Abstract
Background:
Multidrug resistance (MDR) is a major cause of unsuccessful cancer treatment in which drugs are not effective. Therefore, it is necessary to identify the critical mechanisms of the development of MDR and target those with novel compounds. Accordingly, the current study is the first to investigate the combination effect and molecular mechanism of nitazoxanide (NTZ) and oxaliplatin (OXP) on LS174T/OXP-resistant cells.
Methods:
The effect of NTZ on OXP cytotoxicity in LS174T and LS174T/OXP cell lines was evaluated by MTT assay. Changes in expression levels of MDR1, MRP1, CTNNB1, peptidylarginine deiminase (PAD)2, and PAD4 genes and proteins were evaluated by RT-qPCR and western blotting methods, respectively. Lastly, the apoptosis assay was performed by flow cytometer.
Results:
OXP resistant and sensitive cells were identified based on the IC50 values (11567 nM vs. 1745 nM, p<0.05 for 24 h treatment; and 5161 nM vs. 882 nM, p<0.05 for 48 h incubation). The combination of NTZ and OXP for 48 h led to a reduction in IC50 values in resistant cells (2154 nM, p<0.05). The effect of NTZ plus OXP significantly decreased the expression of MDR1 (p<0.001), MRP1 (p<0.05), and CTNNB1 (p<0.001), while PAD2 and PAD4 expression was significantly increased (p<0.001). This combination therapy enhanced the percentage of the sub-G1 population (apoptosed) compared to other groups.
Conclusion:
The results showed that NTZ leads to notable upregulation of PAD2 and PAD4, which can disrupt the Wnt/β-catenin signaling pathway and reverse the MDR by reducing MDR1 and MRP1 expression.
Key Words: Colorectal cancer, nitazoxanide, peptidylarginine deiminase, multidrug resistance, Wnt/β-catenin
Introduction
Colorectal cancer (CRC) is a highly prevalent malignancy categorized as a severely fatal disease (Jing et al., 2021). Chemotherapy is the best choice for CRC, but a phenomenon that scientists call multidrug resistance (MDR) leads to unsuccessful treatment (Hind et al., 1992; Chang et al., 2018). With MDR, cancer cells can become resistant to a wide range of anti-cancer drugs (Robey et al., 2018). One highly probable reason for MDR is the overexpression of adenosine triphosphate binding cassette transporters (ABC-transporters), among which MDR1 and MRP1 are of high importance. These pumps are located in the cell membrane and remove drug from cells (Wang et al., 2021).
The Wnt/β-catenin signaling pathway is involved in several processes such as cell proliferation and transcription regulation. The role of this pathway in the beginning and the development of CRC is well documented (Vaiopoulos et al., 2012; Rahmani et al., 2018). Excessive activity of the Wnt pathway causes β-catenin to accumulate inside the nucleus, later affecting downstream genes. Thus, it seems logical that CRC can be successfully treated by targeting the Wnt/β-catenin pathway (Drost et al., 2015; Ghosh et al., 2019). Nitazoxanide (NTZ), an antiprotozoal and FDA-approved drug, can interfere with the Wnt/β-catenin pathway and has positive effects on cancer therapy (Kahn, 2014; Yu et al., 2020).
One of the first-line drugs used to treat CRC is oxaliplatin (OXP). The function of this medication was applied by intercalation into strands of DNA and then the formation of adducts in GC-reach regions; this mechanism could disrupt replication and transcription processes; however, resistance to OXP remains the main cause of unsuccessful CRC therapy (Liu et al., 2019; Oliveira et al., 2021).
Peptidylarginine deiminases (PADs) are a cluster of enzymes, whose role is to add a citrulline group to target proteins through the deamination of arginine (Mondal and Thompson, 2019). Recently, PADs enzymes have received special attention because of their roles in different cancers. The family of PADs includes four members named from PAD1 to PAD4 (Li et al., 2019). PAD2 and PAD4 have been shown to play a key role in the initiation and progression of numerous cancers (Chang and Han, 2006).
For the first time ever, the present study evaluated the effects of NTZ on OXP resistance in LS174T/OXP cells and determined the IC50 values of LS174T/OXP cells after treatment with NTZ alone, OXP alone, and NTZ and OXP combined. Furthermore, the expression rates of MDR1, MRP1, CTNNB1, PAD2, and PAD4 genes and of MDR1, CTNNB1, and PAD2 at protein levels were evaluated. Finally, the apoptosis cell percentage was evaluated by flow cytometry.
