Abstract
Background:
Higher nigral iron has been reported in Parkinson’s disease (PD).
Objective:
To understand the dynamics of nigral iron accumulation in PD and its association with drug treatment.
Methods:
Susceptibility MRI data was obtained from 79 control, 18 drug-naïve (PDDN), and 87 drug-treated (PDDT) subjects. Regional brain iron in basal ganglia and cerebellar structures was estimated using quantitative susceptibility mapping. Nigral iron was compared among PDDN and PDDT subgroups defined by disease duration [early (PDE, <2 years), middle (PDM, 2-6 years), later (PDL, >6 years)]. Associations with both disease duration and types of antiparkinson drugs were explored using regression analysis.
Results:
Compared to controls, PDDN had lower iron in the substantia nigra (p=0.018), caudate nucleus (p=0.038), and globus pallidus (p=0.01), but not in the putamen or red nucleus. In contrast, PDDT had higher iron in the nigra (p’s < 0.001), but not other regions, compared to either controls or PDDN. Iron in the nigra increased with disease duration [ PDE > PDDN (p=0.001), PDM > PDE (p=0.045)] except for PDM versus PDL (p=0.226). Levodopa usage was associated with higher (p=0.013) nigral iron, whereas lower nigral iron was correlated with selegiline usage (p= 0.030).
Conclusion:
Nigral iron is lower prior to the start of dopaminergic medication, and then increases throughout the disease until it plateaus at late stages, suggesting increased iron may not be an etiological factor. Interestingly, PD medications may have differential associations with iron accumulation that are worthy of further investigation.
Keywords: Substantia nigra, brain iron, susceptibility MRI, drug naïve PD
Introduction
Parkinson’s disease (PD) is marked pathologically by loss of dopamine neurons in the substantia nigra (SN) pars compacta (SNc) of the basal ganglia.1-3 Since Dexter et al.4 first reported increased iron in the SN of 11 post-mortem PD patients in 1987, a number of post-mortem studies have shown higher nigral iron concentrations in PD patients.5-9 In addition, findings from preclinical studies have suggested that excess intracellular iron is associated with oxidative stress, cellular dysfunction, and neuronal death in PD,10,11 possibly playing a role in PD pathogenesis.12,13 As a result, iron chelation therapies have been proposed to modify disease course.14,15 It is unclear, however, if nigral iron accumulates before PD diagnosis, and how it evolves during disease progression. Moreover, it is unknown whether iron is a consequence of the disease itself, and/or is influenced by medical interventions (e.g., antiparkinson drugs).4,16
Recent advances in MRI technology have enabled in vivo assessment of brain iron content in humans.17-20 Susceptibility MRI has been shown to correlate well with post-mortem histological measurements of iron.21,22 Many prior studies,3,17,23 including from our group,19,20,24 have utilized apparent transverse relaxation rate (R2*) and newer quantitative susceptibility mapping (QSM) to understand the role of iron in PD. Consistent with earlier pathological reports, many of these in vivo MRI studies have demonstrated higher nigral iron content in PD patients.3,17,18,20
Interestingly, higher nigral iron has not been found in early-stage PD patients, in either post-mortem reports,4,6,7 or our recent MRI studies.19,24 Furthermore, we found no significant change in nigral iron in these early-stage participants over an 18-month epoch.19 It is important to note that except for one post-mortem study prior to the introduction of levodopa,25 all post-mortem 4,6-9,26 and MRI studies3,17-19,24 investigating nigral iron were from PD patients who were taking antiparkinson medication. In addition, our previous study also demonstrated a non-linear and stage-dependent pattern of progression in the nigral iron content.19 It is, however, unclear if iron accumulation is solely a result of PD progression or is associated with commonly used medications.
The current study employed state-of-the-art brain susceptibility MRI analyses to investigate brain iron deposition in both newly diagnosed drug-naïve (PDDN) and drug-treated (PDDT) PD patients. We tested two hypotheses: 1) newly diagnosed drug-naïve PD patients do not have significantly higher nigral iron content compared to controls or drug-treated PD patients with longer disease duration; and 2) increased iron deposition that occurs after introduction of antiparkinson medication may be associated with the drugs being taken.
Methods
Participants
PD patients [n=105, including 18 drug-naïve patients (PDDN) and 87 drug-treated patients (PDDT)on one or more antiparkinson drugs] and controls (n=78) were recruited as part of an observational PD biomarker study sponsored by the National Institute of Neurological Disorders and Stroke. PD patients were recruited from a tertiary movement disorders clinic, whereas controls were from the spousal population and the local community. PD diagnosis was confirmed based on the Movement Disorder Society criteria.27 All participants were free of major medical issues or neurological conditions other than PD. The Movement Disorder Society Unified PD Rating Scale parts I, II, and III (MDS-UPDRS-I, -II, -III) and the six-item freezing of gait questionnaire (FoG)28 were used to measure PD-related symptom severity. We also included the Montreal Cognitive Assessment (MoCA) for global cognitive function and the Hamilton Depression Rating Scale (HDRS) for depression symptoms. Clinical measures for PDDT patients were obtained in the “ON” state while patients were on clinically optimized antiparkinsonian medications. Detailed demographic and clinical information are provided in Table 1. All participants gave written informed consent that was in accordance with the Declaration of Helsinki, and the protocol was approved by the Penn State Hershey Institutional Review Board.
