Abstract
Background:
The use of opioids for the treatment of pain is a risk versus benefit analysis and metabolic disease is an often overlooked variable in the equation and may lead to increased risk of comorbidities of cardiovascular and cerebrovascular disease and diabetes.
Objectives:
Our objective was to identify and describe abnormalities among the comprehensive metabolic and lipid panels of individuals taking prescription opioids.
Study Design:
We performed a cross-sectional study of the laboratory values with 3 cycles (2011–2016) of the National Health and Nutrition Examination Survey (NHANES) in March 2020.
Setting:
NHANES sampling is conducted using a multistaged, stratified, cluster sampling technique to create a representative sample of the United States.
Methods:
We excluded patients with histories of cancer and under the age of 25 years. Our final sample size was 11,061 (n = 162,547,635), with 797 reportedly using a prescription opioid in the past 30 days—a weighted percent representing 22.95% of the US population. Our analyses identified mean differences in biomarkers between individuals taking prescription opioids and the US population.
Results:
Laboratory values from the comprehensive metabolic panel were all within reference ranges for both groups, with only bilirubin levels being statistically lower in the group currently taking prescription opioids. Values from the lipid panel of both the opioid using and comparison groups were above reference range for total cholesterol and fasting glucose. The opioid using group was also higher than the reference range for triglycerides (mean [M] = 165.4, standard deviation [SD] = 14.2) and insulin (M = 15.5, SD = 2.2), whereas the comparison group was not. The oral glucose measure was within normal ranges for both groups; however, the opioid using group was 13.7 points higher than the comparison group (M = 122.3, SD = 1.8; M = 108.6, SD = 4.0; P < 0.01).
Limitations:
While our study uses a large sample for a robust generalizable analysis it is a correlation study and a longitudinal cohort would provide better evidence linking potential disease states to prescription opioid use.
Conclusions:
Although all Americans should be alarmed at the lipid levels reported in this study, specific combinations of heightened lipid laboratory values among prescription opioid users accelerate the trajectories toward comorbidities—heart disease, cerebrovascular disease, and diabetes—leading to diminished quality of life. Therefore pain management and comprehensive drug recovery programs should include nutritional counseling and physical activity as part of their overall treatment plan.
Keywords: Opioid use, NHANES, pain management, lab values, analgesics
Chronic pain is a significant issue in the United States and affects 1 in 10 citizens (1). Opioids are frequently used for the treatment of pain even though the side effects of opioid use are extensive and include nausea, vomiting, constipation, addiction, and respiratory depression (2). Studies in rats and small cohorts of males have shown opioid use causes significant increases in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, blood urea nitrogen (BUN), and creatinine (3,4). Another small study identified an association between hyperglycemia and acute opioid use (5).
Further, the use of opioids for pain is a risk versus benefit analysis, and metabolic disease is an often overlooked variable in the equation. One study in rats showed alterations in serum concentrations of triglycerides, plus total high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol (6). Moreover, opioid-induced endocrinopathies to include decreased free and total testosterone have recently been discovered and may have an impact on hyperlipidemia (7,8). A cohort study of over 20,000 veterans with long-term opioid use and low testosterone found an increased risk of hyperlipidemia in patients receiving testosterone supplementation. Definitive evidence of lipid aberrancy in human opioid users is, however, lacking.
The use of long-acting opioids to treat opioid use disorder is also on the rise. From 2003 to 2016, opioid use disorder treatment programs increased by 39% (9). Although opioids are increasingly being prescribed for justifiable reasons, such as in the treatment of opioid use disorder, many physicians do not consider the nutritional and metabolic impact of opioid use. One study found that only 7% of residential treatment facilities included the services of dietitians (10). As a result, the need for increased awareness and understanding of metabolic derangements in opioid users is apparent. Despite the attempt to identify laboratory derangements in opioid users by multiple researchers, no study has analyzed these effects in a large, representative US sample. Thus our objective was to identify and describe abnormalities among the comprehensive metabolic and lipid panels of individuals taking prescription opioids.
Methods
We performed a cross-sectional study of the laboratory value derangements among individuals taking prescription opioids using the (2011–2016) National Health and Nutrition Examination Survey (NHANES) dataset. NHANES is a nationally representative, publicly available, health survey of civilian and noninstitutionalized US citizens. The survey is composed of a health questionnaire, physical examination, and biospecimen collection performed by a trained health care professional. Sampling was conducted using a multistaged, stratified, cluster sampling technique with ethnic minority and elder population oversampling to create a representative sample of the United States. In return for participation, travel expenses and childcare were reimbursed. Following biospecimen collection, sera was stored at −70°C until quantitative analysis was performed using methods previously validated by the NHANES (11). The NHANES survey was approved by the National Center for Health Statistics research ethics institutional review board (IRB) and consent was obtained from all patients prior to data collection.