Materials and Methods
Materials
OXP (O9512), thiazolyl blue tetrazolium blue (MTT) (M2128) powder, phosphate buffer saline (PBS) (SLBJ2117V) tablets, and NTZ (N0290-10MG) were obtained from Sigma-Aldrich (St. Louis, MO, USA). OXP and NTZ were dissolved in sterile deionized water and dimethylsulfoxide (DMSO), respectively. Dulbeccoʼs Modified Eagleʼs Medium (DMEM) (1791923) high glucose media, Trypsin/EDTA 0.25% (1726653), and fetal bovine serum (FBS) (42Q7363K) were obtained from Gibco (Maryland, USA). The primary antibodies against MDR1, PAD2, and CTNBB1 proteins and the secondary antibody that was conjugated with horseradish peroxidase (HRP) were purchased from Cell Signaling Technology (Beverly, Massachusetts, USA).
Cell cultures
The LS174T and LS174T/OXP CRC cells (gifted by Dr. Nasser Samadi at Tabriz University of Medical Sciences) were regularly cultured in DMEM-high glucose media enriched with 10% FBS at 37 °C, 5% CO2, and 95% humidity. In order for the cells to maintain their resistance to OXP, they were continuously cultured with increasing doses of OXP.
Assessment of cell viability using MTT assay
Approved drug resistance in LS174T/OXP cells
Cell viability was evaluated by MTT assay. To confirm that the LS174T/OXP cells were resistant, MTT assay was conducted on LS174T and LS174T/OXP cells treated with different concentrations of OXP (0.0-100 μM). Then, LS174T/OXP cells were selected based on enhanced IC50 value compared with LS174T cells (at least three-fold).
Cell viability assessment of NTZ treated cells
LS174T/OXP cells (104 cells/well) were seeded in 96-well plates in triplicate and then treated with different concentrations of NTZ (0.0-100 μM) for 24 and 48 h. To examine the effect of NTZ and OXP combined, resistant cells were pretreated with 40 μM of NTZ for 24 h and then incubated with different concentrations of OXP for 24 h and 48 h. The old media was replaced with fresh media containing 2 mg/ml MTT solution, and the cells were again incubated at 37 °C for 24 h. Afterward, the media was removed, and 200 μl DMSO plus 25 μl Sorenson glycine buffer was added. The mixture was gently shaken for 15 minutes, and the optical density (OD) of the wells was determined in 570 nm using a 96-well plate reader (Labsystems, Finland) (Kumar et al., 2018).
RNA isolation and real-time RT- PCR
To extract total RNA from treated cells, the TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was applied according to the manufacturer’s instructions. For cDNA synthesis from RNA, a cDNA synthesis kit (Takara, Japan) was used. RT-qPCR was performed using SYBR green (Prime Script RT Master Mix, Takara) and MIC PCR (BioMolecular System, Australia) with the following conditions: pre-denaturation at 95 °C for 35 s, followed by 40 cycles of denaturation at 95 °C for 5 s and extension at 62 °C for 1 min. Each sample was prepared in triplicate. Raw data was analyzed using the Pfaffl method, and the GAPDH gene was considered as an internal control (Akbarzadeh et al., 2017). The sequence of primers used is shown in Table 1.
Table 1.
The Sequence of the Primers Used
| Gene | Forwad primer | Revers primer |
|---|---|---|
| MDR1 | AGTCATCTGTGGTCTTTCC | AGTTCTTCTTCTTTGCTCCT |
| MRP1 | AGTTCTGCGGTGCTGTTGTG | TTCGCTGAGTTCCTGCGTAC |
| CTNNB1 | ATG GAA CCA GAC AGA AAAGCG | CAG GAT TGC CTT TAC CACTCA |
| PAD2 | GCACATTGATCCGTGTGACC | TGGAGGAACCTGTGGATTTC |
| PAD4 | GCTCTTCCTCACAGGCCATTG | TCTCGGTCACAGTTCACCAG |
| GAPDH | GGATTTGGTCGTATTGGG | GGAAGATGGTGATGGGATT |
Total protein extraction and Western blotting
Samples were prepared for Western blotting by first lysing the cells on ice for 40 minutes with RIPA buffer containing a protease inhibitor cocktail. Then, the quantity of each specimen was evaluated using the BCA method (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, the proteins were separated by SDS-PAGE and transferred to the activated polyvinylidene difluoride membranes, and 5% skim milk was added. Then, the primary antibodies (anti-MDR1, anti-PAD2, and anti-CTNNB1 proteins) were added to the membranes, and the samples were incubated and gently shaken for 2 h at 4 °C. Afterward, the membranes were incubated with the conjugated secondary antibody. Finally, HRP substrate was added to the membranes to make the protein bands visible. The protein bands were observed using a ChemiDoc MP System (Bio-Rad Laboratories Inc., Hercules, California, USA). GAPDH protein was considered as an internal control.
