Abstract
Background:
The technology enabled distributed model in Kerala is based on an innovative partnership model between Karkinos Healthcare and private health centers. The model is designed to address the barriers to cancer screening by generating demand and by bringing together the private health centers and service providers at various levels to create a network for continued care. This paper describes the implementation process and presents some preliminary findings.
Methods:
The model follows the hub-and-spoke and further spoke framework. In the pilot phases, from July 2021 to December 2021, five private health centers (partners) collaborated with Karkinos Healthcare across two districts in Kerala. Screening camps were organized across the districts at the community level where the target groups were administered a risk assessment questionnaire followed by screening tests at the spoke hospitals based on a defined clinical protocol. The screened positive patients were examined further for confirmatory diagnosis at the spoke centers. Patients requiring chemotherapy or minor surgeries were treated at the spokes. For radiation therapy and complex surgeries the patients were referred to the hubs.
Results:
A total of 2,459 individuals were screened for cancer at the spokes and 299 were screened positive. Capacity was built at the spokes for cancer surgery and chemotherapy. A total of 189 chemotherapy sessions and 17 surgeries were performed at the spokes for cancer patients. 70 patients were referred to the hub.
Conclusion:
Initial results demonstrate the ability of the technology Distributed Cancer Care Network (DCCN) system to successfully screen and detect cancer and to converge the actions of various private health facilities towards providing a continuum of cancer care. The lessons learnt from this study will be useful for replicating the process in other States.
Key Words: Cancer, early detection, screening, technology, India, - Kerala, hub and spoke model, pandemic
Introduction
As of 2016, cancer contributed to 8% of overall deaths in India (Dhillon et al., 2018). While cervical and lip and oral cavity cancer are the most common cancers in India, the cases of breast cancer are increasing rapidly (Chaturvedi et al., 2015; Dhillon et al., 2018; Dsouza et al., 2013b; Mallath et al., 2014). Kerala has emerged as the hotspot with the incidence of cancer cases increasing from 74 per 100,000 in 1990 to 135 per 100,000 in 2016 which is more than all India incidence rate of 81·2 per 100,000 (Dhillon et al., 2018). Notably, while the mortality burden in India due to cancer is expected to increase by 19% over 2011 to 2026, the mortality burden in Kerala will increase by 30%(Dsouza et al., 2013a). The primary reason for increase in cancer cases in Kerala is an ageing population with increase in longevity and unhealthy dietary habits (Kaur et al., 2014; Sugathan et al., 2008) .
Several studies have confirmed the effectiveness of screening in preventing cancer cases (Bretthauer and Kalager, 2013; Gøtzsche and Nielsen, 2009) which could lead to higher detection rates at an early stage (Adab et al. , 2020). Notably, the increase in screening rate is associated with mortality reduction (Jansen et al., 2020; Jun et al., 2017; Sankaranarayanan et al., 2005, Sankaranarayanan et al., 2007). Towards this end, the government of India has introduced mandatory screening for three types of cancer-breast, cervical and oral at the Primary Health Centre (PHC) across several districts through the National Programme for the Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS) (NHM, 2016). Despite these efforts, early detection of cancer remains a key challenge in India as screening rates remain low. As per the recently released NFHS data, the number of women in 15-49 age group screened for cancer remains very low which undermine the effort to control cancer at an early stage (IIPS, 2021). In addition, there is a paucity of data on number of patients screened for cancer in India. The government budget is diverted primarily towards curative services rather than preventive services (Mather et al., 2002; Pramesh et al., 2014).
It is well recognized that primary screening is the first step towards early identification but the other major challenge is the delay in presentation and subsequent follow-ups by the patients (Sharma et al., 2012). The lack of co-ordination between various tiers of the health system and a dilapidated infrastructure which do not have screening and diagnostic tools results in delays in sharing of results, late-stage diagnosis and treatment, inequitable cancer care and higher cost to patients (Pramesh et al., 2014; Sullivan et al., 2015; Vinet and Zhedanov, 2011). The government hospitals especially in rural areas do not have enough specialists which prompt the patients to seek treatment from local practitioners and quacks. Particularly, in case of cancer care even a delay of few months in treatment is associated with transition to an advanced stage and mortality.
