Abstract
Objective:
The objective of this study was to determine the efficacy of curcumin in combination with intralesional dexamethasone with hyaluronidase in the treatment of oral submucous fibrosis (OSF).
Methods:
This randomized, double blind, parallel design, clinical trial was conducted at B.P. Koirala Institute of Health Sciences, Nepal. Thirty-four patients with clinically diagnosed OSF were randomized into two groups (17 participants in each) with baseline treatment of intralesional dexamethasone with hyaluronidase for 6 weeks for the both. Curcumin (2gm/day) was provided to Group A (Test) and Group B (Control) received placebo. Interincisal mouth opening, tongue protrusion, cheek flexibility and visual analogue scale (VAS) scoring of burning sensation of oral mucosa was recorded at baseline, 6, 8 and 12 weeks follow-up and independent t-test was used to compare the improvements in two groups.
Results:
On comparing the 6 weeks and baseline values, in Group A and B the mean difference in mouth opening was 8.82±1.33 mm and 5.53±1.17 mm respectively (p<0.001), in cheek flexibility was 2.94±1.02 mm and 1.94±1.24 mm respectively (p=0.02) and in tongue protrusion was 6.23±1.48 and 3.65±1.37 mm respectively (p<0.001). The findings were consistent in the 8 weeks follow-up. In 12 weeks follow-up, on comparing with the baseline values, in Group A and B, the mean difference in mouth opening was 8.71±1.16 mm and 5.35±1.22 mm respectively (<0.001), ), in cheek flexibility was 2.81±1.01 mm and 1.76±1.35 mm respectively (p=0.02) and in tongue protrusion was 6.06±1.48 and 3.35±1.50 mm respectively (p<0.001). Both the arms showed 100% improvement in burning sensation in 6, 8 and 12 weeks follow-up.
Conclusion:
Curcumin in combination with intralesional dexamethasone with hyaluronidase is efficacious in the treatment of OSF.
Key Words: Curcumin, efficacy, oral submucous fibrosis (OSF), randomized controlled trial, treatment
Introduction
Oral submucous fibrosis (OSF) is a chronic, slowly progressive disease that has been found to be associated with an increased risk of malignant transformation (Rimal and Shrestha, 2015). It is characterized by fibroelastic change in the lamina propria of the oral mucosa, gradually involving the submucosa and muscles of the oral cavity over the course of the disease (Hazarey et al., 2015). The disease then progresses to involve the oropharynx and the upper third of the oesophagus as well (Arakeri and Brennan, 2013; Dua, 2017). The etiology and progression of the disease has been linked mainly with the habit of chewing areca nut, the fourth most addictive substance globally (Hazarey et al., 2015; Rimal et al., 2019; Angadi and Rao, 2011). In a recent study conducted by Tariq et al., (2020), majority of OSF patients consumed betel nut every day, average consumption being six packets per day for nine years (Tariq et al., 2020). Betel nut chewing is carried out due to the lack of awareness on oral cancer and oral potentially malignant disorders (Somathunga et al., 2021).
Clinical presentation of OSF depends on the stage of the disease. Initially, most patients present with a burning sensation with or without vesicles in the mouth. Gradually, fibrosis along with blanching of the oral mucosa and around the pterygomandibular raphe leads to trismus, fibrosis of the eustachian tube may lead to deafness and may involve oesophagus developing dysphagia to solids (Angadi and Rao, 2011; Aziz 1997). Blanched opaque oral mucosa with palpable fibrous bands are the pathognomonic signs of OSF. The overlying epithelium may progress into dysplasia and malignancy in the long term (Angadi and Rao, 2011; Asifali et al., 2014). Till date, though several drugs, surgical therapy, and physiotherapy have been tried, no standardized treatment has been designed to treat OSF (Hazarey et al., 2015; Dayanarayan et al., 2014).
Curcumin (diferuloylmethane) is a polyphenol compound isolated from ground rhizomes of the plant Curcuma longa belonging to family Zingiberaceae (Saran et al., 2018). Various pharmacological actions of curcumin have been studied by various researchers worldwide (Chanani-wu, 2003). It is known to possess anti-inflammatory properties. It has the ability to suppress acute and chronic inflammation by lowering histamine levels and by possibly increasing the production of natural cortisone by adrenal glands (Alok et al., 2015). Moreover, it has been advocated to inhibit lipo-oxygenase and cyclo-oxygenase and also attenuate inflammatory response of Tumor Necrosis Factor-α stimulated human endothelial cells by interfering with nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) (Yahfoufi et al., 2018). Furthermore, curcumin is also capable of preventing platelet derived growth factor and shows scavenging actions against various reactive oxygen species produced by macrophages (Alok et al., 2015; Sandur et al., 2007).
