Abstract
Introduction and Aims.
Transdermal alcohol sensors allow objective, continuous monitoring and have potential to expand current research on adolescent and young adult alcohol use. The purpose of this manuscript is to evaluate the feasibility and reliability of transdermal alcohol sensor use among female adolescents as compared to female young adults.
Design and Methods.
This trial included 59 female adolescents and young adults aged 14–24 years who reported drinking during the previous month. All participants were asked to wear a Giner Wrist Transdermal Alcohol Sensor (WrisTAS)-7 over a 1 month prospective study. Participants came to the research lab weekly to complete a detailed self-report of behaviours, including day of drinking events, amounts and types of alcohol use and length of drinking events. Estimates of blood alcohol concentration (eBAC) were computed from self-report data using the Matthew and Miller, NHTSA and Zhang equations. Daily transdermal alcohol concentration (TAC) peaks and calculated eBAC peak data were analysed with paired-samples t-tests and repeated measures correlations for validity comparisons.
Results.
All participants (100%, n = 59) completed the trial, however, two participants were removed due to greater than 50% of missing transdermal alcohol sensor data. Of the 57 participants, the data included 1,722 days of continuous alcohol monitoring. Missing data was recorded more frequently among female adolescents at about (11.78%) as compared to female young adults (8.59%; χ2 = −18.40, P < 0.001). Participant self-report of drinking occurred with greater frequency (374 events) than detected by the WrisTAS transdermal alcohol sensors (243 events). On days when self-report and sensor data indicated a drinking event, participants’ eBAC was moderately correlated with TAC, after accounting for repeated measures.
Discussion and Conclusions.
This study finds that transdermal alcohol sensors are moderately reliable when sensor data is paired with self-report. This objective data collection method may improve the ability to collect alcohol curves among adolescents.
Keywords: transdermal alcohol sensor, estimates of blood alcohol concentration, female adolescent, female young adult, transdermal alcohol concentration
Introduction
Alcohol is the most common substance of use and abuse by adolescents [1], due to its perceived availability [2] and social acceptability [3]. In the USA, prevalence of alcohol use increases rapidly through adolescence. Among high school students, fewer than one in four first-year secondary school students consumed alcohol in the past month as compared to more than two in five last year secondary school students [1]. Of current drinkers, three of five underage drinkers used alcohol heavily [4]. Notably, the rate of alcohol use among female adolescents converged with male adolescents in 2015, suggesting rates of adolescent alcohol use no longer varies by gender [1]. In the USA, alcohol use has been seen to increase with age and peaks at the legal drinking age [5].
Adolescent alcohol use before the age of 15 is associated with increased risks for lifetime alcohol dependence [6]. Adolescent drinking stems from social expectations associated with drinking, risk-taking, disruptive or hyperactive behaviours, mental health comorbidities and family history [7]. While there is ample research investigating why adolescents and young adults drink, the acute and chronic health effects of alcohol consumption during puberty depend on accurate reports of their drinking tendencies.
Better understanding of the typical quantity and frequency of alcohol use is needed to develop individual-level interventions specifically aimed at limiting escalation of use. Transdermal alcohol sensor technologies may improve the ability to compare interventions to accomplish this feat, across populations sub-groups, including by age. Transdermal alcohol sensors have been used and evaluated for many health behaviours and conditions, including monitoring diabetes [8], sleep patterns [9], physical activity and performance [10] and breast cancer treatments [11]. Advancements in sensors and the normalisation of wearable technology allow confirmation and improvement of self-report [12].
In the field of alcohol research, transdermal alcohol sensors have been developed to make the continuous monitoring of alcohol use feasible [13]. These sensors have the capability to provide continuous alcohol use data while minimising the burden placed among participants within their daily lives [14]. The data collected from these sensors is a measure of transdermal alcohol concentration (TAC), which is the amount of alcohol expiration through the skin [14]. For the purpose of this study, the Wrist Transdermal Alcohol Sensor (WrisTAS) developed by Giner, Inc. will be used to continuously sample ethanol vapour detected near the skin and store the data until downloaded to a computer serial port [15]. The use of transdermal alcohol sensors has been used in other research settings to monitor alcohol use among adults, however, the use of these sensors among female adolescents and young adults has not been assessed.
Transdermal alcohol sensors may have the capacity to expand the knowledge and understanding of typical alcohol use among adolescents beyond the current limitations of self-report and estimated blood alcohol content (eBAC) in real-world settings. TAC from transdermal alcohol sensors provides continuous, longitudinal data that may give researchers valuable information regarding the trajectory and change of TAC over time across a typical drinking event; however, the sensors must be assessed for feasibility and validated against the currently used gold-standard measures in field settings. The purpose of this study will evaluate: (i) the validity of the transdermal alcohol sensors’ measured TAC peaks compared to calculated eBAC peaks from self-reported data among female adolescents and young adults; and (ii) the reliability of the sensors’ ability to record transdermal alcohol by assessing missing and invalid data.
Methods
All methods were approved by the Oklahoma State University Institutional Review Board. The investigators also acquired a certificate of confidentiality from the National Institute of Child Health and Human Development.
Recruitment
Presentations were conducted at two local high schools in February 2016. Study recruitment, however, depended upon respondent-driven sampling. It is typical for private drinking environments to be a mixture of individuals under the minimum legal drinking age (MLDA) and those of legal drinking age. Notably, one study of parties identified that 68% of partygoers were under MLDA, while 32% were at or above the MLDA [13]. Upon referral to study staff, potential participants were directed to the study website (www.TulsaFABstudy.com), which includes a video explaining the study, frequently asked questions and a link to a screening survey. Individuals met the following inclusion criteria to participate in the study: female, ages 14–24 years, lives within a 45-minute drive of the study office, and must have consumed at least four alcoholic drinks in at least one sitting within the past month. Only female participants were recruited because this is part of a larger trial examining the periconceptional behaviours of women of child-bearing potential. If a potential participant met screening criteria, a member of the research team called to re-screen and schedule a consent and baseline appointment.
Between February 2017 and February 2018, the screening survey was accessed and completed 406 times. Each survey response was not necessarily a unique individual. During this same time frame, 141 individuals qualified for the study and gave contact information, while 21 did not qualify for the study and also gave their contact information. Additionally, 73 participants were recruited into the study and completed the 1 month trial; the initial 15 participants are omitted from this manuscript because of changes to data collection between piloting and finalised data collection.
The laboratory in which this study was conducted is located on the Oklahoma State University, Tulsa campus. Participants signed the informed consent at their baseline appointment prior to participation. If a participant was under the age of 18, one parent was required to attend the baseline appointment to partake in the informed consent process. Participants were contacted by a member of the research team and then scheduled for a baseline appointment to begin their participation in the study. Although 141 participants qualified for the study during this time frame, the number of transdermal alcohol sensors we had restricted the number of participants who were able to complete the 1 month trial to 73. Participants were scheduled on different days of the week dependent upon their schedule.