To ensure efficiency in delivery of cancer care services, introduction of technology enabled solutions in the health systems can prove effective in improving the communication between a) patient and providers, b) various providers at different levels c) system and providers and d) support patients (Clauser et al., 2011; Wallace, 2007).
To elaborate, information technology provides patients the opportunity to virtually connect with service providers, discuss symptoms and also manage mild symptoms in remote areas. Advances in information technology has the potential to improve the cancer diagnostic and delivery of services by integrating the tasks of the primary care providers and specialty providers, who usually fail to co-ordinate, by creating a centralized repository of records. Also, efficiency of the system can be vastly improved by keeping a stock of equipment, tools and medicines at various locations based on forecasted needs. Consequently, the patients are empowered when their history of treatment is readily available in the system and could be navigated to the facilities best suited for their needs. As a result, treatment time will come down as patients receive reminders and book appointments digitally.
Realizing the potential of technology to integrate the health systems and improve cancer care, the Karkinos Healthcare, in July 2021, introduced a Distributed Cancer Care Model (DCCM) in Kerala with a pilot in five private health facilities across two districts - Ernakulam and Idukki.
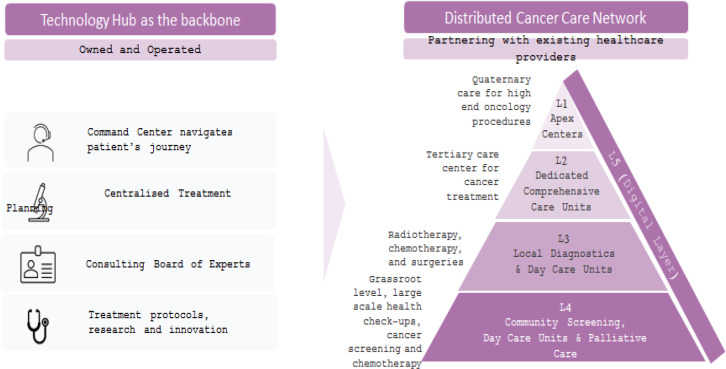
The DCCM is developed with the rationale that using a technology-enabled data platform the knowledge architecture can be centralised and the delivery systems can be democratised and distributed. DCCM intends to create a hierarchy of care that connects various care centers depending on the complexity of care. Starting from a center that can provide care for screening and early detection to progressively more complex care, Karkinos creates a mesh or connected network of experts and care centers that ensures that all levels and aspects of care along with right guidance and support is available to the patient within the network of Karkinos (Figure 1).
Figure 1.
A Distributed Cancer Care Model, Powered by Technology Platform
The DCCM predominantly leverages a tiered hub-and-spoke and further spoke hierarchy model with each spoke providing appropriate cancer care services such as screening, diagnostic and day care services closer to people’s homes. Karkinos Healthcare through its awareness campaigns, digital applications for patient and citizen engagement, network of early detection centers and large-scale community screening programs aims to proactively identify the at-risk population. The focus of the programme was initially was to enrol the eligible population for cancer screening, and provide further care coordination to those screened positive at the spokes for oncologist consultation, diagnosis, counselling and appropriate treatment.
Given this background, the overall aim of this study is to describe the implementation of the distributed cancer care model in selected districts of Kerala and to present some preliminary results in terms of number of cancer screenings completed, detection rates, early diagnosed and existing cancer patients under treatment. Experience of healthcare providers is also presented to highlight the significance of Karkinos DCCM in solving accessibility, delayed diagnosis and information availability gaps in cancer care.
Materials and Methods
Study Setting
Programme description
The DCCM was implemented in two districts of Kerala with Kochi city in Ernakulam district as the geographical hub and smaller towns in Ernakulam and Idukki districts with the spokes and further spokes. The DCCM has a technology and knowledge driven architecture which enables patients to have a standard experience across all centers, get diagnosed early and referred to the right center for continued treatment. DCCM focuses on working with existing private health care providers through private partnerships and vertical investments in machinery (Table 1).
Table 1.
