshold (Ct) values were obtained from specimens from NPS/DTS (whichever was lower) on admission. Studies have shown a good correlation between Ct values and SARS-CoV-2 viral loads [20, 21], such that the lower the Ct values, the higher the viral loads. COVID-19 severity was classified according to the ‘Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition)’ published by the Chinese National Health Commission [22]. For patients treated for COVID-19, one or more of the following were given: clofazimine [23], ribavirin, interferon beta-1b, or remdesivir [24]. Dexamethasone [25] and subcutaneous low-molecular-weight heparin [26] were added at physicians’ discretion as clinically indicated. Interferon beta-1b was given once daily subcutaneously at a dose of 16 million IU [9]. The decision to use interferon beta-1b was not influenced by the baseline thyroid function and antibody levels.
COVID-19 survivors were offered two follow-up visits at a dedicated COVID-19 clinic at three and six months from admission. During the follow-up visits, they were systematically evaluated for symptoms with a standard checklist, and had reassessment thyroid function tests (TFTs) and anti-thyroid antibodies. Long COVID was defined as the presence/persistence of symptoms after acute COVID-19 [27].
Statistical analyses
All statistical analyses were performed with IBM® SPSS® version 26. Two-sided P-values < 0.05 were considered statistically significant. Data were presented as mean±standard deviation, median with interquartile range (IQR) or number with percentage as appropriate. Between-group comparisons were performed with the t-test or Mann-Whitney U test for continuous variables and Chi-square or Fisher’s exact tests for categorical variables as appropriate. General linear models with repeated measures were used to evaluate the changes in anti-thyroid antibody titres over 6 months, with Bonferroni’s adjustment for multiple comparisons. Multivariable logistic regression analysis was performed to identify the independent determinant of long COVID. Variables reaching statistical significance P < 0.05 in univariate analysis were included in the multivariable model. Subgroup analyses were performed according to baseline severity of acute COVID-19. Chi-square test was performed to compare the rate of persistent TFT abnormalities and incident TFT abnormalities across the subgroups of baseline COVID-19 severity. The trends of anti-TPO and anti-Tg titres were compared across the subgroups of baseline COVID-19 severity in the analyses using general linear models with repeated measures.
Results
Baseline characteristics
Two hundred and fifty COVID-19 survivors were included. Most of them were infected by the ancestral strain of SARS-CoV-2, except one patient (a 48-year-old man) infected with the delta variant. None of them received COVID-19 vaccination during the period of follow-up. Their baseline characteristics are shown in Table 1. Their mean age was 52.7 years, with no sex preponderance. 95.2% had non-severe COVID-19 at baseline. The most common COVID-19 treatment was interferon beta-1b therapy (56.8%; most treated for 5 days). The baseline characteristics of COVID-19 survivors by interferon exposure were all comparable (Table 1).
Table 1.
Baseline characteristics of the cohort with stratification according to interferon exposure
| All | Interferon-treated | Interferon-naïve | P value | |
|---|---|---|---|---|
| Number of patients | 250 | 142 | 108 | --- |
| Age (year) | 52.7 ± 15.3 | 53.7 ± 14.9 | 51.4 ± 15.8 | 0.584 |
| Male sex | 126 (50.4%) | 70 (49.3%) | 56 (51.9%) | 0.689 |
| Smoking | 32/213 (15.0%) | 18/119 (15.1%) | 14/94 (14.9%) | 0.962 |
| Drinking | 44/206 (21.4%) | 22/113 (19.5%) | 22/93 (23.7%) | 0.466 |
| Abnormal TFT on admission | 51 (20.4%) | 32 (22.5%) | 19 (17.6%) | 0.337 |
| Baseline anti-TPO positivity | 51/246 (20.4%) | 26/142 (18.3%) | 25/104 (24.0%) | 0.274 |
| Baseline anti-Tg positivity | 25/246 (10.0%) | 16/142 (11.3%) | 9/104 (8.7%) | 0.503 |
| Baseline COVID-19 severity | 0.596 | |||
| Mild | 168 (67.2%) | 99 (69.7%) | 69 (63.9%) | |
| Moderate | 70 (28.0%) | 35 (24.6%) | 35 (32.4%) | |
| Severe | 12 (4.8%) | 8 (5.6%) | 4 (3.7%) | |
| Symptomatic on presentation | 201 (80.4%) | 119 (83.8%) | 82 (75.9%) | 0.120 |
| Comorbidities | ||||
| Hypertension | 60 (24.0%) | 34 (23.9%) | 26 (24.1%) | 0.981 |
| Diabetes | 36 (14.4%) | 22 (15.5%) | 14 (13.0%) | 0.572 |
| IHD/CHF | 11 (4.4%) | 6 (4.2%) | 5 (4.6%) | 0.999 |
| Stroke/TIA | 5 (2.0%) | 3 (2.1%) | 2 (1.9%) | 0.999 |
| Malignancy | 16 (6.4%) | 8 (5.6%) | 8 (7.4%) | 0.570 |
| Pulmonary disease | 13 (5.2%) | 6 (4.2%) | 7 (6.5%) | 0.426 |
| Clinical course | ||||
| Length of hospitalisation (days) | 8 (6–12) | 9 (7–13) | 6 (2–10) | <0.001 |
| Interferon beta-1b therapy | 142 (56.8%) | 142 (100%) | 0 (0%) | --- |
| Dexamethasone | 44 (17.6%) | 28 (19.7%) | 16 (14.8%) | 0.313 |
| Oxygen requirement | 31 (12.4%) | 20 (14.1%) | 11 (10.2%) | 0.354 |
| Intensive care unit admission | 8 (3.2%) | 6 (4.2%) | 2 (1.9%) | 0.472 |
TFT thyroid function test, IHD ischaemic heart disease, CHF congestive heart failure, TIA transient ischaemic attack
Among the 250 patients, 236 (94.4%) came back for 3-month TFT reassessment at a median of 90 days (IQR: 64–102), and 163 (65.2%) came back for 6-month TFT reassessment at a median of 186 days (IQR: 174–201). Patients who did and did not have reassessment at 3 months were similar in terms of sex, baseline abnormal TFT and baseline anti-TPO/Tg positivity, except for younger age among those who received reassessment (52.1 ± 15.1 vs 63.7 ± 14.1 years, P = 0.005). Patients who did and did not receive reassessment at 6 months were similar in terms of sex, abnormal baseline TFT, baseline anti-TPO/Tg positivity, except for older age among those reassessed (54.5 ± 14.5 vs 49.3 ± 16.2 years, P = 0.010).