Apoptosis assay
Cell apoptosis was evaluated through flow cytometry based on previously-described PI staining guidelines (Riccardi and Nicoletti, 2006). Briefly, LS174T/OXP cells were seeded (105 cells/well) and treated with NTZ, OXP, and NTZ plus OXP for 24 h and 48 h in the incubator. Then, cells were harvested and washed twice with PBS. Next, PI lysis solution (1% sodium citrate, 0.1% Triton X-100, 40 μg/ml propidium iodide, and 0.5 mg/ml RNase A) was used to re-suspend the cells, and the cells were incubated for another 30 min at 37 °C. Finally, the cells were analyzed using a MACS Quant flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany).
Statistical analysis
All data was analyzed using SPSS (version 22) and Graph Pad Prism (version 7) software. The results were analyzed by student’s t-test or one-way ANOVA. A value of p <0.05 was considered as statistically significant.
Results
Cell viability
Cell viability of OXP treated cells
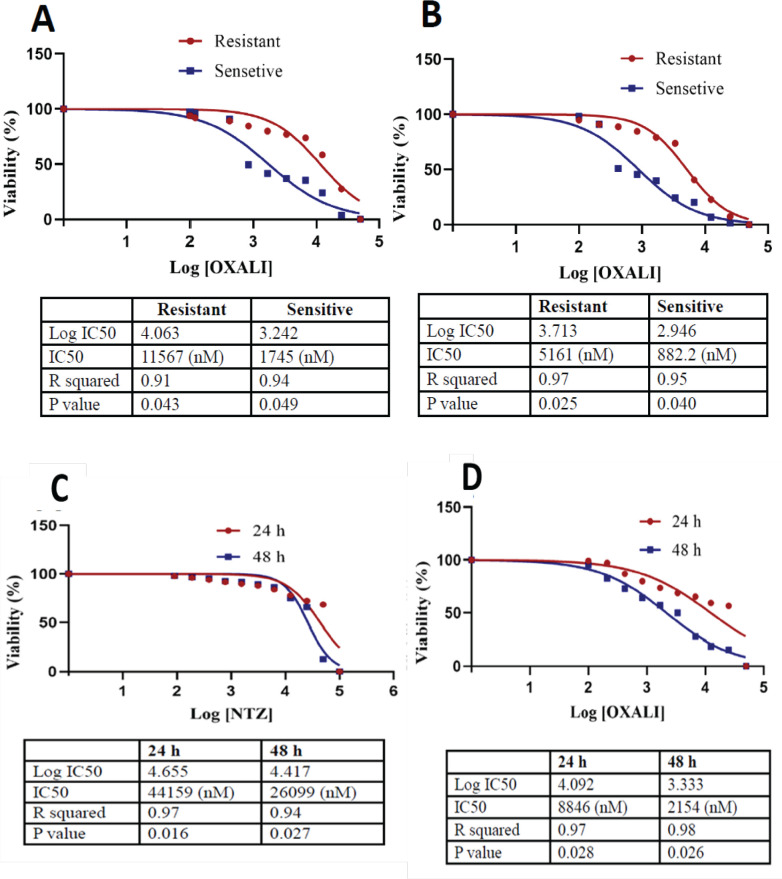
Evaluation of cell cytotoxicity after treatment with OXP during 24 h incubation showed that IC50 values increased from 1745 nM (p <0.05) in LS174T (sensitive) cells to 11567 nM (p <0.05) in LS174T/OXP (resistant) cells. After 48 h incubation, the IC50 values increased from 882 nM (p <0.05) in LS174T cells to 5161 nM (p <0.05) in LS174T/OXP cells (Figure 1A and B). OXP was observed to have more cytotoxic effects on LS174T cells than on LS174T/OXP cells.
Figure 1.