List of Partnerships and Oncology Services Offered
| Community engagement | Early detection and wellness | Diagnosis | Treatment (chemo) | Treatment (radiation oncology) |
Treatment (surgical oncology) |
|
|---|---|---|---|---|---|---|
| Rajagiri Hospital, Ernakulam (L2) | Yes | Yes | Yes | Yes | ||
| Chazhikattu Multi-specialty hospital, Idukki (L3) | Yes | Yes | Yes | Yes | Yes | |
| Mar Besilios Medical Mission hospital, Ernakulam (L3) | Yes | Yes | Yes | Yes | Yes | |
| Munnar Tata hospital, Idukki (L4) | Yes | Yes | Yes | Yes | ||
| SD Tata hospital, Ernakulam (L4) | Yes | Yes |
The proposed DCCM segments cancer care across five levels (L1 to L5) supported by a centralized system (command center) (Figure 1).
Level 5 (L5) is the digital layer, where citizens who have probable symptoms of cancer complete pre-screening using a mobile application and are facilitated through a command center to receive further guidance to visit the nearest screening and diagnostic facility or even teleconsult. The L5 layer connects together all the layers of DCCM.
Level 4 (L4) is the screening and early diagnosis facility at primary health center or medical colleges or private hospitals, where people walk in or referred through digital initiatives for screening, diagnosis and treatment. Chemotherapy and Palliative care are provided at level 4 layer.
Level 3 (L3) centers provides diagnosis using invasive methods, day care services and cancer surgeries for common forms of cancer such as oral, breast, cervical and colorectal cancers.
Level 2 (L2) are dedicated comprehensive care units which handle less complex cancer cases, which have been staged and focus on frequent diagnostic and therapeutic procedures.
Level 1 (L1) are apex centers which are well recognized or established centers providing a range of cancer care services including complex treatments.
L1 is the superhub, L2s are the hub and L3s, L4s and L5s are the spokes and further spokes in the DCCM hierarchy.
Implementation on ground
One referral center (hub) and four pilot centres (spokes) were onboarded sequentially during July 2021 to December 2021, with interventions at different stages advancement. Table 1 below lists current partnerships.
The pilot execution is at its first phase of implementation in two districts of Kerala – Ernakulam and Idukki. The Karkinos Cancer Center in Chazhikattu Multi-Speciality (CMS) Hospital in Idukki District (an L3 center) was launched in July 20, 2021. All the cancer treatments were performed outside the Idduki district before the Karkinos Cancer Center was established in Idukki district.
For regular radiology, pathology tests and some treatment modalities, the patient care is provided at the L4/L3 hospital (shown in Table 1) but for staging purposes, which requires a PET CT to be done, the patients are referred to the nearest L2 which is Rajagiri Hospital in Ernakulam District. The availability of high-end diagnostic equipment for confirmatory diagnosis and staging along with and treatment modalities such as Radiotherapy makes Rajagiri Hospital a referral centre for the cancer centres. Among the hub and spoke centers, the attempt is to ensure a seamless patient experience in terms of the same care coordinator ensuring navigation, one-stop payment system at Karkinos, standardized pricing, and quality of care, driven by technology, thus enabling a distributed cancer care network for cancer care.
Patient Navigation
Command center is the centralized knowledge network across multiple hospitals which supports process workflows for various patient services. It is the central pillar coordinating and orchestrating every step of the care that includes scheduling appointments, reminding the team, distributing patient reports, notifying the right people about the next steps, documenting adequate data and ensuring the accuracy of the patient data recorded at every step of the journey. It is the backbone of patient navigation that enables smooth, transparent communication as well as maintain the human element that is based on personal comfort and empathy.
The Karkinos central command center has supported process workflows for various patient services between the centers. It has ensured end-to-end patient navigation so that the patient drop offs are minimized ensuring patient compliance.
Target Group
The primary target groups include those who are at high risk of developing breast cancer, oral cancer, cervical cancer and colorectal cancer identified from the digital risk assessment questionnaire. The criteria for risk assessment for oral, breast, cervix and colorectal is presented in Table 2 along with clinical examinations for specific age groups for different cancers.
Table 2.