Data was collected from a sample of 59 participants; however, two participants had greater than 50% missing data from their transdermal alcohol sensor. These participants were excluded from these analyses. The final sample included in this manuscript is 57 participants. Capacity of the research lab, including a limited number of WrisTAS devices, was a major bottleneck between potential participant interest in the study to completion of the study.
Incentive structure
Participants received, in total, at least $325 for participating in the 4-week trial. Incentive structure may be an important component of the feasibility of this trial: incentives increased throughout the study period to reward completion. Participants earned $50 for the completion of week 1, $60 for the completion of week 2, $70 for the completion of week 3, $80 for the completion of week 4, and a $65 bonus for returning instrumentation in good, working order. This incentive structure takes into consideration other data collection pieces not required for this manuscript, including weekly blood samples, a hair sample and a saliva sample, among other self-report measures.
Data collection
Participants completed a number of measurements over a 1-month period. This data collection was part of a larger study that included other health habits, environmental conditions and biomarkers of female adolescents and young adults of childbearing potential.
Demographics.
Several demographic factors were collected at baseline, which included race/ethnicity, mothers’ education and current age. Race and ethnicity were assessed by two items: racial identity was assessed via a “check all that apply” option and ethnic identity was assessed as Hispanic or non-Hispanic. These two items were combined into a single racial/ethnic identity. All Hispanics selected “other race” and were therefore categorised as Hispanic. All American Indian participants also selected at least one other race: for the purposes of this analysis they are considered American Indian and not multi-racial. Mother’s level of education was also included as a proxy of socioeconomic status. Taken together, racial/ethnic identity and mother’s education accounts for approximately 10% of the variability in alcohol use during early adolescence and as little as 7% among older adolescents [14]. The female adolescents and young adults in this trial did not have significantly different race/ethnicity, or mother’s educational status (Table 1).
Table 1.
Sample Characteristics
| Total sample n = 57 Mean (SD) or n (%) |
Adolescent sample n = 40 Mean (SD) or n (%) |
Young adult sample n = 17 Mean (SD) or n (%) |
t value (P-value) or χ2 value (P-value) |
|
|---|---|---|---|---|
| Age (n = 57) | 18.82 (2.75) | 17.18 (1.08) | 22.71 (0.92) | −18.40 (P <0.001) |
| Number of drinking events in previous month (n = 56) | 7.41 (3.82) | 6.73 (3.76) | 9.13 (3.54) | −2.20 (P <0.05) |
| Race/ethnicity (n = 57) | ||||
| White | 42 (73.7%) | 30 (75.0%) | 12 (70.6%) | 7.08 (NS) |
| Black | 2 (3.5%) | 0 (0.0%) | 2 (11.8%) | |
| American Indian | 8 (14.0%) | 7 (17.5%) | 1 (5.9%) | |
| Latino | 4 (7.0%) | 2 (2.5%) | 2 (11.8%) | |
| Multiracial | 1 (1.8%) | 1 (2.5%) | 0 (0.0%) | |
| Mother’s education (n = 54) | ||||
| Less than HS | 4 (7.4%) | 4 (10.5%) | 0 (0.0%) | 8.25 (NS) |
| HS graduate | 7 (13.0%) | 5 (13.2%) | 2 (12.5%) | |
| Some college | 12 (22.2%) | 9 (23.7% | 3 (18.8%) | |
| Community college/tech school | 4 (7.4%) | 3 (7.9%) | 1 (6.3%) | |
| College graduate | 18 (33.3%) | 14 (36.8%) | 4 (25.0%) | |
| Graduate or professional degree | 9 (16.7%) | 3 (7.9%) | 6 (37.5%) | |
| Age at first drink (n = 56) | ||||
| 14 or younger | 18 (32.1%) | 16 (40.0%) | 2 (12.5%) | 3.96 (P < 0.05) |
| 15 or older | 38 (67.9%) | 24 (60.0%) | 14 (87.5%) | |
HS, high school; NS, not significant.
Participants’ ages were collected at baseline. Participants ranged in age from 14 to 24 years old. For the purposes of the current study, participants up to age 20 were grouped as adolescents, and participants 21-years old and over were grouped as young adults. These criteria are based on MLDA, rather than on development. Examination of age as a dichotomous variable will allow direct comparison of the validity and use of transdermal sensors in adolescent and young adult females. Overall, the mean sample age was 18.82 (SD = 2.75; Table 1). The sample consisted of female adolescents (n = 40) with a mean age of 17.18 (SD = 1.08) and female young adults (n = 17) with a mean age of 22.71 (SD = 0.92). We split this study into two groups to assess the feasibility and reliability of the transdermal alcohol sensors between the groups.
Estimated blood alcohol concentration.
Each week, participants completed a retrospective report of alcohol and other drug use, following a timeline follow-back approach [15]. Participants were asked to report on the number of standard drinks of beer, wine, mixed drinks and shots consumed each day during the previous week. Participants were also asked to estimate the length of the drinking occasion. These self-reports were used to calculate a daily eBAC via three distinct calculations: Matthews and Miller [16], the National Highway Traffic Safety Administration (NHTSA) calculation [17] and a modified NHTSA calculation by Zhang [18]. Each calculation was conducted with and without alcohol metabolism, because the metabolic component would result in a calculation of eBAC at the end of a drinking event and not the peak of a drinking event, as was theoretically collected via the transdermal alcohol sensor. The equations vary slightly, and the equations for females are presented as follows:
Wherein c is the self-reported number of standard drinks consumed; w is the weight of the participant in kilograms as collected for this study objectively by trained research assistants; t is time spent drinking and β60 is the rate of alcohol metabolism or 0.017 g/dL. Estimated BAC, as calculated, will serve as the comparison measure to assess the transdermal alcohol sensors in field settings.
Transdermal alcohol sensor data
As an application of Henry’s law, [19] provided evidence that there was no significant difference between measured eBAC and insensible perspiration through the skin. Wrist Transdermal Alcohol Sensor (WrisTAS; Giner Inc; Newton, USA), manufactured by Giner Incorporated is a proton exchange membrane sensor capable of measuring the concentration of ethanol molecules in solution [20]. [Correction added on 30 March 2020, after first online publication: the preceding sentence has been amended.] WrisTAS devices demonstrate convergent validity with other transdermal measures [21] and self-report [22] among adult populations. In order to develop interventions using these emerging technologies, their use among adolescent and young adult populations must be feasible and reliable.