Data extracted included sociodemographics, prescription opioid use in the past 30 days, and the following laboratory values: ALT, AST, total bilirubin, alkaline phosphatase, BUN, creatinine, carbon dioxide, chloride, sodium, potassium, calcium, total protein, albumin, globulin, total cholesterol, LDL, HDL, triglycerides, fasting glucose, 2-hour oral glucose, and fasting insulin. Male and female patients younger than 25 years were excluded from the analysis due to low prevalence of reported opioid usage. Patients with a history of cancer were excluded. Prescription opioid use was self-reported. Generic opioid names were extracted and collated into a single category for statistical analysis.
Statistical analysis was performed using Stata 16.1 (StataCorp, College Station, TX) in March 2020. Appropriate weighting for the statistical analysis of the subsamples were modified to accommodate 3 cycles of data using the oral glucose weighting for oral glucose test; the fasting subsample weighting for glucose, triglycerides, and LDL tests; and the Mobile Examination Center weighting for insulin, total and HDL cholesterol. To analyze the association of opioid use on the laboratory values from comprehensive metabolic and lipid panels, a multiple linear regression model was constructed to obtain mean differences of laboratory values between groups and marginal means for each group — adjusted for age, gender, race, and body mass index (BMI). Effect sizes were calculated from the standardized coefficients of the binary opioid use variable in the regression model (12) and transformed to Cohen’s d. This study complied with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines. Data utilized in this study were deidentified and not eligible for IRB approval.
Results