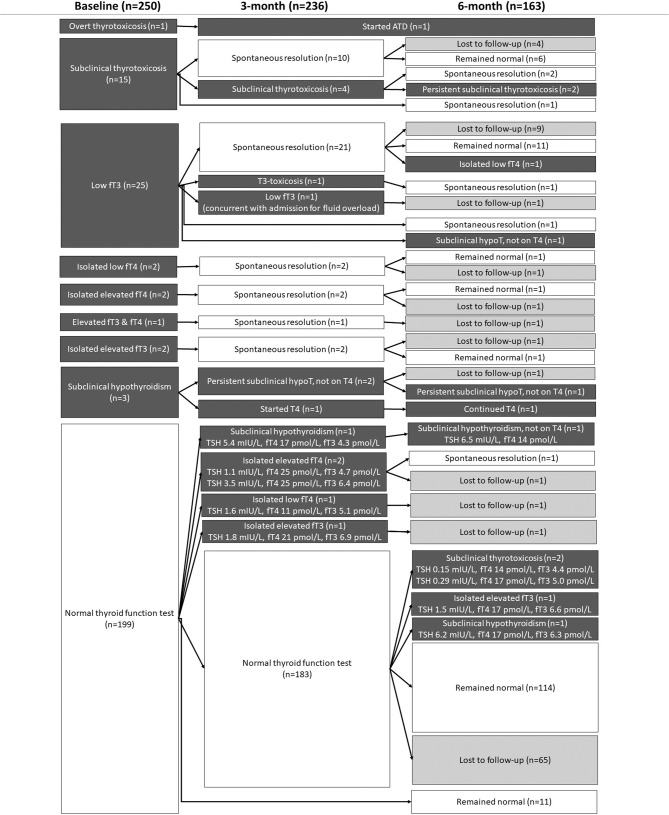
Figure 1 summarises the changes in TFT status in all 250 patients included in this study.
Fig. 1.
Evolution of thyroid function among 250 COVID-19 survivors over 6 months. ATD anti-thyroid drug, hypoT hypothyroidism
Outcomes of patients with abnormal TFT in acute COVID-19
Fifty-one patients had abnormal TFT in acute COVID-19: most (n = 42; 82.4%) resolved spontaneously upon follow-up, and two, with Graves’ disease and Hashimoto’s thyroiditis respectively, were rendered euthyroid after initiation of specific treatment. Their evolution is described below.
Overt thyrotoxicosis
One patient (34-year-old man) was diagnosed with Graves’ disease, presenting with tachycardia and diffuse goitre. TFT showed fT4 51 pmol/L and fT3 15pmol/L with suppressed TSH. His anti-TSHR titre was 3.6 IU/L (normal: <1 IU/L). Technetium thyroid scan showed diffuse uptake. His thyrotoxicosis was brought under control with carbimazole.
Subclinical thyrotoxicosis
Fifteen patients had abnormal TFT suggestive of subclinical thyrotoxicosis (low TSH with normal fT4 and fT3) during acute COVID-19. Most (12 of 15) were negative for both anti-TPO and anti-Tg. Their fT3/fT4 ratio of <0.3 in the context of thyrotoxicosis was more suggestive of thyroiditis than Graves’ disease. Subsequent reassessment over the next 3–6 months showed resolution in 13 (86.7%) of them. The remaining two still had mild subclinical thyrotoxicosis at 6-month follow-up: one (56-year-old man) had the latest TFT showing TSH 0.30 mIU/L, fT4 17pmol/L, fT3 5.4 pmol/L, with still positive anti-TPO and anti-TSHR mildly elevated at 1.9 IU/L, raising the possibility of mild subclinical hyperthyroidism due to Graves’ disease; the other patient (61-year-old woman) had the latest TFT showing TSH 0.29 mIU/L, fT4 14 pmol/L, fT3 4.9 pmol/L, with negative anti-TPO/Tg and anti-TSHR, raising the possibility of toxic thyroid nodule(s). Unfortunately, both patients did not show up for subsequent investigations.
Low fT3 (NTIS)
Among the 25 patients with low fT3 on admission, the classical indicator of NTIS, 21 (84%) had spontaneous resolution at 3 months upon recovery from acute COVID-19, and another one had spontaneous resolution at 6 months. The remaining three patients experienced the following TFTs changes: one patient experienced a transient T3-toxicosis at 3 months which spontaneously normalized at the 6-month follow-up (suggesting painless thyroiditis); another patient had a low fT3 at the 3-month reassessment coinciding with a hospitalization for fluid overload (suggesting NTIS); the last patient (73-year-old man) developed subclinical hypothyroidism (TSH 7.1 mIU/L, fT4 15 pmol/L, fT3 4.0 pmol/L). This patient already had strongly positive anti-TPO (5302 units) and anti-Tg (692 units) at the moment of acute COVID-19, suggesting possible pre-existing thyroid autoimmunity.
Subclinical hypothyroidism
Three patients had subclinical hypothyroidism on presentation. All three of them had positive anti-TPO status. One of them (75-year-old woman) was put on thyroxine replacement since TSH was 11 mIU/L – her anti-TPO was strongly positive (18719 units), suggestive of pre-existing Hashimoto’s thyroiditis. The other two also had moderately positive anti-TPO suggestive of pre-existing Hashimoto’s thyroiditis. One of them was a 75-year-old woman who had TSH 7.9 mIU/L on admission. As she did not have convincing symptoms of hypothyroidism, she was not put on thyroxine replacement [28]. Subsequent 3-month follow-up showed transiently worsening in subclinical hypothyroidism (TSH 15 mIU/L, fT4 17 pmol/L), which improved spontaneously upon 6-month assessment (TSH 5.6 mIU/L, fT4 17 pmol/L). The other patient was a 30-year-old man who had TSH 5.0 mIU/L and fT4 16 pmol/L on admission. He was not put on thyroxine replacement in view of no convincing symptoms of hypothyroidism [28]. His 3-month reassessment showed similar degree of subclinical hypothyroidism with TSH 6.3 mIU/L and fT4 17 pmol/L. He defaulted subsequent 6-month follow-up.