Curcumin has been found to possess anti-cancer properties (Sood and Nagpal, 2013). Its anti-cancer activity can be explained by its ability to lower the activity of smokeless tobacco extractor nicotine derived nitrosamine ketone induced NFκB and cyclooxygenase (COX-2) in oral premalignant and cancer cells (Das et al., 2012; Liu et al., 2021). Apart from it, the downregulation of growth factors, protein kinases, oncogenic molecules and various signaling pathways also comes under the mechanism of action of curcumin. (Zoi et al., 2021). Investigations have shown specific inhibitory effect of COX- 2 by dietary curcumin in human colon cancer cells and human breast carcinoma cells. Because of its ability to control carcinogenesis without causing adverse effects, curcumin has also been used effectively as a chemosensitizer agent in combination with chemotherapeutic drugs (Khameneh et al., 2018). It has also been found to be useful in minimizing the side effects of chemotherapeutic agents when used in combination with them (Sarkhosh et al., 2021). Recently, PAC (3,5-Bis(4-hydroxyl-3-methoxybenzylidine)-N-methyl-4- piperidone), a new analogue of curcumin has been found to have preferential killing ability, i.e., ability to act on cancer cells without causing cytotoxic effects to normal cells (Semlali et al., 2021). Curcumin also exhibits fibrinolytic property due to its ability to inhibit lipid peroxidation and check cellular proliferation, which results in the reduction in the rate of collagen synthesis (Das et al., 2012). It has therefore been studied for treatment of OSF by some researchers to observe its efficacy in the treatment of OSF.
Dexamethasone is the widely used steroid because of its ability to inhibit the production of inflammatory factors and increased apoptosis of inflammatory cells providing relief from burning sensation. It also inhibits the proliferation of fibroblasts while causing up-regulation of collagenase synthesis and down-regulation of collagen production (Dayanarayan et al., 2014). Hyaluronidase is often used as an adjunct to dexamethasone treatment. It acts by breaking down hyaluronic acid thereby lowering the viscosity of the intercellular cement substances and also decreases collagen formation (Rajendran et al., 1994; Veedu et al., 2015).
Thus, this randomized controlled trial was designed to assess the efficacy of curcumin in combination with intralesional dexamethasone with hyaluronidase in the treatment of OSF. The null hypothesis being curcumin in combination with intralesional dexamethasone with hyaluronidase is not efficacious in the treatment of OSF.
Materials and Methods
This was a randomized, double-blind, 12-weeks parallel-design clinical trial that included 34 patients diagnosed clinically with OSF with patient enrollment starting from May, 2019. Patients were recruited from the Department of Oral Medicine and Radiology, College of Dental Surgery, B.P. Koirala Institute of Health Sciences (BPKIHS), Dharan, Nepal.
Ethical consideration and trial registration
The study was approved by the Institutional Review Committee of BPKIHS (IRC/1306/018) and registered in www.ctri.nic.in (CTRI/2019/05/019451). The study followed the standard ethical principles and was conducted in compliance with the Helsinki Declaration 1964 and its later amendments. Informed consent was received from all participants in the written form.
Patient’s selection and sample size calculation
Patients aged 18 years and above falling under any grade from Grade 1 to 3 of OSF as per the grading system provided by Kerr et al., (2011) were included in the trial which are listed below (Kerr et al., 2011).
Grade 1: Mild: Any features of the disease triad for OSF (burning, depapillation, blanching or leathery mucosa) may be reported and inter-incisal opening> 35mm
Grade 2: Moderate: Above features of OSF + interincisal opening 20-35mm
Grade 3- Severe: Above features of OSF + interincisal opening< 20mm
The exclusion criteria included patients with debilitating diseases such as carcinoma, patients contraindicated for steroids, patients with other mucosal disorders with clinical features same as OSF (such as: trismus due to Temporomandibular Joint Dysfunction, pericoronitis, space infection, history of surgery or trauma, systemic sclerosis, amyloidosis, iron deficiency anemia, radiation therapy), patients who were under treatment or have had already undergone treatment for OSF and the patients with history of hypersensitivity to hyaluronidase. Demographic parameters including age, gender and occupation were recorded. Patient’s chief complains, habit of areca nut consumption and type of areca nut product (areca nut, betel quid with tobacco, betel quid without tobacco, commercial products) were recorded along with other deleterious habits like cigarette, bidi and alcohol.