After excluding patients with a historical diagnosis of cancer and those under the age of 25 years, the remaining sample size was 11,061 (n = 162,547,635). Of the respondents, 797 had reported using a prescription opioid in the past 30 days with a weighted percent representing 22.95% of this portion of the US population. Laboratory values from the comprehensive metabolic panel were all within reference ranges for both groups, with only bilirubin levels being statistically lower in the group currently taking prescription opioids (mean [M] = 0.6, standard deviation [SD] = 0.0) from the comparison group (M = 0.7, SD = 0.0) (Fig. 1).
Fig. 1.
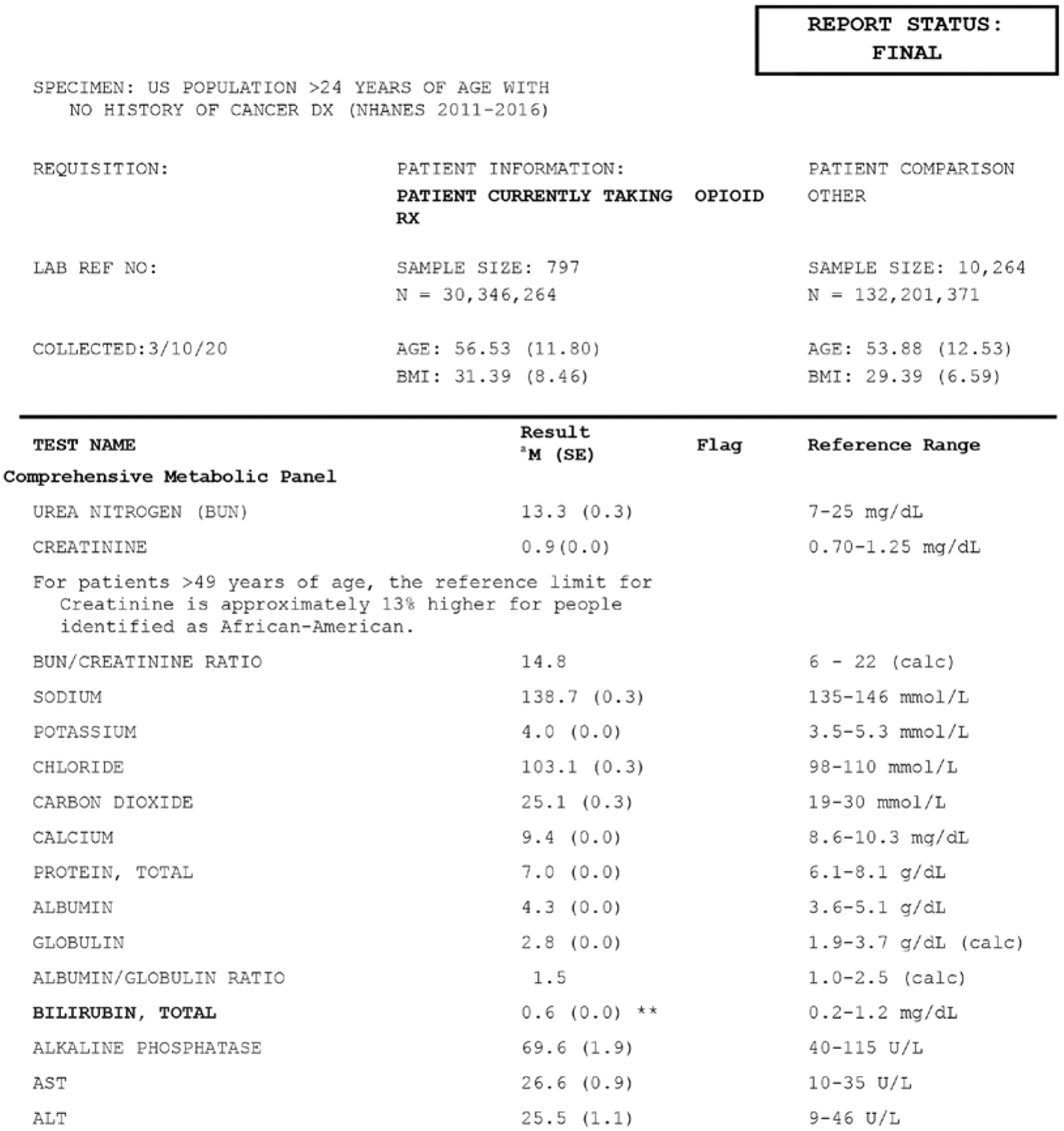
Adjusted means of comprehensive metabolic panel values between individuals currently taking opioid prescriptions and those without in the US over 24 years of age, excluding those with a cancer diagnosis (NHANES 2011–2016).
a Marginal means from regression models adjusted for age, race, gender, and BMI with statistical significance noted as (*) represents P < 0.05 and (**) as P < 0.01 compared NHANES participants over 25 years of age with no cancer diagnosis using mobile examination center (MEC) subsample and weighting modified for 3 cycles.
Values from the glucose and lipid panel s h o w e d that both the opioid using and comparison groups were above reference range for total cholesterol (M = 208.8, SD = 3.3; M = 201.1, SD = 1.2; P < 0.05, Cohen’s d = 0.19) and fasting glucose (M = 108.6, SD = 3.4; M = 108.0, SD = 1.2) (Fig. 2). The opioid using group was also higher than the reference range for triglycerides (M = 165.4, SD = 14.2; M = 136.3, SD = 4.3; P < 0.05) and insulin (M = 15.5, SD = 2.2; M = 13.1, SD = 0.29), whereas the comparison group was not. The oral glucose measure was within normal ranges for both groups; however, the opioid using group was 13.7 points higher than the comparison group (M = 122.3, SD = 1.8; M = 108.6, SD = 4.0; P < 0.01).
Fig. 2.