Others
Seven patients had patterns of thyroid dysfunction which could represent different phases of acute thyroiditis. Isolated borderline low fT4 (11 pmol/L) was seen in two patients on admission and normalised upon reassessments. Another two patients had isolated borderline elevated fT4 (24 pmol/L) on admission which normalised upon reassessments. One patient had mildly elevated fT4 and fT3 (TSH 2.2 mIU/L, fT4 25 pmol/L, fT3 6.6 pmol/L) which had resolved by the 3-month assessment. Another two patients with borderline elevated fT3 (6.7 pmol/L) on admission (one of them had mildly elevated anti-TPO of 109 units, and the other was negative for anti-TPO) had normal TFTs upon reassessment.
Incident abnormal TFTs
One hundred and ninety-nine patients had normal TFT in acute COVID-19. At 3 months, only 5 patients (2.5%) had abnormal TFT. One patient (38-year-old woman) developed mild subclinical hypothyroidism at 3-month assessment, which persisted at 6-month assessment, with negative anti-TPO and anti-Tg. She was put on observation as she did not have any convincing symptom of hypothyroidism. The remaining four patients had fT4 or fT3 just outside the reference range, where assay variability could not be entirely excluded. Among the four patients, only one attended the 6-month follow-up, showing normalisation of TFT.
Among the 183 patients with normal TFT at 3-month follow-up, 118 (64.5%) were reassessed at 6 months. Among them, four had abnormal TFT: (i) two patients developed subclinical thyrotoxicosis, with negative anti-TPO/Tg and anti-TSHR except for one having mildly positive anti-TPO (125units), but both defaulted subsequent investigations and follow-up; (ii) one developed subclinical hypothyroidism, which normalised upon reassessment another 9 months later; and (iii) the remaining one had isolated mild elevation in fT3, but lost to subsequent follow-up.
In summary, among 199 patients with normal TFT at baseline, only 9 (4.5%) had abnormal TFTs at either 3-month or 6-month assessments, with none clinically overt. Among all the baseline characteristics, only interferon treatment in acute COVID-19 was associated with incident abnormal TFT upon serial follow-up (interferon-treated 8/110 [7.3%] vs interferon-naïve 1/89 [1.1%], P = 0.044). Dexamethasone treatment in acute COVID-19 was not associated with incident abnormal TFT upon serial follow up (p = 0.127). The only interferon-naïve patient with incident abnormal TFT had mildly elevated fT4 at 3 months (TSH 3.5 mIU/L, fT4 25 pmol/L, fT3 6.4 pmol/L), where assay variability could not be entirely excluded. Moreover, he was anti-TPO/Tg negative at baseline and 3 months. These observations raised the possibility that those 8 interferon-treated patients may represent individuals with interferon-associated thyroiditis, which may occur as a result of non-autoimmune or autoimmune mechanisms [29, 30].
Among all 250 survivors, abnormal TFTs were more likely to be observed upon the latest reassessment among those with abnormal TFTs in acute COVID-19: 9/51 (17.6%) among those with abnormal TFTs in acute COVID-19 had abnormal TFTs on reassessment, compared with 8/199 (4.0%) among those with normal TFTs in acute COVID-19 (P = 0.001). The pattern of evolution of TFTs was similar across different baseline COVID-19 severity: the rate of persistent TFT abnormalities among COVID-19 survivors with abnormal TFTs in acute COVID-19 was similar (mild: 5/28 [17.9%], moderate: 3/18 [16.7%], severe: 1/5 [20.0%], P = 0.974), so was the rate of incident TFT abnormalities (mild: 5/140 [3.6%], moderate: 3/52 [5.8%], severe: 0/7 [0%], P = 0.817). (Supplementary Fig. 1–3) The only patient infected by the delta variant of SARS-CoV-2 had normal thyroid function in acute COVID-19, which remained normal at 3-month reassessment.
Anti-thyroid antibody assessments
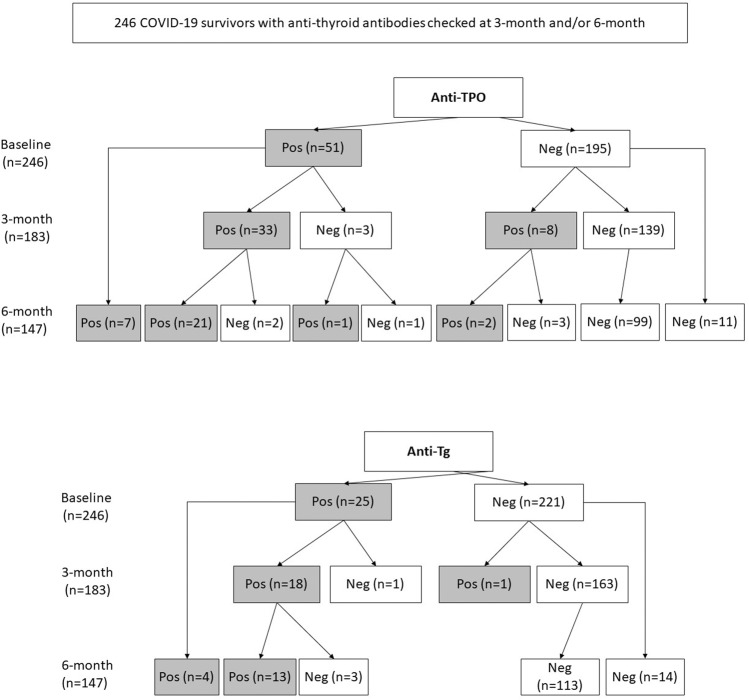
Of the 250 patients included, 246 patients (98.4%) had anti-thyroid antibody titres reassessed at 3-month or 6-month (183 patients at 3-month; 147 patients at 6-month). Figure 2 summarises the anti-TPO and anti-Tg positivity status over the 6 months. Incident anti-TPO positivity occurred at a rate of 1.7% (2 out of 115 assessed) at 6 months. Incident anti-Tg positivity was rare – only one observed at 3 months (0.6%, 1 out of 164 assessed), and none at 6 months. Table 2 shows the TFT and anti-thyroid antibody profiles of the patients who developed incident anti-TPO or anti-Tg positivity upon reassessment. Notably, most cases of incident anti-thyroid antibody positivity were interferon-treated patients. Subgroup analyses showed similar patterns of evolution of anti-thyroid antibody positivity across different baseline COVID-19 severity (Supplementary Fig. 4–6).