In reference to the previous study conducted by Yadav et al., (2014), considering improvement in the VAS score for burning sensation, at 80% power and 95% confidence interval, taking 10% non-response rate, the sample size was calculated as 34 with 17 patients in each arm (Yadav et al., 2014).
Randomization, clinical assessment, intervention and blinding
The eligible patients were randomized into two groups based on computer generated block randomization. Allocation concealment was accomplished by sealed envelopes holding the randomization number created by a non-involved member and intervention assigned to patients by another person not involved in the research. Before the commencement of intervention and during each follow-up visit, thorough intraoral examination was done and parameters measured were measurement of interincisal mouth opening between central incisors tip to tip of the same side with the help of vernier calliper, measurement of cheek flexibility, measurement of tongue protrusion (Reddy et al., 2011; Patil et al., 2015). Visual Analogue Scale (VAS) Scoring of burning sensation of oral mucosa was recorded. Both the groups received baseline treatment of intralesional injection dexamethasone 4mg/ml, 2ml twice a week for first 4 visits and intralesional injection dexamethasone (Dexona® manufactured by Zydus Healthcare Ltd, India) 4mg/ml, 2ml with hyaluronidase (Hynidase® manufactured by Shreya Life Sciences Pvt Ltd, India) 1500 IU twice a week for next 8 visits. One of the groups was provided with commercially available Curcumin extract (Capsule Adcumin® manufactured by Rishi Herbal Products, Haryana, India), 2 gm daily in 4 divided dosage for 6 weeks. Another group was provided with placebo capsules having similar appearance to the Curcumin capsules, 1 capsule four times daily for 6 weeks. Drug provided to both the groups were dispensed in similar opaque, sealed bottles with coded labels. The investigator, patients and the statistician were masked about the drug they were receiving. The physiotherapy was indicated for both the groups by mouth exercise device in the form of acrylic screw for 12 weeks. The patients were advised to exercise for 10 minutes each side with mouth exercise device, three times daily (Hazarey et al., 2015).
Statistical analysis
The data collected was entered in Microsoft excel sheet. It was then transferred into SPSS version 11.5 (Statistical Package for Social Sciences) for statistical analysis. Coding was assigned to enter the data. Weekly monitoring of the data was done. The independent variables were age, gender and other demographic variables and the dependent variables were interincisal mouth opening, tongue protrusion, cheek flexibility and VAS score of burning sensation. Mean, standard deviation and percentage were used for descriptive statistics. Chi-square test or Fischer’s exact test was used to compare the distribution of patients in the test and control group according to demographic features and clinical presentations. Independent t test was applied to compare the mean of interincisal mouth opening, cheek flexibility and tongue protrusion at baseline and mean of difference in interincisal mouth opening, tongue protrusion and cheek flexibility at baseline to 6 weeks, 8 weeks and 12 weeks follow-up in test and control group.
Results
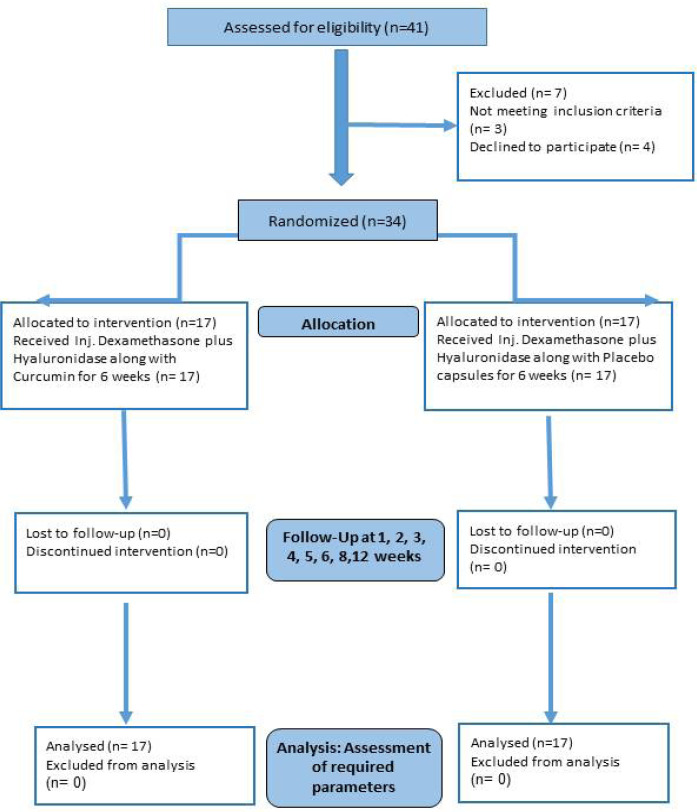
Of all the 41 patients assessed for eligibility, three didn’t meet the inclusion criteria (OSF with carcinoma) and four declined to participate in the study. Thirty-four patients were randomized into two groups, Group A (Test) receiving Curcumin and Group B (Control) receiving Placebo. (Figure 1)
Figure 1.