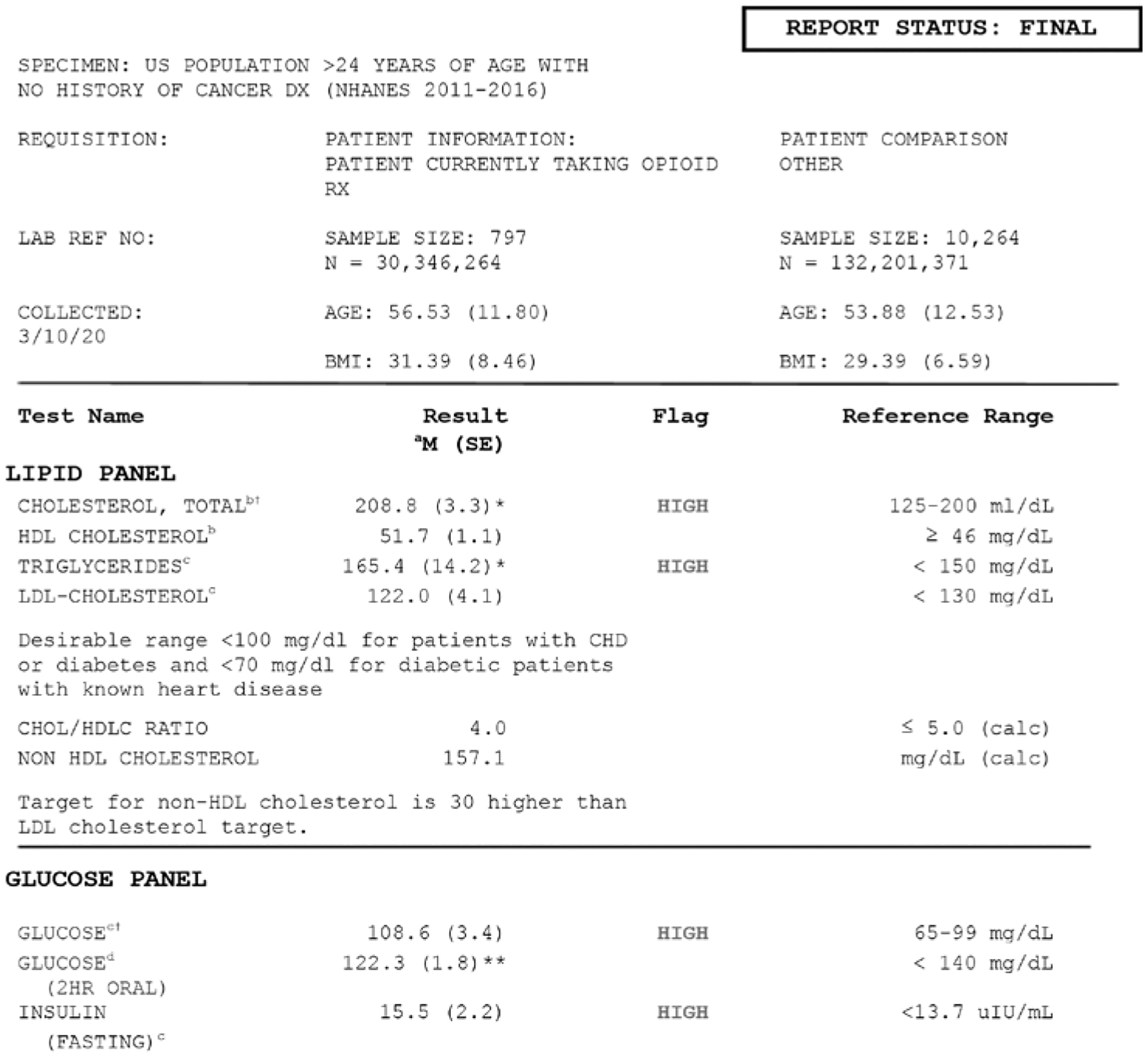
Adjusted means of lipid panel values between individuals currently taking opioid prescriptions and those without in the US over 24 years of age, excluding those with a cancer diagnosis (NHANES 2011–2016).
aMarginal means from regression models adjusted for age, race, gender, and DMI with statistical significance noted as (*) represents P < 0.05 and (**) as P < 0.01 compared NHANES participants over 25 years of age with no cancer diagnosis.
bUsing mobile examination center (MEC) subsample and weighting modified for 3 cycles.
cUsed Fasting subsample and weighting modified for 3 cycles.
dUsed Oral Glucose subsample and weighting modified for 3 cycles.
†Comparison group also higher than reference range.
Discussion
Results from our analysis show that on average all US individuals over age 24 years and without cancer (controlling for age, gender, and BMI) have high cholesterol and glucose levels, whereas individuals taking prescription opioids also have high triglycerides and insulin compared with the reference ranges. Although all Americans should be alarmed at the average lipid levels reported in this study, specific combinations of heightened lipid laboratory values in the prescription opioids users lead to multiple comorbidities.
One concerning combination of high lipid values is that of fasting glucose and high insulin. Together, glucose and insulin work together to provide energy to the body; however, prolonged (13) imbalance may lead to weight gain, insulin resistance, prediabetes, and type II diabetes mellitus. Previous research has linked opium (14) to increased serum glucose and heroin to increased insulin sensitivity (15), although there are several small studies exploring this relationship with mixed results over the past 40 years.
Another problematic combination of lipid values among opioid users is that of high triglycerides and total cholesterol. This is a known risk factor for atherosclerosis, which can lead to ischemic heart disease and further to myocardial infarction (16) or cerebrovascular disease increasing potential risk for stroke (17). Although this is not a new conclusion, it has yet to be identified within this population.