Participants wore the transdermal alcohol sensors for at least 28 days. These devices continuously sample and store the following data: ethanol vapour detected near the skin, temperature and skin conductivity data. Temperature and skin conductivity data can be used to check that participants wore the sensors for at least 21 hours per day, as required for the study. Participants were taught how to adjust the velcro straps on the sensor for comfort. Participants were instructed to remove the sensor to exercise or shower to prevent exposure to water, as the sensors are water resistant but not water proof. Additionally, participants were instructed to avoid putting lotion or perfumes at the site where they would wear the sensor.
While these sensors are designed for use on wrists, the manufacturer’s website states ‘you can wear it discretely on your wrist, or on just about any part of your body where blood vessels are close to the skin surface’ [23]. Due to the sizing of the velcro straps on the transdermal alcohol sensor, the ability for participants to wear it on their ankle (which has three main arteries – peroneal, posterior tibial and the anterior tibial artery) for comfort and most secure fit was critical. Transdermal alcohol sensors placed on the ankle may be worn more discreetly than the wrist as they can be covered by pants or boots. Moreover, the research staff showed the participants how to place the transdermal alcohol sensor on their ankle at their initial appointment of the study.
Each week, the participant wore a different transdermal alcohol sensor, due to the need to service the sensors weekly. Each sensor could then be maintained, including changing batteries and adding deionized water when necessary. WrisTAS devices sample at a preselected interval: 10 s, 2 min, 5 min or 10 min. For this study, the transdermal alcohol sensors were set to record every 5 min throughout the study period. The data is stored in the unit, and requires serial port connection to a computer to download the data. The research team downloaded data weekly throughout the study period; the data was then reduced as described below.
Data smoothing
Because transdermal alcohol sensor data is often noisy, we used a locally estimated scatterplot smoothing (LOESS) technique. This technique was used to smooth the TAC data utilising both R 3.40 and R-Studio programs. LOESS is a non-parametric regression technique, which allows the model to be adjusted by baseline, and smoothed by the parameter of α, or span. The span is the percent of overall data to be estimated for each local data point [24]. The other parameter required for LOESS is the degree of polynomial represented by lambda, λ. For this data, we are evaluating local linearity, λ = 1. While LOESS has not been used to smooth TAC data, it has been shown to successfully smooth other continuous physiological monitoring [25] and has been established in comparisons of other measurement techniques of eBAC [26].
The transdermal alcohol sensors were rotated on a weekly basis for each participant. Therefore, LOESS models were computed each week to establish consistent baseline values. The span for LOESS smoothing was set at 0.0125. This parameter was chosen to fit the model over approximately 25 data points, or about 2 hours. This provided adequate smoothing while also maintaining enough specificity to model drinking events. Baseline measures for the data were estimated by averaging the values of non-drinking sensor readings below 0.015 and then subtracting that mean from the predicted LOESS values. To accommodate the missing values, the average baseline measure was imputed.
Two independent coders on the research team then evaluated the smoothed curves by week for each participant. These independent coders read the smoothed data and determined the number of actual peaks in the data. Despite the data having been smoothed, some relatively short-term peaks still existed. The coders assessed whether it was physiologically possible for a participant to reach a certain peak within a short period of time. For example, the coders agreed that a peak TAC of 0.10 could not be attained over a 10 min drinking event. However, such peak value was present after the smoothing technique had been completed. The independent coders evaluated each of the 4 weeks for each of the 59 participants, or 236 weeks. Then, the coders discussed weeks where there was divergence until they agreed on 100% of the peak values from the transdermal alcohol sensors. Figure 1 shows LOESS smoothing over the period of 1 week, with three distinct drinking events. Although the smoothed line eliminates it, raw data overlaid on this figure shows the notable right-handed tail as alcohol is metabolized, which is characteristic of drinking events. Figure 2 shows a week of data wherein the LOESS smoothing identified >30 peaks; the coders, however, determined that this week of data was far too noisy to retain in the dataset and is coded as missing.
Figure 1.
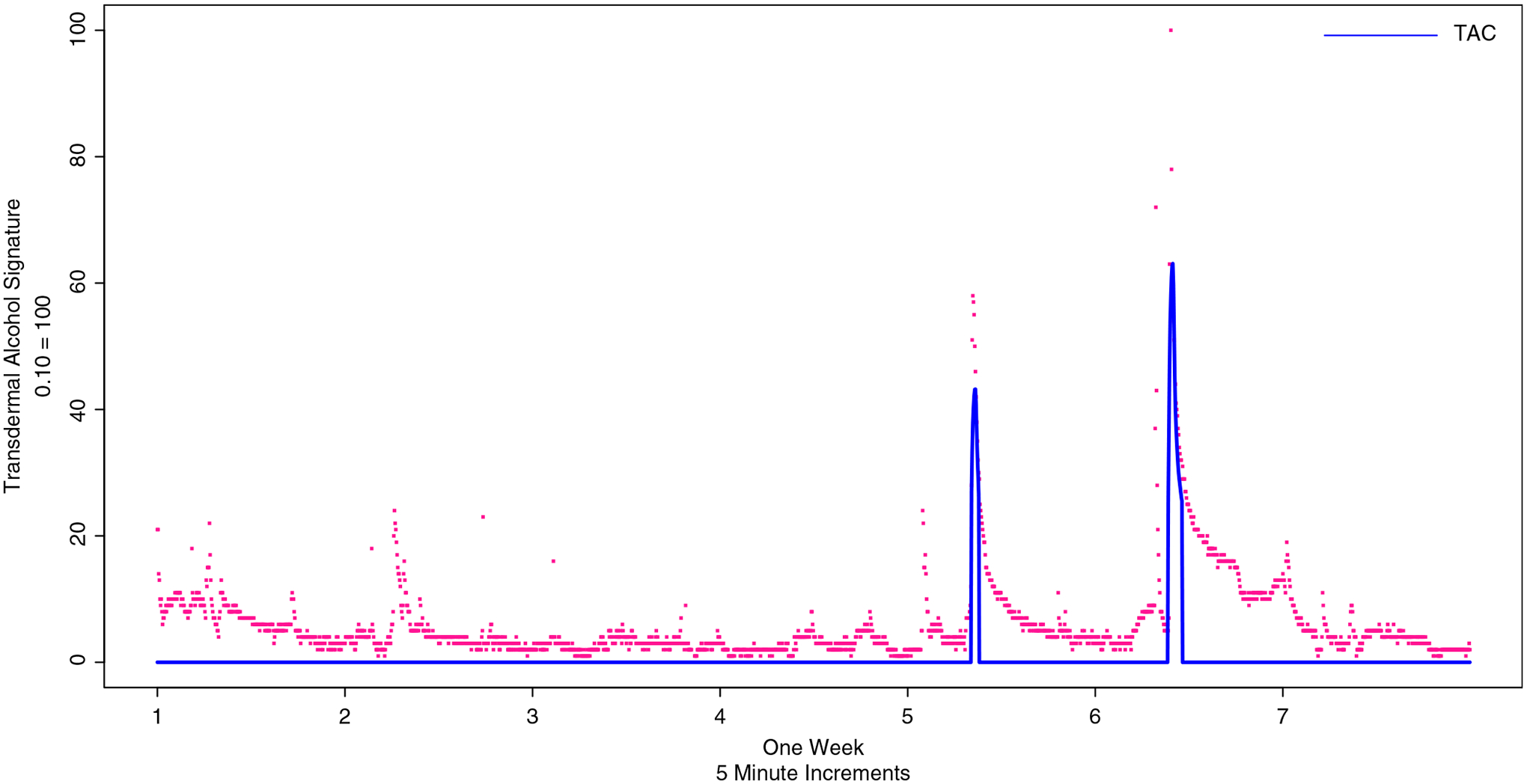
Transdermal alcohol sensor curves, showing adjustment for baseline and locally estimated scatterplot smoothing and prediction in blue and raw data points in red.