Fig. 2.
Evolution of anti-thyroid antibody positivity status among 246 COVID-19 survivors over 6 months
Table 2.
Thyroid function and antibody profile of patients with incident anti-TPO or anti-Tg positivity at reassessment
| Patient number | Sex/Age | On admission | COVID-19 treatment | 3-month assessment | 6-month assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSHa | fT4a | fT3a | Anti-TPOb | Anti-Tgb | TSHa | fT4a | fT3a | Anti-TPOb | Anti-Tgb | TSHa | fT4a | fT3a | Anti-TPOb | Anti-Tgb | |||
| 1 | M/58 | 0.55 | 16 | 2.9 | 64.9 | 2957 | IFN + RIB + DEX | 0.02 | 21 | 7.2c | 100.1 | 2794 | 1.9 | 14 | 5.4 | 112.2 | 2819 |
| 2 | F/61 | 0.61 | 19 | 4.1 | 44.4 | 5.1 | IFN + RIB | 0.45 | 18 | 4.7 | 129.7 | 5.62 | 0.60 | 18 | 5.1 | 131.9 | 5.7 |
| 3 | F/67 | 1.3 | 18 | N/A | 45.5 | 4.3 | IFN + RIB | 1.7 | 17 | 5.3 | 148.2 | 6.6 | 1.1 | 16 | 6.1 | 56.8 | 7.2 |
| 4 | F/61 | 1.2 | 17 | 3.5 | 6.9 | 4.9 | IFN + RIB | 2.8 | 20 | 5.0 | 141.2 | 8.3 | 1.9 | 16 | 4.3 | 27.2 | 20.0 |
| 5 | M/51 | 2.2 | 16 | 4.6 | 97.1 | 4.8 | IFN + RIB | 1.7 | 20 | 5.6 | 122.4 | 3.7 | 2.8 | 17 | 5.7 | 68.0 | 6.4 |
| 6 | M/55 | 1.6 | 15 | 3.3 | 89.3 | 21.1 | REM | 3.7 | 14 | 3.7 | 197.9 | 38.0 | N/A | ||||
| 7 | M/21 | 1.1 | 17 | 4.4 | 99.9 | 6.6 | IFN + RIB | 0.47 | 19 | 5.2 | 128.5 | 9.7 | N/A | ||||
| 8 | M/45 | 1.6 | 13 | 4.9 | 82.9 | 9.0 | IFN + REM + DEX | 1.6 | 11 | 5.1 | 108.1 | 6.2 | N/A | ||||
| 9 | F/62 | 0.98 | 14 | 3.3 | 198.9 | 43.5 | Nil | 1.2 | 15 | 4.2 | 264.3 | 839 | N/A | ||||
M male, F female, TSH thyroid-stimulating hormone (mIU/L), fT4 free thyroxine (pmol/L), fT3 free triiodothyronine (pmol/L), anti-TPO anti-thyroid peroxidase antibody (units), anti-Tg anti-thyroglobulin antibody (units), COVID-19 coronavirus disease 2019, IFN interferon beta-1b, RIB ribavirin, REM remdesivir, DEX dexamethasone, N/A not available
Age expressed in years
aReference ranges: TSH 0.35–4.8 mIU/L, fT4 12–23 pmol/L, fT3 3.2–6.5 pmol/L
bPositive anti-TPO defined by >100 units; positive anti-Tg defined by >100 units
cHis anti-TSHR titre was 0.8 IU/L, i.e. negative
Values out of reference ranges are in bold
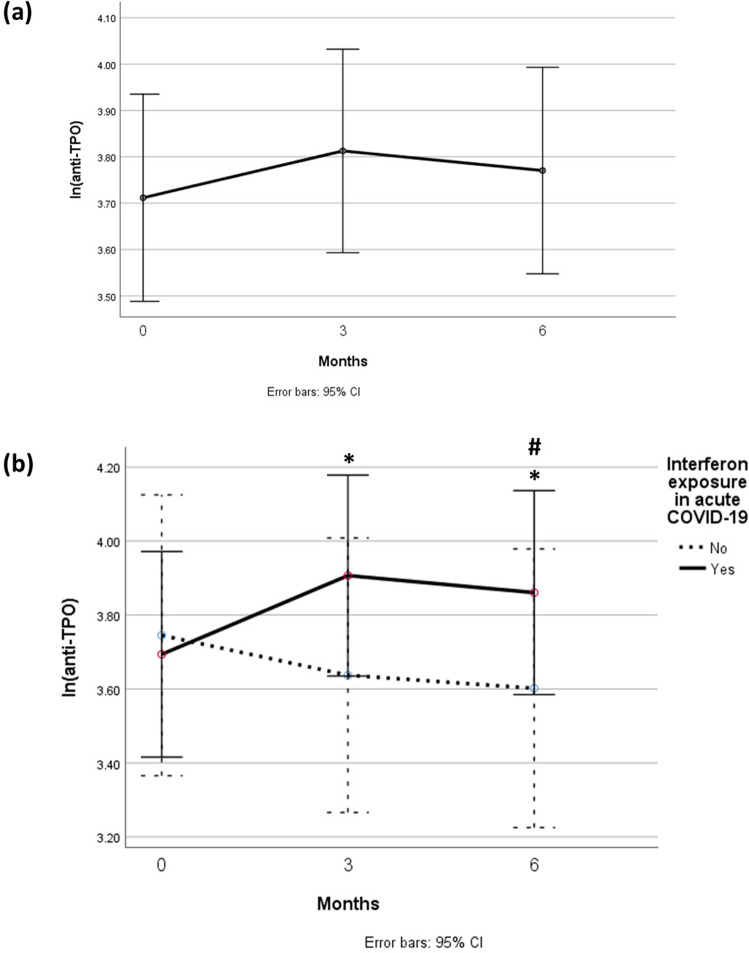
In total, 129 patients had serial anti-thyroid antibody assessments from baseline, 3 months to 6 months. Among the 129 patients, there was no statistically significant change in anti-TPO titres over the 6 months (baseline: 30.5 units [IQR: 18.0–63.3] vs 3-month: 29.7 [IQR: 19.1–80.4] vs 6-month: 29.8 [IQR: 19.1–71.4]; P = 0.070) (Fig. 3a). We further stratified the analysis of anti-TPO titres by interferon exposure in acute COVID-19 (Fig. 3b). Among the 129 patients, 84 (65.1%) were exposed to interferon beta-1b treatment during acute COVID-19, while 45 (34.9%) were interferon-naïve. Among the 84 interferon-treated patients, there was a statistically significant, though modest, increase in anti-TPO titres from baseline (30.5 units [IQR: 15.4–68.2]) to 3 months (39.2 units [IQR: 19.3–95.2], P = 0.001 vs baseline), which persisted at 6 months (33.4 units [IQR: 20.0–75.2], P = 0.006 vs baseline). Among the 45 interferon-naïve patients, there was no change in anti-TPO titres from baseline (30.5 units [IQR: 18.7–57.5]) to 3 months (25.4 units [IQR: 18.5–51.2], P = 0.109), and there was even a mild decrease in anti-TPO at 6 months that marginally reached statistical significance (25.9 units [IQR: 18.7–37.2], P = 0.049 vs baseline).