Diagnostic Tests for Identifying Cancer Suspects
| Site | Age/Eligibility | Screening modality |
|---|---|---|
| Breast | Asymptomatic: | Awareness with periodic breast self-examination |
| 30-74 years | Screening clinical breast examination | |
| High risk of breast cancer: age not a criterion | Awareness with periodic breast self-examination Screening clinical breast examination Bilateral Mammogram and / or USG |
|
| Cervix | 30-65 years | HPV test |
| Oral | 30-74 years with risk factors /symptoms | COE (Clinical Oral Examination) |
| Colon | 45-74 years | Fecal Immuno-chemical Test |
| Symptomatic patients/ High risk | Fecal Immuno-chemical Test |
Outcome variable and Analysis
We present only aggregate results in this study since the programme is still in the pilot mode. Primary outcomes include the number of people who were screened and underwent further diagnosis. Secondary outcomes include patients who received treatment such as surgery and chemotherapy at the spoke centers and number of patient referred to the hub center for further treatment. In addition, we also provide anecdotal evidence based on discussion with health service providers at the various facilities.
Ethics and Dissemination
Written consent was taken from all the participants before administering the questionnaire , screening and collecting the information. The identity of patients and healthcare providers will not be disclosed and no identifiable data will be published.
Results
Table 2 presents the programmatic indicators. Between July to December 2021, a total of 2,459 individuals were screened for cancer (oral, breast, cervical and colorectal cancers): 904 at CMS Hospital, 97 people at Mar Besilios Medical Mission (MBMM) Hospital, 1312 at Munnar Tata (MT) Hospital and 146 at SD Tata (SDT) Hospital.
299 were screened positive: 107 suspects at CMS Hospital, Idukki, 50 at MBMM Hospital, 123 at MT Hospital and 19 at SDT Hospital.
Out of 299 suspects, 145 patients were asked to perform further diagnostic tests and 130 patients were kept under regular follow up period (1, 3 or 6 months) as per the doctors instructions. Out of 145 patients, 46 patients performed the investigations. Regular efforts are being made to ensure compliance to further clinical investigations in order to improve outcomes.
Existing patients who would have otherwise travelled to cities inside or outside Kerala, received care at the spoke hospitals nearer to their homes. While 11 surgeries were done in CMS Hospital and 6 surgeries were done in MBMM Hospital, a total of 189 chemotherapy sessions were done at all the spoke centers. In total, 70 patients were referred either from the spoke centers or directly from the command center to Rajagiri Hospital for consultation, diagnosis and treatment including radiotherapies.
The key outcomes of the Karkinos DCCM based on the discussion with various health workers are as follows:
Enhance access to cancer diagnosis and treatment
There are several issues that arise due to inaccessibility of cancer care services. Most of the tertiary cancer care centres in India are in the cities. There is a huge delay in diagnosis and/or treatment during presentation. The availability of cancer care closer to homes has helped in early detection, diagnosis and treatment of cancer, leading to improved outcomes.
Treatment outcomes
Karkinos Healthcare engages with the patient in the early stages of the disease, provides contextualized and comprehensive patient demographic and clinical information. This enables clinicians to arrive at a comprehensive approach to disease treatment and progression monitoring quickly and avoid errors and duplications in prescriptions and procedures, ensuring better treatment outcomes for the patient. Expert oncology guidance from world renowned oncologists through the virtual tumor board is creating benefit of scale by pooling cases across DCCN partners.
Improved patient experience
The central command center of Karkinos Healthcare has ensured efficient patient navigation and visit management, which has helped accelerate the overall workflow of healthcare delivery to reduce patient wait times while enabling better utilization of health professionals’ time and hospital resources.
Following are some of the success stories based on the discussion with the health personnel
Case 1
A 50 year old postmenopausal lady who screened positive for breast cancer at SDTT was referred to Rajagiri Hospital for confirmatory tests. She was diagnosed with Invasive lobular Carcinoma of the Left Breast Underwent Surgery in Rajagiri Hosptial and is now on chemotherapy at SDT Hospital.
Case 2
Patient who had a history of vaginal infection and discharge was detected HPV test positive at MBMM Hospital. For further diagnoses, colposcopy was done at MBMM Hospital and biopsy taken from posterior lip of cervix and right lateral fornix was send to Karkinos lab. Result is positive and patient will now undergo loop electrical excision procedure.
Case 3
Patient with no symptoms and none expressed medical complaint tested HPV positive. Colposcopy was performed at SDT hospital. Punch biopsy taken from cervix was sent to Karkinos Lab. Impression – hyperplastic squamous mucosa with chronic cervicitis. Patient will now undergo Loop electrical excision procedure.