Figure 2.
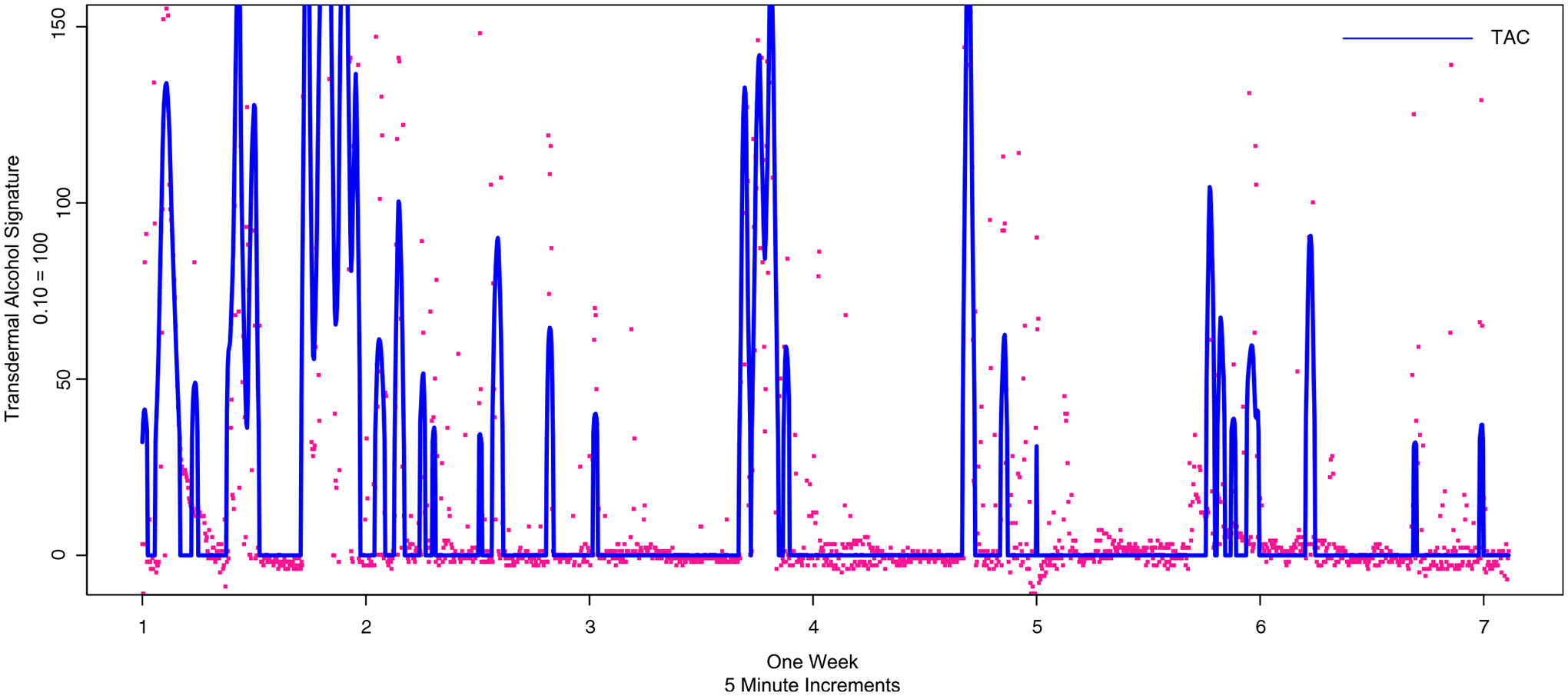
One week of transdermal alcohol data, assessed by coders as noisy sensor data, therefore, coded as missing data. As with Figure 1, adjustment for baseline and locally estimated scatterplot smoothing and prediction in blue and raw data points in red.
After two independent coders assessed graphical peaks from the smoothed data output, peak values were calculated. TAC peaks were identified after the baseline TAC reading was adjusted and after LOESS smoothing; values at or above 0.015 were considered a drinking event and quantified for each day of transdermal alcohol sensor use. Amongst the 57 participants, 471,625 data points were recorded from the transdermal alcohol sensors.
Data analysis
The data to conduct this study included measures described in the sections above. A sub-group of this data with paired eBAC and TAC datapoints were also analysed. Paired-samples t-tests were used to determine differences in demographics and drinking events by age category. Then, paired-samples t-tests were conducted to determine mean differences between computed estimations of eBAC peaks from each of the formulas (Matthews & Miller, Zhang, and NHTSA with and without the metabolic time components) and TAC peaks by age group.
Repeated measures correlations were calculated using the package rmcorr [27] to determine the relationship between peak eBAC and TAC. This correlation calculation accounts for the repeated values by day, clustered by the participant. Using a Fisher r-to-z transformation, we tested if the correlations between the adolescent and young adult groups were significantly different. A linear mixed model was conducted with age group as a predictor, with the method of eBAC measurement nested in participants by day to assess agreement. This was done via the intraclass correlation coefficient (ICC) to determine if age group is a significant predictor of eBAC. A linear mixed model was used to determine the agreement among measures greater than the legal-limit (0.08). Using the legal-limit as a cut-point, accuracy, specificity and sensitivity were analysed between measures, with TAC above 0.08 as the referent positive. Finally, to provide a visual representation of agreement among eBAC and TAC peaks, the best performing formula was plotted with the TAC values using a Bland–Altman Plot.