Table 1. Demographic and clinical measures.
| Control | PDDN | PDDT | p-values* | PDE | PDM | PDL | p§ | |
|---|---|---|---|---|---|---|---|---|
| # | 79 | 18 | 87 | 27 | 26 | 34 | ||
| Sex (F/M) | 41/38 | 12/6 | 41/46 | 0.335 | 14/13 | 16/10 | 11/23 | 0.069 |
| Age (y) | 66.4 ± 10.3 | 64.8 ± 11.4 | 67.1 ± 9.1 | 0.666 | 65.0 ± 9.4 | 67.7 ± 9.8 | 68.2 ± 8.2 | 0.251 |
| Disease Duration (y) | - | 0.2 ± 1.0 | 5.9 ± 5.4 | <0.001 | 0.9 ± 0.5 | 3.5 ± 1.1 | 11.6 ± 4.2 | <0.001 |
| MDS-UPDRS I | - | 7.1 ± 5.2 | 8.8 ± 6.6 | 0.291 | 6.9 ± 4.2 | 7.6 ± 5.8 | 11.6 ± 4.2 | 0.019 |
| MDS-UPDRS II | - | 7.1 ± 5.0 | 9.0 ± 8.6 | 0.360 | 5.4 ± 5.0 | 6.7 ± 6.1 | 11.3 ± 8.0 | <0.001 |
| MDS-UPDRS III | - | 25.4 ± 13.1 | 25.5 ± 15.7 | 0.985 | 20.9 ± 12.5 | 23.6 ± 10.6 | 30.7 ± 19.9 | 0.041 |
| HY Stage | - | 1.6 ± 0.9 | 1.9 ± 0.8 | 0.145 | 1.6 ± 0.6 | 1.7 ± 0.7 | 2.2 ± 0.8 | 0.003 |
| FoG | 3.8 ± 4.9 | 4.6 ± 5.2 | 0.653 | 2.7 ± 4.35 | 3.8 ± 4.5 | 6.8 ± 5.6 | 0.006 | |
| LEDD | - | - | 698 ± 447 | - | 419 ± 221 | 553 ± 298 | 1030 ± 471 | <0.001 |
| MoCA | 25.5 ± 2.4 | 24.8 ± 3.8 | 24.2 ± 3.5 | 0.026 | 24.4 ± 2.3 | 25.5 ± 3.6 | 23.0 ± 4.0 | 0.024 |
| HDRS | 2.9 ± 3.4 | 5.3 ± 4.4 | 5.7 ± 4.8 | <0.001 | 4.8 ± 3.7 | 4.4 ± 4.5 | 7.6 ± 5.4 | 0.019 |
Data presented as mean ± SD. Abbreviations: PDDN = drug naïve PD; PDDT = drug treated PD patients; PDE = early-stage PD; PDM = middle-stage PD; PDL = late-stage PD; HY = Hoehn & Yahr; FoG = Freezing of Gait.
indicates p-values between controls, PDDN, and PD.
indicates p-values between PDE, PDM, and PDL.
Subgroup definition
PD duration was defined based on the time since PD first was diagnosed by a neurologist. To investigate when nigral iron increased along the disease course, we subgrouped drug-treated PD patients into three stages. 1) Early-stage PD (PDE) patients were defined as having disease duration <2 years; this subgroup provided a good clinical match for the PDDN group. 2) Middle-stage PD (PDM) patients were defined as those having disease duration 2-6 years; this subgroup captured the “honeymoon” period when PD patients are relatively “stable” clinically.29,30 3) Later-stage PD (PDL) patients were defined as those with disease duration >6 years; this subgroup reflected those whose clinical “honeymoon period: had ended. Detailed demographic and clinical information for each group are provided in Table 1.
PD medications
Levodopa equivalent daily dosage (LEDD) was calculated using a published method.31 Patient drug status was coded as on/off for each drug category depending on whether a patient was on the specific medication at the visit. Drug categories included dopamine replacement therapy [levodopa (of any formulation) and/or dopamine agonists (including ropinirole, pramipexole, or rotigotine)] and/or monoamine oxidase-B (MAO-B) inhibitors (selegiline or rasagiline). Although selegiline and rasagiline are both effective MAO-B inhibitors,32 they have different pathways of metabolism. Selegiline, but not rasagiline, can be bioconverted to amphetamine and methamphetamine,33 the latter of which have been associated with neurotoxic effects on the striatal dopamine system.34 Thus, we investigated the two drugs individually as opposed to considering them a single class.