Case 4
Patient with no symptoms but tested positive for colon cancer. Command Center navigated patient to Rajagiri hospital for colonoscopy procedure. Colonoscopy was done at Rajagiri Hospital followed by polypectomy. Specimen was taken for biopsy in Rajagiri hospital but for a second opinion, slide was reviewed at Karkinos lab. Follow-up visit in clinic is advised after 3 months.
Case 5
Patient enquired Karkinos command center about mammogram appointment as she found lump in breasts. Appointment for bilateral mammogram was taken by command center at Rajagiri Hospital, as this was the nearest Karkinos center from her residence. Mammogram reported positive (BIRADS IV). Patient visited Karkinos expert for discussing further treatment plan who advised Trucut biopsy of left UIQ breast lesion. USG guided trucut biopsy of right UOQ breast lesion was done. Biopsy and Whole body PET-CT scan done at Rajagiri Hospital. Breast Surgery was done at Rajagiri Hospital (Right modified radical mastectomy, sentinal lymph node biopsy and left modified radical mastectomy ; and axillary lymph node dissection). Adjuvant chemotherapy and hormone therapy was advised post-surgery. Patient on chemotherapy at SDTT center.
Table 3.
Programmatic Indicators for the Spoke Hospitals, July to December 2021
| No of people screened | No of screened positives | Chemotherapy sessions | Surgery | |
|---|---|---|---|---|
| Chazhikattu Multi-specialty hospital | 904 | 107 | 84 | 11 |
| Mar Besilios Medical Mission hospital | 97 | 50 | 76 | 6 |
| Munnar Tata Hospital | 1312 | 123 | 8 | 0 |
| SD Tata hospital, Ernakulam | 146 | 19 | 21 | 0 |
Discussion
Introduction of cancer screening programme is associated with reduction in cancer related mortality rates as detection and care during early stage vastly improves the chances of survival (Hofvind et al., 2013; Jansen et al., 2020). In this study we have elaborated on the implementation process and success of a distributed model in Kerala to not only improve cancer screening but also provide an easy and accessible pathway for the screened positive with the help of patient navigation from the command center. Some of previous studies (Clauser et al., 2011; Wallace, 2007) emphasizes the role of technology to improve the delivery of services and patient experience, there is limited evidence on implementation outcomes of a centralized process which aims to integrate the facilities providing services with help of a technology support system.
An important achievement, therefore, in the distributed cancer care model envisaged has been the ability of Karkinos to integrate with the partners’ HIS. We have taken a technology-first approach across the patients and providers touchpoints. Inputs from the the risk assessment and screening tests is collected digitally and stored at the Karkinos command center. A central patient orchestration is deployed to ensure information access to the patients, seamless navigation, and real time feedback to improve the quality of care. The command center staff communicates the results back to the patients and provides further navigation for the screened postive at the nearest Karkinos center for a consultation. The information to the partner hospital’s about the patients’ visit is communicated directly into the Hospital Information System (HIS) and an appointment is scheduled. All the longitudinal data of the patient is also passed on the HIS for the consulting doctor to review. Bi-laterally, the information from the partners’ HIS about the patients consultation and tests is communicated back to the Karkinos command center for record and further patient navigation or counselling, treatment planning, programme monitoring etc. as required.
The initial data and experiences indicate that the platform has been useful for those in need. For many patients who have previously gone through multiple consultations, the distributed cancer care model has been instrumental in saving time as well as reducing out-of-pocket expenditure due to closer access to care. Generally, cancer suspected or diagnosed patients rely on cancer centers located at the cities. This creates accessibility issues which is one of the reasons for delay in diagnosis and treatment, and the COVID-19 pandemic made this situation worse across India, particularly in smaller towns and rural areas (Pramesh et al., 2021) such as Idukki district with no cancer centers nearby.
One of the key findings which has emerged from the study is the improve in access to cancer care in smaller towns during the COVID-19 pandemic. The frequent lockdowns, restricted travels and fear of contracting COVID-19 prevented patents from travelling to cancer centers for diagnosis, treatment and follow-up (Ranganathan et al., 2021). The risk of delays could potentially lead to tumor progression and poorer outcomes.
In addition, tele-consultation mode through the command center was leveraged to serve the patients residing in remote areas and also to provide guidance to junior oncologists. In fact, technology enabled system has ensured disease treatment and progression monitoring quickly. The model has also resulted in minimizing errors and duplications in prescriptions and procedures thereby ensuring better treatment outcomes for the patient.