Results
Participant compliance
Two participants were excluded for large amounts of missing data; suggesting they were not compliant during the trial. Both participants were female adolescents and represented 3.4% of the trial population. All female young adults completed the trial with less than 50% of data missing. One transdermal alcohol sensor was lost by an adolescent participant during this time period; however, she quickly notified the research team, who gave her a new transdermal alcohol sensor to continuously monitor her alcohol use. The 57 participants, who were recruited and consented to participate, completed the trial and were compliant with the study methodology and demonstrated device acceptability having worn the transdermal alcohol sensor over a 1 month study period.
Feasibility
Of the 471,625 data points of continuous alcohol monitoring, 35,803 data points were missing or corrupted. Therefore, the overall percentage of missing or corrupted data points was 7.59%. This equated to 186 whole or partial days of missing data, representing 10.8% of the data collected by day. Missing data varied by the participant age group. Missing data was recorded more frequently among adolescent females at about (11.78% of the daily data collected, 141 missing days/1,197 days collected, n = 40) than young adult females (8.59% of the daily data collected, 45 days missing/524 days collected, n = 17), which was statistically significant even while the composition of the study had more than twice as many younger participants (χ2 = −18.40, P < 0.001).
Missing data from the sensors occurred in 34 days when participants reported drinking events, 1.92% of the total days of monitoring. This occurred in 2% of days (n = 24 days) within the younger group and 1.91% of days (n = 10 days) in the older group. Typical data from a drinking participant appears as clear curves, particularly after the LOESS procedure (see Figure 1). Researchers can identify if sensors have been damaged during data download. The file resulting from a sensor error, if graphed, looks like rain or sporadic data points in Figure 2.
Reliability
Among all participants, WrisTAS collected 243 drinking events, while there were 374 self-reported drinking events. Using weekly totals, participants reported having more drinking events than were recorded by WrisTAS (MSelf-Report = 1.17, SE = 0.048; MWrisTAS = 0.760, SE = 0.064; t = −4.32, df = 7, P = 0.002).
Among all observations, the daily Peak TAC mean differed from nearly every calculated eBAC estimation (Table 2). Furthermore, the repeated measures correlation between TAC and eBAC ranged from 0.20 to 0.24; among adolescents, this range was 0.083 to 0.10 and among young adults 0.37 to 0.39 (Table 2), which were significantly different by age group (P < 0.001). For all observations, the linear mixed model, using the NHTSA (without time/metabolism) indicated that age group was a statistically significant predictor of eBAC (β = 0.013, SE = 0.004, t[df = 66.35] = 3.085, P = 0.002), when clustered by measurement method, date and participant. The ICC for this model was 0.16, indicating poor agreement. The Bland–Altman plot shows that there are several datapoints in which TAC or eBAC was present without the other method; but the difference over increasing measurements is stable and relatively good [Figure 3a].
Table 2.
Analyses of all observed participant days with repeated measures correlations between biosensor detected TAC and self-reported eBAC peaks, correlations difference test between adolescent and young adult, and paired-samples t-test identifying best performing eBAC formula(s) compared to peak TAC values
| Total sample n = 1525, RMC df = 1467 |
Adolescent sample n = 1046, RMC df = 1005 |
Young adult sample n = 479, RMC df = 461 |
Fisher r-to-z transformation correlation difference test between groups | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | RMC | Mean (SD) | RMC | Mean (SD) | RMC | Z, P-value | |
| TAC | 0.017 (0.051) | 0.013 (0.045) | 0.024 (0.062) | ||||
| Matthews & Miller | 0.023 (0.063)** | 0.228** | 0.018 (0.056)* | 0.086** | 0.034 (0.075)** | 0.402** | −6.03, <0.001 |
| Matthews & Miller without metabolism | 0.033 (0.082)** | 0.240** | 0.024 (0.070)** | 0.096** | 0.052 (0.100)** | 0.404** | −5.89, <0.001 |
| Zhang | 0.013 (0.041)* | 0.200** | 0.011 (0.040) | 0.083** | 0.017 (0.044)* | 0.366** | −5.33, <0.001 |
| Zhang without metabolism | 0.023 (0.059)** | 0.228** | 0.017 (0.053)* | 0.100** | 0.035 (0.068)** | 0.389** | −5.51, <0.001 |
| NHTSA | 0.017 (0.047) | 0.201** | 0.013 (0.045) | 0.086** | 0.021 (0.050) | 0.372** | −5.40, <0.001 |
| NHTSA without metabolism | 0.025 (0.064)** | 0.228** | 0.019 (0.058)** | 0.099** | 0.039 (0.075) | 0.389** | −5.52, <0.001 |
Statistically significant difference in TAC peaks from paired-samples t-test at P < 0.05.
Statistically significant difference in TAC peaks from paired-samples t-test at P < 0.01.
P-value for repeated measures correlation tests for H0: r ≠ 0. Bold formulas indicate that all t-test components (n = 3) are not statistically significantly different from TAC means. eBAC, estimate of blood alcohol concentration; NHTSA, National Highway Traffic Safety Administration; RMC, repeated measures correlation; TAC, transdermal alcohol concentration.
Figure 3.