Fig. 3.
a Evolution of anti-TPO titres over 6 months (p = 0.070); b Evolution of anti-TPO titres over 6 months, stratified by interferon exposure in acute COVID-19. *p < 0.05 compared with baseline among interferon-treated patients; #p < 0.05 compared with baseline among interferon-naïve patients
Among the 129 patients, there was no statistically significant change in anti-Tg titres over the 6 months (Baseline: 9.0 units [IQR: 5.7–16.6] vs 3-month: 9.5 [IQR: 6.6–16.0] vs 6-month: 8.8 [IQR: 6.8–19.3]; P = 0.060). We further stratified the analysis of anti-Tg titres by interferon exposure in acute COVID-19. Among the 84 interferon-treated patients, there was a statistically significant, though modest, increase in anti-Tg titres from baseline (8.6 units [IQR: 5.5–18.5]) to 3 months (9.2 units [IQR: 6.6–17.6], P = 0.007 vs baseline), which persisted at 6 months (9.2 units [IQR: 6.7–20.7], P = 0.004 vs baseline). Among the 45 interferon-naïve patients, there was no change in anti-Tg titres from baseline (9.7 units [IQR: 7.5–13.3]) to 3 months (9.9 units [IQR: 6.7–14.4]) and 6 months (8.7 units [IQR: 6.9–11.8]) (P = 0.704).
The evolution of anti-thyroid antibody titres over 6 months did not differ according to baseline COVID-19 severity (P = 0.550 for anti-TPO; P = 0.620 for anti-Tg).
Long COVID: the role of thyroid function and autoimmunity
At 3-month follow-up, 46 (19.5%) of the 236 patients had symptoms defining long COVID: most commonly dyspnoea (n = 17), cough (n = 17), anosmia (n = 13) and fatigue (n = 7). Table 3 shows the comparison between patients with and without long COVID at 3 months. Patients with long COVID were younger, more likely to be female and had higher SARS-CoV-2 viral loads in acute COVID-19. Baseline COVID-19 severity was comparable. Thyroid function and anti-thyroid antibodies were comparable. Multivariable logistic regression analysis showed that the independent determinants of long COVID at 3 months were age (adjusted OR 0.97, 95%CI 0.95–0.99, P = 0.008), female (adjusted OR 2.48, 95%CI 1.23–5.00, P = 0.011) and baseline SARS-CoV-2 PCR Ct value (adjusted OR 0.25, 95%CI 0.08–0.79, P = 0.018).
Table 3.
Comparison between patients with and without long COVID at 3 months
| Long COVID | No Symptom | P value | |
|---|---|---|---|
| Number | 46 | 190 | --- |
| Baseline characteristics | |||
| Age (year) | 47.2 ± 16.0 | 53.2 ± 14.7 | 0.016 |
| Female sex | 29 (63.0%) | 87 (45.8%) | 0.036 |
| Charlson comorbidity index | 0 (0–1) | 0 (0–0) | 0.286 |
| Baseline SARS-CoV-2 PCR Ct value | 20.9 (17.2–25.6) | 25.5 (19.2–30.6) | 0.016 |
| Baseline COVID-19 severity | 0.453 | ||
| Mild | 33 (71.7%) | 128 (67.4%) | |
| Moderate | 12 (26.1%) | 53 (27.9%) | |
| Severe | 1 (2.2%) | 9 (4.7%) | |
| TSH (mIU/L) | 1.10 (0.81–1.60) | 1.15 (0.72–1.70) | 0.460 |
| fT4 (pmol/L) | 17.3 ± 2.8 | 17.5 ± 3.7 | 0.755 |
| fT3 (pmol/L) | 4.0 ± 0.7 | 4.1 ± 1.1 | 0.650 |
| Anti-TPO positivity | 5/44 (11.4%) | 41/188 (21.8%) | 0.118 |
| Anti-Tg positivity | 3/44 (6.8%) | 19/188 (10.1%) | 0.775 |
| 3-month assessment | |||
| TSH (mIU/L) | 1.60 (0.94–2.1) | 1.40 (0.99–1.90) | 0.344 |
| fT4 (pmol/L) | 17.4 ± 2.6 | 17.0 ± 2.9 | 0.460 |
| fT3 (pmol/L) | 4.7 ± 0.6 | 4.8 ± 0.6 | 0.234 |
| Anti-TPO positivity | 4/35 (11.4%) | 37/151 (24.5%) | 0.093 |
| Anti-Tg positivity | 2/35 (5.7%) | 17/151 (11.3%) | 0.536 |
Ct cycle threshold, TSH thyroid-stimulating hormone, fT4 free thyroxine, fT3 free triiodothyronine, TPO thyroid peroxidase, Tg thyroglobulin
Values reaching statistical significance are in bold
At 6-month follow-up, 17 (10.4%) of 163 patients had symptoms defining long COVID. The most common symptoms were dyspnoea (n = 6), cough (n = 5), anosmia (n = 5) and fatigue (n = 4). Comparison between patients with and without long COVID at 6 months (Table 4) did not reveal significant differences, including baseline COVID-19 severity, and thyroid function and anti-thyroid antibodies at 6 months.