MRI image acquisition and analysis
For each participant, T1-weighted, T2-weighted, and multi-gradient-echo MR images were acquired on a 3T Siemens scanner (Erlangen, Germany). A magnetization-prepared rapid acquisition gradient echo sequence was used to obtain T1-weighted images with repetition time/echo time = 1540/2.34 milliseconds; inversion time = 807 milliseconds; field of view = 256×256; slice thickness, 1 mm (with no gap); and slice number = 176. A 3-dimensional T2-weighted SPACE (sampling perfection with application optimized contrast using different angle evolution) sequence was used to obtain T2-weighted images with repetition time/echo time = 2500/316 milliseconds and the same spatial resolution settings as the T1-weighted images. T1- and T2-weighted images were acquired sagittally to save scan time. T2*-weighted images were acquired using a multi-gradient-echo sequence with 8 echoes (echo times evenly spaced from 6.2 to 49.6 milliseconds); and repetition time = 55 milliseconds; flip angle = 15°; field of view = 240×240; matrix = 256×256; slice thickness = 2 mm; slice number = 64; and voxel size = 0.9×0.9×2 mm3. T2*-weighted images were acquired transversely. All images were inspected offline, and deemed free of severe artifacts or any major structural abnormalities. Quantitative susceptibility maps (QSM) were generated using morphology enabled dipole inversion employing an automated uniform cerebrospinal fluid zero reference (MEDI+0) with a nonlinear formation of the magnetic field to source.35,36
Regions-of-interest segmentation
Regions-of-interest (ROIs) included in the study were basal ganglia [substantia nigra pars compacta (SNc), putamen (PUT), caudate (CN), and globus pallidus (GP)] and a related cerebellar structure [red nucleus (RN)], all of which are known to be iron rich. Frontal white matter (FWM) was included as a reference region that is not known for high iron content in the brain. The ROIs were segmented using automatic atlas-based parcellation, followed by manual correction by an neuroimager (GD) with >15 years of experience and blinded to group information to minimize any segmentation errors at the boundary.19 This semi-automated approach improves the accuracy and precision of the segmentation. It consists of the following four steps:
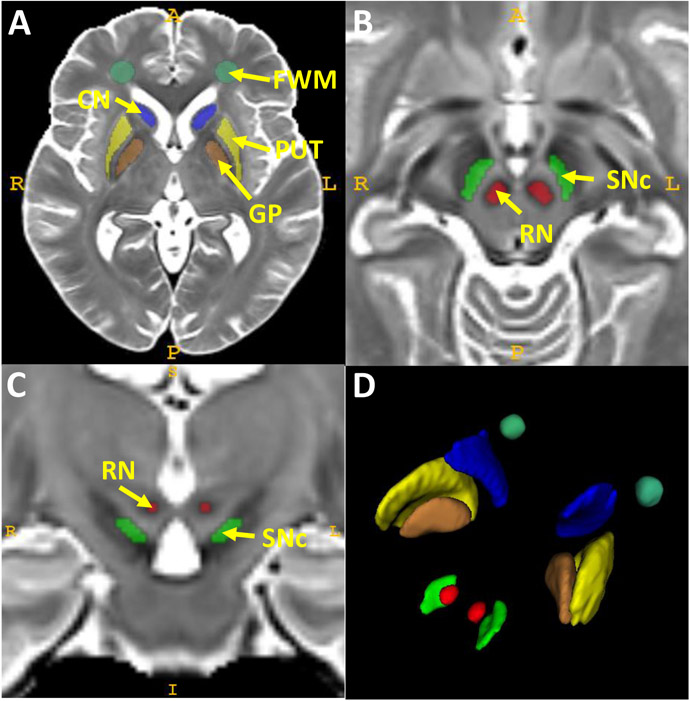
Step1: T1- and T2-weighted images from all participants were used to construct a cohort-specific template using an unbiased atlas construction algorithm in the Advanced Normalization Tools (ANTs) package.37,38 Since most of these structures have high iron content and are seen best in T2-weighted images, the atlas of ROIs was defined manually on the constructed T2-weighted template according to previous studies.19 Figure 1 shows the exact location of these five structures on the T2-weighted template and their 3D view. There are inconsistent definitions of the SNc in the literature.17,24,39,40 We utilized the findings from our previous QSM voxel-wise analysis20 and anatomical information derived from prior neuromelanin-sensitive MRI studies to delineate the SNc.40-42 Namely, the SNc was defined on six transverse slices starting superiorly one slice below the level of the largest RN radius. This resulted in a 4 × 10 mm kidney-shaped region between the RN and the medial side of the hypointense band [that includes both the SN pars reticulata (lateral half) and SNc (medial half); Figure 1B and 1C].
Figure 1. Regions of interest (ROIs) defined on the T2-weighted cohort template.