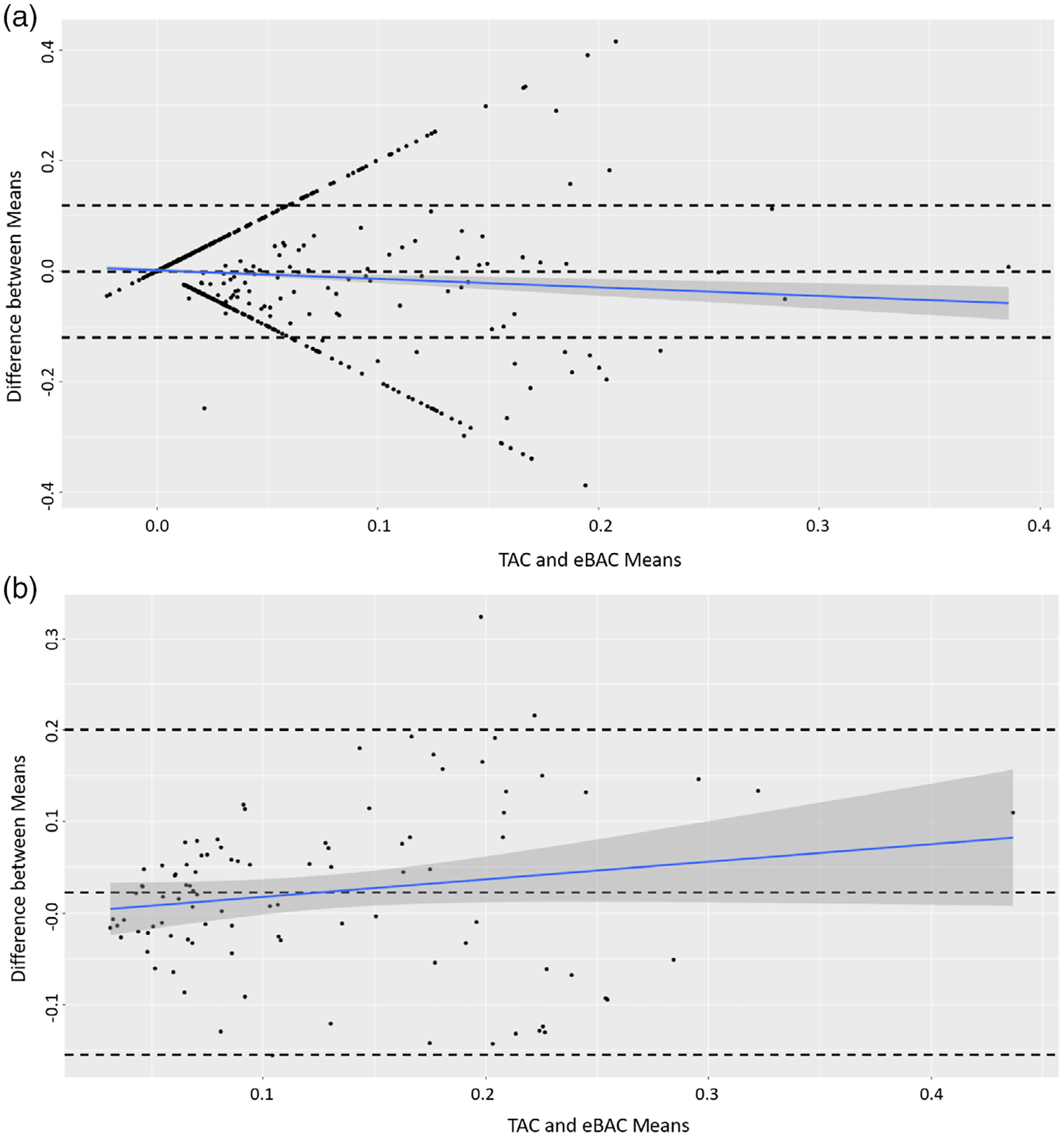
(a) Bland–Altman plot for estimated blood alcohol concentration (eBAC) and transdermal alcohol concentration (TAC) measures. The Bland–Altman plot shows the difference between measures of TAC and eBAC means. This figure uses all daily-recorded measures of TAC and eBAC, which shows little change with increased alcohol measures. (b) Bland–Altman plot showing the difference between measures of TAC and eBAC means. This figure shows only matched events where the sensors detected alcohol concentration in addition to the participant reporting alcohol consumption. This plot shows that the agreement between methods lessened when blood alcohol concentration rose above ~0.2.
Using the dataset with co-occurring method data subset, the mean TAC peak value was closer to eBAC means among all formula estimations. The NHTSA (without time/metabolism) formula’s estimated mean peak values were non-statistically significantly different from the mean TAC peaks among all participants, or by age group (Table 3). Repeated measures correlations for TAC and eBAC were moderate and ranged from 0.49 to 0.57, with the young adult groups’ repeated measures correlations being stronger in all eBAC estimations compared to the adolescent group. Using this data, the linear mixed model’s age group predictor was not statistically significant (β = 0.018, SE = 0.017, t[df = 41.29] = 1.033, P = 0.308), with an ICC of 0.41 showing moderate agreement between measures. The Bland–Altman plot among matched method datapoints shows moderate agreement overall, but as the mean eBAC increases, so does the difference between method measures [Figure 3b].
Table 3.
Analyses of days where matched biosensor detected and self-reported events align with repeated measures correlations between TAC and estimate of blood alcohol concentration peaks, correlations difference test between adolescent and young adult, and paired-samples t-test identifying best performing estimate of blood alcohol concentration formula(s) compared to peak TAC values
| Total sample | Adolescent sample | Young adult sample | Fisher r-to-z transformation, Correlation difference test between groups | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | RMC | Mean (SD) | RMC | Mean (SD) | RMC | Z, P-value | |
| TAC | 0.115 (0.084) n = 96 | 0.103 (0.066) n = 31 | 0.121 (0.092) n = 65 | ||||
| Matthews & Miller | 0.130 (0.107) n = 94 | 0.569** df = 57 | 0.125 (0.108)* n = 31 | 0.214 df = 10 | 0.133 (0.123) n = 63 | 0.607** df = 46 | −1.26, 0.104 |
| Matthews & Miller without metabolism | 0.181 (0.126)** n = 96 | 0.547** df = 59 | 0.170 (0.115)** n = 31 | 0.254 df = 10 | 0.190 (0.132)** n = 65 | 0.581** df = 48 | −1.06, 0.147 |
| Zhang | 0.077 (0.072)** n = 91 | 0.486** df = 55 | 0.087 (0.087) n = 30 | 0.153 df = 10 | 0.072 (0.064)** n = 61 | 0.538** df = 44 | −1.16, 0.123 |
| Zhang without metabolism | 0.125 (0.089) n = 96 | 0.510** df = 59 | 0.127 (0.092)** n = 31 | 0.228 df = 10 | 0.124 (0.088) n = 65 | 0.553** df = 48 | −1.02, 0.154 |
| NHTSA | 0.089 (0.081)** n = 92 | 0.498** df = 56 | 0.099 (0.096) n = 30 | 0.163 df = 10 | 0.084 (0.073)** n = 62 | 0.550** df = 48 | −1.18, 0.119 |
| NHTSA without metabolism | 0.134 (0.097) n = 96 | 0.510** df = 59 | 0.139 (0.101) n = 31 | 0.228 df = 10 | 0.137 (0.097) n = 65 | 0.553** df = 45 | −1.01, 0.156 |
Statistically significant difference in TAC peaks from paired-samples t-test at P < 0.05.
Statistically significant difference in TAC peaks from paired-samples t-test at P < 0.01.
P-value for repeated measures correlation tests for H0: r ≠ 0. Bold formulas indicate that all t-test components (n = 3) are not statistically significantly different from TAC means. NHTSA, National Highway Traffic Safety Administration; RMC, repeated measures correlation; TAC, transdermal alcohol concentration.
The accuracy of capturing true positive events (n = 39), where TAC and eBAC values were greater than 0.08, and true negative events (n = 1005), where both values were less than 0.08, was 85.7%. The specificity among observations was 87.9% with a low rate of false positives (a self-reported event in which the sensor did not register); while sensitivity was 40.0%. Accordingly, the linear mixed model comparing measurement methods had an ICC of 0.71 when eBAC was greater than the legal limit; however, the ICC was much lower (0.06) when eBAC measures were below 0.08.