Further, the role of cholesterol in opioid uptake may be exploited in this population. Previous research shows that total cholesterol levels and dosage of opioids, especially initial doses, were inversely related—people with higher cholesterol were more likely to respond to lower doses of opioids (18). Given the increased activity of opioids and opioid receptors within cholesterol-rich environments (19,20), and that US citizens, on average, have high cholesterol levels, this may be an unexplored linkage in the opioid addiction crisis.
Our analyses also showed that neither group was out of the reference ranges of the comprehensive metabolic panel tests, which is in line with previous small studies (21); however, the group currently taking prescription opioids had a statistically lower value for bilirubin. Although lower bilirubin levels are not generally of concern, this may be due to intake of nonsteroidal anti-inflammatory drugs (22) or caffeine (23), and is associated with other conditions such as atherosclerosis (24,25), diabetes (26–28), or ulcerative colitis (29).
Recommendations for all persons with hyperlipidemia include eating a heart-healthy diet – limiting sugar-sweetened beverages and increasing intake of fruits and vegetables, nuts, whole grains, fish and lean poultry (30), eliminating smoking, and improving cardiorespiratory fitness (31). Individuals who may be limited in movement by pain (or other condition) need to consult their physician prior to starting an exercise regimen, but should try to meet physical activity guidelines with modifications and professional guidance as needed (32).
This information highlights the need for comprehensive drug recovery programs to include nutritional counseling and physical activity as part of their overall treatment plan. Not only would improved diet and exercise reduce risk factors associated with heart disease and stroke, but significant preclinical data shows promising effects of exercise on drug self-administration (33). In addition, gold-standard medication-assisted treatment might perpetuate nutritional risks factors thus further emphasizing the need for nutritional consultation during treatment (34). Further research is needed to show how mitigating these risks factors affects relapse rates.
To our knowledge, this study was the first to use NHANES data to explore the laboratory values of individuals currently taking opioid prescriptions, which provides for a robust generalizable analysis; however, a longitudinal cohort would provide better evidence linking potential disease states to prescription opioid use. Future research should explore associations between cholesterol and the addictive properties of opioids, which may lead to novel preventive recommendations or changes in clinical practice guidelines regarding opioid prescriptions.
Conclusions
The elevated lipid panel values illustrate a critical need for all US citizens to be aware of the implications—heart disease, cerebrovascular disease, and diabetes—all leading to diminished quality of life. Individuals currently taking opioids may have an elevated risk of and an accelerated trajectory toward these comorbidities.
Footnotes
Publisher's Disclaimer: Disclaimer: The study was funded by Grünenthal Pharma, Spain. The sponsor was involved in design and conduct of the study. Data management and biostatistical analyses were performed by Medicxact (Spain), and paid for by the sponsor. Writing and editorial assistance was provided by Pablo Vivanco, Elke Grosselindemann, and Birgit Brett and was paid for by the sponsor.
Conflict of interest: AEE is an employee of Grünenthal Pharma, Spain.
References
- 1.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015; 16:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregorian RS Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: A trade-off analysis with patients and physicians. J Pain 2010; 11:1095–1108. [DOI] [PubMed] [Google Scholar]
- 3.Atici S, Cinel I, Cinel L, Doruk N, Eskandari G, Oral U. Liver and kidney toxicity in chronic use of opioids: An experimental long term treatment model. J Biosci 2005; 30:245–252. [DOI] [PubMed] [Google Scholar]
- 4.Elmanama AA, Abu NE, Essawaf HN. Tramadol-induced liver and kidney toxicity among abusers in Gaza Strip, Palestine. Jordan J Biol Sci 2015; 8:133–137. [Google Scholar]
- 5.Solis E Jr, Cameron-Burr KT, Shaham Y, Kiyatkin EA. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro 2017; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solin AV, Lyashev AY, Lyashev YD. Effects of opioid peptides on changes in lipid metabolism in rats subjected to swimming stress. Bull Exp Biol Med 2017; 162:313–315. [DOI] [PubMed] [Google Scholar]
- 7.Khademi H, Kamangar F, Brennan P, Malekzadeh R. Opioid therapy and its side effects: A review. Arch Iran Med 2016; 19:870–876. [DOI] [PubMed] [Google Scholar]
- 8.Gudin JA, Laitman A, Nalamachu S. Opioid related endocrinopathy. Pain Med 2015; 16:S9–S15. [DOI] [PubMed] [Google Scholar]
- 9.Alderks CE. Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (Update). In: The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2017. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29200242. Accessed 03/14/2020. [PubMed] [Google Scholar]
- 10.Wiss DA, Schellenberger M, Prelip ML. Rapid assessment of nutrition services in Los Angeles substance use disorder treatment centers. J Community Health 2019; 44:88–94. [DOI] [PubMed] [Google Scholar]
- 11.Standard Biochemistry Profile. National Health and Nutrition Examination Survey. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/BIOPRO_H.htm#Laboratory_Method_Files. Accessed 02/21/20.