Table 4.
Comparison between patients with and without long COVID at 6 months
| Long COVID | No Symptom | P value | |
|---|---|---|---|
| Number | 17 | 146 | --- |
| Baseline characteristics | |||
| Age (year) | 49.4 ± 16.7 | 55.1 ± 14.2 | 0.122 |
| Female sex | 10 (58.8%) | 71 (48.6%) | 0.426 |
| Charlson comorbidity index | 0 (0–1) | 0 (0–0) | 0.460 |
| Baseline SARS-CoV-2 PCR Ct value | 23.9 (17.6–30.0) | 22.7 (17.6–28.5) | 0.357 |
| Baseline COVID-19 severity | 0.878 | ||
| Mild | 12 (70.6%) | 95 (65.1%) | |
| Moderate | 4 (23.5%) | 43 (29.5%) | |
| Severe | 1 (5.9%) | 8 (5.5%) | |
| 6-month assessment | |||
| TSH (mIU/L) | 1.20 (0.83–1.90) | 1.40 (1.00–1.90) | 0.703 |
| fT4 (pmol/L) | 17.1 ± 1.6 | 16.5 ± 2.4 | 0.382 |
| fT3 (pmol/L) | 4.8 ± 0.6 | 4.8 ± 0.6 | 0.973 |
| Anti-TPO positivity | 2/13 (15.4%) | 29/136 (21.3%) | 0.999 |
| Anti-Tg positivity | 0/13 (0%) | 17/136 (12.5%) | 0.364 |
Ct cycle threshold, TSH thyroid-stimulating hormone, fT4 free thyroxine, fT3 free triiodothyronine, TPO thyroid peroxidase, Tg thyroglobulin
Discussion
Our study addressed several important issues regarding thyroid function and autoimmunity among COVID-19 survivors. We showed that over 6 months of follow-up, most abnormal TFTs in acute COVID-19 resolved, and no clinically significant incident thyroid dysfunction was observed among COVID-19 survivors. While SARS-CoV-2 infection per se did not lead to changes in thyroid autoimmunity, interferon beta-1b treatment in acute COVID-19 was associated with a modest increase in anti-thyroid antibody titres. Nonetheless, this was not associated with a significant incidence of anti-thyroid antibody positivity. Thyroid function and anti-thyroid antibodies did not explain the occurrence of long COVID. Our results have provided insights on the strategies of thyroid function monitoring among COVID-19 survivors.
Our 6-month prospective follow-up data confirmed that SARS-CoV-2 infection did not lead to clinically significant thyroid dysfunction. Our results also indicated that SARS-CoV-2 infection has a minor role in inducing thyroid autoimmunity. These findings were consistent across different baseline COVID-19 severity. Prior to our study, Khoo et al. showed in a cohort of 55 patients with COVID-19 that the lower TSH during COVID-19 recovered upon follow-up at a median of 2.5 months, with no changes in fT4 levels, suggesting no evidence of SARS-CoV-2-related thyroiditis. [31] Clarke et al. evaluated 68 COVID-19 survivors at a median of 7 months after acute COVID-19, showing TSH, fT4 and fT3 within the normal range in all [5]. These two cohorts mainly included hospitalised patients and COVID-19 patients with at least moderate severity. In contrast, Goyal et al. studied a cohort of 240 women with a history of hyperglycaemia or normoglycaemia during pregnancy who came for postpartum follow-up [6]. Using SARS-CoV-2 seropositivity as a surrogate of past SARS-CoV-2 infection, 109 (45.4%) were classified as COVID-19 survivors. Goyal et al. reported 9.9% progression of thyroid dysfunction into subclinical/overt hypothyroidism: the proportion of progressors was comparable between COVID-19 survivors and the non-infected group. Incident anti-TPO positivity occurred in 1.7% and was comparable between COVID-19 survivors and the non-infected group. Our results are consistent with the existing literature and are more generalisable as our cohort of COVID-19 survivors is representative of those in the community, having mostly non-severe COVID-19 in the acute phase. Among the 199 patients with initially normal TFTs in acute COVID-19, only 4.5% had abnormal TFTs upon reassessment. Importantly, none was clinically overt. The rate of abnormal TFTs appears to be comparable to that identified upon screening of TFTs in a general population [32]. Our further evaluation of the anti-thyroid antibody titres showed no significant changes after SARS-CoV-2 infection.
Little is known about the outcomes of COVID-19 survivors who had abnormal TFTs in acute COVID-19. Pizzocaro et al. reported outcomes of 29 COVID-19 survivors who had thyrotoxicosis in acute COVID-19 (58.6% overt; 41.4% subclinical): 28 became euthyroid at a median of 3 months, and one became overtly hypothyroid (who had subclinical thyrotoxicosis in acute COVID-19) [33]. In our cohort, among the 15 patients with subclinical thyrotoxicosis, 86.7% became euthyroid upon reassessment. However, we did not observe any progression into hypothyroidism. The difference may be explained by the fact that our cohort comprised mainly non-severe COVID-19 patients, in contrast to Pizzocaro’s cohort consisting of mainly severe COVID-19 patients. Also, consistent with the study by Khoo et al. [31], we showed that NTIS recovered upon resolution of COVID-19 in most patients. Hence, we may conclude that COVID-19, especially non-severe disease, is unlikely associated with long-term sequelae on the thyroid.