CONSORT Participant Flow Diagram
The distribution of patients according to demographic features and clinical presentations is depicted in Table 1. The mean interincisal mouth opening in Group A at the time of first presentation was 25.47±4.50 mm and in Group B was 25.35±5.7 mm. The difference was not statistically significant. (p=0.07). The mean baseline cheek flexibility in Group A and B was 12.76±2.80 mm and 13.47±2.88 mm respectively, the difference was not found statistically significant (p=0.50). The mean baseline tongue protrusion in Group A was 14.88±3.22 mm and in Group B was 14.47±2.48 mm, the difference was not statistically significant (p=0.34) (Table 2).
Table 1.
Distribution of Patients According to Demographic Features and Clinical Presentations
| Group | Group A (N) | Group B (N) | p-value | |
|---|---|---|---|---|
| Age (Mean± Standard Deviation) | 36.06± 12.56 | 34.53± 13.29 | 0.73*** | |
| Sex | Male | 13 | 11 | 0.45** |
| Female | 4 | 6 | ||
| Occupation | Student | 5 | 3 | 0.67* |
| Business | 5 | 6 | ||
| Farmer | 3 | 5 | ||
| Housewife | 2 | 3 | ||
| Service | 2 | 0 | ||
| Chief complain | Burning sensation | 10 | 7 | 0.08* |
| Difficulty in mouth opening | 7 | 5 | ||
| Both | 0 | 5 | ||
| Habit of areca nut consumption | Areca nut | 1 | 0 | 0.56* |
| Betel quid without tobacco | 2 | 1 | ||
| Betel quid with tobacco | 2 | 0 | ||
| Commercial product | 6 | 7 | ||
| Two or more | 6 | 9 | ||
| Deleterious habits | Cigarette | 2 | 7 | 0.11* |
| Bidi | 2 | 0 | ||
| Alcohol | 2 | 0 | ||
| Cigarette and alcohol | 1 | 2 | ||
| None | 10 | 8 | ||
| Clinical grading of OSF | Grade 1 | 1 | 0 | 0.60* |
| Grade 2 | 15 | 14 | ||
| Grade 3 | 1 | 3 |
***Independent t test, p<0.05: statistically significant; **Chi-square test, p<0.05: statistically significant; *Fischer's exact test, p<0.05: statistically significant
Table 2.
Comparison of Baseline Variables of Group A and B
| Outcome | Group A | Group B | p* | 95% Confidence interval | ||
|---|---|---|---|---|---|---|
| N | Mean ± SD (mm) | N | Mean ± SD (mm) | |||
| IO | 17 | 25.47± 4.50 | 17 | 25.35± 5.80 | 0.95 | -3.51, 3.74 |
| CF | 17 | 12.76± 2.80 | 17 | 13.47± 2.88 | 0.47 | 2.70 , 1.28 |
| TP | 17 | 14.88± 3.22 | 17 | 14.47± 2.48 | 0.68 | -1.60-2.42 |
IO, interincisal mouth opening; CF, cheek flexibility; TP, tongue protrusion; *Independent t test, p<0.05: statistically significant
The mean improvement in interincisal mouth opening at the time of presentation and at the time of completion of intervention i.e., 6 weeks was 8.82±1.33 mm in Group A and 5.53±1.17 mm in Group B, the difference being statistically significant (p<0.001). The mean interincisal mouth opening in 8 weeks follow-up was same as 6 weeks in both the groups. The mean improvement in interincisal mouth opening at first visit and 12 weeks follow-up was 8.71±1.26 mm in Group A and 5.29±1.31 mm in Group B. This difference was statistically significant (p<0.001)(Table 3).
Table 3.