- 12.Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol 2005; 90:175–181. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla F, Guerrini A, Guastalla A, Beretta P, Maio DD. Glucose-insulin metabolism in herion addicts. Neuropsychobiology 1976; 2:341–349. [DOI] [PubMed] [Google Scholar]
- 14.Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol 2004; 9:53–58. [DOI] [PubMed] [Google Scholar]
- 15.Ghodse AH. Evaluation of blood glucose, insulin, growth hormone and cortisol response in heroin addicts. Pahlavi Med J 1977; 8:141–156. [PubMed] [Google Scholar]
- 16.Pocock SJ, Shaper AG, Phillips AN. Concentrations of high density lipoprotein cholesterol, triglycerides, and total cholesterol in ischaemic heart disease. BMJ 1989; 298:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen city heart study. BMJ 1994; 309;11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Z, Liang L, Li L, et al. Opioid doses required for pain management in lung cancer patients with different cholesterol levels: Negative correlation between opioid doses and cholesterol levels. Lipids Health Dis 2016; 15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaibelet G, Millot C, Lebrun C, et al. Cholesterol content drives distinct pharmacological behaviours of μ-opioid receptor in different microdomains of the CHO plasma membrane. Mol Membr Biol 2008; 25:423–435. [DOI] [PubMed] [Google Scholar]
- 20.Lagane B, Gaibelet G, Meilhoc E, Masson JM, Cézanne L, Lopez A. Role of sterols in modulating the human mu-opioid receptor function in Saccharomyces cerevisiae. J Biol Chem 2000; 275:33197–33200. [DOI] [PubMed] [Google Scholar]
- 21.Sood A, Thakur V, Ahuja MM. Effect of chronic opioid administration on glycosylated haemoglobin levels in heroin addicts. Indian J Med Res 1989; 90:51–54. [PubMed] [Google Scholar]
- 22.Schmeltzer PA, Kosinski AS, Kleiner DE, et al. Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int 2016; 36:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modi AA, Feld JJ, Park Y, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology 2010; 51:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: A prospective study in Korean men and women. Stroke 2009; 40:3422–3427. [DOI] [PubMed] [Google Scholar]
- 25.Gupta N, Singh T, Chaudhary R, et al. Bilirubin in coronary artery disease: Cytotoxic or protective? World J Gastrointest Pharmacol Ther 2016; 7:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karuppannasamy D, Venkatesan R, Thankappan L, Andavar R, Devisundaram S. Inverse association between serum bilirubin levels and retinopathy in patients with type 2 diabetes mellitus. J Clin Diagn Res 2017; 11:NC09–NC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim ES, Lee SW, Mo EY, Moon SD, Han JH. Inverse association between serum total bilirubin levels and diabetic peripheral neuropathy in patients with type 2 diabetes. Endocrine 2015; 50:405–412. [DOI] [PubMed] [Google Scholar]
- 28.Kim ES, Mo EY, Moon SD, Han JH. Inverse association between serum bilirubin levels and arterial stiffness in Korean women with type 2 diabetes. PLoS One 2014; 9:e109251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schieffer KM, Bruffy SM, Rauscher R, Koltun WA, Yochum GS, Gallagher CJ. Reduced total serum bilirubin levels are associated with ulcerative colitis. PLoS One 2017; 12:e0179267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114:82–96. [DOI] [PubMed] [Google Scholar]
- 31.Farrell SW, Finley CE, Grundy SM. Cardiorespiratory fitness, LDL cholesterol, and CHD mortality in men. Med Sci Sports Exerc 2012; 44:2132–2137. [DOI] [PubMed] [Google Scholar]
- 32.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, Lippincott Williams & Wilkins, 2017: pp. 472. [Google Scholar]
- 33.Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: Evidence from preclinical studies. Front Psychiatry 2011; 2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez MN, Rigg KK. Nutritional implications of opioid use disorder: A guide for drug treatment providers. Psychol Addict Behav 2020; 34:699–707. [DOI] [PubMed] [Google Scholar]