Interferon beta-1b is one of the immunomodulatory treatments of COVID-19, which helps to stimulate viral defence to combat the initial viraemia. [7] The clinical benefits of interferon beta-1b have been shown in two different RCTs [15, 34], similarly shown in another two trials using interferon-based therapies [35, 36]. While the large multicentre trial coordinated by the World Health Organization (WHO) failed to demonstrate clinical benefits with interferon treatment in hospitalized COVID-19 patients [37], it could be explained by the delayed treatment. Hence, it is clinically relevant to evaluate the safety of interferon treatment, including thyroid-specific outcomes, which inform the potential need for surveillance, as our previous 3-month follow-up study demonstrated a modest increase in anti-thyroid antibody titres among interferon-treated patients [9]. Interferon-beta has also been used in multiple sclerosis as chronic maintenance therapy and reported to be associated with incident thyroid dysfunction and autoimmunity, mainly during the first year of treatment [38]. Proposed mechanisms of the impact of interferon therapy on the thyroid include non-autoimmune and autoimmune mechanisms [29, 30]. In contrast to the usual regimen of 8 million IU every other day as a chronic therapy in multiple sclerosis, we evaluated the effect of a much shorter treatment duration (median of 5 days) and a more intense dosing regimen (16 million IU/day), revealing a modest increase in anti-thyroid antibody titres, which persisted at 6 months, among interferon-treated COVID-19 survivors. Nonetheless, with the modest increase in anti-thyroid antibody titres, incident anti-TPO or anti-Tg positivity was uncommon. Indeed, some patients who developed incident anti-TPO positivity at 3 months reverted to anti-TPO negative status at 6 months, suggesting that the phenomenon may be transient. Although we found that incident thyroid function abnormalities were more likely with interferon-treated patients, none of them were clinically overt.
Whether thyroid function plays a role in long COVID has only been addressed in two studies. Our previous 3-month follow-up showed no significant difference in TFTs between patients with and without long COVID [17]. Similarly, Clarke et al. showed no discernible difference in TFTs between patients with and without fatigue in 68 COVID-19 survivors evaluated at a median of 7 months after acute COVID-19 [5]. Our current 6-month follow-up has confirmed that both thyroid function and autoimmunity do not play a major role in long COVID.
We found that patients who had abnormal TFTs upon reassessment were more likely to have abnormal TFTs in acute COVID-19. Taken together, our results showed that while abnormal TFTs in acute COVID-19 should be reassessed to ensure resolution, routine thyroid function monitoring among COVID-19 survivors is probably unnecessary, in line with the current recommendations [39], except perhaps those who have been treated with interferon in acute COVID-19.
The strength of our study was the systematic reassessment of both thyroid function and anti-thyroid antibodies over 6 months. The fact that the COVID-19 severity was non-severe for most patients in our cohort means that our results are generalisable. However, there are certain limitations in this study. First, most patients were infected with the ancestral strains of SARS-CoV-2. Results may not apply to other variants. Second, these patients did not receive any COVID-19 vaccination during the entire follow-up period. This study did not address whether COVID-19 survivors who receive COVID-19 vaccination may have different trajectories of thyroid function and autoimmunity. Nonetheless, we recently showed that COVID-19 vaccination did not lead to a significant change in thyroid function or autoimmunity [40, 41]. Third, this was a single-centre study. Although there was no significant difference in the evolution of thyroid function or autoimmunity across subgroups of baseline COVID-19 severity, the size of the subgroup especially that of severe COVID-19 was relatively small. Also, our results on the role of thyroid function and autoimmunity in long COVID may require further validation from larger or multicentric studies. Last but not least, as a non-COVID-19 control group was not available, some potential confounding factors, such as the possible influence of seasonality on thyroid function and autoimmunity, could not be evaluated [42, 43].
In conclusion, most abnormal TFTs in acute COVID-19 resolved upon 6-month follow-up, with no significant incident thyroid dysfunction seen among COVID-19 survivors. SARS-CoV-2 infection did not lead to a change in thyroid autoimmunity, while interferon beta-1b treatment was associated with a modest increase in anti-thyroid antibody titres. Thyroid function and anti-thyroid antibodies did not play a significant role in long COVID. Our results suggest routine thyroid function measurements are unnecessary among COVID-19 survivors except for those with initial thyroid function abnormalities in acute COVID-19 and those treated with interferon beta-1b.
Supplementary Information
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1007/s12020-022-03281-8.
References
- 1.Clarke SA, Abbara A, Dhillo WS. Impact of COVID-19 on the Endocrine System: A Mini-review. Endocrinology. 2022;163:bqab203. doi: 10.1210/endocr/bqab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poma AM, Basolo A, Bonuccelli D, et al. Activation of Type I and Type II Interferon Signaling in SARS-CoV-2-Positive Thyroid Tissue of Patients Dying from COVID-19. Thyroid. 2021;31:1766–1775. doi: 10.1089/thy.2021.0345. [DOI] [PubMed] [Google Scholar]
- 3.Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Invest. 2021;44:1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coperchini F, Ricci G, Croce L, et al. Modulation of ACE-2 mRNA by inflammatory cytokines in human thyroid cells: a pilot study. Endocrine. 2021;74:638–645. doi: 10.1007/s12020-021-02807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke SA, Phylactou M, Patel B, et al. Normal Adrenal and Thyroid Function in Patients Who Survive COVID-19 Infection. J. Clin. Endocrinol. Metab. 2021;106:2208–2220. doi: 10.1210/clinem/dgab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal A, Gupta Y, Kalaivani M, Tandon N. Mild and asymptomatic SARS-CoV-2 infection is not associated with progression of thyroid dysfunction or thyroid autoimmunity. Clin. Endocrinol. (Oxf.) 2022;5:7–9. doi: 10.1111/cen.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022;28:39–50. doi: 10.1038/s41591-021-01643-9. [DOI] [PubMed] [Google Scholar]