Comparison of Mean Improvement in Interincisal Opening, Cheek Flexibility and Tongue Protrusion between Baseline and Follow-up
| Outcome | Comparison | Group A | Group B | p* | Mean difference ( 95% Confidence interval) |
||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD (mm) | n | Mean ± SD (mm) | ||||
| IO | Baseline vs 6 weeks | 17 | 8.82 ± 1.33 | 17 | 5.53 ± 1.18 | <0.001 | 3.29 (2.41, 4.17) |
| Baseline vs 8 weeks | 17 | 8.82 ± 1.33 | 17 | 5.53 ± 1.18 | <0.001 | 2.29 (2.41, 4.17) | |
| Baseline vs 12 weeks | 17 | 8.71 ± 1.16 | 17 | 5.35 ± 1.22 | <0.001 | 3.35 (2.48, 4.22) | |
| CF | Baseline vs 6 weeks | 17 | 2.94 ± 1.03 | 17 | 1.94 ± 1.25 | 0.02 | 1.00 (0.20, 1.80) |
| Baseline vs 8 weeks | 17 | 2.94 ± 1.03 | 17 | 1.94 ± 1.25 | 0.02 | 1.00 (0.20, 1.80) | |
| Baseline vs 12 weeks | 17 | 2.82 ± 1.01 | 17 | 1.76 ± 1.35 | 0.02 | 1.06 (0.22, 1.89) | |
| TP | Baseline vs 6 weeks | 17 | 6.24 ± 1.48 | 17 | 3.65 ± 1.37 | <0.001 | 2.60 (1.59, 3.58) |
| Baseline vs 8 weeks | 17 | 6.24 ± 1.48 | 17 | 3.65 ± 1.37 | <0.001 | 2.60 (1.59, 3.58) | |
| Baseline vs 12 weeks | 17 | 6.06 ± 1.48 | 17 | 3.35 ± 1.50 | <0.001 | 2.71 (1.67, 3.75) | |
IO, interincisal mouth opening; CF, cheek flexibility; TP, tongue protrusion; *Independent t test, p<0.05: statistically significant
The mean improvement in cheek flexibility at the time of presentation and at the time of completion of intervention i.e., 6 weeks was 2.94±1.03 mm in Group A and 1.94±1.25 mm in Group B, the difference being statistically significant (p=0.02). The mean cheek flexibility in 8 weeks follow-up was same as 6 weeks in both the groups. The mean improvement in cheek flexibility at first visit and 12 weeks follow-up was 2.82±1.01 mm in Group A and 1.76±1.35 mm in Group B. This difference was statistically significant (p=0.02)(Table 3).
The mean improvement in tongue protrusion at the time of presentation and at the time of completion of intervention i.e., 6 weeks was 6.23±1.48 mm in Group A and 3.64±1.37 mm in Group B, the difference being statistically significant (p<0.001). The mean tongue protrusion in 8 weeks follow-up was same as 6 weeks in both the groups. The mean improvement in tongue protrusion at first visit and 12 weeks follow-up was 6.06±1.48 mm in Group A and 3.34±1.50 mm in Group B. This difference was statistically significant (p<0.001) (Table 3).
All the patients in Group A and B had baseline VAS score greater than or equal to 7. There was 100% reduction in VAS score at the end of intervention in both the groups. The VAS score of zero remained constant in subsequent follow-up in 8 and 12 weeks in both the groups.
All the patients in the study tolerated the treatment regimens well. None of the patients reported any allergic or abnormal reaction, rashes or gastrointestinal disturbances.
Discussion
Curcumin has been found to bring improvement even at the later stages of the disease by many studies, while steroids with hyaluronidase have been found to be helpful mostly for symptomatic relief (Hazarey et al., 2015; Yadav et al., 2014). Our study is an attempt to combine the effects of all these agents and observe the change in the quality of life of patients thereby. Our patients had also been advised to carry out mouth opening exercise in order to modify tissue remodeling through physical movements (Hazarey et al., 2015; Kerr et al., 2011).
Though the etiopathogenesis of this disease is yet poorly understood, most studies have pointed towards a masticatory substance, areca nut as a causative agent (Reddy et al., 2011; Patil et al., 2015). Chewing areca nut with or without tobacco is a common habit, mostly in Asian countries (Rimal et al., 2019; Kerr et al., 2011; Kopuri et al., 2016). All patients in our study also gave a positive history of areca nut chewing in the raw form as a quid or in a commercial preparation such as gutkha or pan masala.
The mean age group of patients in our study was in 30s and there was male predominance (75%). These findings are similar to those reported by Piyush (2019) (Piyush et al., 2019). Increased incidence of this disease in young males can be attributed to their increased tendency of substance abuse including areca nut as a form of recreation (Yadav et al., 2014).
In our study, 100% improvement was seen in burning sensation in both the test and control group at the end of intervention. No relapse of burning sensation was seen in 8 weeks and 12 weeks follow-up. Similar complete resolution of burning sensation was noted in studies conducted by Yadav et al., (2014), Saran et al., (2018) and Srivastava et al., (2021) on administration of curcumin (Yadav et al., 2014; Saran et al., 2018; Srivastava et al., 2021).