The locations of the CN, PUT, GP, and FWM are shown in A in an axial slice that captures all four structures. The SNc and RN regions are displayed in B (axial slice) and C (coronal slice) at the midbrain level. A 3D view of all ROIs is shown in D.
Abbreviations: CN: caudate nucleus; FWM: frontal white matter; GP: globus pallidus; PUT: putamen; RN: red nucleus; SN: substantia nigra.
Step 2: Using the cohort-specific atlas generated from Step 1, an atlas-based segmentation pipeline (AutoSeg v3.0; University of North Carolina Neuro Image Analysis Laboratory) then was employed to segment the ROIs in individual space based on the T2-weighted images for each subject.43
Step 3: An affine registration algorithm was used to bring the ROIs from the T2-weighted images to the QSM images by co-registering the T2-weighted images to the QSM images.
Step 4: Automated segmentation data from all subjects were inspected visually and corrected manually on the QSM images by a rater blinded to group information. Finally, mean QSM values from each ROI (averaging both sides of each structure) were calculated for individual subjects.
Statistical analyses
Demographic data were compared between control, PDDN, and PDDT groups using the Fisher’s exact test for sex and one-way analysis of variance for age. Disease duration, MDS-UPDRS-I, -II, -III scores; Hoehn-Yahr stage as a part in MDS-UPDRS-III score; FoG; LEDD; MoCA; and HDRS were compared among PDDN and PDDT using one-way analyses-of-covariance (ANCOVA) with adjustments for age and sex. Group differences in demographics and clinical measures between PD subgroups were assessed using the same approaches. One-way ANCOVA with age and sex as covariates also was used to compare QSM values in different ROIs among groups. The Bonferroni method was used for multiple comparison correction for the main group.
Because SNc is the key pathological loci for PD and showed the most dynamic changes between different groups, we specifically compared nigral QSM among controls, PDDN and different PD subgroups, with Bonferroni correction for multiple comparisons. In addition, a multiple regression analysis was performed to explore factors that may be associated with iron accumulation in the SNc. A step-wise variable selection method was used to identify important factors that independently were associated with nigral iron content. The factors of interest include medications use for PD (levodopa, dopamine agonists, selegiline, or rasagiline), disease duration, LEDD, age, and sex. Clinical measures (MDS-UPDRS-I, II, III, MoCA, and FoG) were excluded from the multiple regression analysis because they likely are causal consequences of higher nigral iron. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Demographic and clinical information
The demographic and clinical data yielded no significant differences among control, PDDN, and PDDT subjects for sex and age (Table 1). Although PDDN patients had a shorter disease duration compared to drug-treated patients (p=0.001), they did not differ either in MDS-UPDRS scores, FoG, or Hoehn-Yahr stages. PDDT patients had lower MoCA scores (p=0.026) than controls or PDDN, and both PDDN and PDDT patients had significantly higher HDRS scores than controls (p<0.001).
Group comparison of regional QSM values
Compared to controls, PDDN patients had lower QSM values in the SNc (p=0.018), caudate (p=0.038), and globus pallidus (p=0.010), but not in other regions. In contrast, PDDT patients had significantly higher QSM values only in the SNc (p<0.001). Compared to PDDN, PDDT had significantly higher QSM values in the SNc (p<0.001) and a trend in the red nucleus (p=0.052 after Bonferroni correction) (Table 2).
Table 2.
Group comparisons of regional QSM values.
| Controls | PDDN | PDDT | Overall | C vs PDDN | C vs PDDT |
PDDN vs PDDT |
|
|---|---|---|---|---|---|---|---|
| SNc | 609 ± 42 | 575 ± 34 | 638 ± 44 | <0.001 | 0.018 | <0.001 | <0.001 |
| RN | 627 ± 47 | 604 ± 31 | 633 ± 44 | 0.185 | 0.222 | 1.0 | 0.052 |