Characterization of LS174T (sensitive) and LS174T/OXP (resistant) Cells Under OXP (0.0-100 µM) Treatment. IC50 values for OXP in the sensitive and resistant cells after 24 (A) and 48 h (B) incubation. As well, treatment of LS174T/OXP (resistant) cells with different doses of NTZ (0.0-100 µM). the IC50 values for NTZ after 24 and 48 h (C). Also, treatment of LS174T/OXP cells with NTZ (40 µM) + OXP (different doses). IC50 values for each group after 24 h and 48 h incubation with OXP + NTZ (D) measured by MTT assay. OXP, oxaliplatin; NTZ, nitazoxanide
Decreased cell viability by NTZ
NTZ had the most toxic effect in concentrations higher than 44 μM (p <0.05) at 24 h and 26 μM (p <0.05) and 48 h incubation (Figure 1C). The effect of NTZ plus OXP combined showed that IC50 values were 8846 nM (p <0.05) at 24 h and 2154 nM (p <0.05) at 48 h incubation (Figure 1D).
Gene expression levels
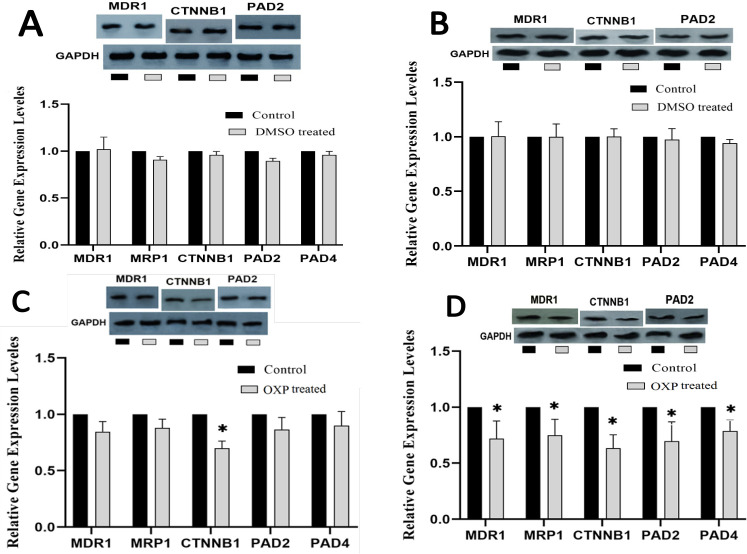
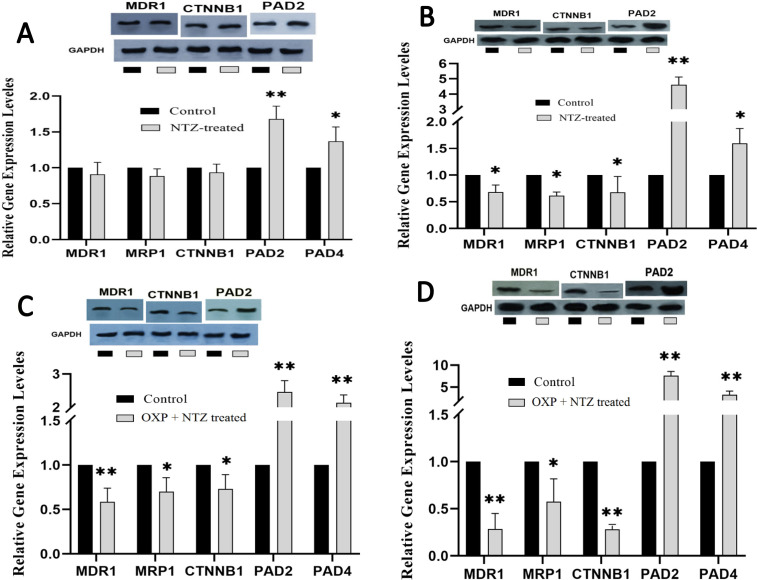
The results of MDR1, MRP1, CTNNB1, PAD2, and PAD4 expression levels achieved by RT-qPCR showed that after 24 h incubation, OXP led to a marked decrease in CTNNB1 (p <0.05) (Figure 2C), and 48 h incubation with OXP caused a significant decrease in all genes (p <0.05) (Figure 2D). Furthermore, NTZ-treated cells for 24 and 48 h showed significantly enhanced expression levels of PAD2 (p <0.001) and PAD4 (p <0.05) genes (Figure 3A and B). The NTZ plus OXP treatment led to significant changes; after 24 and 48 h incubation, MDR1, MRP1, and CTNNB1 were decreased, but PAD2 and PAD4 (p <0.5 and p <0.001, respectively) were increased (Figure 3C and D).