Curcumin improves burning sensation by its anti-inflammatory action as it is known to inhibit the production of inflammatory products such as prostaglandins and leukotrienes by blocking both cycloxygenase and lipoxygenase pathways of inflammation (Das et al., 2012). Curcumin has scavenging effect on superoxide radicals, hydroxyl radicals and lipid peroxidation (Hazarey et al., 2015). The improvement in burning sensation in both the groups in our study can also be attributed to the baseline treatment with dexamethasone and hyaluronidase. Dexamethasone exerts anti- inflammatory activity and hyaluronidase has the ability to breakdown abnormal collagen. Because of this property, dexamethasone is considered to decrease burning sensation inhibiting the inflammatory process and hyaluronidase is attributed to play more important role in the improvement in pain sensation during mouth opening (Dayanarayana et al., 2014; Veedu et al., 2015).
In our study, mean interincisal improvement in the curcumin group (Group A) was high compared to the control group (Group B) after the completion of intervention. This result is consistent with that of Yadav et al., (2014), Hazarey et al., (2015), Kopuri et al., (2016), and Piyush et al., (2019) (Yadav et al., 2014; Hazarey et al., 2015; Kopuri et al., 2016; Piyush et al., 2019). Similarly, Saran et al., (2018) showed improvement in mouth opening with the use of curcumin along with piperine (Saran et al., 2018). Also, in the study by Das et al., (2012), patients receiving curcumin capsules and those receiving turmeric oil showed a statistically significant increase in mouth opening (Das et al., 2012). However, Agrawal et al., (2017), showed no significant improvement in mouth opening with low dose of curcumin (Agrawal et al., 2017). The mean improvement in tongue protrusion at first visit and 6 weeks follow-up was higher in Group A compared to that in Group B. These findings are consistent with those of Yadav et al., (2014), Piyush et al., (2019) and Srivastava et al., (2021) (Yadav et al., 2014; Piyush et al., 2019; Srivastava et al., 2021). However, in the study by Das et al., (2012), patients receiving turmeric oil showed better increase in tongue protrusion when compared to those receiving curcumin tablets (Das et al., 2012). The mean improvement in cheek flexibility at the time of presentation and at the time of completion of intervention was more in Group A compared to Group B. Piyush et al., (2019) have also shown an improvement in cheek flexibility with the use of curcumin (Piyush et al., 2019).
The increase in mouth opening, tongue protrusion and cheek flexibility brought about by the curcumin can be attributed to its anti-inflammatory, antioxidant and fibrinolytic properties (Chattopadhyay et al., 2004; Borra et al., 2014). Curcumin has also been used as a fibrinolytic agent in Chinese medicine (Das et al., 2012). The fibrinolytic action of curcumin is due to its ability to inhibit lipid peroxidation, check cellular proliferation and inhibit callogenesis (Kopuri et al., 2016). More improvement in all the parameters in our study can be attributed to the use of higher dosage of curcumin along with administration of dexamethasone and hyaluronidase twice a week. Similar was the outcome in the study by Hazarey et al., (2014) which may be due to the higher dosage of curcumin (Hazarey et al., 2014). Since curcumin has been proven to be non-toxic even at higher dosages, it can be administered safely for the treatment of OSF. All the patients in our study tolerated the treatment regimens well without any adverse reaction or signs of toxicity. This is in agreement with other studies that have mentioned curcumin as a safe substance even at higher dosages (Das et al., 2012; Kopuri et al., 2016).
The statistically significant improvement in all the parameters in our study support better efficacy of curcumin in combination with intralesional dexamethasone and hyaluronidase in the treatment of OSF than using it alone. Corticosteroids are capable of causing the suppression of the fibroproductive inflammation found in OSF lesions (Hazarey et al, 2015). They have an ability to upregulate collagenase synthesis and downregulate collagen production. They provide symptomatic relief during early stages of the disease; however, they were found to be less useful in reversing the fibrosis and restoring elasticity of the oral mucosa in the long term (Dayaynarayana et al., 2014). Hyaluronidase, on the other hand, improves mouth opening and relieves tightness by decreasing the viscosity of intercellular substance, inhibiting callogenesis as well as helping in collagenolysis (Veedu et al., 2015). It also facilitates the proper diffusion of injected fluids into the affected area. Thus, combined use of dexamethasone and hyaluronidase shows better efficacy than the use of either agent alone (Dayanarayana et al., 2014).