| Put | 538 ± 20 | 534 ± 19 | 535 ± 19 | 1.0 | 1.0 | 1.0 | 1.0 |
| CN | 541 ± 20 | 529 ± 11 | 538 ± 18 | 0.145 | 0.038 | 1.0 | 0.139 |
| GP | 608 ± 40 | 581 ± 29 | 598 ± 26 | 0.029 | 0.010 | 0.435 | 0.206 |
| FWM | 493 ± 5 | 491 ± 6 | 490 ± 32 | 1.0 | 1.0 | 1 | 1.0 |
Data presented are mean ± SD. P-values are corrected using the Bonferroni method.Abbreviations: PDDN = drug naïve PD, PDDT = drug treated PD, C = controls
Subgroup analysis of nigral iron content
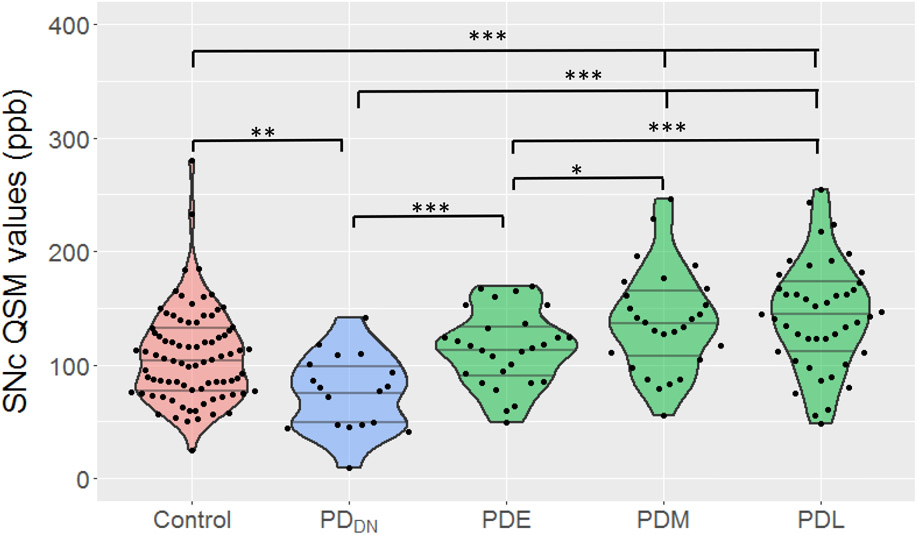
SNc QSM values were greater in PDE patients versus those in PDDN subjects (p=0.002), but not different than controls (p=0.375). SNc QSM values in PDM patients were greater than controls (p=0.001), PDDN (p<0.001), and PDE (p=0.045) subjects. Whereas, SNc QSM values in PDL were not different than PDM patients (p = 0.226) (Figure 2).
Figure 2. Subgroup comparison of SNc QSM values between PDDN, early-, middle-, and later-stages of PD.
Medications and disease duration that may be associated with nigral iron content
Levodopa, selegiline, and disease duration emerged as the only features that survived the stepwise variable selection process (Table 3). The multiple regression analysis showed that disease duration was a significant contributor to SNc QSM values (p=0.035) (Table 3). In addition, PD patients taking levodopa had higher SNc QSM values than those not taking levodopa [mean difference = 28.4 ppb (p=0.013)]. This difference is similar to the mean difference in QSM values (29 ppb) between PDDT and controls after controlling for disease duration. Conversely, patients taking selegiline had SNc QSM values that were 19 units lower than patients not taking this drug (Table 3).
Table 3.
Stepwise variable selection and multiple regression analyses for important clinical factors that affect nigral iron in PDDT.
| Variable Selected | Multiple regression analysis | ||||
|---|---|---|---|---|---|
| β | SEM | F values | P values | ||
| Age | No | ||||
| Sex | No | ||||
| Dopamine agonists | No | ||||
| Rasagiline | No | ||||
| LEDD | No | ||||
| Disease duration (y) | Yes | 2.09 | 0.8 | 4.58 | 0.035 |
| Levodopa (11 vs. 76) | Yes | 28.4 | 13.3 | 6.52 | 0.013 |
| Selegiline (39 vs. 48) | Yes | -19.0 | 8.6 | 4.89 | 0.030 |
Step-wise variable selection was used to explore potential clinical measures and drug effects that may affect QSM values in the SNc. The factors explored included age, sex, disease duration, levodopa (On-Off), dopamine agonists (On-Off), rasagiline (On-Off), selegiline (On-Off), and levodopa equivalent daily dosage (LEDD). We first indicate whether the factor was selected in the variable selection process and then present the results from the multiple regression analysis with the selected factors.
Discussion
Consistent with previous post-mortem observations and recent MRI studies, we found that nigral iron concentrations (as represented by QSM) were greater in drug-treated PD patients. In addition, we confirmed previous reports8 19 that nigral iron was not significantly higher in early-stage PD patients. Most importantly, our data indicate that that drug-naïve PD patients have significantly lower nigral iron compared to either controls or early-stage PD patients. In addition, increasing iron accumulation in early- and middle-stage patients plateaus in later-stage patients who are at the end of the levodopa clinical “honeymoon period” (i.e., defined by increasing fluctuations and the emergence of dyskinesias29). Lastly, our study yielded tantalizing preliminary data that nigral iron accumulation during PD may be associated with the type of antiparkinson medication being taken. These new findings are inconsistent with the current hypothesis that higher nigral iron leads to initiation of PD pathology, and the notion that increased iron causes disease progression. Further studies that delineate the dynamic changes of brain iron in living PD patients may have important scientific and clinical impact.