Figure 2.
Comparative Gene and Protein Expressions Profiling of MDR, MRP1 (210 kDa), CTNNB1, PAD2, and PAD4 in LS174T/OXP treated with DMSO 24 h (A), DMSO 48 h (B), OXP 24 h (C), and OXP 48 h (D) incubated. Concentration of NTZ, 40 µM; and OXP, 5 µM. MDR1(150 kDa), MRP1(210 kDa), PAD2 (101 kDa). Each point represents the mean ± S.D. * p < 0.05, ** p < 0.001 vs control
Figure 3.
Comparative Gene and Proteins Expression Profiling of MDR1, MRP1, CTNNB1, PAD2, and PAD4 in LS174T/OXP Treated with NTZ for 24 h (A), NTZ for 48 h (B), NTZ + OXP for 24 h (C), and NTZ + OXP for 48 h (D) Incubation. Concentration of NTZ, 40 µM; and OXP, 5 µM. MDR1(150 kDa), MRP1(210 kDa), PAD2 (101 kDa). Each point represents the mean ± S.D. * p < 0.05, ** p < 0.001 vs control
Western blot analysis
The effects of NTZ on the expression of MDR1 (P-gp protein), CTNNB1 (β-catenin protein), and PAD2 at the protein levels were assessed using the Western blotting test. The results showed that OXP treatment for 24 h decreased CTNNB1 significantly, and after 48 h, it led to a significant decrease in MDR1, CTNNB1, and PAD2 proteins (Figure 2C and D). After 24 and 48 h, treatment with NTZ led to a remarkable increase in PAD2 protein, and it reduced MDR1 and CTNNB1 proteins after 48 h (Figure 3A and B). Finally, co-treatment of LS174T/OXP cells with NTZ plus OXP for 24 h and 48 h caused a notable increase in PAD2 protein and a significant decrease in MDR1 and CTNNB1 proteins (Figure 3C and D).
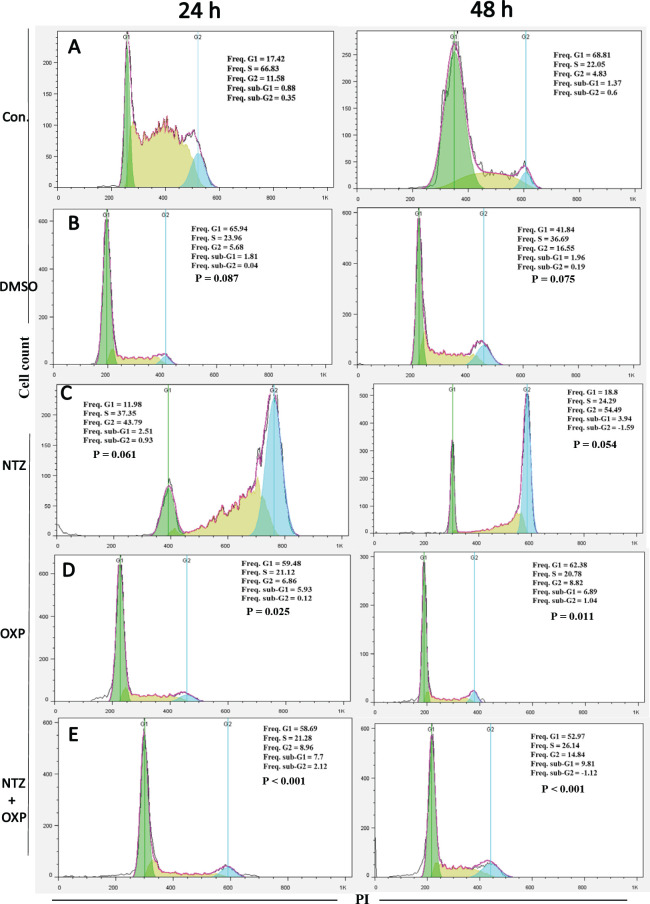
Combination of NTZ and OXP enhanced the level of cell apoptosis
The effects of NTZ combined with OXP on cell apoptosis was evaluated through PI staining flow cytometry analysis. The results showed that treatment with NTZ plus OXP for 24 h and 48 h had high sub-G1 population percentages in comparison with the other groups (Figure 4A and B). In the 24 h and 48 h treatment groups, the sup-G1 population percentage for NTZ plus OXP was remarkably higher than those of NTZ (Figure 4C) and OXP (Figure 4D) alone. Moreover, the percentage of sub-G1 population was significantly higher in the OXP-alone treatment than in the NTZ-alone treatment. However, no significant cellular apoptosis was observed in the NTZ-alone treatment compared to the control group (Figure 4E).