The variations in success rates with curcumin in different studies can be due to the variations in dosages and formulations of curcumin used in different studies. Also, it can be due to the heterogeneity in clinical staging and severity of OSF in different patients.
In conclusion, this study showed that curcumin in combination with intralesional dexamethasone with hyaluronidase is efficacious in the treatment of OSF in terms of improvement in interincisal mouth opening, cheek flexibility and tongue protrusion. However, due to the small sample size and shorter duration of the study, larger studies with bigger sample size and longer duration may be needed for the generalization of the result. Most of our patients were in clinical stage Grade 2 during the study and thus the effect of drugs on all stages could not be tested upon equally.
Author Contribution Statement
The authors confirm contribution to the paper as follows: Study concept and design: Sagar Adhikari and Jyotsna Rimal; study conduction: Sagar Adhikari; resources: Sagar Adhikari; formal analysis: Sagar Adhikari and Ashish Shrestha; draft writing, review and editing: Sagar Adhikari, Jyotsna Rimal, Iccha Kumar Maharjan and Ashish Shrestha; and funding acquisition: Sagar Adhikari.
Acknowledgements
The authors would like to acknowledge Dr. Pragya Regmee, Assistant Professor, Department of Oral Medicine and Radiology and Dr. Santosh Kumari Agrawal, Assistant Professor, Department of Public Health Dentistry, BPKIHS for their intellectual support in designing of research, statistical analysis and manuscript writing.
Funding statement
This research was partially funded by Nepal Health Research Council, Nepal under the post-graduate thesis grant scheme.
Study Approval and Ethical Declaration
This was a post-graduate thesis research approved by the Institutional Review Committee of B.P. Koirala Institute of Health Sciences, Dharan, Nepal (IRC/1306/018)
Data Availability
The data is available through contact with the corresponding author.
Study Registration
This trial has been registered in www.ctri.nic.in (CTRI/2019/05/019451).
Conflict of Interest
The authors declare no potential conflict of interest.
References
- Agrawal A, Kaushal Y, Vaidya S, Shrivastava K. Medical management of oral submucous fibrosis. Int J Otorhinolaryngol Head Neck Surg. 2017;3:628–31. [Google Scholar]
- Alok A, Singh ID, Singh S, Kishore M, Jha PC. Curcumin – Pharmacological actions and its role in oral submucous fibrosis: A review. J Clin Diagnostic Res. 2015;9:ZE01–3. doi: 10.7860/JCDR/2015/13857.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: Revisited. Oral Maxillofac Surg. 2011;15:1–9. doi: 10.1007/s10006-010-0219-8. [DOI] [PubMed] [Google Scholar]
- Arakeri G, Brennan PA. Oral submucous fibrosis: An overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg. 2013;51:587–93. doi: 10.1016/j.bjoms.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Asifali S, Khan M, Khan S, Mehdi H, Sawani A. Frequency and clinical presentation of oral submucous fibrosis. Pak J Med Dent. 2014;3:48–53. [Google Scholar]
- Aziz S. Oral submucous fibrosis: an unusual disease. J N J Dent Assoc. 1997;18:17–9. [PubMed] [Google Scholar]
- Borra SK, Mahendra J, Gurumurthy P, et al. Effect of curcumin against oxidation of biomolecules by hydroxyl radicals. J Clin Diagnostic Res. 2014;8:1–5. doi: 10.7860/JCDR/2014/8517.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin. J Altern Complement Med. 2003;9:161–8. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- Das AD, Balan A, Sreelatha KT. Comparative study of the efficacy of curcumin and Turmeric Oil as chemopreventive agents in oral submucous fibrosis: A Clinical and Histopathological Evaluation. J Indian Acad Oral Med Radiol. 2012;22:88–92. [Google Scholar]
- Dayanarayana U, Doggalli N, Patil K, Shankar J KPM, Sanjay S. Non surgical approaches in treatment of OSF. IOSR J Dent Med Sci. 2014;13:63–9. [Google Scholar]
- Dua A. Oral submucous fibrosis-A case report. Adv Cytol Pathol. 2017;2:21–3. [Google Scholar]
- Hazarey V, Sakrikar A, Ganvir S. Efficacy of curcumin in the treatment for oral submucous fibrosis - A randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145–52. doi: 10.4103/0973-029X.164524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, Warnakulasuriya S, Mighell A, et al. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 2011;17:42–57. doi: 10.1111/j.1601-0825.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- Khameneh ZR, Mohammadian M, Rasouli MA, et al. Effects of curcumin in combination with doxorubicin in human colorectal cancer cell line. Asian Pac J Cancer Biol. 2018;3:89–92. [Google Scholar]