History of iron-PD link and our unique focus on drug-naïve PD patients
The first report of iron accumulation in PD was a single case study in 1924 that observed increased iron deposition in the globus pallidus.44 It was nearly a half-century before the next report that involved a study of 11 post-mortem PD cases in which iron was measured using x-ray fluorescent spectroscopy, albeit without a predesigned control group.25 Later, Dexter et al.4 reported much higher nigral iron post-mortem in 11 PD vs, 13 controls. Additional post-mortem5-9 and susceptibility MRI3,17,20,23,45,46 studies confirmed these initial findings in patients who all were taking antiparkinson drugs (primarily levodopa). No study to date, however, has investigated brain iron in drug-naïve PD patients in vivo.
Interestingly, a post-mortem cases series of eight PD patients did not find increased nigral iron.8 The average disease duration in that report was 7.5 y, the patients had mild pathological changes, and had taken relatively low doses of antiparkinson medication (i.e., LEDD=375 mg).8 Our prior published MRI studies of early-stage PD patients (disease duration ≤ 1 year) 19,24 also did not find increased nigral iron cross-sectionally or longitudinally. To our best knowledge, the current study is the first to focus on brain iron content of PD patients in drug-naïve stage by leveraging the availability of susceptibility MRI.
Rapid eye movement behavior disorder (RBD) has been considered a prodromal phase of PD. In a recent study using QSM MRI, Sun et al. have demonstrated that idiopathic RBD patients had higher SN iron content compared to controls, and lower iron content compared to PD patients.47 In addition, Pyatigorskaya et al. reported that asymptomatic carriers of two common PD risk genes have higher SN iron content compared to controls estimated using the apparent relaxation rate (R2*).48 Together, this work suggested that SN iron content already is increased in prodromal or at-risk PD subjects. Our results, however, are inconsistent with the hypothesis, and suggested the opposite. This inconsistency in large part may be attributable to the fact that not all “prodromal” or “at-risk” subjects will develop classic PD. Future studies with longitudinal designs to follow these prodromal models will be critically important that may provide insight into whether iron status may predict future disease diagnosis/conversion.
The finding of lower nigral iron in drug-naïve PD patients
Based on both post-mortem and MRI studies in early PD patients, as well as the large body of basic science literature linking higher iron to PD etiology,13,49-51 we hypothesized that drug-naïve PD patients would not differ from controls in nigral iron. Yet the results were that drug-naïve PD patients have significantly lower nigral iron compared to controls, which actually is consistent with several large epidemiological studies that have reported lower iron may be related to increased PD risk.52-54 Using Mendelian randomization to control for iron-regulating genes, Pichler et al.52 reported higher serum iron is associated with lower PD risk in a large cohort with ~20k PD patients and ~88k controls. Furthermore, a meta-analysis by Wang et al.54 reported an association between anemia and higher PD risk. Thus, in some way lower iron may be a risk factor in PD etiology, much as it is in restless leg syndrome,55 possibly because of its role in dopamine metabolism.56,57
Nigral iron increases from early- to mid-stage drug-treated PD patients before plateauing in later-stages
The current study replicates previous findings that showed nigral iron concentration is increased to a similar extent in middle- and late-stage drug-treated PD patients.17,20,24 It is unclear if this is a result of the progression of pathophysiological processes (e.g., dopaminergic cell loss) or if it is associated with antiparkinson medication. Consistent with the former hypothesis, the number of surviving dopamine neurons also plateaus with disease progression. If true, nigral iron accumulation measured by QSM may be a surrogate marker for the remaining neurons. Consistent with the latter hypothesis, levodopa itself may be neurotoxic, although this is controversial and a major study yielded data inconsistent with this notion.58
Relevance and limitations of our findings
There are several ongoing clinical trials testing iron chelation as a treatment for PD 14,59 based on the premise that iron accumulation may be linked etiologically to PD and/or propel disease progression. These trials have targeted new PD patients with the assumption that iron accumulation in the SN occurs before symptom onset and levodopa treatment. In a small Phase II trial of 22 early-stage PD patients (disease duration <5 years, within the clinical honeymoon period, and on standard PD medications),14 iron chelation using deferiprone (20 mg/kg or 30 mg/kg) was compared to placebo. Neither dose had an effect on motor scores, but did lower iron concentrations of iron in the caudate and dentate nuclei but not the SN.14 The finding of lower nigral iron in drug-naïve PD patients in the current study makes the results from the deferiprone trial unsurprising, and argues future use of iron chelating agents as adjuvant in middle- or late-stage PD patients along with levodopa.
Our study has a few limitations. First, the sample size for the drug-naïve PD group is small relative to those of the control and other PD groups. Nonetheless, we demonstrate significant group differences when comparing drug-naïve PD to both controls and drug-treated PD groups. The difference persisted even when drug-naïve PD patients were compared to early-stage PD patients with a comparable sample size, disease duration. Second, the analyses were cross-sectional in nature and the progression pattern was postulated from the baseline characteristics of the individual PD subgroups having different disease durations. Despite these limitations, our study provides initial evidence indicating drug-naïve PD have lower nigral iron compared to controls and drug-treated PD patients. Consistent with earlier studies, our study also demonstrated that nigral iron increases with disease progression that plateaus in later-stage patients. Our investigation regarding factors that might affect iron accumulation is limited by the explorative and associative nature of the study. The resulting data, however, are tantalizing and hint that antiparkinson treatments may interact with disease and nigral iron progression in a complex fashion that may have clinical relevance. Validation of these findings with longitudinal follow-up and using independent cohorts is warranted. If confirmed, the mechanisms underlying this dynamic pattern of nigral iron change in PD will be of great interest.