Figure 4.
Cell Cycle Distribution after Treated LS174T/OXP Cells by DMSO, NTZ, OXP, and Combination of NTZ Plus OXP for 24 and 48 h. Flow cytometry histograms indicating cell cycle distribution and apoptosis (sub-G1) after treated the resistant cells with NTZ (40 µM) and OXP (5 µM) or combination them. Data are reported as means ± SD of three independent assays
Discussion
CRC is one of the most aggressive malignancies among all types of cancers. Two major challenges, i.e. multidrug resistance and targeting key molecules in CRC treatment, still exist (Costea et al., 2020). Therefore, it seems necessary to identify and target these important molecules. Therefore, the current study evaluated for the first time the effects of NTZ on the reversal of MDR by targeting the Wnt/β-catenin signaling pathway.
The current results showed that the co-treatment of LS174T/OXP cells with NTZ plus OXP had a positive effect on the elevated cell sensitivity to OXP. Other results showed that the treatment of LS174T/OXP cells with NTZ plus OXP led to a significant increase in PAD2 and PAD4 but reduced MDR1, MRP1, and CTNNB1 at the gene and protein levels. These results can support the theory that by affecting the Wnt/β-catenin signaling pathway, NTZ leads to the reversal of drug resistance in LS174T/OXP cells. This idea was confirmed by the data obtained from flow cytometry analysis, which showed a high rate of cell apoptosis after combination therapy compared to NTZ and OXP treatments alone. Effectively, the Wnt/β-catenin signaling has different functional proteins that are activated as a cascade to up- or downregulate downstream genes to change cell functions. In line with our theory and the results, Yi Qu et al. showed in their high-quality study that NTZ can block Wnt/β-catenin signaling by targeting β-catenin and directing it to proteasome (Qu et al., 2018). Routinely in the Wnt signaling pathway, β-catenin as a key functional protein can enter the nucleus and change gene expression. The β-catenin protein can be phosphorylated by GSK3β and APC in Wnt signaling, leading to the degradation of β-catenin in the proteasome (Clevers and Nusse, 2012).
Although it has been well documented that NTZ plays a positive role against bacteria, viruses, and cancer, little information is available about the targets with which NTZ can interact directly in males (Di Santo and Ehrisman, 2014). However, chemoproteomic approaches have helped identify several candidate goals that can directly bind to NTZ, such as PADs, ENOA, CEP89, CALR, RNF115, and IKZF1. The NTZ effects are dependent upon a target protein; for example, ENOA protein is a c-Myc promoter binding protein and a transcriptional repressor. Thus, in cancer cells treated with NTZ, c-Myc is downregulated (Hsu et al., 2008; Senkowski et al., 2015). It will certainly be exciting to investigate the function of these NTZ direct binding proteins in various fields. Fresh and novel data has highlighted the role of NTZ in the treatment of cancer, but its exact mechanism is not yet clear (Müller et al., 2008; Fan-Minogue et al., 2013; Senkowski et al., 2015). The data further confirmed that in the pathogenesis of CRC, the Wnt signaling pathway is disturbed. Thus, targeting this pathway in CRC therapy is important. It has been demonstrated that NTZ can strongly inhibit the Wnt signaling pathway. In effect, by depleting the β-catenin protein, NTZ is useful to CRC and MDR therapy in which the CTNNB1 gene is overexpressed (Qu et al., 2018).
The solution to reducing the relationship between NTZ and β-catenin lies in the function of a protein known as PAD. NTZ has been reported to enhance the activity of PAD2, which leads to the depletion of β-catenin; however, the current study showed that NTZ not only leads to stabilizing, but also increases the gene and protein expression of PADs. It has been well described that PAD proteins are able to citrullinate target proteins (here, β-catenin) (Stadler et al., 2013; Deplus et al., 2014). Given the fact that citrulline groups have a negative charge and arginine residues have a positive charge on protein (Bang et al., 2007), extensive citrullination on the target protein disrupts intramolecular interactions and leads to protein dysfolding; eventually, these processes facilitate the breakdown of proteins in the proteasome (Vossenaar et al., 2003; Wu et al., 2015). Furthermore, GSK3β as another major protein in the Wnt/β-catenin pathway is a target to PAD4, but citrullination of GSK3β by PAD4 leads to its stabilization (Stadler et al., 2013). Normally, GSK3β by phosphorylation of β-catenin leads to the degradation of β-catenin and inhibits Wnt signaling. However, the stabilization of GSK3β by PAD4, which is a result of NTZ function, can be another lever of pressure to block the Wnt pathway in CRC treatment (Qu et al., 2018).