The prospects of developing cancer care facilities in Low-and-Middle-Income Countries (LMICs), given the economic constraints, are challenging, decentralizing the delivery of cancer care is emerging as a popular mechanism to meet the growing need for cancer care. AMPATH-Oncology program in sub-Saharan Africa highlights the successful application of the hub-and-spoke model in a resource-constrained setting to deliver cancer care at a relatively low cost (Strother et al., 2013). Where availability of high skilled manpower in oncology is a major challenge in High-Income Countries (HICs), technological advancements have enabled to bridge this gap. Tele-oncology has further facilitated the development of a distributed model of cancer care. The Queensland Remote Chemotherapy Supervision (QReCS) and Townsville Teleoncology Network have implemented teleoncology models where chemotherapy is administered to patients in rural towns under supervision by health professionals from larger centers using teleoncology (supervised 327 cycles of chemotherapy and systemic therapy regimens for 62 patients). The result of implementing such a model of cancer care delivery has enhanced access to chemotherapy services (MacDonald et al., 2010; Sabesan et al., 2018). Munson Healthcare’s hub and spoke model utilizes advanced practice providers (APPs) – trained nurse practitioners and physician assistants – to provide assistance throughout a patient’s treatment journey (LaRaia and Worden, 2020).
The findings suggests that DCCM has decentralized cancer care through a hub-and-spoke and further model of service delivery. It has been successful in addressing the several issues that arise due to information assymmetry and inaccessibility of cancer care services.
Limitations
This study has certain limitations. First, rigorous analysis of data has not been undertaken since the model is being still implemented. Although, we have presented preliminary findings based on data of five facilities the process of integrating other facilities is being carried on. It remains a future endeavor to follow the patient trajectory and mortality outcomes. Also, the study design does not consider the situation prior to implementation of the model which might have provided valuable insights in the number of cases being detected at various stages of cancer at different facilities. As a priori, it is expected that the number of patients being detected at initial stages should be higher in the post implementation phase. Finally, the study does not elaborate much on the challenges experienced during the implementation process of the DCCN model.
In conclusion, the proposed model could be replicated across in several States of India which are reporting high burden of cancer. Developing a structured network of cancer care delivery requires a combination of clinical aspects of cancer as well as an integrated approach to building capability and capacity which can be facilitated using right technology platforms. The referrals from the Karkinos patient engagement applications and network of early detection, diagnostic and treatment centers can significantly reduce the load at tertiary centers.
Technology led cancer screening interventions are very important steps in controlling the rising cancer cases. Providing technological support to the health facilities enable them to serve the cancer patients more efficiently. This research brings evidence about the manner in which technology could be used to enhance both patient and provider level experience. The research also lays the foundation to design and conduct studies aimed at evaluating the impact of such models which would improve the implementation process in future.
Author Contribution Statement
Conception and design: TNA, MA, RS, MAK, RK, SR, AP and RV conceptualized and designed the study; Implementation and data collection: AP, BKS, KO, SR, RSK, MS, MAK were part of the implementation team; Data analysis and interpretation: SP, RV, AP, AK and KO; Manuscript writing: RV wrote the first draft of the manuscript. AP and AK contributed to the writing of the manuscript and revised the draft. BKS, KO, SR, RSK, MS, MAK and RS wrote sections of the manuscript. TNA, MA, RS, MAK and RK provided the overall supervision.
Acknowledgment
Study approval
This study is a part of doctoral thesis of R Venkataramanan. The thesis synopsis and methods were approved by the research committee constituted at the University of Warwick.
Ethics Statement
Written consent was taken from all the participants before administering the questionnaire and collecting the information. The identity of patients and healthcare providers will not be disclosed and no identifiable data will be published.
Disclosure Statement
No potential conflict of interest declared.