Acknowledgements:
This work was supported in part by the National Institute of Neurological Disorders and Stroke (NS060722 to XH) and Parkinson’s Disease Biomarker Program (NS082151 and NS112008 to XH), the Hershey Medical Center General Clinical Research Center (National Center for Research Resources, Grant UL1 RR033184 that is now at the National Center for Advancing Translational Sciences, Grant UL1 TR000127), the PA Department of Health Tobacco CURE Funds, the Penn State College of Medicine Translational Brain Research Center, and the Michael J. Fox Foundation for Parkinson’s Research (18078 to GD).We express gratitude to all of the participants who volunteered for this study and study personnel who contributed to its success.
Footnotes
Conflict of interest/financial disclosures: The authors have no financial disclosures/conflict to report.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114 ( Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 2.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013;136(Pt 8):2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham JM, Paley MN, Grunewald RA, Hoggard N, Griffiths PD. Brain iron deposition in Parkinson's disease imaged using the PRIME magnetic resonance sequence. Brain 2000;123 Pt 12:2423–2431. [DOI] [PubMed] [Google Scholar]
- 4.Dexter DT, Wells FR, Agid F, et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet 1987;2(8569):1219–1220. [DOI] [PubMed] [Google Scholar]
- 5.Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991;114 ( Pt 4):1953–1975. [DOI] [PubMed] [Google Scholar]
- 6.Dexter DT, Wells FR, Lees AJ, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem 1989;52(6):1830–1836. [DOI] [PubMed] [Google Scholar]
- 7.Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MBH. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. Journal of neurochemistry 1991;56(3):978–982. [DOI] [PubMed] [Google Scholar]
- 8.Riederer P, Sofic E, Rausch WD, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 1989;52(2):515–520. [DOI] [PubMed] [Google Scholar]
- 9.Sofic E, Riederer P, Heinsen H, et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 1988;74(3):199–205. [DOI] [PubMed] [Google Scholar]
- 10.Castellani RJ, Siedlak SL, Perry G, Smith MA. Sequestration of iron by Lewy bodies in Parkinson's disease. Acta Neuropathol 2000;100(2):111–114. [DOI] [PubMed] [Google Scholar]
- 11.Paris I, Martinez-Alvarado P, Cardenas S, et al. Dopamine-dependent iron toxicity in cells derived from rat hypothalamus. Chem Res Toxicol 2005;18(3):415–419. [DOI] [PubMed] [Google Scholar]
- 12.Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J Neurochem 2011;118(6):939–957. [DOI] [PubMed] [Google Scholar]
- 13.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014;13(10):1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Bastida A, Ward RJ, Newbould R, et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson's disease. Sci Rep 2017;7(1):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grolez G, Moreau C, Sablonniere B, et al. Ceruloplasmin activity and iron chelation treatment of patients with Parkinson's disease. BMC Neurol 2015;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in Neurodegeneration - Cause or Consequence? Front Neurosci 2019;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 2008;70(16 Pt 2):1411–1417. [DOI] [PubMed] [Google Scholar]
- 18.Langkammer C, Pirpamer L, Seiler S, et al. Quantitative Susceptibility Mapping in Parkinson's Disease. PLoS One 2016;11(9):e0162460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du G, Lewis MM, Sica C, et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson's patients. Mov Disord 2018;33(9):1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du G, Liu T, Lewis MM, et al. Quantitative susceptibility mapping of the midbrain in Parkinson's disease. Mov Disord 2016;31(3):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 2012;62(3):1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 2010;257(2):455–462. [DOI] [PubMed] [Google Scholar]
- 23.Peran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 2010;133(11):3423–3433. [DOI] [PubMed] [Google Scholar]
- 24.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov Disord 2012;27(13):1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earle KM. Studies on Parkinson's disease including x-ray fluorescent spectroscopy of formalin fixed brain tissue. J Neuropathol Exp Neurol 1968;27(1):1–14. [DOI] [PubMed] [Google Scholar]
- 26.Dexter D, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991;114(4):1953–1975. [DOI] [PubMed] [Google Scholar]
- 27.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 28.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord 2000;6(3):165–170. [DOI] [PubMed] [Google Scholar]
- 29.Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson's disease? Eur Neurol 2010;63(5):257–266. [DOI] [PubMed] [Google Scholar]