A vital issue that needs special attention is explaining the mechanism of NTZ for stabilizing PADs. It is known that the fate of a protein in the body is influenced by a set of factors and is not a one-factor process. However, according to the available information, it is attributed to the PADs’ allosteric effects. In effect, the binding site of NTZ with PADs is where calcium ions can bind. Moreover, the conformation of PAD proteins at calcium-binding sites is constantly transitioning between the Apo and Holo forms, which are important to their remaining stable or being degraded (Slade et al., 2015).
In conclusion, PAD2 and probably PAD4, through the citrullination of β-catenin and GSK3β, can block the Wnt/β-catenin signaling pathway. NTZ can be an agent for enhancing the stability of PADs in cells. However, the current study has illustrated that the expression of PAD2 and PAD4 at the gene level was increased. Given that CTNBB1 is an important gene in Wnt signaling, by blocking this gene expression and degrading β-catenin, NTZ can downregulate the main ABC-transporters, finally reverse the resistance of LS174T/OXP cells, and elevate the sensitivity of cells to OXP.
Author Contribution Statement
Dr. Hemmati-Dinarvand and Dr. Mostafazadeh performed experimental work and analysis and drafted the manuscript. Professor Seghatoleslam designed the study, designed the primer sets and provided supervision, edited the manuscript, and conceptualized the study. Ms. Kheirandish and Mr. Khodadadian assisted in the experimental design, and analysis and co-wrote the manuscript.
Acknowledgments
The authors gratefully acknowledge Department of Clinical Biochemistry, Faculty of Medicine, Shiraz University of Medical Sciences for financial suport. Furthermore, the authors gratefully acknowledge Department of Clinical Biochemistry, Faculty of Medicine, Tabriz University of Medical Sciences and Department of Clinical Biochemistry, Faculty of Medicine, Lorestan University of Medical Sciences for technical support.
Funding
This study was funded by Shiraz University of Medical sciences as PhD thesis of M. Hemmati-Dinarvand (Funding No: 99-01-01-22832).
Ethics approval
The investigation was approved by the Committee of Research Ethical at Shiraz University of Medical Sciences, Iran (Ethical code: IR.SUMS.REC.1399.1267).
Availability of Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Consent for publication
All authors have reviewed and approved the submitted version of the manuscript.
Conflicts of interest
The authors have no financial or other conflict of interests to declare.
References
- Akbarzadeh M, Rahbarghazi R, Nabat E, et al. The impact of different extracellular matrices on melatonin effect in proliferation and stemness properties of ovarian cancer cells. Biomed Pharmacother. 2017;87:288–95. doi: 10.1016/j.biopha.2016.12.119. [DOI] [PubMed] [Google Scholar]
- Bang H, Egerer K, Gauliard A, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007;56:2503–11. doi: 10.1002/art.22817. [DOI] [PubMed] [Google Scholar]
- Chang H, Yu X, Xiao WW, et al. Neoadjuvant chemoradiotherapy followed by surgery in patients with unresectable locally advanced colon cancer: a prospective observational study. Onco Targets Ther. 2018;11:409–18. doi: 10.2147/OTT.S150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol Carcinog. 2006;45:183–96. doi: 10.1002/mc.20169. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Costea T, Vlad OC, Miclea LC, et al. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplus R, Denis H, Putmans P, et al. Citrullination of DNMT3A by PADI4 regulates its stability and controls DNA methylation. Nucleic Acids Res. 2014;42:8285–96. doi: 10.1093/nar/gku522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo N, Ehrisman J. A functional perspective of nitazoxanide as a potential anticancer drug. Mutat Res. 2014;768:16–21. doi: 10.1016/j.mrfmmm.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Drost J, van Jaarsveld RH, Ponsioen B, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–7. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- Fan-Minogue H, Bodapati S, Solow-Cordero D, et al. A c-Myc activation sensor-based high-throughput drug screening identifies an antineoplastic effect of nitazoxanide. Mol Cancer Ther. 2013;12:1896–905. doi: 10.1158/1535-7163.MCT-12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N, Hossain U, Mandal A, et al. The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci U S A. 2019;1443:54–74. doi: 10.1111/nyas.14027. [DOI] [PubMed] [Google Scholar]