References
- Adab P, McGhee SM, Yanova J, Wong CM, Hedley AJ. Effectiveness and efficiency of opportunistic cervical cancer screening comparison with organized screening. Med Care. 2004;42:600–9. doi: 10.1097/01.mlr.0000128007.04494.29. [DOI] [PubMed] [Google Scholar]
- Bretthauer M, Kalager M. Principles, effectiveness and caveats in screening for cancer. Br J Surg. 2013;100:55–65. doi: 10.1002/bjs.8995. [DOI] [PubMed] [Google Scholar]
- Chaturvedi M, Vaitheeswaran K, Satishkumar K, et al. Time trends in breast cancer among Indian women population: An analysis of population based cancer registry data. Indian J Surg Oncol. 2015;6:427–34. doi: 10.1007/s13193-015-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauser SB, Wagner EH, Aiello Bowles EJ, Tuzzio L, Greene SM. Improving modern cancer care through information technology. Am J Prev Med. 2011:40. doi: 10.1016/j.amepre.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dsouza NDR, Murthy NS, Aras RY. Projection of burden of cancer mortality for india, 2011-2026. Asian Pac J Cancer Prev. 2013a;14:4387–92. [PubMed] [Google Scholar]
- Dsouza NDR, Murthy NS, Aras RY. Projection of cancer incident cases for india - till 2026. Asian Pac J Cancer Prev. 2013b;14:4379–86. [PubMed] [Google Scholar]
- Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2009:4. doi: 10.1002/14651858.CD001877.pub3. [DOI] [PubMed] [Google Scholar]
- Hofvind S, Ursin G, Tretli S, Møller B. Breast cancer mortality in participants of the Norwegian Breast Cancer Screening Program. Cancer. 2013;19:3106–12. doi: 10.1002/cncr.28174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EEL, Zielonke N, Gini A, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. 2020;2020:207–23. doi: 10.1016/j.ejca.2019.12.013. [DOI] [PubMed] [Google Scholar]
- Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology. 2017;152:1319–28. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Kaur J, Singh S, Kaur K. Impact of age on the prevalence of chronic diseases in geriatric population. Int Res J Biol Sci. 2014;3:79–85. [Google Scholar]
- LaRaia K, Worden KG. A rural healthcare system expands cancer care with a “Hub and Spoke” model. Oncol Issues. 2020;35:38–44. [Google Scholar]
- Mallath MK, Taylor DG, Badwe RA, et al. The growing burden of cancer in India: Epidemiology and social context. Lancet Oncol. 2014;15 : 70115 –9. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- Mather I, Ramaiah S, Crowley P. Private health care in developing countries. BMJ. 2002;324:47. [PMC free article] [PubMed] [Google Scholar]
- Pramesh CS, Badwe RA, Borthakur BB, et al. Delivery of affordable and equitable cancer care in India. Lancet Oncol. 2014:15 . doi: 10.1016/S1470-2045(14)70117-2. [DOI] [PubMed] [Google Scholar]
- Sabesan S, Senko C, Schmidt A, et al. Enhancing chemotherapy capabilities in rural hospitals: Implementation of a telechemotherapy model (QReCS) in North Queensland, Australia. J Oncol Pract. 2018;14:429–37. doi: 10.1200/JOP.18.00110. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Esmy PO, Rajkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Ramadas K, Thomas G, et al. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–33. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- Sharma K, Costas A, Shulman LN, Meara JG. A systematic review of barriers to breast cancer care in developing countries resulting in delayed patient presentation. J Oncol. 2012;2012 doi: 10.1155/2012/121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaram S, Majumdar G, Perin D, et al. Population-based cancer screening programmes in low-income and middle-income countries: regional consultation of the International Cancer Screening Network in India. Lancet Oncol. 2018;19:113–22. doi: 10.1016/S1470-2045(18)30003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother RM, Asirwa FC, Busakhala NB, et al. AMPATH-Oncology: A model for comprehensive cancer care in sub-Saharan Africa. J Cancer Policy. 2013;1:42–8. [Google Scholar]
- Sugathan TN, Soman CR, Sankaranarayanan K. Behavioural risk factors for non communicable diseases among adults in Kerala, India. Indian J Med Res. 2008;127:555–63. [PubMed] [Google Scholar]
- Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16:1193–1224. doi: 10.1016/S1470-2045(15)00223-5. [DOI] [PubMed] [Google Scholar]
- Wallace PJ. Reshaping cancer learning through the use of health information technology. Health Aff. 2007;26 doi: 10.1377/hlthaff.26.2.w169. https://doi.org/10.1377/hlthaff.26.2.w169. [DOI] [PubMed] [Google Scholar]