Discussion
Overall, this study provides proof-of-concept for transdermal alcohol sensors in young women, but suggests that results are less reliable among adolescent females. Adolescents may benefit from other methods of collecting self-report data, like the use of ecological momentary assessments. The context of the study affects social desirability bias. When adolescent participants know that a research team is largely interested in the effects of alcohol use, social desirability biases may lead to increased reporting of alcohol use. By contrast, if self-reported assessments of alcohol use are conducted within school systems, social desirability biases may support under-reporting of alcohol use.
Drinking event frequency was more frequently captured via self-report when compared to the transdermal alcohol sensor. Low-peak events may not have been detected because ethanol transport through the skin has been known to be affected by factors such as skin thickness, temperature and the hydration state of the skin [28]. Because of these factors, lower peak events may not have been detected as smoothing may contribute to or confound this problem. Due to low-peak events not being detected, future studies may take into account that WrisTAS gave a more conservative reported drinking frequency. For this reason, it may be beneficial to depend upon self-reported frequency of use in order to determine ways to reduce the frequency of alcohol use. These results support the use of protocols for considering a combination of self-report and transdermal alcohol sensor data to improve the quality of both measurements [21]. Notably, in the event of agreement between self-report and transdermal alcohol sensor for a drinking event, the TAC and eBAC values correlated moderately (r = 0.49–0.57); exceeding the correlations of previous breath and eBAC studies (r = 0.35) [29]. From these results, we hypothesize that self-report may be better for frequency of drinking events than transdermal alcohol sensors.
Transdermal alcohol sensors may give a more accurate estimation than eBAC as calculated by self-report. Self-reported measures are well tolerated by research participants, and offer insights into frequency and type of alcohol that cannot be ascertained by other technological methods. However, in natural drinking environments, particularly those in private events, eBAC calculations have been inconsistent [29,30]. Participants are also poor judges of alcohol volume [31], an error which varies by drink vessel size [32]. Individuals are not accurate reporters of alcohol use [30,33], in part due to the memory consequences associated with alcohol use, particularly at heavy use levels [34]. Likewise, self-report of alcohol consumption is inaccurate even when recall bias is minimised by inquiring about alcohol consumption within the context of a drinking event [35]. Estimates of eBAC based on self-report also assume that participants are drinking on an empty stomach, which is an additional source of error as alcohol consumption with a meal will disrupt and impede absorption of alcohol [36].
Use of transdermal alcohol sensors is a promising way to improve estimations of peak eBAC which can be compared across developmental periods. This feasibility trial demonstrated that female adolescents had higher rates of missing data than young adults. Greater than 88% of data collected on adolescents and 91% of data on young adults provided continuous, longitudinal data mapping substance use and abuse; which was previously unavailable. This may be due to the recruitment of the present study, which included videos and other visual presentations of what to anticipate. It may also be due to the incentive structure, which rewarded completion of the study by increasing the incentive each week and an incentive for the return of all sensors without damage. We anticipate that missing data and negligence would have been higher without this incentive.
This research demonstrates consistent reliability with previous studies of adults for the use of the WrisTAS in combination with self-report. The repeated measures correlation of peaks from matched self-report and TAC detected drinking events from our sample of adolescent and young adult females are consistent with previous assessments of the WrisTAS monitors of adults in clinical dosing settings (r = 0.610) [37] and self-report by daily diary (r = 0.59) [38]. Likewise, they are in the acceptable range for other self-reported health behaviours (r = 0.55–0.57) [39].
These findings indicate that adolescent females have slightly higher rates of missing data from these sensors compared to the young adult group. Moreover, the total missing rate of 10.8% is much lower than expected given NHTSA’s technical report [12] stating the WrisTAS provided missing or erratic data nearly 67% of the time. These results also provided higher rates of sensor and self-report converge of drinking events from the sensor as previous research had a true-positive rate of 24% [28].
Additionally, the WrisTAS has adjustable Velcro straps, which may have contributed to errors in transdermal alcohol sensor data due to poor fit. Also, the adjustable straps may have influenced the ability to comply with wearing the transdermal alcohol sensor for a whole month, as compared to recent trials of single day transdermal alcohol sensor wear [40].
Future engineering improvements may also positively influence the quality of data collected. Improvements in the technology, including waterproofing and rechargeable batteries, will likely reduce problems associated with missing data among this population. As the technology improves, feasibility and reliability of use among adolescent females should be reassessed. Moreover, the sensors may be more regularly integrated into free-living adolescents, as they are for the assessment of physical activity [41].
Future studies may need to plan for higher rates of missing data or provide additional usage instruction to adolescents, as they may be less careful with the transdermal alcohol sensors than young adults. Lifestyle factors of adolescents, including less access to transportation, may impact the missing data rate. Due to limited access to transportation, these participants may be several days late to participate in their weekly appointments, which increased the potential for loss of battery charge. Therefore, this directly impacted the missing data rate. Moreover, adolescents may have fewer structured daily activities, leading to confusion over days of week of self-reported alcohol use.
Our study indicates promise and the need for additional methodological studies of transdermal alcohol sensors by adolescents and young adults.
Acknowledgements
Research reported in this publication was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant number [P20GM109097].