- Hind R, Rew DR, Johnson CD. Surgical excision alone is adequate treatment for primary colorectal cancer. Ann R Coll Surg Engl. 1992;74:63–7. [PMC free article] [PubMed] [Google Scholar]
- Hsu KW, Hsieh RH, Lee YH, et al. The activated Notch1 receptor cooperates with alpha-enolase and MBP-1 in modulating c-myc activity. Mol Cell Biol. 2008;28:4829–42. doi: 10.1128/MCB.00175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z, Xi Y, Yin J, et al. Biological roles of piRNAs in colorectal cancer. Gene. 2021;769:145063. doi: 10.1016/j.gene.2020.145063. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018:2018. doi: 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
- Li F, Miao L, Xue T, et al. Inhibiting PAD2 enhances the anti-tumor effect of docetaxel in tamoxifen-resistant breast cancer cells. J Exp Clin Cancer Res. 2019;38:414. doi: 10.1186/s13046-019-1404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang X, Du L, et al. Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Mol Cancer. 2019;18:43. doi: 10.1186/s12943-019-0981-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mondal S, Thompson PR. Protein arginine deiminases (PADs): Biochemistry and Chemical Biology of Protein Citrullination. Acc Chem Res. 2019;52:818–32. doi: 10.1021/acs.accounts.9b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Sidler D, Nachbur U, et al. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int J Cancer. 2008;123:1797–806. doi: 10.1002/ijc.23755. [DOI] [PubMed] [Google Scholar]
- Oliveira A, Zerillo L, Cruz LJ, et al. Maximizing the potency of oxaliplatin coated nanoparticles with folic acid for modulating tumor progression in colorectal cancer. Mater Sci Eng C Mater Biol Appl. 2021;120:111678. doi: 10.1016/j.msec.2020.111678. [DOI] [PubMed] [Google Scholar]
- Qu Y, Olsen JR, Yuan X, et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat Chem Biol. 2018;14:94–101. doi: 10.1038/nchembio.2510. [DOI] [PubMed] [Google Scholar]
- Rahmani F, Avan A, Hashemy SI, et al. Role of Wnt/β-catenin signaling regulatory microRNAs in the pathogenesis of colorectal cancer. J Cell Physiol. 2018;233:811–7. doi: 10.1002/jcp.25897. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Robey RW, Pluchino KM, Hall MD, et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–64. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski W, Zhang X, Olofsson MH, et al. Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol Cancer Ther. 2015;14:1504–16. doi: 10.1158/1535-7163.MCT-14-0792. [DOI] [PubMed] [Google Scholar]
- Slade DJ, Fang P, Dreyton CJ, et al. Protein arginine deiminase 2 binds calcium in an ordered fashion: implications for inhibitor design. ACS Chem Biol. 2015;10:1043–53. doi: 10.1021/cb500933j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler SC, Vincent CT, Fedorov VD, et al. Dysregulation of PAD4-mediated citrullination of nuclear GSK3β activates TGF-β signaling and induces epithelial-to-mesenchymal transition in breast cancer cells. Proc Natl Acad Sci U S A. 2013;110:11851–6. doi: 10.1073/pnas.1308362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiopoulos AG, Kostakis ID, Koutsilieris M, et al. Colorectal cancer stem cells. Stem Cells. 2012;30:363–71. doi: 10.1002/stem.1031. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, et al. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang Z, Wang K, et al. Metabolomics analysis of multidrug resistance in colorectal cancer cell and multidrug resistance reversal effect of verapamil. Biomed Chromatogr. 2021;35:e4976. doi: 10.1002/bmc.4976. [DOI] [PubMed] [Google Scholar]
- Wu HY, Chen SF, Hsieh JY, et al. Structural basis of antizyme-mediated regulation of polyamine homeostasis. Proc Natl Acad Sci U S A. 2015;112:11229–34. doi: 10.1073/pnas.1508187112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yang K, Zheng J, et al. Synergistic tumor inhibition of colon cancer cells by nitazoxanide and obeticholic acid, a farnesoid X receptor ligand. Cancer Gene Ther. 2020:2020. doi: 10.1038/s41417-020-00239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.