- 30.Lewis MM, Harkins E, Lee EY, et al. Clinical Progression of Parkinson's Disease: Insights from the NINDS Common Data Elements. J Parkinsons Dis 2020;10(3):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schade S, Mollenhauer B, Trenkwalder C. Levodopa Equivalent Dose Conversion Factors: An Updated Proposal Including Opicapone and Safinamide. Mov Disord Clin Pract 2020;7(3):343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar Am O, Amit T, Youdim MB. Contrasting neuroprotective and neurotoxic actions of respective metabolites of anti-Parkinson drugs rasagiline and selegiline. Neurosci Lett 2004;355(3):169–172. [DOI] [PubMed] [Google Scholar]
- 33.Palhagen S, Heinonen E, Hagglund J, Kaugesaar T, Maki-Ikola O, Palm R. Selegiline slows the progression of the symptoms of Parkinson disease. Neurology 2006;66(8):1200–1206. [DOI] [PubMed] [Google Scholar]
- 34.Moszczynska A, Fitzmaurice P, Ang L, et al. Why is parkinsonism not a feature of human methamphetamine users? Brain 2004;127(Pt 2):363–370. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn Reson Med 2018;79(5):2795–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med 2013;69(2):467–476. [DOI] [PubMed] [Google Scholar]
- 37.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergsland N, Zivadinov R, Schweser F, Hagemeier J, Lichter D, Guttuso T Jr. Ventral posterior substantia nigra iron increases over 3 years in Parkinson's disease. Mov Disord 2019;34(7):1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langley J, He N, Huddleston DE, et al. Reproducible detection of nigral iron deposition in 2 Parkinson's disease cohorts. Mov Disord 2019;34(3):416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaillancourt DE, Mitchell T. Parkinson's disease progression in the substantia nigra: location, location, location. Brain 2020;143(9):2628–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson's disease. Brain 2020;143(9):2757–2770. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Vachet C, Rumple A, et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front Neuroinform 2014;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lhermitte J, Kraus WM, McAlpine D. Original Papers: On the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localization. J Neurol Psychopathol 1924;5(19):195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du G, Lewis MM, Styner M, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov Disord 2011;26(9):1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP. Parkinson's disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov Disord 2017;32(3):441–449. [DOI] [PubMed] [Google Scholar]
- 47.Sun J, Lai Z, Ma J, et al. Quantitative evaluation of iron content in idiopathic rapid eye movement sleep behavior disorder. Mov Disord 2020;35(3):478–485. [DOI] [PubMed] [Google Scholar]
- 48.Pyatigorskaya N, Sharman M, Corvol JC, et al. High nigral iron deposition in LRRK2 and Parkin mutation carriers using R2* relaxometry. Mov Disord 2015;30(8):1077–1084. [DOI] [PubMed] [Google Scholar]
- 49.Zucca FA, Segura-Aguilar J, Ferrari E, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol 2017;155:96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen MS, Andersen MV, Christoffersen PR, Jensen MD, Lichota J, Moos T. Neurodegeneration with inflammation is accompanied by accumulation of iron and ferritin in microglia and neurons. Neurobiol Dis 2015;81:108–118. [DOI] [PubMed] [Google Scholar]
- 51.Hare DJ, Double KL. Iron and dopamine: a toxic couple. Brain 2016;139(Pt 4):1026–1035. [DOI] [PubMed] [Google Scholar]
- 52.Pichler I, Del Greco MF, Gogele M, et al. Serum iron levels and the risk of Parkinson disease: a Mendelian randomization study. PLoS medicine 2013;10(6):e1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong CT, Huang YH, Liu HY, Chiou HY, Chan L, Chien LN. Newly Diagnosed Anemia Increases Risk of Parkinson's disease: A Population-Based Cohort Study. Sci Rep 2016;6:29651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang YC, Huang AP, Yuan SP, et al. Association between Anemia and Risk of Parkinson Disease. Behav Neurol 2021;2021:8360627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen RP, Earley CJ. The role of iron in restless legs syndrome. Mov Disord 2007;22 Suppl 18:S440–448. [DOI] [PubMed] [Google Scholar]
- 56.Pino JMV, da Luz MHM, Antunes HKM, Giampa SQC, Martins VR, Lee KS. Iron-Restricted Diet Affects Brain Ferritin Levels, Dopamine Metabolism and Cellular Prion Protein in a Region-Specific Manner. Front Mol Neurosci 2017;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dichtl S, Haschka D, Nairz M, et al. Dopamine promotes cellular iron accumulation and oxidative stress responses in macrophages. Biochem Pharmacol 2018;148:193–201. [DOI] [PubMed] [Google Scholar]
- 58.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. The New England journal of medicine 2004;351(24):2498–2508. [DOI] [PubMed] [Google Scholar]
- 59.Ward RJ, Dexter DT, Martin-Bastida A, Crichton RR. Is Chelation Therapy a Potential Treatment for Parkinson's Disease? International journal of molecular sciences 2021;22(7). [DOI] [PMC free article] [PubMed] [Google Scholar]