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- [1].Chen CM, Yoon Y, Faden VB. NIAAA Surveillance report #107: Trends in underage drinking the U.S. 1991–2015. Rockville, M.D.: Alcohol Res, 2017. [Google Scholar]
- [2].Paschall MJ, Grube JW, Black C, Ringwalt CL. Is commercial alcohol availability related to adolescent alcohol sources and alcohol use? Findings from a multi-level study. J Adolesc Health 2007;41:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Epstein JA, Botvin GJ, Diaz T, Schinke SP. The role of social factors and individual characteristics in promoting alcohol use among inner-city minority youths. J Stud Alcohol 1995;56:39–46. [DOI] [PubMed] [Google Scholar]
- [4].Substance Abuse and Mental Health Services Administration. Key indicators in the US: Results from the 2016 National Survey on Drug Use and Health. (HHS publication number SMA 17–5044, NSDUH series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, 2017. [Google Scholar]
- [5].Substance Abuse and Mental Health Services Administration. (2011). Results from the 2010 National Survey on drug use and health: summary of National Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11–4658. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- [6].Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 2011;32:2149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Health and Human Services. Underage drinking: why do adolescents drink, what are the risks, and how can underage drinking be prevented. Alcohol Alert 2006;67:1–7. [Google Scholar]
- [8].Turner A, Pickup J. Diabetes mellitus: biosensors for research and management. Biosensors 1985;1:85–115. [DOI] [PubMed] [Google Scholar]
- [9].Jortberg E, Silva I, Bhatkar V et al. A novel adhesive biosensor system for detecting respiration, cardiac, and limb movement signals during sleep: validation with polysomnography. Nat Sci Sleep 2018;10:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pilehvar S, Wilhelm A, Wilhelm A, King K, Emaminejad S. Emerging wearable technologies for personalized health and performance monitoring. In: George T, Dutta AK, Saif Islam M, eds. Micro-and nanotechnology sensors, systems, and applications X, Vol. 10639. Bellingham, WA: International Society for Optics and Photonics, 2018:106391B. [Google Scholar]
- [11].Ikegwuonu T, Haddow G, Tait J, Murray AF, Kunkler IH. Horizon scanning implanted biosensors in personalising breast cancer management: first pilot study of breast cancer patients views. Health Sci Rep 2018;1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Appelboom G, Camacho E, Abraham ME et al. Smart wearable body sensors for patient self-assessment and monitoring. Arch Public Health 2014;72:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clapp JD, Min JW, Trim RS et al. Predictors of error in estimates of blood alcohol concentration: a replication. J Stud Alcohol Drugs 2009; 70:683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blum RW, Beuhring T, Shew ML, Bearinger LH, Sieving RE, Resnick MD. The effects of race/ethnicity, income, and family structure on adolescent risk behaviors. Am J Public Health 2000;90:1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sobell LC, Sobell MB. Timeline follow-back. In: Measuring alcohol consumption. Totowa, NJ: Humana Press, 1992:41–72. [Google Scholar]
- [16].Matthew DB, Miller WR. Estimating blood alcohol concentration: two computer programs and their applications in therapy and research. Addict Behav 1979;4:55–60. [DOI] [PubMed] [Google Scholar]
- [17].National Highway Transportation Safety Administration. Computing a BAC estimate. Washington D.C.: Department of Transportation, 1994. [Google Scholar]
- [18].Zhang Y, Wu C, Wan J. Development and validation of a model to predict blood alcohol concentrations: updating the NHTSA equation. Addict Behav 2017;71:46–53. [DOI] [PubMed] [Google Scholar]
- [19].Brown DJ. A method for determining the excretion of volatile substances through skin. Methods Find Exp Clin Pharmacol 1985;7:269–74. [PubMed] [Google Scholar]
- [20].Marques PR., McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res 2009;33:703–71. [DOI] [PubMed] [Google Scholar]
- [21].Leffingwell TR, Cooney NJ, Murphy JG et al. Continuous objective monitoring of alcohol use: twenty-first century measurement using transdermal sensors. Alcohol Clin Exp Res 2013;37:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Simons JS, Wills TA, Emery NN, Marks RM. Quantifying alcohol consumption: self-report, transdermal assessment, and prediction of dependence symptoms. Addict Behav 2015;50:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giner Labs. Wrist Transdermal Alcohol Sensor – WrisTAS. Available at: https://www.ginerinc.com/wrist-transdermal-alcohol-sensor (accessed November 2018).
- [24].Cleveland WS, Devlin SJ. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1998;83:596–610. [Google Scholar]
- [25].Hertzberg V, Mac V, Elon L et al. Novel analytic methods needed for real-time continuous core body temperature data. West J Nurs Res 2016; 39:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carpenter RW, Trela CJ, Lane SP, Wood PK, Piasecki TM, Trull TJ. Elevated rate of alcohol consumption in borderline personality disorder patients in daily life. Psychopharmacology (Berl) 2017;234:3395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marques PR, McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res 2009;33:703–11. [DOI] [PubMed] [Google Scholar]
- [29].Clapp JD, Min JW, Shillington AM, Reed MB, Lange JE, Holmes MR. Environmental and individual predictors of error in field estimates of blood alcohol concentration: a multilevel analysis. J Stud Alcohol 2006; 67:620–7. [DOI] [PubMed] [Google Scholar]
- [30].Clapp JD, Reed MB, Min JW et al. Blood alcohol concentrations among bar patrons: a multi-level study of drinking behavior. Drug Alcohol Depend 2009;102:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaskutas LA, Graves K. An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse 2000;12:67–78. [DOI] [PubMed] [Google Scholar]
- [32].White AM, Kraus CL, McCracken LA, Swartzwelder HS. Do college students drink more than they think? Use of a free-pour paradigm to determine how college students define standard drinks. Alcohol Clin Exp Res 2003;27:1750–6. [DOI] [PubMed] [Google Scholar]
- [33].Clapp JD, Holmes MR, Reed MB, Shillington AM, Freisthler B, Lange JE. Measuring college students’ alcohol consumption in natural drinking environments: field methodologies for bars and parties. Eval Rev 2007;31:469–89. [DOI] [PubMed] [Google Scholar]
- [34].Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. J Psychopharmacol 2003;165:306–12. [DOI] [PubMed] [Google Scholar]
- [35].Clapp JD, Ketchie JM, Reed MB, Shillington AM, Lange JE, Holmes MR. Three exploratory studies of college theme parties. Drug Alcohol Rev 2008;27:509–18. [DOI] [PubMed] [Google Scholar]
- [36].National Institute of Alcohol Abuse and Alcoholism. Alcohol alert: alcohol metabolism. No. 35, PH 371. Bethesda, MD: The Institute, 1997. [Google Scholar]
- [37].Swift RM, Martin C, Swette L, LaConti A, Kackley N. Studies on a wearable, electronic, transdermal alcohol sensor. Alcohol Clin Exp Res 1992;16:721–5. [DOI] [PubMed] [Google Scholar]
- [38].Swift RM, Grenga A, Tempelman L, Moeller M. Transdermal alcohol detection vs. self report for determining alcohol drinking in non-alcoholic, alcohol drinkers. Alcohol Clin Exp Res 2010;34:326A Abstract no. 901. [Google Scholar]
- [39].Kristal AR, Feng Z, Coates RJ, Oberman A, George V. Associations of race/ethnicity, education, and dietary intervention with the validity and reliability of a food frequency questionnaire: the Women’s health trial feasibility study in minority populations. Am J Epidemiol 1997;146:856–69. [DOI] [PubMed] [Google Scholar]
- [40].Clapp JD, Madden DR, Mooney DD, Dahlquist KE. Examining the social ecology of a bar-crawl: an exploratory pilot study. PLoS One 2017;12:e0185238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schneider M, Chau L. Validation of the Fitbit zip for monitoring physical activity among free-living adolescents. BMC Res Notes 2016; 9:448. [DOI] [PMC free article] [PubMed] [Google Scholar]