- Kopuri RKC, Chakravarthy C, Sunder S, et al. A comparative study of the clinical efficacy of lycopene and curcumin in the treatment of oral submucous fibrosis using ultrasonography. J Int Oral Heal. 2016;8:687–91. [Google Scholar]
- Liu T, Long T, Li H. Curcumin suppresses the proliferation of oral squamous cell carcinoma through a specificity protein 1/nuclear factor κB dependent pathway. Exp Ther Med. 2021;21:1–8. doi: 10.3892/etm.2021.9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S, Sahu R, Sghaireen M, Kunsi S, Maheshwari S. Comparative study of the efficacy of lycopene and aloe vera in the treatment of oral submucous fibrosis. Int J Heal Allied Sci. 2015;4:13–7. [Google Scholar]
- Piyush P, Mahajan A, Singh K, Ghosh S, Gupta S. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis. 2019;25:73–9. doi: 10.1111/odi.12947. [DOI] [PubMed] [Google Scholar]
- Rajendran R. Oral submucous fibrosis: Etiology, pathogenesis, and future research. Bull World Health Organ. 1994;72:985–96. [PMC free article] [PubMed] [Google Scholar]
- Reddy V, Wanjari PV, Banda NR, Reddy P. Oral Submucous Fibrosis: Correlation of Clinical Grading to various habit factors. Int J Dent Clin. 2011;3:21–4. doi: 10.4103/jispcd.JISPCD_92_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimal J, Shrestha A. Validation of Nepalese Oral Health Impact Profile14 and assessment of its impact in patients with oral submucous fibrosis in Nepal. J Nepal Health Res Counc. 2015;13:43–9. [PubMed] [Google Scholar]
- Rimal J, Shrestha A, Maharjan IK, Shrestha S, Shah P. Risk assessment of smokeless tobacco among oral precancer and cancer patients in eastern developmental region of Nepal. Asian Pac J Cancer Prev. 2019;20:411–5. doi: 10.31557/APJCP.2019.20.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran G, Umapathy D, Misra N, et al. A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: A randomized clinical trial. Indian J Dent Res. 2018;29:303–12. doi: 10.4103/ijdr.IJDR_551_16. [DOI] [PubMed] [Google Scholar]
- Sarkhosh H, Mahmoudi R, Malekpour M, Ahmadi Z, Khiyavi AA. The effect of curcumin in combination chemotherapy with 5-FU on non-malignant fibroblast cells. Asian Pac J Cancer Care. 2019;4:7–10. [Google Scholar]
- Semlali A, Contant C, Al-Otaibi B, Al-Jammaz I, Chandad F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci Rep. 2021;11:1–5. doi: 10.1038/s41598-021-90754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somathunga ES, Dissanayaka DS, Ratnayake DD, Jayasinghe RD. Awareness of oral cancer and OPMDs among patients attending the University Dental Hospital, Peradeniya, Sri Lanka. Asian Pac J Cancer Care. 2021;6:47–51. [Google Scholar]
- Sood S, Nagpal M. Role of curcumin in systemic and oral health: An overview. J Nat Sci Biol Med. 2013;4:3–7. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Kundu A, Pradhan D, et al. A comparative study to evaluate the efficacy of curcumin lozenges (TurmNova®) and intralesional corticosteroids with hyaluronidase in management of oral submucous fibrosis. J Contemp Dent Pract. 2021;22:751–5. [PubMed] [Google Scholar]
- Tariq H, Ahmed S, Naz M, Naureen A. Frequency of oral sub mucous fibrosis and its correlation with the level of education in patients coming to a tertiary care hospital of Karachi from January 2018 to December 2018. Asian Pac J Cancer Prev. 2020;5:157–60. [Google Scholar]
- Veedu RA, Balan A, Sankar SP. A randomized double-blind, multiple-arm trial comparing the efficacy of submucosal injections of hyaluronidase, dexamethasone, and combination of dexamethasone and hyaluronidase in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:588–93. doi: 10.1016/j.oooo.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Yadav M, Aravinda K, Saxena VS, et al. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis - A randomized, open-label interventional study. J Oral Biol Craniofacial Res. 2014;4:169–73. doi: 10.1016/j.jobcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10:1–23. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoi V, Galani V, Lianos GD, et al. The role of curcumin in cancer treatment. Biomedicines. 2021;9:1086–105. doi: 10.3390/biomedicines9091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available through contact with the corresponding author.