- 8.Ngo BT, Marik P, Kory P, et al. The time to offer treatments for COVID-19. Expert Opin. Investig. Drugs. 2021;30:505–518. doi: 10.1080/13543784.2021.1901883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lui DTW, Hung IFN, Lee CH, et al. The Impact of Interferon Beta-1b Therapy on Thyroid Function and Autoimmunity Among COVID-19 Survivors. Front Endocrinol. (Lausanne) 2021;12:746602. doi: 10.3389/fendo.2021.746602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siriwardhane T, Krishna K, Ranganathan V, et al. Significance of Anti-TPO as an Early Predictive Marker in Thyroid Disease. Autoimmune Dis. 2019;2019:1684074. doi: 10.1155/2019/1684074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.H.M. Rando, T.D. Bennett, J.B. Byrd, et al. (2021) Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv. 10.1101/2021.03.20.21253896
- 13.Murray T. Unpacking “long COVID.”. CMAJ. 2021;193:E318–E319. doi: 10.1503/cmaj.1095923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lui DTW, Lee CH, Chow WS, et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2020;106:e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.I.F.N. Hung, K.C. Lung, E.Y.K. Tso et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (Lond., Engl.) 395, 1695–1704 (2020). 10.1016/S0140-6736(20)31042-4 [DOI] [PMC free article] [PubMed]
- 16.Online appointments for Universal Community Testing Programme to begin tomorrow. https://www.info.gov.hk/gia/general/202008/28/P2020082800837.htm. Accessed 3 Mar 2021
- 17.Lui DTW, Lee CH, Chow WS, et al. Long COVID in Patients with Mild to Moderate Disease: Do Thyroid Function and Autoimmunity Play a Role. Endocr. Pr. 2021;27:894–902. doi: 10.1016/j.eprac.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.W.M. Chan, J.D. Ip, A.W.H. Chu et al. Identification of nsp1 gene as the target of SARS-CoV-2 real-time RT-PCR using nanopore whole-genome sequencing. J. Med Virol. 92, 2725–2734 (2020). 10.1002/jmv.26140 [DOI] [PMC free article] [PubMed]
- 19.Lui D.T.W., Lee C.H., Chow W.S., et al. (2021) Role of non-thyroidal illness syndrome in predicting adverse outcomes in COVID-19 patients predominantly of mild-to-moderate severity. Clin Endocrinol (Oxf). 10.1111/cen.14476 [DOI] [PMC free article] [PubMed]
- 20.Yu F, Yan L, Wang N, et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. 2020;71:793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tom MR, Mina MJ. To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value. Clin. Infect. Dis. 2020;71(16):2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed 23 Aug 2020
- 23.Yuan S, Yin X, Meng X, et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021;593:418–423. doi: 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- 24.Hung IFN. Treatment of coronavirus disease 2019. Curr. Opin. HIV AIDS. 2020;15:336–340. doi: 10.1097/COH.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 25.Crisan Dabija R, Antohe I, Trofor A, Antoniu SA. Corticosteroids in SARS-COV2 infection: certainties and uncertainties in clinical practice. Expert Rev. Anti Infect. Ther. 2021;19:1553–1562. doi: 10.1080/14787210.2021.1933437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barcellona D, Fanni D, Gerosa C, et al. Heparins and 2019-nCoV infection: a narrative review. Eur. Rev. Med Pharm. Sci. 2021;25:3594–3606. doi: 10.26355/eurrev_202105_25842. [DOI] [PubMed] [Google Scholar]
- 27.Amenta EM, Spallone A, Rodriguez-Barradas MC, et al. Postacute covid-19: An overview and approach to classification. Open Forum Infect. Dis. 2020;7:ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur. Thyroid J. 2013;2:215–228. doi: 10.1159/000356507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monzani F, Caraccio N, Casolaro A, et al. Long-term interferon β-1b therapy for MS: Is routine thyroid assessment always useful? Neurology. 2000;55:549–552. doi: 10.1212/WNL.55.4.549. [DOI] [PubMed] [Google Scholar]
- 30.Frisullo G, Calabrese M, Tortorella C, et al. Thyroid autoimmunity and dysfunction in multiple sclerosis patients during long-term treatment with interferon beta or glatiramer acetate: An Italian multicenter study. Mult. Scler. J. 2014;20:1265–1268. doi: 10.1177/1352458514521311. [DOI] [PubMed] [Google Scholar]
- 31.Khoo B, Tan T, Clarke SA, et al. Thyroid Function Before, During, and After COVID-19. J. Clin. Endocrinol. Metab. 2021;106:e803–e811. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasagi K, Takahashi N, Inoue G, et al. Thyroid function in Japanese adults as assessed by a general health checkup system in relation with thyroid-related antibodies and other clinical parameters. Thyroid. 2009;19:937–944. doi: 10.1089/thy.2009.0205. [DOI] [PubMed] [Google Scholar]
- 33.Pizzocaro A, Colombo P, Vena W, et al. Outcome of Sars-COV-2-related thyrotoxicosis in survivors of Covid-19: a prospective study. Endocrine. 2021;73:255–260. doi: 10.1007/s12020-021-02758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A.R. Tam, R.R. Zhang, K.C. Lung, et al. (2022) Early treatment of high-risk hospitalized COVID-19 patients with a combination of interferon beta-1b and remdesivir: a phase 2 open-label randomized controlled trial. Clin Infect Dis. 10.1093/cid/ciac523 [DOI] [PMC free article] [PubMed]
- 35.Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 2021;9:498–510. doi: 10.1016/S2213-2600(20)30566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021;9:196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caraccio N, Dardano A, Manfredonia F, et al. Long-term follow-up of 106 multiple sclerosis patients undergoing interferon-β 1a or 1b therapy: Predictive factors of thyroid disease development and duration. J. Clin. Endocrinol. Metab. 2005;90:4133–4137. doi: 10.1210/jc.2004-2326. [DOI] [PubMed] [Google Scholar]
- 39.Pal R, Joshi A, Bhadada SK, et al. Endocrine Follow-up During Post-Acute COVID-19: Practical Recommendations Based on Available Clinical Evidence. Endocr. Pr. 2022;28:425–432. doi: 10.1016/j.eprac.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lui DTW, Lee CH, Cheung CYY, et al. Effect of COVID-19 Vaccines on Thyroid Function and Autoimmunity and Effect of Thyroid Autoimmunity on Antibody Response. J. Clin. Endocrinol. Metab. 2022;107:e3781–e3789. doi: 10.1210/clinem/dgac355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong CKH, Lui DTW, Xiong X, et al. Risk of thyroid dysfunction associated with mRNA and inactivated COVID-19 vaccines: a population-based study of 2.3 million vaccine recipients. BMC Med. 2022;20:339. doi: 10.1186/s12916-022-02548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thvilum M, Brandt F, Brix TH, Hegedüs L. Month of birth is associated with the subsequent diagnosis of autoimmune hypothyroidism. A nationwide Danish register-based study. Clin. Endocrinol. (Oxf.) 2017;87(6):832–837. doi: 10.1111/cen.13425. [DOI] [PubMed] [Google Scholar]
- 43.De Grande LA, Goossens K, Van Uytfanghe K, et al. Using “big data” to describe the effect of seasonal variation in thyroid-stimulating hormone. Clin. Chem. Lab Med. 2017;55:e34––e36. doi: 10.1515/cclm-2016-0500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.